Rationalization of In-Situ Synthesized Plasmonic Paper for Colorimetric Detection of Glucose in Ocular Fluids
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. In-Situ Synthesis of Gold Nanospheres on Paper Platform
- 2 mM of gold (III) chloride aqueous solution (5 μL) was dropped on a hydrophilic circle and dried;
- Glucose-spiked solution or artificial tear sample (5 μL) was taken and likewise dried on the reaction zone;
- An initiator composing of sodium hydroxide (60 mM) and sodium chloride (200 mM and then titrated by 2-folded dilution) was applied to initialize the reaction.
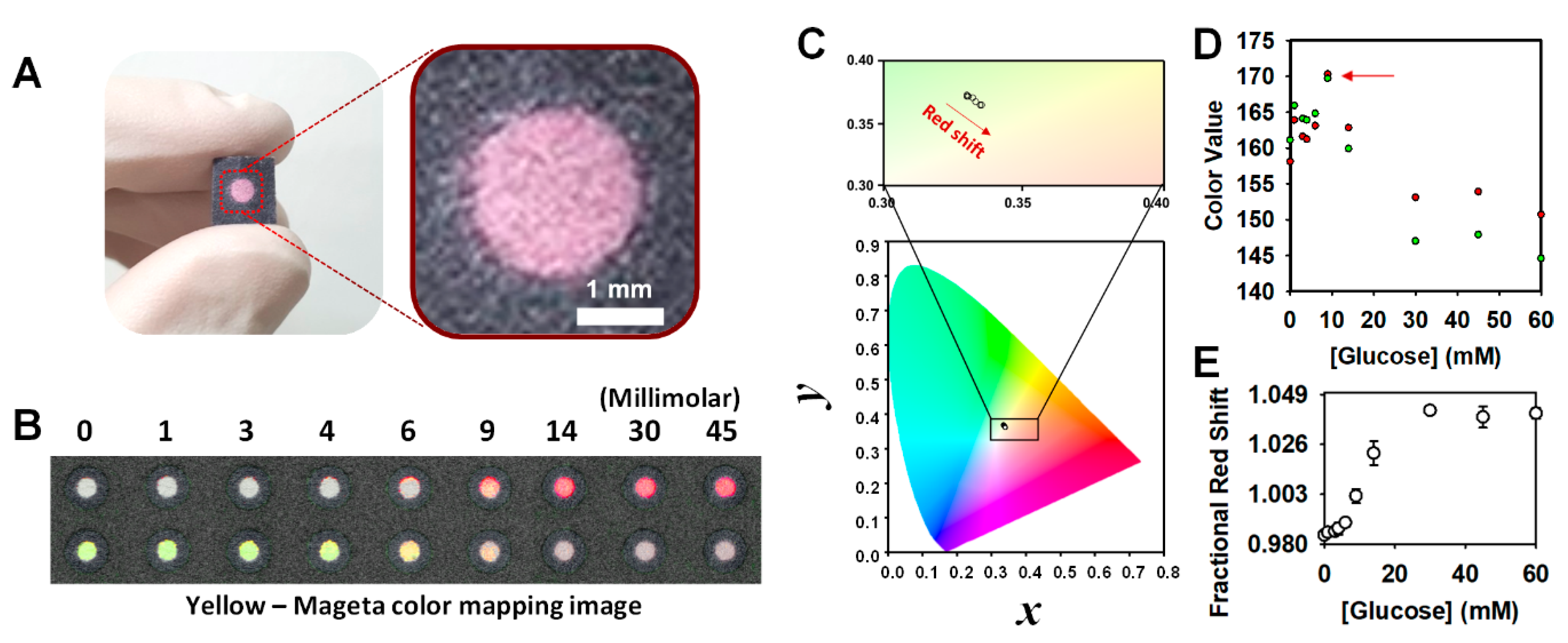
2.3. Chromatic Characterization and Colorimetric Assay
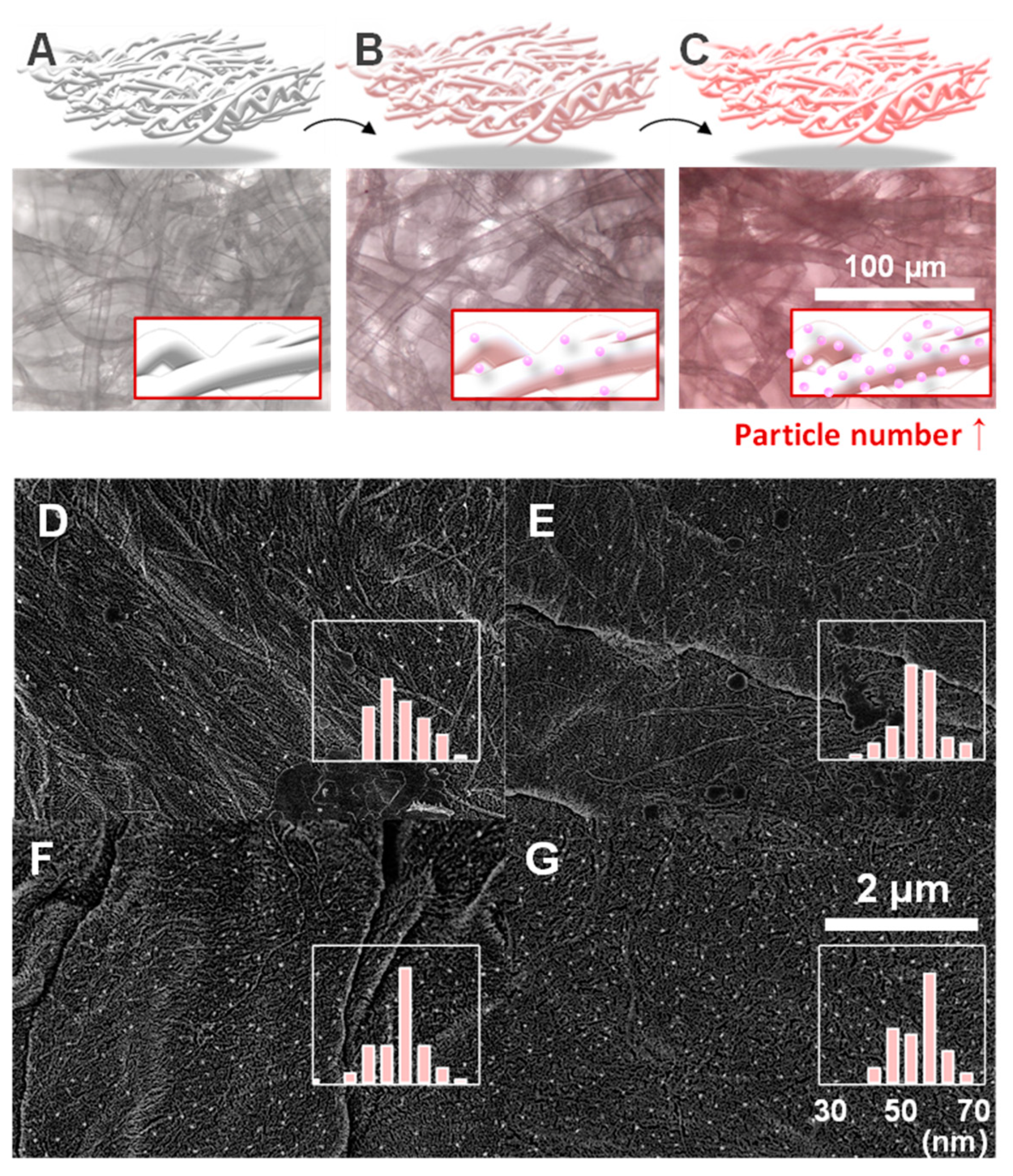
2.4. Microscopy and Morphologic Analysis
2.5. UV-vis Spectrometry
2.6. Statistics
3. Results and Discussion
3.1. Determination of In Situ Synthesis of Gold Nanospheres on Paper Cellulose
3.1.1. Chromatic Property and Theoretical Assumption
3.1.2. Characterization of In Situ Synthesized Gold Nanospheres
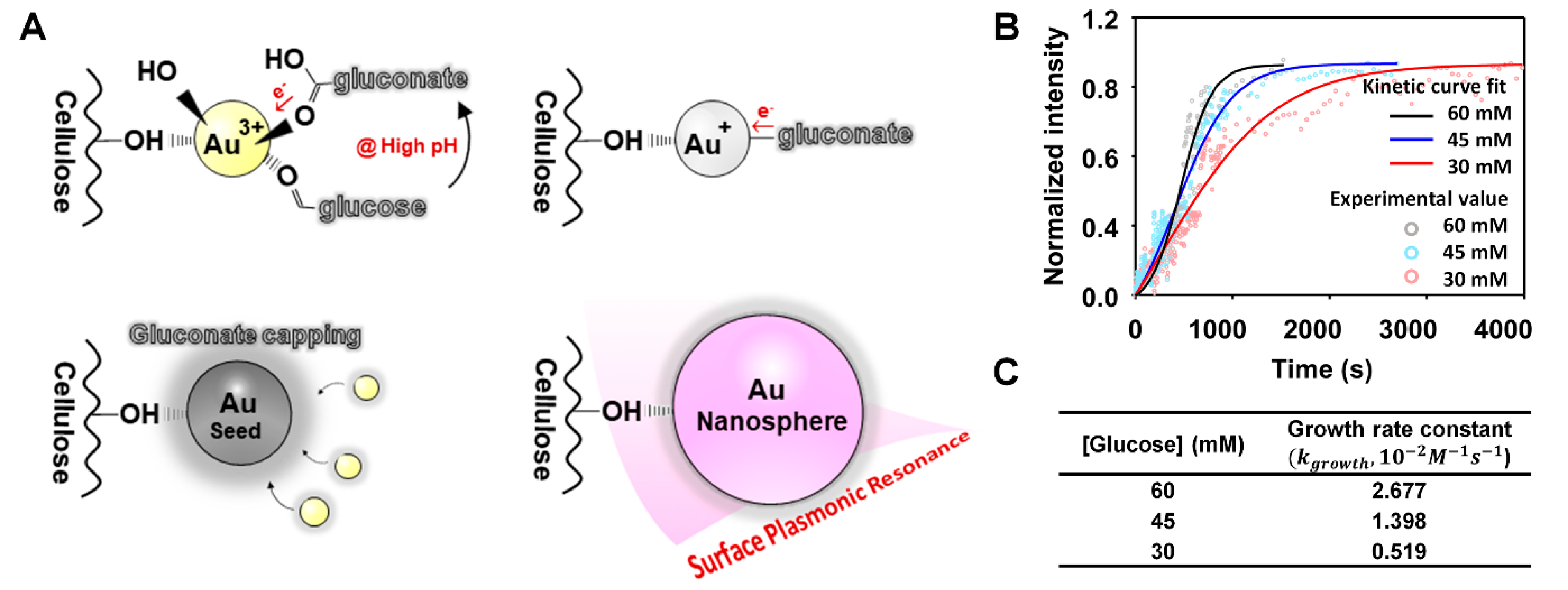
3.1.3. Projected Mechanism
3.2. Tear Glucose Sensing
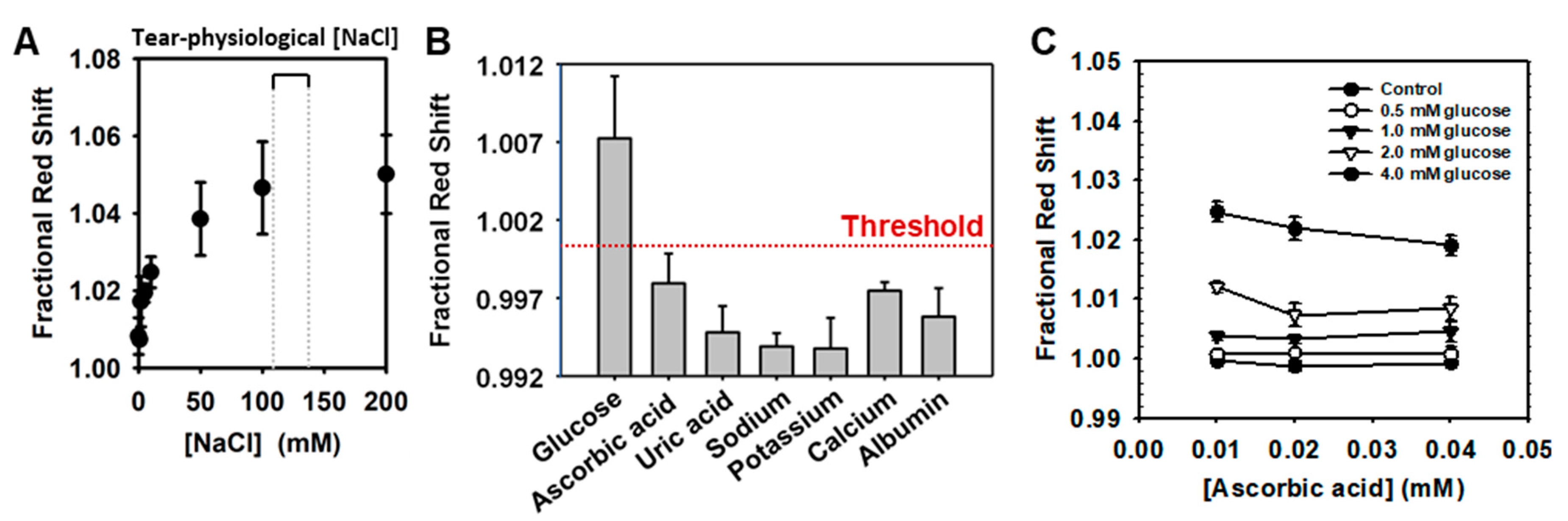
3.2.1. Matrix Components of Tears
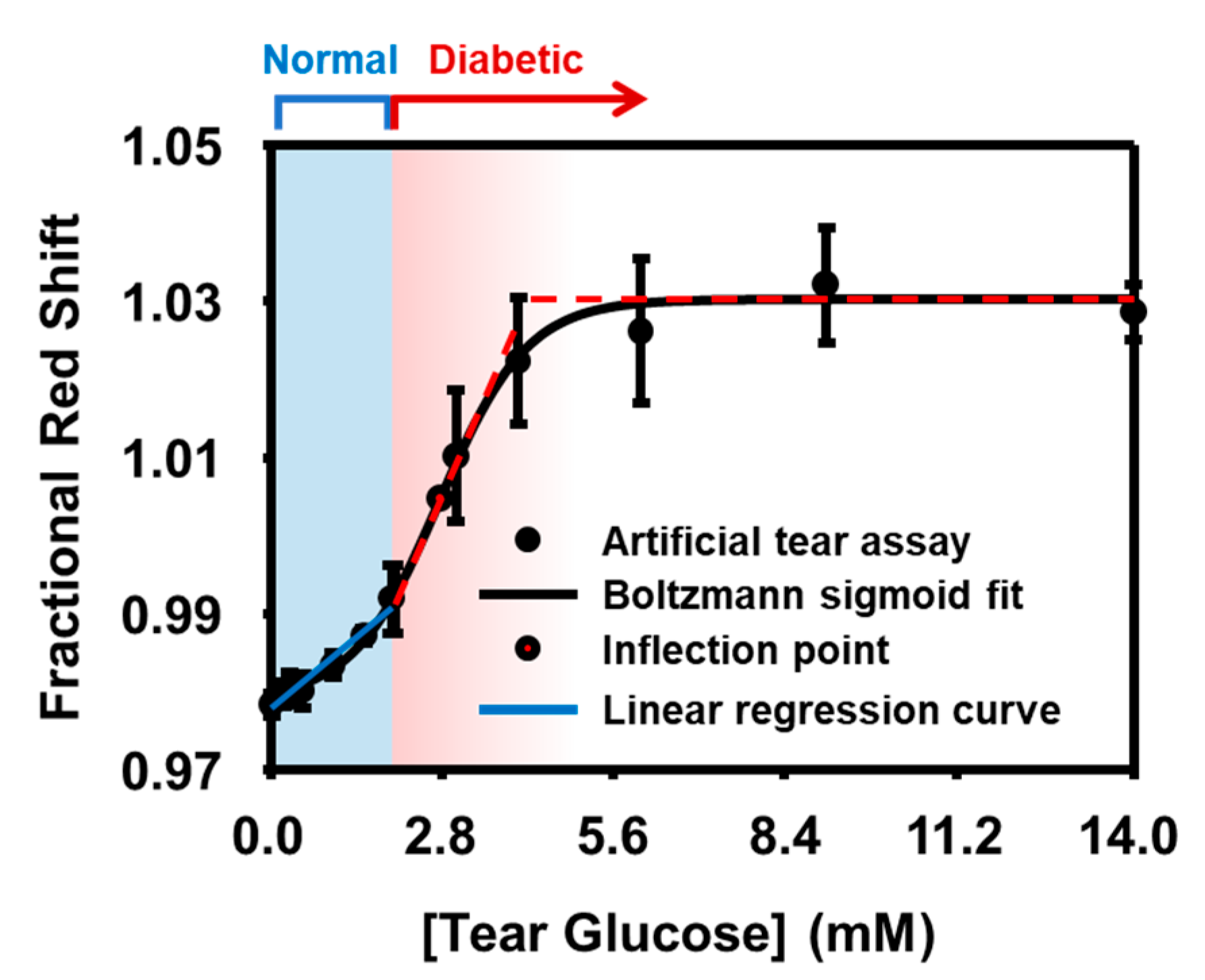
3.2.2. Artificial Tear Assay
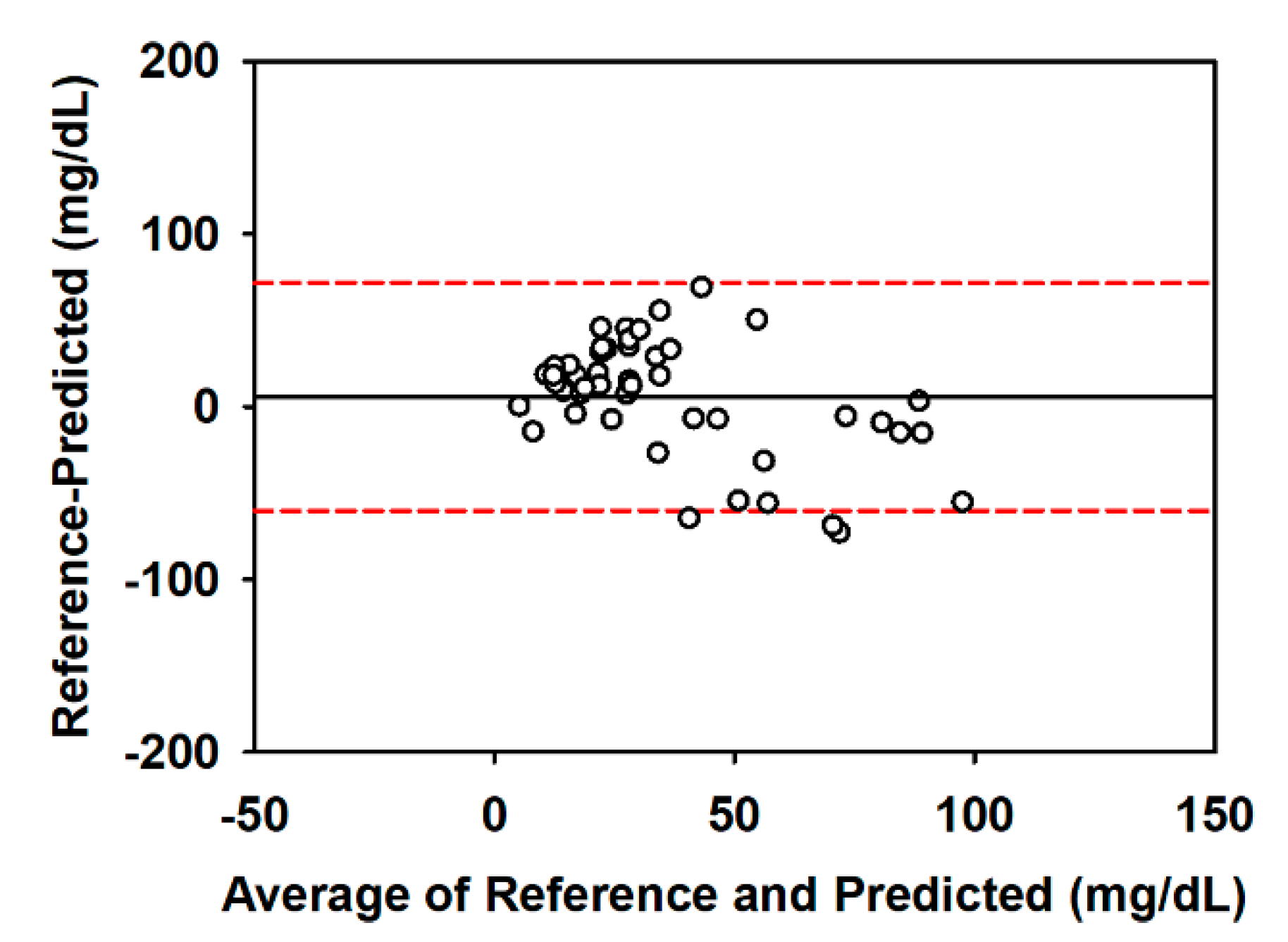
3.2.3. Statistical Validation
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Heineman, W.R.; Jensen, W.B. Leland C. Clark Jr. (1918–2005). Biosens. Bioelectron. 2006, 21, 1403–1404. