Electrochemical Hybrid Methods and Sensors for Antioxidant/Oxidant Activity Monitoring and Their Use as a Diagnostic Tool of Oxidative Stress: Future Perspectives and Challenges
Abstract
:1. Introduction
- the current state of sensor development to determine AOA/OA (concentration of determined compounds);
- terminology, importance of AOA/OA units expression standardization;
- hybrid variants of chronopotentiometry and chronoamperometry as a tool for AOA/OA determination
- invasive and non-invasive electrochemical methods and sensors for estimation of AOA/OA of biological fluids and tissue (blood/serum (plasma), semen, sweat, saliva and skin.
- application of sensors for testing of the AOA/OA state of healthy volunteers and patients with various diseases in compare with clinical and medical features;
- AOA of plants, food, nutrients, raw materials, drugs and cosmetics; the future perspectives of AOA/OA electrochemical estimation.
- (a)
- voltammetric and amperometric sensors, in which the current is measured: in these cases, chemically inert or active and modified electrodes are used.
- (b)
- potentiometric sensors in which the potential of the indicator electrode is measured against a reference electrode [16].
2. Methods
- The soluble mediator (signal generating) system is introduced into the solution to be analyzed
- The signal generating system containing sparingly soluble compounds is placed on the surface of the electrode.
- The increment of K4[Fe(CN)6] oxidation current, resulting from the interaction of the AO, containing in the sample, with the oxidized form of the mediator system K3[Fe(CN)6] previously introduced into the solution (chronoamperometric version) [17].
2.1. Invasive and Non-Invasive Electrochemical Methods in Estimation of AOA/OA of Bioliquids (Blood/Serum (Plasma), Semen, Sweat, Saliva) and Tissue (Skin)
2.2. Sensors
2.3. Measuring Means
Sensors of AOA in Medicine
2.4. Sensors Application in Determination of Antioxidant Activity of Plants, Nutrients, Food, Drugs and Cosmetics
2.5. Future Perspectives and Challenges
Funding
Conflicts of Interest
References
- Lomonosov, M.V. A word about the benefits of chemistry, in the public meeting of the imperial academy of sciences in 6 September 1751, spoken by Mikhail Lomonosov. In Complete Works. Works on Physics and Chemistry. 