Polymer Nanoparticle Identification and Concentration Measurement Using Fiber-Enhanced Raman Spectroscopy
Abstract
1. Introduction
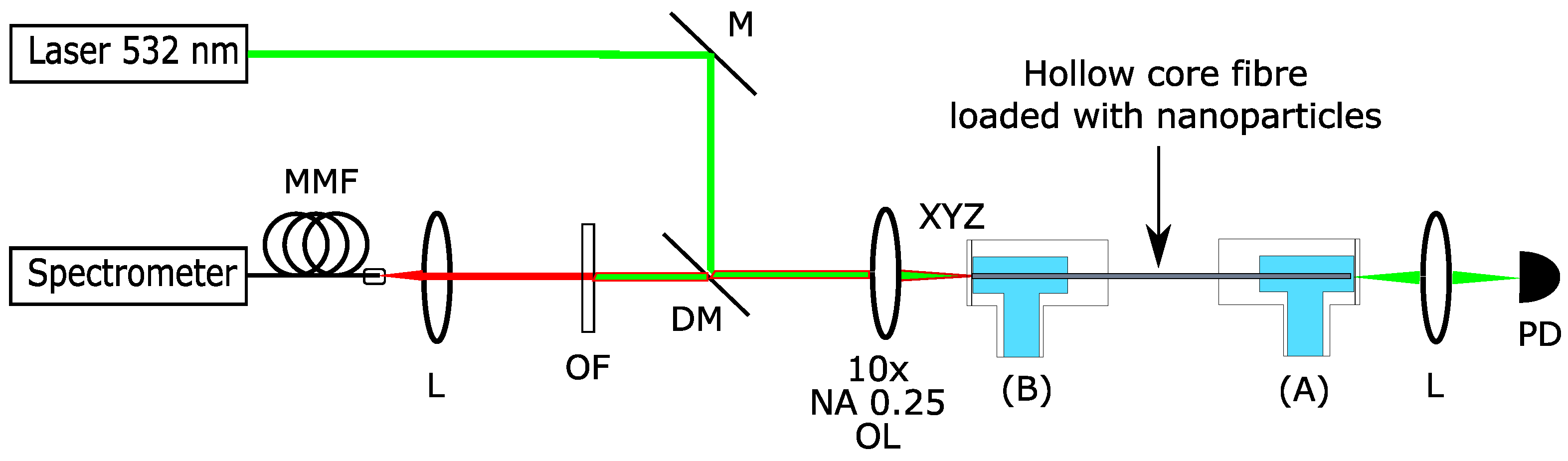
2. Materials and Methods
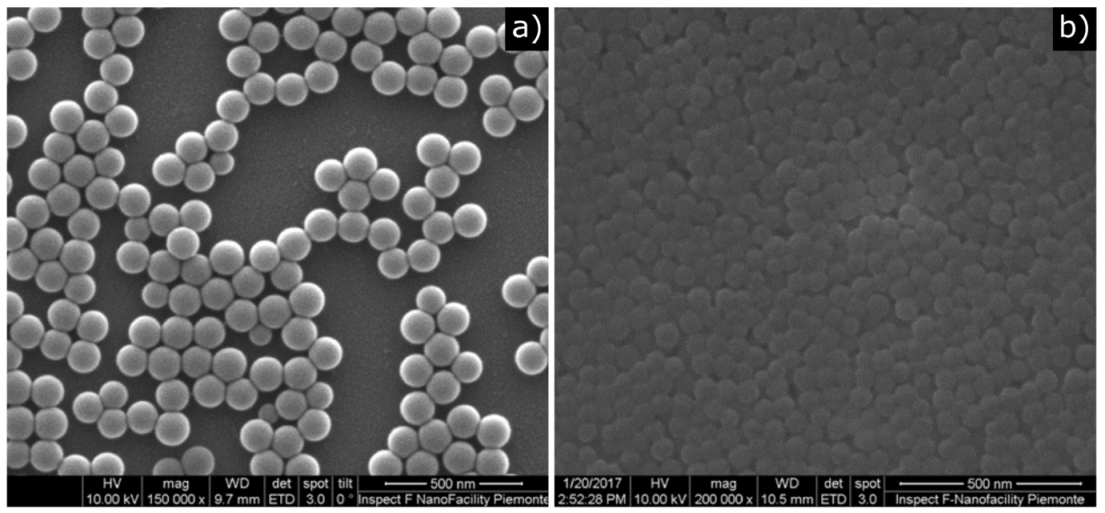
2.1. Nanoparticle Preparation
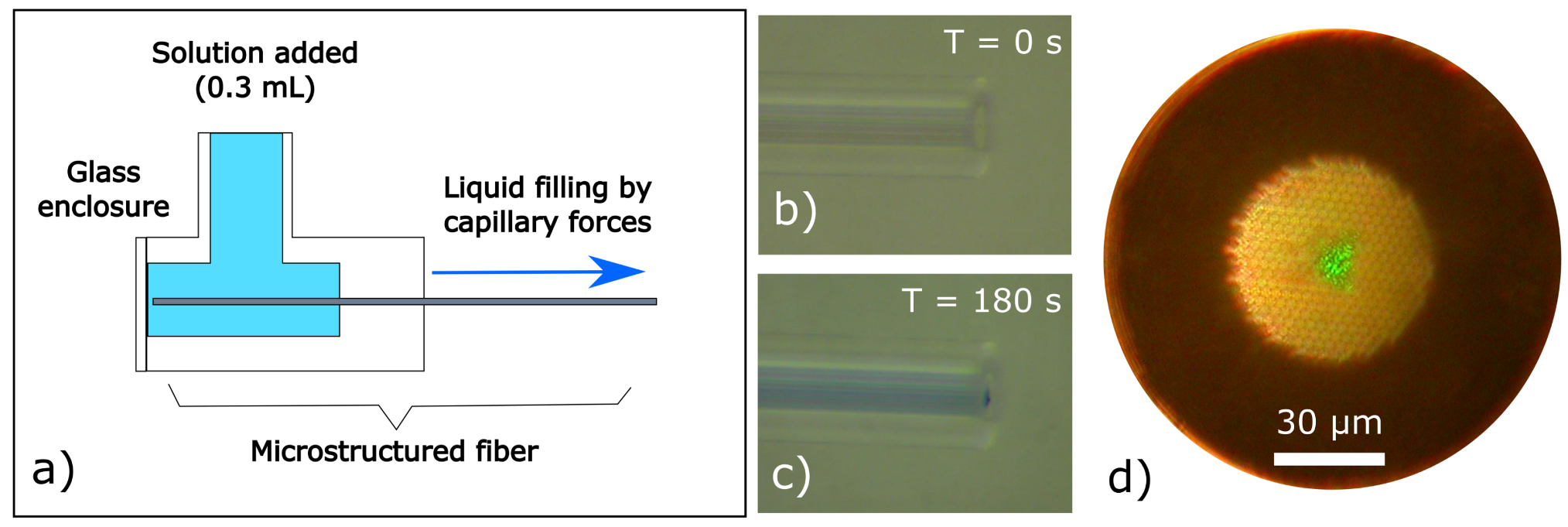
2.2. Fiber Preparation and Filling
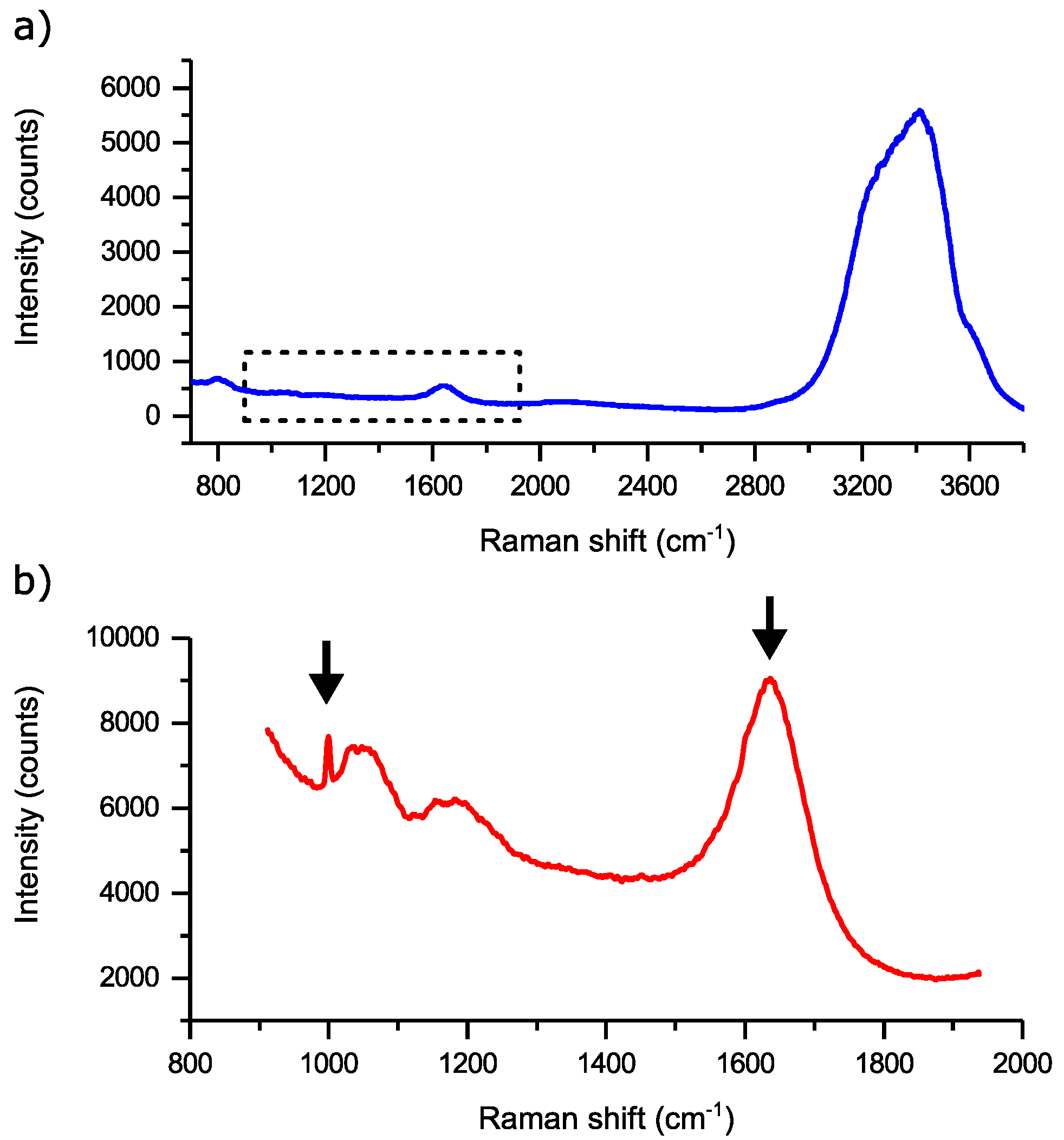
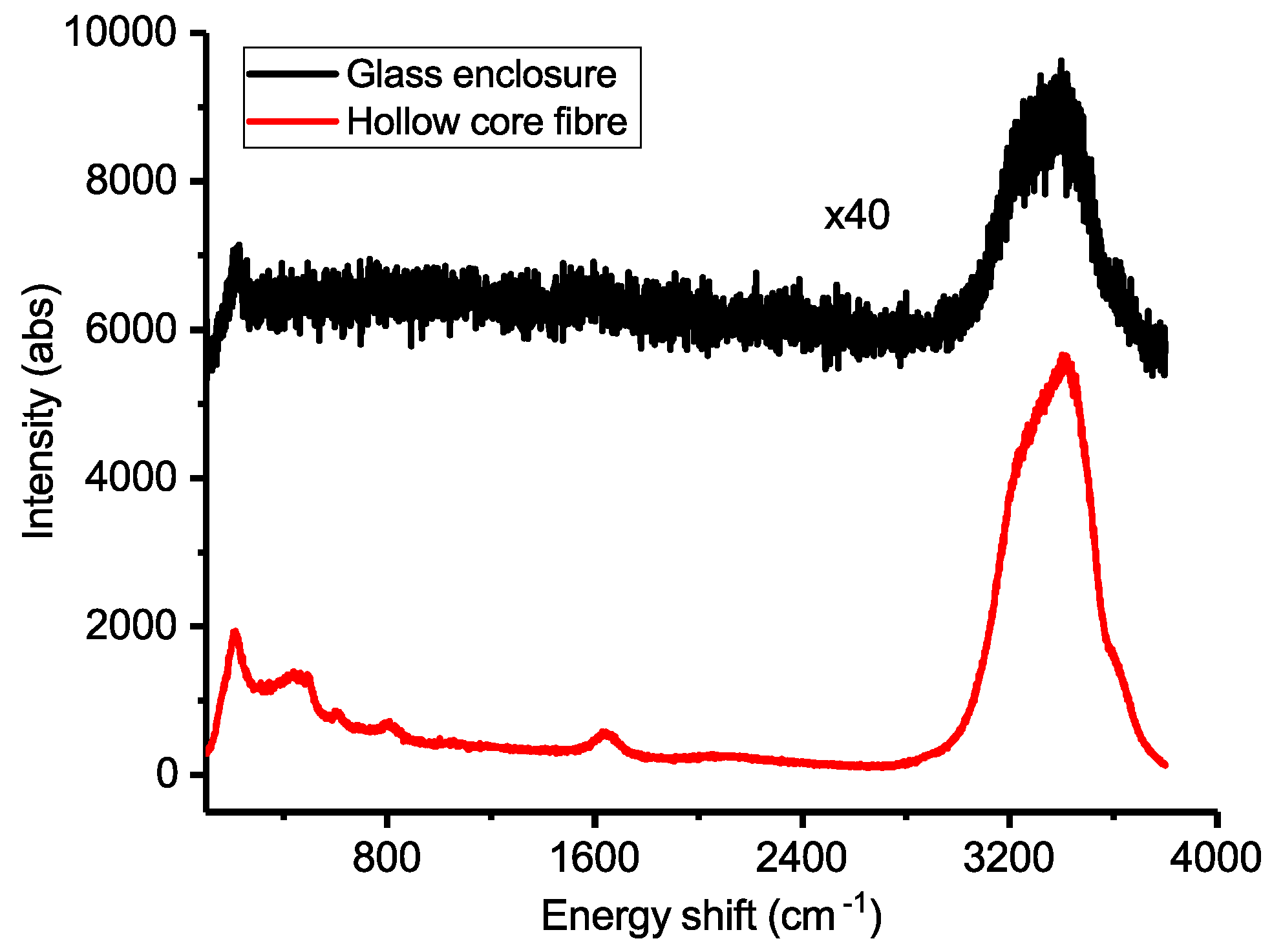
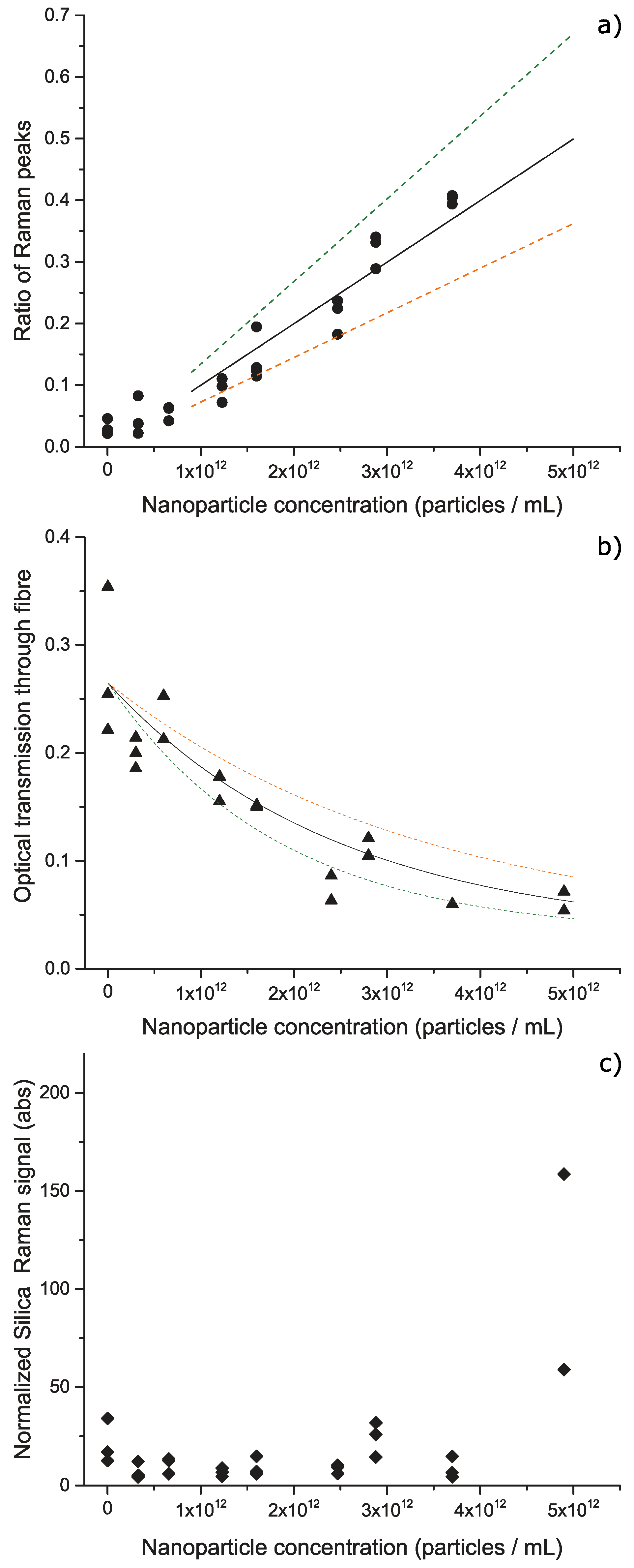
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lue, J.-T. A review of characterization and physical property studies of metallic nanoparticles. J. Phys. Chem. Solids 2001, 62, 1599–1612. [Google Scholar] [CrossRef]
- Nasir, A.; Kausar, A.; Younus, A. A Review on Preparation, Properties and Applications of Polymeric Nanoparticle-Based Materials. Polym. Plast. Technol. Eng. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Shirasaki, Y.; Supran, G.J.; Bawendi, M.G.; Bulovic, V. Emergence of colloidal quantum-dot light-emitting technologies. Nat. Photonics 2013, 7, 13–23. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, X.; Huang, Q.; Xu, Q.; Song, W. Preparation of solid silver nanoparticles for inkjet printed flexible electronics with high conductivity. Nanoscale 2014, 6, 1622–1628. [Google Scholar] [CrossRef] [PubMed]