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Carrilho, E.; Thomas, S.W.; Sindi, H.; Whitesides, G.M. Simple telemedicine for developing regions: Camera phones and paper-based microfluidic devices for real-time, off-site diagnosis. Anal. Chem. 2008, 80, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, K.; Stobiecka, M. High-performance modified cellulose paper-based biosensors for medical diagnostics and early cancer screening: A concise review. Carbohydr. Polym. 2020, 229, 115463. [Google Scholar] [CrossRef] [PubMed]
- Bergamonti, L.; Potenza, M.; Haghighi Poshtiri, A.; Lorenzi, A.; Sanangelantoni, A.M.; Lazzarini, L.; Lottici, P.P.; Graiff, C. Ag-functionalized nanocrystalline cellulose for paper preservation and strengthening. Carbohydr. Polym. 2020, 231, 115773. [Google Scholar] [CrossRef]
- Yang, H.; Deng, M.; Tang, Q.; He, W.; Hu, C.; Xi, Y.; Liu, R.; Wang, Z.L. A Nonencapsulative Pendulum-Like Paper–Based Hybrid Nanogenerator for Energy Harvesting. Adv. Energy Mater. 2019, 9. [Google Scholar] [CrossRef]
- Jin, Q.; Ma, L.; Zhou, W.; Shen, Y.; Fernandez-Delgado, O.; Li, X.J. Smart paper transformer: New insight for enhanced catalytic efficiency and reusability of noble metal nanocatalysts. Chem. Sci. 2020, 11, 2915–2925. [Google Scholar] [CrossRef]
- Tran, C.D. Subnanogram Detection of Dyes on Filter Paper by Surface-Enhanced Raman Scattering Spectrometry. Anal. Chem. 1984, 56, 824–826. [Google Scholar] [CrossRef]
- He, J.; Kunitake, T.; Nakao, A. Facile In Situ Synthesis of Noble Metal Nanoparticles in Porous Cellulose Fibers. Chem. Mater. 2003, 15, 4401–4406. [Google Scholar] [CrossRef]
