All-Solid-State Calcium Sensors Modified with Polypyrrol (PPY) and Graphene Oxide (GO) as Solid-Contact Ion-to-Electron Transducers
Abstract
1. Introduction
2. Experimental
2.1. Apparatus
2.2. Materials and Reagents
2.3. Sensors’ Construction
2.4. Electrochemical Impedance Spectroscopy and Chronopotentiometry
2.5. Calcium Assessment in Real Samples
3. Results and Discussion
3.1. Performance Characteristics of the Proposed Sensors
3.2. Chronopotentiometry
3.3. Electrochemical Impedance Spectroscopy
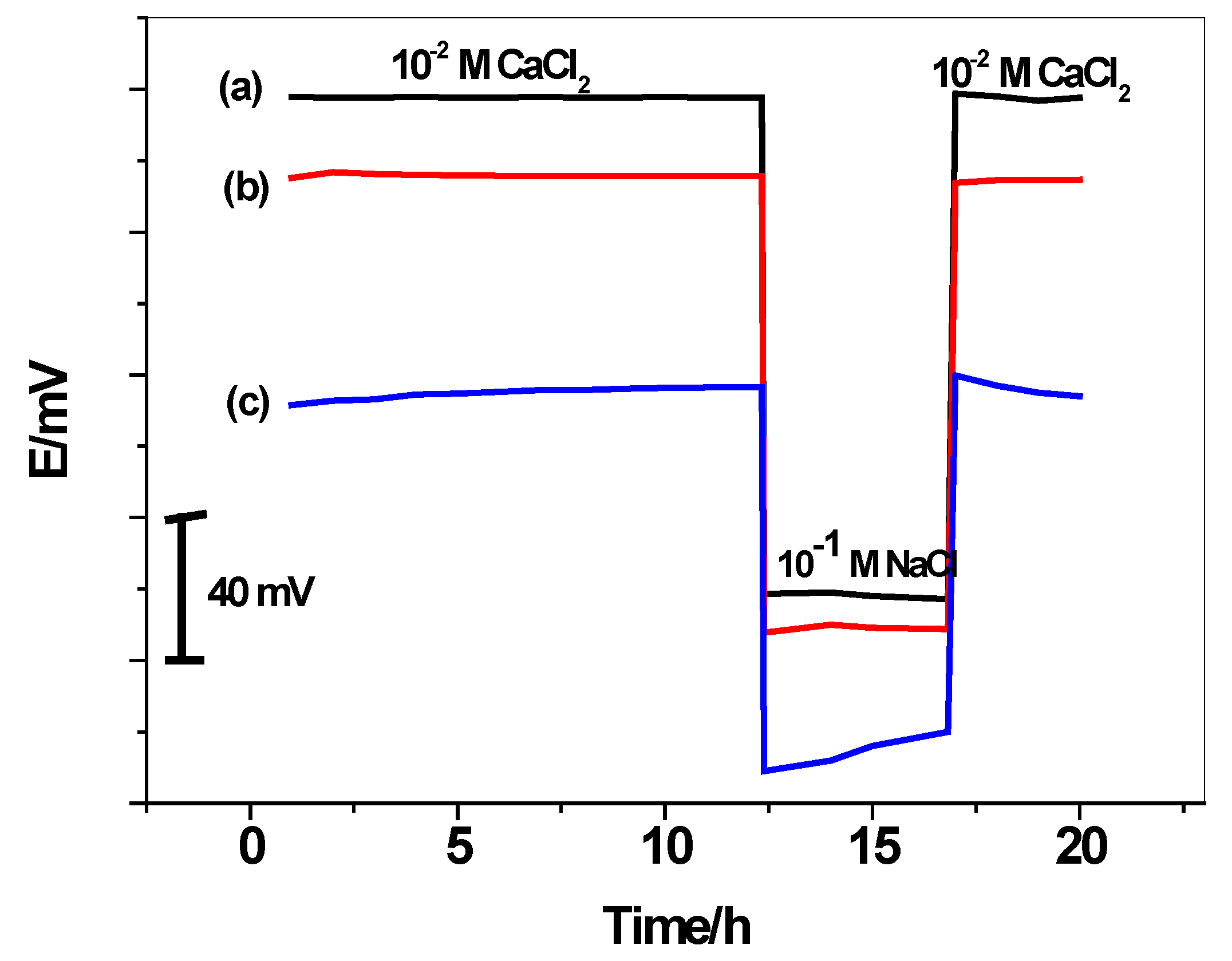
3.4. Water-Layer Test
3.5. Analytical Applications
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Statement
References
- Grishin, S.N. Transmembrane calcium current: Mechanism, registration procedures, calcium-mediated modulators of synaptic transmission. Biochem. (Moscow) Suppl. Ser. Membr. Cell Biol. 2014, 8, 213–224. [Google Scholar] [CrossRef]
- Byrnes, M.C.; Huynh, K.; Helmer, S.D.; Stevens, C.; Dort, J.M.; Smith, R.S. A comparison of corrected serum calcium levels to ionized calcium levels among critically ill surgical patients. Am. J. Surg. 2005, 189, 310–314. [Google Scholar] [CrossRef]
- Kochegarov, A.A. Pharmacological modulators of voltage-gated calcium channels and their therapeutical application. Cell Calcium 2003, 33, 145–162. [Google Scholar] [CrossRef]
- Robertson, W.G. Measurement of Ionised Calcium in Body Fluids—A Review. Annals Clin. Biochem. Int. Lab. Med. 1976, 13, 540–548. [Google Scholar] [CrossRef]
- Ross, J.W. Calcium-selective electrode with liquid ion exchanger. Science 1967, 156, 1378–1379. [Google Scholar] [CrossRef]
- Young, C.C. Evolution of blood chemistry analyzers based on ion selective electrodes. J. Chem. Educ. 1997, 74, 177–182. [Google Scholar] [CrossRef]