1747–1752 (in Russian); Publishing House of the Academy of Sciences of the USSR: Moscow, Leningrad, 1951; Volume 2, p. 362. [Google Scholar]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Ivanova, A.V.; Sharafutdinova, E.N.; Lozovskaya, E.L.; Shkarina, E.I. Potentiometry as a method of antioxidant activity investigation. Talanta 2007, 71, 13–84. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Alyoshina, L.V.; Gerasimova, E.L.; Kazakov, Y.E.; Ivanova, A.V.; Beykin, Y.B.; Belyaeva, S.V.; Usatova, T.I.; Khodos, M.Y. New electrochemical method of determining blood and blood fractions antioxidant activity. Electroanalysis 2009, 21, 618–624. [Google Scholar] [CrossRef]
- Ivanova, A.V.; Gerasimova, E.L.; Brainina, K.Z. Potentiometric study of antioxidant activity: Development and prospects. Crit. Rev. Anal. Chem. 2015, 45, 311–322. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Varzakova, D.P.; Kazakov, Y.E.; Vidrevich, M.B. Noninvasive electrochemical antioxidant activity estimation: Saliva analysis. Biointerface Res. Appl. Chem. 2018, 8, 3381–3387. [Google Scholar]
- Brainina, K.; Stozhko, N.; Vidrevich, M. Antioxidants: Terminology, Methods, and Future Considerations. Antioxidants 2019, 8, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazakov, Y.; Tarasov, A.; Alyoshina, L.; Brainina, K. Interplay between antioxidant activity, health and disease. Biointerface Res. Appl. Chem. 2020, 10, 4893–4901. [Google Scholar]
- Brainina, K.Z.; Tarasov, A.V.; Vidrevich, M.B. Silver Chloride/Ferricyanide-Based Quasi-Reference Electrode for Potentiometric Sensing Applications. Chemosensors 2020, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Brainina, K.; Tarasov, A.; Khamzina, E.; Kazakov, Y.; Stozhko, N. Disposable potentiometric sensory system for skin antioxidant activity evaluation. Sensors 2019, 19, 2586. [Google Scholar] [CrossRef] [Green Version]
- Brainina, K.; Tarasov, A.; Khamzina, E.; Stozhko, N.; Vidrevich, M. Contact hybrid potentiometric method for on-site and in situ estimation of the antioxidant activity of fruits and vegetables. Food Chem. 2020, 309, 125703. [Google Scholar] [CrossRef]
- Kazakov, Y.; Khodos, M.; Vidrevich, M.; Brainina, K. Potentiometry as a tool for monitoring of antioxidant activity and oxidative stress estimation in medicine. Crit. Rev. Anal. Chem. 2019, 49, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Sochor, J.; Dobes, J.; Krystofova, O.; Ruttkay-Nedecky, B.; Babula, P.; Pohanka, M.; Jurikova, T.; Zitka, O.; Adam, V.; Klejdus, B.; et al. Electrochemistry as a Tool for Studying Antioxidant Properties. Int. J. Electrochem. Sci. 2013, 8, 8464–8489. [Google Scholar]
