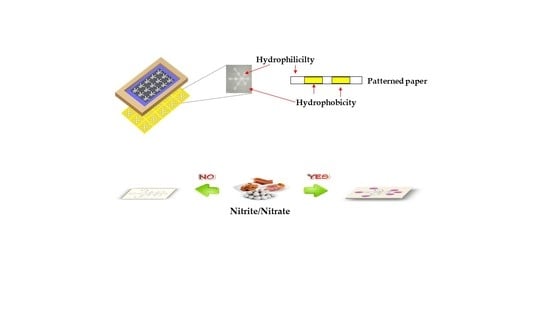
One-Step Polylactic Acid Screen-Printing Microfluidic Paper-Based Analytical Device: Application for Simultaneous Detection of Nitrite and Nitrate in Food Samples
Abstract
1. Introduction
2. Materials and Methods
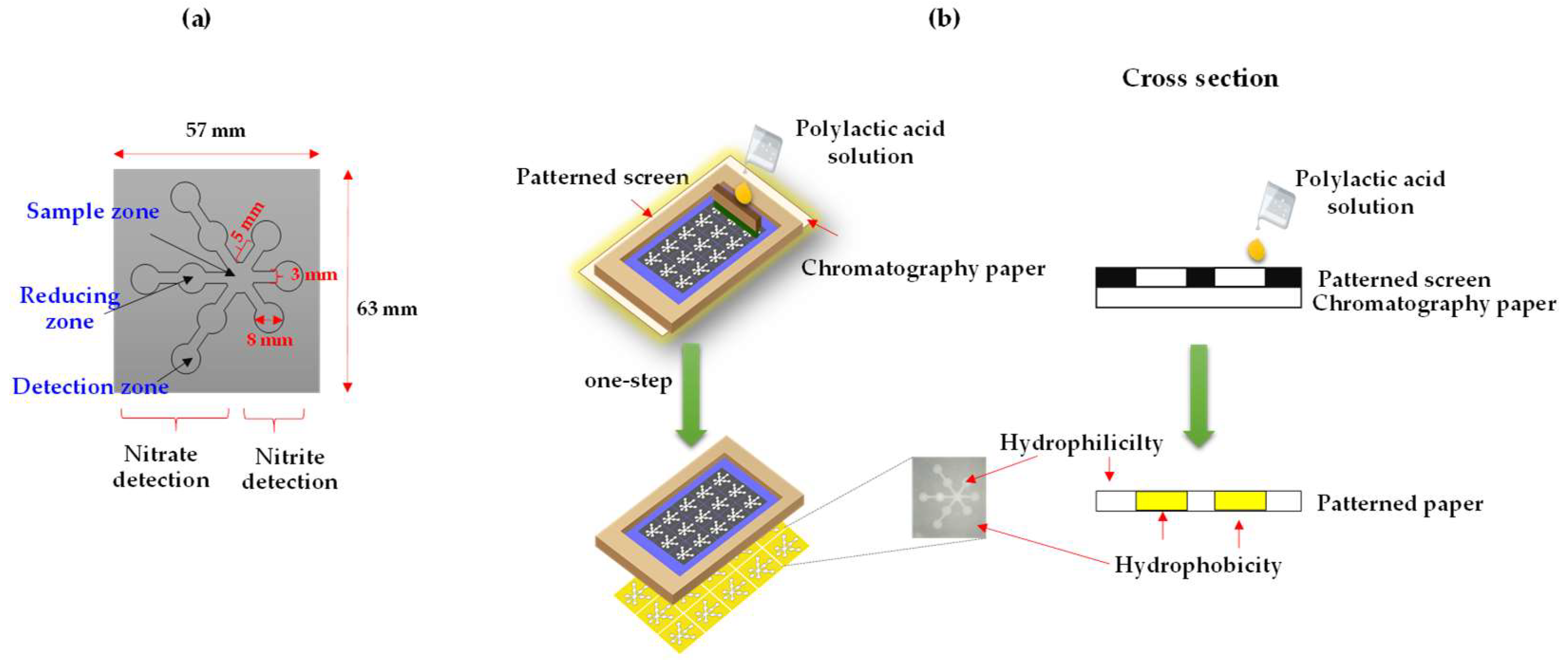
2.1. Paper-Based Design and Fabrication
2.2. Detection of Nitrite and Nitrate with the µPAD
2.3. Samples Preparation
2.4. The Detection of Nitrite and Nitrate Using Spectrophotometric as a Reference Method
