Novel 1D/2D KWO/Ti3C2Tx Nanocomposite-Based Acetone Sensor for Diabetes Prevention and Monitoring
Abstract
:1. Introduction
2. Experiments
2.1. Material Synthesis
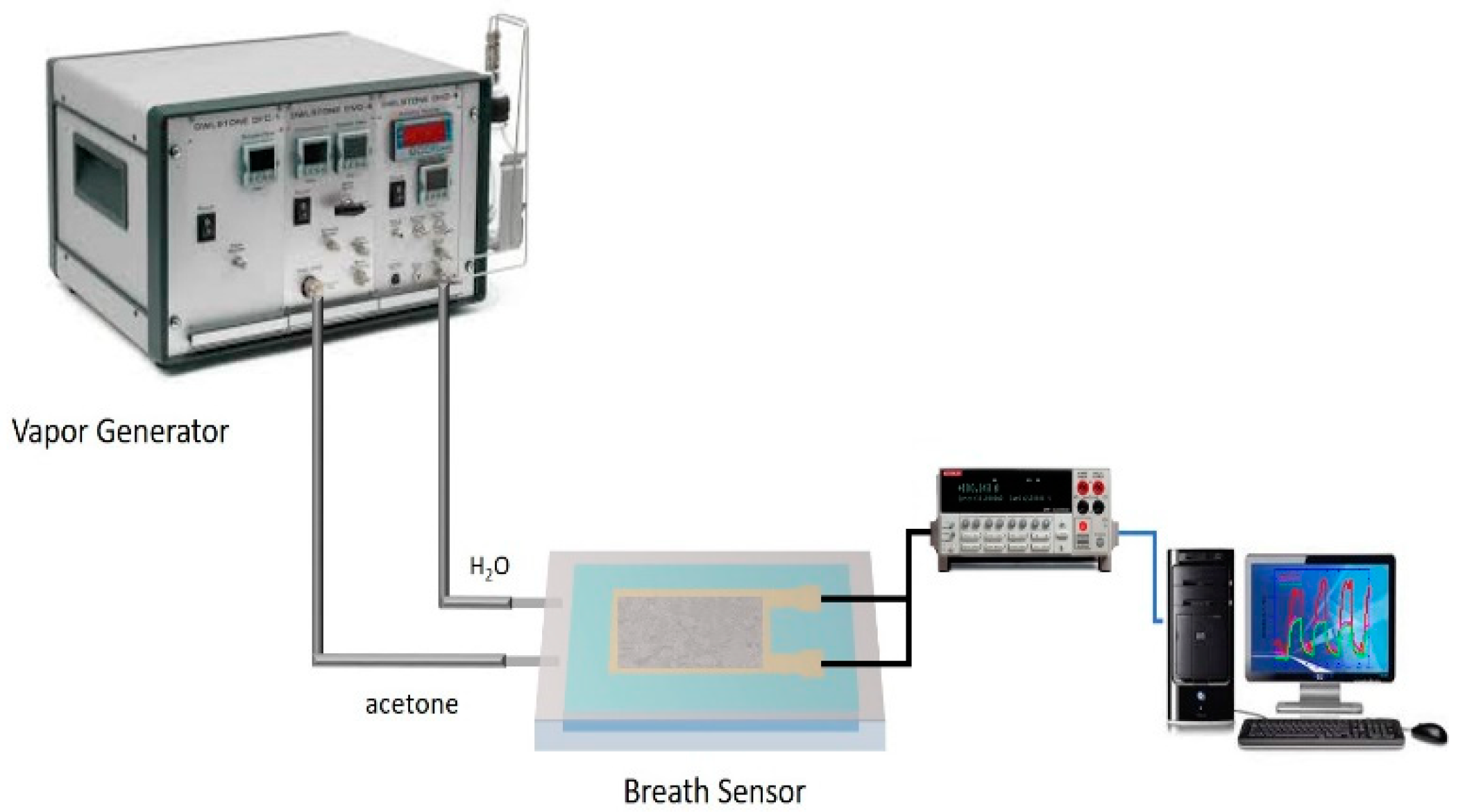
2.2. Sensing Test System
3. Results and Discussion
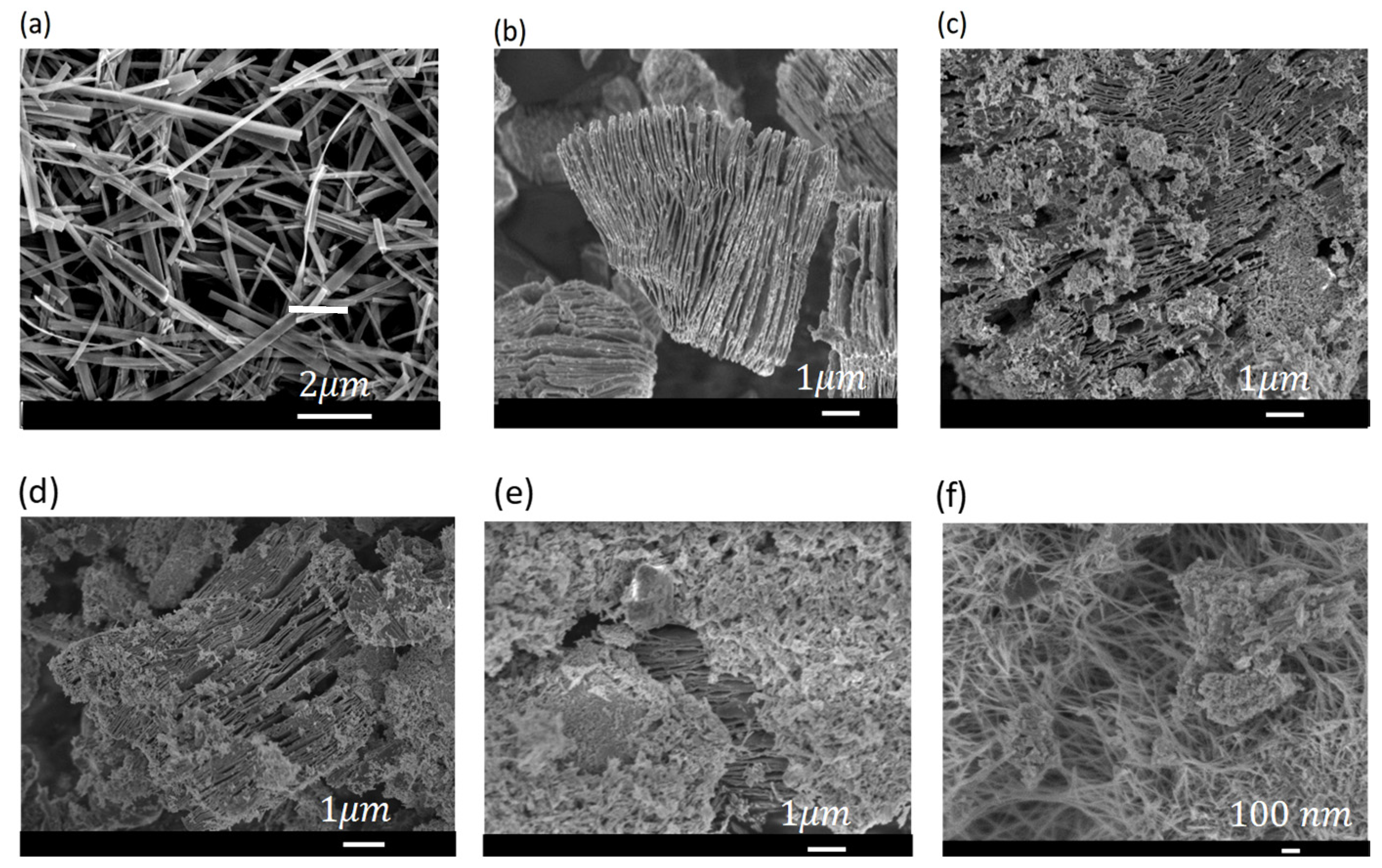
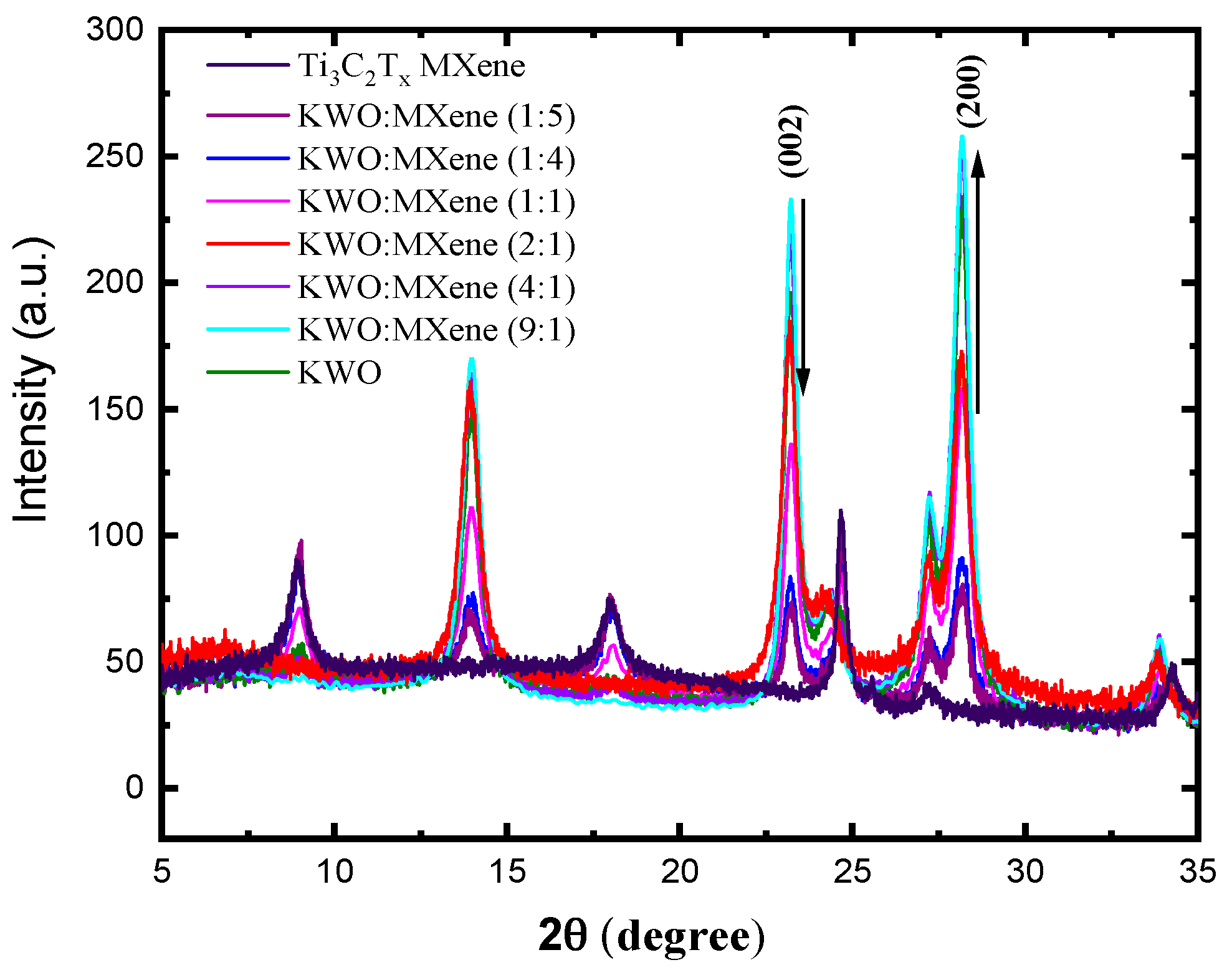
3.1. Characterization
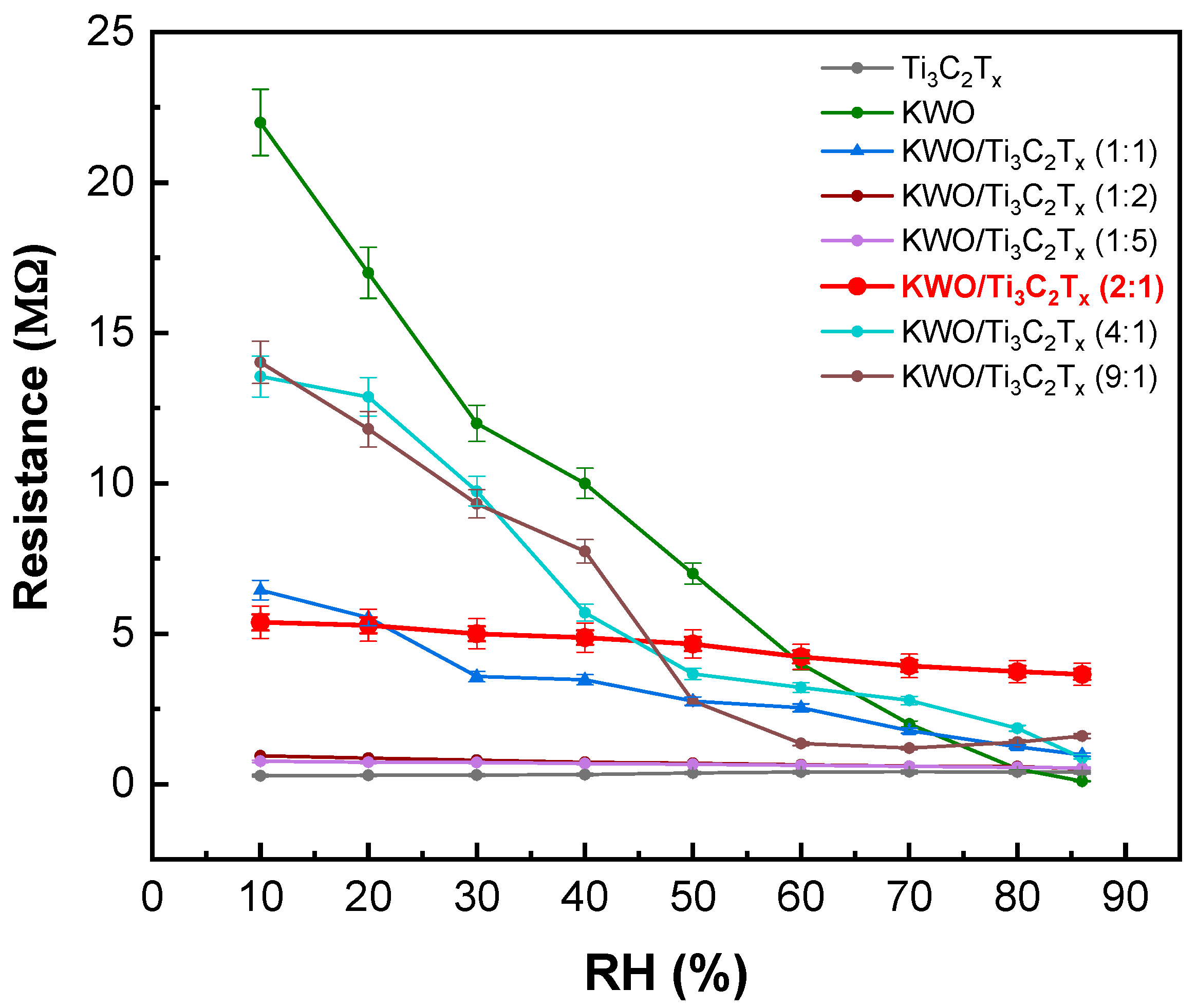
3.2. Sensing Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation (IDF). Diabetes Atlas; Hoorens Printing NV: Brussels, Belgium, 2006. [Google Scholar]
- Saasa, V.; Beukes, M.; Lemmer, Y.; Mwakikunga, B. Blood Ketone Bodies and Breath Acetone Analysis and Their Correlations in Type 2 Diabetes Mellitus. Diagnostics 2019, 9, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, A.D. Application of electronic-nose technologies and VOC-biomarkers for the noninvasive early diagnosis of gastrointestinal diseases. Sensors 2018, 18, 2613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruzsanyi, V.; Kalapos, M.P. Breath acetone as a potential marker in clinical practice. J. Breath Res. 2017, 11, 024002. [Google Scholar] [CrossRef] [PubMed]
- Rydosz, A. A negative correlation between blood glucose and acetone measured in healthy and type 1 diabetes mellitus patient breath. J. Diabetes Sci. Technol. 2015, 9, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhao, X.; Yin, H.; Wang, Z.; Jiang, C.; Liu, W.; Chen, Z.; Yuan, Y.; Li, Y.; Wang, C. Study of breath acetone and its correlations with blood glucose and blood beta-hydroxybutyrate using an animal model with lab-developed type 1 diabetic rats. RSC Adv. 2015, 5, 71002–71010. [Google Scholar] [CrossRef]