- Schauermann, S.; Nilius, N.; Shaikhutdinov, S.; Freund, H.-J. Nanoparticles for Heterogeneous Catalysis: New Mechanistic Insights. Acc. Chem. Res. 2013, 46, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Sheth, P.; Sandhu, H.; Singhal, D.; Malick, W.; Shah, N.; Kislalioglu, M.S. Nanoparticles in the pharmaceutical industry and the use of supercritical fluid technologies for nanoparticle production. Curr. Drug Deliv. 2012, 9, 269–284. [Google Scholar] [CrossRef]
- Byrne, J.D.; Betancourt, T.; Brannon-Peppas, L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 1615–1626. [Google Scholar] [CrossRef]
- Lisinger, T.P.J.; Roebben, G.; Gilliland, D.; Calzolai, L.; Rossi, F.; Gibson, N.; Klein, C. Requirements on Measurements for the Implementation of the European Commission Definition of the Term ‘Nanomaterial’; Publications Office of the European Union: Brussels, Belgium, 2012; p. JRC73260. [Google Scholar]
- Brown, S.C.; Boyko, V.; Meyers, G.; Voetz, M.; Wohlleben, W. Toward advancing nano-object count metrology: A best practice framework. Environ. Health Perspect. 2013, 121, 1282–1291. [Google Scholar] [CrossRef]
- Domingos, R.F.; Baalousha, M.A.; Ju-Nam, Y.; Reid, M.M.; Tufenkji, N.; Lead, J.R.; Leppard, G.G.; Wilkinson, K.J. Characterizing Manufactured Nanoparticles in the Environment: Multimethod Determination of Particle Sizes. Environ. Sci. Technol. 2009, 43, 7277–7284. [Google Scholar] [CrossRef]
- Proulx, K.; Wilkinson, K.J. Separation, detection and characterisation of engineered nanoparticles in natural waters using hydrodynamic chromatography and multi-method detection (light scattering, analytical ultracentrifugation and single particle ICP-MS). Environ. Chem. 2014, 11, 392–401. [Google Scholar] [CrossRef]
