Tungsten-Based Cost-Effective Gas Sensors for H2S Detection
Abstract
:1. Introduction
2. Materials and Methods
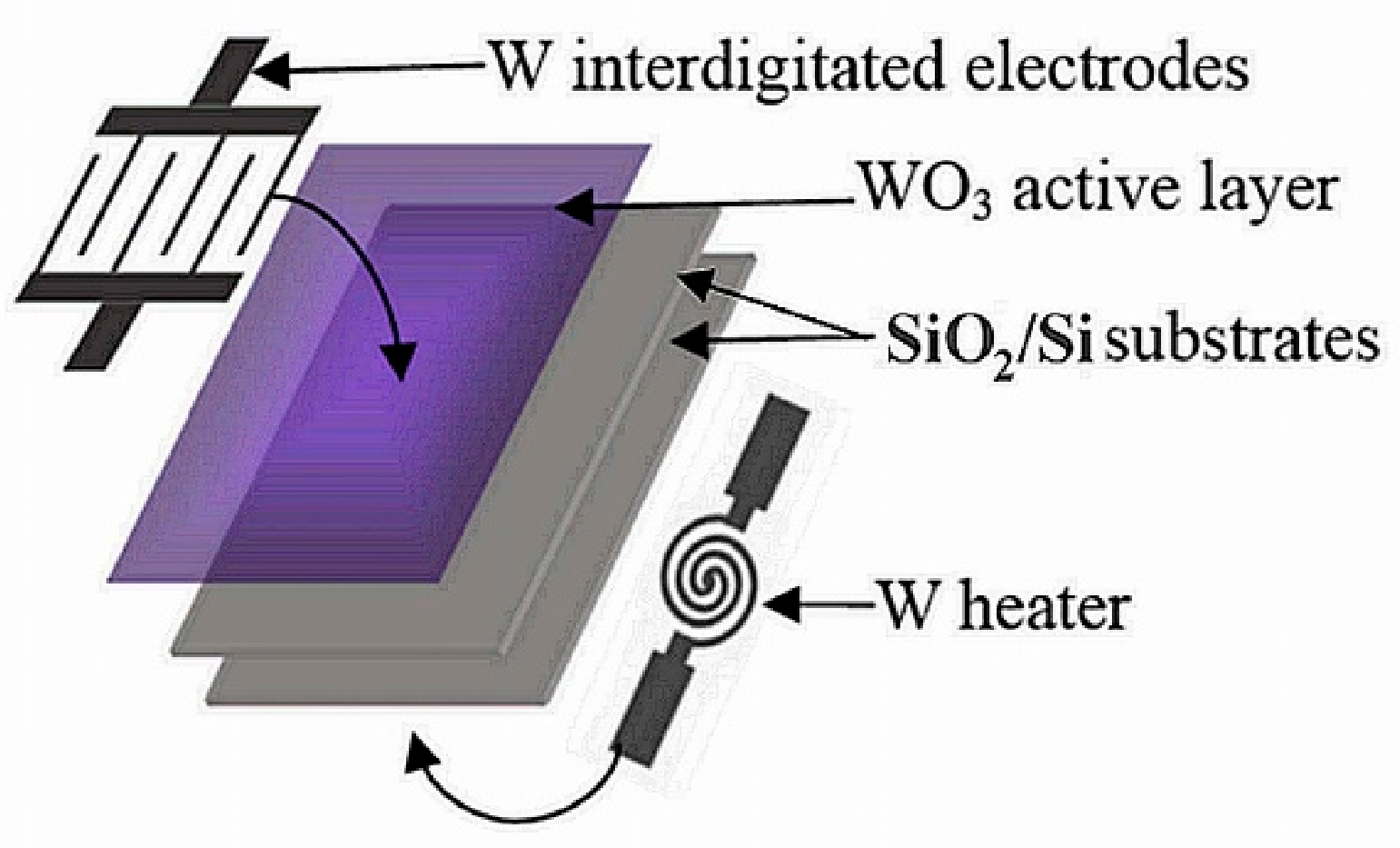
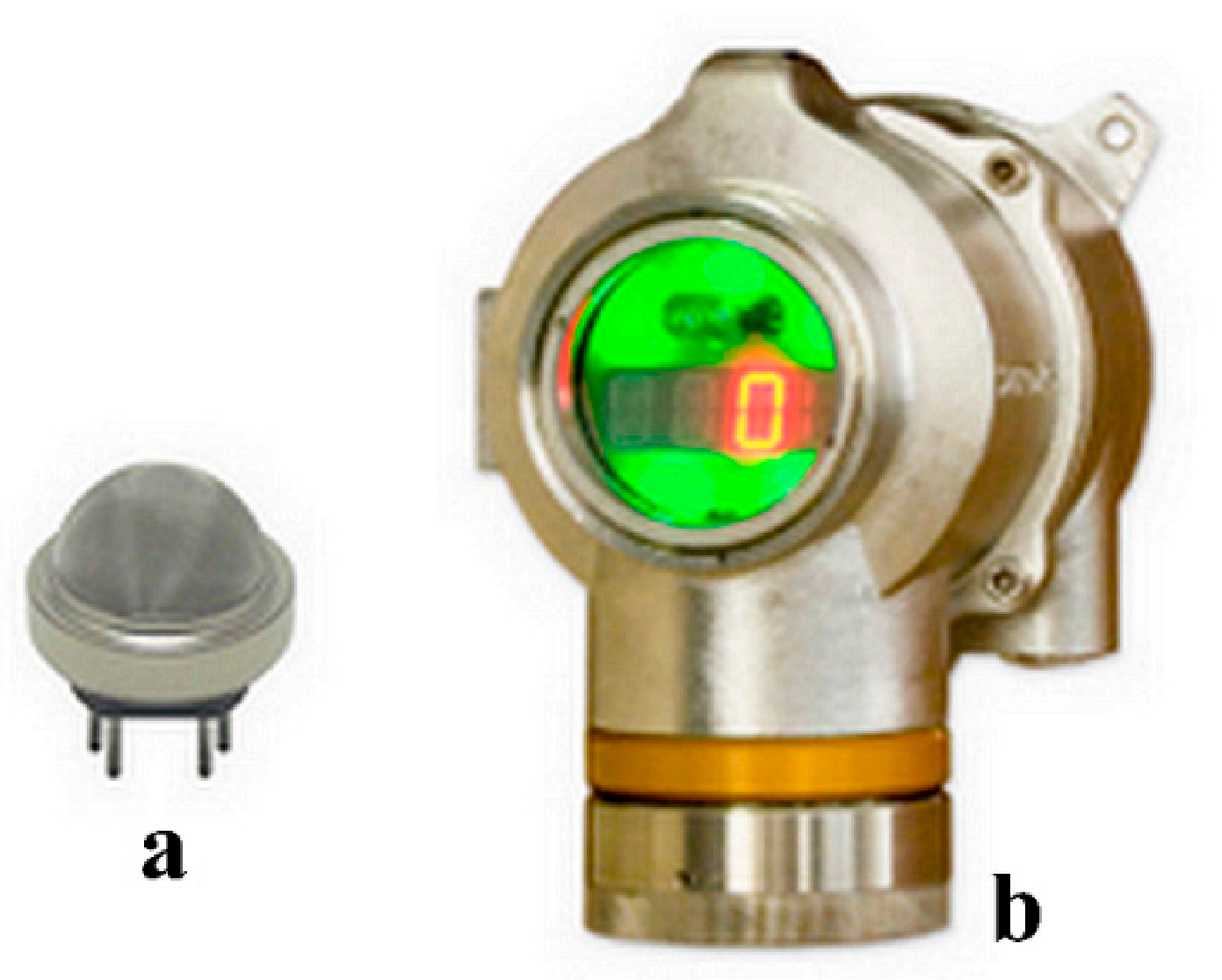
2.1. Sensor Fabrication
2.1.1. Heating Element
2.1.2. Sensing Element
2.2. Physical and Electrical Characterizations
3. Results and Discussion
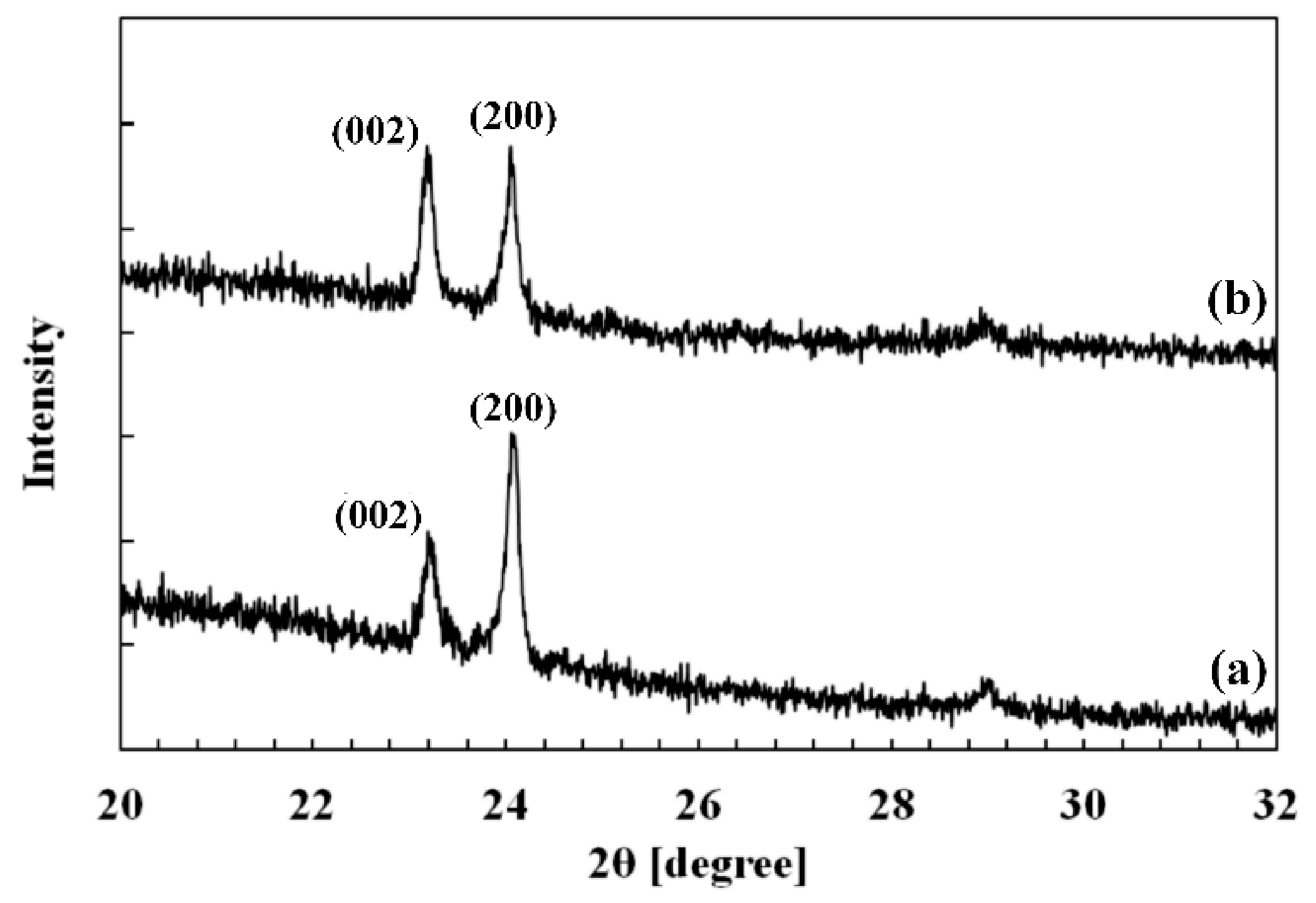
3.1. Crystallographic Structure of WO3 Films
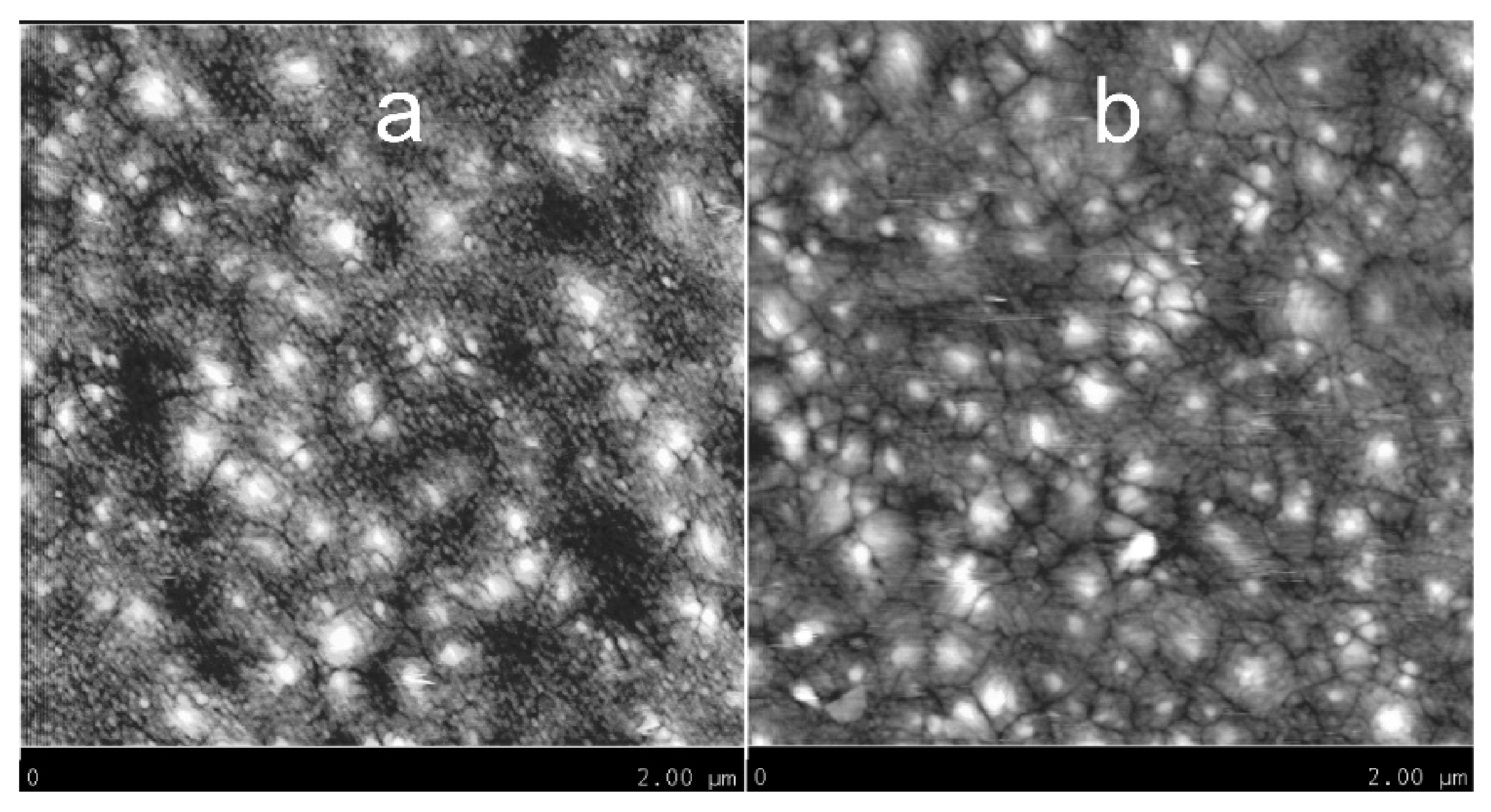
3.2. Surface Morphology of WO3 Films
3.3. Electrical Properties
3.4. Measurements under H2S
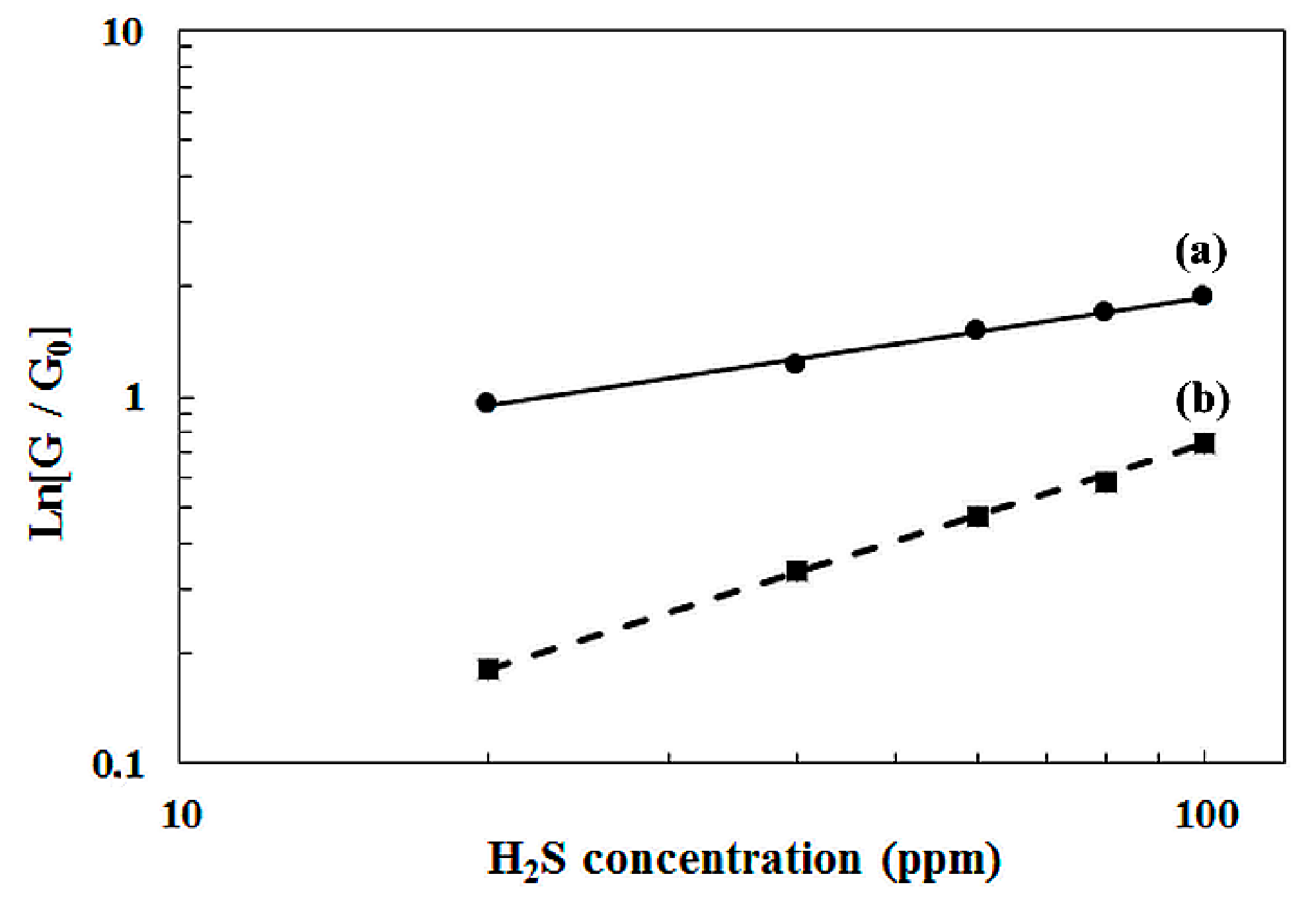
4. Modeling of the Sensitivity Evolution as a Function of the H2S Concentration
5. Dynamic Response of the Sensors under H2S and Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pandey, S.K.; Kim, K.-H.; Tang, K.-T. A review of sensor-based methods for monitoring hydrogen sulfide. TrAC Trends Anal. Chem. 2012, 32, 87–99. [Google Scholar] [CrossRef]
- Moseley, P. Materials selection for semiconductor gas sensors. Sens. Actuators B Chem. 1992, 6, 149–156. [Google Scholar] [CrossRef]
- Heiland, G. Homogeneous semiconducting gas sensors. Sens. Actuators 1982, 2, 343–361. [Google Scholar] [CrossRef]
