Towards an Integrated System as Point-of-Care Device for the Optical Detection of Sepsis Biomarkers
Abstract
1. Introduction
2. Materials and Methods
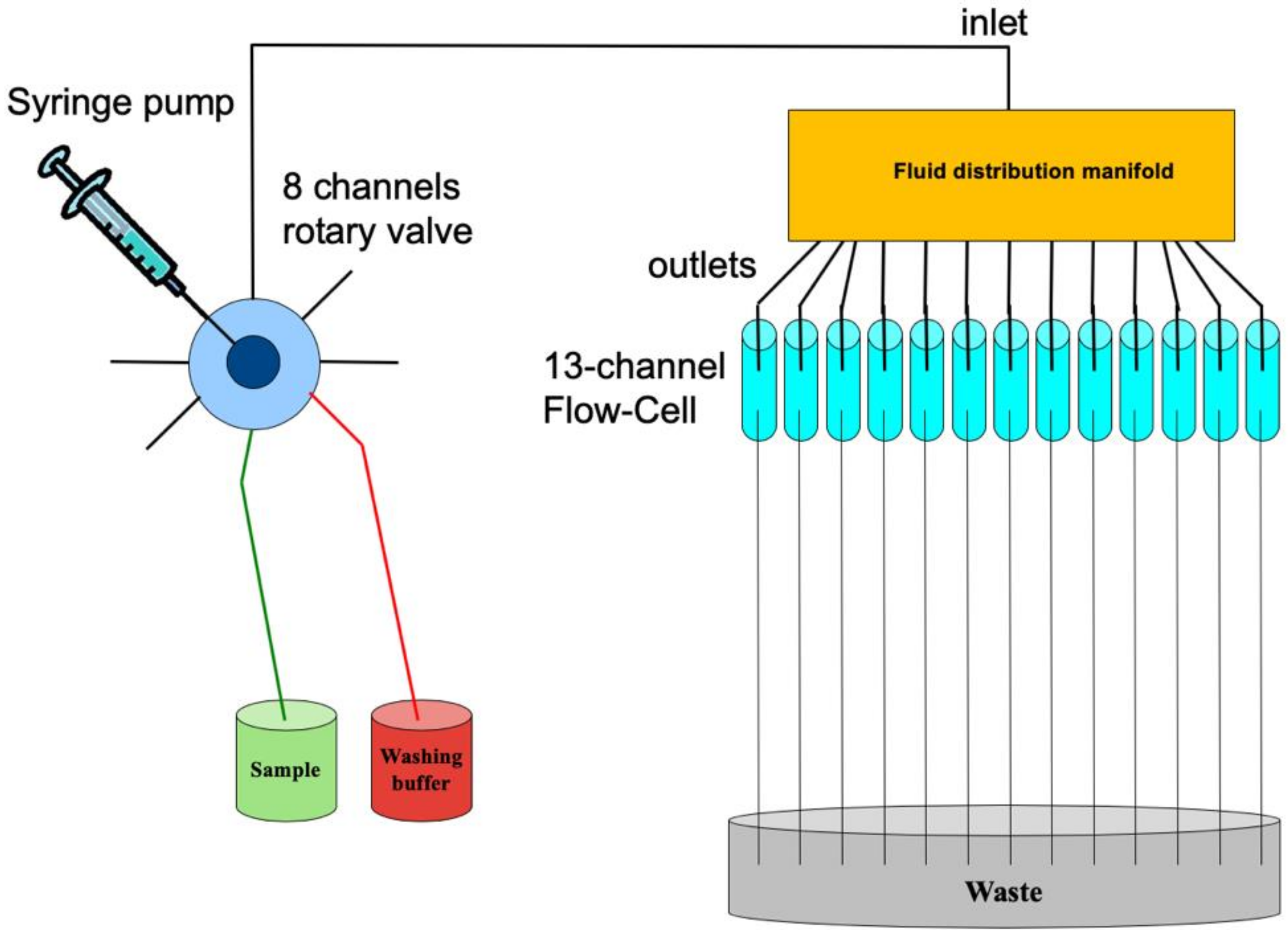
2.1. The Multi-Channel Chip
2.2. The Immunoassay
- -
- CRP was quantified by rate nephelometry (BN ProSpec, Siemens Healthcare Diagnostics, Italy);
- -
- NP was measured by using a commercial ELISA kit (DRG International Inc., Mountainside, NJ, USA).