- Pisoschi, A.M.; Cimpeanu, C.; Predoi, G. Electrochemical Methods for Total Antioxidant Capacity and its Main Contributors Determination: A review. Open Chem. 2015, 13, 824–856. [Google Scholar] [CrossRef] [Green Version]
- Kozitsina, A.N.; Svalova, T.S.; Malysheva, N.N.; Okhokhonin, A.V.; Vidrevich, M.B.; Brainina, K.Z. Sensors Based on Bio and Biomimetic Receptors in Medical Diagnostic, Environment, and Food Analysis. Biosensors 2018, 8, 35. [Google Scholar] [CrossRef] [Green Version]
- Hulanicki, A.; Glab, S.; Ingman, F. Chemical sensors: Definitions and classification. Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Varzakova, D.P.; Gerasimova, E.L. A chronoamperometric method for determining total antioxidant activity. J. Anal. Chem. 2012, 67, 364–369. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Karyakina, E.E.; Gorton, L. On the mechanism of H2O2 reduction at Prussian Blue modified electrodes. Electrochem. Commun. 1999, 1, 78–82. [Google Scholar] [CrossRef]
- Komkova, M.; Zarochintsev, A.; Karyakina, E.; Karyakin, A. Electrochemical and sensing properties of Prussian Blue based nanozymes “artificial peroxidase”. J. Electroanal. Chem. 2020, 114048. [Google Scholar] [CrossRef]
- Komkova, M.A.; Karyakina, E.E.; Marken, F.; Karyakin, A.A. Hydrogen Peroxide Detection in Wet Air with a Prussian Blue Based Solid Salt Bridged Three Electrode System. Anal. Chem. 2013, 85, 2574–2577. [Google Scholar] [CrossRef]
- Mokrushina, A.V.; Heim, M.; Karyakina, E.E.; Kuhn, A.; Karyakin, A.A. Enhanced hydrogen peroxide sensing based on Prussian Blue modified macroporous microelectrodes. Electrochem. Commun. 2013, 29, 78–80. [Google Scholar] [CrossRef]
- Sitnikova, N.A.; Mokrushina, A.V.; Karyakin, A.A. Iron triad-mate hexacyanoferrates as Prussian Blue stabilizers: Toward the advanced hydrogen peroxide transducer. Electrochim. Acta 2014, 122, 173–179. [Google Scholar] [CrossRef]
- Karpova, E.V.; Karyakina, E.E.; Karyakin, A.A. Iron–nickel hexacyanoferrate bilayer as an advanced electrocatalyst for H2O2 reduction. RSC Adv. 2016, 6, 103328–103331. [Google Scholar] [CrossRef] [Green Version]
- Karpova, E.V.; Shcherbacheva, E.V.; Galushin, A.A.; Vokhmyanina, D.V.; Karyakina, E.E.; Karyakin, A.A. Noninvasive Diabetes Monitoring through Continuous Analysis of Sweat Using Flow-Through Glucose Biosensor. Anal. Chem. 2019, 91, 3778–3783. [Google Scholar] [CrossRef] [PubMed]