- Morrison, S.R. Semiconductor gas sensors. Sens. Actuators 1982, 2, 329–341. [Google Scholar] [CrossRef]
- Verbrugghe, N.; Fasquelle, D.; Duponchel, B.; Deputier, S. Study of tungsten films deposited by DC sputtering dedicated to integrated heaters. J. Vac. Sci. Technol. B 2017, 35, 031204. [Google Scholar] [CrossRef]
- Antonik, M.; Schneider, J.; Wittman, E.; Snow, K.; Vetelino, J.; Lad, R. Microstructural effects in WO3 gas-sensing films. Thin Solid Films 1995, 256, 247–252. [Google Scholar] [CrossRef]
- Sberveglieri, G.; Depero, L.; Groppelli, S.; Nelli, P. WO3 sputtered thin films for NOx monitoring. Sens. Actuators B Chem. 1995, 26, 89–92. [Google Scholar] [CrossRef]
- Moseley, P.T.; Norris, J.O.W.; Williams, D.E. Techniques and Mechanisms in Gas Sensing; 1991; pp. 46–60. Available online: https://www.scienceopen.com/document?vid=7883bc3a-29bc-4934-bab8-0f9dccf51c1a (accessed on 9 September 2021).
- Cantalini, C.; Sun, H.T.; Faccio, M.; Pelino, M.; Santucci, S.; Lozzi, L.; Passacantando, M. NO2 sensitivity of WO3 thin film ob-tained by high vacuum thermal evaporation. Sens. Actuators B 1996, 31, 81–87. [Google Scholar] [CrossRef]
