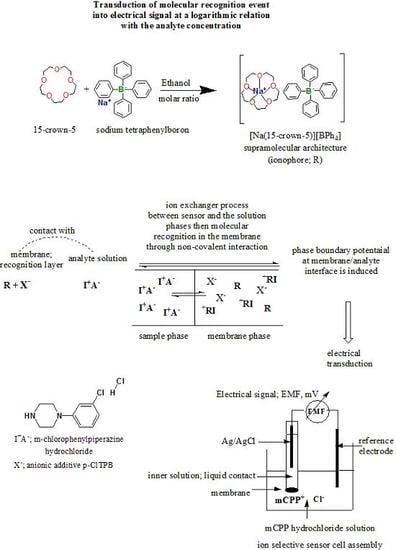
Potentiometric Signal Transduction for Selective Determination of 1-(3-Chlorophenyl)piperazine “Legal Ecstasy” Through Biomimetic Interaction Mechanism
Abstract
1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. Apparatus
2.3. Synthesis and Characterization of Sensing Elements
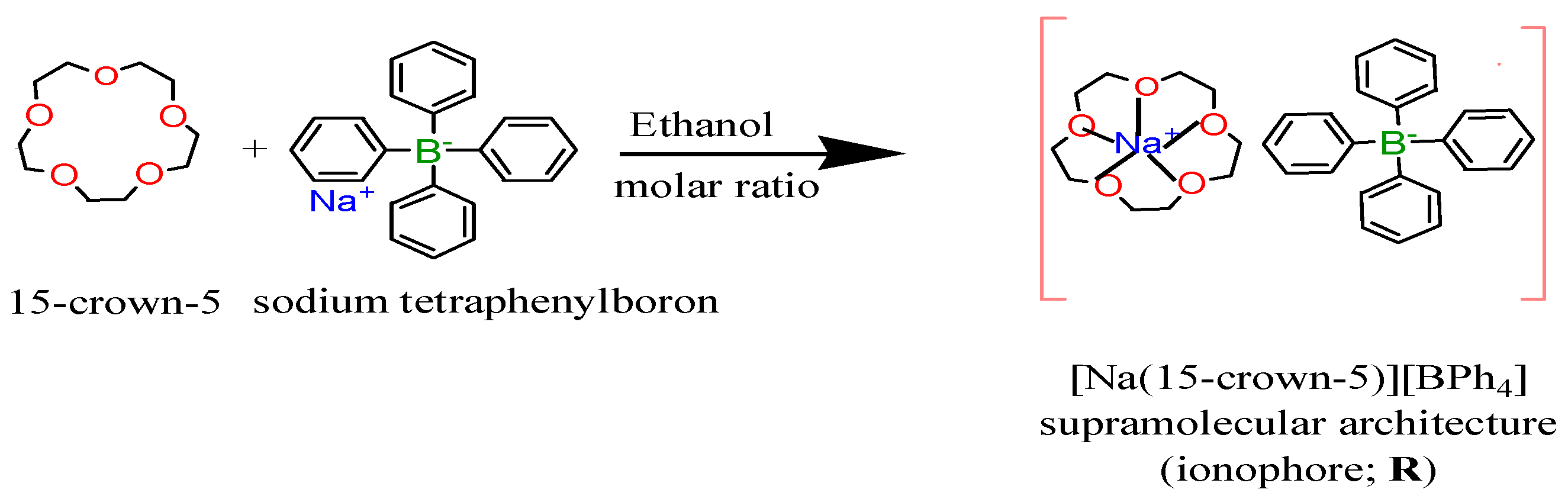
2.3.1. 15-Crown-5•Sodium Tetraphenylboron; [Na(15-crown-5)][BPh4] Supramolecular Architecture; (CE•NaTPB)
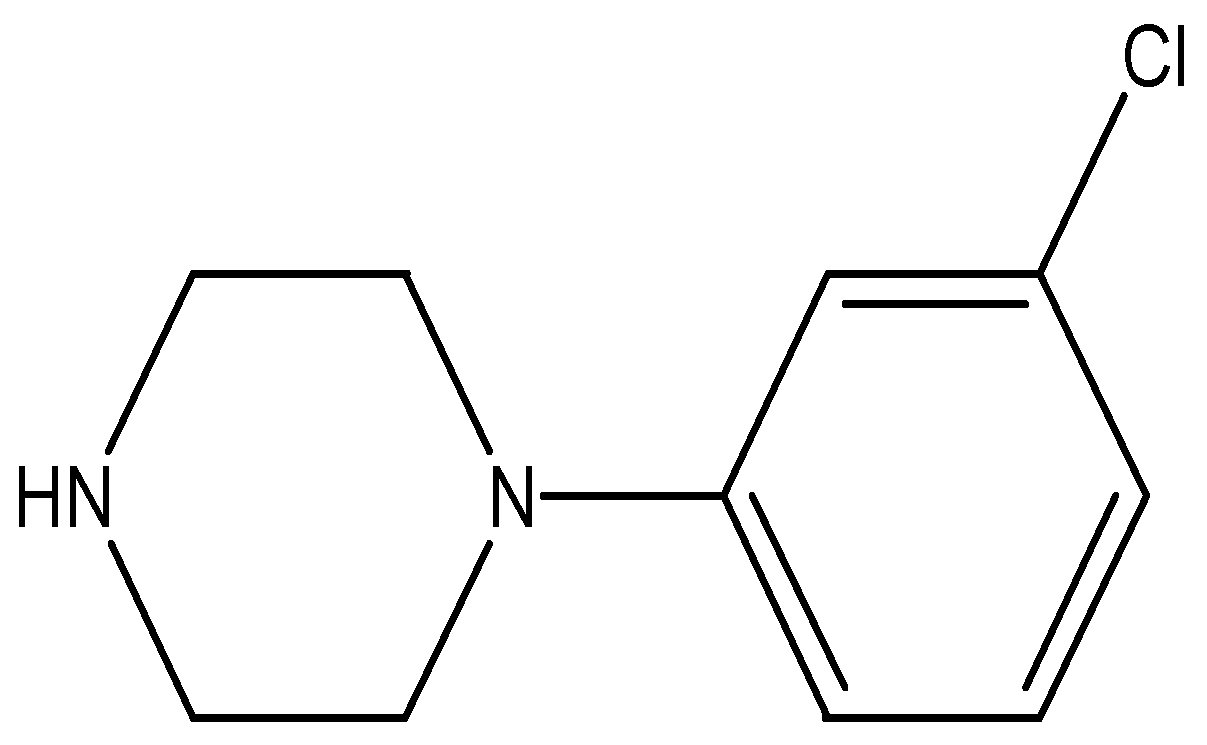
2.3.2. Sodium Tetraphenylboron:m-Chlorophenylpiperazine [mCPP]+:[TPB]− Ion Pair
2.4. Membrane and Sensor Preparation
2.5. EMF Measurement and Sensors Calibration
3. Results and Discussion
3.1. Performance Characteristics of m-Chlorophenylpiperazine Sensors
3.1.1. Membrane Response and Sensitivity of the Sensors
3.1.2. Optimization of the Membrane Composition
3.2. Response Time and pH Effect
3.3. Selectivity
3.4. Analytical Applications
3.5. Method Validation
4. Conclusions
Conflicts of Interest
References
