1. Introduction
Volatile organic compounds (VOCs) are contained in gasoline, diesel fuel and various petroleum-based products and originate from industrial effluents, sewage disposal, oil storage wastes, and oil tanker accidents. Many of them are highly toxic, mutagenic, and carcinogenic. Due to their production and widespread use, VOCs are one of the most commonly detected organic pollutants in water. Releases into the environment may occur resulting in damages to humans and the environment [
1,
2,
3,
4,
5].
Monitoring of pollutants, particularly VOCs, in global waters including seawater, river and lake water, ground, surface and drinking water is important to maintain public health and protect the environment. The most commonly analytical techniques for detection of VOCs are chromatographic methods, i.e., gas chromatography and high-performance liquid chromatography [
3,
6,
7,
8,
9]. However, these methods include bulky measurement equipment and are therefore, confined to laboratory use. Furthermore, trained personnel are needed due to complex sample pretreatment steps. Therefore, these methods are very time-and cost-intensive. Hence, these techniques are not appropriate for monitoring in real-time and on-site.
Sensors based on ATR-FTIR spectroscopy are emerging one of the most suitable methods for detection of pollutants. Specific vibrations (i.e., stretching, bending and rotating) of the molecules in the “fingerprint” region (1500–500 cm
−1), enable direct qualitative analysis to distinguish different analytes [
10]. These sensors offer continuous on-site and in real-time monitoring of pollutants. Therefore, the sample does not have to be transported to the laboratory. Hence, falsified results due to the volatility of the analytes and point-sampling are prevented, time is saved, and costs are reduced.
In ATR-FTIR spectroscopy, the IR beam is totally internally reflected and an evanescent field at every reflection is established. According to Snell’s law, the incident angle has to be larger than the critical angle
as described in Equation (1) with the refractive indices of the waveguide
and surrounding medium, i.e., polymer membrane,
n2.
Detection directly in aqueous systems is enabled due to hydrophobic polymer-coated waveguides. Hydrophobic analyte molecules (i.e., VOCs) are enriched, whereas water is excluded from the penetration depth of the evanescent field. Therefore, high interfering water absorptions are prevented. The penetration depth
dp can be calculated as follows:
With the incident wavelength
, the angle of incidence
at the interface between waveguide and the surrounding medium (i.e., polymer membrane). The evanescent field intensity is dependent on the distance to the surface waveguide
x and the penetration depth [
11].
ATR-FTIR waveguide materials include crystals, e.g., zinc selenide (ZnSe), zinc sulfide (ZnS), silicon (Si), and germanium (Ge) and thin film waveguides based on gallium arsenide/aluminum gallium arsenide (GaAs/AlGaAs), mercury-cadmium-telluride (MCT), and diamond [
12,
13,
14,
15]. Alternatively, optical fiber waveguide materials were already reported, e.g., chalcogenide, silver and tellurium halide [
10,
16,
17,
18,
19,
20,
21,
22]. In particular, silver halide fibers are one of the most promising materials due to their flexibility and transparence in the entire mid-infrared (MIR) spectral regime [
23]. However, silver halide fibers decompose upon contact with seawater due to the high amount of chloride ions, furthermore, silver halide fibers are susceptible to ultraviolet radiation. Since a sensor withstanding in difficult matrices, i.e., seawater with its high ionic strength and high content of organic matter, is envisaged, the optical fiber has to be resistant to ever-changing conditions. Hence, a protective coating of the silver halide fiber is strictly necessary.
In order to detect VOCs in aqueous solutions, the waveguide has to be coated with a hydrophobic polymer membrane. The hydrophobic pollutants diffuse into the polymer layer, whereas the water matrix is excluded. At the same time, this polymer layer acts as protective coating of the silver halide fiber [
24,
25,
26]. Various types of hydrophobic polymer membranes were already investigated, e.g., low-density polyethylene (LDPE) [
17,
27,
28], Teflon
®AF [
29,
30,
31], poly(dimethylsiloxane) (PDMS) [
32,
33,
34], polyisobutylene (PIB) [
17,
35,
36,
37], and E/P-co polymer [
16,
35,
38,
39,
40,
41]. However, within harsh environments the polymer coating can come off. Hence, sufficient adhesion of the polymer to the waveguide surface has to be ensured.