- Haugland, R.P.; Johnson, I.D. Intracellular ion indicators. In Fluorescent and Luminescent Probes for Biological Activity; Mason, W.T., Ed.; Academic Press: London, UK, 1999; pp. 40–50. [Google Scholar]
- Dimeski, G.; Badrick, T.; John, A.S. Ion selective electrodes (ISEs) and interferences—A review. Clin. Chim. Acta 2010, 411, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Ashmawy, N.H.; Almehizia, A.A.; Youssef, T.A.; El-Galil, E.A.A.; Al-Omar, M.A.; Kamel, A.H. Novel Carbon/PEDOT/PSS-Based screen-printed biosensors for acetylcholine neurotransmitter and acetylcholinesterase detection in human serum. Molecules 2019, 24, 1539. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.M.; Elnemma, E.M.; Mohamed, A.H.K. Novel biomedical sensors for flow injection potentiometric determination of creatinine in human serum. Electroanalysis 2005, 17, 2246–2253. [Google Scholar] [CrossRef]
- Kamel, A.H.; Hassan, A.M.E. Solid contact potentiometric sensors based on host-tailored molecularly imprinted polymers for creatine assessment. Int. J. Electrochem. Sci. 2016, 11, 8938–8949. [Google Scholar] [CrossRef]
- Abdalla, N.S.; Amr, A.E.G.E.; El-Tantawy, A.S.M.; Al-Omar, M.A.; Kamel, A.H.; Khalifa, N.M. Tailor-madespecific recognition of cyromazine pesticide integrated in a potentiometric strip cell for environmental andfood analysis. Polymers 2019, 11, 1526. [Google Scholar] [CrossRef] [PubMed]
- GalalEldin, A.; Amr, E.; El-Galil, A.; Kamel, A.H.; Hassan, S.S.M. Screen-printed Microsensors UsingPolyoctyl-thiophene (POT) conducting polymer as solid transducer for ultratrace determination of Azides. Molecules 2019, 24, 1392. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, L.; d’Angremont, E.; Olthuis, W. Solid contact potassium selective electrodes for biomedical applications—A review. Talanta 2016, 160, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Lindfors, T.; Ivaska, A. All-solid-state calcium-selective electrode prepared of soluble electrically conducting polyaniline and di(2-ethylhexyl)phosphate with tetraoctylammonium chloride as cationic additive. Anal. Chim. Acta 2000, 404, 111–119. [Google Scholar] [CrossRef]
- Sundfors, F.; Bereczki, R.; Bobacka, J.; Toth, K.; Ivaska, A.; Gyurcsanyi, R.E. Microcavity based solid-contact ion-selective microelectrodes. Electroanalysis 2006, 18, 1372–1378. [Google Scholar] [CrossRef]
- Zachara, J.E.; Toczylowska, R.; Pokrop, R.; Zagórska, M.; Dybko, A.; Wróblewski, W. Miniaturized all-solid-state potentiometric ion sensors based on PVC-membranes containing conducting polymers. Sens. Actuators B 2004, 101, 207–212. [Google Scholar] [CrossRef]
- Zine, N.; Bausells, J.; Teixidor, F.; Vinas, C.; Masalles, C.; Samitier, J.; Errachid, A. All solid-state hydrogen sensing microelectrodes based on novel PPy[3,3′-Co(1,2-C2B9H11)2] as a solid internal contact. Mater. Sci. Eng. C 2006, 26, 399–404. [Google Scholar] [CrossRef]
- Pandey, P.C.; Prakash, R. Polyindole modified potassium ion-sensor using dibenzo-18-crown-6 mediated PVC matrix membrane. Sens. Actuators B 1998, 46, 61–65. [Google Scholar] [CrossRef]
- Zine, N.; Bausells, J.; Vocanson, F.; Lamartine, R.; Asfari, Z.; Teixidor, F.; Crespo, E.; de Oliveira, I.A.M.; Samitier, J.; Errachid, A. Potassium-ion selective solid contact microelectrode based on a novel 1,3-(di-4-oxabutanol)-calix[4]arene-crown-5 neutral carrier. Electrochim. Acta 2006, 51, 5075–5079. [Google Scholar] [CrossRef]
