Evaluation of a Reduced Graphene Oxide-Sb Nanoparticles Electrochemical Sensor for the Detection of Cadmium and Lead in Chamomile Tea
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
2.2. Synthesis of the Nanocomposites
2.3. Procedures
2.4. Electrodes Preparation
2.5. Real Tea Samples
3. Results and Discussion
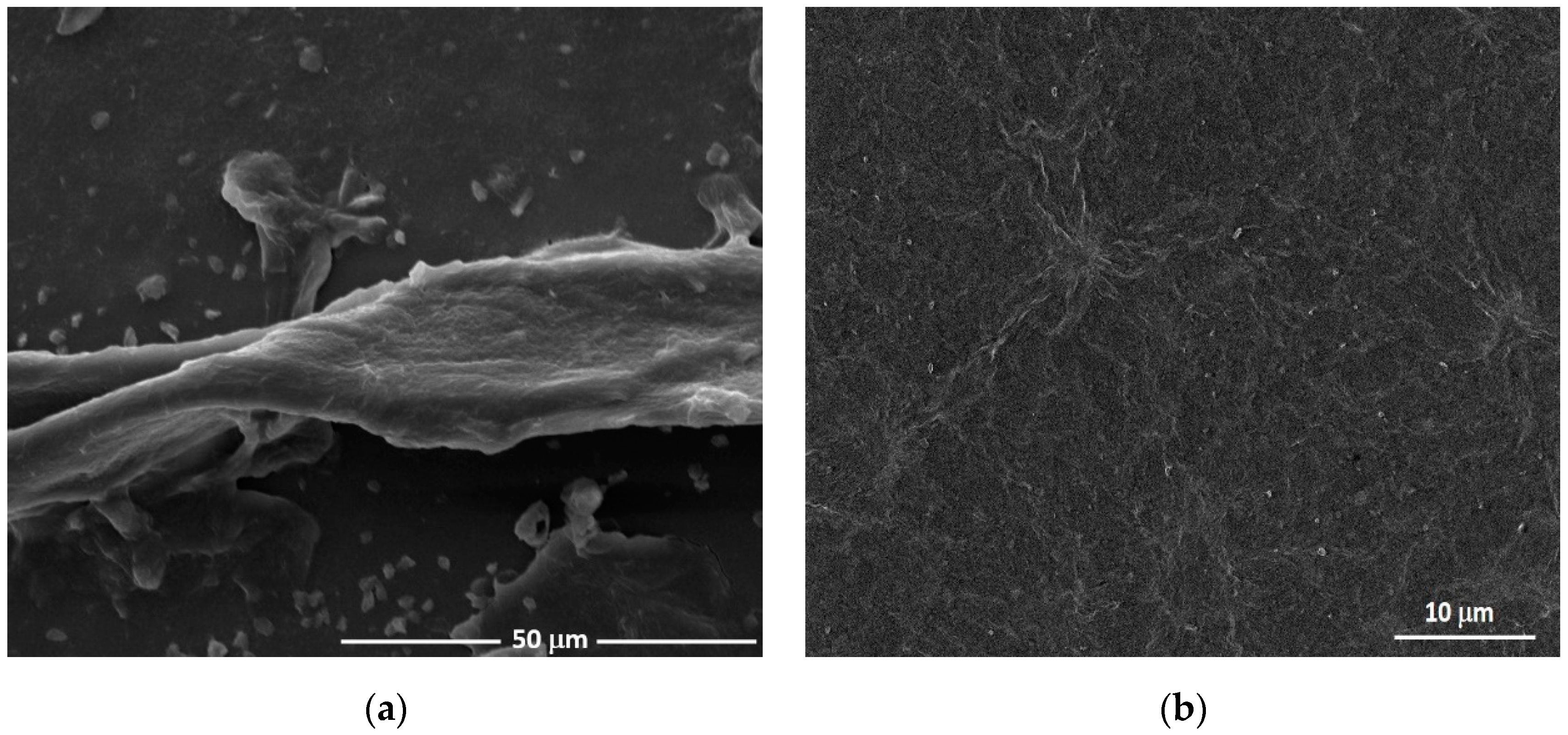
3.1. Morphologic Characterization of Nanocomposites
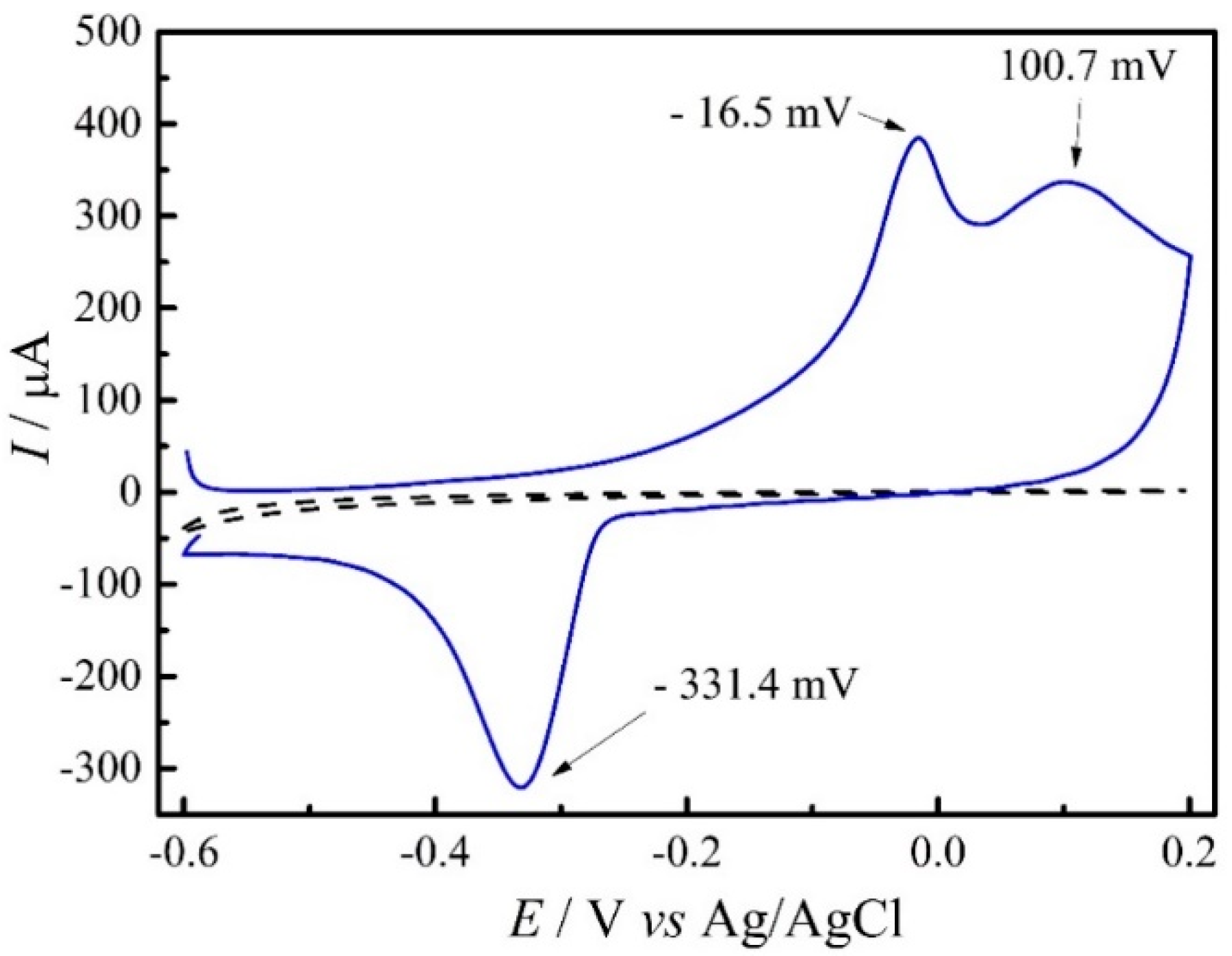
3.2. Electrochemical Characterization
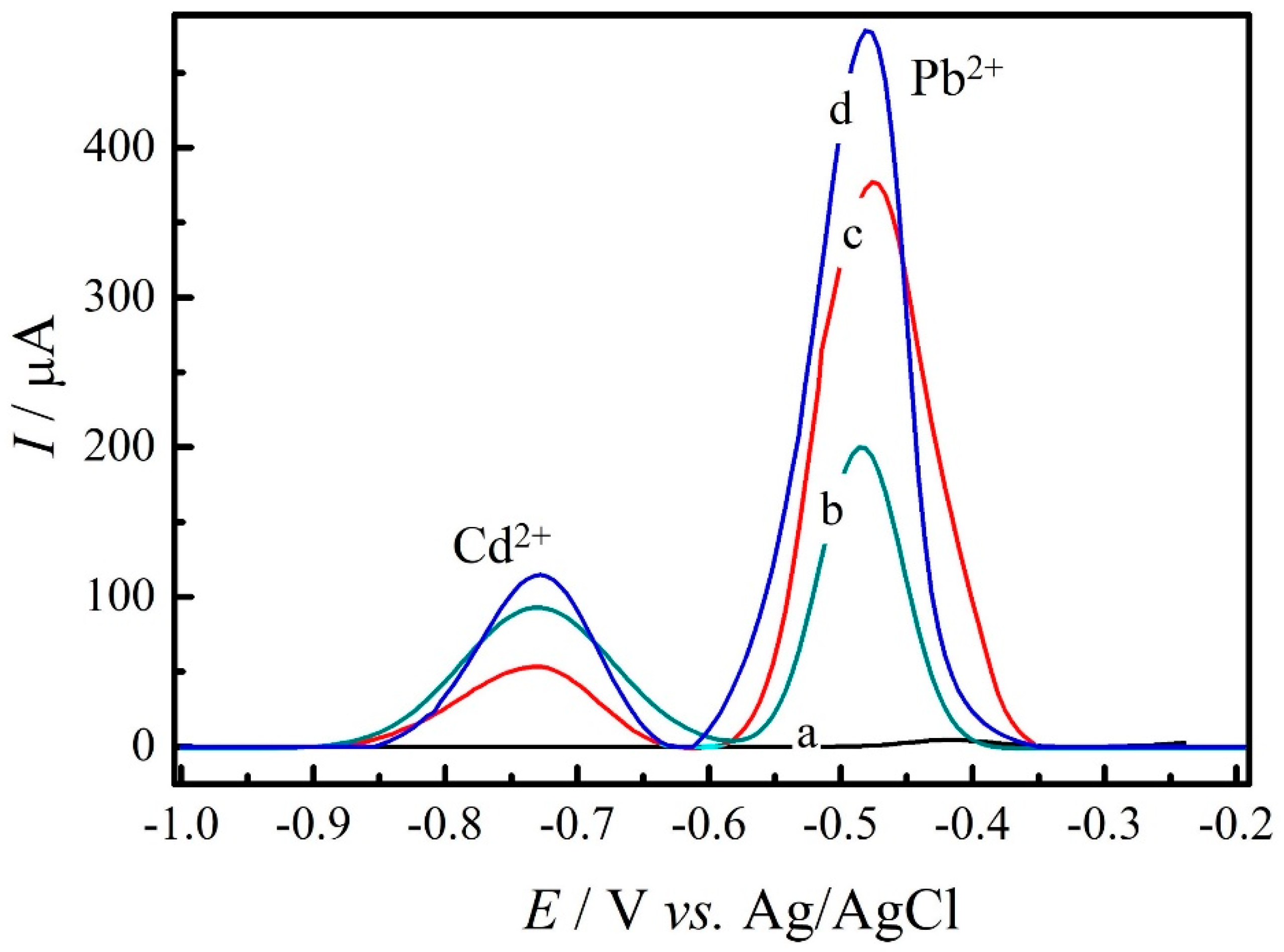
3.3. Performance of GC/rGO-SbNPs Sensor for the Analysis of Cd2+ and Pb2+
3.4. Optimization of Experimental Parameters
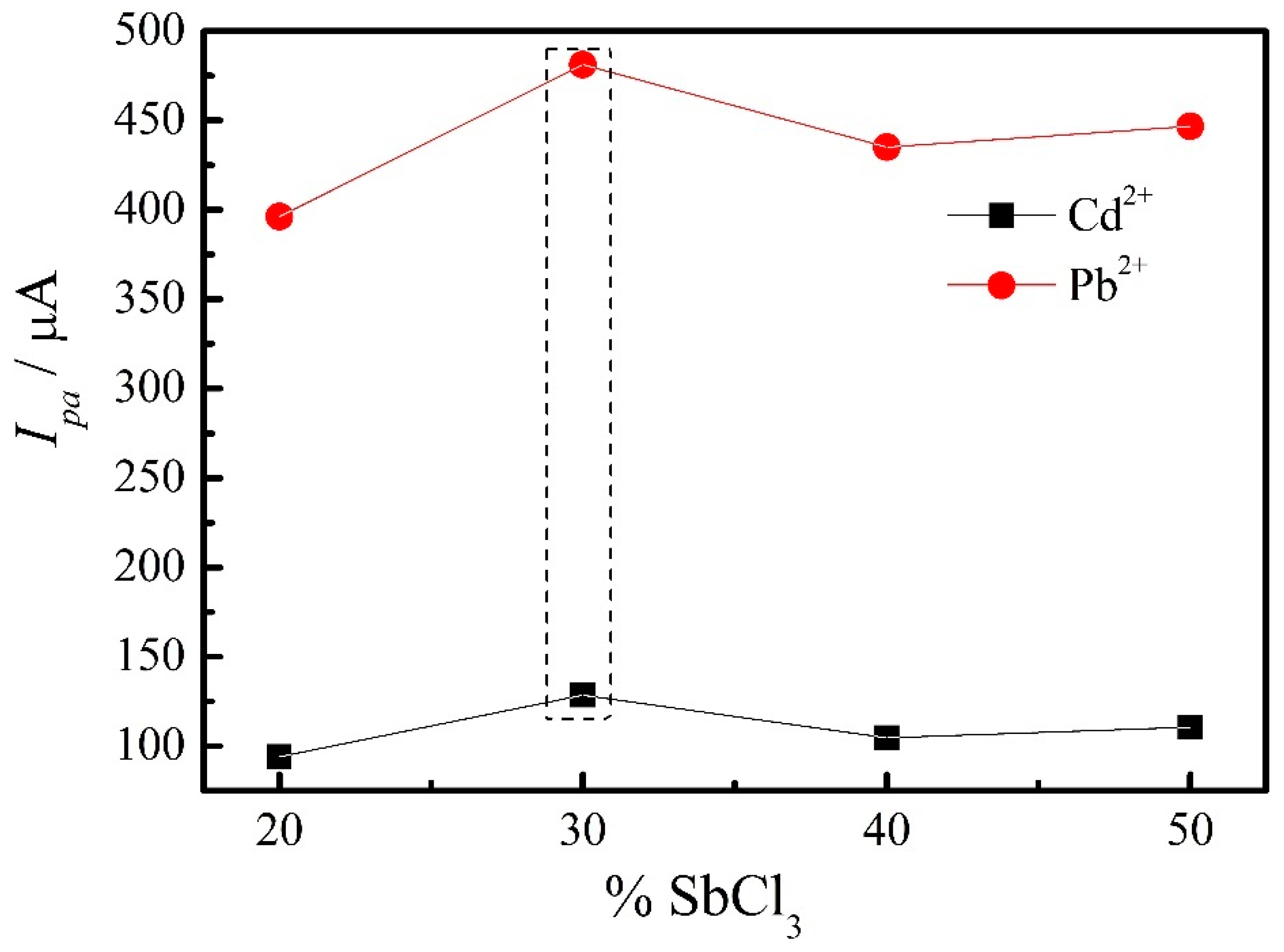
3.4.1. Electrode Film Optimization
3.4.2. SWASV and Supporting Electrolyte Optimization
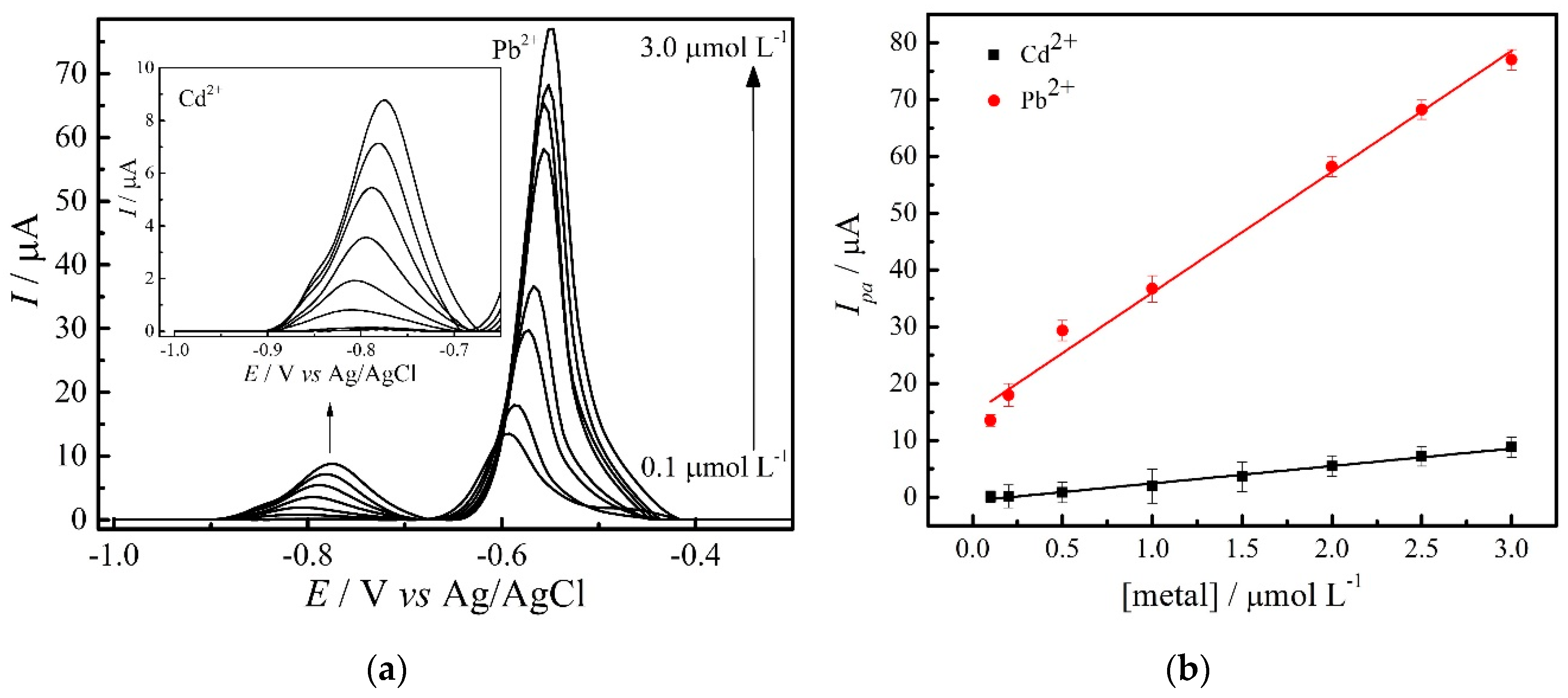
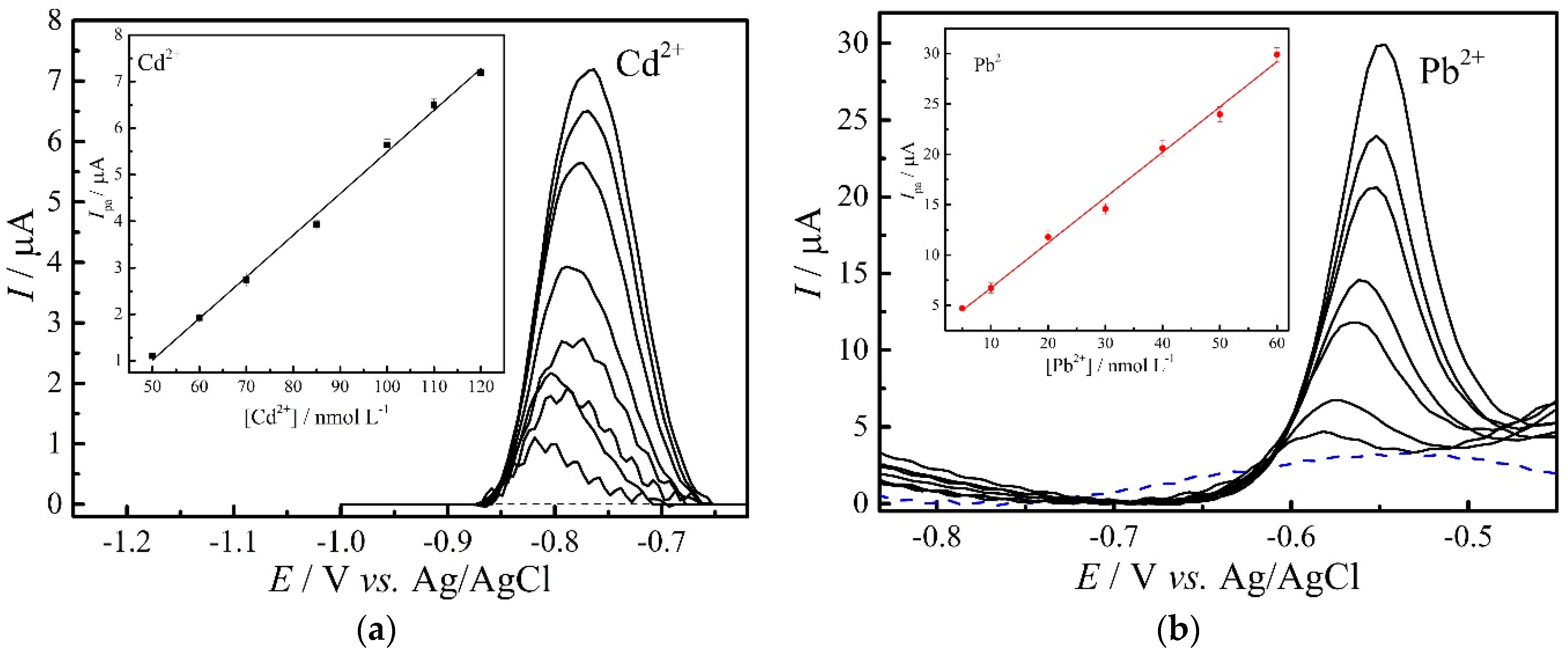
3.5. Calibration Curves