- Ozer, N.; Lampert, C. Electrochromic performance of sol-gel deposited WO3–V2O5 films. Thin Solid Films 1999, 349, 205–211. [Google Scholar] [CrossRef]
- de Tacconi, N.R.; Chenthamarakshan, C.R.; Wouters, K.L.; MacDonnell, F.M.; Rajeshwar, K. Composite WO3-TiO2 films pre-pared by pulsed electrodeposition: Morphological aspects and electrochromic behavior. J. Electroanal. Chem. 2004, 566, 249–256. [Google Scholar] [CrossRef]
- Moulzolf, S.C.; Ding, S.-A.; Lad, R.J. Stoichiometry and microstructure effects on tungsten oxide chemiresistive films. Sens. Actuators B Chem. 2001, 77, 375–382. [Google Scholar] [CrossRef]
- Khatko, V.; Vallejos, S.; Calderer, J.; Llobet, E.; Vilanova, X.; Correig, X. Gas sensing properties of WO3 thin films deposited by rf sputtering. Sens. Actuators B Chem. 2007, 126, 400–405. [Google Scholar] [CrossRef]
- Acosta, M.; González, D.; Riech, I. Optical properties of tungsten oxide thin films by non-reactive sputtering. Thin Solid Films 2009, 517, 5442–5445. [Google Scholar] [CrossRef]