- Komkova, M.A.; Pasquarelli, A.; Andreev, E.A.; Galushin, A.A.; Karyakin, A.A. Prussian Blue modified boron-doped diamond interfaces for advanced H2O2 electrochemical sensors. Electrochim. Acta 2020, 339, 135924. [Google Scholar] [CrossRef]
- Puganova, E.A.; Karyakin, A.A. New materials based on nanostructured Prussian blue for development of hydrogen peroxide sensors. Sens. Actuators B Chem. 2005, 109, 167–170. [Google Scholar] [CrossRef]
- Suprun, E.V.; Karpova, E.V.; Radko, S.P.; Karyakin, A.A. Advanced electrochemical detection of amino acids and proteins through flow injection analysis and catalytic oxidation on Prussian Blue. Electrochim. Acta 2020, 331, 135289. [Google Scholar] [CrossRef]
- Karyakina, E.E.; Vokhmyanina, D.V.; Sizova, N.V.; Sabitov, A.N.; Borisova, A.V.; Sazontova, T.G.; Arkhipenko, Y.V.; Tkachuk, V.A.; Zolotov, Y.A.; Karyakin, A.A. Kinetic approach for evaluation of total antioxidant activity. Talanta 2009, 80, 749–753. [Google Scholar] [CrossRef]
- Nasri, Z.; Kahlert, H.; Scholz, F. A chronopotentiometric sensor for assays of redox-active compounds. Electrochem. Commun. 2014, 49, 18–20. [Google Scholar] [CrossRef]
- Kohen, R.; Fanberstein, D.; Tirosh, O. Non-Invasive Device and Method for Quantitative Determination of Oxidants and/or Antioxidants in the Skin. U.S. Patent No. 6,108,570, 10 October 1995. [Google Scholar]
- Kh, Z.; Brainina, E.L.; Gerasimova, M.Y. Khodos, Method of Noninvasive Potentiometric Determination of Oxidant/Antioxidant Activity of Biological Tissues and Device for Its Realization. RF Patent RU 2433405 C1, 23 June 2010. [Google Scholar]
- Brainina, K.Z.; Khodos, M.Y.; Sudakova, L.A.; Chernov, V.I. Device for Non-invasive Potentiometric Determination of Oxidant/Antioxidant Activity of Biological Tissues. RF Patent RU 2552942C2, 28 October 2013. [Google Scholar]
- Brajnina, K.Z.; Khodos, M.Y.; Zakharov, A.S. Method of Determining Integral Antioxidant/Oxidant Activity of Organic Condensed Media. RF Patent RU 2595814C1, 17 June 2015. [Google Scholar]
- Brainina, K.Z.; Galperin, L.G.; Gerasimova, E.L.; Khodos, M.Y. Noninvasive potentiometric method of determination of skin oxidant/antioxidant activity. IEEE Sens. J. 2012, 12, 527–532. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Markina, M.G.; Stozhko, N.Y. Optimized potentiometric assay for non-invasive investigation of skin antioxidant activity. Electroanalysis 2018, 30, 2405–2412. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Gerasimova, E.L.; Varzakova, D.P.; Kazakov, Y.E.; Galperin, L.G. Noninvasive method of determining skin antioxidant/oxidant activity: Clinical and cosmetics applications. Anal. Bioanal. Electrochem. 2013, 5, 528–542. [Google Scholar]
