A Review of Nanostructured Resistive-Based Vanadium Oxide Gas Sensors
Abstract
1. Introduction
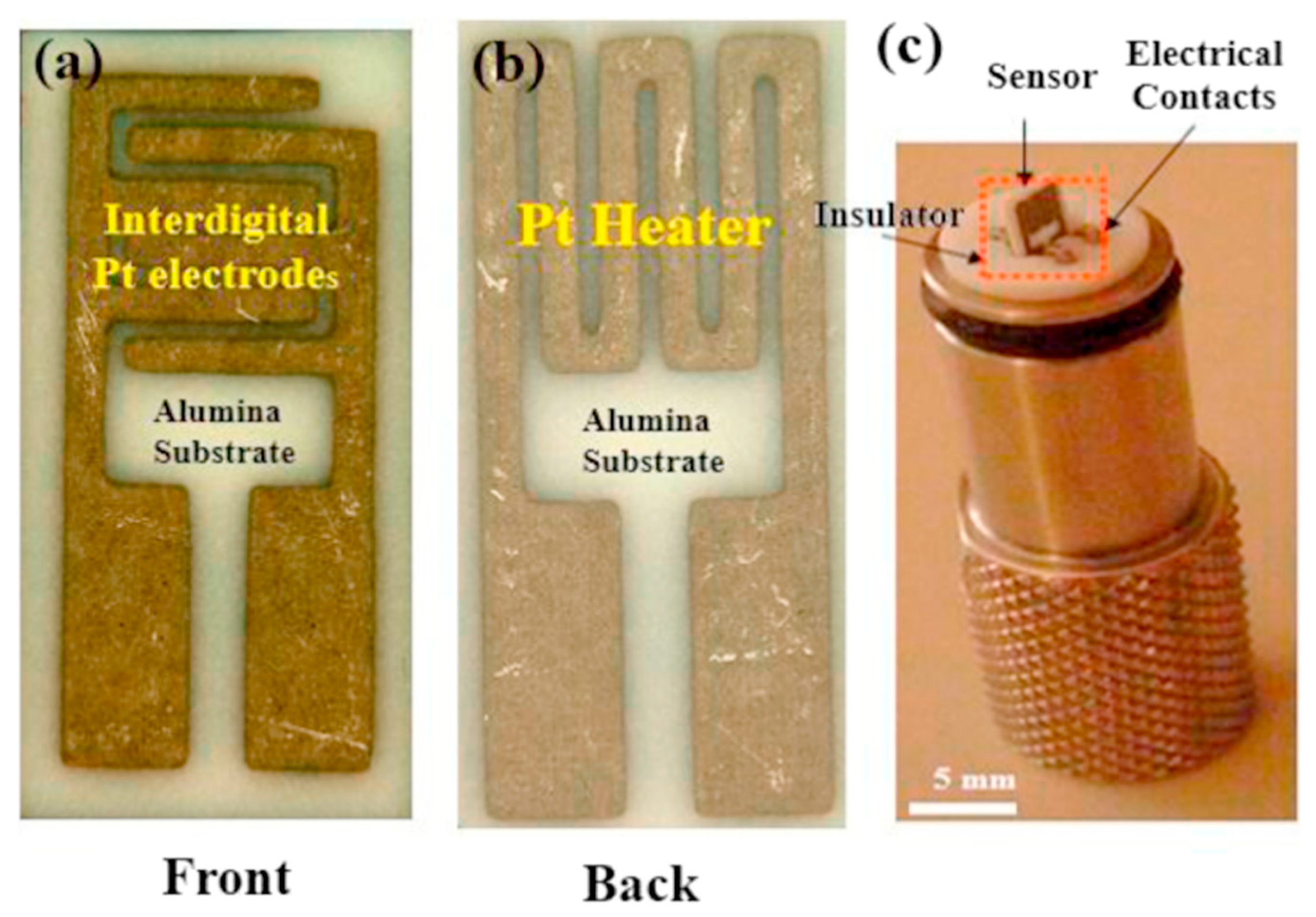
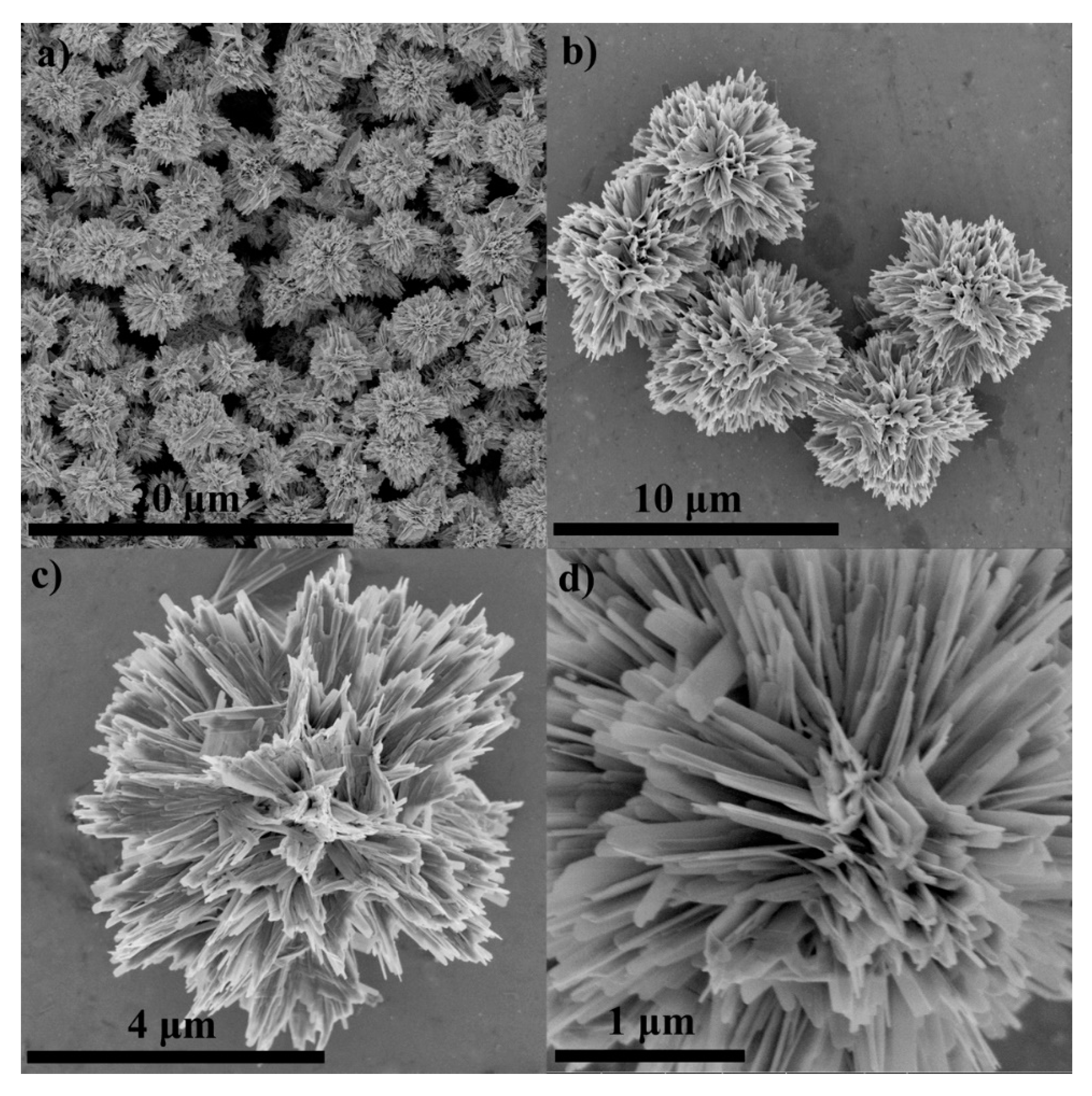
