Development of Portable E-Nose System for Fast Diagnosis of Whitefly Infestation in Tomato Plant in Greenhouse
Abstract
1. Introduction
2. Methods and Materials
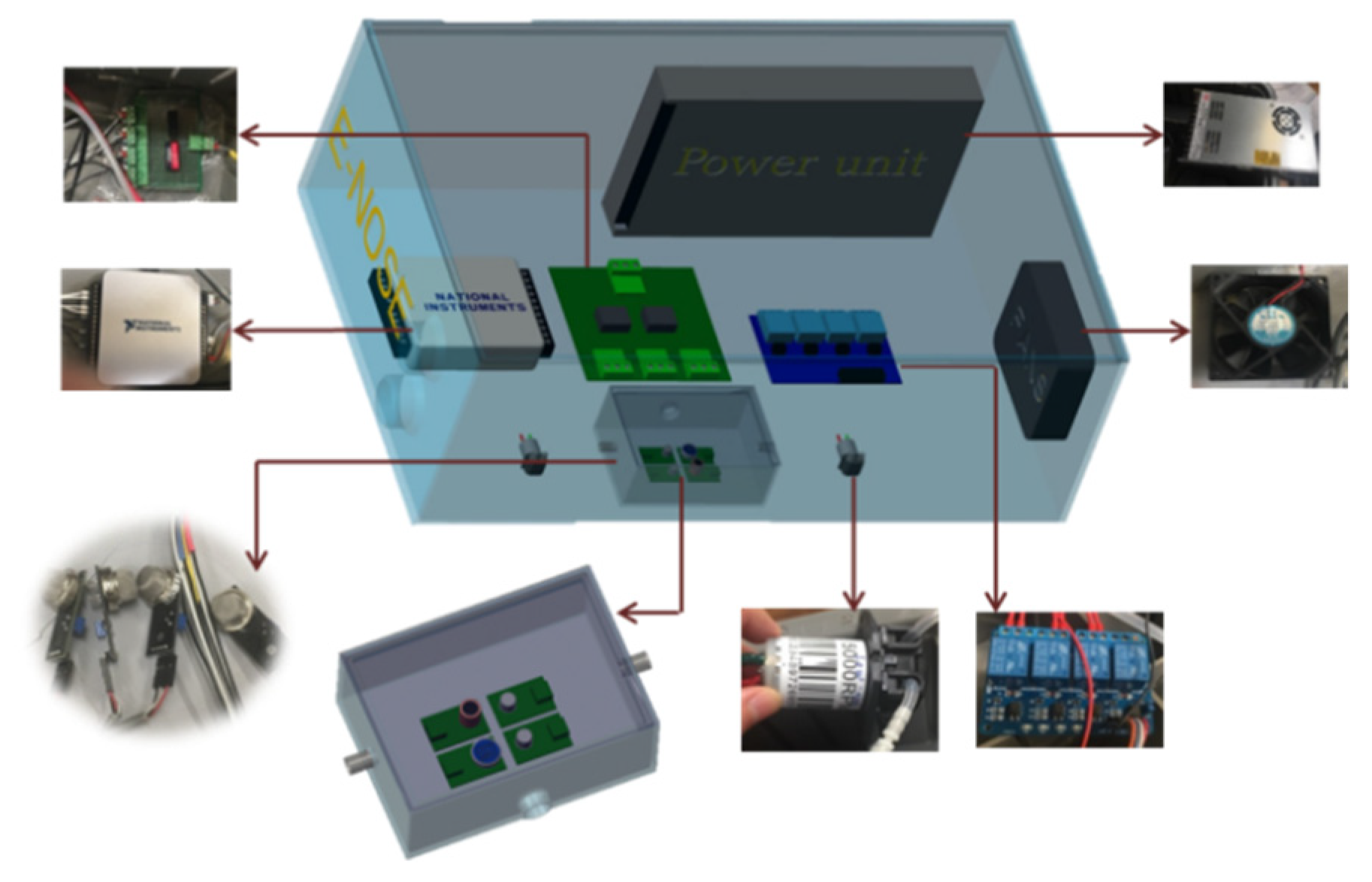