- United Nations Office on Drugs and Crime (UNODC), the Challenge of New Psychoactive Substances, A Report from the Global SMART Programme March 2013. Available online: http://www.unodc.org/documents/scientific/NPS_2013_SMART. PDF (accessed on 28 October 2018).
- de Boer, D.; Bosman, I.J.; Hidvégi, E.; Manzoni, C.; Benko, A.A.; dos Reys, L.J.A.L.; Maes, R.A.A. Piperazine-like compounds: A new group of designer drugs-of-abuse on the european market. Forensic Sci. Int. 2001, 121, 47–56. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) 2009. BZP and Other Piperazines. Available online: http://www.emcdda.europa.eu/publications-/drug-profiles/bzp (accessed on 17 January 2019).
- World Health Organization. 1-(3-chlorophenyl) Piperazine (mCPP) Pre-Review Report, Expert Committee on Drug Dependence. In Proceedings of the Thirty-fifth Meeting, Hammamet, Tunisia, 4–8 June 2012. [Google Scholar]
- European Police Office, Europol-EMCDDA Joint Report on A New Psychoactive Substance: 1-(3-chlorophenyl) Piperazine (mCPP), European Monitoring Center on Drugs and Drug Addiction. 2005. Available online: http://www.emcdda.europa.eu/attachements.cfm /att_33256 EN_Final _Europol EMCDDA_Active_Monitoring_Report_mCPP_290307.Pdf (accessed on 1 December 2018).