- Galassetti, P.R.; Novak, B.; Nemet, D.; Rose-Gottron, C.; Cooper, D.M.; Meinardi, S.; Newcomb, R.; Zaldivar, F.; Blake, D.R. Breath ethanol and acetone as indicators of serum glucose levels: An initial report. Diabetes Technol. Ther. 2005, 7, 115–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, M.; Greenberg, J. Ion-trap detection of volatile organic compounds in alveolar breath. Clin. Chem. 1992, 38, 60–65. [Google Scholar] [CrossRef]
- Trotter, M.D.; Sulway, M.J.; Trotter, E. The rapid determination of acetone in breath and plasma. Clin. Chim. Acta 1971, 35, 137–143. [Google Scholar] [CrossRef]
- Ueta, I.; Saito, Y.; Hosoe, M.; Okamoto, M.; Ohkita, H.; Shirai, S.; Tamura, H.; Jinno, K. Breath acetone analysis with miniaturized sample preparation device: In-needle preconcentration and subsequent determination by gas chromatography–mass spectroscopy. J. Chromatogr. B 2009, 877, 2551–2556. [Google Scholar] [CrossRef]
- Lehnert, A.S.; Behrendt, T.; Ruecker, A.; Pohnert, G.; Trumbore, S.E. SIFT-MS optimization for atmospheric trace gas measurements at varying humidity. Atmos. Meas. Tech. 2020, 13, 3507–3520. [Google Scholar] [CrossRef]
- Usman, F.; Dennis, J.O.; Ahmed, A.Y.; Meriaudeau, F.; Ayodele, O.B.; Rabih, A.A. A review of biosensors for non-invasive diabetes monitoring and screening in human exhaled breath. IEEE Access 2018, 7, 5963–5974. [Google Scholar] [CrossRef]