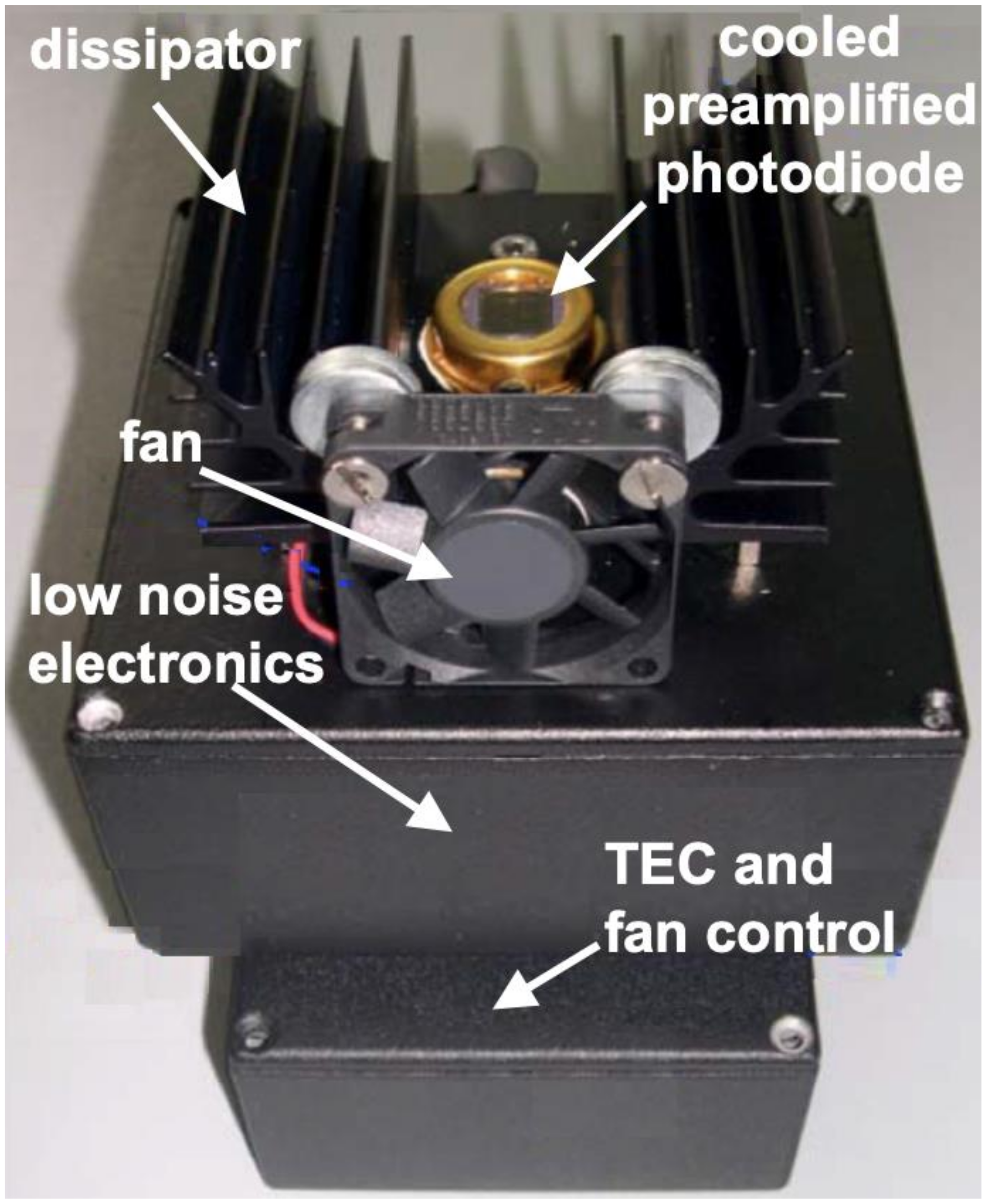
2.3. The Integrated Optical Detection System
2.4. The Software
3. Results and Discussion
3.1. The Integrated POCT-Prototype
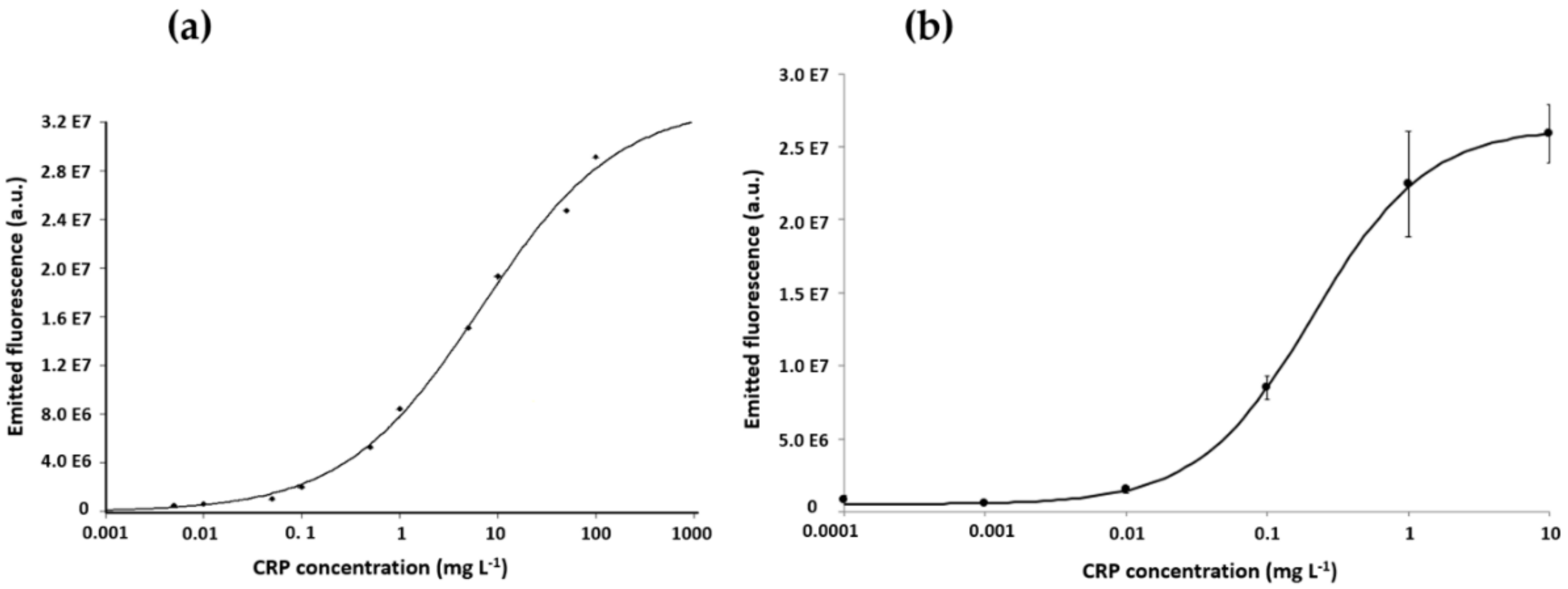
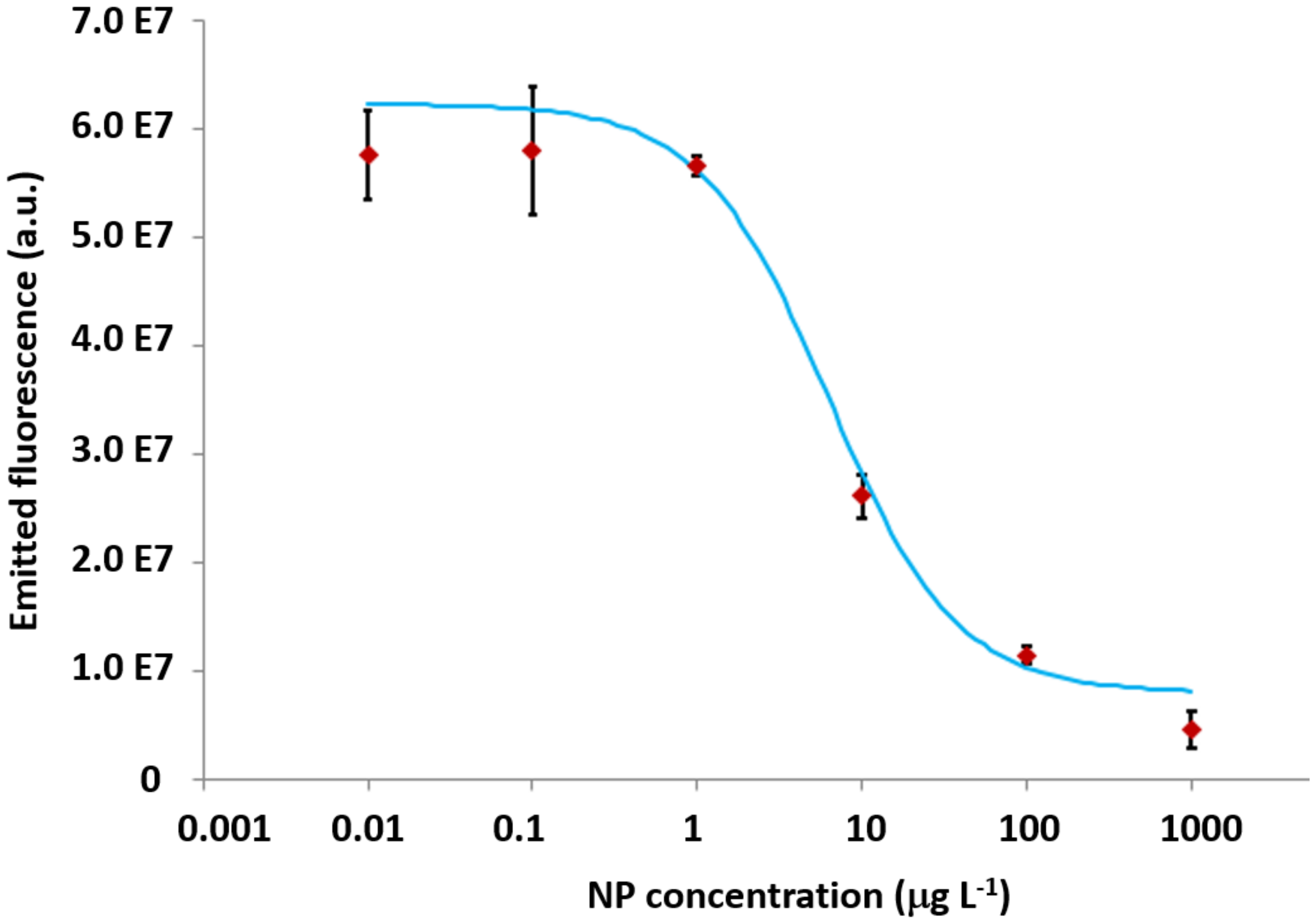
3.2. The Calibration Curves for CRP and NP
3.3. The Measurements on Human Samples
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kost, G.; Tran, N.; Louie, R.; Tang, Z.; Woodruff, A.; Albertson, T.; Derlet, R.; Azari, R. Rapid diagnosis of sepsis: Point-of-care testing, nucleic acid testing, and the value model. Point Care 2003, 2, 163–171. [Google Scholar]
- Biron, B.M.; Ayala, A.; Lomas-Neira, J.L. Biomarkers for Sepsis: What Is and What Might Be? Biomark. Insights 2015, 10, 7–17. [Google Scholar]
- Fagerström, J.; Bång, M.; Wilhelms, D.; Chew, M.S. Lisep lstm: A machine learning algorithm for early detection of septic shock. Sci. Rep. 2019, 9, 1–8. [Google Scholar]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.; Coopersmith, C.M.; et al. The Third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar]
- De La Rica, A.S.; Gilsanz, F.; Maseda, E. Epidemiologic trends of sepsis in western countries. Ann. Transl. Med. 2016, 4, 325. [Google Scholar] [CrossRef]
- Raith, E.P.; Udy, A.A.; Bailey, M.; McGloughlin, S.; MacIsaac, C.; Bellomo, R.; Pilcher, D.V. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA J. Am. Med. Assoc. 2017, 317, 290–300. [Google Scholar]
- Oeschger, T.; McCloskey, D.; Kopparthy, V.; Singh, A.; Erickson, D. Point of care technologies for sepsis diagnosis and treatment. Lab Chip 2019, 19, 728–737. [Google Scholar]