- Tancer, M.E.; Johanson, C.-E. The subjective effects of MDMA and mCPP in moderate MDMA users. Drug Alcohol Depend. 2001, 65, 97–101. [Google Scholar] [CrossRef]
- Baumann, M.H.; Clark, R.D.; Budzynski, A.G.; Partilla, J.S.; Blough, B.E.; Rothman, R.B. N-substituted piperazines abused by humans mimic the molecular mechanism of 3,4-methylenedioxymethamphetamine (MDMA, or Ecstasy). Neuropsychopharmacology 2005, 30, 550–560. [Google Scholar] [CrossRef]
- Brunt, T.M.; Nagy, C.; Bücheli, A.; Martins, D.; Ugarte, M.; Beduwe, C.; Vilamala, M.V. Drug testing in Europe: Monitoring results of the Trans European Drug Information (TEDI) project. Drug Test. Anal. 2017, 9, 188–198. [Google Scholar] [CrossRef]
- Romão, W.; Lalli, P.M.; Franco, M.F.; Sanvido, G.; Schwab, N.V.; Lanaro, R.; Costa, J.L.; Sabino, B.D.; Bueno, M.I.M.S.; de Sa, G.F.; et al. Chemical profile of meta chlorophenylpiperazine (m-CPP) in ecstasy tablets by easy ambient sonic-spray ionization, X-ray fluorescence, ion mobility mass spectrometry and NMR. Anal. Bioanal. Chem. 2011, 400, 3053–3064. [Google Scholar] [CrossRef] [PubMed]
- Bossong, M.G.; Brunt, T.M.; Van Dijk, J.P.; Rigter, S.M.; Hoek, J.; Goldschmidt, H.M.J.; Niesink, R.J.M. mCPP: An undesired addition to the ecstasy market. J. Psychopharmacol. 2010, 24, 1395–1401. [Google Scholar] [CrossRef]
- Kahn, R.S.; Wetzler, S. m-Chlorophenylpiperazine as a probe of serotonin function. Biol. Psychiatry 1991, 30, 1139–1166. [Google Scholar] [CrossRef]
- Patel, B.N.; Sharma, N.; Sanyal, M.; Shrivastav, P.S. High throughput and sensitive determination of trazodone and its primary metabolite, m-chlorophenylpiperazine, in human plasma by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2008, 871, 44–54. [Google Scholar] [CrossRef]
- Lendoiro, E.; Jiménez-Morigosa, C.; Cruz, A.; Páramo, M.; López-Rivadulla, M.; de Castro, A. An LC-MS/MS methodological approach to the analysis of hair for amphetamine-type stimulant (ATS) drugs, including selected synthetic cathinones and piperazines. Drug Test. Anal. 2017, 9, 96–105. [Google Scholar] [CrossRef]
- Moreno, I.E.D.; da Fonseca, B.M.; Barroso, M.; Costa, S.; Queiroz, J.A.; Gallardo, E. Determination of piperazine-type stimulants in human urine by means of microextraction in packed sorbent and high performance liquid chromatography-diode array detection. J. Pharm. Biomed. Anal. 2012, 61, 93–99. [Google Scholar] [CrossRef]