- Christodouleas, D.C.; Simeone, F.C.; Tayi, A.; Targ, S.; Weaver, J.C.; Jayaram, K.; Fernández-Abedul, M.T.; Whitesides, G.M. Fabrication of Paper-Templated Structures of Noble Metals. Adv. Mater. Technol. 2017, 2. [Google Scholar] [CrossRef]
- Faraday, M. LIX. Experimental relations of gold (and other metals) to light.—The bakerian lecture. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1857, 14, 512–539. [Google Scholar] [CrossRef]
- Lu, X.; Rycenga, M.; Skrabalak, S.E.; Wiley, B.; Xia, Y. Chemical synthesis of novel plasmonic nanoparticles. Annu. Rev. Phys. Chem. 2009, 60, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Campion, A.; Kambhampati, P. Surface-enhanced Raman scattering. Chem. Soc. Rev. 1998, 27, 241–250. [Google Scholar] [CrossRef]
- Quesada-González, D.; Stefani, C.; González, I.; de la Escosura-Muñiz, A.; Domingo, N.; Mutjé, P.; Merkoçi, A. Signal enhancement on gold nanoparticle-based lateral flow tests using cellulose nanofibers. Biosens. Bioelectron. 2019, 141, 111407. [Google Scholar] [CrossRef] [PubMed]
- De La Rica, R.; Stevens, M.M. Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nat. Nanotechnol. 2012, 7, 821–824. [Google Scholar] [CrossRef]
- Stobiecka, M.; Ratajczak, K.; Jakiela, S. Toward early cancer detection: Focus on biosensing systems and biosensors for an anti-apoptotic protein survivin and survivin mRNA. Biosens. Bioelectron. 2019, 137, 58–71. [Google Scholar] [CrossRef]
- Keleştemur, S.; Avci, E.; Çulha, M. Raman and Surface-Enhanced Raman Scattering for Biofilm Characterization. Chemosensors 2018, 6, 5. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, P.; Wang, Y.; Li, S.; Zhang, L.; Zhang, Y.; Ge, S.; Yu, J. Paper-based sandwich type SERS sensor based on silver nanoparticles and biomimetic recognizer. Sens. Actuators B Chem. 2020, 313, 127989. [Google Scholar] [CrossRef]