- Riech, I.; Acosta, M.; Pena, J.L.; Bartolo-Perez, P. Effects of working pressure on physical properties of tungsten-oxide thin films sputtered from oxide target. J. Vac. Sci. Technol. A 2010, 28, 329. [Google Scholar] [CrossRef]
- Woodward, P.M.; Sleight, A.W. Ferroelectric Tungsten Trioxide. J. Solid State Chem. 1997, 131, 9–17. [Google Scholar] [CrossRef]
- Lefkowitz, I.; Dowell, M.; Shields, M. Phase transitions in tungsten trioxide at low temperatures. J. Solid State Chem. 1975, 15, 24–39. [Google Scholar] [CrossRef]
- Khojier, K.; Savaloni, H.; Habashi, N.; Sadi, M. On the influence of temperature gradient of annealing process on the nano-structure and sensing properties of WO3 thin films to NO2 gas and relative humidity. Mater. Sci. Semicond. Process. 2016, 41, 177–183. [Google Scholar] [CrossRef]
- Fasquelle, D.; Verbrugghe, N.; Laasri, H.A.; Tachafine, A.; Duponchel, B.; Députier, S.; Costa, L.C.; Elaatmani, M.; Outzourhit, A.; Carru, J.-C. Promising Performance of Lead-Free Functional thin films and ceramics. J. Phys. Conf. Ser. 2019, 1153, 012064. [Google Scholar] [CrossRef]
- Cora, F.; Stachiotti, M.G.; Catlow, C.R.A.; Rodriguez, C.O. Transition Metal Oxide Chemistry: Electronic Structure Study of WO3, ReO3, and NaWO3. J. Phys. Chem. 1997, 101, 3945–3952. [Google Scholar] [CrossRef]