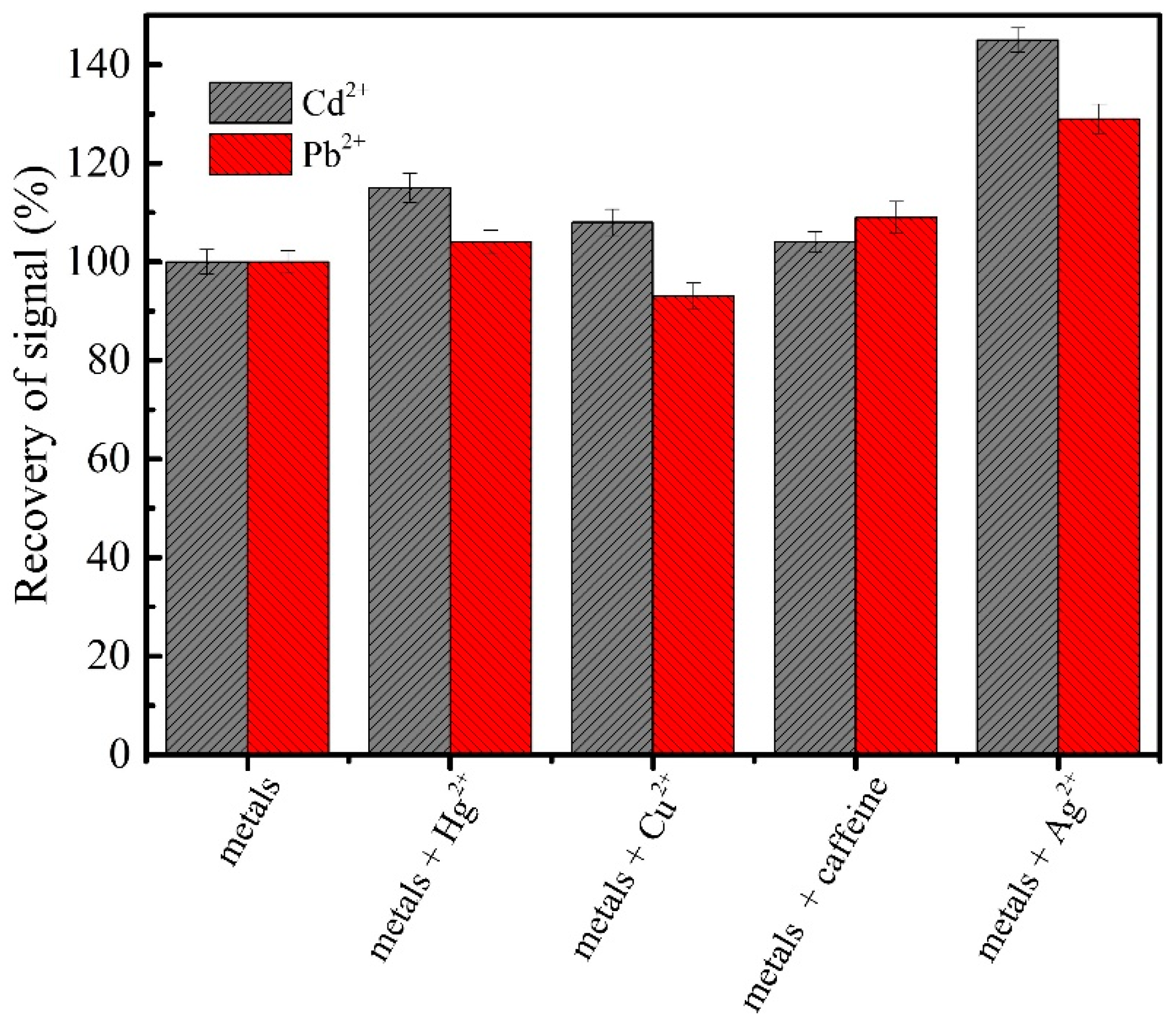
3.6. Interference Study
3.7. Real Samples Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar]
- Chen, Y.; Wang, C.; Wang, Z. Residues and source identification of persistent organic pollutants in farmland soils irrigated by effluents from biological treatment plants. Environ. Int. 2005, 31, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Kachenko, A.G.; Singh, B. Heavy metals contamination in vegetables grown in urban and metal smelter contaminated sites in Australia. Water Air Soil Pollut. 2006, 169, 101–123. [Google Scholar] [CrossRef]
- Alloway, B.J. Sources of Heavy Metals and Metalloids in Soils. In Heavy Metals in Soils; Springer: Amsterdam, The Netherlands, 2013; pp. 11–50. [Google Scholar]