- Yang, S.; Taflove, A.; Backman, V. Experimental confirmation at visible light wavelengths of the backscattering enhancement phenomenon of the photonic nanojet. Opt. Express 2011, 19, 7084–7093. [Google Scholar] [CrossRef] [PubMed]
- Wheaton, S.; Gelfand, R.M.; Gordon, R. Probing the Raman-active acoustic vibrations of nanoparticles with extraordinary spectral resolution. Nat. Photonics 2015, 9, 68–72. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Ferning, D.G. Determination of Size and Concentration of Gold Nanoparticles from UV-Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Brar, S.K.; Verma, M. Measurement of nanoparticles by light-scattering techniques. TrAC Trends Anal. Chem. 2011, 30, 4–17. [Google Scholar] [CrossRef]
- Russel, P. Photonic Crystal Fibers. Science 2003, 299, 358–362. [Google Scholar] [CrossRef]
- Nielsen, K.; Noordegraaf, D.; Sørensen, T.; Bjarklev, A.; Hansen, T.P. Selective filling of photonic crystal fibres. J. Opt. A Pure Appl. Opt. 2005, 7, L13–L20. [Google Scholar] [CrossRef]
- Zuber, A.; Purdey, M.; Schartner, E.; Forbes, C.; van der Hoek, S.; Giles, D.; Abell, A.; Monro, T.; Ebendorff-Heidepriem, H. Detection of gold nanoparticles with different sizes using absorption and fluorescence based method. Sens. Actuator B-Chem. 2016, 227, 117–127. [Google Scholar] [CrossRef]
