Design of A Low-Cost and Disposable Paper-Based Immunosensor for the Rapid and Sensitive Detection of Aflatoxin B1
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents
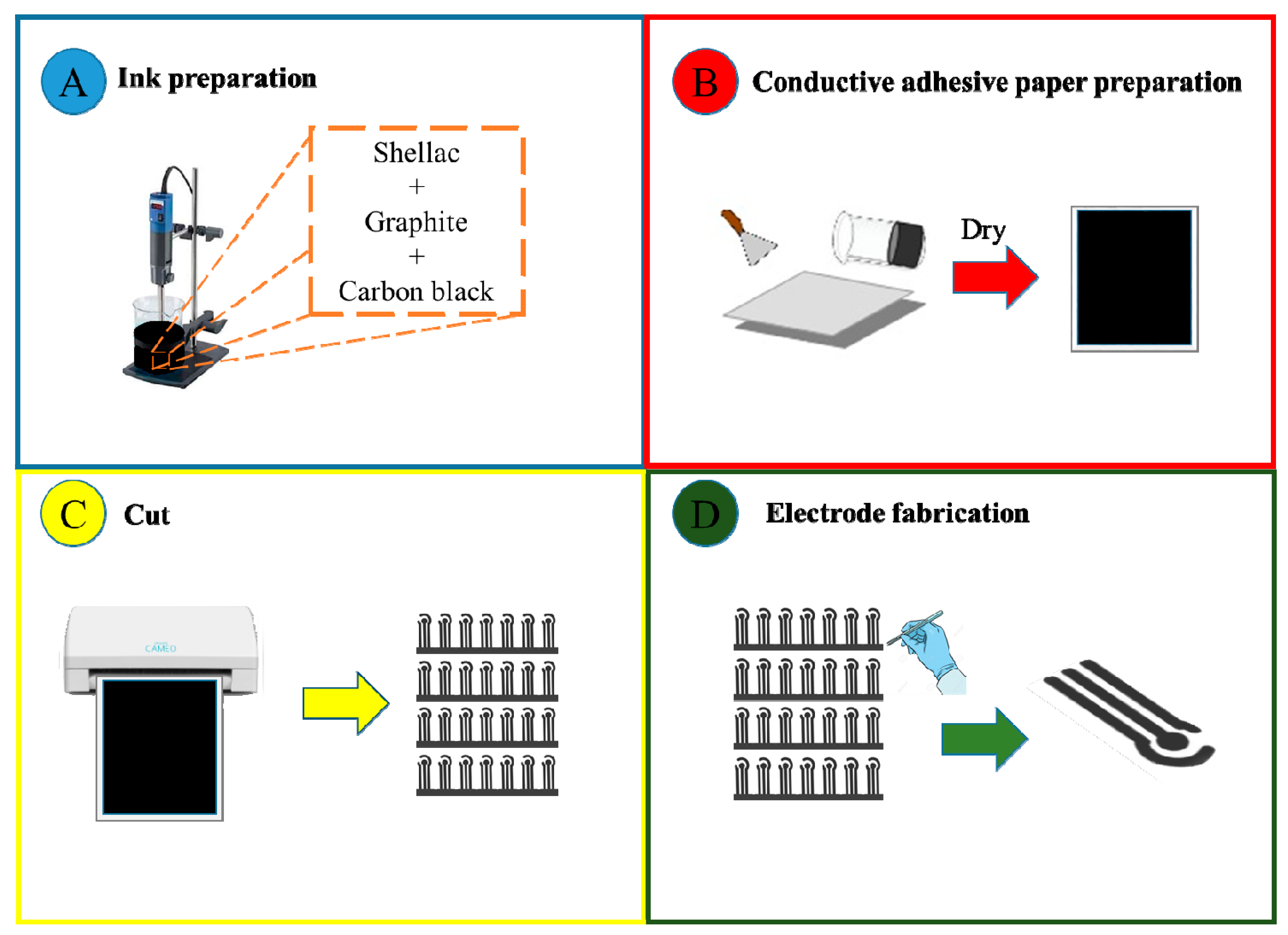
2.2. Fabrication of Disposable Printed Electrodes
2.3. Functionalization of Printed Electrodes and Antibody Immobilization
2.4. Morphological, Structural, and Electrochemical Characterization of Sensing Platforms