Films of DLC are composed of a sp
3-and sp
2-hybridized carbon. Hence, DLC offers a high robustness and chemical inertness with transparency in the entire IR spectral range [
42,
43,
44]. Janotta et al. have successfully demonstrated the feasibility of DLC films as protective coating for detecting strongly oxidizing agents in aqueous matrix [
45]. DLC films have the advantage of having an amorphous structure offering the possibility of surface termination with e.g., hydrogen, oxygen, or fluorine tailoring the features of the waveguide surface [
46]. Therefore, a fluorine-termination reveals a highly hydrophobic surface offering a strong connection to the applied hydrophobic polymer coating. DLC films would enhance the long-term durability hindering the decomposition of the silver halide fiber, and therefore, preventing the attenuation of the transmitted IR radiation [
47]. DLC in combination with MIR sensing was already presented by several research teams [
43,
45,
48,
49,
50], and application within changing conditions, i.e., high temperature and humidity, was already shown [
51]. Therefore, application in harsh environments and sufficient protection of the waveguide is enabled. To our knowledge, there are no literature reports on the use of DLC-coated waveguides combined with hydrophobic polymers for IR spectroscopic detection of VOCs in water.
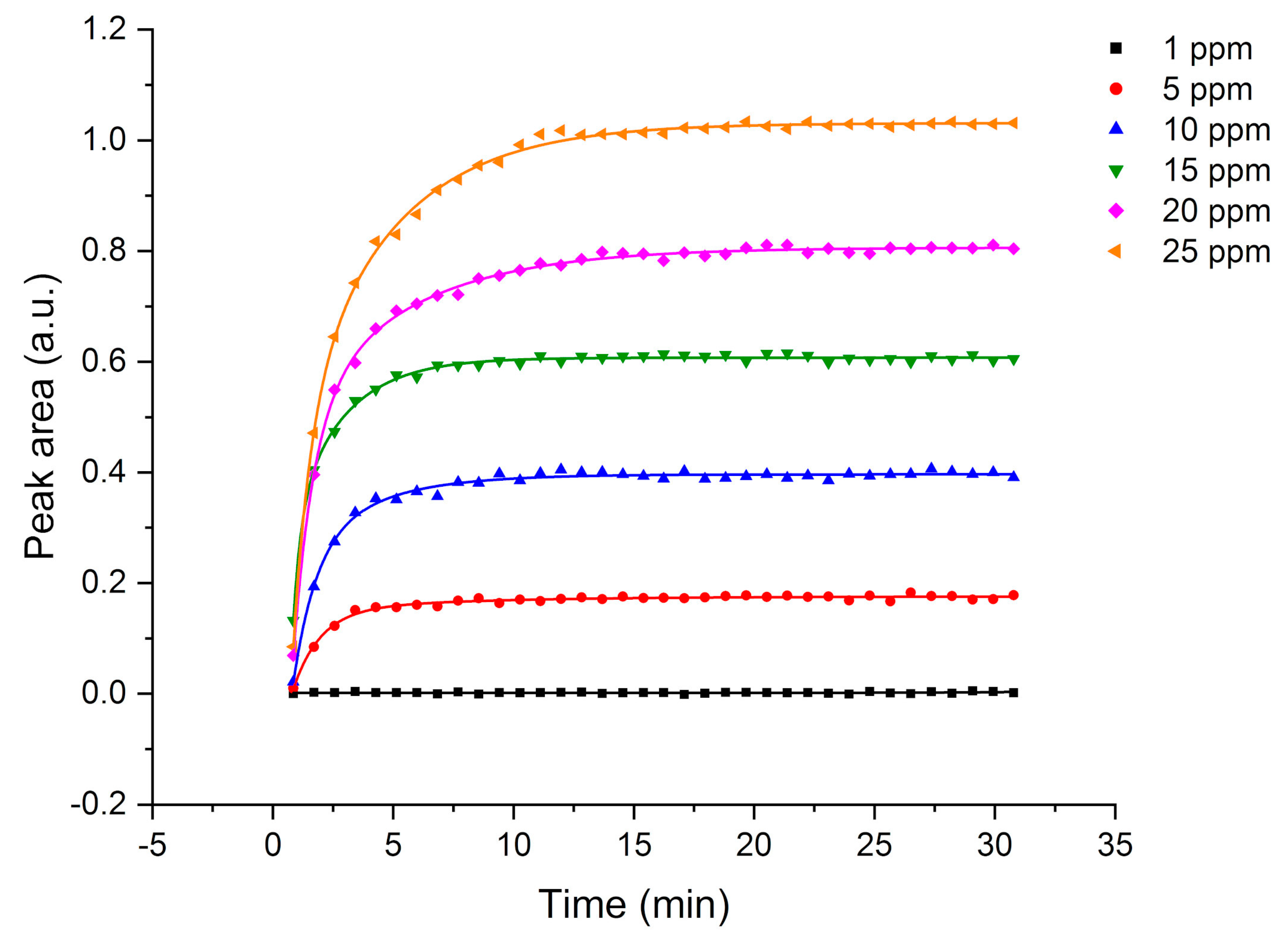
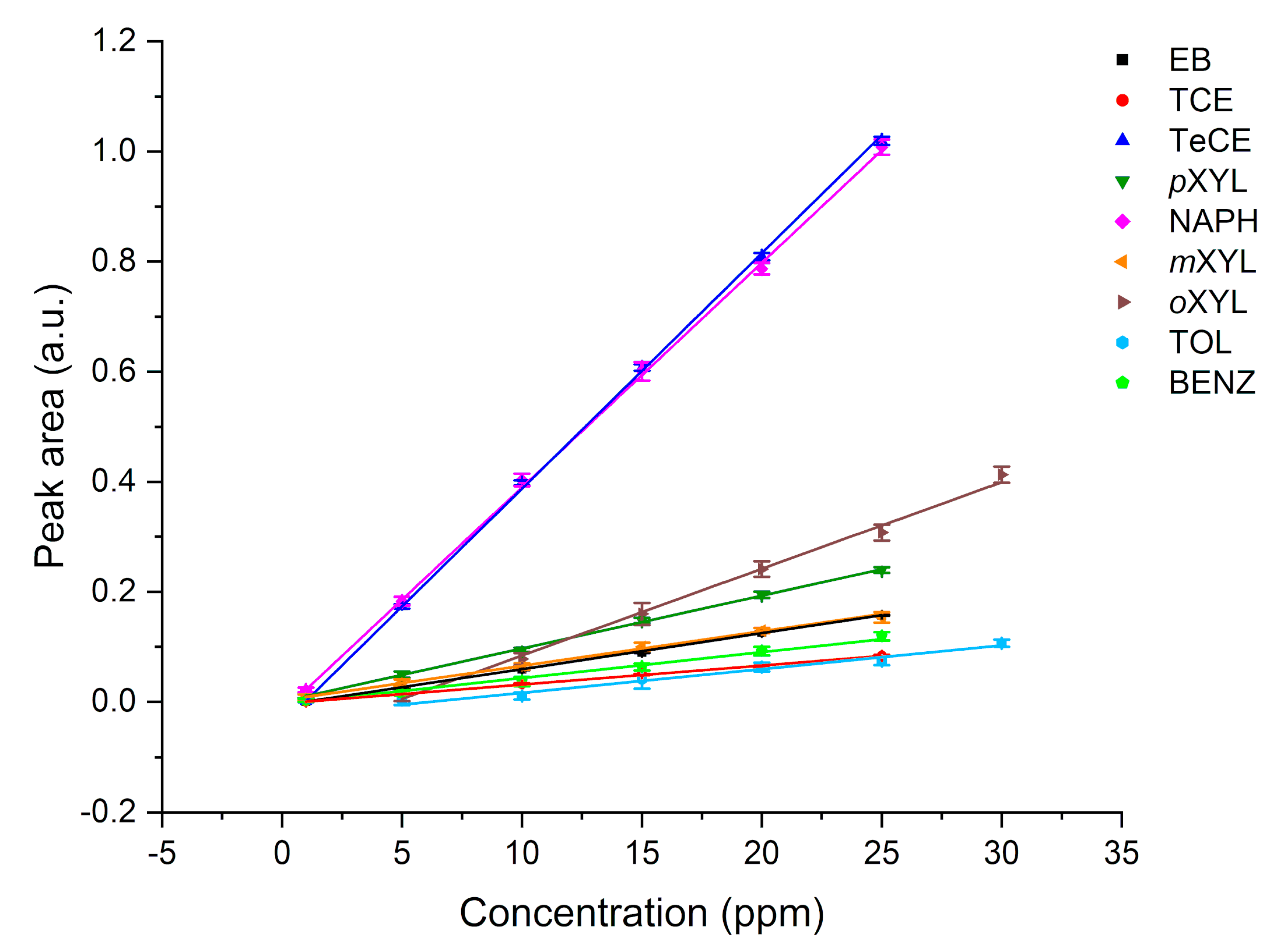
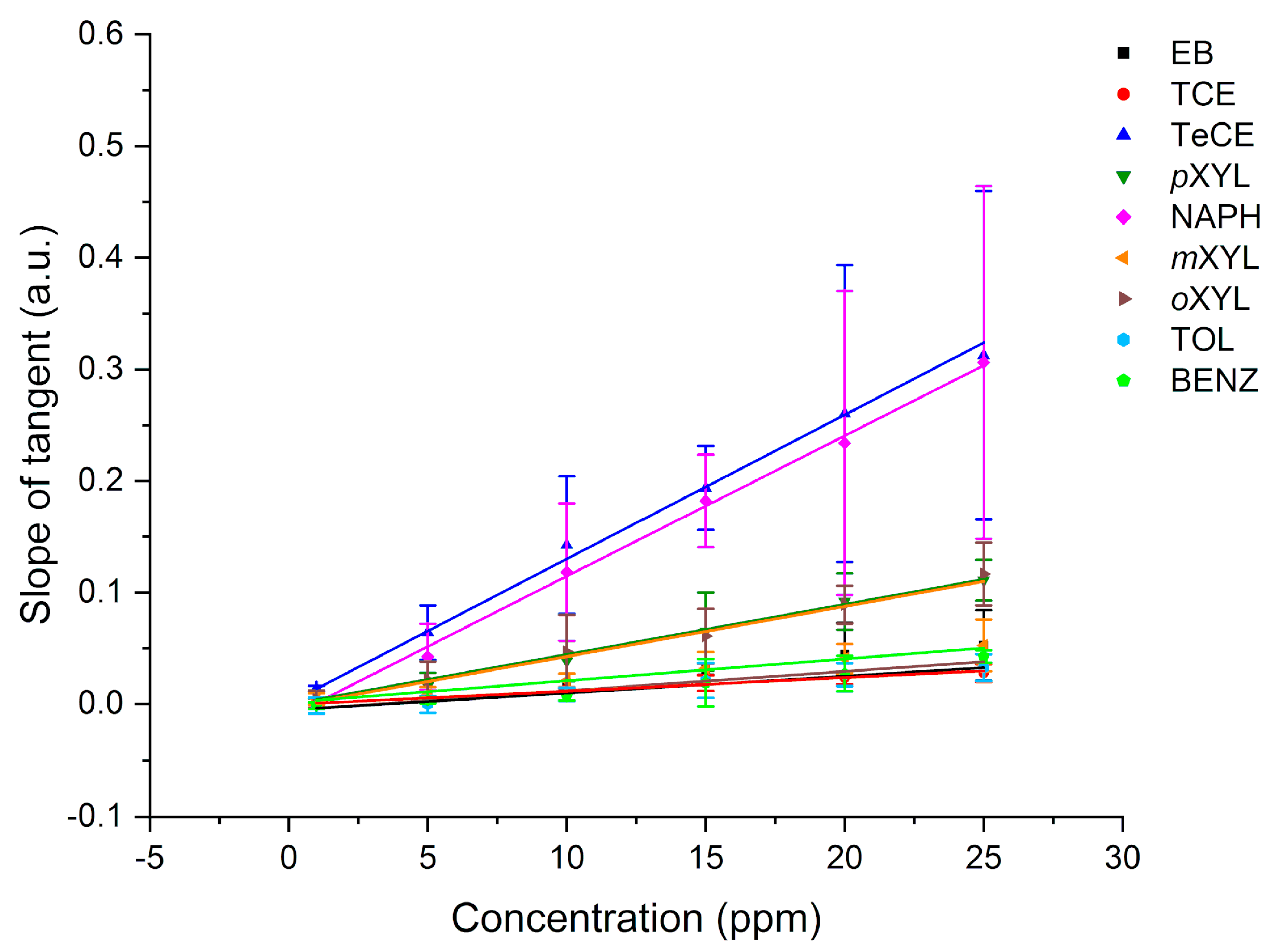
In this study, the potential of F-terminated DLC-coated Si wafers in combination with a hydrophobic E/P-co polymer layer for pollution monitoring, i.e., direct detection of VOCs, via ATR-FTIR spectroscopy is demonstrated. The fluorine surface termination of the DLC layer enables strong binding to the polymer membrane. Therefore, water molecules are efficiently rejected from the evanescent field while analyte molecules diffuse inside the membrane. Nine VOCs could be simultaneously detected providing detection limits in the ppb to ppm concentration range. Multiple regenerations with an aqueous methanol solution did not influence the adhesion of the polymer layer to the DLC-coated waveguide. The sensor responds very fast due to direct change in peak area of the respective evaluated analyte peak, i.e., using the kinetic method by evaluating the slope of the tangent, qualitative results are obtained within the first minutes after starting the measurement. After a short period of 5 to 10 min partition equilibrium has been achieved and evaluation can be performed via equilibrium method for more precise results, This is very important for a fast detection of leaks, i.e., underground fuel storage tanks and pipelines, and real-time monitoring of occurring VOCs in wastewater in order to protect human and animal health and the environment.