- Colthup, N.B.; Daly, L.H.; Wiberly, S.E. Introduction to Infrared and Raman Spectroscopy; Academic Press: New York, NY, USA, 1990. [Google Scholar]
- Das, R.S.; Agrawal, Y.K. Raman spectroscopy: Recent advancements, techniques and applications. Vib. Spectrosc. 2011, 57, 163–176. [Google Scholar] [CrossRef]
- Kumar, C.S.S.R. Raman Spectroscopy for Nanomaterials Characterization; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Ye, K.; Li, K.; Lu, Y.; Guo, Z.; Ni, N.; Liu, H.; Huang, Y.; Ji, H.; Wang, P. An overview of advanced methods for the characterization of oxygen vacancies in materials. Trends Anal. Chem. 2019, 116, 102–108. [Google Scholar] [CrossRef]
- Vanden-Hehir, S.; Tipping, W.J.; Lee, M.; Brunton, V.G.; Williams, A.; Hulme, A.N. Raman Imaging of Nanocarriers for Drug Delivery. Nanomaterials 2019, 9, 341. [Google Scholar] [CrossRef] [PubMed]
- Mak, J.S.W.; Rutledge, S.A.; Abu-Ghazalah, R.M.; Eftekhari, F.; Irizar, J.; Tam, N.C.M.; Zheng, G.; Helmy, A.S. Recent developments in optofluidic-assisted Raman spectroscopy. Prog. Quantum Electron. 2013, 37, 1–50. [Google Scholar] [CrossRef]
- Schmidt, M.S.; Hübner, J.; Boisen, A. Large Area Fabrication of Leaning Silicon Nanopillars for Surface Enhanced Raman Spectroscopy. Adv. Mater. 2012, 24, OP11–OP18. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.-K.; Jeon, K.-S.; Kim, Y.M.; Nam, J.