- Markina, M.; Lebedeva, E.; Neudachina, L.; Stozhko, N.; Brainina, K. Determination of antioxidants in human skin by capillary zone electrophoresis and potentiometry. Anal. Lett. 2016, 49, 1804–1815. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Tarasov, A.V.; Kazakov, Y.E.; Vidrevich, M.B. Platinum electrode regeneration and quality control method for chronopotentiometric and chronoamperometric determination of antioxidant activity of biological fluids. J. Electroanal. Chem. 2018, 808, 14–20. [Google Scholar] [CrossRef]
- Lee, G.-J.; Lee, S.-K.; Kim, J.-M.; Rhee, C.K.; Lee, Y.-K.; Brainina, K.Z.; Kazakov, Y.E. Application Feasibility of Antioxidant Activity Evaluation using Potentiometry in Major Depressive Disorder. Electrochemistry 2014, 82, 264–266. [Google Scholar] [CrossRef] [Green Version]
- Brainina, K.Z.; Bukharinova, M.A.; Stozhko, N.Y.; Sokolkov, S.V.; Tarasov, A.V.; Vidrevich, M.B. Electrochemical Sensor Based on a Carbon Veil Modified by Phytosynthesized Gold Nanoparticles for Determination of Ascorbic Acid. Sensors 2020, 20, 1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stozhko, N.; Bukharinova, M.; Galperin, L.; Brainina, K. A nanostructured sensor based on gold nanoparticles and nafion for determination of uric acid. Biosensors 2018, 8, 21. [Google Scholar] [CrossRef] [Green Version]
- Brainina, K.; Stozhko, N.; Bukharinova, M.; Vikulova, E. Nanomaterials: Electrochemical Properties and Application in Sensors. In Nanoanalytics. Nanoobjects and Nanotechnologies in Analytical Chemistry; Chapter 6; Shtykov, S., Ed.; De Gruyter: Berlin, Germany, 2018; pp. 165–222. [Google Scholar] [CrossRef]
- Botelho, C.; Pereira, N.; Silva, G.; Menezes, A.; Bezerra, C.; Damos, F.; Luz, R. Photoelectrochemical-assisted determination of caffeic acid exploiting a composite based carbon nanotubes, cadmium telluride quantum dots, and titanium dioxide. Anal. Methods 2019, 11, 4775–4784. [Google Scholar] [CrossRef]
- David, M.; Serban, A.; Radulescu, C.; Danet, A.F.; Florescu, M. Bioelectrochemical evaluation of plant extracts and gold nanozyme-based sensors for total antioxidant capacity determination. Bioelectrochemistry 2019, 129, 124–134. [Google Scholar] [CrossRef]