- Byrska, B.; Zuba, D.; Stanaszek, R. Determination of piperazine derivatives in “legal highs”. Probl. Forensic Sci. 2010, 81, 101–113. [Google Scholar]
- Kuleya, C.; Hall, S.; Gautam, L.; Cole, M.D. An optimized gas chromatographic-mass spectrometric method for the chemical characterization of benzylpiperazine and 1-arylpiperazine based drugs. Anal. Methods 2014, 6, 156–163. [Google Scholar] [CrossRef]
- Ŝiroká, J.; Polesel, D.N.; Costa, J.L.; Lanaro, R.; Tavares, M.F.M.; Polášek, M. Separation and determination of chlorophenylpiperazine isomers in confiscated pills by capillary electrophoresis. J. Pharm. Biomed. Anal. 2013, 84, 140–147. [Google Scholar] [CrossRef]
- Asturias-Arribas, L.; Alonso-Lomillo, M.A.; Domínguez-Renedo, O.; Arcos-Martínez, M.J. Sensitive and selective cocaine electrochemical detection using disposable sensors. Anal. Chim. Acta 2014, 834, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Tadini, M.C.; Balbino, M.A.; Eleoterio, I.C.; de Oliveira, L.S.; Dias, L.G.; Demets, G.J.-F.; de Oliveira, M.F. Developing electrodes chemically modified with cucurbit [6] uril to detect 3,4-methylenedioxymethamphetamine (MDMA) by voltammetry. Electrochim. Acta 2014, 121, 188–193. [Google Scholar] [CrossRef]
- Balbino, M.A.; Oiye, É.N.; Ribeiro, M.F.M.; Júnior, J.W.C.; Eleotério, I.C.; Ipólito, A.J.; de Oliveira, M.F. Use of screen-printed electrodes for quantification of cocaine and -THC: Adaptions to portable systems for forensic purposes. J. Solid State Electrochem. 2016, 20, 2435–2443. [Google Scholar] [CrossRef]
- Khairy, M.; Mahmoud, B.G.; Banks, C.E. Simultaneous determination of codeine and its co-formulated drugs acetaminophen and caffeine by utilizing cerium oxide nanoparticles modified screen-printed electrodes. Sens. Actuators B Chem. 2018, 259, 142–154. [Google Scholar] [CrossRef]
- Shaw, L.; Dennany, L. Applications of electrochemical sensors: Forensic drug analysis. Curr. Opin. Electrochem. 2017, 3, 23–28. [Google Scholar] [CrossRef]
- Smith, J.P.; Metters, J.P.; Khreit, O.I.G.; Sutcliffe, O.B.; Banks, C.E. Forensic electrochemistry applied to the sensing of new psychoactive substances: Electroanalytical sensing of synthetic cathinones and analytical validation in the quantification of seized street samples. Anal. Chem. 2014, 86, 9985–9992. [Google Scholar] [CrossRef]