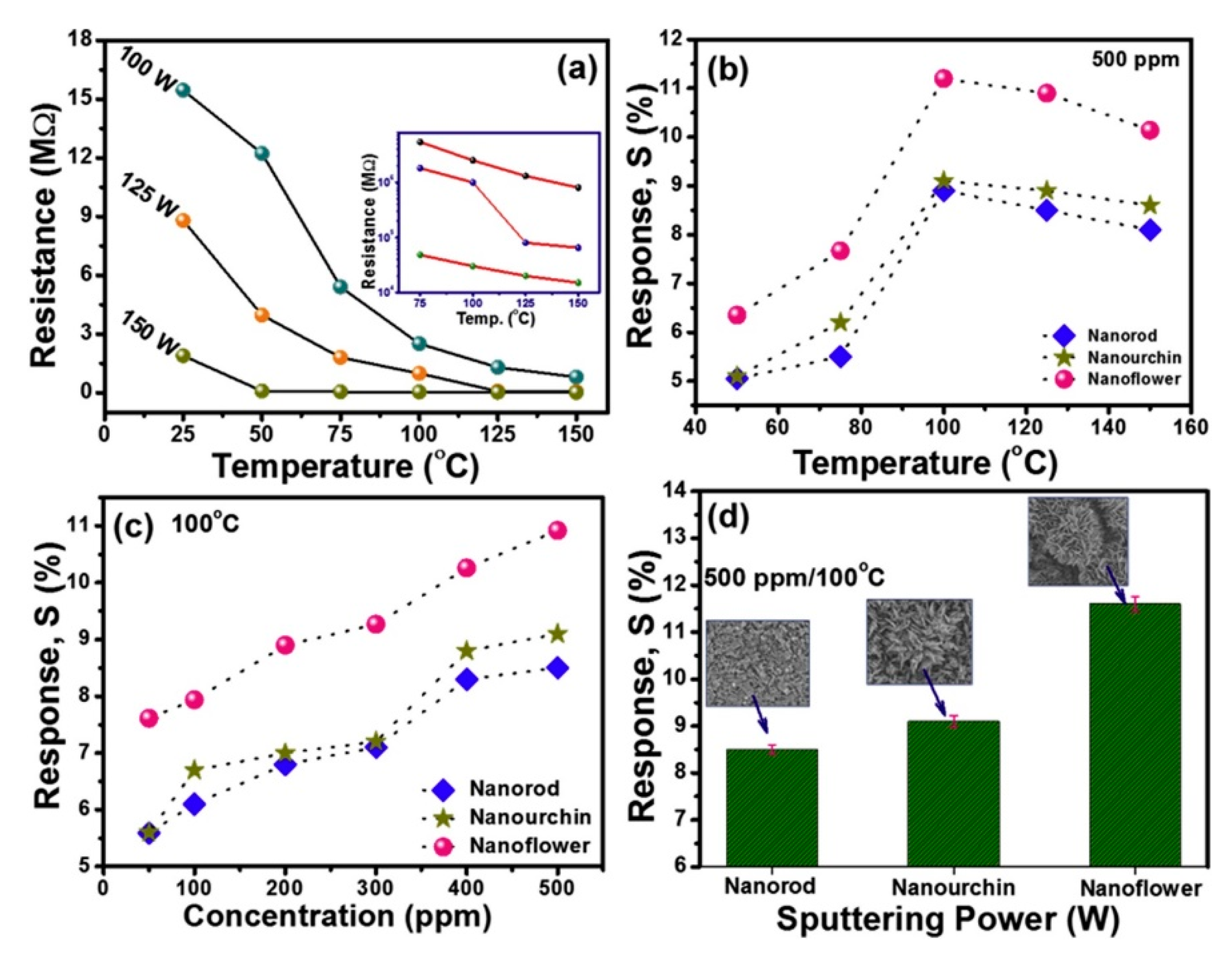
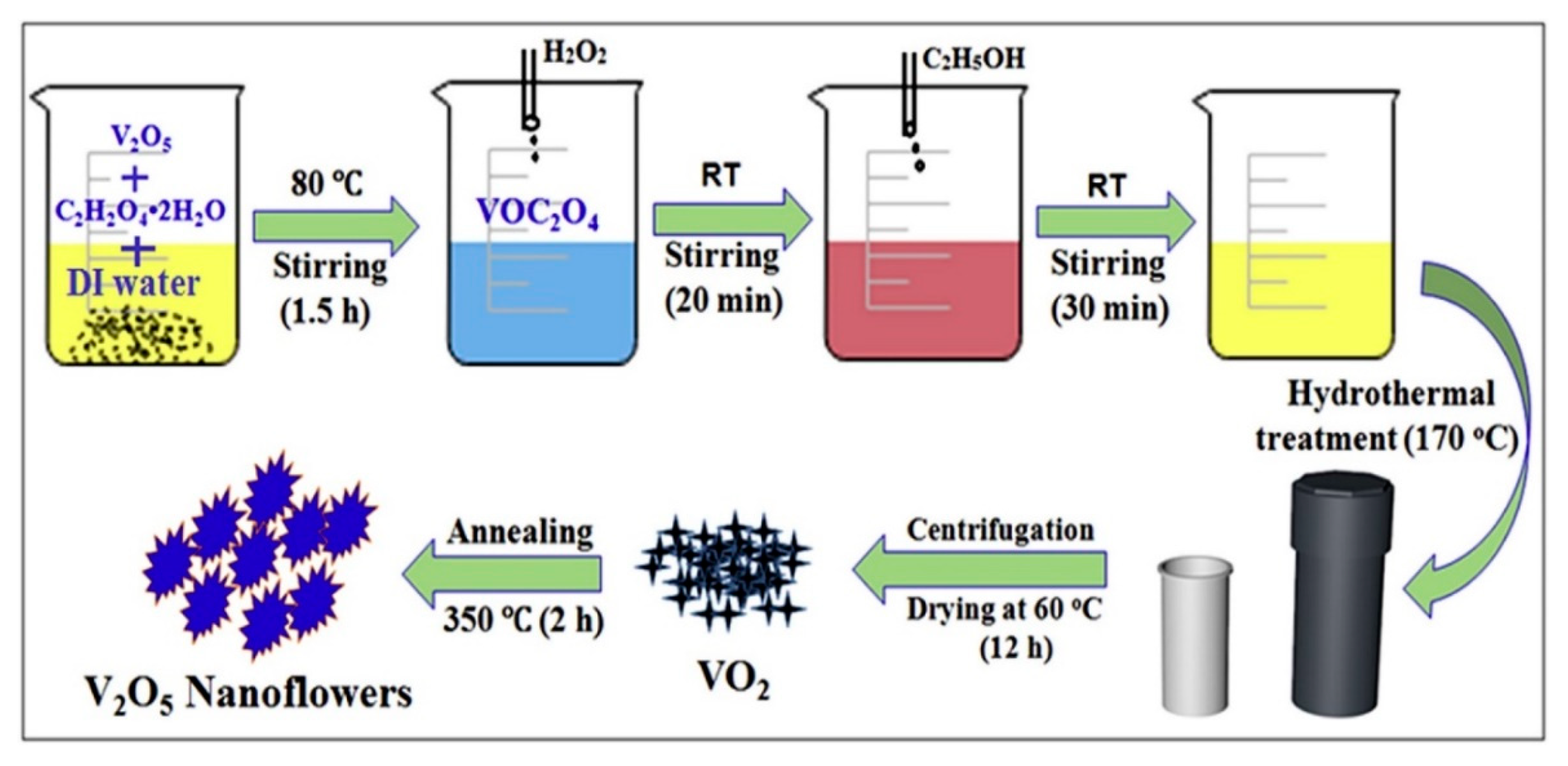
2. Pristine Nanostructured V2O5 Gas Sensors
 N bond energy and high electron cloud density around N atoms in TMA.