- Kim, W.; Lee, S.H.; Kim, J.H.; Ahn, Y.J.; Kim, Y.H.; Yu, J.S.; Choi, S. Paper-Based Surface-Enhanced Raman Spectroscopy for Diagnosing Prenatal Diseases in Women. ACS Nano 2018, 12, 7100–7108. [Google Scholar] [CrossRef]
- Jeon, J.; Lee, S.H.; Joung, Y.; Kim, K.; Choi, N.; Choo, J. Improvement of reproducibility and thermal stability of surface-enhanced Raman scattering-based lateral flow assay strips using silica-encapsulated gold nanoparticles. Sens. Actuators B Chem. 2020, 321, 128521. [Google Scholar] [CrossRef]
- Della Pelle, F.; Scroccarello, A.; Scarano, S.; Compagnone, D. Silver nanoparticles-based plasmonic assay for the determination of sugar content in food matrices. Anal. Chim. Acta 2019, 1051, 129–137. [Google Scholar] [CrossRef]
- Andreu-Navarro, A.; Fernández-Romero, J.M.; Gómez-Hens, A. Determination of antioxidant additives in foodstuffs by direct measurement of gold nanoparticle formation using resonance light scattering detection. Anal. Chim. Acta 2011, 695, 11–17. [Google Scholar] [CrossRef]
- Palazzo, G.; Facchini, L.; Mallardi, A. Colorimetric detection of sugars based on gold nanoparticle formation. Sens. Actuators B Chem. 2012, 161, 366–371. [Google Scholar] [CrossRef]
- Aihara, M.; Kubota, N.; Kadowaki, T. Study of the Correlation between Tear Glucose Concentrations and Blood Glucose Concentrations. Diabetes 2018, 67, 944. [Google Scholar] [CrossRef]