- Lee, J.E.; Lim, C.K.; Park, H.J.; Song, H.; Choi, S.Y.; Lee, D.S. ZnO–CuO Core-Hollow Cube Nanostructures for Highly Sensitive Acetone Gas Sensors at the ppb Level. ACS Appl. Mater. Interfaces 2020, 12, 35688–35697. [Google Scholar] [CrossRef] [PubMed]
- Kao, K.W.; Hsu, M.C.; Chang, Y.H.; Gwo, S.; Yeh, J.A. A sub-ppm acetone gas sensor for diabetes detection using 10 nm thick ultrathin InN FETs. Sensors 2012, 12, 7157–7168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Ghosh, S.; Kumar, R.; Bag, A.; Biswas, D. Highly sensitive acetone sensor based on Pd/AlGaN/GaN resistive device grown by plasma-assisted molecular beam epitaxy. IEEE Trans. Electron Devices 2017, 64, 4650–4656. [Google Scholar] [CrossRef]
- Qiu, Z.; Hua, Z.; Li, Y.; Wang, M.; Huang, D.; Tian, C.; Zhang, C.; Tian, X.; Li, E. Acetone sensing properties and mechanism of Rh-Loaded WO3 nanosheets. Front. Chem. 2018, 6, 385. [Google Scholar] [CrossRef]
- Kim, N.H.; Choi, S.J.; Yang, D.J.; Bae, J.; Park, J.; Kim, I.D. Highly sensitive and selective hydrogen sulfide and toluene sensors using Pd functionalized WO3 nanofibers for potential diagnosis of halitosis and lung cancer. Sens. Actuators B 2014, 193, 574–581. [Google Scholar] [CrossRef]
- Tomer, V.K.; Singh, K.; Kaur, H.; Shorie, M.; Sabherwal, P. Rapid acetone detection using indium loaded WO3/SnO2 nanohybrid sensor. Sens. Actuators B 2017, 253, 703–713. [Google Scholar] [CrossRef]