N bond energy and high electron cloud density around N atoms in TMA. 3. Decorated/Doped V2O5 Gas Sensors
4. Nanocomposites/Nanohybrids of V2O5 Gas Sensors
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Le, T.K.; Kang, M.; Kim, S.W. A review on the optical characterization of v2o5 micro-nanostructures. Ceram. Int. 2019, 45, 15781–15798. [Google Scholar] [CrossRef]
- Mane, A.A.; Maldar, P.S.; Dabhole, S.H.; Nikam, S.A.; Moholkar, A.V. Effect of substrate temperature on physicochemical and gas sensing properties of sprayed orthorhombic v2o5 thin films. Measurement 2019, 131, 223–234. [Google Scholar] [CrossRef]
- Bouzidi, A.; Benramdane, N.; Bresson, S.; Mathieu, C.; Desfeux, R.; Marssi, M.E. X-ray and raman study of spray pyrolysed vanadium oxide thin films. Vib. Spectrosc. 2011, 57, 182–186. [Google Scholar] [CrossRef]
- Su, Q.; Lan, W.; Wang, Y.Y.; Liu, X.Q. Structural characterization of β-v2o5 films prepared by dc reactive magnetron sputtering. Appl. Surf. Sci. 2009, 255, 4177–4179. [Google Scholar] [CrossRef]
- Ramana, C.; Hussain, O.; Naidu, B.S.; Reddy, P. Spectroscopic characterization of electron-beam evaporated v2o5 thin films. Thin Solid Film 1997, 305, 219–226. [Google Scholar] [CrossRef]
- Laubach, S.; Schmidt, P.C.; Thißen, A.; Fernandez-Madrigal, F.J.; Wu, Q.-H.; Jaegermann, W.; Klemm, M.; Horn, S. Theoretical and experimental determination of the electronic structure of v2o5, reduced v2o5− x and sodium intercalated nav2o5. Phys. Chem. Chem. Phys. 2007, 9, 2564–2576. [Google Scholar] [CrossRef]
- Aawani, E.; Memarian, N.; Dizaji, H.R. Synthesis and characterization of reduced graphene oxide–v2o5 nanocomposite for enhanced photocatalytic activity under different types of irradiation. J. Phys. Chem. Solids 2019, 125, 8–15. [Google Scholar] [CrossRef]
- Rathika, R.; Kovendhan, M.; Joseph, D.P.; Pachaiappan, R.; Kumar, A.S.; Vijayarangamuthu, K.; Venkateswaran, C.; Asokan, K.; Jeyakumar, S.J. Tailoring the properties of spray deposited v2o5 thin films using swift heavy ion beam irradiation. Nucl. Eng. Technol. 2020. [Google Scholar] [CrossRef]
- Hou, T.-F.; Johar, M.A.; Boppella, R.; Hassan, M.A.; Patil, S.J.; Ryu, S.-W.; Lee, D.-W. Vertically aligned one-dimensional zno/v2o5 core–shell hetero-nanostructure for photoelectrochemical water splitting. J. Energy Chem. 2020, 49, 262–274. [Google Scholar] [CrossRef]
- Slewa, L.H.; Abbas, T.A.; Ahmed, N.M. Effect of sn doping and annealing on the morphology, structural, optical, and electrical properties of 3d (micro/nano) v2o5 sphere for high sensitivity ph-egfet sensor. Sens. Actuators B Chem. 2020, 305, 127515. [Google Scholar] [CrossRef]
- Abd-Alghafour, N.M.; Ahmed, N.M.; Hassan, Z.; Almessiere, M.A.; Bououdina, M.; Al-Hardan, N.H. High sensitivity extended gate effect transistor based on v2o5 nanorods. J. Mater. Sci. Mater. Electron. 2017, 28, 1364–1369. [Google Scholar] [CrossRef]
- Yin, Z.; Xu, J.; Ge, Y.; Jiang, Q.; Zhang, Y.; Yang, Y.; Sun, Y.; Hou, S.; Shang, Y.; Zhang, Y. Synthesis of v2o5 microspheres by spray pyrolysis as cathode material for supercapacitors. Mater. Res. Express 2018, 5, 036306. [Google Scholar] [CrossRef]
- Deepak Raj, P.; Gupta, S.; Sridharan, M. Studies on nanostructured v2o5/v/v2o5 films for un-cooled ir detector application. J. Mater. Sci. Mater. Electron. 2016, 27, 7494–7500. [Google Scholar] [CrossRef]
- Abd-Alghafour, N.M.; Ahmed, N.M.; Hassan, Z. Fabrication and characterization of v2o5 nanorods based metal–semiconductor–metal photodetector. Sens. Actuators A Phys. 2016, 250, 250–257. [Google Scholar] [CrossRef]
- Kim, D.; Yun, J.; Lee, G.; Ha, J.S. Fabrication of high performance flexible micro-supercapacitor arrays with hybrid electrodes of mwnt/v2o5 nanowires integrated with a sno2 nanowire uv sensor. Nanoscale 2014, 6, 12034–12041. [Google Scholar] [CrossRef]
- Singh, N.; Umar, A.; Singh, N.; Fouad, H.; Alothman, O.Y.; Haque, F.Z. Highly sensitive optical ammonia gas sensor based on sn doped v2o5 nanoparticles. Mater. Res. Bull. 2018, 108, 266–274. [Google Scholar] [CrossRef]
- Santos, M.C.; Hamdan, O.H.C.; Valverde, S.A.; Guerra, E.M.; Bianchi, R.F. Synthesis and characterization of v2o5/pani thin films for application in amperometric ammonia gas sensors. Org. Electron. 2019, 65, 116–120. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Yuan, Y.; Wang, B.; Huang, J.; Xia, F.; Zhang, H.; Xiao, J. Effects of sintering temperature on sensing properties of v2o5-wo3-tio2 electrode for potentiometric ammonia sensor. Sens. Actuators B Chem. 2017, 241, 268–275. [Google Scholar] [CrossRef]
- Alam, M.M.; Uddin, M.T.; Asiri, A.M.; Rahman, M.M.; Islam, M.A. Development of reproducible thiourea sensor with binary sno2/v2o5 nanomaterials by electrochemical method. Arab. J. Chem. 2020, 13, 5406–5416. [Google Scholar] [CrossRef]