2. Materials and Methods
2.1. Chemicals and Reagents
TCE (≥99.5%, CAS number: 79-01-6), TeCE (≥99.9%, CAS number: 127-18-4), pXYL (≥99.0%, CAS number: 106-42-3), mXYL (≥99%, CAS number: 108-38-3), oXYL (≥98.0%, CAS number: 95-47-6), NAPH (≥99.0%, CAS number: 91-20-3), EB (≥99.0%, CAS number: 104-41-4), TOL (≥99.8%, CAS number: 108-88-3), BENZ (≥99.9%, CAS number: 71-43-2) and methanol (MeOH, ≥99.8%, CAS number: 67-56-1) were purchased from Sigma-Aldrich Sweden AB (Stockholm, Sweden). E/P-co polymer (60:40) (CAS number: 9010-79-1) was obtained from Aldrich Chemical Company (Milwaukee, WI, USA). All chemicals were used without further purification steps.
2.2. Sample Preparation
Si wafers were cleaned using standard RCA 1 and 2 procedure. Subsequently, the wafers were dipped into aqueous HF solution (1:50). The cleaned Si wafers were treated with a pulsed filtered cathodic arc deposition with a high-purity graphite cathode in a deposition chamber (base pressure: 10−5 Pa) obtaining a DLC layer (tetragonal amorphous carbon, ta-C) with a thickness of 30 nm. At the end of the DLC deposition step, SF6 was induced into the deposition chamber (pressure 1 Pa) for surface termination of the DLC revealing a highly hydrophobic surface (F:a-C).
Subsequently, the F-DLC-coated Si wafer was coated with E/P-co polymer. Therefore, solid E/P-co was dissolved under reflux in
n-hexane resulting in a 1% E/P-co solution. 20 µL of the solution were dip-coated using an Eppendorf pipette. After evaporation of the solvent a thin film of E/P-co with a thickness of 8.23 ± 0.22 µm, determined via differential weighing [