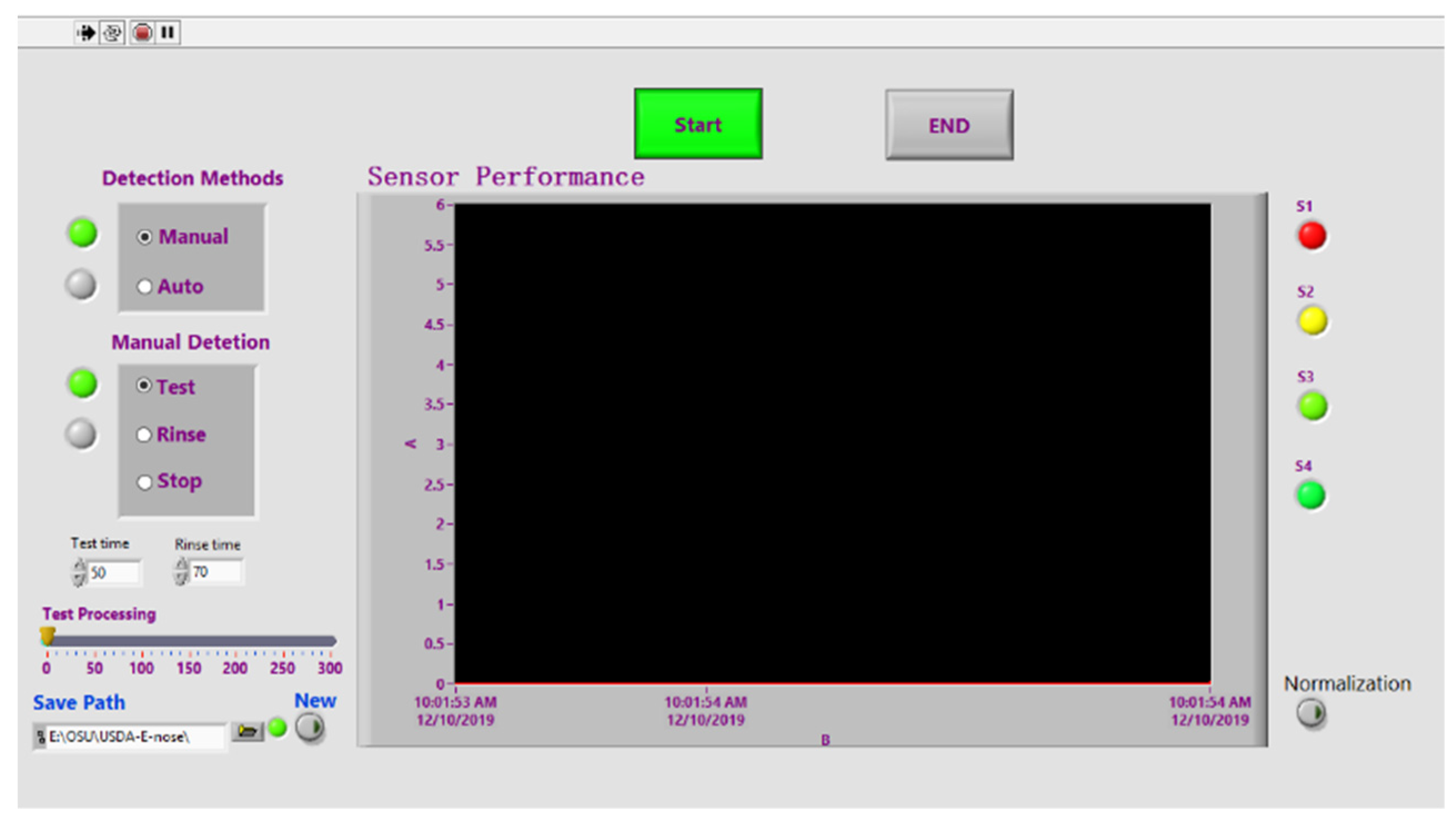
2.1. Fabrication of E-Nose System
2.2. Plant and Insects
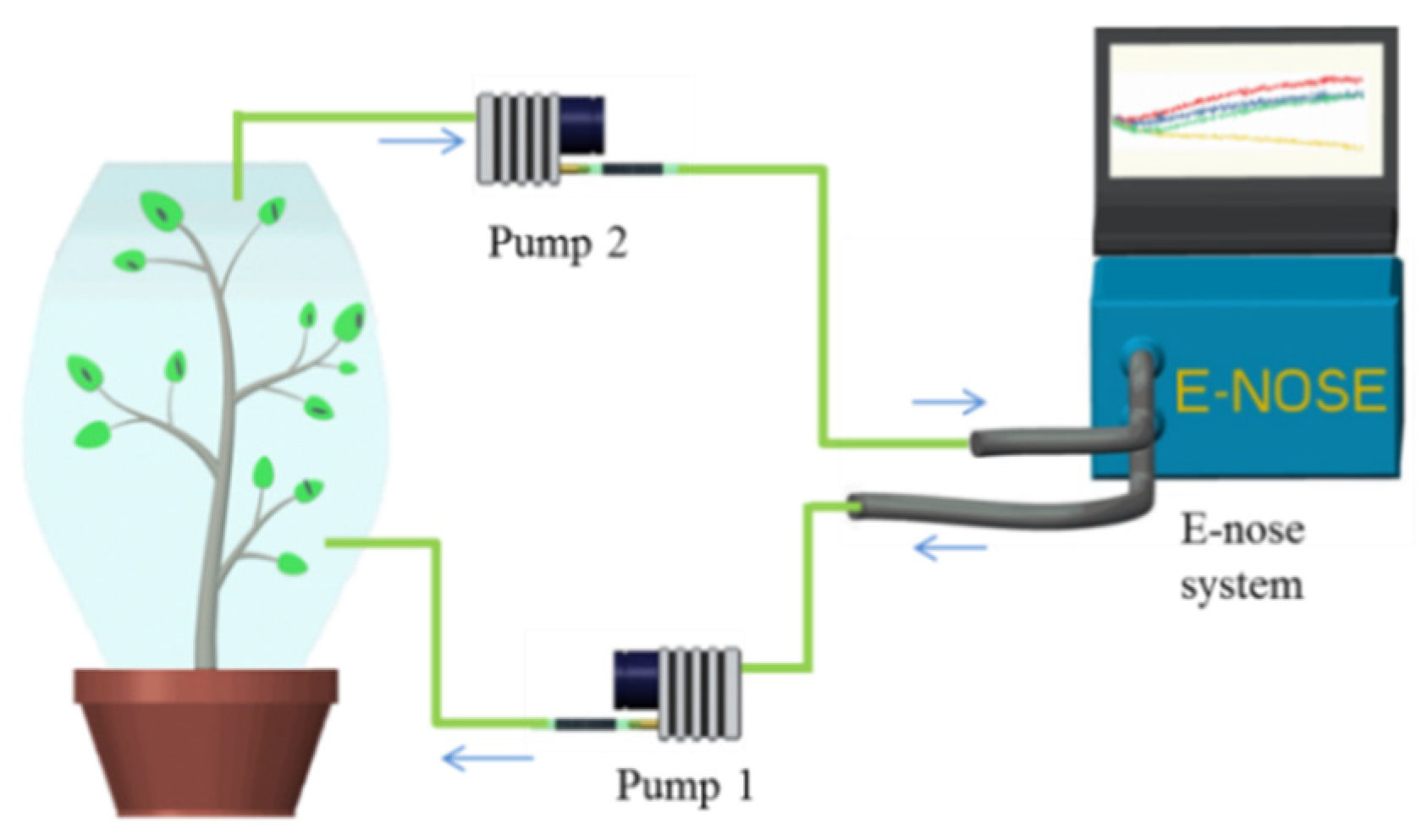
2.3. Experimental Design
2.4. GC-MS Tests