- Al Mohammad, A.; Gillet, M. Phase transformations in WO3 thin films during annealing. Thin Solid Films 2002, 408, 302–309. [Google Scholar] [CrossRef]
- Gillet, M.; Aguir, K.; Bendahan, M.; Mennini, P. Grain size effect in sputtered tungsten trioxide thin films on the sensitivity to ozone. Thin Solid Films 2005, 484, 358–363. [Google Scholar] [CrossRef]
- Wang, S.-H.; Chou, T.-C.; Liu, C.-C. Nano-crystalline tungsten oxide NO2 sensor. Sens. Actuators B Chem. 2003, 94, 343–351. [Google Scholar] [CrossRef]
- Geistlinger, H. Electron theory of thin-film gas sensors. Sens. Actuators B Chem. 1993, 17, 47–60. [Google Scholar] [CrossRef]
- McAleer, J.F.; Moseley, P.T.; Norris, J.O.W.; Williams, D.E. Tin dioxide gas sensors. Part 1.—Aspects of the surface chemistry revealed by electrical conductance variations. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1987, 83, 1323–1346. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Lal, P.; Dwivedi, R.; Srivastava, S. Sensing mechanism in tin oxide-based thick-film gas sensors. Sens. Actuators B Chem. 1994, 21, 213–218. [Google Scholar] [CrossRef]