16], was obtained. A schematic of the F-DLC E/P-co polymer coated Si wafer is illustrated in
Figure 1. Prior to measurement, the polymer membrane was exposed to water for at least 24 h for polymer conditioning.
Since the analytes are highly hydrophobic, and therefore, poorly soluble in water, stock solutions in MeOH with a concentration of 2000 ppm each, were prepared in 20 mL headspace vials. A specific amount of the stock solution was dissolved in deionized water obtaining the respective concentration. MeOH acts simultaneously as a solubility mediator, but do not influence the measurement performance [
51]. The concentration of MeOH was kept at 1% (
v/v).
2.3. Instrumentation and Data Acquisition
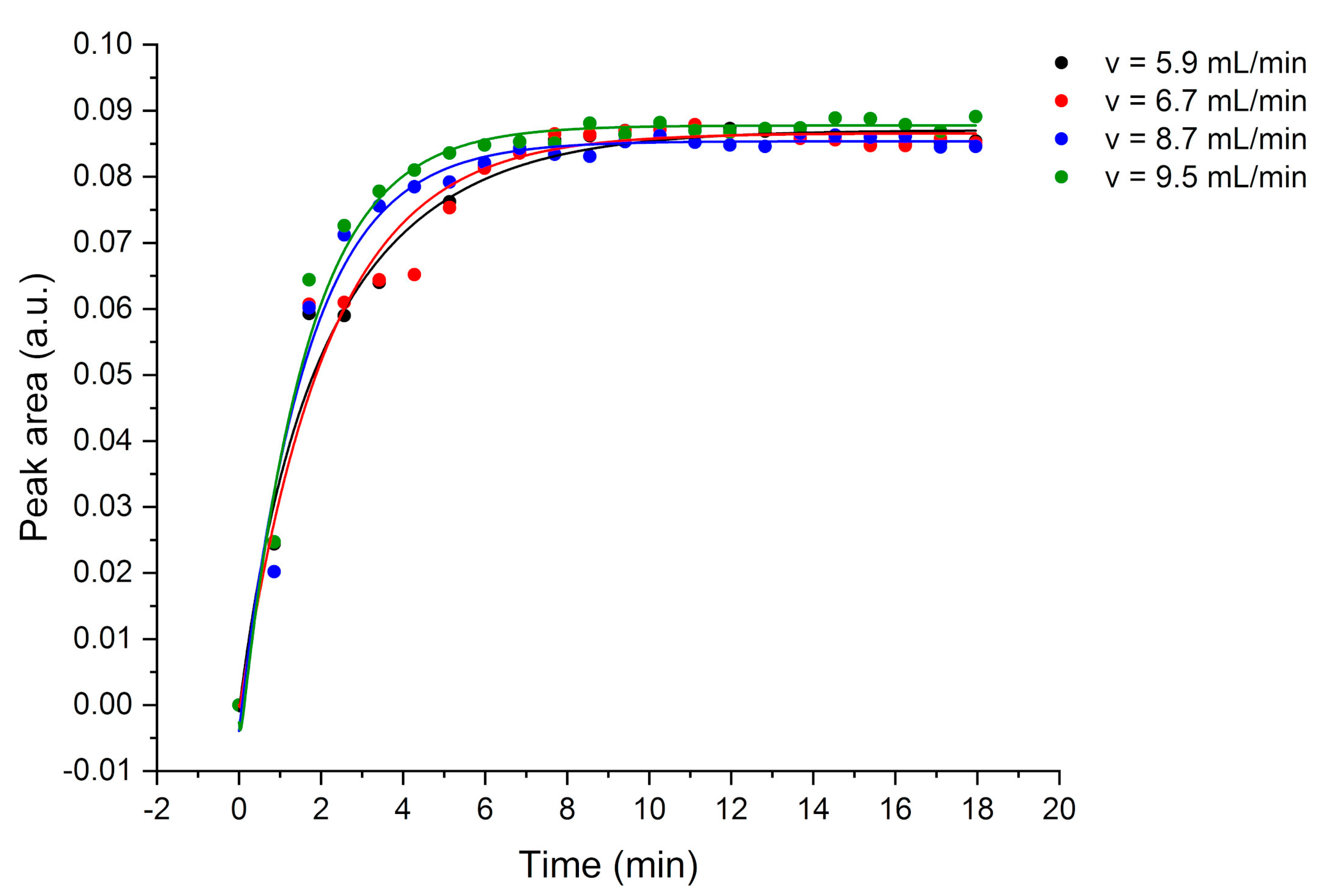
ATR-FTIR spectroscopic measurements were performed with a commercial vacuum-pumped Bruker IFS 66v/S FTIR spectrometer (Bruker Optics, Ettlingen, Germany) in combination with a BioATR II cell and a liquid nitrogen-cooled MCT detector. The BioATR II cell was equipped with an E/P-co polymer coated F-DLC-coated Si ATR chip with 13 internal reflections. The analyte solution containing the nine analytes was flushed through the cell using a peristaltic pump (GE Healthcare, Chicago, IL, USA). The flow rate was set to 6 mL/min. IR spectra were recorded in the MIR spectral range from 4000 to 600 cm−1 averaging 128 scans and a resolution of 2 cm−1. Deionized water was used as background spectrum. All measurements were performed at room temperature. After each measurement, the E/P-co polymer layer was regenerated using a mixture of MeOH and water (1:10, v/v). In order to remove residual MeOH, pure deionized water was flushed through the BioATR cell II.