2.5. Data Analysis
3. Results and Discussion
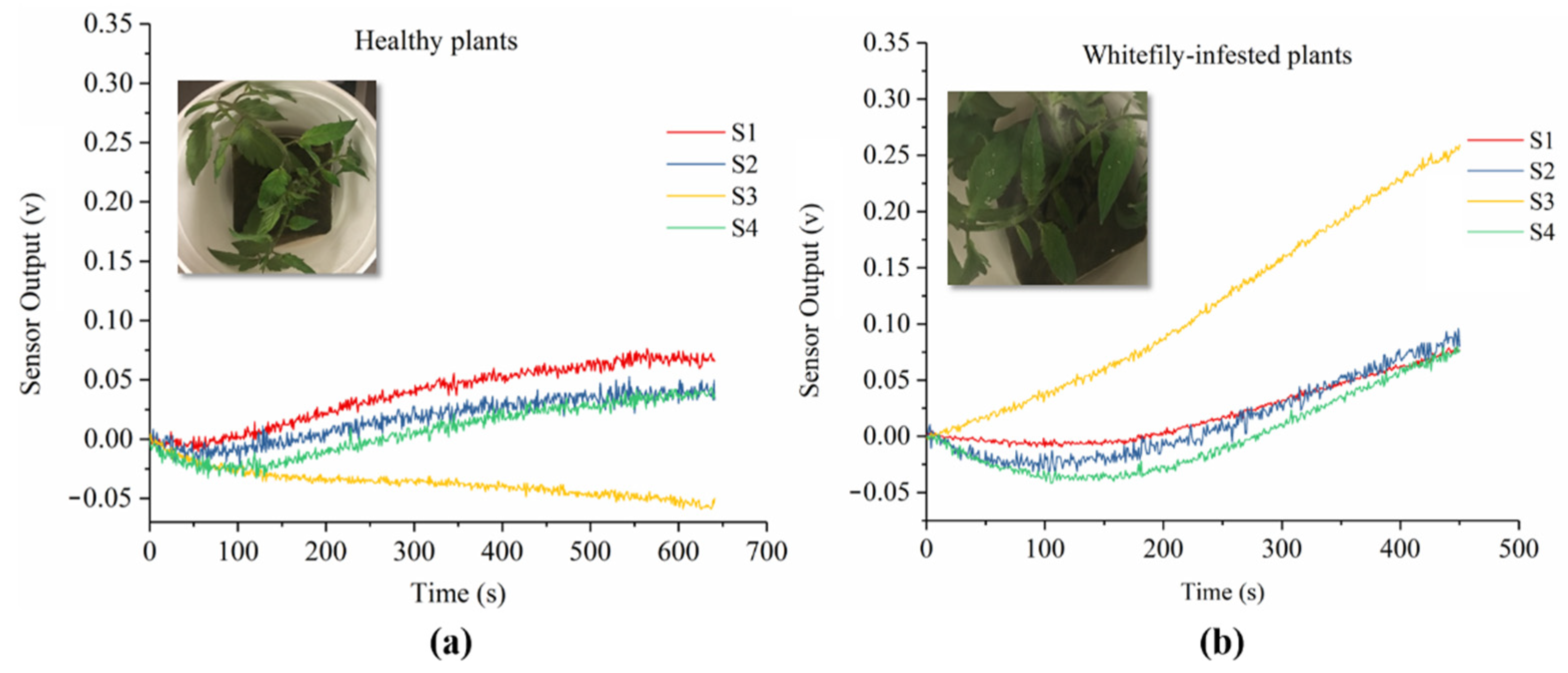
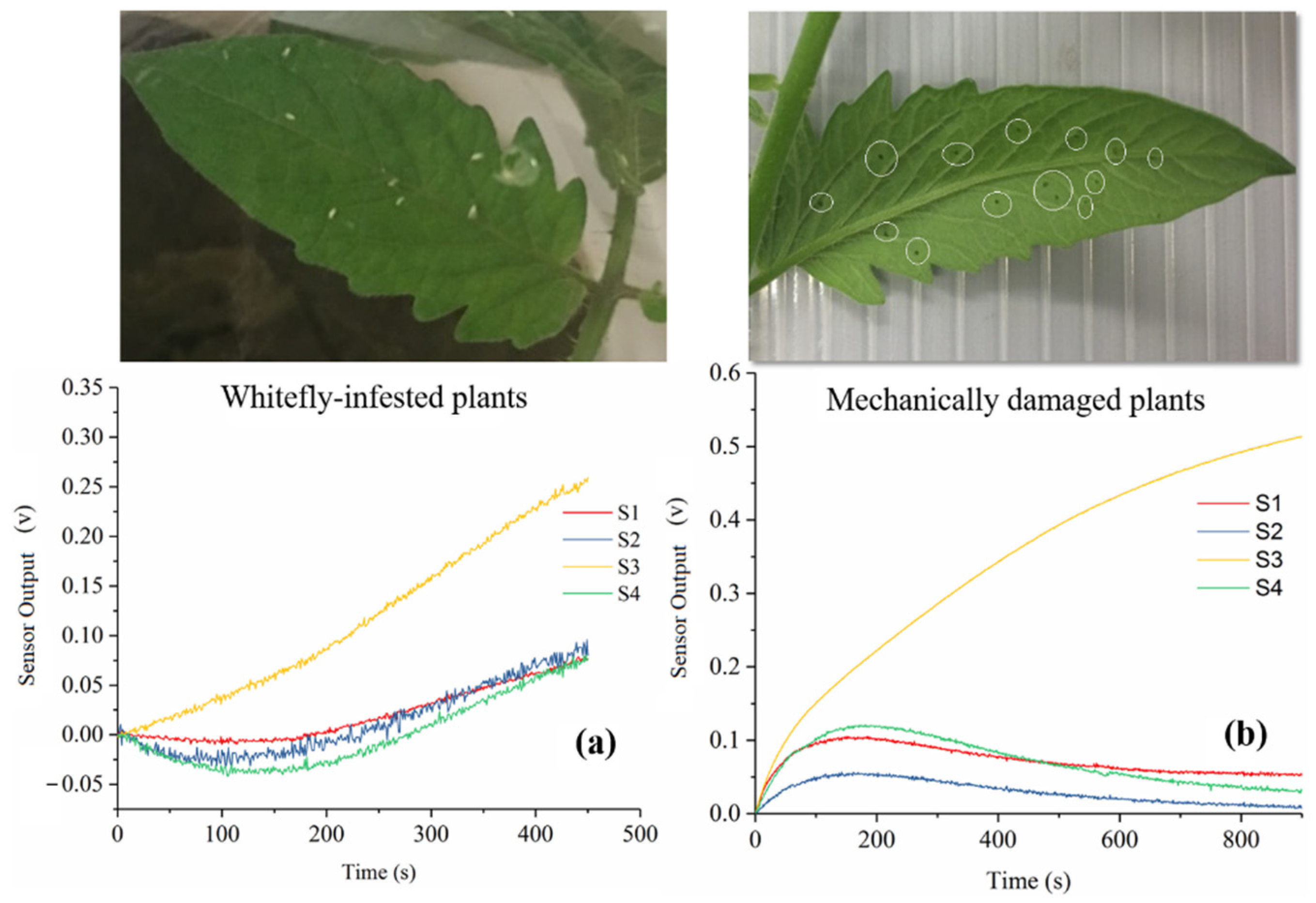
3.1. Sensor Response
3.2. E-Nose Performance