2.5. Electrochemical Detection of AFB1
2.6. Data Analysis with Information Visualization Techniques
2.7. Application of the Immunosensors for Real Food Sample Analysis
3. Results and Discussion
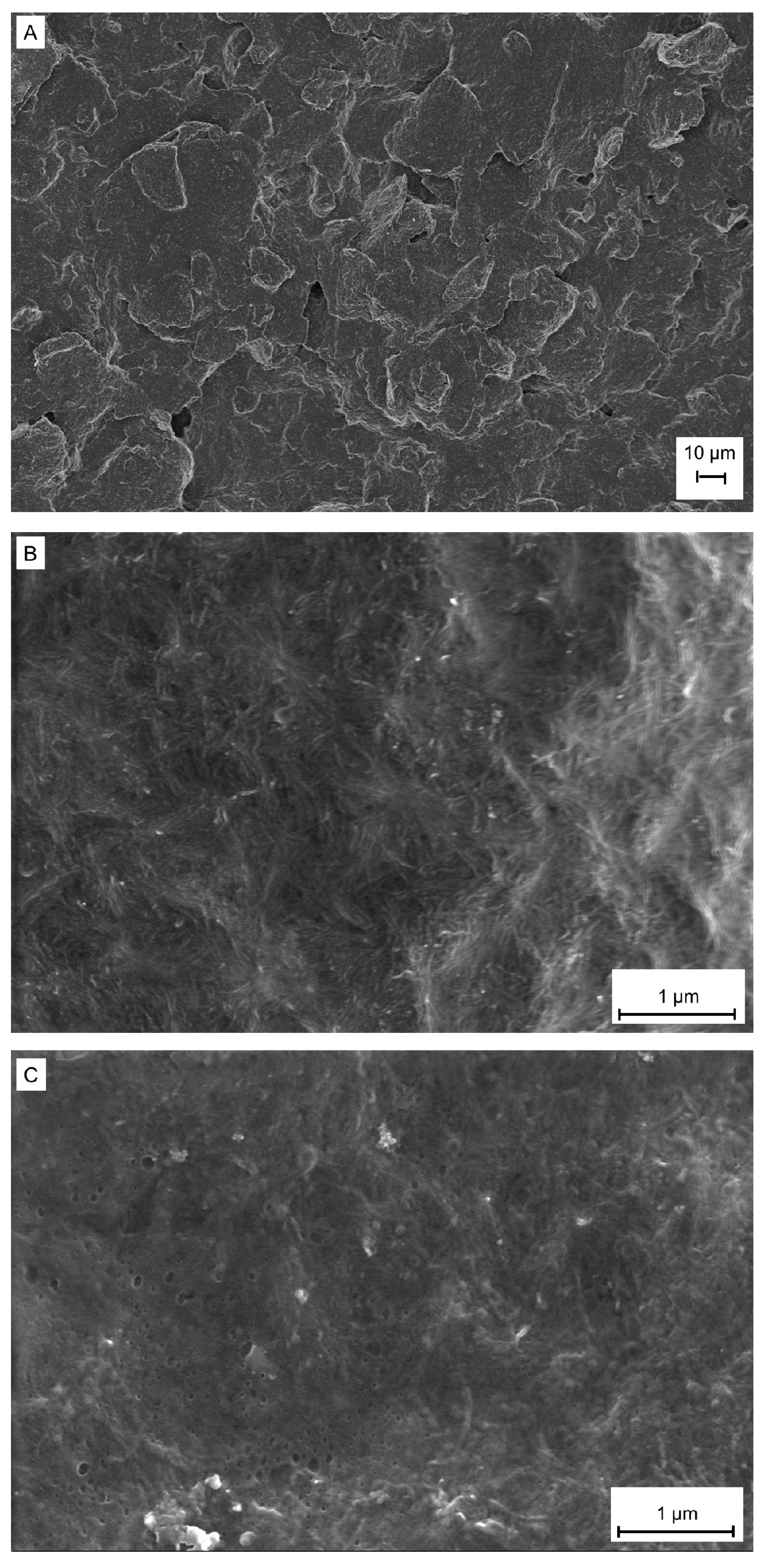
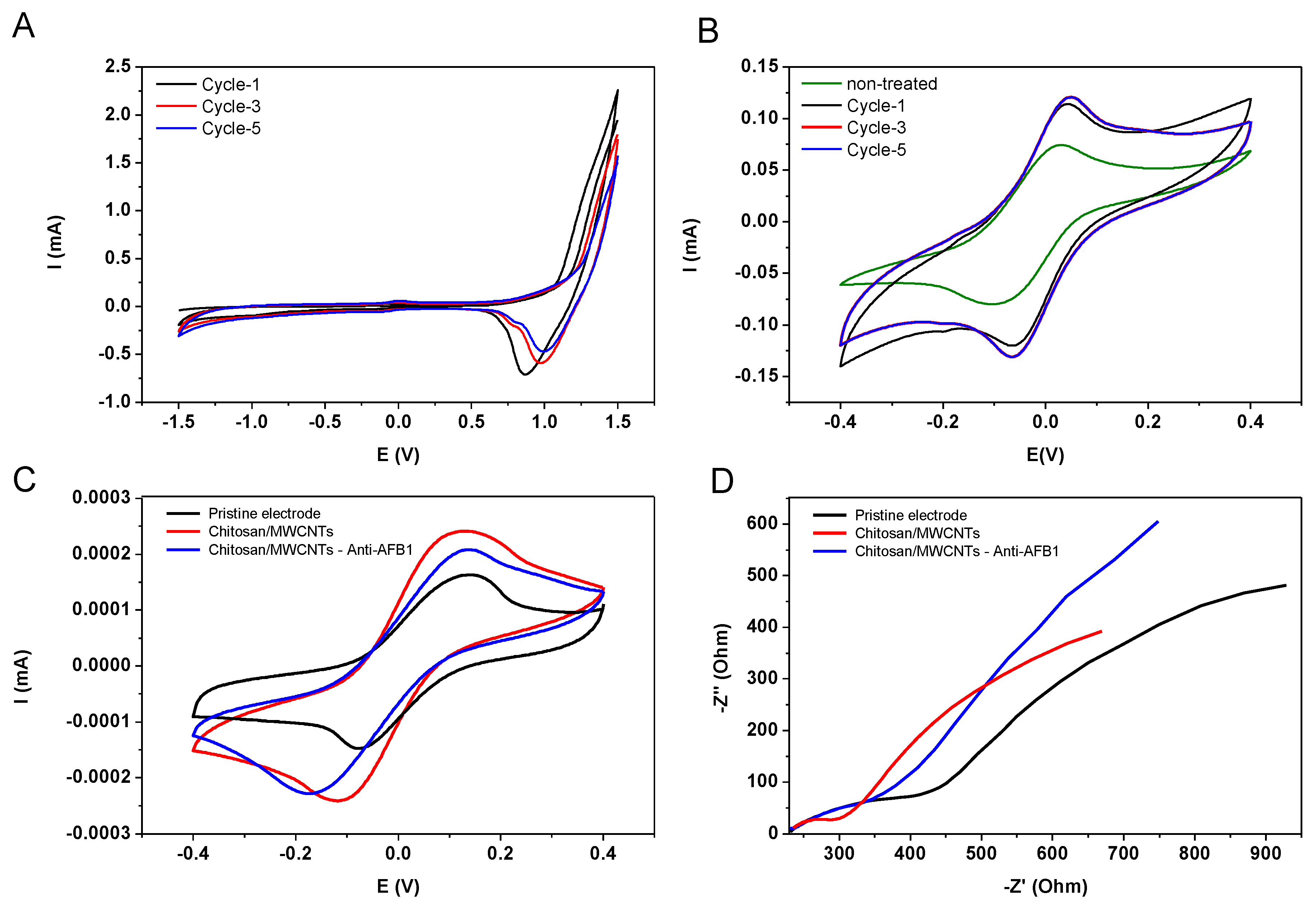
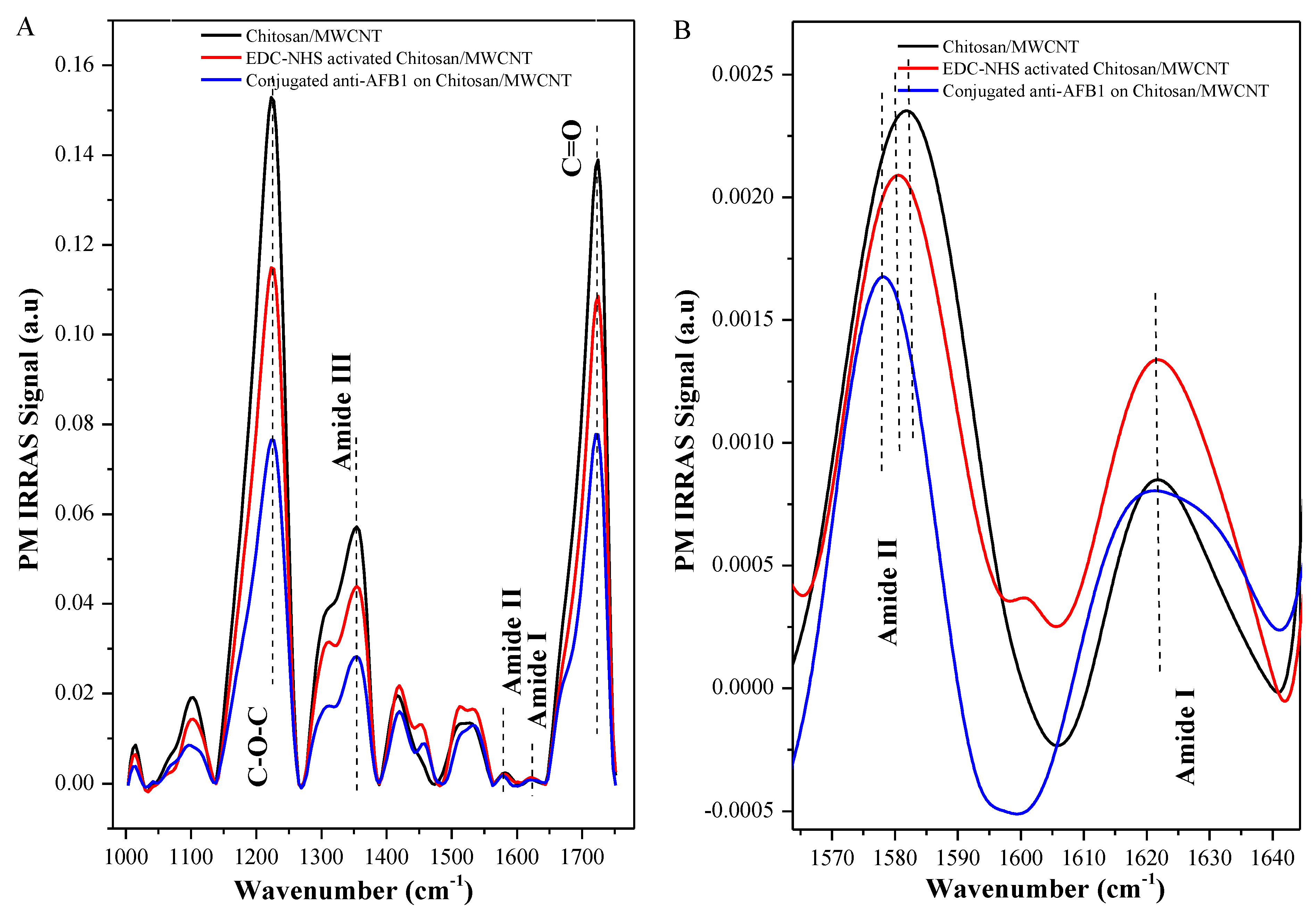
3.1. Morphological, Electrochemical, and Spectral Characterization of Electrodes
3.2. Electrochemical Detection of Mycotoxin AFB1