- Correia, D.P.A.; Magalhaes, J.M.C.S.; Machado, A.A.S.C. Array of potentiometric sensors for simultaneous analysis of urea and potassium. Talanta 2005, 67, 773–782. [Google Scholar] [CrossRef]
- Lindfors, T.; Ivaska, A. All-solid-state calcium-selective electrode prepared of soluble electrically conducting polyaniline and di(2-ethylhexyl)phosphate with ETH1001 as neutral carrier. Anal. Chim. Acta 2000, 404, 101–110. [Google Scholar] [CrossRef]
- Tymecki, L.; Glab, S.; Koncki, R. Miniaturized, planar ion-selective electrodes fabricated by means of thick-film technology. Sensors 2006, 6, 390–396. [Google Scholar] [CrossRef]
- Singh, A.K.; Mehtab, S. Calcium(II)-selective potentiometric sensor based on α-furildioxime as neutral carrier. Sens. Actuators B 2007, 123, 429–436. [Google Scholar] [CrossRef]
- Wang, S.H.; Chou, T.C.; Liu, C.C. Development of a solid-state thick film calcium ion-selective electrode. Sens. Actuators B 2003, 96, 709–716. [Google Scholar] [CrossRef]
- Sutor, D.J.; Wilkie, L.I. The crystalline salts of calcium bilirubinate in human gallstones. Clin. Sci. Mol. Med. 1977, 53, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Black, B.E.; Carr, S.H.; Ostrow, J.D. Equilibrium swelling of pigment gallstones: Evidence for network polymer structure. Biopolymers 1982, 21, 601–610. [Google Scholar] [CrossRef]
- Bakker, E.; Pretsch, E.; Bühlman, P. Selectivity of potentiometric ion sensors. Anal. Chem. 2000, 72, 1127–1133. [Google Scholar] [CrossRef]
- Bobacka, J. Potential stability of all-solid-state ion-selective electrodes using conducting polymers as ion-to-electron transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef]
- Kou, L.; Fu, M.; Liang, R. Solid-contact Ca2+-selective electrodes based on two-dimensional black phosphorus as ion-to-electron transducers. RSC Adv. 2017, 7, 43905–43908. [Google Scholar] [CrossRef]