- Sun, H.-T.; Cantalini, C.; Lozzi, L.; Passacantando, M.; Santucci, S.; Pelino, M. Microstructural effect on NO2 sensitivity of WO3 thin film gas sensors. Thin Solid Films 1996, 287, 258–265. [Google Scholar] [CrossRef]
- Takács, M.; Pap, A. Gas Sensitivity of Sol-gel Prepared Mesoporous WO3 thin Film. Procedia Eng. 2016, 168, 289–292. [Google Scholar] [CrossRef]
- Poongodi, S.; Kumar, P.S.; Mangalaraj, D.; Ponpandian, N.; Meena, P.; Masuda, Y.; Lee, C. Electrodepositon of WO3 nanostruc-tured thin films for electrochromic and H2S gas sensor applications. J. Alloys Compd. 2017, 719, 71–81. [Google Scholar] [CrossRef]
- Kim, W.-S.; Kim, H.-C.; Hong, S.-H. Gas sensing properties of MoO3 nanoparticles synthesized by solvothermal method. J. Nanopart. Res. 2009, 12, 1889–1896. [Google Scholar] [CrossRef]
- Balouria, V.; Kumar, A.; Samanta, S.; Singh, A.; Debnath, A.; Mahajan, A.; Bedi, R.; Aswal, D.; Gupta, S. Nano-crystalline Fe2O3 thin films for ppm level detection of H2S. Sens. Actuators B Chem. 2013, 181, 471–478. [Google Scholar] [CrossRef]