- Khokhra, R.; Bharti, B.; Lee, H.N.; Kumar, R. Visible and UV photo-detection in ZnO nanostructured thin films via simple tuning of solution method. Sci. Rep. 2017, 7, 15032. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Qin, H.; Pei, J.; Chen, Y.; Li, L.; Xie, J.; Hu, J. Sensing performances to low concentration acetone for palladium doped LaFeO3 sensors. J. Rare Earths 2016, 34, 704–710. [Google Scholar] [CrossRef]
- Wang, F.; Yang, C.; Duan, M.; Tang, Y.; Zhu, J. TiO2 nanoparticle modified organ-like Ti3C2 MXene nanocomposite encapsulating hemoglobin for a mediator-free biosensor with excellent performances. Biosens. Bioelectron. 2015, 74, 1022–1028. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, D. Room temperature acetone sensor based on nanostructured K2W7O22. In Proceedings of the IEEE SENSORS 2016, Orlando, FL, USA, 30 October–3 November 2016; pp. 1–3. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Fundamentals of Metal Oxide Gas Sensors. In Proceedings of the 14th International Meeting on Chemical Sensors-IMCS 2012, Nuremberg, Germany, 20–23 May 2012; pp. 618–621. [Google Scholar]
- Varghese, O.K.; Grimes, C.A. Metal oxide nanoarchitectures for environmental sensing. J. Nanosci. Nanotechnol. 2003, 3, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Q.; Hossain, M.R.; Johnson, M. High sensitive breath sensor based on nanostructured K2W7O22 for detection of type 1 diabetes. IEEE Sens. J. 2018, 18, 4399–4404. [Google Scholar] [CrossRef]
- Hossain, M.R.; Zhang, Q.; Johnson, M.; Wang, D. Highly Sensitive Room-Temperature Sensor Based on Nanostructured K2W7O22 for Application in the Non-Invasive Diagnosis of Diabetes. Sensors 2018, 18, 3703. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.E.; Zhang, Q. KxWO Is a Novel Ferroelectric Nanomaterial for Application as a Room Temperature Acetone Sensor. Nanomaterials 2020, 10, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, M.; Zhang, Q.; Wang, D. Room-temperature ferroelectric K2W7O22 (KWO) nanorods as a sensor material for the detection of acetone. Med. Devices Sens. 2019, 2, e10044. [Google Scholar] [CrossRef]
- Hossain, M.R.; Zhang, Q.F.; Johnson, M.; Ama, O.; Wang, D.L. Investigation of Different Materials as Acetone Sensors for Application in Type-1 Diabetes Diagnosis. Biomed. J. Sci. Tech. Res. 2019, 14, 10940–10945. [Google Scholar]
- Hossain, M.R.; Zhang, Q.; Johnson, M.; Wang, D. Investigation of humidity cross-interference effect on acetone breath sensor based on nanostructured K2W7O22. Eng. Press 2017, 1, 30–34. [Google Scholar]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Huang, K.; Li, Z.; Lin, J.; Han, G.; Huang, P. Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chem. Soc. Rev. 2018, 47, 5109–5124. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.; Arai, M.; Sasaki, T.; Ranjbar, A.; Liang, Y.; Yunoki, S. OH-terminated two-dimensional transition metal carbides and nitrides as ultralow work function materials. Phys. Rev. B 2015, 92, 075411. [Google Scholar] [CrossRef] [Green Version]
- Yuan, W.; Yang, K.; Peng, H.; Li, F.; Yin, F. A flexible VOCs sensor based on a 3D Mxene framework with a high sensing performance. J. Mater. Chem. A 2018, 6, 18116–18124. [Google Scholar] [CrossRef]