- Razavipanah, I.; Alipour, E.; Deiminiat, B.; Rounaghi, G.H. A novel electrochemical imprinted sensor for ultrasensitive detection of the new psychoactive substance “Mephedrone”. Biosens. Bioelectron. 2018, 119, 163–169. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Norouzi, P.; Rezapour, M.; Faridbod, F.; Pourjavid, M.R. Supramolecular based membrane sensors. Sensors 2006, 6, 1018–1086. [Google Scholar] [CrossRef]
- Bűhlmann, P.; Pretsch, E.; Bakker, E. Carrier-based ion-selective electrodes and bulk optodes. 2. ionophores for potentiometric and optical sensors. Chem. Rev. 1998, 98, 1593–1687. [Google Scholar] [CrossRef] [PubMed]
- El-Naby, E.H. Selective ketamine recognition based on membrane potential changes induced by a hybrid organic/inorganic supramolecular assembly. Anal. Methods 2014, 6, 900–906. [Google Scholar] [CrossRef]
- El-Naby, E.H.; Kamel, A.H. Potential transducers based man-tailored biomimetic sensors for selective recognition of dextromethorphan as an antitussive drug. Mater. Sci. Eng. C 2015, 54, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.-J.; Liu, S.-X.; Tian, Z.-F.; Ren, X.-M. A high temperature reversible phase transition in a supramolecular complex of 15-crown-5 with tetraphenylboron sodium. New J. Chem. 2018, 42, 14943–14948. [Google Scholar] [CrossRef]
- Lehn, J.-M. Toward self-organization and complex matter. Science 2002, 295, 2400–2403. [Google Scholar] [CrossRef]
- Zarra, S.; Wood, D.M.; Roberts, D.A.; Nitschke, J.R. Molecular containers in complex chemical systems. Chem. Soc. Rev. 2015, 44, 419–432. [Google Scholar] [CrossRef]
- Lavigne, J.J.; Anslyn, E.V. Sensing a paradigm shift in the field of molecular recognition: From selective to differential Receptors. Angew. Chem. Int. Ed. 2001, 40, 3118–3130. [Google Scholar] [CrossRef]
- Parsons, D.G.; Truter, M.R.; Wingfield, J.N. Alkali metal tetraphenyl-borate complexes with some macrocyclic, “crown”, polyethers. Inorg. Chim. Acta 1975, 14, 45–48. [Google Scholar] [CrossRef]
- Kiviniemi, S.; Nissinen, M.; Alaviuhkola, T.; Rissanen, K.; Pursiainen, J. The complexation of tetraphenylboron with organic N-heteroaromaticcations. J. Chem. Soc. Perkin Trans. 2001, 2, 2364–2369. [Google Scholar] [CrossRef]