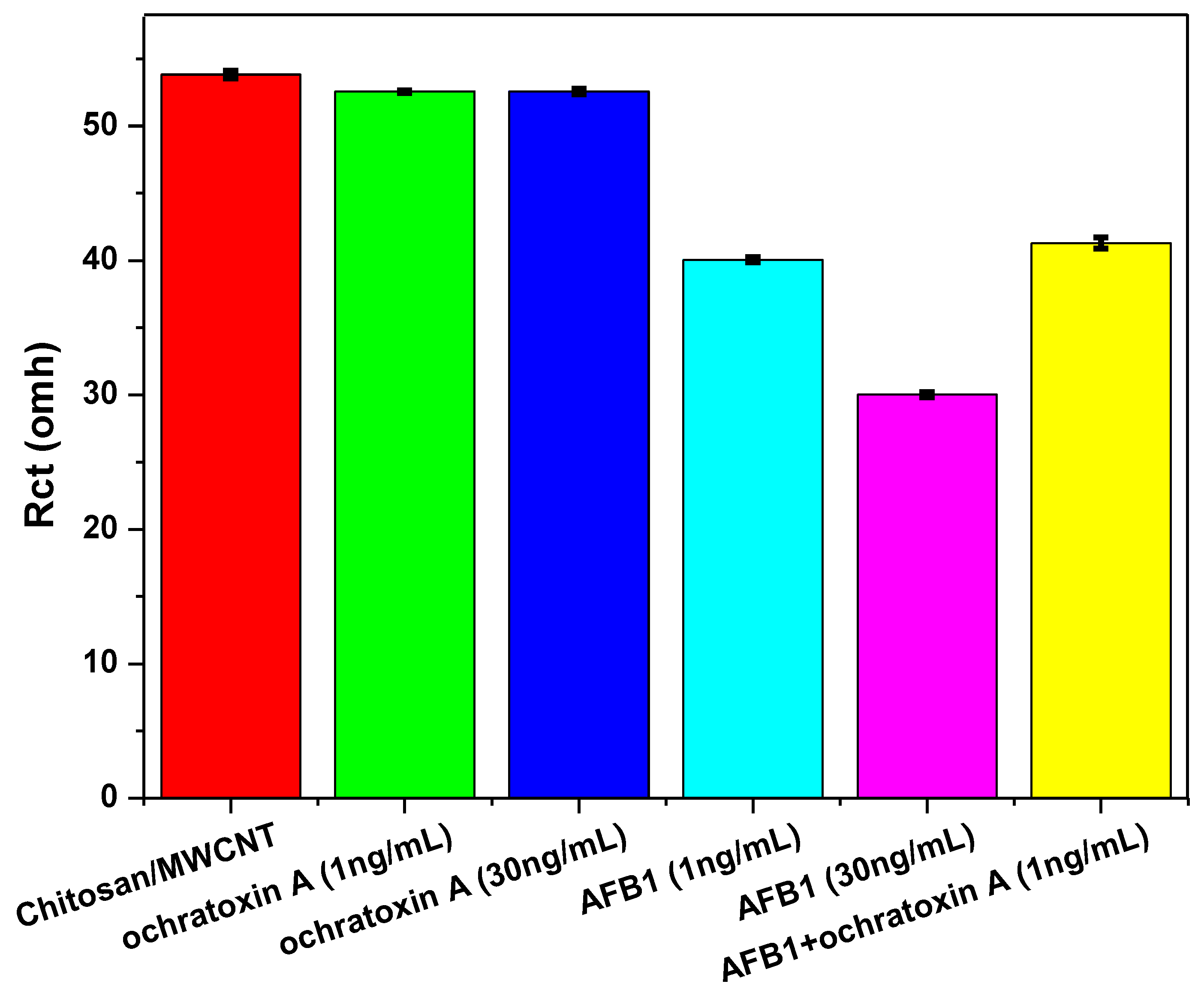
3.3. Interference Studies and Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xue, Z.; Zhang, Y.; Yu, W.; Zhang, J.; Wang, J.; Wan, F.; Kim, Y.; Liu, Y.; Kou, X. Recent advances in aflatoxin B1 detection based on nanotechnology and nanomaterials-A review. Anal. Chim. Acta 2019, 1069, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Sergeyeva, T.; Yarynka, D.; Piletska, E.; Lynnik, R.; Zaporozhets, O.; Brovko, O.; Piletsky, S.; El’skaya, A. Fluorescent sensor systems based on nanostructured polymeric membranes for selective recognition of Aflatoxin B1. Talanta 2017, 175, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, J.Y.; You, S.; Song, G.; Lim, W. Neurotoxic Effects of Aflatoxin B1 on Human Astrocytes In Vitro And on Glial Cell Development in Zebrafish In Vivo; Elsevier B.V.: Amsterdam, The Netherlands; Volume 386, ISBN 8223290499.