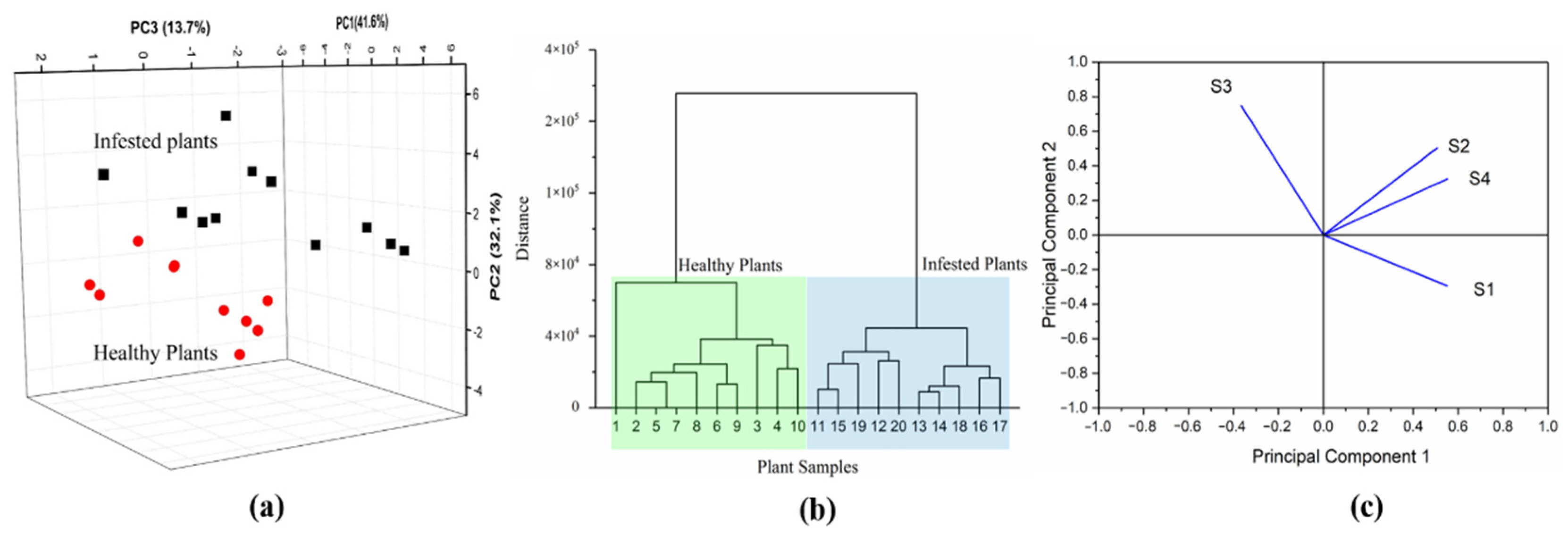
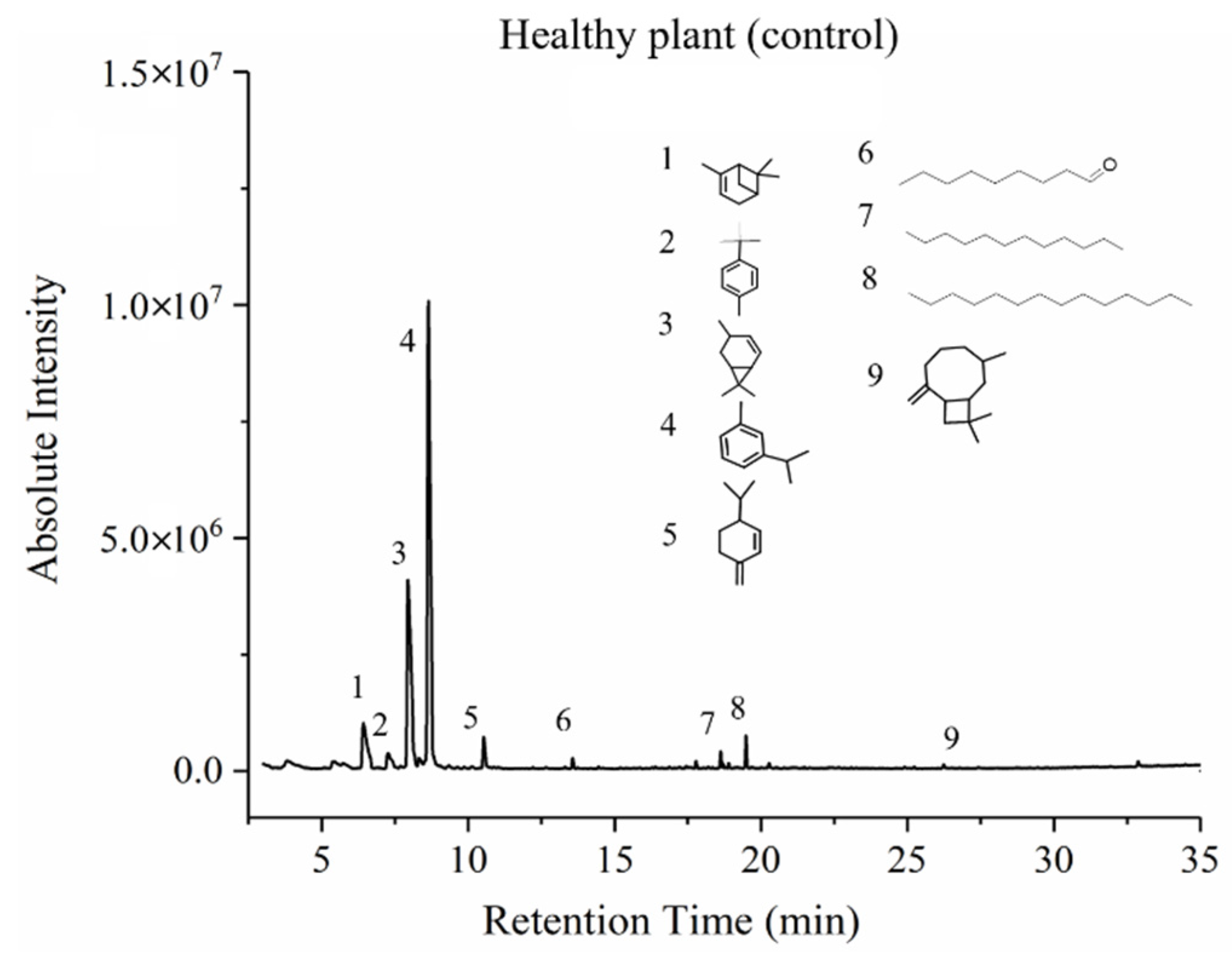
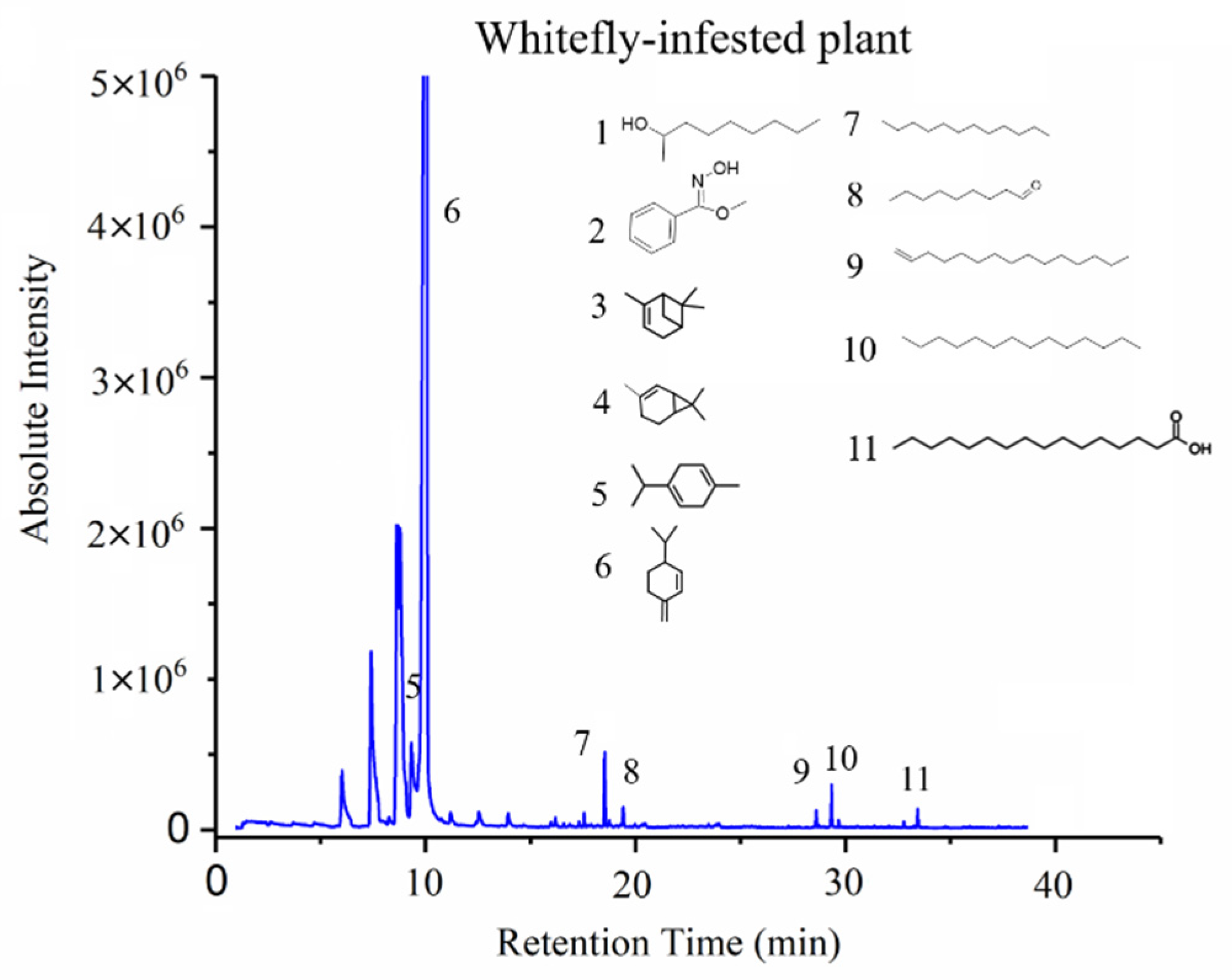
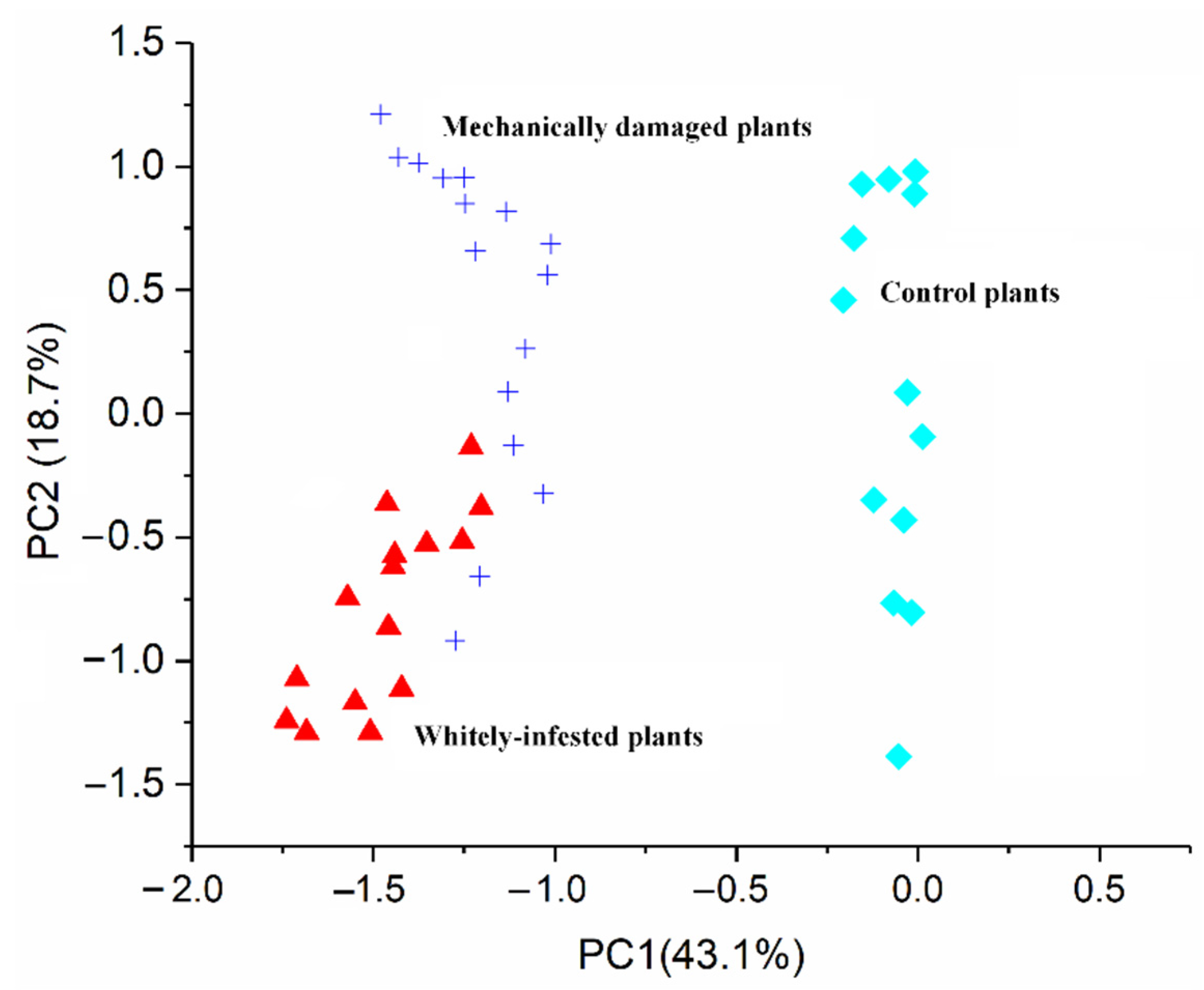
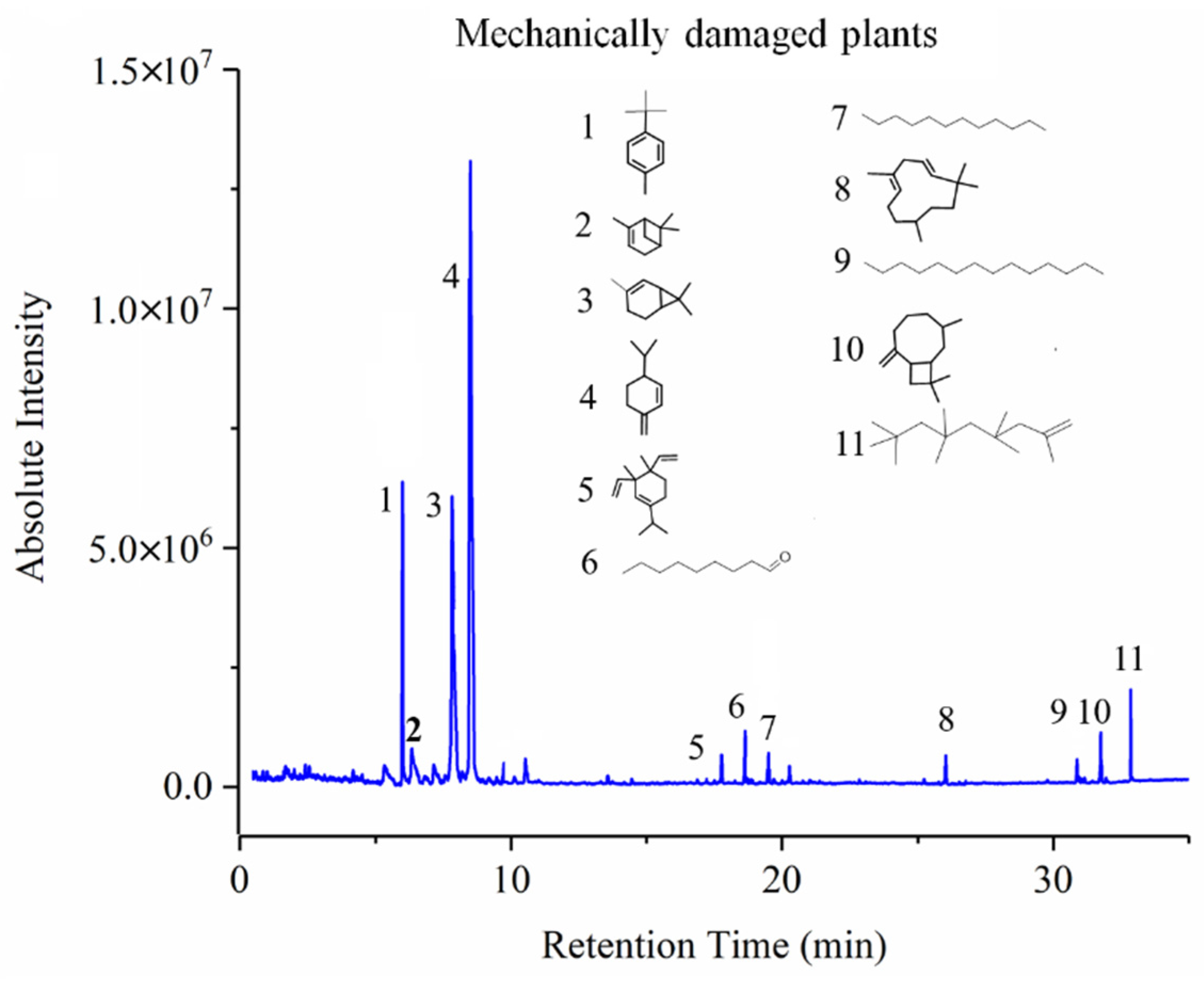
3.3. GC-MS Results
3.4. Sensor Performance between Mechanical Damage and Whitefly Infestation
3.5. Relationship between VOCs Composition and Sensor Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guan, Z.F.; Biswas, T.; Wu, F. The US Tomato Industry: An Overview of Production and Trade. 2018. Available online: https://edis.ifas.ufl.edu/fe1027 (accessed on 17 October 2021).