- Bakker, E.; Bühlmann, P.; Pretsch, E. The phase-boundary potential model. Talanta 2004, 63, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Strauss, S.H. The search for larger and more weakly coordinating anions. Chem. Rev. 1993, 93, 927–942. [Google Scholar] [CrossRef]
- Andreeva, N.A.; Chaban, V.V. Understanding weakly coordinating anions: Tetrakis (pentafluorophenyl) borate paired with inorganic and organic cations. J. Mol. Model. 2017, 23, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Kharitonov, S.V. Membrane drotaverine-selective electrodes based on tetraphenyl-borate derivatives: Electrochemical, adsorption, and transport properties and analytical application. J. Anal. Chem. 2006, 61, 902–911. [Google Scholar] [CrossRef]
- Horton, D.A.; Bourne, G.T.; Smythe, M.L. The combinatorial synthesis of bicyclic privileged structures or privileged substructures. Chem. Rev. 2003, 103, 897–930. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, L.; Xu, L.; Chen, R.; Yang, Y. Interesting organic supramolecular structures constructed by piperazine/N, N′-dimethylpiperazine with aromatic multicomponent acids: Synthon cooperation and structural diversity. CrystEngComm 2012, 14, 6998–7008. [Google Scholar] [CrossRef]
- Zlatović, M.V.; Šukalović, V.V.; Schneider, C.; Roglić, G.M. Interaction of arylpiperazine ligands with the hydrophobic part of the 5-HT1A receptor binding site. Bioorg. Med. Chem. 2006, 14, 2994–3001. [Google Scholar] [CrossRef]
- Zhao, X.F.; Wang, J.; Liu, G.X.; Fan, T.P.; Zhang, Y.J.; Yu, J.; Wang, S.X.; Li, Z.J.; Zhang, Y.Y.; Zheng, X.H. Binding mechanism of nine N-phenylpiperazine derivatives and α1A-adrenoceptor using site directed molecular docking and high performance affinity chromatography. RSC Adv. 2015, 5, 57050–57057. [Google Scholar] [CrossRef]
- Lenik, J.; Nieszporek, J. Construction of a glassy carbon ibuprofen electrode modified with multi-walled carbon nanotubes and cyclodextrins. Sens. Actuators B Chem. 2018, 255, 2282–2289. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Yang, Z.-X.; Chen, Y.; Ding, F.; Liu, Y. Molecular binding and assembly behavior of β-cyclodextrin with piperazine and 1,4-dioxane in aqueous solution and solid state. Cryst. Growth 2012, 12, 1370–1377. [Google Scholar] [CrossRef]