- Reddy, B.; Hassan, U.; Seymour, C.; Angus, D.C.; Isbell, T.S.; White, K.; Weir, W.; Yeh, L.; Vincent, A.; Bashir, R. Point-of-care sensors for the management of sepsis. Nat. Biomed. Eng. 2018, 2, 640–648. [Google Scholar]
- Belushkin, A.; Yesilkoy, F.; González-López, J.J.; Ruiz-Rodríguez, J.C.; Ferrer, R.; Fàbrega, A.; Altug, H. Rapid and digital detection of inflammatory biomarkers enabled by a novel portable nanoplasmonic imager. Small 2019, 16, 1906108. [Google Scholar] [CrossRef]
- Pfäfflin, A.; Schleicher, E. Inflammation markers in point-of-care testing (POCT). Anal. Bioanal. Chem. 2009, 393, 1473–1480. [Google Scholar]
- Baldini, F.; Bolzoni, L.; Giannetti, A.; Kess, M.; Krämer, P.; Kremmer, E.; Porro, G.; Senesi, F.; Trono, C. A new procalcitonin optical immunosensor for POCT applications. Anal. Bioanal. Chem. 2009, 393, 1183–1190. [Google Scholar]
- Kemmler, M.; Sauer, U.; Schleicher, E.; Preininger, C.; Brandenburg, A. Sensors and Actuators B: Chemical Biochip point-of-care device for sepsis diagnostics. Sens. Actuators B. Chem. 2014, 192, 205–215. [Google Scholar]
- Niemz, A.; Ferguson, T.M.; Boyle, D.S. Point-of-care nucleic acid testing for infectious diseases. Trends Biotechnol. 2011, 29, 240–250. [Google Scholar]
- Buchegger, P.; Sauer, U.; Toth-Székély, H.; Preininger, C. Miniaturized protein microarray with internal calibration as point-of-care device for diagnosis of neonatal sepsis. Sensors 2012, 12, 1494–1508. [Google Scholar]