-M.; Suh, Y.D. Nanogap-engineerable Raman-active nanodumbbells for single-molecule detection. Nat. Mater. 2010, 9, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Verma, P. Tip-Enhanced Raman Spectroscopy: Technique and Recent Advances. Chem. Rev. 2017, 117, 6447–6466. [Google Scholar] [CrossRef]
- Irizar, J.; Dinglasan, J.; Goh, J.B.; Khetani, A.; Anis, H.; Anderson, D.; Goh, C.; Helmy, A.S. Raman Spectroscopy of Nanoparticles Using Hollow-Core Photonic Crystal Fibers. IEEE J. Sel. Top. Quantum Electron. 2008, 14, 1214–1222. [Google Scholar] [CrossRef]
- Mak, J.S.W.; Farah, A.A.; Chen, F.; Helmy, A.S. Photonic crystal fiber for efficient Raman scattering of CdTe quantum dots in aqueous solution. ACS Nano 2011, 5, 3823–3830. [Google Scholar] [CrossRef]
- Yan, D.; Popp, J.; Pletz, M.W.; Frosch, T. Highly Sensitive Broadband Raman Sensing of Antibiotics in Step-Index Hollow-Core Photonic Crystal Fibers. ACS Photonics 2017, 4, 138–145. [Google Scholar] [CrossRef]
- Yan, D.; Frosch, T.; Kobelke, J.; Bierlich, J.; Popp, J.; Plets, M.W.; Frosch, T. Fiber-Enhanced Raman Sensing of Cefuroxime in Human Urine. Anal. Chem. 2018, 90, 13243–13248. [Google Scholar] [CrossRef]
- Boisde, G.; Harmer, A. Chemical and Biochemical Sensing With Optical Fibers and Waveguides; Artech House: Boston, MA, USA, 1996. [Google Scholar]
- Xiao, L.; Jin, W.; Demokan, M.S.; Ho, H.L.; Hoo, Y.L.; Zhao, C. Fabrication of selective injection microstructured optical fibers with a conventional fusion splicer. Opt. Express 2005, 13, 3412–3417. [Google Scholar] [CrossRef]