- Allah, A.E.; Wang, J.; Kaneti, Y.V.; Li, T.; Farghali, A.A.; Khedr, M.H.; Nanjundan, A.K.; Ding, B.; Dou, H.; Zhang, X.; et al. Auto-programmed heteroarchitecturing: Self-assembling ordered mesoporous carbon between two-dimensional Ti3C2Tx MXene layers. Nano Energy 2019, 65, 103991. [Google Scholar] [CrossRef]
- Johnson, M.; Zhang, Q.; Wang, D. Titanium carbide MXene: Synthesis, electrical and optical properties and their applications in sensors and energy storage devices. Nanomater. Nanotechnol. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Michael, J.; Wang, D.L.; Zhang, Q.F. Synthesis of high yield, pure Ti3C2 MXene using high temperature etching. Nanomaterials 2020, in press. [Google Scholar]
- Cao, M.; Wang, F.; Wang, L.; Wu, W.; Lv, W.; Zhu, J. Room temperature oxidation of Ti3C2 MXene for supercapacitor electrodes. J. Electrochem. Soc. 2017, 164, A3933. [Google Scholar] [CrossRef]
- Kim, S.J.; Koh, H.J.; Ren, C.E.; Kwon, O.; Maleski, K.; Cho, S.Y.; Anasori, B.; Kim, C.K.; Choi, Y.K.; Kim, J.; et al. Metallic Ti3C2Tx MXene Gas Sensors with Ultrahigh Signal-to-Noise Ratio. ACS Nano 2018, 12, 986–993. [Google Scholar] [CrossRef] [Green Version]

| Material | Principle of Operation Device Type | Lowest Concentration Detected (ppm) | Response Time | Operation Temperature |
|---|---|---|---|---|
| ZnO-CuO [13] | Resistance change Core-hollow cube | 0.04 ppm | 5.59 s for 0.5 ppm | 200 °C |
| In2O3 [14] | Resistance change Nanowire | 25 ppm | ~10 s (in N2) | 400 °C |
| InN [15] | Resistance change Thin Films | 0.4 ppm | 150 s for 10 ppm (in air) | 200 °C |
| GaN [16] | Resistance change thin Films | 500 ppm | 10 s for 1000 ppm (in air) | 350 °C |