- Faix, J.D. Biomarkers of sepsis. Crit. Rev. Clin. Lab. Sci. 2013, 50, 23–36. [Google Scholar]
- Sapa, A.; Rak, A.; Wybieralska, M.; Machoń, J.; Krzywonos-Zawadzka, A.; Zawadzki, K.; Wełna, M.; Woźniak, M. Diagnostic usefulness of sCD163, procalcitonin & neopterin for sepsis risk assessment in critically ill patients. Adv. Clin. Exp. Med. 2017, 26, 101–108. [Google Scholar]
- Shapiro, N.I.; Trzeciak, S.; Hollander, J.E.; Birkhahn, R.; Otero, R.; Osborn, T.M.; Moretti, E.; Nguyen, H.B.; Gunnerson, K.J.; Milzman, D.; et al. A prospective, multicenter derivation of a biomarker panel to assess risk of organ dysfunction, shock, and death in emergency department patients with suspected sepsis. Crit. Care Med. 2009, 37, 96–104. [Google Scholar]
- Pepys, M.B.; Baltz, M.L. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A Protein. Adv. Immunol. 1983, 34, 141–212. [Google Scholar]
- Ridker, P.M. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003, 107, 363–369. [Google Scholar]
- Berdowska, A.; Zwirska-Korczala, K. Neopterin measurement in clinical diagnosis. J. Clin. Pharm. Ther. 2001, 26, 319–329. [Google Scholar]
- Hensler, T.; Sauerland, S.; Lefering, R.; Nagelschmidt, M.; Bouillon, B.; Andermahr, J.; Neugebauer, E.A.M. The clinical value of procalcitonin and neopterin in predicting sepsis and organ failure after major trauma. Shock 2003, 20, 420–426. [Google Scholar]
- Baydar, T.; Yuksel, O.; Sahin, T.T.; Dikmen, K.; Girgin, G.; Sipahi, H.; Kurukahvecioglu, O.; Bostanci, H.; Sare, M. Neopterin as a prognostic biomarker in intensive care unit patients. J. Crit. Care 2009, 24, 318–321. [Google Scholar]