- Rattan, R.K.; Datta, S.P.; Chhonkar, P.K.; Suribabu, K.; Singh, A.K. Long-term impact of irrigation with sewage effluents on heavy metal content in soils, crops and groundwater—A case study. Agric. Ecosyst. Environ. 2005, 109, 310–322. [Google Scholar] [CrossRef]
- Morel, F.M.M.; Kraepiel, A.M.L.; Amyot, M. The chemical cycle and bioaccumulation of mercury. Annu. Rev. Ecol. Syst. 1998, 29, 543–566. [Google Scholar] [CrossRef]
- Volesky, B.; Holan, Z.R. Biosorption of Heavy Metals. Biotechnol. Prog. 1995, 11, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.J.; Leonard, S.S.; Shi, X. Conference overview: Molecular mechanisms of metal toxicity and carcinogenesis. Mol. Cell. Biochem. 2005, 279, 3–15. [Google Scholar] [CrossRef]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Zhou, S.; Luoma, S.E.; St. Armour, G.E.; Thakkar, E.; Mackay, T.F.C.; Anholt, R.R.H. A drosophila model for toxicogenomics: Genetic variation in susceptibility to heavy metal exposure. PLoS Genet. 2017, 13, e1006907. [Google Scholar] [CrossRef]
- Shahbandeh, M. Tea Production and Exports Worldwide, 2018|Statista. Available online: https://www.statista.com/statistics/264183/global-production-and-exports-of-tea-since-2004/ (accessed on 26 June 2020).
- Bennetzen, J. Culturing better tea research. Nature 2019, 566, S5. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Shankar, E.; Gupta, S. Chamomile: A herbal medicine of the past with a bright future (review). Mol. Med. Rep. 2010, 3, 895–901. [Google Scholar] [PubMed]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phyther. Res. 2006, 20, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, W.; Yun, J.M. Luteolin Inhibits hyperglycemia-induced proinflammatory cytokine production and its epigenetic mechanism in human monocytes. Phyther. Res. 2014, 28, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Sebai, H.; Jabri, M.A.; Souli, A.; Rtibi, K.; Selmi, S.; Tebourbi, O.; El-Benna, J.; Sakly, M. Antidiarrheal and antioxidant activities of chamomile (Matricaria recutita L.) decoction extract in rats. J. Ethnopharmacol. 2014, 152, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Saeed Khan, S.; Najam, R.; Anser, H.; Riaz, B.; Alam, N. Chamomile tea: Herbal hypoglycemic alternative for conventional medicine. Pak. J. Pharm. Sci. 2014, 27, 1509–1514. [Google Scholar]
- Chaves, P.F.P.; Iacomini, M.; Cordeiro, L.M.C. Chemical characterization of fructooligosaccharides, inulin and structurally diverse polysaccharides from chamomile tea. Carbohydr. Polym. 2019, 214, 269–275. [Google Scholar] [CrossRef]
- Nováková, L.; Vildová, A.; Mateus, J.P.; Gonalves, T.; Solich, P. Development and application of UHPLC-MS/MS method for the determination of phenolic compounds in Chamomile flowers and Chamomile tea extracts. Talanta 2010, 82, 1271–1280. [Google Scholar] [CrossRef]
- Karak, T.; Bora, K.; Paul, R.K.K.; Das, S.; Khare, P.; Dutta, A.K.K.; Boruah, R.K.K. Paradigm shift of contamination risk of six heavy metals in tea (Camellia sinensis L.) growing soil: A new approach influenced by inorganic and organic amendments. J. Hazard. Mater. 2017, 338, 250–264. [Google Scholar] [CrossRef]
- Vulcano, I.R.C.; Silveira, J.N.; Alvarez-Leite, E.M. Teores de chumbo e cádmio em chás comercializados na região metropolitana de Belo Horizonte. Rev. Bras. Ciências Farm. 2008, 44, 425–431. [Google Scholar] [CrossRef][Green Version]
- Brasil Ministério da Saúde Agência Nacional de Vigilância Sanitária; RDC 42/2013. Available online: http://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2013/rdc0042_29_08_2013.html (accessed on 9 July 2020).