- Bakker, E.; Bühlmann, P.; Pretsch, E. Carrier-Based Ion-Selective Electrodes and Bulk Optodes. 1. General Characteristics. Chem. Rev. 1997, 97, 3083–3132. [Google Scholar] [CrossRef] [PubMed]
- Gehrig, P.; Morf, W.E.; Welti, M.; Pretsch, E.; Simon, W. Catalysis of Ion Transfer by tetraphenylborons in Neutral Carrier-Based Ion-Selective Electrodes. Helv. Chem. Acta 1990, 73, 203–212. [Google Scholar] [CrossRef]
- Petrukhin, O.M.; Kharitonov, A.B.; Frakiisky, E.V.; Urusov, Y.I.; Zhukov, A.F.; Shipway, A.N.; Baulin, V.E. The effect of lipophilic anionic additives on detection limits of ion-selective electrodes based on ionophores with phosphoryl complexing groups. Sens. Actuators B Chem. 2001, 76, 653–659. [Google Scholar] [CrossRef]
- Janata, J. Principles of Chemical Sensors, 2nd ed.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; London, UK; New York, NY, USA, 2009; pp. 14–17. [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC). International Quality Assurance Programme (IQAP); International Collaborative Exercises (ICE) Summary Report Seized Materials; The Laboratory and Scientific Section UNODC, International Centre: Vienna, Austria, 2014. [Google Scholar]
- Tohda, K.; Dragoe, D.; Shibata, M.; Umezawa, Y. Studies on the matched potential method for determining the selectivity coefficients of ion-selective electrodes based on neutral ionophores: Experimental and theoretical verification. Anal. Sci. 2001, 17, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, Y.; Bűhlmann, P.; Umezawa, K.; Tohda, K.; Amemiya, S. Potentiometric selectivity coefficients of ion-selective electrodes part I. inorganic cations. Pure Appl. Chem. 2000, 72, 1851–2082. [Google Scholar] [CrossRef]
- Schneider, H.-J.; Yatsimirsky, A.K. Selectivity of chemical receptors. In Artificial Receptors for Chemical Sensors, 1st ed.; Mirsky, V.M., Yatsimirsky, A.K., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp. 17–65. [Google Scholar]
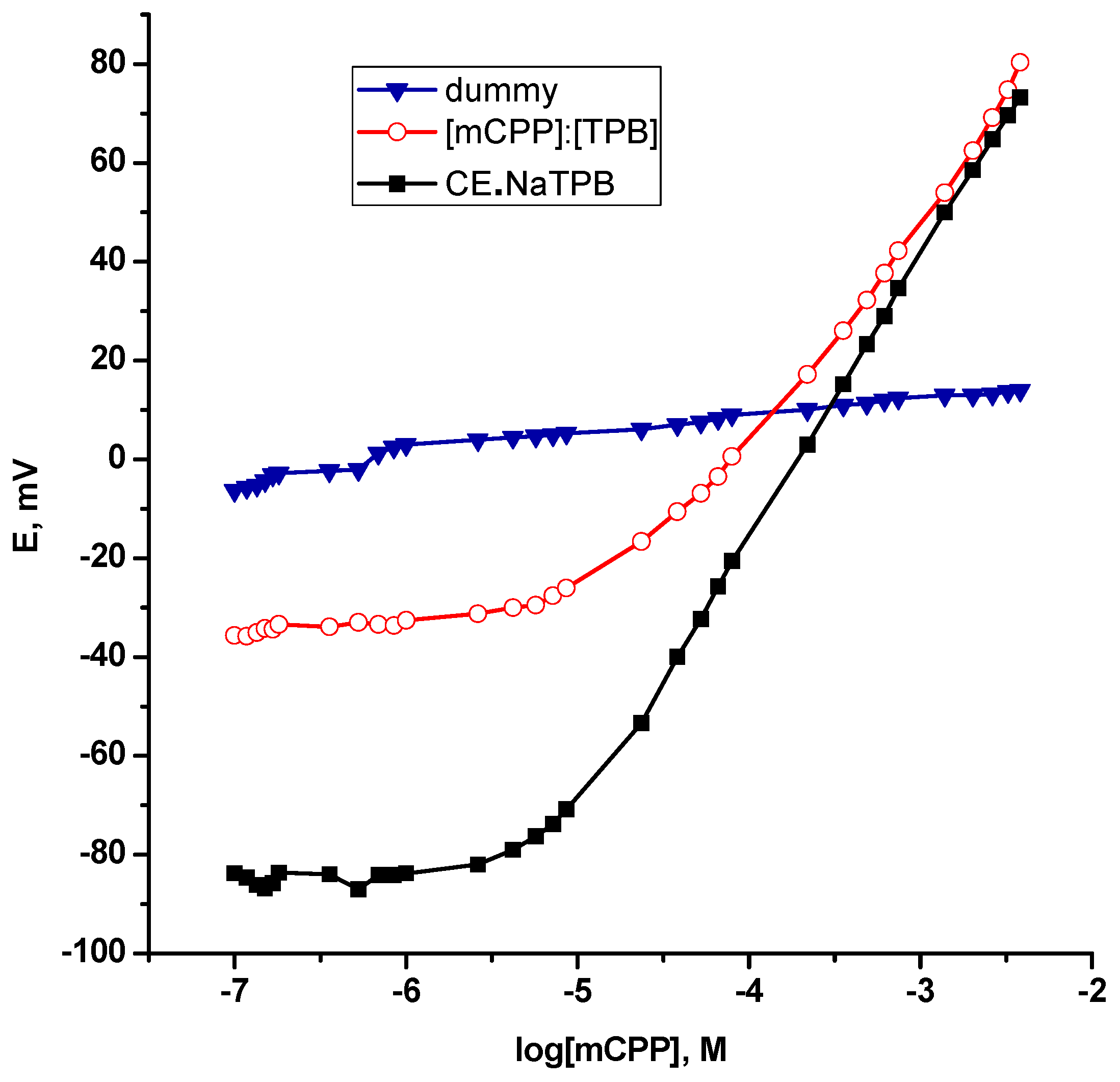
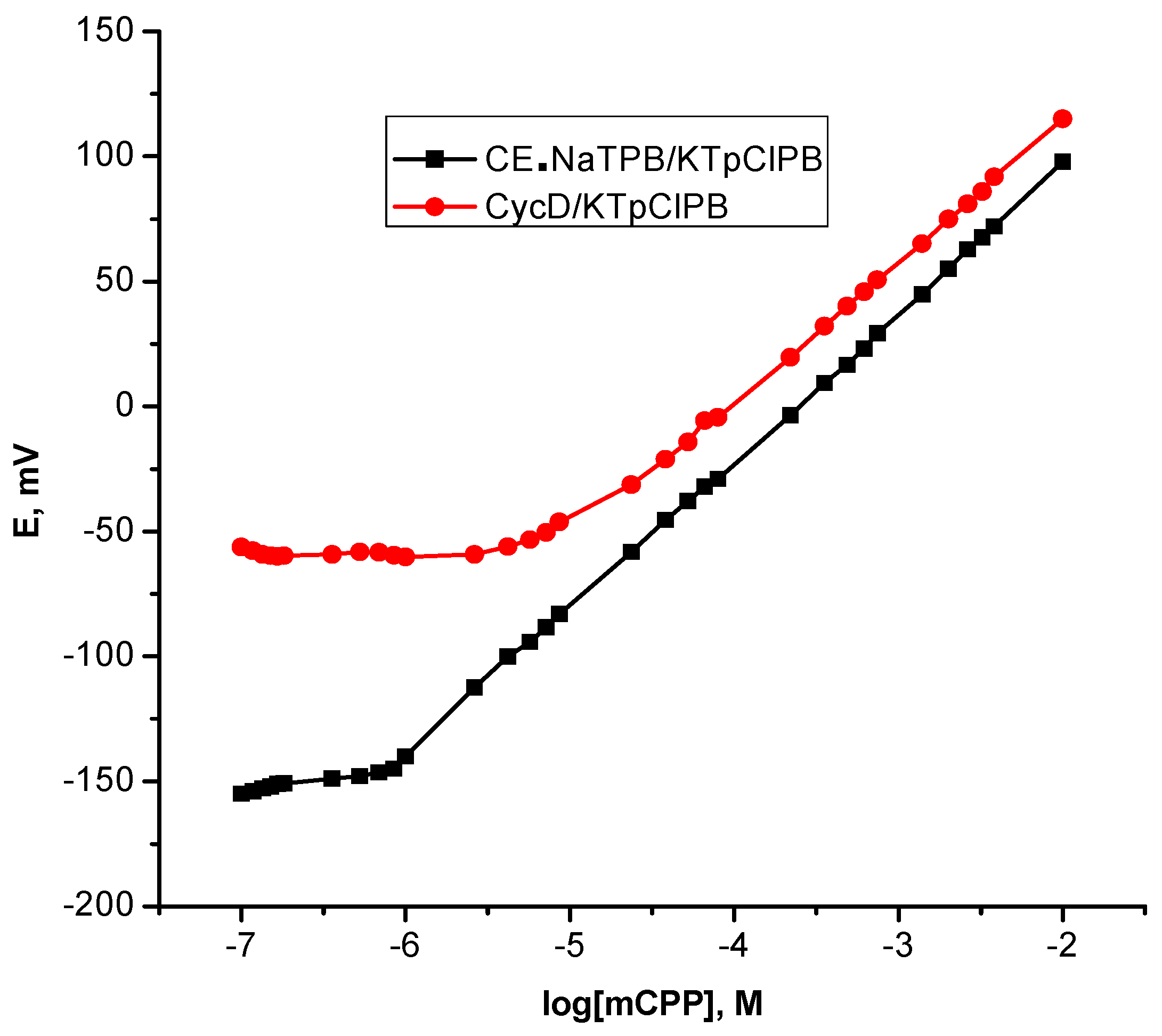
| Parameter | CE•NaTPB/KTpClPB | β-CycD/KTpClPB | mCPP:TPB |
|---|---|---|---|
| Slope, mV/decade * | 58.9 ± 0.43 | 55.2 ± 0.60 | 46.96 ± 2.93 |
| Correlation coefficient (r2) | 0.9998 | 0.9997 | 0.9985 |
| Linear range, M | 1.0 × 10−6–1.0 × 10−2 | 2.4 × 10−5–1.0×10−2 | 2.2 × 10−4–2.6 × 10−3 |