- Arman, A.; Üzer, A.; Sağlam, Ş.; Erçağ, E.; Apak, R. Indirect electrochemical determination of antioxidant capacity with hexacyanoferrate(III) reduction using a gold nanoparticle-coated o-phenylenediamine-aniline copolymer electrode. Anal. Lett. 2019, 52, 1282–1297. [Google Scholar] [CrossRef]
- Markina, M.; Stozhko, N.; Krylov, V.; Vidrevich, M.; Brainina, K. Nanoparticle-based paper sensor for thiols evaluation in human skin. Talanta 2017, 165, 563–569. [Google Scholar] [CrossRef]
- Apak, R.; Çekiç, S.D.; Üzer, A.; Çelik, S.E.; Bener, M.; Bekdeşer, B.; Can, Z.; Saglam, Ş.; Önem, A.N.; Erçag, E. Novel spectroscopic and electrochemical sensors and nanoprobes for the characterization of food and biological antioxidants. Sensors 2018, 18, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choleva, T.G.; Kappi, F.A.; Giokas, D.L.; Vlessidis, A.G. Paper-based assay of antioxidant activity using analyte-mediated on-paper nucleation of gold nanoparticles as colorimetric probes. Anal. Chim. Acta 2015, 860, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.E.; Li, X.; Yin, Y.; Indrisek, N. Determination of antioxidant activities in fruit juices based on rapid colorimetric measurement and characterization of gold nanoparticles. Int. J. Environ. Anal. Chem. 2015, 95, 531–541. [Google Scholar] [CrossRef]
- Antuña-Jiménez, D.; González-García, M.B.; Hernández-Santos, D.; Fanjul-Bolado, P. Screen-Printed Electrodes Modified with Metal Nanoparticles for Small Molecule Sensing. Biosensors 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komkova, M.A.; Karyakina, E.E.; Karyakin, A.A. Method for Determining Concentration of Hydrogen Peroxide in Solution. RF Patent RU 2682568 C1, 3 November 2017. [Google Scholar]
- Sharafutdinova, E.N.; Ivanova, A.V.; Matern, A.I.; Brainina, H.Z. Food quality and antioxidant activity. Anal. Control. 2011, 15, 281–286. (In Russian) [Google Scholar]
- Brainina, K.; Stozhko, N.; Bukharinova, M.; Khamzina, E.; Vidrevich, M. Potentiometric method of plant microsuspensions antioxidant activity determination. Food. Chem. 2019, 278, 653–658. [Google Scholar] [CrossRef]
- Pramanik, P.K.D.; Nayyar, A.; Pareek, G. WBAN: Driving e-healthcare Beyond Telemedicine to Remote Health Monitoring: Architecture and Protocols. In Telemedicine Technologies. Big Data, Deep Learning, Robotics, Mobile and Remote Applications for Global Healthcare; Jude, H.D., Balas, V.E., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 89–119. ISBN 9780128169483. [Google Scholar] [CrossRef]