- Rashid, M.H.; Fardous, Z.; Chowdhury, M.A.Z.; Alam, M.K.; Bari, M.L.; Moniruzzaman, M.; Gan, S.H. Determination of heavy metals in the soils of tea plantations and in fresh and processed tea leaves: An evaluation of six digestion methods. Chem. Cent. J. 2016, 10, 7. [Google Scholar] [CrossRef]
- Li, L.; Fu, Q.L.; Achal, V.; Liu, Y. A comparison of the potential health risk of aluminum and heavy metals in tea leaves and tea infusion of commercially available green tea in Jiangxi, China. Environ. Monit. Assess. 2015, 187, 228. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, M.; Zheng, P.; Xue, S. Quantitative Analysis of Lead in Tea Samples by Laser-Induced Breakdown Spectroscopy. J. Appl. Spectrosc. 2017, 84, 188–193. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, X.; Niyungeko, C.; Zhou, J.; Xu, J.; Tian, G.; Yuanyuan, L.; Xinqiang, L.; Niyungeko, C.; Junjie, Z.; et al. A review of the identification and detection of heavy metal ions in the environment by voltammetry. Talanta 2017, 178, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Analytical Electrochemistry; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2000; p. 232. ISBN 0471282723. [Google Scholar]
- Wallace, P.R. The band theory of graphite. Phys. Rev. 1947, 71, 622–634. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Ruoff, R.S.; Bielawski, C.W. From conception to realization: An historial account of graphene and some perspectives for its future. Angew. Chem. Int. Ed. 2010, 49, 9336–9344. [Google Scholar] [CrossRef]
- Huang, K.J.; Niu, D.J.; Liu, X.; Wu, Z.W.; Fan, Y.; Chang, Y.F.; Wu, Y.Y. Direct electrochemistry of catalase at amine-functionalized graphene/gold nanoparticles composite film for hydrogen peroxide sensor. Electrochim. Acta 2011, 56, 2947–2953. [Google Scholar] [CrossRef]
- Cesarino, I.; Cincotto, F.H.; Machado, S.A.S. A synergistic combination of reduced graphene oxide and antimony nanoparticles for estriol hormone detection. Sens. Actuators B Chem. 2015, 210, 453–459. [Google Scholar] [CrossRef]
- Zhang, L.N.; Deng, H.H.; Lin, F.L.; Xu, X.W.; Weng, S.H.; Liu, A.L.; Lin, X.H.; Xia, X.H.; Chen, W. In situ growth of porous platinum nanoparticles on graphene oxide for colorimetric detection of cancer cells. Anal. Chem. 2014, 86, 2711–2718. [Google Scholar] [CrossRef]
- Silva, M.K.L.; Cesarino, I. Electrochemical sensor based on Sb nanoparticles/reduced graphene oxide for heavy metal determination. Int. J. Environ. Anal. Chem. 2020, 1–15. [Google Scholar] [CrossRef]
- Anderson, K. Analytical Techniques for Inorganic Contaminants; AOAC International: Rockville, MD, USA; Gaithersburg, MD, USA, 1999; p. 193. ISBN 9780935584653. [Google Scholar]
- Hocevar, S.B.; Švancara, I.; Ogorevc, B.; Vytřas, K. Antimony film electrode for electrochemical stripping analysis. Anal. Chem. 2007, 79, 8639–8643. [Google Scholar] [CrossRef]
- Moraes, F.C.; Cesarino, I.; Cesarino, V.; Mascaro, L.H.; MacHado, S.A.S. Carbon nanotubes modified with antimony nanoparticles: A novel material for electrochemical sensing. Electrochim. Acta 2012, 85, 560–565. [Google Scholar] [CrossRef]
- da Silva, M.K.L.; Vanzela, H.C.; Defavari, L.M.; Cesarino, I. Determination of carbamate pesticide in food using a biosensor based on reduced graphene oxide and acetylcholinesterase enzyme. Sens. Actuators B Chem. 2018, 277, 555–561. [Google Scholar] [CrossRef]