- Rajesh, K.; Santhanalakshmi, J. Design and development of graphene intercalated v2o5 nanosheets based electrochemical sensors for effective determination of potentially hazardous 3,5–dichlorophenol. Mater. Chem. Phys. 2017, 199, 497–507. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Hu, J.; Cai, P.; Lv, Y. A cataluminescence gas sensor based on nanosized v2o5 for tert-butyl mercaptan. Talanta 2010, 82, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, P.S.; Dhivya, P.; Deepak Raj, P.; Sridharan, M. V2o5 thin film for 2-propanol vapor sensing. Mater. Today Proc. 2016, 3, 1510–1516. [Google Scholar] [CrossRef]
- Imawan, C.; Steffes, H.; Solzbacher, F.; Obermeier, E. Structural and gas-sensing properties of v2o5–moo3 thin films for h2 detection. Sens. Actuators B Chem. 2001, 77, 346–351. [Google Scholar] [CrossRef]
- Chen, C.L.; Dong, C.L.; Ho, Y.K.; Chang, C.C.; Wei, D.H.; Chan, T.C.; Chen, J.L.; Jang, W.L.; Hsu, C.C.; Kumar, K.; et al. Electronic and atomic structures of gasochromic v2o5 films. EPL (Europhys. Lett.) 2013, 101, 17006. [Google Scholar] [CrossRef]
- Tadeo, I.J.; Parasuraman, R.; Krupanidhi, S.B.; Umarji, A.M. Enhanced humidity responsive ultrasonically nebulised v2o5 thin films. Nano Express 2020, 1, 010005. [Google Scholar] [CrossRef]
- Schneider, K.; Lubecka, M.; Czapla, A. V2o5 thin films for gas sensor applications. Sens. Actuators B Chem. 2016, 236, 970–977. [Google Scholar] [CrossRef]
- Vasanth Raj, D.; Ponpandian, N.; Mangalaraj, D.; Viswanathan, C. Effect of annealing and electrochemical properties of sol–gel dip coated nanocrystalline v2o5 thin films. Mater. Sci. Semicond. Process. 2013, 16, 256–262. [Google Scholar] [CrossRef]
- Gandasiri, R.; Sreelatha, C.J.; Nagaraju, P.; Vijayakumar, Y. Effect of annealing temperature on micro-structural, optical and electrical characterization of nanostructured v2o5 thin films prepared by spray pyrolysis technique. Phys. B Condens. Matter 2019, 572, 220–224. [Google Scholar] [CrossRef]
- Thangarasu, R.; Thangavel, E.; Chandrasekaran, J.; Balasundaram, O.N. Synthesis, characterization and gas sensing performance of v2o5 nano-structure on pet substrate. J. Mater. Sci. Mater. Electron. 2019, 30, 4238–4249. [Google Scholar] [CrossRef]
- Yıldırım, M.A.; Tuna Yıldırım, S.; Çağirtekin, A.O.; Karademir, M.; Karaduman Er, I.; Coşkun, A.; Ateş, A.; Acar, S. The effect of deposition time on the structural, morphological and h2s gas sensing properties of the v2o5 nanostructures deposited by hydrothermal method. J. Mater. Sci. Mater. Electron. 2019, 30, 12215–12223. [Google Scholar] [CrossRef]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (vocs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Mirzaei, A.; Neri, G. Microwave-assisted synthesis of metal oxide nanostructures for gas sensing application: A review. Sens. Actuators B Chem. 2016, 237, 749–775. [Google Scholar] [CrossRef]
- Amiri, V.; Roshan, H.; Mirzaei, A.; Neri, G.; Ayesh, A.I. Nanostructured metal oxide-based acetone gas sensors: A review. Sensors 2020, 20, 3096. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Lee, J.-H.; Majhi, S.M.; Weber, M.; Bechelany, M.; Kim, H.W.; Kim, S.S. Resistive gas sensors based on metal-oxide nanowires. J. Appl. Phys. 2019, 126, 241102. [Google Scholar] [CrossRef]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonyani, M.; Leonardi, S.G.; Neri, G. A novel gas sensor based on ag/fe2o3 core-shell nanocomposites. Ceram. Int. 2016, 42, 18974–18982. [Google Scholar] [CrossRef]
- Mounasamy, V.; Mani, G.K.; Madanagurusamy, S. Vanadium oxide nanostructures for chemiresistive gas and vapour sensing: A review on state of the art. Microchim. Acta 2020, 187, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, X.; Gao, S.; Cheng, X.; Rong, Z.; Xu, Y.; Zhao, H.; Huo, L. Construction of monodisperse vanadium pentoxide hollow spheres via a facile route and triethylamine sensing property. CrystEngComm 2013, 15, 10123–10131. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Whang, W.-T.; Chen, C.-H. Hollow v2o5 nanoassemblies for high-performance room-temperature hydrogen sensors. ACS Appl. Mater. Interfaces 2015, 7, 8480–8487. [Google Scholar] [CrossRef]
- Hakim, S.A.; Liu, Y.; Zakharova, G.S.; Chen, W. Synthesis of vanadium pentoxide nanoneedles by physical vapour deposition and their highly sensitive behavior towards acetone at room temperature. RSC Adv. 2015, 5, 23489–23497. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Zhang, J.; Liang, X.; Zhang, X.; Qi, Q. Deposition of in2o3 nanofibers on polyimide substrates to construct high-performance and flexible trimethylamine sensor. Chin. Chem. Lett. 2019. [Google Scholar] [CrossRef]
- Zhang, J.; Song, P.; Li, Z.; Zhang, S.; Yang, Z.; Wang, Q. Enhanced trimethylamine sensing performance of single-crystal moo3 nanobelts decorated with au nanoparticles. J. Alloys Compd. 2016, 685, 1024–1033. [Google Scholar] [CrossRef]