- Xu, H.X.; Qian, L.X.; Wang, X.W.; Shao, R.X.; Hong, Y.; Liu, S.S.; Wang, X.W. A salivary effector enables whitefly to feed on host plants by eliciting salicylic acid-signaling pathway. Proc. Natl. Acad. Sci. USA 2019, 116, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Moodley, V.; Gubba, A.; Mafongoya, P.L. A survey of whitefly-transmitted viruses on tomato crops in South Africa. J. Crop Prot. 2019, 123, 21–29. [Google Scholar] [CrossRef]
- Nault, B.A.; Speese, J. Major insect pests and economics of fresh market tomato in eastern Virginia. J. Crop Prot. 2002, 21, 359–366. [Google Scholar] [CrossRef]
- Pico, B.; Diez, M.J.; Nuez, F. Viral diseases causing the greatest economic losses to the tomato crop. II. The tomato yellow leaf curl virus—A review. Sci. Hortic. 1996, 67, 151–196. [Google Scholar] [CrossRef]
- Jesus, N.C.; Elvira, F.O.; Sonia, S.C. Emerging Virus Disease Transmitted by whiteflies. Annu. Rev. Phytopathol. 2011, 49, 219–248. [Google Scholar]
- Gantz, V.M.; Akbari, O.S. Gene editing technologies and applications for insects. Curr. Opin. Insect Sci. 2018, 28, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Kaur, A. Control of insect pests in crop plants and stored food grains using plant saponins: A review. LWT 2018, 87, 93–101. [Google Scholar] [CrossRef]
- Lombardo, L.; Coppola, G.; Zelasco, S. New technologies for insect-resistant and herbicide tolerant plants. Trends Biotechnol. 2016, 34, 49–57. [Google Scholar] [CrossRef]
- Bhatia, V.; Uniyal, P.L.; Bhattacharya, R. Aphid resistance in Brassica crops: Challenges, biotechnological progress and emerging possibilities. Biotechnol. Adv. 2011, 29, 879–888. [Google Scholar] [CrossRef]
- Kumar, S.; Atri, C.; Sangha, M.K.; Banga, S.S. Screening of wild crucifers for resistance to mustard aphid, Lipaphis erysimi (Kaltenbach) and attempt at introgression of resistance genes(s) from Brassica fruticalosa to Brassica juncea. Euphytica 2011, 3, 461–470. [Google Scholar] [CrossRef]
- Matsui, K. A portion of plant airborne communication is endorsed by uptake and metabolism of volatile organic compounds. Curr. Opin. Plant Biol. 2016, 32, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Oilkawa, P.Y.; Lerdau, M.T. Catabolism of volatile organic compounds influences plant survival. Trends Plant Sci. 2013, 18, 695–703. [Google Scholar] [CrossRef]
- Maffei, M.E. Sites of synthesis, biochemistry and functional role of plant volatiles. S. Afr. J. Bot. 2010, 76, 612–631. [Google Scholar] [CrossRef]
- Noman, A.; Aqeel, M.; Qasim, M.; Haider, I.; Lou, Y. Plant-insect-microbe interaction: A love triangel between enemies in ecosystem. Sci. Total Environ. 2020, 699, 134181. [Google Scholar] [CrossRef]
- Maffei, M.E.; Mithofer, A.; Boland, W. Insects feeds on plants: Rapid signals and response preceding the induction of phytochemucal release. Phytochemistry 2007, 68, 2946–2959. [Google Scholar] [CrossRef]
- Chang, K.P.P.; Zakaria, A.; Nasir, A.S.A.; Yusuf, N.; Thriumani, R.; Shakaff, A.Y.M.; Adom, A.H. Analysis and Feasibility Study of Plant Disease using E-Nose. In Proceedings of the 2014 IEEE International Conference on Control System, Computing and Engineering (ICCSCE 2014), Penang, Malaysia, 28–30 November 2014. [Google Scholar]
- Lan, Y.B.; Zheng, X.Z.; Westbrook, J.K.; Lopez, J.; Lacey, R.; Hoffmann, W.C. Identification of stink bugs using an electronic nose. J. Bionic Eng. 2008, 5, 72–180. [Google Scholar] [CrossRef]
- Khatoon, Z.; Fouad, H.; Alothman, O.Y.; Hashem, M.; Ansari, Z.A.; Ansari, S.A. Doped SnO2 Nanomaterials for E-nose based electrochemical sensing of biomarkers of lung cancer. ACS Omega 2020, 5, 27645–27654. [Google Scholar] [CrossRef]
- Cui, S.Q.; Yang, L.C.; Wang, J.; Wang, X.L. Fabrication of a sensitive gas sensor based on PPy/TiO2 nanocomposites films by layer-by-layer self-assembly and its application in food storage. Sens. Actuators B. Chem. 2016, 233, 337–346. [Google Scholar] [CrossRef]
- Tastan, M.; Gokozan, H. Real-time monitoring of indoor air qualifty with internet of things-based E-nose. Appl. Sci. 2019, 9, 3435. [Google Scholar] [CrossRef]
- Cui, S.Q.; Ling, P.; Zhu, H.P.; Keener, H.M. Plant pest detection using an artificial nose system: A review. Sensors 2018, 18, 378. [Google Scholar] [CrossRef]
- Cellini, A.; Blasioli, S.; Biondi, E.; Bertaccini, A.; Braschi, I.; Spinelli, F. Potential applications and limitations of electronic nose devices for plant disease diagnosis. Sensors 2017, 17, 2596. [Google Scholar] [CrossRef]
- Okur, S.; Sarheed, M.; Hubber, R.; Zhang, Z.; Heinke, L.; Kanbar, A.; Woll, C.; Nick, P.; Lemmer, U. Identification of mint scents using a QCM based E-nose. Chemosensors 2021, 9, 31. [Google Scholar] [CrossRef]
- Cheng, S.-M.; Wang, J.; Wang, Y.-W.; Wei, Z.-B. Discrimination of Different Types Damage of Tomato Seedling by Electronic Nose. In Proceedings of the ITM Web of Conferences, Wuhan, China, 24–26 March 2017. [Google Scholar]
- Cui, S.Q.; Inocnete, E.A.A.; Acosta, N.; Keener, H.M.; Zhu, H.P.; Ling, P.P. Development of E-nose system for fast diagnosis of aphids-stressed tomato plants at early stage. Sensors 2019, 19, 3480. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Yang, J.; Nie, Q.; Huang, D.; Yu, C.; Zheng, L.; Cai, M.; Thomashow, L.S.; Weller, D.M.; Yu, Z.; et al. Volatile organic compounds from paehibacillus polymyxa KM2501-1 control meloidogyne incognita by multiple strategies. Sci. Rep. 2017, 7, 16213–16225. [Google Scholar] [CrossRef]
- Wang, R.; Shen, X.; Wang, C.; Ge, R.; Zhang, Z.; Guo, X. Analysis of leaf volatiles of crabapple (Malus sp.) individuals in different aphids’ resistance. Am. J. Plant Sci. 2014, 5, 3295–3301. [Google Scholar] [CrossRef]