- Ferraro, J.R.; Nakamoto, K.; Brown, C.W. Introductory Raman Spectroscopy; Academic Press: San Diego, CA, USA, 2003. [Google Scholar]
- Eftekhari, F.; Irizar, J.; Hulbert, L.; Helmy, A.S. A comparative study of Raman enhancement in capillaries. J. Appl. Phys. 2011, 109, 113104. [Google Scholar] [CrossRef]
- Weik, M.H. Fibre Optics Standard Dictionary; Chapman and Hall: New York, NY, USA, 1997. [Google Scholar]
- Lan, G.-L.; Banerjee, P.K.; Mitra, S.S. Raman scattering in optical fibers. J. Raman Spectrosc. 1981, 11, 416–423. [Google Scholar] [CrossRef]
- Carey, D.M.; Korenowski, G.M. Measurement of the Raman spectrum of liquid water. J. Chem. Phys. 1998, 108, 2669–2675. [Google Scholar] [CrossRef]
- Jones, C.H.; Wesley, I.J. A preliminary study of the Fourier transform Raman spectra of polystyrenes. Spectrochim. Acta A 1991, 47, 1293–1298. [Google Scholar] [CrossRef]
- Nielsen, L. Evaluation of measurements by the method of least squares. In Algorithms for Approximation IV; Levesley, J., Anderson, I.J., Mason, J.C., Eds.; University of Huddersfield: Huddersfield, UK, 2002; pp. 170–186. [Google Scholar]
- Mätzler, C. MATLAB functions for Mie scattering and absorption, version 2. IAP Res. Rep 2002, 8, 1–24. [Google Scholar]
- Rugeland, P.; Sterner, C.; Margulis, W. Visible light guidance in silica capillaries by antiresonant reflection. Opt. Express 2013, 21, 29217–29222. [Google Scholar] [CrossRef]