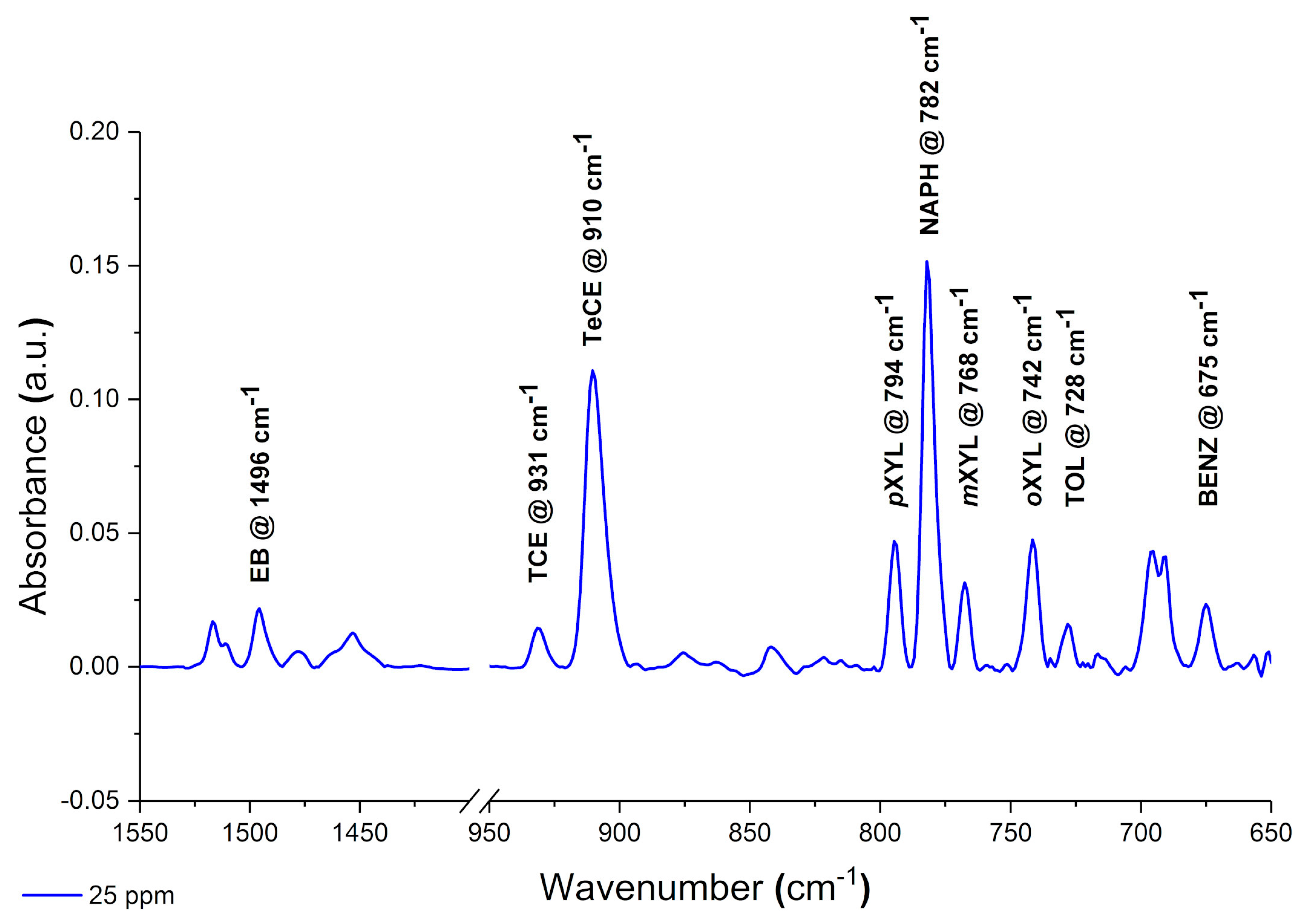
Data acquisition and processing was performed using the software OPUS and Essential FTIR, respectively. The obtained spectra were manually baseline corrected and evaluated using the peak area of the respective analyte.
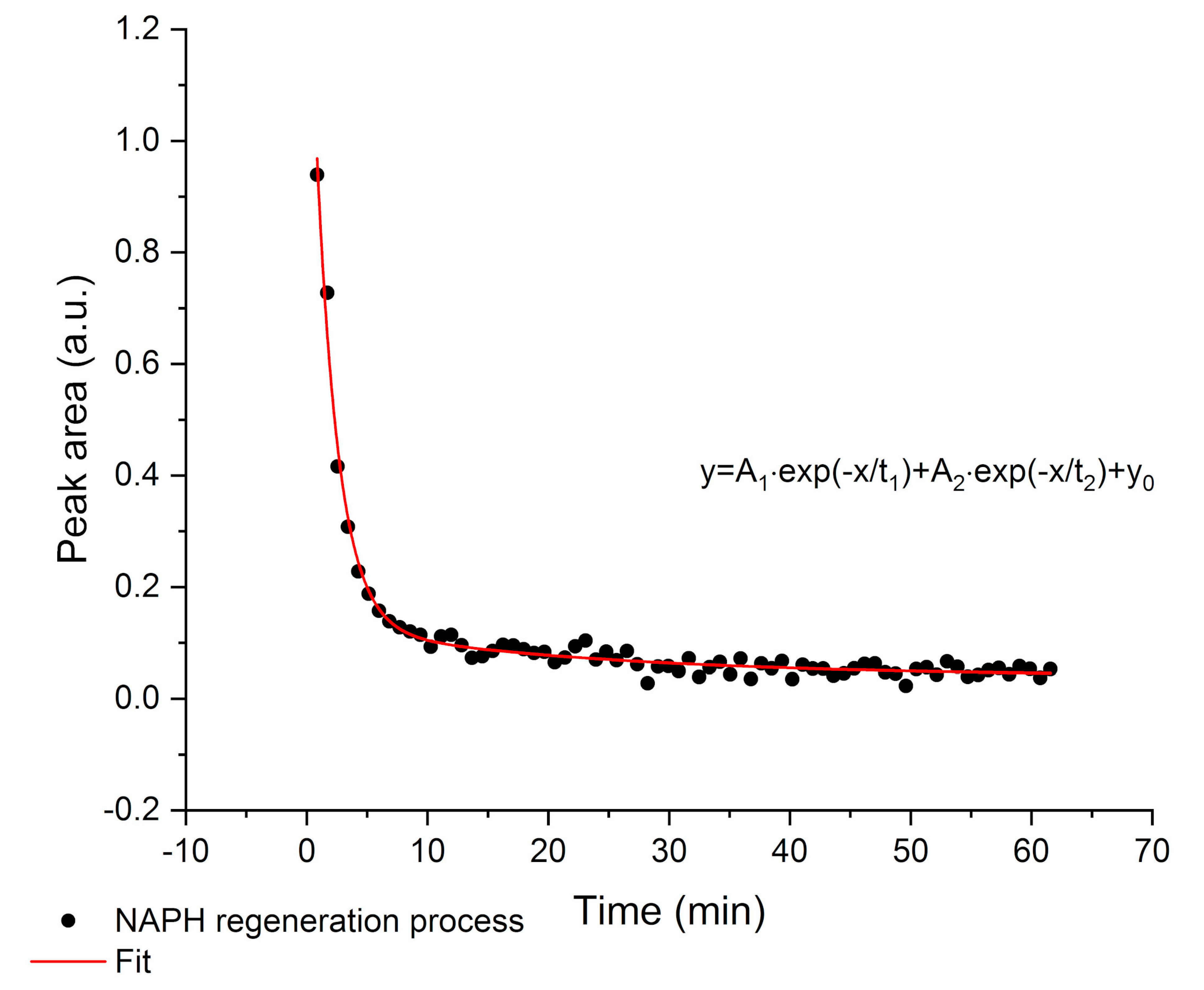
4. Conclusions
This study shows the potential of employing a F-DLC coated Si wafer in combination with an E/P-co polymer as enrichment membrane for analysis of VOCs and pollutants in aqueous matrices via ATR-FTIR spectroscopy. The DLC coating was not influencing the IR absorption features, therefore, nine different VOCs were simultaneously detected, and the results showed a high sensitivity in the ppb to ppm concentration range. Detection is very fast due to direct response of the sensor, i.e., change in peak area, if the analyte reaches the polymer layer. Depending on the evaluation method, i.e., evaluating the concentration at steady-state conditions (equilibrium method) or the slope of the tangent of the enrichment curve (kinetic method), results are obtained within 5-10 min or 2-3 min, respectively. If a high accuracy is required, the equilibrium method should be applied. If a fast detection of pollutants is required, to detect leaking pipelines or underground fuel tanks the kinetic method is advantageous since accurate results are not necessary. Mainly the detection of pollutants is important. Hence, real-time monitoring at sewage disposal or near effluents of industries is enabled. The polymer can be reversibly recovered using a MeOH/water mixture within 10-30 min. The long-term stability of E/P-co polymer in combination with F-DLC was successfully maintained despite a variety of regeneration processes containing MeOH. Silver halide fibers used as sensing elements offers an increased number of internal reflections, and therefore, a higher sensitivity. If the Si wafer is replaced by a silver halide fiber in future environmental applications, the stability over a long period of time of E/P-co polymer is very important since the silver halide fiber decomposes when exposed to light or in contact with chloride ions contained in seawater matrix. Within this study, the combination of F-terminated DLC film with hydrophobic polymer layers providing a sufficient protection of the waveguide due to strong adhesion was shown. Future investigations therefore include, implementation of F-DLC coated silver halide fibers with real seawater matrices for long-term monitoring applications. In conclusion, the study showed promising results for the potential of DLC coatings in combination with a hydrophobic polymer as protection layer for silver halide fibers for future integration in sensing applications via ATR-FTIR spectroscopy for environmental analysis.