- Hernandez, R.; Riu, J.; Rius, F.X. Determination of calcium ion in sap using carbon nanotube-based ion-selective electrodes. Analyst 2010, 135, 1979–1985. [Google Scholar] [CrossRef]
- Abramova, N.; Moral-Vico, J.; Soley, J.; Ocaña, C.; Bratov, A. Solid contact ion sensor with conducting polymer layer copolymerized with the ion-selective membrane for determination of calcium in blood serum. Anal. Chim. Acta 2016, 943, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Blaz, T.; Migdalski, J.; Lewenstam, A. Polypyrrole–calcion film as a membrane and solid-contact in an indicator electrode for potentiometric titrations. Talanta 2000, 52, 319–328. [Google Scholar] [CrossRef]
- He, N.; Hofler, L.; Latonen, R.; Lindfors, T. Influence of hydrophobization of the polyazulene ion-to-electron transducer on the potential stability of calcium-selective solid-contact electrodes. Sens. Actuators B 2015, 207, 918–925. [Google Scholar] [CrossRef]
- Lindfors, T.; Sundfors, F.; Hofler, L.; Gyurcsanyi, R.E. The water uptake of plasticized Poly(vinyl chloride) solid-contact calcium-selective electrodes. Electroanalysis 2011, 23, 2156–2163. [Google Scholar] [CrossRef]
- Michalska, A.; Konopka, A.; Maj-Zurawska, M. All-solid-state calcium solvent polymeric membrane electrode for low-level concentration measurements. Anal. Chem. 2003, 75, 141–144. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Yang, X.; Luo, Z.; Zhang, J.; Li, G. All-solid-state blood calcium sensors based on screen-printed poly(3,4-ethylenedioxythiophene) as the solid contact. Sens. Actuators B 2012, 173, 630–635. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Y.; Luo, Z.; Pan, Y. A miniature all-solid-state calciumelectrode applied to in situ seawater measurement. Meas. Sci. Technol. 2013, 24, 5105. [Google Scholar] [CrossRef]