- Eisenhut, M. Neopterin in diagnosis and monitoring of infectious diseases. J. Biomark. 2013, 2013, 10. [Google Scholar] [CrossRef]
- Giannetti, A.; Trono, C.; Baldini, F.; Bolzoni, L.; Porro, G. The Channel Array Interrogation (CAI) Instrument for C-Reactive Protein Analysis. In Proceedings of the IEEE 2011 International Workshop on Biophotonics, Parma, Italy, 8–10 June 2011. [Google Scholar]
- Cernoch, I.; Schleicher, E.; Franek, M. Production and analytical characterization of neopterin immunoreagents for biosensor developments. Anal. Bioanal. Chem. 2011, 399, 979–986. [Google Scholar]
- Baldini, F.; Bolzoni, L.; Giannetti, A.; Porro, G.; Senesi, F.; Trono, C. A Fluorescent Immunoassay for the Determination of Procalcitonin and C-Reactive Protein. In Proceedings of the Spie Optics + Optoelectronics, Prague, Czech Republic, 20–23 April 2009; Volume 7356. [Google Scholar]
- Baldini, F.; Bolzoni, L.; Giannetti, A.; Porro, G.; Trono, C. A portable instrument for the optical interrogation of a novel biochip. In Proceedings of the (Ewofs’10) Fourth European Workshop on Optical Fibre Sensors, Porto, Portugal, 8–10 September 2010; Volume 7653. [Google Scholar]
- Baldini, F.; Carloni, A.; Giannetti, A.; Porro, G.; Trono, C. An optical PMMA biochip based on fluorescence anisotropy: Application to C-reactive protein assay. Sens. Actuators B Chem. 2009, 139, 64–68. [Google Scholar]
- Rowe, C.A.; Tender, L.M.; Feldstein, M.J.; Golden, J.P.; Scruggs, S.B.; MacCraith, B.D.; Cras, J.J.; Ligler, F.S. Array biosensor for simultaneous identification of bacterial, viral, and protein analytes. Anal. Chem. 1999, 71, 3846–3852. [Google Scholar]