- Wang, C.; Li, Y.; Zhao, Q. A signal-on electrochemical aptasensor for rapid detection of aflatoxin B1 based on competition with complementary DNA. Biosens. Bioelectron. 2019, 144, 111641. [Google Scholar] [CrossRef] [PubMed]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- Solanki, P.R.; Singh, J.; Rupavali, B.; Tiwari, S.; Malhotra, B.D. Bismuth oxide nanorods based immunosensor for mycotoxin detection. Mater. Sci. Eng. C 2017, 70, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Uludag, Y.; Esen, E.; Kokturk, G.; Ozer, H.; Muhammad, T.; Olcer, Z.; Basegmez, H.I.O.; Simsek, S.; Barut, S.; Gok, M.Y.; et al. Lab-on-a-chip based biosensor for the real-time detection of aflatoxin. Talanta 2016, 160, 381–388. [Google Scholar] [CrossRef]
- Li, X.; Cao, L.; Zhang, Y.; Yan, P.; Kirk, D.W. Fabrication and Modeling of an Ultrasensitive Label Free Impedimetric Immunosensor for Aflatoxin B1 based on Protein A Self-assembly Modified Gold 3D Nanotube Electrode ensembles. Electrochim. Acta 2017, 247, 1052–1059. [Google Scholar] [CrossRef]
- Myndrul, V.; Viter, R.; Savchuk, M.; Koval, M.; Starodub, N.; Silamiķelis, V.; Smyntyna, V.; Ramanavicius, A.; Iatsunskyi, I. Gold coated porous silicon nanocomposite as a substrate for photoluminescence-based immunosensor suitable for the determination of Aflatoxin B1. Talanta 2017, 175, 297–304. [Google Scholar] [CrossRef]
- Tan, Y.; Wei, X.; Zhang, Y.; Wang, P.; Guo, L.; Lin, Z.; Yang, H. Exonuclease-Catalyzed Target Recycling Amplification and Immobilization Free Electrochemical Aptasensor Exonuclease-Catalyzed Target Recycling Amplification and Immobilization Free Electrochemical Aptasensor. Anal. Chem. 2015. [Google Scholar] [CrossRef]
- Sergeyeva, T.; Yarynka, D.; Piletska, E.; Linnik, R.; Zaporozhets, O.; Brovko, O.; Piletsky, S.; El’skaya, A. Development of a smartphone-based biomimetic sensor for aflatoxin B1 detection using molecularly imprinted polymer membranes. Talanta 2019, 201, 204–210. [Google Scholar] [CrossRef]
- Barandun, G.; Soprani, M.; Naficy, S.; Grell, M.; Kasimatis, M.; Chiu, K.L.; Ponzoni, A.; Güder, F. Cellulose Fibers Enable Near-Zero-Cost Electrical Sensing of Water-Soluble Gases. ACS Sensors 2019, 4, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Arduini, F.; Cinti, S.; Mazzaracchio, V.; Scognamiglio, V.; Amine, A.; Moscone, D. Carbon black as an outstanding and affordable nanomaterial for electrochemical (bio)sensor design. Biosens. Bioelectron. 2020, 156, 112033. [Google Scholar] [CrossRef] [PubMed]
- Razzino, C.A.; Serafín, V.; Gamella, M.; Pedrero, M.; Montero-Calle, A.; Barderas, R.; Calero, M.; Lobo, A.O.; Yáñez-Sedeño, P.; Campuzano, S.; et al. An electrochemical immunosensor using gold nanoparticles-PAMAM-nanostructured screen-printed carbon electrodes for tau protein determination in plasma and brain tissues from Alzheimer patients. Biosens. Bioelectron. 2020, 112238. [Google Scholar] [CrossRef] [PubMed]
- Moro, G.; Bottari, F.; Van Loon, J.; Du Bois, E.; De Wael, K.; Moretto, L.M. Disposable electrodes from waste materials and renewable sources for (bio)electroanalytical applications. Biosens. Bioelectron. 2019, 146, 111758. [Google Scholar] [CrossRef] [PubMed]
- Kurbanoglu, S.; Ozkan, S.A.; Merkoçi, A. Nanomaterials-based enzyme electrochemical biosensors operating through inhibition for biosensing applications. Biosens. Bioelectron. 2017, 89, 886–898. [Google Scholar] [CrossRef]
- de Oliveira, T.R.; Fonseca, W.T.; de Oliveira Setti, G.; Faria, R.C. Fast and flexible strategy to produce electrochemical paper-based analytical devices using a craft cutter printer to create wax barrier and screen-printed electrodes. Talanta 2019, 195, 480–489. [Google Scholar] [CrossRef]