- Pongpaiboonkul, S.; Phokharatkul, D.; Hodak, J.H.; Wisitsoraat, A.; Hodak, S.K. Enhancement of H2S-sensing performances with Fe-doping in CaCu3Ti4O12 thin films prepared by a sol–gel method. Sens. Actuators B Chem. 2016, 224, 118–127. [Google Scholar] [CrossRef]




| Element | Value |
|---|---|
| Substrate dimensions W film thickness of the heater | 8 mm × 4 mm 565 nm ± 35 nm |
| Heater electrical resistance at 20 °C | 50 Ω ± 10 Ω |
| W film thickness of the electrodes Thickness of WO3 films annealed at 400 and 500 °C Electrical resistance at room temperature of WO3 films annealed at 400 and 500 °C | 300 nm ± 10 nm 120 nm ± 10 nm 5 MΩ ± 1 MΩ |
| Annealing Temperature (°C) | 2θ (°) | d (Å) | Lhkl (nm) | (hkl) Plane |
|---|---|---|---|---|
| 400 | 23.26 ± 0.02 | 3.82 ± 0.01 | 63 ± 2 | (002) |
| 24.12 ± 0.02 | 3.68 ± 0.01 | 64 ± 2 | (200) | |
| 500 | 23.27 ± 0.02 | 3.82 ± 0.01 | 61 ± 2 | (002) |
| 24.14 ± 0.02 | 3.68 ± 0.01 | 77 ± 2 | (200) |
| Gas Sensing Material | Operating Temperature | Sensitivity G/G0 | Response Time (s) | Recovery Time (s) | H2S (ppm) | Refs. |
|---|---|---|---|---|---|---|
| Mesoporous WO3 Sol–gel | 260 °C | 1.5 | 200 | 60 | 100 | [28] |
| Nanostructured WO3 electrodeposition | 300 °C | 0.85 | 50 | 100 | 10 | [29] |