- Wang, D.; Gu, K.; Zhao, Q.; Zhai, C.; Yang, T.; Lu, Q.; Zhang, J.; Zhang, M. Synthesis and trimethylamine sensing properties of spherical v2o5 hierarchical structures. New J. Chem. 2018, 42, 14188–14193. [Google Scholar] [CrossRef]
- Meng, D.; Si, J.; Wang, M.; Wang, G.; Shen, Y.; San, X.; Meng, F. In-situ growth of v2o5 flower-like structures on ceramic tubes and their trimethylamine sensing properties. Chin. Chem. Lett. 2019. [Google Scholar] [CrossRef]
- Akande, A.A.; Mosuang, T.; Ouma, C.N.M.; Benecha, E.M.; Tesfamichael, T.; Roro, K.; Machatine, A.G.J.; Mwakikunga, B.W. Ammonia gas sensing characteristics of v2o5 nanostructures: A combined experimental and ab initio density functional theory approach. J. Alloys Compd. 2020, 821, 153565. [Google Scholar] [CrossRef]
- Dhayal Raj, A.; Pazhanivel, T.; Suresh Kumar, P.; Mangalaraj, D.; Nataraj, D.; Ponpandian, N. Self assembled v2o5 nanorods for gas sensors. Curr. Appl. Phys. 2010, 10, 531–537. [Google Scholar] [CrossRef]
- Dhayal Raj, A.; Suresh Kumar, P.; Yang, Q.; Mangalaraj, D. Synthesis and gas sensors behavior of surfactants free v2o5 nanostructure by using a simple precipitation method. Phys. E Low-Dimens. Syst. Nanostructures 2012, 44, 1490–1494. [Google Scholar] [CrossRef]
- Yang, T.; Yu, H.; Xiao, B.; Li, Z.; Zhang, M. Enhanced 1-butylamine gas sensing characteristics of flower-like v2o5 hierarchical architectures. J. Alloys Compd. 2017, 699, 921–927. [Google Scholar] [CrossRef]
- Raible, I.; Burghard, M.; Schlecht, U.; Yasuda, A.; Vossmeyer, T. V2o5 nanofibres: Novel gas sensors with extremely high sensitivity and selectivity to amines. Sens. Actuators B Chem. 2005, 106, 730–735. [Google Scholar]
- Yang, X.H.; Xie, H.; Fu, H.T.; An, X.Z.; Jiang, X.C.; Yu, A.B. Synthesis of hierarchical nanosheet-assembled v2o5 microflowers with high sensing properties towards amines. RSC Adv. 2016, 6, 87649–87655. [Google Scholar] [CrossRef]
- Modafferi, V.; Panzera, G.; Donato, A.; Antonucci, P.L.; Cannilla, C.; Donato, N.; Spadaro, D.; Neri, G. Highly sensitive ammonia resistive sensor based on electrospun v2o5 fibers. Sens. Actuators B Chem. 2012, 163, 61–68. [Google Scholar] [CrossRef]
- Gross, P.-A.; Jaramillo, T.; Pruitt, B. Cyclic-voltammetry-based solid-state gas sensor for methane and other voc detection. Anal. Chem. 2018, 90, 6102–6108. [Google Scholar] [CrossRef] [PubMed]
- Mounasamy, V.; Mani, G.K.; Ponnusamy, D.; Tsuchiya, K.; Reshma, P.R.; Prasad, A.K.; Madanagurusamy, S. Investigation on ch4 sensing characteristics of hierarchical v2o5 nanoflowers operated at relatively low temperature using chemiresistive approach. Anal. Chim. Acta 2020, 1106, 148–160. [Google Scholar] [CrossRef]
- Kim, B.-Y.; Ahn, J.H.; Yoon, J.-W.; Lee, C.-S.; Kang, Y.C.; Abdel-Hady, F.; Wazzan, A.A.; Lee, J.-H. Highly selective xylene sensor based on nio/nimoo4 nanocomposite hierarchical spheres for indoor air monitoring. ACS Appl. Mater. Interfaces 2016, 8, 34603–34611. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Gui, X.; Navale, S.T.; Han, S.; Xu, W.; Fang, M.; Liu, X.; Zeng, Y.; Liu, W.; Zhu, D.; et al. Design of flower-like v2o5 hierarchical nanostructures by hydrothermal strategy for the selective and sensitive detection of xylene. J. Alloys Compd. 2020, 815, 152378. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Bhattacharya, S. Highly sensitive v2o5·1.6h2o nanostructures for sensing of helium gas at room temperature. Mater. Lett. 2018, 217, 83–87. [Google Scholar] [CrossRef]
- Mane, A.A.; Suryawanshi, M.P.; Kim, J.H.; Moholkar, A.V. Fast response of sprayed vanadium pentoxide (v2o5) nanorods towards nitrogen dioxide (no2) gas detection. Appl. Surf. Sci. 2017, 403, 540–550. [Google Scholar] [CrossRef]
- Vijayakumar, Y.; Mani, G.K.; Ponnusamy, D.; Shankar, P.; Kulandaisamy, A.J.; Tsuchiya, K.; Rayappan, J.B.B.; Ramana Reddy, M.V. V2o5 nanofibers: Potential contestant for high performance xylene sensor. J. Alloys Compd. 2018, 731, 805–812. [Google Scholar] [CrossRef]
- Jin, W.; Yan, S.; An, L.; Chen, W.; Yang, S.; Zhao, C.; Dai, Y. Enhancement of ethanol gas sensing response based on ordered v2o5 nanowire microyarns. Sens. Actuators B Chem. 2015, 206, 284–290. [Google Scholar] [CrossRef]
- Panahi, N.; Shirazi, M.; Hosseinnejad, M.T. Fabrication, characterization and hydrogen gas sensing performance of nanostructured v2o5 thin films prepared by plasma focus method. J. Mater. Sci. Mater. Electron. 2018, 29, 13345–13353. [Google Scholar] [CrossRef]
- Mane, A.A.; Moholkar, A.V. Effect of film thickness on no2 gas sensing properties of sprayed orthorhombic nanocrystalline v2o5 thin films. Appl. Surf. Sci. 2017, 416, 511–520. [Google Scholar] [CrossRef]
- Fu, H.; Jiang, X.; Yang, X.; Yu, A.; Su, D.; Wang, G. Glycothermal synthesis of assembled vanadium oxide nanostructures for gas sensing. J. Nanopart. Res. 2012, 14, 871. [Google Scholar] [CrossRef]