- Boeva, Z.A.; Lindfors, T. Few-layer graphene and polyaniline composite as ion-to-electron transducer in silicone rubber solid-contact ion-selective electrodes. Sens. Actuators B 2016, 224, 624–631. [Google Scholar] [CrossRef]
- Trudeau, D.L.; Freier, E.F. Determination of calcium in urine and serum by atomic absorption spectrophotometry (AAS). Clin. Chem. 1967, 13, 101–114. [Google Scholar] [CrossRef]
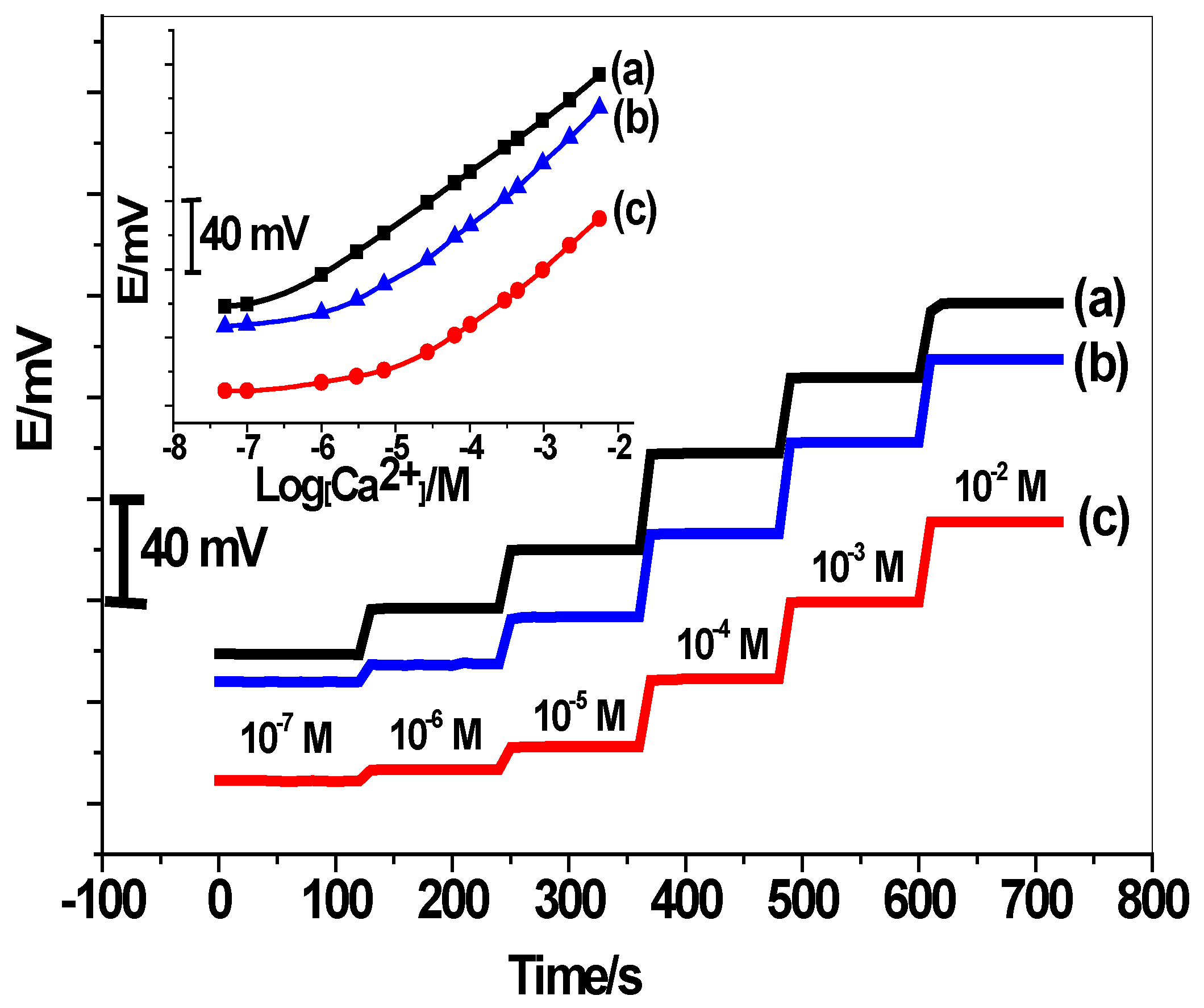

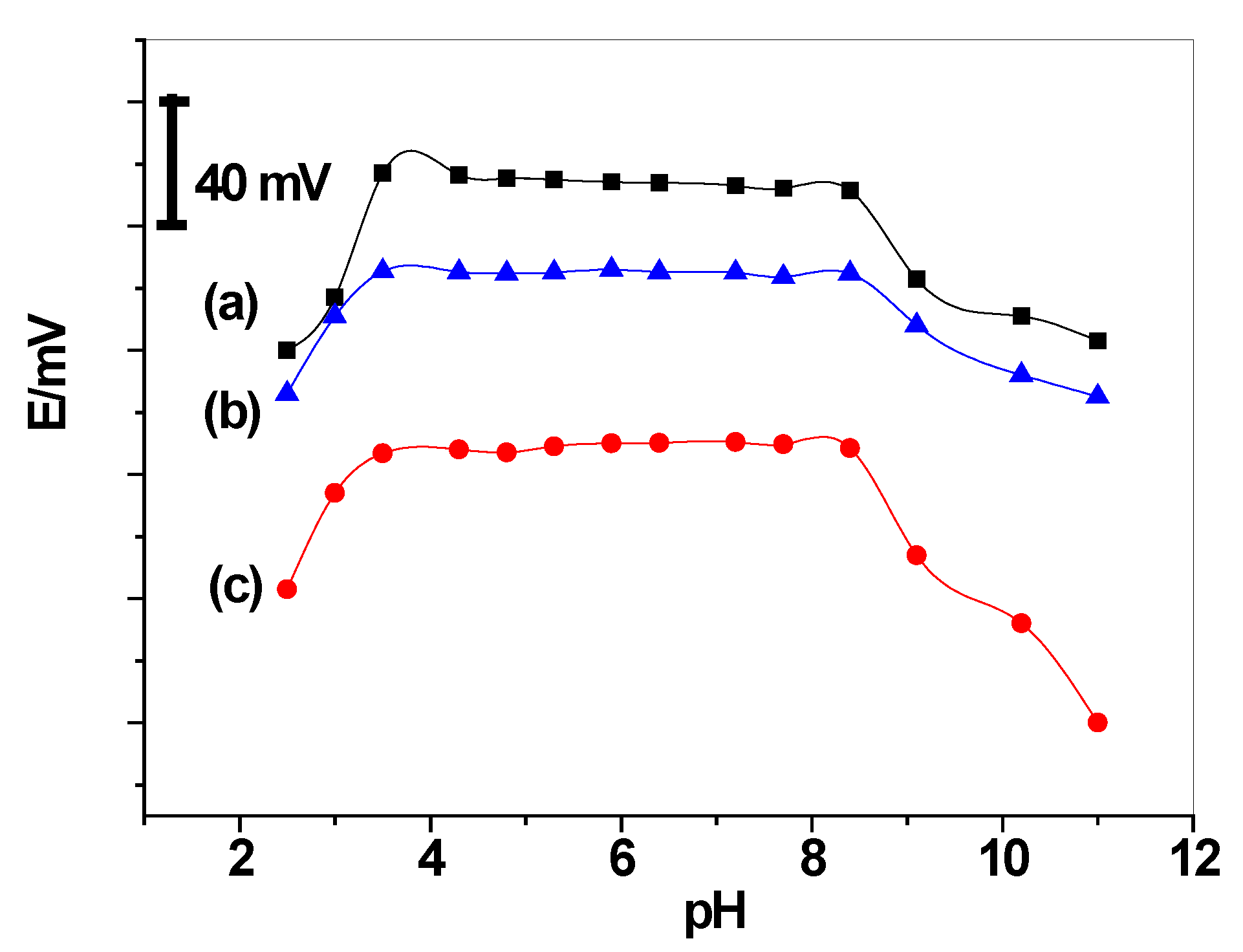
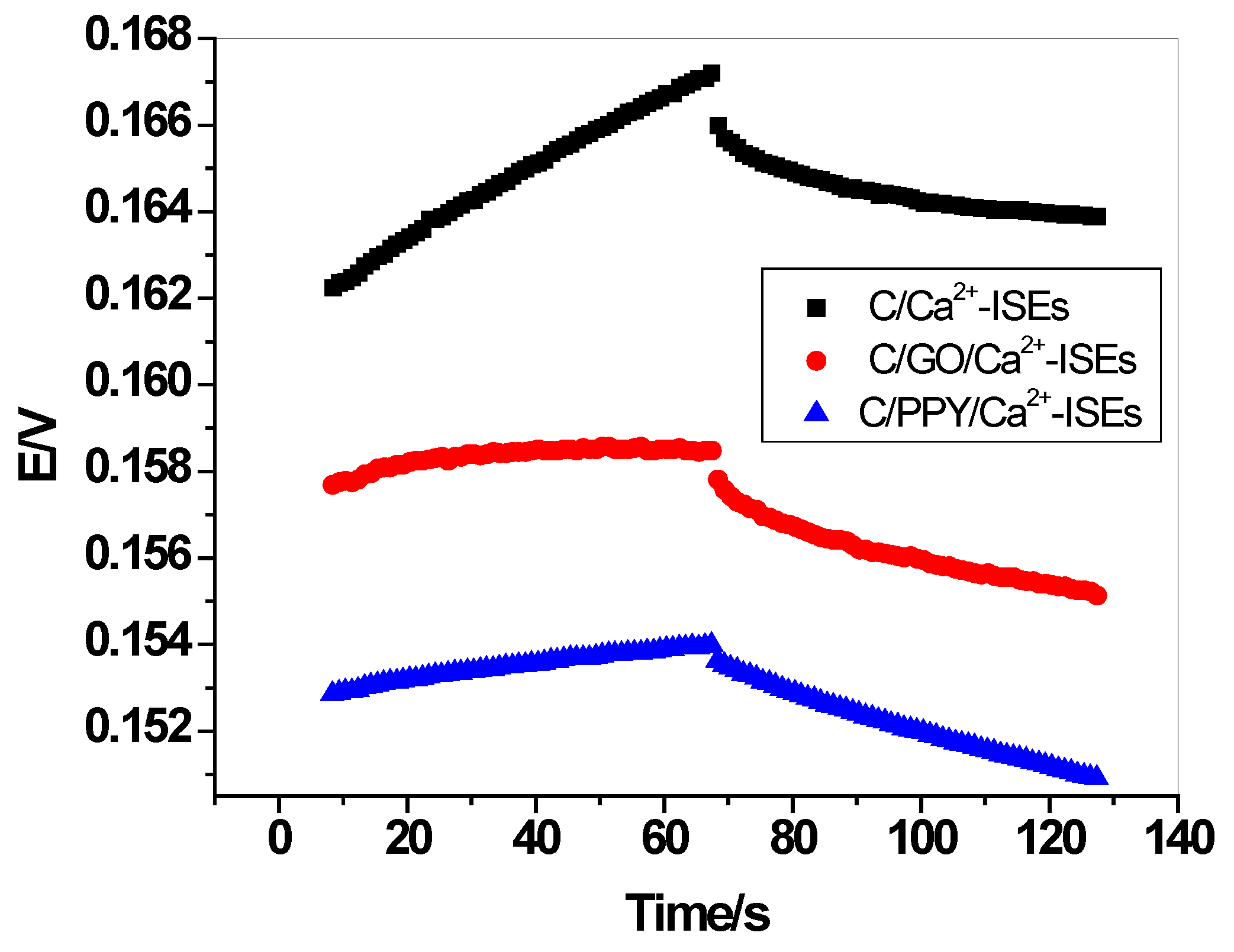
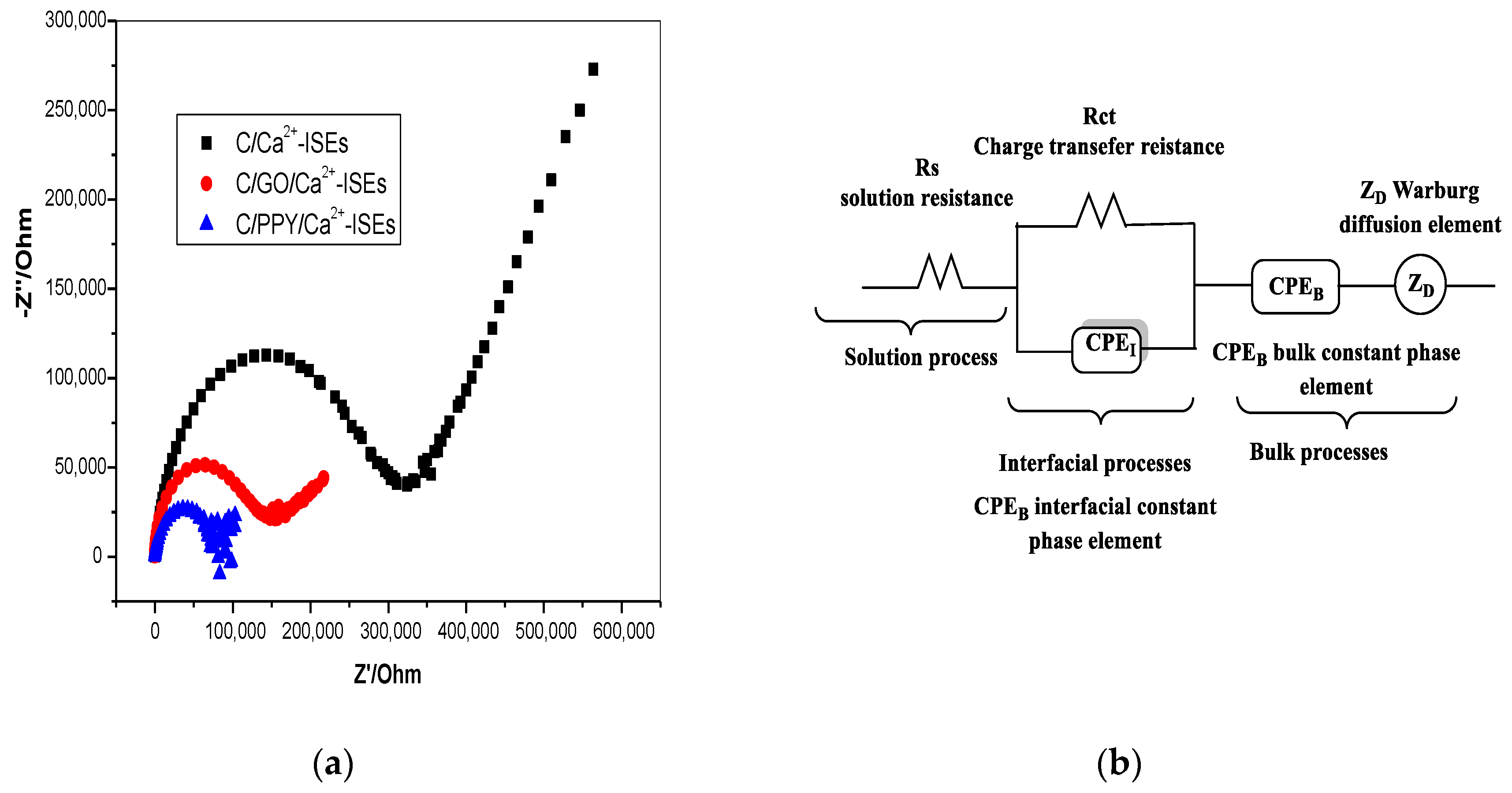
| Parameter | C/PPY/Ca2+-ISEs | C/GO/Ca2+-ISEs | C/Ca2+-ISEs |
|---|---|---|---|
| Slope (mV/decade) | 38.1 ± 0.4 | 31.1 ± 0.6 | 34.7 ± 0.3 |
| Correlation coefficient (r2) | 0.996 | 0.999 | 0.995 |
| Linear range (M) | 7.0 × 10−6–1.0 × 10−2 | 7.0 × 10−7–1.0 × 10−2 | 1.0 × 10−5–1.0 × 10−2 |
| Detection limit (M) | 3.8 × 10−6 | 2.3 × 10−7 | 8.0 × 10−6 |
| Working acidity range (pH) | 4.0–7.4 | 3.5–8.4 | 3.5–8.4 |
| Response time (s) | <5 | <5 | <5 |
| Accuracy (%) | 99.1 | 99.4 | 98.8 |
| Precision (%) | 1.3 | 0.7 | 1.1 |
| Trueness (%) | 99.2 | 99.3 | 98.7 |
| Bias (%) | 0.6 | 0.4 | 0.9 |
| Within-day repeatability (%) | 0.8 | 0.5 | 1.1 |
| Between-days variations (%) | 1.1 | 0.8 | 1.2 |
| Sensor Type | Log KpotCa2+,J | ||||||
|---|---|---|---|---|---|---|---|
| Na+ | K+ | NH4+ | Mg2+ | Ba2+ | Fe2+ | Li+ | |
| C/PPY/Ca2+-ISEs | −4.3 ± 0.3 | −3.7 ± 0.5 | −3.9 ± 0.8 | −5.1 ± 0.3 | −5.8 ± 0.2 | −3.9 ± 0.4 | −6.3 ± 0.2 |
| C/GO/Ca2+-ISEs | −4.4 ± 0.2 | −3.8 ± 0.7 | −3.8 ± 0.7 | −5.3 ± 0.1 | −5.7 ± 0.4 | −3.5 ± 0.7 | −6.1 ± 0.5 |