- Kim, S.; Jeon, H.J.; Park, S.; Lee, D.Y.; Chung, E. Tear Glucose Measurement by Reflectance Spectrum of a Nanoparticle Embedded Contact Lens. Sci. Rep. 2020, 10, 8254. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Brazaca, L.C.; García-Carmona, L.; Bolat, G.; Campbell, A.S.; Martin, A.; Tang, G.; Shah, R.; Mishra, R.K.; Kim, J.; et al. Eyeglasses-based tear biosensing system: Non-invasive detection of alcohol, vitamins and glucose. Biosens. Bioelectron. 2019, 137, 161–170. [Google Scholar] [CrossRef]
- Jang, H.; Noh, H. Chemiluminescent detection of tear glucose on paper microfluidic devices. Macromol. Res. 2015, 23, 493–495. [Google Scholar] [CrossRef]
- Google. Official Google Blog: Introducing Our Smart Contact Lens Project. Available online: https://googleblog.blogspot.com/2014/01/introducing-our-smart-contact-lens.html (accessed on 13 July 2020).
- Park, S.; Boo, H.; Chung, T.D. Electrochemical non-enzymatic glucose sensors. Anal. Chim. Acta 2006, 556, 46–57. [Google Scholar] [CrossRef]
- Van Haeringen, N.J. Clinical biochemistry of tears. Surv. Ophthalmol. 1981, 26, 84–96. [Google Scholar] [CrossRef]
- Carrilho, E.; Martinez, A.W.; Whitesides, G.M. Understanding wax printing: A simple micropatterning process for paper-based microfluidics. Anal. Chem. 2009, 81, 7091–7095. [Google Scholar] [CrossRef]
- Choy, C.K.M.; Benzie, I.F.F.; Cho, P. Ascorbic acid concentration and total antioxidant activity of human tear fluid measured using the FRASC assay. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3293–3298. [Google Scholar]