- Wang, X.; Sun, J.; Tong, J.; Guan, X.; Bian, C.; Xia, S. Paper-based sensor chip for heavy metal ion detection by SWSV. Micromachines 2018, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Cesarino, I.; Cavalheiro, É.T.G.; Brett, C.M.A. Simultaneous determination of cadmium, lead, copper and mercury ions using organofunctionalized SBA-15 nanostructured silica modified graphite -polyurethane composite electrode. Electroanalysis 2010, 22, 61–68. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, T.; Chou, K.C.; Chen, J.; Su, L.; Hou, X. The effective determination of Cd(II) and Pb(II) simultaneously based on an aluminum silicon carbide-reduced graphene oxide nanocomposite electrode. Analyst 2017, 142, 2741–2747. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.M.; Chen, Z.; Li, L.; Liu, E. Reduced graphene oxide decorated with tin nanoparticles through electrodeposition for simultaneous determination of trace heavy metals. Electrochim. Acta 2015, 174, 207–214. [Google Scholar] [CrossRef]
- Sang, S.; Li, D.; Zhang, H.; Sun, Y.; Jian, A.; Zhang, Q.; Zhang, W. Facile synthesis of AgNPs on reduced graphene oxide for highly sensitive simultaneous detection of heavy metal ions. RSC Adv. 2017, 7, 21618–21624. [Google Scholar] [CrossRef]
- Liu, G.; Chen, J.; Hou, X.; Huang, W. A highly-sensitive electrochemical sensor for the simultaneous detection of Cd2+ and Pb2+ using liquid phase-exfoliated graphene. Anal. Methods 2014, 6, 5760–5765. [Google Scholar] [CrossRef]
- Wickremasinghe, R.L. Tea. In Advances in Food Research; Academic Press: Cambridge, MA, USA, 1978; pp. 229–286. [Google Scholar]
- Bansod, B.K.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef]
| Parameter | Evaluated Range | Optimized Value |
|---|---|---|
| Frequency | 10 to 90 Hz | 40 Hz |
| Pulse amplitude | 15 to 100 mV | 100 mV |
| Accumulation time | 45 to 150 s | 150 s |
| Deposition potential | −1.3 to −0.8 V | −1.2 V |
| pH of supporting electrolyte | 2.0 to 6.0 | 3.0 |
| Metals | Method | Calibration Curve | Linear Range | R2 | LOD (nmol L−1) |
|---|---|---|---|---|---|
| Cd2+ | Simultaneous | Ipa = 0.64 + 3.06 CCd2+ | 0.1–3.0 µmol L−1 | 0.9901 | 70.03 |
| Individual | Ipa = 3.45 + 0.09 CCd2+ | 50–120 nmol L−1 | 0.9963 | 20.50 | |
| Pb2+ | Simultaneous | Ipa = 14.70 + 21.30 CPb2+ | 0.1–3.0 µmol L−1 | 0.9898 | 45.50 |
| Individual | Ipa = 2.24 + 0.45 CPb2+ | 5–60 nmol L−1 | 0.9932 | 2.01 |
| Metals | Added (µmol L−1) | Recovery (µmol L−1) | Error (%) 1 |
|---|---|---|---|
| Cd2+ | 0.5 | 0.4874 | −2.6 |
| 0.5376 | 7.0 | ||
| 0.5152 | 3.0 | ||
| Mean ± SD | 0.5134 ± 0.0250 | ||
| Pb2+ | 0.5 | 0.5159 | 3.1 |
| 0.4976 | −0.5 | ||
| 0.5085 | 1.7 | ||
| Mean ± SD | 0.5073 ± 0.0090 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, E.W.; Silva, M.K.L.; Cesarino, I. Evaluation of a Reduced Graphene Oxide-Sb Nanoparticles Electrochemical Sensor for the Detection of Cadmium and Lead in Chamomile Tea. Chemosensors 2020, 8, 53. https://doi.org/10.3390/chemosensors8030053
Nunes EW, Silva MKL, Cesarino I. Evaluation of a Reduced Graphene Oxide-Sb Nanoparticles Electrochemical Sensor for the Detection of Cadmium and Lead in Chamomile Tea. Chemosensors. 2020; 8(3):53. https://doi.org/10.3390/chemosensors8030053
Chicago/Turabian StyleNunes, Erik W., Martin K. L. Silva, and Ivana Cesarino. 2020. "Evaluation of a Reduced Graphene Oxide-Sb Nanoparticles Electrochemical Sensor for the Detection of Cadmium and Lead in Chamomile Tea" Chemosensors 8, no. 3: 53. https://doi.org/10.3390/chemosensors8030053
APA StyleNunes, E. W., Silva, M. K. L., & Cesarino, I. (2020). Evaluation of a Reduced Graphene Oxide-Sb Nanoparticles Electrochemical Sensor for the Detection of Cadmium and Lead in Chamomile Tea. Chemosensors, 8(3), 53. https://doi.org/10.3390/chemosensors8030053