| C/Ca2+-ISEs | −4.2 ± 0.5 | −3.7 ± 0.4 | −3.6 ± 0.6 | −5.4 ± 0.2 | −5.6 ± 0.3 | −3.1 ± 0.8 | −6.2 ± 0.3 |
| Ionophore | Functional Materials | Conductive Substrate | Detection Limit, M | Slope, mV/decade | Potential Drift, µV/s | Interfacial Capacitance, µF | Ref. |
|---|---|---|---|---|---|---|---|
| Calcium ionophore IV (ETH 5234) | Black phosphorus | GC | 4.0 × 10−7 | 28.3 ± 0.7 | 72 | - | [30] |
| Calcium ionophore II (ETH129) | SWCNTs | Cu | 6.3 × 10−7 | 28.7 | 930 | 5.37 | [31] |
| Calcium ionophore II | P-NPEDMA | Au | 3.2 × 10−6 | 30.2 ± 0.5 | - | - | [32] |
| Calcichrome | Polypyrrole | Pt or GC | 1.0 × 10−5 | 29.1 ± 0.8 | - | - | [33] |
| Calcium ionophore IV (ETH 5234) | Polyazulene | Pt/ZnSe | - | - | 0.14 | 874 | [34] |
| Calcium ionophore IV (ETH 5234) | POT | Pt/ZnSe | 2.0 × 10−8 | 27.3 ± 0.1 | - | - | [35] |
| Calcium ionophore I (ETH-1001) | POT | GC | 1.0 × 10−6 | 25.0 ± 0.9 | - | - | [36] |
| Calcium ionophore II (ETH129) | PEDOT/PSS | Carbon | 1.0 × 10−4 | 30.0 ± 1.0 | - | - | [37] |
| Calcium ionophore II (ETH129) | PEDOT/PSS | Pt | 1.0 × 10−6 | 27.7 | - | - | [38] |
| Calcium ionophore I (ETH-1001) | Graphene/Polyaniline | GC | 5.0 × 10−8 | 28.7 ± 0.1 | 87 | 11 | [39] |
| Bilirubin | Polypyrrol | Carbon | 3.8 × 10−6 | 38.1 ± 0.4 | 18.3 | 54.6 | This work |
| Graphene oxide | 2.3 × 10−7 | 31.1 ± 0.6 | 11.4 | 88.4 |
| Sample | Labeled Amount mg/tab. or mg/g | Potentiometry | AAS | bF-Test | ||
|---|---|---|---|---|---|---|
| mg/tab. or Cap. | a Mean% ± SD | mg/tab. or Cap. | a Mean% ± SD | |||
| Pharmaceutical formulations | ||||||
| Calcimate (ADWIC, Egypt) | 500 mg/tablet | 497.3 ± 1.2 | 99.4 ± 0.7 | 499.2 ± 0.2 | 99.8 ± 0.1 | 4.46 |
| Vitacid calcium (CID, Egypt) | 800 mg/tablet | 787.3 ± 3.1 | 98.4 ± 1.1 | 795.1 ± 0.7 | 99.4 ± 0.2 | 3.72 |