- Luensmann, D.; Jones, L. Albumin adsorption to contact lens materials: A review. Contact Lens Anterior Eye 2008, 31, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV-Vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Basu Ray, G.; Chakraborty, I.; Moulik, S.P. Pyrene absorption can be a convenient method for probing critical micellar concentration (cmc) and indexing micellar polarity. J. Colloid Interface Sci. 2006, 294, 248–254. [Google Scholar] [CrossRef]
- Watzky, M.A.; Finke, R.G. Transition metal nanocluster formation kinetic and mechanistic studies. A new mechanism when hydrogen is the reductant: Slow, continuous nucleation and fast autocatalytic surface growth. J. Am. Chem. Soc. 1997, 119, 10382–10400. [Google Scholar] [CrossRef]
- Namin, A.H.; Leboeuf, K.; Muscedere, R.; Wu, H.; Ahmadi, M. Efficient hardware implementation of the hyperbolic tangent sigmoid function. In Proceedings of the 2019 IEEE International Symposium on Circuits and Systems, Taipei, Taiwan, 24–27 May 2009; pp. 2117–2120. [Google Scholar]
- Mampallil, D.; Burak Eral, H. Historical perspective A review on suppression and utilization of the coffee-ring effect. Adv. Colloid Interface Sci. 2018, 252, 38–54. [Google Scholar] [CrossRef]
- Marsden, J.; House, L. The Chemistry of Gold Extraction, 2nd ed.; SME: Englewood, CO, USA, 2006; ISBN 978-0-87335-240-6. [Google Scholar]
- Amendola, V.; Meneghetti, M. Size evaluation of gold nanoparticles by UV-vis spectroscopy. J. Phys. Chem. C 2009, 113, 4277–4285. [Google Scholar] [CrossRef]