- Zhu, G.; Yin, X.; Jin, D.; Zhang, B.; Gu, Y.; An, Y. Paper-based immunosensors: Current trends in the types and applied detection techniques. TrAC Trends Anal. Chem. 2019, 111, 100–117. [Google Scholar] [CrossRef]
- Huang, X.; Shi, W.; Li, J.; Bao, N.; Yu, C.; Gu, H. Determination of salivary uric acid by using poly(3,4-ethylenedioxythipohene) and graphene oxide in a disposable paper-based analytical device. Anal. Chim. Acta 2020, 1103, 75–83. [Google Scholar] [CrossRef]
- Cincotto, F.H.; Fava, E.L.; Moraes, F.C.; Fatibello-Filho, O.; Faria, R.C. A new disposable microfluidic electrochemical paper-based device for the simultaneous determination of clinical biomarkers. Talanta 2019, 195, 62–68. [Google Scholar] [CrossRef]
- Orzari, L.O.; Cristina de Freitas, R.; Aparecida de Araujo Andreotti, I.; Gatti, A.; Janegitz, B.C. A novel disposable self-adhesive inked paper device for electrochemical sensing of dopamine and serotonin neurotransmitters and biosensing of glucose. Biosens. Bioelectron. 2019, 138, 111310. [Google Scholar] [CrossRef]
- Orzari, L.O.; de Araujo Andreotti, I.A.; Bergamini, M.F.; Marcolino, L.H.; Janegitz, B.C. Disposable electrode obtained by pencil drawing on corrugated fiberboard substrate. Sensors Actuators B Chem. 2018, 264, 20–26. [Google Scholar] [CrossRef]
- Agrisuelas, J.; González-Sánchez, M.I.; Valero, E. Hydrogen peroxide sensor based on in situ grown Pt nanoparticles from waste screen-printed electrodes. Sensors Actuators B Chem. 2017, 249, 499–505. [Google Scholar] [CrossRef]
- Qin, Q.; Bai, X.; Hua, Z. Electropolymerization of a conductive β-cyclodextrin polymer on reduced graphene oxide modified screen-printed electrode for simultaneous determination of ascorbic acid, dopamine and uric acid. J. Electroanal. Chem. 2016, 782, 50–58. [Google Scholar] [CrossRef]
- Kampeera, J.; Pasakon, P.; Karuwan, C.; Arunrut, N.; Sappat, A.; Sirithammajak, S.; Dechokiattawan, N.; Sumranwanich, T.; Chaivisuthangkura, P.; Ounjai, P.; et al. Point-of-care rapid detection of Vibrio parahaemolyticus in seafood using loop-mediated isothermal amplification and graphene-based screen-printed electrochemical sensor. Biosens. Bioelectron. 2019, 132, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.A.; Cardoso, T.M.G.; Chagas, C.L.S.; Oliveira, V.X.G.; Munoz, R.A.A.; Henry, C.S.; Santana, M.H.P.; Paixão, T.R.L.C.; Coltro, W.K.T. Detection of Analgesics and Sedation Drugs in Whiskey Using Electrochemical Paper-based Analytical Devices. Electroanalysis 2018, 30, 2250–2257. [Google Scholar] [CrossRef]
- Hernández-Ibáñez, N.; García-Cruz, L.; Montiel, V.; Foster, C.W.; Banks, C.E.; Iniesta, J. Electrochemical lactate biosensor based upon chitosan/carbon nanotubes modified screen-printed graphite electrodes for the determination of lactate in embryonic cell cultures. Biosens. Bioelectron. 2016, 77, 1168–1174. [Google Scholar] [CrossRef]
- Ochiai, L.M.; Agustini, D.; Figueiredo-Filho, L.C.S.; Banks, C.E.; Marcolino-Junior, L.H.; Bergamini, M.F. Electroanalytical thread-device for estriol determination using screen-printed carbon electrodes modified with carbon nanotubes. Sensors Actuators B Chem. 2017, 241, 978–984. [Google Scholar] [CrossRef]
- Serafín, V.; Valverde, A.; Martínez-García, G.; Martínez-Periñán, E.; Comba, F.; Garranzo-Asensio, M.; Barderas, R.; Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Graphene quantum dots-functionalized multi-walled carbon nanotubes as nanocarriers in electrochemical immunosensing. Determination of IL-13 receptor A2 in colorectal cells and tumor tissues with different metastatic potential. Sensors Actuators B Chem. 2019, 284, 711–722. [Google Scholar] [CrossRef]