| WO3 [16] | Resistance change Nanoparticles | 0.2 ppm | ~3.5 m | 400 °C |
| WO3 Fibers w/Pt [17] | Resistance change Nanoparticles | 0.12 ppm | 5 min (in air) | 300 °C |
| In/WO3-SnO2 [18] | Resistance change thin films | Verify | Verify | 200 °C |
| ZnO [19] | Resistance change thin film | 100 ppm | 30 s | 200 °C |
| Fe2O3 [20] | Resistance change Thin Film | 500 ppm | 33 s (in air) | 275 °C |
| TiO2 [21] | Resistance change Thin Film | 1 ppm | 10 s (in air) | 500 °C |
| K2W7O22 [22] | Resistance change Thin Film | 0.5 ppm | ~30 s * | 25 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ama, O.; Sadiq, M.; Johnson, M.; Zhang, Q.; Wang, D. Novel 1D/2D KWO/Ti3C2Tx Nanocomposite-Based Acetone Sensor for Diabetes Prevention and Monitoring. Chemosensors 2020, 8, 102. https://doi.org/10.3390/chemosensors8040102
Ama O, Sadiq M, Johnson M, Zhang Q, Wang D. Novel 1D/2D KWO/Ti3C2Tx Nanocomposite-Based Acetone Sensor for Diabetes Prevention and Monitoring. Chemosensors. 2020; 8(4):102. https://doi.org/10.3390/chemosensors8040102
Chicago/Turabian StyleAma, Obinna, Mahek Sadiq, Michael Johnson, Qifeng Zhang, and Danling Wang. 2020. "Novel 1D/2D KWO/Ti3C2Tx Nanocomposite-Based Acetone Sensor for Diabetes Prevention and Monitoring" Chemosensors 8, no. 4: 102. https://doi.org/10.3390/chemosensors8040102
APA StyleAma, O., Sadiq, M., Johnson, M., Zhang, Q., & Wang, D. (2020). Novel 1D/2D KWO/Ti3C2Tx Nanocomposite-Based Acetone Sensor for Diabetes Prevention and Monitoring. Chemosensors, 8(4), 102. https://doi.org/10.3390/chemosensors8040102