| Detection limit, M | 5.0 × 10−7 | 9.0 × 10−6 | 4.0 × 10−5 |
| Working range (pH) | 4–8 | 4–8 | 4–8 |
| Response time (s) ≥ 10−4 M | 5.0 | 15 | 50 |
| Life span (week) | 8.0 | 5.0 | 1.0 |
| Precision CVw (%) | 1.10 | 1.14 | 6.23 |
| Between-day variability CVb (%) | 0.53 | 0.70 | 1.66 |
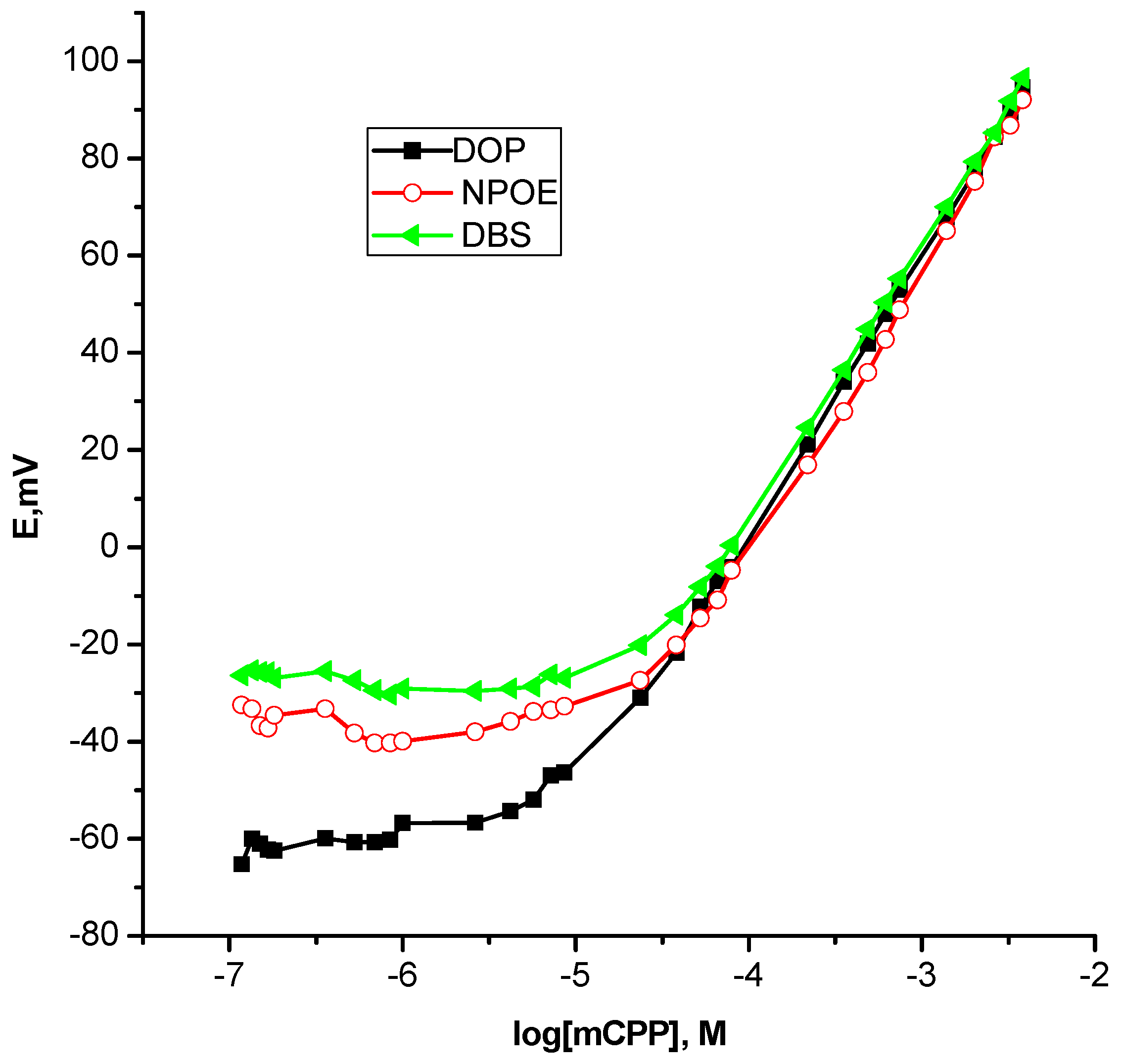
| Plasticizer/Dielectric Constant, ε | Slope, mV/decade | Linear Range, M | Detection Limit, M |
|---|---|---|---|
| DOP, 5.10 | 55.1 | 2.4 × 10−5–3.9 × 10−3 | 7.5 × 10−6 |
| DBS, 4.46 | 55.6 | 3.8 × 10−5–3.9 × 10−3 | 3.0 × 10−5 |
| NPOE, 24.0 | 61.8 | 2.2 × 10−4–3.9 × 10−3 | 5.0 × 10−5 |
| Interferent | CE•NaTPB/KTpClPB | β-CycD/KTpClPB |
|---|---|---|
| m-chlorophenylpiperazine | 0.0 | 0.0 |
| morphine | 2.91 | 1.60 |
| codeine | 2.37 | 0.72 |
| ephedrine | 2.02 | 0.90 |
| norephedrine | 2.62 | 1.65 |
| dextromethorphan | 2.16 | 0.42 |
| caffeine | 2.70 | 0.89 |
| Interferent | CE•NaTPB/KTpClPB | β-CycD/KTpClPB |
|---|---|---|
| phenylalanine | 3.09 | 1.59 |
| glycine | 3.07 | 1.72 |
| Na+ | 3.19 | 1.78 |
| 3.57 | 2.06 | |
| Ca2+ | 3.40 | 1.89 |
| K+ | 3.21 | 1.89 |
| Weighted, mg | Found, mg ± SD | Recovery, % |
|---|---|---|
| 10.0 | 10.25 ± 0.05 | 102.5 |
| 35.0 | 34.83 ± 0.40 | 99.5 |
| 100.0 | 100.3 ± 0.14 | 100.3 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Naby, E.H. Potentiometric Signal Transduction for Selective Determination of 1-(3-Chlorophenyl)piperazine “Legal Ecstasy” Through Biomimetic Interaction Mechanism. Chemosensors 2019, 7, 46. https://doi.org/10.3390/chemosensors7030046
El-Naby EH. Potentiometric Signal Transduction for Selective Determination of 1-(3-Chlorophenyl)piperazine “Legal Ecstasy” Through Biomimetic Interaction Mechanism. Chemosensors. 2019; 7(3):46. https://doi.org/10.3390/chemosensors7030046
Chicago/Turabian StyleEl-Naby, Eman H. 2019. "Potentiometric Signal Transduction for Selective Determination of 1-(3-Chlorophenyl)piperazine “Legal Ecstasy” Through Biomimetic Interaction Mechanism" Chemosensors 7, no. 3: 46. https://doi.org/10.3390/chemosensors7030046
APA StyleEl-Naby, E. H. (2019). Potentiometric Signal Transduction for Selective Determination of 1-(3-Chlorophenyl)piperazine “Legal Ecstasy” Through Biomimetic Interaction Mechanism. Chemosensors, 7(3), 46. https://doi.org/10.3390/chemosensors7030046