- Ganbat, K.; Pan, D.; Chen, K.; Ning, Z.; Xing, L.; Zhang, Y.; Shen, Y. One-pot electrografting preparation of bifunctionalized carbon nanotubes for sensitive electrochemical immunosensing. J. Electroanal. Chem. 2020, 860, 113906. [Google Scholar] [CrossRef]
- Atashbar, M.Z.; Bejcek, B.; Singamaneni, S.; Santucci, S. Carbon nanotube based biosensors. Proc. IEEE Sens. 2004, 2, 1048–1051. [Google Scholar] [CrossRef]
- Gupta, S.; Murthy, C.N.; Prabha, C.R. Recent advances in carbon nanotube based electrochemical biosensors. Int. J. Biol. Macromol. 2018, 108, 687–703. [Google Scholar] [CrossRef] [PubMed]
- Thapa, A.; Soares, A.C.; Soares, J.C.; Awan, I.T.; Volpati, D.; Melendez, M.E.; Fregnani, J.H.T.G.; Carvalho, A.L.; Oliveira, O.N. Carbon Nanotube Matrix for Highly Sensitive Biosensors to Detect Pancreatic Cancer Biomarker CA19-9. ACS Appl. Mater. Interfaces 2017, 9, 25878–25886. [Google Scholar] [CrossRef]
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sakthivel, R.; Liu, W.C.; Huang, C.W.; Li, J.; Xu, C.; Wu, Y.; Song, L.; He, W.; Chung, R.J. Ultra-highly sensitive organophosphorus biosensor based on chitosan/tin disulfide and British housefly acetylcholinesterase. Food Chem. 2020, 324, 126889. [Google Scholar] [CrossRef]
- Kaur, N.; Bharti, A.; Batra, S.; Rana, S.; Rana, S.; Bhalla, A.; Prabhakar, N. An electrochemical aptasensor based on graphene doped chitosan nanocomposites for determination of Ochratoxin A. Microchem. J. 2019, 144, 102–109. [Google Scholar] [CrossRef]
- dos Santos, D.M.; Bukzem, A.D.L.; Campana-Filho, S.P. Response surface methodology applied to the study of the microwave-assisted synthesis of quaternized chitosan. Carbohydr. Polym. 2016, 138, 317–326. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Hu, C.; Wu, H.; Yang, Y.; Huang, C.; Jia, N. Highly sensitive electrochemical impedance spectroscopy immunosensor for the detection of AFB 1 in olive oil. FOOD Chem. 2015, 176, 22–26. [Google Scholar] [CrossRef]
- Ma, H.; Sun, J.; Zhang, Y.; Xia, S. Disposable amperometric immunosensor for simple and sensitive determination of aflatoxin B1 in wheat. Biochem. Eng. J. 2016, 115, 38–46. [Google Scholar] [CrossRef]
- Ma, H.; Sun, J.; Zhang, Y.; Bian, C.; Xia, S.; Zhen, T. Label-free immunosensor based on one-step electrodeposition of chitosan-gold nanoparticles biocompatible film on Au microelectrode for determination of aflatoxin B1 in maize. Biosens. Bioelectron. 2016, 80, 222–229. [Google Scholar] [CrossRef]
- Paulovich, F.V.; Moraes, M.L.; Maki, R.M.; Ferreira, M.; Oliveira, O.N.; de Oliveira, M.C.F. Information visualization techniques for sensing and biosensing. Analyst 2011, 136, 1344–1350. [Google Scholar] [CrossRef]
- Minghim, R.; Paulovich, F.V.; de Andrade Lopes, A. Content-based text mapping using multi-dimensional projections for exploration of document collections. Vis. Data Anal. 2006, 6060, 60600S. [Google Scholar] [CrossRef]
- Bernalte, E.; Marín-Sánchez, C.; Pinilla-Gil, E.; Brett, C.M.A. Characterisation of screen-printed gold and gold nanoparticle-modified carbon sensors by electrochemical impedance spectroscopy. J. Electroanal. Chem. 2013, 709, 70–76. [Google Scholar] [CrossRef]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Mallamace, F.; Corsaro, C.; Mallamace, D.; Vasi, S.; Vasi, C.; Dugo, G. The role of water in protein’s behavior: The two dynamical crossovers studied by NMR and FTIR techniques. Comput. Struct. Biotechnol. J. 2015, 13, 33–37. [Google Scholar] [CrossRef]
- Gao, Y.; Kyratzis, I. Covalent Immobilization of Proteins on Carbon Nanotubes Using the. Bioconjug. Chem. 2008, 19, 1945–1950. [Google Scholar] [CrossRef]