- Lont, M.; Milosevic, D.; van Roermund, A.H.M. Wake-up Receiver Based Ultra-Low-Power WBAN; Springer: Berlin, Germany, 2014; pp. 7–28. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Guo, H.; Li, J.; Bandodkar, A.J.; John, A. Body-Interfaced Chemical Sensors for Noninvasive Monitoring and Analysis of Biofluids. Trends Chem. 2019, 1, 559–571. [Google Scholar] [CrossRef]
- Fan, R.; Andrew, T.L. Perspective—Challenges in Developing Wearable Electrochemical Sensors for Longitudinal Health Monitoring. J. Electrochem. Soc. 2020, 167, 037542. [Google Scholar] [CrossRef] [Green Version]
- Seshadri, D.R.; Li, R.T.; Voos, J.E.; Rowbottom, J.R.; Alfes, C.M.; Zorman, C.A.; Drummond, C.K. Wearable sensors for monitoring the physiological and biochemical profile of the athlete. NPJ Digit. Med. 2019, 2, 72. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Song, Y.; Bo, X.; Min, J.; Pak, O.S.; Zhu, L.; Wang, M.; Tu, J.; Kogan, A.; Zhang, H.; et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 2020, 38, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Bandodkar, A.J.; Jia, W.; Wang, J. Tattoo-based wearable electrochemical devices: A Review. Electroanalysis 2015, 27, 562–572. [Google Scholar] [CrossRef]
- Alizadeh, A.; Burns, A.; Lenigk, R.; Gettings, R.; Ashe, J.; Porter, A.; McCaul, M.; Barrett, R.; Diamond, D.; White, P.; et al. A wearable patch for continuous monitoring of sweat electrolytes during exertion. Lab. Chip 2018, 18, 2632–2641. [Google Scholar] [CrossRef] [PubMed]
- Sempionatto, J.R.; Jeerapan, I.; Krishnan, S.; Wang, J. Wearable Chemical Sensors: Emerging Systems for On-body Analytical Chemistry. Anal. Chem. 2020, 92, 378–396. [Google Scholar] [CrossRef] [PubMed]
- Goud, K.Y.; Moonla, C.; Mishra, R.K.; Yu, C.; NarayanIrene, R.; Litvan, I.; Wang, J. Wearable Electrochemical Microneedle Sensor for Continuous Monitoring of Levodopa: Toward Parkinson Management. ACS Sens. 2019, 4, 2196–2204. [Google Scholar] [CrossRef]
- Montiel, V.R.V.; Sempionatto, J.R.; Campuzano, S.; Pingarrón, J.M.; de Ávila, B.E.F.; Wang, J. Direct electrochemical biosensing in gastrointestinal fluids. Anal. Bioanal. Chem. 2019, 411, 4597–4604. [Google Scholar] [CrossRef] [PubMed]
- Barfidokht, A.; Mishra, R.K.; Seenivasan, R.; Liu, S.; Hubble, L.J.; Wang, J.; Hall, D.A. Wearable electrochemical glove-based sensor for rapid and on-site detection of fentanyl. Sens. Actuators B Chem. 2019, 296, 126422. [Google Scholar] [CrossRef]