- Qiang, X.; Hu, M.; Zhou, L.; Liang, J. Pd nanoparticles incorporated porous silicon/v2o5 nanopillars and their enhanced p-type no2-sensing properties at room temperature. Mater. Lett. 2018, 231, 194–197. [Google Scholar] [CrossRef]
- Bolokang, A.S.; Motaung, D.E. Reduction-oxidation of v2o5-wo3 nanostructured by ball milling and annealing: Their improved h2s gas sensing performance. Appl. Surf. Sci. 2019, 473, 164–173. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Cheng, Y.-R. Combinational physical synthesis methodology and crystal features correlated with oxidizing gas detection ability of one-dimensional zno–vox crystalline hybrids. CrystEngComm 2015, 17, 5801–5807. [Google Scholar] [CrossRef]
- Birajdar, S.N.; Hebalkar, N.Y.; Pardeshi, S.K.; Kulkarni, S.K.; Adhyapak, P.V. Ruthenium-decorated vanadium pentoxide for room temperature ammonia sensing. RSC Adv. 2019, 9, 28735–28745. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, Y.; Zhuo, M.; Yang, T.; Liang, J.; Zhang, M.; Ma, J.; Duan, H.; Li, Q. Diethylamine gas sensor using v2o5-decorated α-fe2o3 nanorods as a sensing material. RSC Adv. 2016, 6, 6511–6515. [Google Scholar] [CrossRef]
- Ko, W.C.; Kim, K.M.; Kwon, Y.J.; Choi, H.; Park, J.K.; Jeong, Y.K. Ald-assisted synthesis of v2o5 nanoislands on sno2 nanowires for improving no2 sensing performance. Appl. Surf. Sci. 2020, 509, 144821. [Google Scholar] [CrossRef]
- Ozdemir, S.; Gole, J.L. The potential of porous silicon gas sensors. Curr. Opin. Solid State Mater. Sci. Semicond. Process. 2007, 11, 92–100. [Google Scholar] [CrossRef]
- Yan, W.; Hu, M.; Wang, D.; Li, C. Room temperature gas sensing properties of porous silicon/v2o5 nanorods composite. Appl. Surf. Sci. 2015, 346, 216–222. [Google Scholar] [CrossRef]
- Chatterjee, S.G.; Chatterjee, S.; Ray, A.K.; Chakraborty, A.K. Graphene–metal oxide nanohybrids for toxic gas sensor: A review. Sens. Actuators B Chem. 2015, 221, 1170–1181. [Google Scholar] [CrossRef]
- Meng, F.-L.; Guo, Z.; Huang, X.-J. Graphene-based hybrids for chemiresistive gas sensors. TrAC Trends Anal. Chem. 2015, 68, 37–47. [Google Scholar] [CrossRef]
- Toda, K.; Furue, R.; Hayami, S. Recent progress in applications of graphene oxide for gas sensing: A review. Anal. Chim. Acta 2015, 878, 43–53. [Google Scholar] [CrossRef]
- Wang, T.; Huang, D.; Yang, Z.; Xu, S.; He, G.; Li, X.; Hu, N.; Yin, G.; He, D.; Zhang, L. A review on graphene-based gas/vapor sensors with unique properties and potential applications. Nano-Micro Lett. 2016, 8, 95–119. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Ocola, L.E.; Chen, J. Reduced graphene oxide for room-temperature gas sensors. Nanotechnology 2009, 20, 445502. [Google Scholar] [CrossRef]
- Amarnath, M.; Heiner, A.; Gurunathan, K. Surface bound nanostructures of ternary r-go/ mn3o4/v2o5 system for room temperature selectivity of hydrogen gas. Ceram. Int. 2020, 46, 7336–7345. [Google Scholar] [CrossRef]
- Bhati, V.S.; Sheela, D.; Roul, B.; Raliya, R.; Biswas, P.; Kumar, M.; Roy, M.S.; Nanda, K.K.; Krupanidhi, S.B.; Kumar, M. No2 gas sensing performance enhancement based on reduced graphene oxide decorated v2o5 thin films. Nanotechnology 2019, 30, 224001. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Lee, J.-H.; Kim, J.-H.; Mirzaei, A.; Woo Kim, H.; Kim, S.S. Realization of h2s sensing by pd-functionalized networked cuo nanowires in self-heating mode. Sens. Actuators B Chem. 2019, 299, 126965. [Google Scholar] [CrossRef]
- Wu, J.; Xing, X.; Zhu, Z.; Zheng, L.; Chen, J.; Wang, C.; Yang, D. Electrospun hollow cuo modified v2o5 nano-string of pearls with improved acetone sensitivity. Chem. Phys. Lett. 2019, 727, 19–24. [Google Scholar] [CrossRef]
- Yeh, B.-Y.; Jian, B.-S.; Wang, G.-J.; Tseng, W.J. Cuo/v2o5 hybrid nanowires for highly sensitive and selective h2s gas sensor. RSC Adv. 2017, 7, 49605–49612. [Google Scholar] [CrossRef]
- Wang, R.; Yang, S.; Deng, R.; Chen, W.; Liu, Y.; Zhang, H.; Zakharova, G.S. Enhanced gas sensing properties of v2o5 nanowires decorated with sno2 nanoparticles to ethanol at room temperature. RSC Adv. 2015, 5, 41050–41058. [Google Scholar] [CrossRef]
- Jin, W.; Dong, B.; Chen, W.; Zhao, C.; Mai, L.; Dai, Y. Synthesis and gas sensing properties of fe2o3 nanoparticles activated v2o5 nanotubes. Sens. Actuators B Chem. 2010, 145, 211–215. [Google Scholar] [CrossRef]
- Avansi, W.; Catto, A.C.; da Silva, L.F.; Fiorido, T.; Bernardini, S.; Mastelaro, V.R.; Aguir, K.; Arenal, R. One-dimensional v2o5/tio2 heterostructures for chemiresistive ozone sensors. ACS Appl. Nano Mater. 2019, 2, 4756–4764. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, J.-H.; Park, Y.; Kim, J.-Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Toluene- and benzene-selective gas sensors based on pt- and pd-functionalized zno nanowires in self-heating mode. Sens. Actuators B Chem. 2019, 294, 78–88. [Google Scholar] [CrossRef]