| MoO3 micro-sized commercial powder | 375 °C | 33 | 10 | >800 | 20 | [30] |
| Nano-crystalline Fe2O3 E-beam evaporation | 250 °C | 6.2 | 64 | >9000 | 50 | [31] |
| Fe-doped CaCu3Ti4012 Sol–gel | 250 °C | 125 | 5 | 500–1100 | 10 | [32] |
| WO3 sensor-400 RF sputtering | 350 °C | 2.6–6.6 | 30–20 | 240–420 | 20–100 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fasquelle, D.; Verbrugghe, N.; Députier, S. Tungsten-Based Cost-Effective Gas Sensors for H2S Detection. Chemosensors 2021, 9, 295. https://doi.org/10.3390/chemosensors9110295
Fasquelle D, Verbrugghe N, Députier S. Tungsten-Based Cost-Effective Gas Sensors for H2S Detection. Chemosensors. 2021; 9(11):295. https://doi.org/10.3390/chemosensors9110295
Chicago/Turabian StyleFasquelle, Didier, Nathalie Verbrugghe, and Stéphanie Députier. 2021. "Tungsten-Based Cost-Effective Gas Sensors for H2S Detection" Chemosensors 9, no. 11: 295. https://doi.org/10.3390/chemosensors9110295
APA StyleFasquelle, D., Verbrugghe, N., & Députier, S. (2021). Tungsten-Based Cost-Effective Gas Sensors for H2S Detection. Chemosensors, 9(11), 295. https://doi.org/10.3390/chemosensors9110295