- Baldini, F.; Carloni, A.; Giannetti, A.; Trono, C.; Porro, G.; Tedeschi, L. An Optical Platform Based on Fluorescence Anisotropy for C—Reactive Protein Assay. In Proceedings of the 2008 IEEE Sensors, Lecce, Italy, 26–29 October 2008. [Google Scholar]
- Baldini, F.; Giannetti, A.; Trono, C.; Bolzoni, L.; Porro, G. The CAI instrument: A novel optical device for sepsis analysis for POCT applications. In Proceedings of the 2011 International Quantum Electronics Conference (IQEC) and Conference on Lasers and Electro-Optics (CLEO) Pacific Rim Incorporating the Australasian Conference on Optics, Lasers and Spectroscopy and the Australian Conference on Optical Fibre Technology, Sydney, Australia, 28 August–1 September 2011. [Google Scholar]
- Pradhan, S.; Ghimire, A.; Bhattarai, B.; Khanal, B.; Pokharel, K.; Lamsal, M.; Koirala, S. The role of C-reactive protein as a diagnostic predictor of sepsis in a multidisciplinary Intensive Care Unit of a tertiary care center in Nepal. Indian J. Crit. Care Med. 2016, 20, 417–420. [Google Scholar]
- Castelli, G.P.; Pognani, C.; Meisner, M.; Stuani, A.; Bellomi, D.; Sgarbi, L. Procalcitonin and C-reactive protein during systemic inflammatory response syndrome, sepsis and organ dysfunction. Crit. Care 2004, 8, R234. [Google Scholar] [CrossRef]
- Centi, S.; Tombelli, S.; Puntoni, M.; Domenici, C.; Franek, M.; Palchetti, I. Detection of biomarkers for inflammatory diseases by an electrochemical immunoassay: The case of neopterin. Talanta 2015, 134, 48–53. [Google Scholar]