- Pacławski, K.; Streszewski, B.; Jaworski, W.; Luty-Błocho, M.; Fitzner, K. Gold nanoparticles formation via gold(III) chloride complex ions reduction with glucose in the batch and in the flow microreactor systems. Colloids Surf. A Physicochem. Eng. Asp. 2012, 413, 208–215. [Google Scholar] [CrossRef]
- MacLaurin, D.J.; Green, J.W. Carbohydrates in alkaline systems. II. Kinetics of the transformation and degradation reactions of cellobiose, cellobiulose, and 4-O-β-D-glucopyranosyl-D-mannose in 1 M sodium hydroxide at 22 °C. Can. J. Chem. 1969, 47, 3957–3964. [Google Scholar] [CrossRef]
- Liu, J.; Qin, G.; Raveendran, P.; Ikushima, Y. Facile “green” synthesis, characterization, and catalytic function of β-D-glucose-stabilized Au nanocrystals. Chem. A Eur. J. 2006, 12, 2131–2138. [Google Scholar] [CrossRef]
- Pfeiffer, C.; Rehbock, C.; Hühn, D.; Carrillo-Carrion, C.; De Aberasturi, D.J.; Merk, V.; Barcikowski, S.; Parak, W.J. Interaction of colloidal nanoparticles with their local environment: The (ionic) nanoenvironment around nanoparticles is different from bulk and determines the physico-chemical properties of the nanoparticles. J. R. Soc. Interface 2014, 11, 20130931. [Google Scholar] [CrossRef]
- Shang, L.; Wang, Y.; Jiang, J.; Dong, S. PH-dependent protein conformational changes in albumin:Gold nanoparticle bioconjugates: A spectroscopic study. Langmuir 2007, 23, 2714–2721. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Jeong, S.; Noh, H. Quantitative Determination of Tear Glucose Using Paper Based Microfluidic Devices Hyun-Jae. J. Korean Chem. Soc. 2015, 59. [Google Scholar] [CrossRef]
- Garg, S.K.; Hirsch, I.B. Self-Monitoring of Blood Glucose. Diabetes Technol. Ther. 2015, 17, S3–S11. [Google Scholar] [CrossRef]