- Frosch, T.; Yan, D.; Popp, J. Ultrasensitive Fiber Enhanced UV Resonance Raman Sensing of Drugs. Anal. Chem. 2013, 85, 6264–6271. [Google Scholar] [CrossRef]
- Kerdoncuff, H.; Pollard, M.R.; Westergaard, P.G.; Lassen, M. Compact and versatile laser system for polarization-sensitive stimulated Raman spectroscopy. Opt. Express 2017, 25, 5618–5625. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pollard, M.R.; Sparnacci, K.; Wacker, L.J.; Kerdoncuff, H. Polymer Nanoparticle Identification and Concentration Measurement Using Fiber-Enhanced Raman Spectroscopy. Chemosensors 2020, 8, 21. https://doi.org/10.3390/chemosensors8010021
Pollard MR, Sparnacci K, Wacker LJ, Kerdoncuff H. Polymer Nanoparticle Identification and Concentration Measurement Using Fiber-Enhanced Raman Spectroscopy. Chemosensors. 2020; 8(1):21. https://doi.org/10.3390/chemosensors8010021
Chicago/Turabian StylePollard, Mark R., Katia Sparnacci, Lars J. Wacker, and Hugo Kerdoncuff. 2020. "Polymer Nanoparticle Identification and Concentration Measurement Using Fiber-Enhanced Raman Spectroscopy" Chemosensors 8, no. 1: 21. https://doi.org/10.3390/chemosensors8010021
APA StylePollard, M. R., Sparnacci, K., Wacker, L. J., & Kerdoncuff, H. (2020). Polymer Nanoparticle Identification and Concentration Measurement Using Fiber-Enhanced Raman Spectroscopy. Chemosensors, 8(1), 21. https://doi.org/10.3390/chemosensors8010021