- Zhang, X.; Chen, Q.; Ni, S.; Xiang, Z.; Zhou, X.; Huang, Y. Serum neopterin and its significance as biomarker in differentiation of mods from sepsis. Pteridines 2018, 29, 201–205. [Google Scholar]
- Yao, Y.M. Elevated serum neopterin level: Its relation to endotoxaemia and sepsis in patients with major burns. Eur. J. Clin. Investig. 1996, 26, 224–230. [Google Scholar]
- Svoboda, P.; Ko, S.H.; Cho, B.L.; Yoo, S.H.; Choi, S.W.; Ye, S.K.; Kasai, H.; Chung, M.H. Neopterin, a marker of immune response, and 8-hydroxy-2′-deoxyguanosine, a marker of oxidative stress, correlate at high age as determined by automated simultaneous high-performance liquid chromatography analysis of human urine. Anal. Biochem. 2008, 383, 236–242. [Google Scholar]
- Pourakbari, B.; Mamishi, S.; Zafari, J.; Khairkhah, H.; Ashtiani, M.H.; Abedini, M.; Afsharpaiman, S.; Rad, S.S. Evaluation of procalcitonin and neopterin level in serum of patients with acute bacterial infection. Braz. J. Infect. Dis. 2010, 14, 252–255. [Google Scholar]



| Analyte | Capture mAb | Conjugated Antigen | Detection mAb * | Label |
|---|---|---|---|---|
| CRP | Clone C5 | Clone C6 | DY647 (λexc = 635 nm, λem = 675 nm) | |
| NP | NP-BSA | Clone 3E2 |
| Healthy Volunteers | Patients with Sepsis | References | |
|---|---|---|---|
| CRP (mg L−1) | 1–10 | 20–500 | [31,32,33] |
| NP (µg L−1) | 1–5 | 24–105 | [16,34,35,36,37,38] |
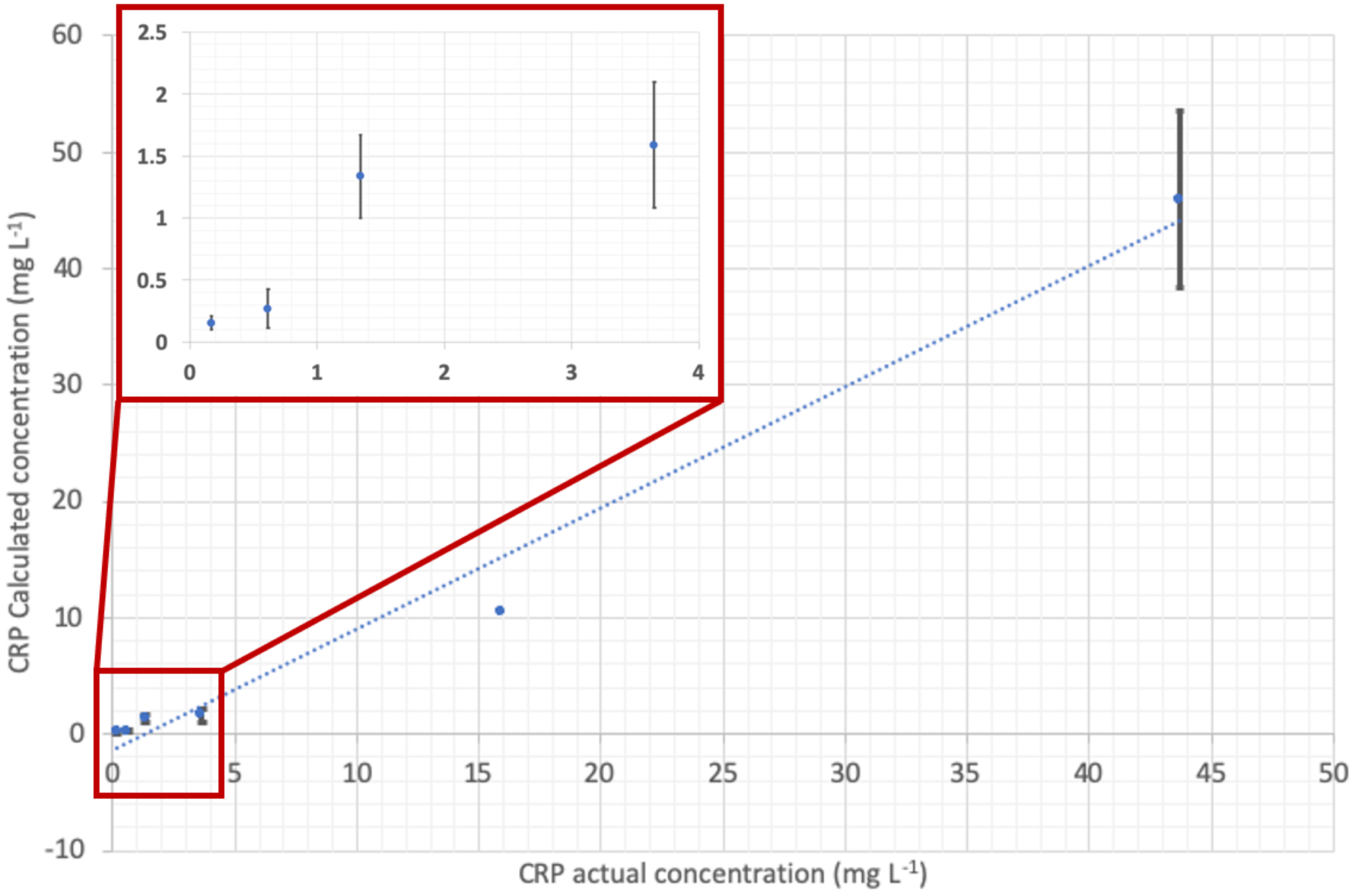