- Wentholt, I.M.E.; Hart, A.A.M.; Hoekstra, J.B.L.; DeVries, J.H. How to Assess and Compare the Accuracy of Continuous Glucose Monitors? Diabetes Technol. Ther. 2008, 10, 57–68. [Google Scholar] [CrossRef]
| Glucose Concentration (mM) | Progress in Diabetic Patient 1 | Linearity | R2 | Analytical Function |
|---|---|---|---|---|
| [tear glucose] < 2 | normal | y = 3.69 × 10 4 x + 0.978 2 | 0.965 | quantitative (LoD = 0.29 mM) |
| 2 ≤ [tear glucose] < 4.2 | suspected or diabetic | y = 1.81 × 10 2 x + 0.978 3 | 0.994 4 | quantitative |
| 4.2 ≤ [tear glucose] | diabetic | N/A | N/A | qualitative |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.J.; Hyung, J.; Noh, H. Rationalization of In-Situ Synthesized Plasmonic Paper for Colorimetric Detection of Glucose in Ocular Fluids. Chemosensors 2020, 8, 81. https://doi.org/10.3390/chemosensors8030081
Kim HJ, Hyung J, Noh H. Rationalization of In-Situ Synthesized Plasmonic Paper for Colorimetric Detection of Glucose in Ocular Fluids. Chemosensors. 2020; 8(3):81. https://doi.org/10.3390/chemosensors8030081
Chicago/Turabian StyleKim, Hyeok Jung, Jiwoo Hyung, and Hyeran Noh. 2020. "Rationalization of In-Situ Synthesized Plasmonic Paper for Colorimetric Detection of Glucose in Ocular Fluids" Chemosensors 8, no. 3: 81. https://doi.org/10.3390/chemosensors8030081
APA StyleKim, H. J., Hyung, J., & Noh, H. (2020). Rationalization of In-Situ Synthesized Plasmonic Paper for Colorimetric Detection of Glucose in Ocular Fluids. Chemosensors, 8(3), 81. https://doi.org/10.3390/chemosensors8030081