- Yang, X.; Wang, W.; Wang, C.; Xie, H.; Fu, H.; An, X.; Jiang, X.; Yu, A. Synthesis of au decorated v2o5 microflowers with enhanced sensing properties towards amines. Powder Technol. 2018, 339, 408–418. [Google Scholar] [CrossRef]
- Sanger, A.; Kumar, A.; Kumar, A.; Jaiswal, J.; Chandra, R. A fast response/recovery of hydrophobic pd/v2o5 thin films for hydrogen gas sensing. Sens. Actuators B Chem. 2016, 236, 16–26. [Google Scholar] [CrossRef]
- Suematsu, K.; Kodama, K.; Ma, N.; Yuasa, M.; Kida, T.; Shimanoe, K. Role of vanadium oxide and palladium multiple loading on the sensitivity and recovery kinetics of tin dioxide based gas sensors. RSC Adv. 2016, 6, 5169–5176. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. Resistive-based gas sensors for detection of benzene, toluene and xylene (btx) gases: A review. J. Mater. Chem. C 2018, 6, 4342–4370. [Google Scholar] [CrossRef]
- Feng, C.; Li, X.; Wang, C.; Sun, Y.; Zheng, J.; Lu, G. Facile synthesis benzene sensor based on v2o5-doped sno2 nanofibers. RSC Adv. 2014, 4, 47549–47555. [Google Scholar] [CrossRef]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Neri, G. Metal-core@metal oxide-shell nanomaterials for gas-sensing applications: A review. J. Nanopart. Res. 2015, 17, 371. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. How shell thickness can affect the gas sensing properties of nanostructured materials: Survey of literature. Sens. Actuators B Chem. 2018, 258, 270–294. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Low power-consumption co gas sensors based on au-functionalized sno2-zno core-shell nanowires. Sens. Actuators B Chem. 2018, 267, 597–607. [Google Scholar] [CrossRef]
- Shah, A.H.; Liu, Y.; Nguyen, V.T.; Zakharova, G.S.; Mehmood, I.; Chen, W. Enhanced ultra-stable n-propylamine sensing behavior of v2o5/in2o3 core–shell nanorods. RSC Adv. 2015, 5, 54412–54419. [Google Scholar] [CrossRef]
- Mane, A.A.; Nikam, S.A.; Moholkar, A.V. No2 gas sensing properties of sprayed composite porous moo3-v2o5 thin films. Mater. Chem. Phys. 2018, 216, 294–304. [Google Scholar] [CrossRef]
- Mane, A.A.; Maldar, P.S.; Desai, S.P.; Moholkar, A.V. Gas sensing properties of (moo3)0.4(v2o5)0.6 microsheets: Effect of pd sensitization. Vacuum 2017, 144, 135–144. [Google Scholar] [CrossRef]
- Diniz, M.O.; Golin, A.F.; Santos, M.C.; Bianchi, R.F.; Guerra, E.M. Improving performance of polymer-based ammonia gas sensor using poma/v2o5 hybrid films. Org. Electron. 2019, 67, 215–221. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Kong, Z.; Lv, K.; Teng, C.; Zhu, Y. Conducting-polymer-based materials for electrochemical energy conversion and storage. Adv. Mater. 2017, 29, 1703044. [Google Scholar] [CrossRef]
- Zhang, J.; Ouyang, J.; Ye, Y.; Li, Z.; Lin, Q.; Chen, T.; Zhang, Z.; Xiang, S.J. Mixed-valence cobalt (ii/iii) metal–organic framework for ammonia sensing with naked-eye color switching. ACS Appl. Mater. Interfaces 2018, 10, 27465–27471. [Google Scholar] [CrossRef]
- Naderi, H.; Hajati, S.; Ghaedi, M.; Dashtian, K.; Sabzehmeidani, M.M. Sensitive, selective and rapid ammonia-sensing by gold nanoparticle-sensitized v2o5/cuwo4 heterojunctions for exhaled breath analysis. Appl. Surf. Sci. 2020, 501, 144270. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Dong, J.; Qin, N.; Xu, J. Selective btex sensor based on a sno2/v2o5 composite. Sens. Actuators B Chem. 2013, 186, 126–131. [Google Scholar] [CrossRef]
- Modafferi, V.; Trocino, S.; Donato, A.; Panzera, G.; Neri, G. Electrospun v2o5 composite fibers: Synthesis, characterization and ammonia sensing properties. Thin Solid Film 2013, 548, 689–694. [Google Scholar] [CrossRef]
- Xiao, B.; Huang, H.; Yu, X.; Song, J.; Qu, J. Facile synthesis of layered v2o5/znv2o6 heterostructures with enhanced sensing performance. Appl. Surf. Sci. 2018, 447, 569–575. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Meng, C.; Gao, Z.; Cao, X.; Li, X.; Xu, L.; Zhu, W.; Peng, X.; Zhang, B.; et al. A high-response ethanol gas sensor based on one-dimensional tio2/v2o branched nanoheterostructures. Nanotechnology 2016, 27, 425503. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, P.; Vijayakumar, Y.; Reddy, M.V.R.; Deshpande, U.P. Effect of vanadium pentoxide concentration in zno/v2o5 nanostructured composite thin films for toluene detection. RSC Adv. 2019, 9, 16515–16524. [Google Scholar] [CrossRef]
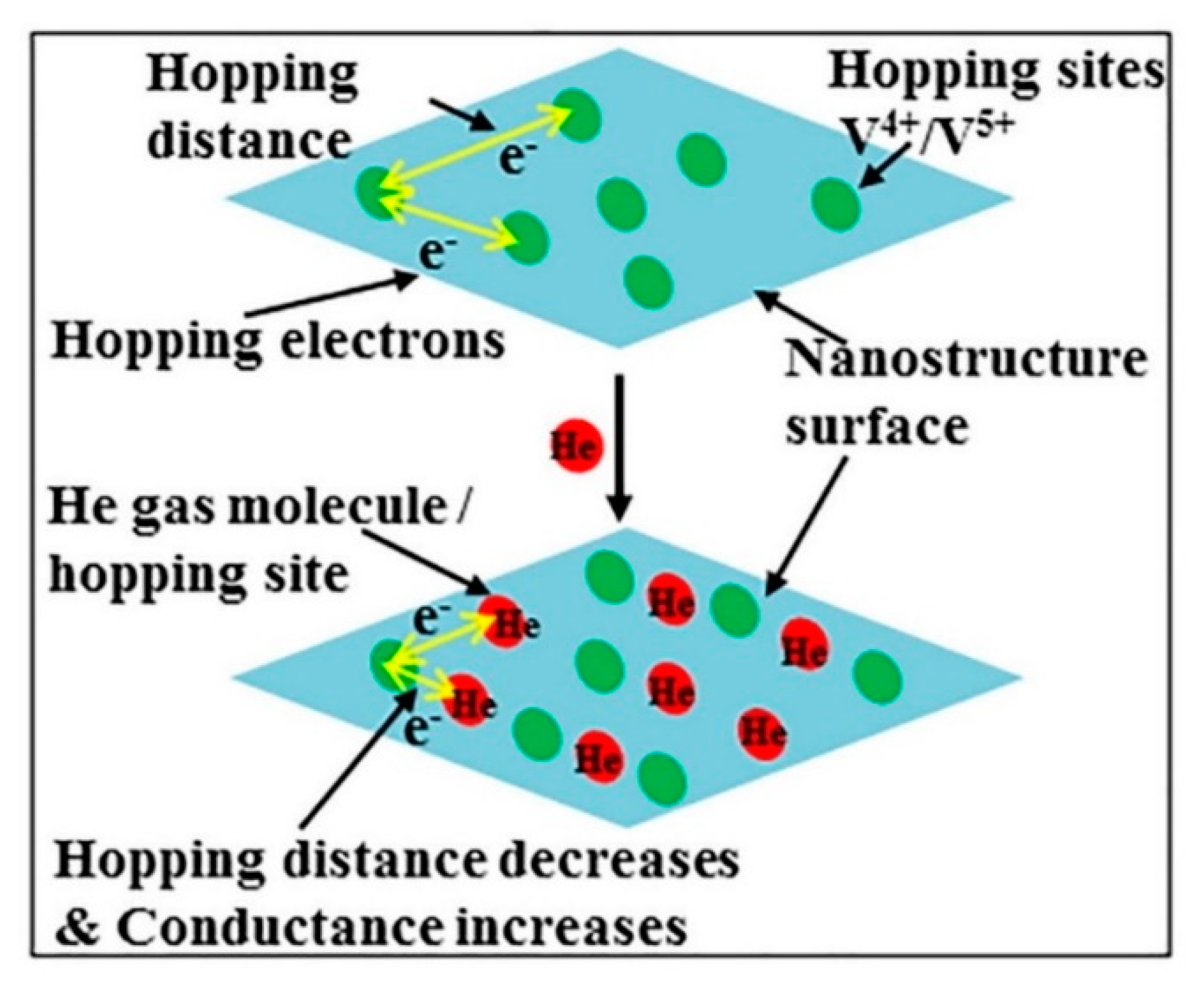
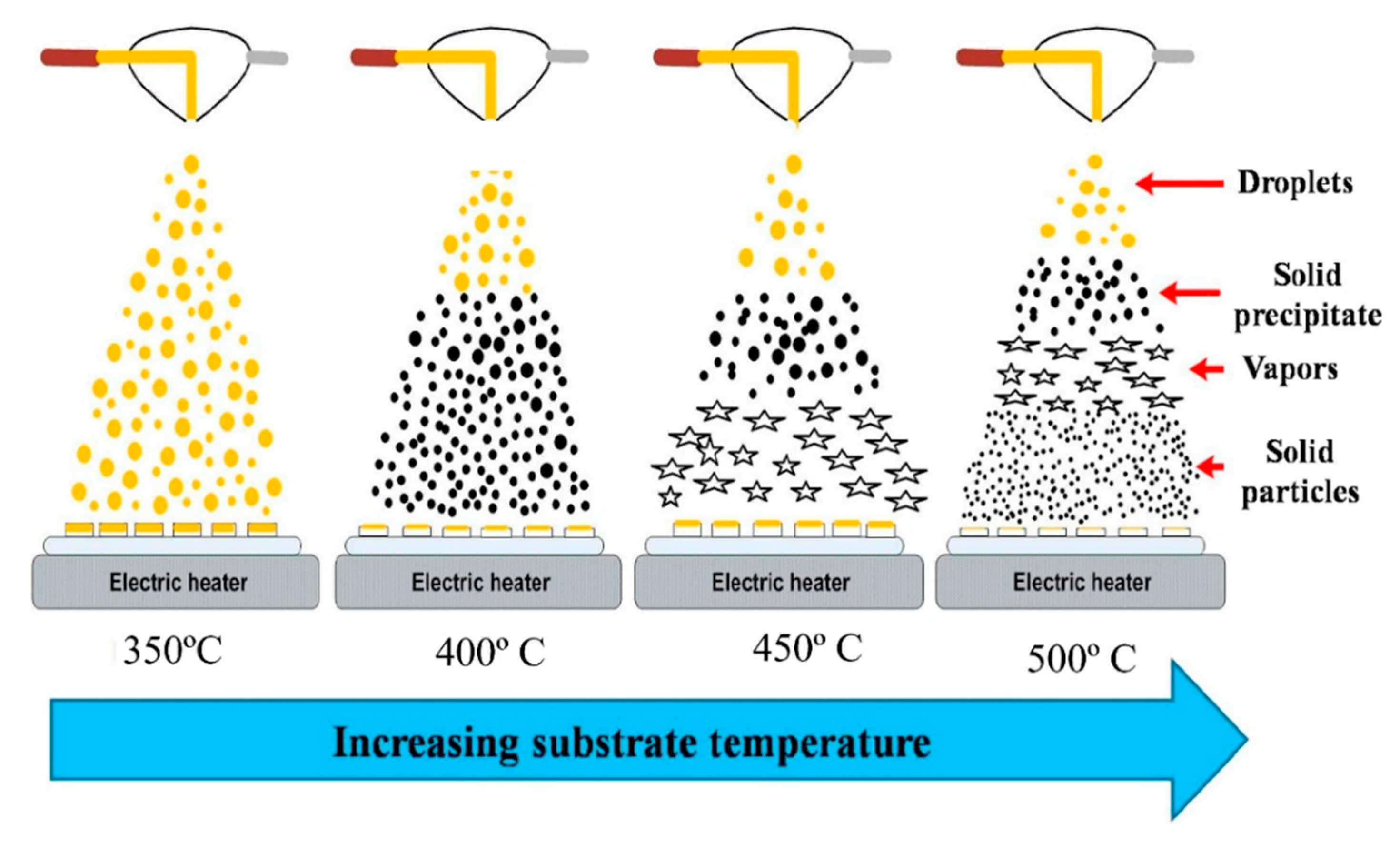
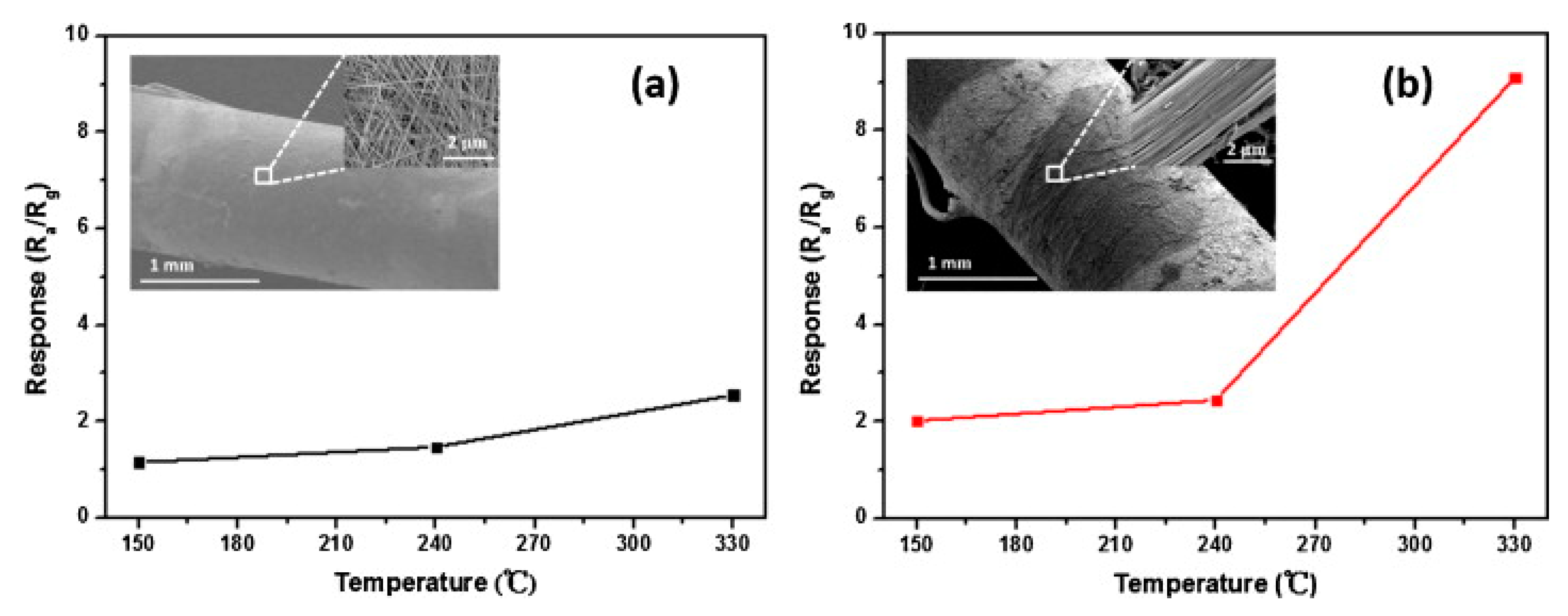

| V2O5 Morphology | Synthesis Method | Target Gas | Conc. (ppm) | Response (Ra/Rg) or (Rg/Ra) | T (°C) | Response time/Recovery Time(s) | Ref. |
|---|---|---|---|---|---|---|---|
| Hollow spheres Solid spheres | Solvothermal | C3H9N | 500 500 | 9.7 3.08 | 370 | 20/83 45/150 | [37] |
| Hollow spheres | Chemical synthesis | H2 | 200 | 2.8 | RT | 50/10 | [38] |