| Patient Identifying N. | CRP (mg L−1) Actual Concentration (Standard Method) | CRP (mg L−1) Calculated Concentration (POCT Prototype) | NP (µg L−1) Actual Concentration (Standard Method) | NP (µg L−1) Calculated Concentration (POCT Prototype) |
|---|---|---|---|---|
| 1 | 3.65 | 1.59 | not available | |
| 2 | <0.168 | 0.15 | not available | |
| 3 | 1.34 | 1.34 | not available | |
| 4 | 43.7 | 45.89 | not available | |
| 5 | 15.9 | 10.5 | 12.4 | 13.32 |
| 6 | 0.614 | 0.27 | 6.58 | 5.60 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannetti, A.; Trono, C.; Porro, G.; Domenici, C.; Puntoni, M.; Baldini, F. Towards an Integrated System as Point-of-Care Device for the Optical Detection of Sepsis Biomarkers. Chemosensors 2020, 8, 12. https://doi.org/10.3390/chemosensors8010012
Giannetti A, Trono C, Porro G, Domenici C, Puntoni M, Baldini F. Towards an Integrated System as Point-of-Care Device for the Optical Detection of Sepsis Biomarkers. Chemosensors. 2020; 8(1):12. https://doi.org/10.3390/chemosensors8010012
Chicago/Turabian StyleGiannetti, Ambra, Cosimo Trono, Giampiero Porro, Claudio Domenici, Mariarita Puntoni, and Francesco Baldini. 2020. "Towards an Integrated System as Point-of-Care Device for the Optical Detection of Sepsis Biomarkers" Chemosensors 8, no. 1: 12. https://doi.org/10.3390/chemosensors8010012
APA StyleGiannetti, A., Trono, C., Porro, G., Domenici, C., Puntoni, M., & Baldini, F. (2020). Towards an Integrated System as Point-of-Care Device for the Optical Detection of Sepsis Biomarkers. Chemosensors, 8(1), 12. https://doi.org/10.3390/chemosensors8010012