- McCormick, A.C.; Unsicker, S.B.; Gershenzon, J. The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci. 2012, 17, 303–310. [Google Scholar] [CrossRef]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant volatiles: Recent advances and future perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Piesik, D.; Łyszczarz, A.; Tabaka, P.; Lamparski, R.; Bocianowski, J.; Delaney, K. Volatile induction of three cereals: Influence of mechanical injury and insect herbivory on injured plants and neighbouring uninjured plants. Ann. Appl. Biol. 2010, 157, 425–434. [Google Scholar] [CrossRef]
- Kant, M.R.; Ament, K.; Sabelis, M.W.; Haring, M.A.; Schuurink, R.C. Differential timing of spider mite induced direct and indirect defenses in tomato plants. Plant Physiol. 2004, 135, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Rodiguez-saona, C.; Crafts-brandner, S.J.; Canas, L.A. Volatile emissions triggered by multiple herbivore damage: Beet armyworm and whitefly feeding on cotton plants. J. Chem. Ecol. 2003, 29, 2539–2550. [Google Scholar] [CrossRef] [PubMed]
- Ángeles López, Y.I.; Martínez-Gallardo, N.A.; Ramírez-Romero, R.; López, M.G.; Sánchez-Hernández, C.; Délano-Frier, J.P. Cross-kingdom effects of plant-plant signaling via volatile organic compounds emitted by tomato (Solanum lycopersicum) plants infested by the greenhouse whitefly (Trialeurodes vaporariorum). J. Chem. Ecol. 2012, 38, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Frey, M.; Stettner, C.; Paré, P.W.; Schmelz, E.A.; Tumlinson, J.H.; Gierl, A. An herbivore elicitor activates the gene for indole emission in maize. Proc. Natl. Acad. Sci. USA 2000, 97, 14801–14806. [Google Scholar] [CrossRef]
- Quintana-Rodriguez, E.; Duran-Flores, D.; Heil, M.; Camacho-Coronel, X. Damage-associated molecular patterns (DAMPs) as future plant vaccines that protect crops from pests. Sci. Hortic. 2018, 237, 207–220. [Google Scholar] [CrossRef]
- Meents, A.K.; Mithofer, A. Plant-plant communication: Is there a role for volatile damage-associated molecular patterns? Front. Plant Sci. 2020, 11, 1538–1550. [Google Scholar] [CrossRef]
- Liu, P.P.; Von Dahl, C.C.; Park, S.W.; Klessig, D.F. Interconnection between methyl salicylate and lipid-based longdistance signaling during the development of systemic acquired resistance in Arabidopsis and tobacco. Plant Physiol. 2011, 155, 1762–1768. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yi, H.S.; Heil, M.; Adame-alvarez, R.M.; Ballhorn, D.; Ryu, C.M. Airborne induction and priming of plant resistance to a bacterial pathogen. Plant Physiol. 2009, 151, 2152–2161. [Google Scholar] [CrossRef] [PubMed]
| No. | Sensor | Sensing Gases |
|---|---|---|
| 1 | MQ-3 (S1) | Alcohol |
| 2 | MQ-138 (S2) | Volatile compounds (aldehydes, alcohols, ketones, and aromatic compounds) |
| 3 | TGS 2602 (S3) | Volatile organic compounds and odorous gases |
| 4 | MQ-135 (S4) | Benzene, alcohol |
| Compound Name | VOCs Identification | ||||||
|---|---|---|---|---|---|---|---|
| No | Infested Plants | Healthy Plants | Mechanically Damaged Plants | ||||
| RT (min) * | Area (%) | RT (min) * | Area (%) | RT(min) * | Area (%) | ||
| 1 | 2-Nonanol | 6.413 | 2.17 | -- | -- | -- | -- |
| 2 | Oxime-, methoxy-phenyl | 7.254 | 14.66 | -- | -- | -- | -- |
| 3 | Benzene, tert-butyl- | 7.451 | 0.73 | 7.508 | 2.43 | 7.681 | 19.6 |
| 4 | Trimethylbicyclo-hept-2-ene | 7.702 | 1.54 | 7.702 | 7.06 | 7.994 | 3.47 |
| 5 | 4-Carene | 7.954 | 17.67 | 8.016 | 28.05 | 8.429 | 23.15 |
| 6 | 1,3-Cyclohexadiene, 1-methyl-4-(methylethyl)- | 8.335 | 2.06 | 8.409 | 0.91 | -- | -- |
| 7 | Beta-phellandrene | 8.674 | 30.65 | 8.811 | 53.7 | 9.322 | 37.78 |
| 8 | Cyclohexene, 4-ethenyl-4-methyl-3-(1-methylethenyl)-1-(1-methylethyl)-, (3R-trans)- | 17.467 | 0.17 | -- | -- | 18.147 | 0.47 |
| 9 | Dodecane | 18.029 | 3.17 | 18.019 | 0.14 | 20.413 | 0.35 |
| 10 | Nonanal | 18.433 | 1.32 | 18.891 | 2.52 | 19.032 | 0.97 |
| 11 | Caryophyllene | 19.505 | 0.96 | 20.017 | 0.85 | -- | -- |
| 12 | Humulene | -- | -- | 28.912 | 0.13 | 27.432 | 0.51 |
| 13 | 1-Pentadecene | 29.126 | 1.12 | -- | -- | -- | -- |
| 14 | Tetradecane | 30.565 | 1.56 | -- | -- | 30.895 | 0.46 |
| 15 | Caryophyllene | 31.609 | 0.96 | 31.903 | 1.43 | 31.011 | 2.69 |
| 16 | 2,3,4,4,6,6,8,8-Heptamethyl-1-nonene | 32.423 | 0.84 | -- | -- | 32.096 | 5.76 |
| 17 | Dodecane, 4,6-dimethyl | -- | -- | -- | -- | 33.763 | 0.91 |
| 18 | n-Hexadecanoic acid | 33.331 | 1.26 | -- | -- | -- | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, S.; Cao, L.; Acosta, N.; Zhu, H.; Ling, P.P. Development of Portable E-Nose System for Fast Diagnosis of Whitefly Infestation in Tomato Plant in Greenhouse. Chemosensors 2021, 9, 297. https://doi.org/10.3390/chemosensors9110297
Cui S, Cao L, Acosta N, Zhu H, Ling PP. Development of Portable E-Nose System for Fast Diagnosis of Whitefly Infestation in Tomato Plant in Greenhouse. Chemosensors. 2021; 9(11):297. https://doi.org/10.3390/chemosensors9110297
Chicago/Turabian StyleCui, Shaoqing, Lin Cao, Nuris Acosta, Heping Zhu, and Peter P. Ling. 2021. "Development of Portable E-Nose System for Fast Diagnosis of Whitefly Infestation in Tomato Plant in Greenhouse" Chemosensors 9, no. 11: 297. https://doi.org/10.3390/chemosensors9110297
APA StyleCui, S., Cao, L., Acosta, N., Zhu, H., & Ling, P. P. (2021). Development of Portable E-Nose System for Fast Diagnosis of Whitefly Infestation in Tomato Plant in Greenhouse. Chemosensors, 9(11), 297. https://doi.org/10.3390/chemosensors9110297