| Nanoneedles | PVD | C3H6O | 140 | 2.35 | RT | 67/- | [39] |
| Hierarchical nanostructures | Hydrothermal | C3H9N | 10–200 | 1.13-3.57 | 240 | 5/28 | [42] |
| Flower-like | Hydrothermal | 5 | 2.25 | 200 | 13/13 | [43] | |
| Nanorods | CVD | NH3 | 100 | 235 * | 400 | -/- | [44] |
| Nanorods | Solvothermal | C2H5OH NH3 | 500 | 1.04 1.02 | RT | -/- | [45] |
| Spherical | Precipitation | C2H5OH NH3 | 1.04 1.06 | -/- | [46] | ||
| Flower-like | Hydrothermal | 1-butylamine | 100 | 2.6 | 140 | 9/49 | [47] |
| Nanofibers | Electrospinning | 9.5 | 500 * | RT | -/- | [48] | |
| Flower-like Sheet-like | Hydrothermal | 100 | 3.6 2.8 | 300 | 25/14 17/14 | [49] | |
| Nanofibers | Sol–gel | NH3 | 2.1 | 11 * | 200 | 50/350 | [50] |
| Flower-like | DC sputtering | CH4 | 500 | 100 | 206/247 | [52] | |
| Hydrothermal | C8H10 | 3 | 300 | 44/74 | [54] | ||
| Nano stars | Hydrothermal | He | 300 | 53 * | RT | 9/10 | [55] |
| Nanorods | Chemical spray pyrolysis | NO2 | 100 | 24.2 * | 200 | 13/140 | [56] |
| Nanofibers | Chemical spray pyrolysis | C8H10 | 27 | RT | 80/50 | [57] | |
| Nanowires | Melt quenching | C2H5OH | 1000 | 9.09 | 330 | -/- | [58] |
| Thin film | Plasma focus method | H2 | 50 * | 275 | -/- | [59] | |
| Chemical spray pyrolysis | NO2 | 100 | 41 * | 200 | 20/150 | [60] |
| Sensor | Synthesis Method | Target Gas | Conc. (ppm) | Response (Ra/Rg) or (Rg/Ra) | Working Temp. (°C) | Response Time/Recovery Time(s) | Ref. |
|---|---|---|---|---|---|---|---|
| Pd decorated porous Si/V2O5 nanopillars | DC sputtering | NO2 | 2 | 4.5 | RT | -/- | [62] |
| Ru-decorated layer structure V2O5 | Hydrothermal | NH3 | 130 | 4 * | RT | ~2/~12 | [65] |
| V2O5-decorated α-Fe2O3 nanorods | Electrospinning | C4H11N | 300 | 9 | 350 | 2/40 | [66] |
| V2O5 decorated SnO2 NWs | VLS/ALD | NO2 | 200 ppb | 3.6 | 250 | -/- | [67] |
| Porous Si/V2O5 NR composite | Galvanostatic electrochemical etching | NO2 | 2 | 7.4 | RT | -/- | [69] |
| rGO/Mn3O4/V2O5 nanocomposite | Hydrothermal | H2 | 50 | 175 | RT | 82/92 | [75] |
| Pd-decorated CuO NWs | UV irradiation | H2S | 100 | 1.962 | 100 | -/- | [76] |
| V2O5/CuO nano-string of pearls | Electrospinning | C3H6O | 500 | 9 | 440 | ~40/~100 | [78] |
| CuO-decorated V2O5 NWs | Hydrothermal and wet-deposition | H2S | 23 | 31.86 | 220 | 130/218 | [79] |
| SnO2 NP-decorated V2O5 NWs | Hydrothermal | C2H5OH | 1000 | 1.3 | RT | -/- | [80] |
| Fe2O3 activated V2O5 nanotubes | Hydrolysis | C2H5OH | 1000 | 2.2 | 270 | -/- | [81] |
| TiO2-decorated V2O5 NWs | Hydrothermal | O3 | 1.25 | 2.6 * | 300 | ~180/~180 | [82] |
| RGO-decorated V2O5 thin film | Reactive sputtering and drop casting | NO2 | 100 | 50.7 | 150 | -/- | [76] |
| Au NP-decorated V2O5 | Two-step in-situ reduction of Au and thermal oxidization as V2O5 | Amines | 100 | 7.5 | 240 | 90/35 | [84] |
| Pd-decorated V2O5 thin film | DC magnetron reactive sputtering | H2 | 100 | 5.7 | 100 | ~6/14.8 | [85] |
| V2O5- doped SnO2 NFs | Electrospinning | C6H6 | 25 | 6.32 | 325 | 3/47 | [88] |
| Sensing Material | Synthesis Method | Target Gas | Conc. (Ppm) | Response (Ra/Rg) Or (Rg/Ra) | T (°C) | Response Time/Recovery Time(S) | Ref. |
|---|---|---|---|---|---|---|---|
| V2O5/In2O3 core–shells | Hydrothermal | n-propylamine | 200 | 4 | 190 | 48/121 | [92] |
| MoO3-V2O5 thin films | Chemical spray pyrolysis | NO2 | 120 | 80 * | 200 | 118/1182 | [93] |
| (MoO3)0.4(V2O5)0.6 sheet composite | Chemical spray pyrolysis | 100 | 115 | 39/453 | [94] | ||
| Au/V2O5/CuWO4 composite | Hydrothermal | NH3 | 5 | 2.7 | 150 | 35/33 | [98] |
| SnO2/V2O5 composite | Sol-gel | C6H6 | 200 | 10.5 | 275 | -/- | [99] |
| V2O5/polyvinyl acetate NF composite | Electrospinning | NH3 | 0.8 | 6 * | 260 | -/- | [100] |
| V2O5/ZnV2O6 nanobelt composite | Chemical route | C2H5OH | 2000 | 16.5 | 240 | -/- | [101] |
| TiO2/V2O5 NF composite | Electrospinning | 100 | 24.6 | 350 | 6/7 | [102] | |
| ZnO/V2O5 thin films | Spray pyrolysis | C7H8 | 400 | 2.3 | 27 | 23/28 | [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amiri, V.; Roshan, H.; Mirzaei, A.; Sheikhi, M.H. A Review of Nanostructured Resistive-Based Vanadium Oxide Gas Sensors. Chemosensors 2020, 8, 105. https://doi.org/10.3390/chemosensors8040105
Amiri V, Roshan H, Mirzaei A, Sheikhi MH. A Review of Nanostructured Resistive-Based Vanadium Oxide Gas Sensors. Chemosensors. 2020; 8(4):105. https://doi.org/10.3390/chemosensors8040105
Chicago/Turabian StyleAmiri, Vahid, Hossein Roshan, Ali Mirzaei, and Mohammad Hossein Sheikhi. 2020. "A Review of Nanostructured Resistive-Based Vanadium Oxide Gas Sensors" Chemosensors 8, no. 4: 105. https://doi.org/10.3390/chemosensors8040105
APA StyleAmiri, V., Roshan, H., Mirzaei, A., & Sheikhi, M. H. (2020). A Review of Nanostructured Resistive-Based Vanadium Oxide Gas Sensors. Chemosensors, 8(4), 105. https://doi.org/10.3390/chemosensors8040105