- Jin, W.J.; Yang, G.J.; Shao, H.X.; Qin, A.J. A novel label-free impedimetric immunosensor for detection of semicarbazide residue based on gold nanoparticles-functional chitosan composite membrane. Sensors Actuators B Chem. 2013, 188, 271–279. [Google Scholar] [CrossRef]
- Almeida, D.A.L.; Edwards, E.R.; Ferreira, N.G. Self-sustaining hybrid electrode prepared from polyaniline, carbon nanotubes, and carbon fibers: Morphological, structural, and electrochemical analyses. J. Solid State Electrochem. 2018, 22, 69–80. [Google Scholar] [CrossRef]
- Comité de Direcção ICH Validation of Analytical Procedures: Text and Methodology Q2(R1). Int. Conf. Harmon. 2005, 1994, 17.
- Currie, L.A. International Union of Pure and Applied Chemistry Nomenclature in Evaluation of Analytical Methods Including Detection and Quantification Capabilities. Pure Appl. Chem. 1995, 67, 1699–1723. [Google Scholar] [CrossRef]
- Ren, M.; Xu, H.; Huang, X.; Kuang, M.; Xiong, Y.; Xu, H.; Xu, Y.; Chen, H.; Wang, A. Immunochromatographic assay for ultrasensitive detection of aflatoxin B1 in maize by highly luminescent quantum dot beads. ACS Appl. Mater. Interfaces 2014, 6, 14215–14222. [Google Scholar] [CrossRef]
- Krittayavathananon, A.; Sawangphruk, M. Impedimetric Sensor of ss-HSDNA/Reduced Graphene Oxide Aerogel Electrode toward Aflatoxin B1 Detection: Effects of Redox Mediator Charges and Hydrodynamic Diffusion. Anal. Chem. 2017, 89, 13283–13289. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Chu, Y.; Ma, H.; Li, Y.; Wu, D.; Du, B.; Wei, Q. Disposable competitive-type immunoassay for determination of aflatoxin B1 via detection of copper ions released from Cu-apatite. Talanta 2016, 147, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Goud, K.Y.; Hayat, A.; Catanante, G.; Satyanarayana, S.M.; Gobi, K.V.; Marty, J.L. An electrochemical aptasensor based on functionalized graphene oxide assisted electrocatalytic signal amplification of methylene blue for aflatoxin B1 detection. Electrochim. Acta 2017, 244, 96–103. [Google Scholar] [CrossRef]
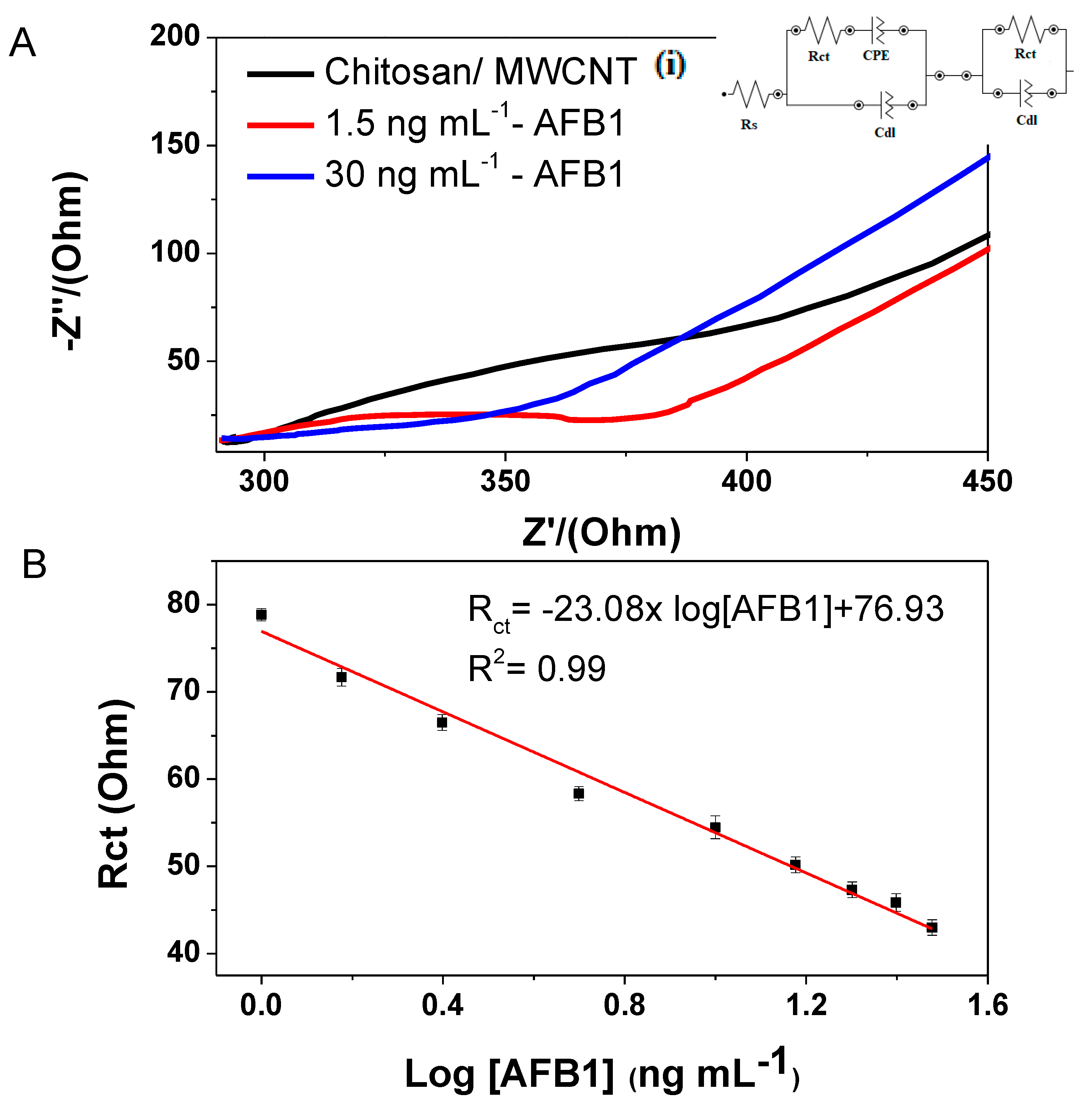
| Immunoelectrode | Linear Range (ng mL−1) | Detection Limit (ng mL−1) | Reference |
|---|---|---|---|
| BSA/anti-AFB1/AuNPs | 0.001–100 | 0.0002 | 53 |
| BSA/anti-AFB1/chitosan-AuNPs | 0.1–1; 1–30 | 0.06 | 40 |
| BSA/anti-AFB1/chitosan-AuNPs | 0.2–2; 2–30 | 0.12 | 39 |
| BSA/anti-AFB1/GO | 0.05–6 | 0.05 | 54 |
| BSA/anti-AFB1/chitosan/MWCNT | 1–30 | 0.62 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migliorini, F.L.; Santos, D.M.d.; Soares, A.C.; Mattoso, L.H.C.; Oliveira, O.N., Jr.; Correa, D.S. Design of A Low-Cost and Disposable Paper-Based Immunosensor for the Rapid and Sensitive Detection of Aflatoxin B1. Chemosensors 2020, 8, 87. https://doi.org/10.3390/chemosensors8030087
Migliorini FL, Santos DMd, Soares AC, Mattoso LHC, Oliveira ON Jr., Correa DS. Design of A Low-Cost and Disposable Paper-Based Immunosensor for the Rapid and Sensitive Detection of Aflatoxin B1. Chemosensors. 2020; 8(3):87. https://doi.org/10.3390/chemosensors8030087
Chicago/Turabian StyleMigliorini, Fernanda L., Danilo M. dos Santos, Andrey C. Soares, Luiz H. C. Mattoso, Osvaldo N. Oliveira, Jr., and Daniel S. Correa. 2020. "Design of A Low-Cost and Disposable Paper-Based Immunosensor for the Rapid and Sensitive Detection of Aflatoxin B1" Chemosensors 8, no. 3: 87. https://doi.org/10.3390/chemosensors8030087
APA StyleMigliorini, F. L., Santos, D. M. d., Soares, A. C., Mattoso, L. H. C., Oliveira, O. N., Jr., & Correa, D. S. (2020). Design of A Low-Cost and Disposable Paper-Based Immunosensor for the Rapid and Sensitive Detection of Aflatoxin B1. Chemosensors, 8(3), 87. https://doi.org/10.3390/chemosensors8030087