- Hubble, L.J.; Wang, J. Sensing at Your Fingertips: Glove-based Wearable Chemical Sensors. Electroanalysis 2019, 31, 428. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable Biosensors for Healthcare Monitoring. Nat. Biotechnol. 2019, 37, 389. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Martin, A.; García-Carmona, L.; Barfidokht, A.; Kurniawan, J.F.; Moreto, J.R.; Tang, G.; Shin, A.; Liu, X.; Escarpa, A.; et al. Skin-worn soft microfluidic potentiometric detection system. Electroanalysis 2019, 31, 239–245. [Google Scholar] [CrossRef]
- Zhai, Q.; Wang, Y.; Gong, S.; Ling, Y.; Yap, L.W.; Liu, Y.; Wang, J.; Simon, G.P.; Cheng, W. Vertical Gold Nanowires Stretchable Electrochemical Electrodes. Anal. Chem. 2018, 90, 13498–13505. [Google Scholar] [CrossRef]
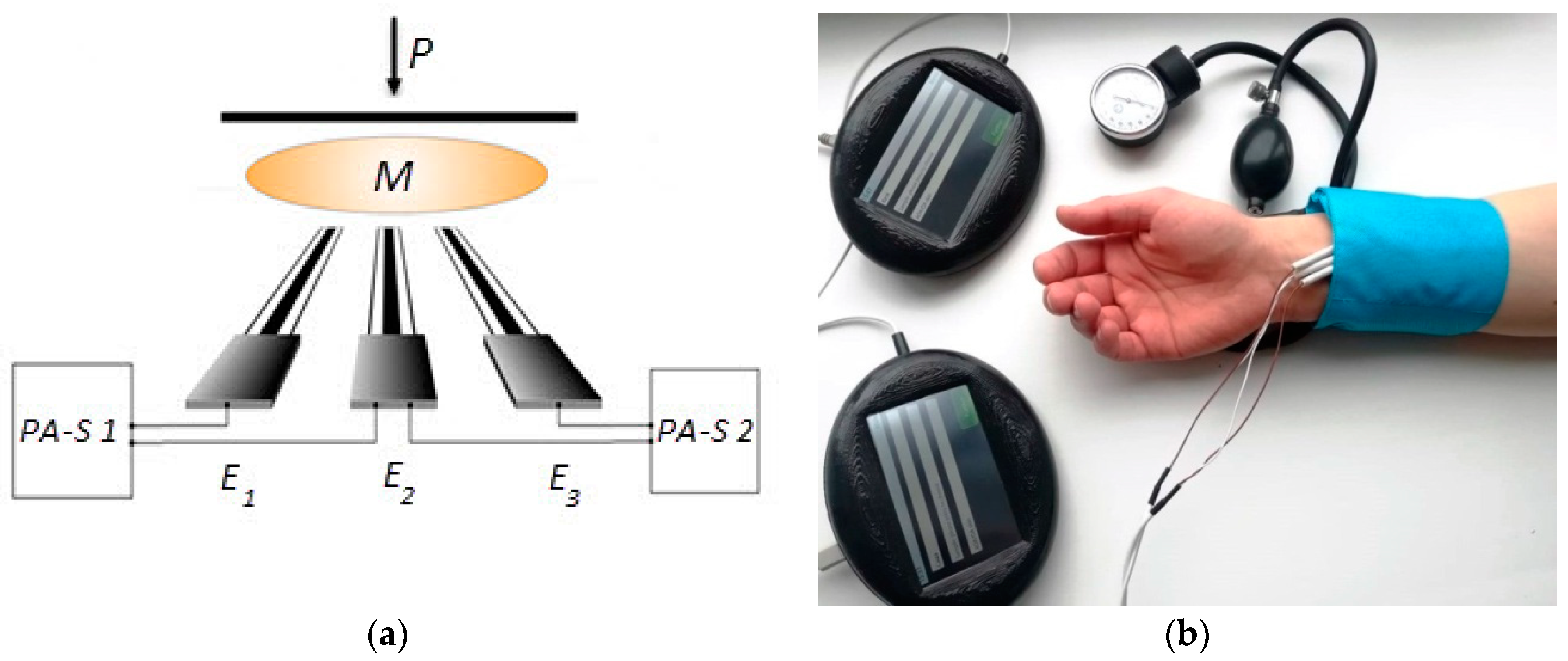
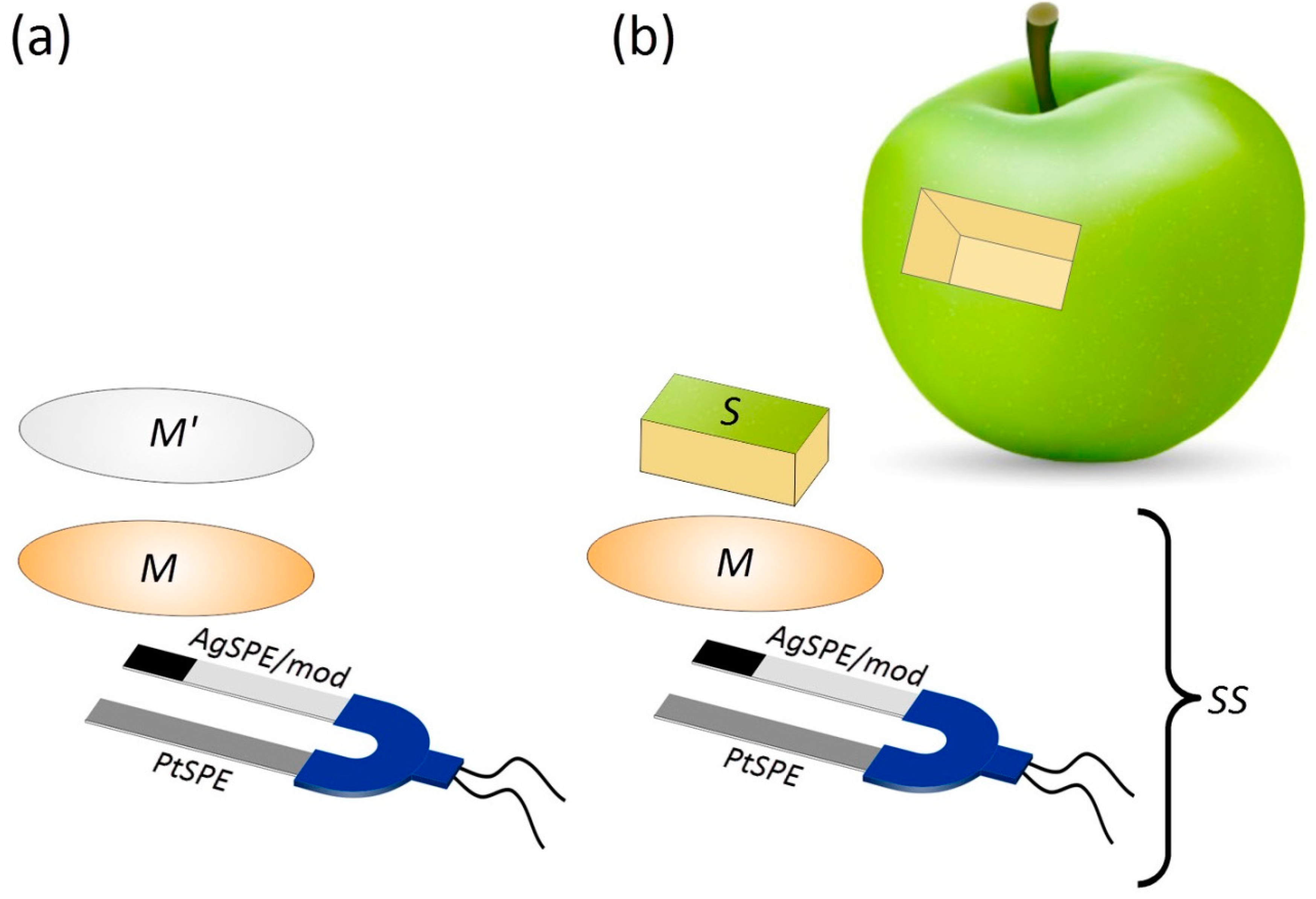
| Subgroup | AOA, mM-eq | t-Test |
|---|---|---|
| Men (n = 42) | 1.21 ± 0.26 | non-significantly difference |
| Women (n = 68) | 1.22 ± 0.30 | |
| Time of blood sampling: | - | - |
| without starvation (n = 36) | 1.42 ± 0.29 | significantly difference |
| after starvation period (n = 74) | 1.12 ± 0.23 |
| Fruit or Vegetable | CHPM (μM-eq/g) | |
|---|---|---|
 | garlic | 21.1 |
 | beet | 12.8 |
 | tomato | 10.0 |
 | peach | 8.6 |
 | pear | 7.3 |
 | melon | 7.1 |
 | cucumber | 3.8 |
 | apple | 3.5 |
 | pumpkin | 2.9 |
 | plum | 2.1 |
 | carrot | 1.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brainina, K.Z.; Kazakov, Y.E. Electrochemical Hybrid Methods and Sensors for Antioxidant/Oxidant Activity Monitoring and Their Use as a Diagnostic Tool of Oxidative Stress: Future Perspectives and Challenges. Chemosensors 2020, 8, 90. https://doi.org/10.3390/chemosensors8040090
Brainina KZ, Kazakov YE. Electrochemical Hybrid Methods and Sensors for Antioxidant/Oxidant Activity Monitoring and Their Use as a Diagnostic Tool of Oxidative Stress: Future Perspectives and Challenges. Chemosensors. 2020; 8(4):90. https://doi.org/10.3390/chemosensors8040090
Chicago/Turabian StyleBrainina, Khiena Z., and Yan E. Kazakov. 2020. "Electrochemical Hybrid Methods and Sensors for Antioxidant/Oxidant Activity Monitoring and Their Use as a Diagnostic Tool of Oxidative Stress: Future Perspectives and Challenges" Chemosensors 8, no. 4: 90. https://doi.org/10.3390/chemosensors8040090
APA StyleBrainina, K. Z., & Kazakov, Y. E. (2020). Electrochemical Hybrid Methods and Sensors for Antioxidant/Oxidant Activity Monitoring and Their Use as a Diagnostic Tool of Oxidative Stress: Future Perspectives and Challenges. Chemosensors, 8(4), 90. https://doi.org/10.3390/chemosensors8040090