| Baby-food products | ||||||
| Cerelac + rice | 4.3 mg/g | 3.9 ± 0.3 | 90.7 ± 1.2 | 4.1 ± 0.5 | 95.3 ± 2.1 | 1.76 |
| Cerelac + wheat + 4 fruits | 5.0 mg/g | 4.7 ± 0.6 | 94 ± 2.2 | 5.04 ± 0.4 | 100.8 ± 0.4 | 2.23 |
| Nestle® NIDO® FORTIFIED Milk Powder Tin 900 g | 8.6 mg/g | 8.8 ± 0.4 | 102.3 ± 0.4 | 8.7 ± 0.2 | 101.1 ± 0.7 | 3.34 |
| Sample | * Calcium Content, mg/dL | aF-Test | |
|---|---|---|---|
| Potentiometry | AAS | ||
| Volunteer 1 | 9.3 ± 1.5 | 9.5 ± 0.7 | 4.12 |
| Volunteer 2 | 10.5 ± 0.9 | 10.3 ± 0.3 | 3.36 |
| Volunteer 3 | 7.8 ± 0.6 | 8.4 ± 0.2 | 1.43 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-Rabboh, H.S.M.; H. Kamel, A.; Amr, A.E.-G.E. All-Solid-State Calcium Sensors Modified with Polypyrrol (PPY) and Graphene Oxide (GO) as Solid-Contact Ion-to-Electron Transducers. Chemosensors 2020, 8, 93. https://doi.org/10.3390/chemosensors8040093
Abd-Rabboh HSM, H. Kamel A, Amr AE-GE. All-Solid-State Calcium Sensors Modified with Polypyrrol (PPY) and Graphene Oxide (GO) as Solid-Contact Ion-to-Electron Transducers. Chemosensors. 2020; 8(4):93. https://doi.org/10.3390/chemosensors8040093
Chicago/Turabian StyleAbd-Rabboh, Hisham S. M., Ayman H. Kamel, and Abd El-Galil E. Amr. 2020. "All-Solid-State Calcium Sensors Modified with Polypyrrol (PPY) and Graphene Oxide (GO) as Solid-Contact Ion-to-Electron Transducers" Chemosensors 8, no. 4: 93. https://doi.org/10.3390/chemosensors8040093
APA StyleAbd-Rabboh, H. S. M., H. Kamel, A., & Amr, A. E.-G. E. (2020). All-Solid-State Calcium Sensors Modified with Polypyrrol (PPY) and Graphene Oxide (GO) as Solid-Contact Ion-to-Electron Transducers. Chemosensors, 8(4), 93. https://doi.org/10.3390/chemosensors8040093