3. Results and Discussion
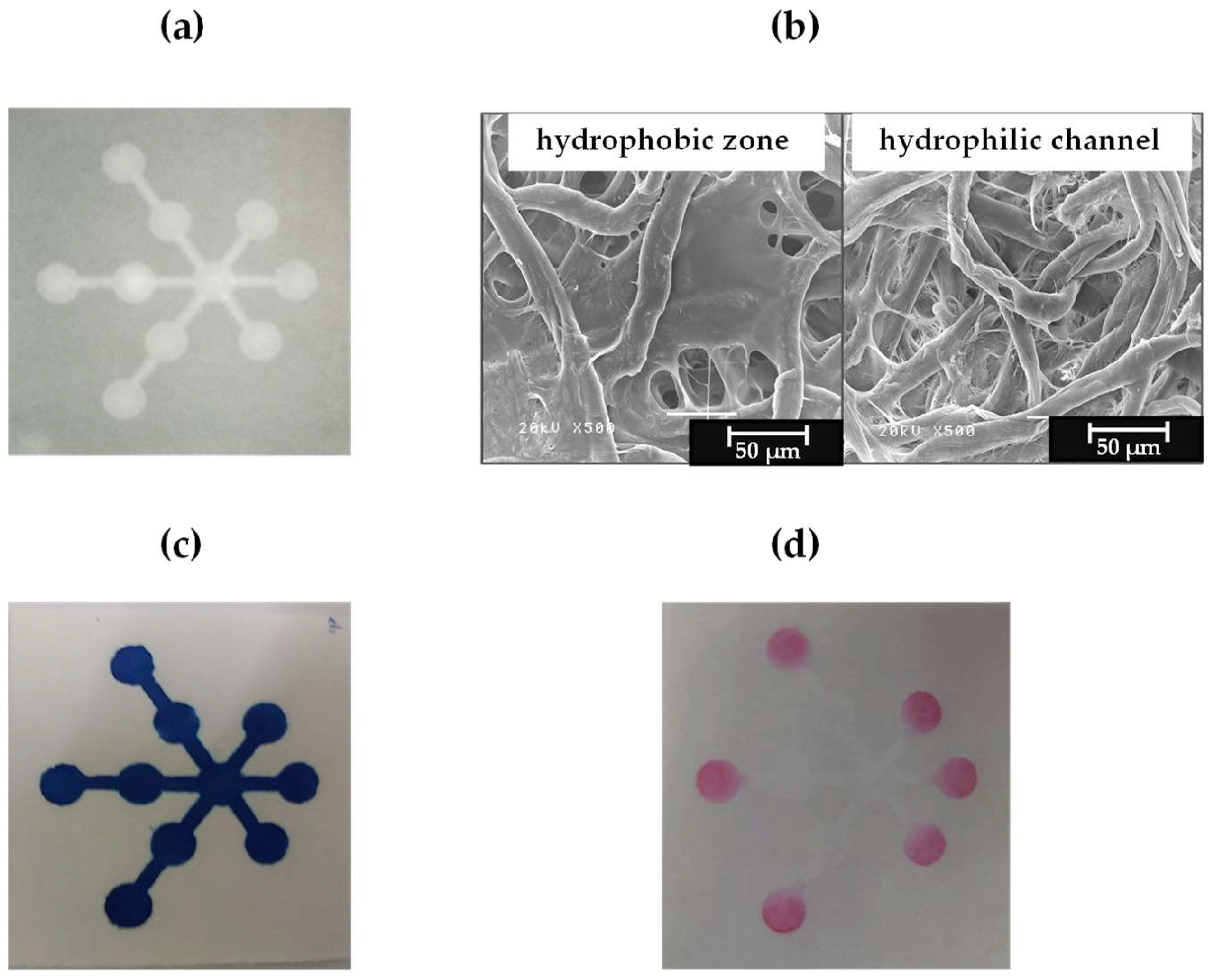
3.1. Fabrication of the µPAD Using the Single-Step Polylactic Acid Screen-Printing Method
3.2. Reproducibility and Stability of the Fabricated µPADs
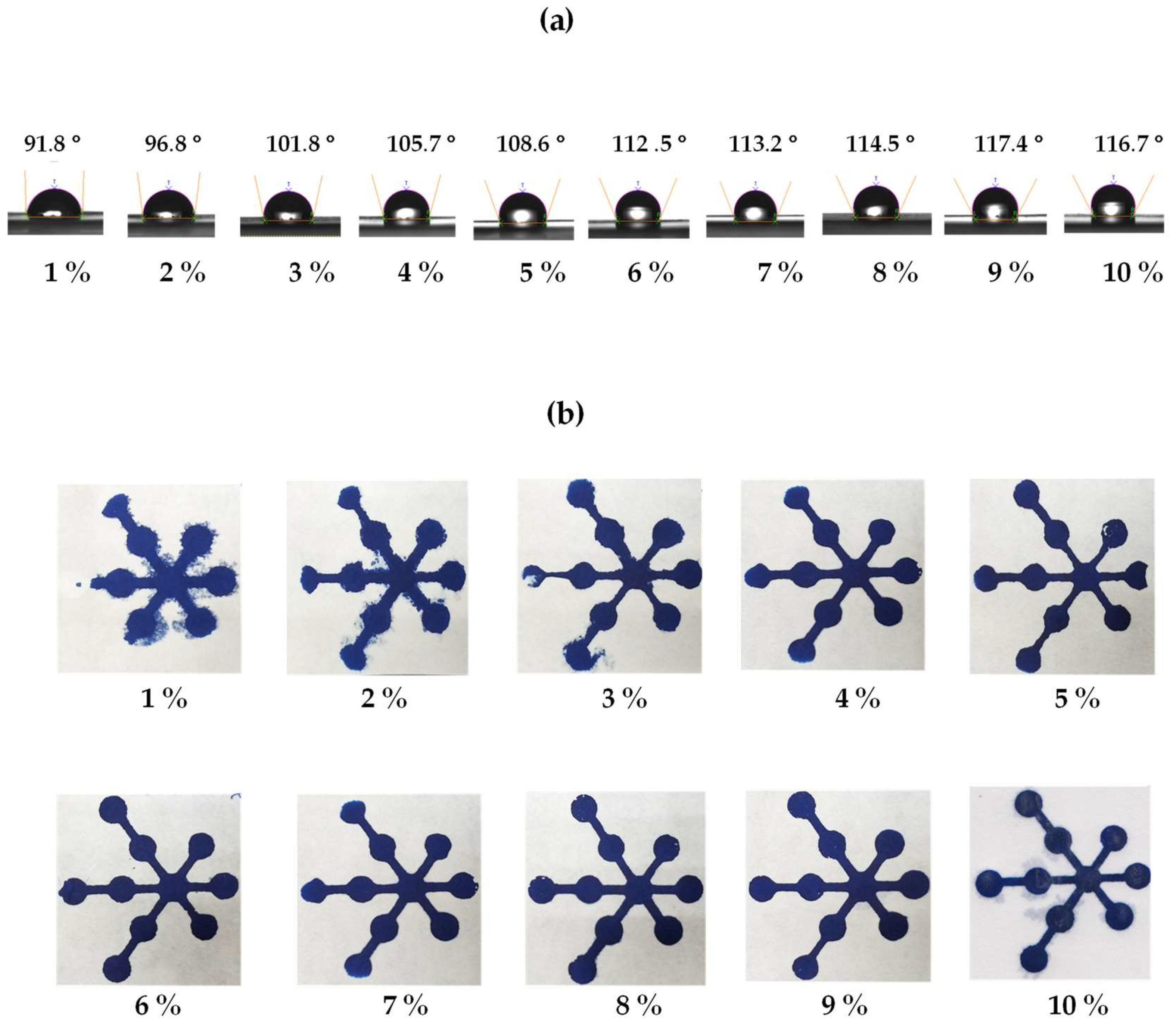
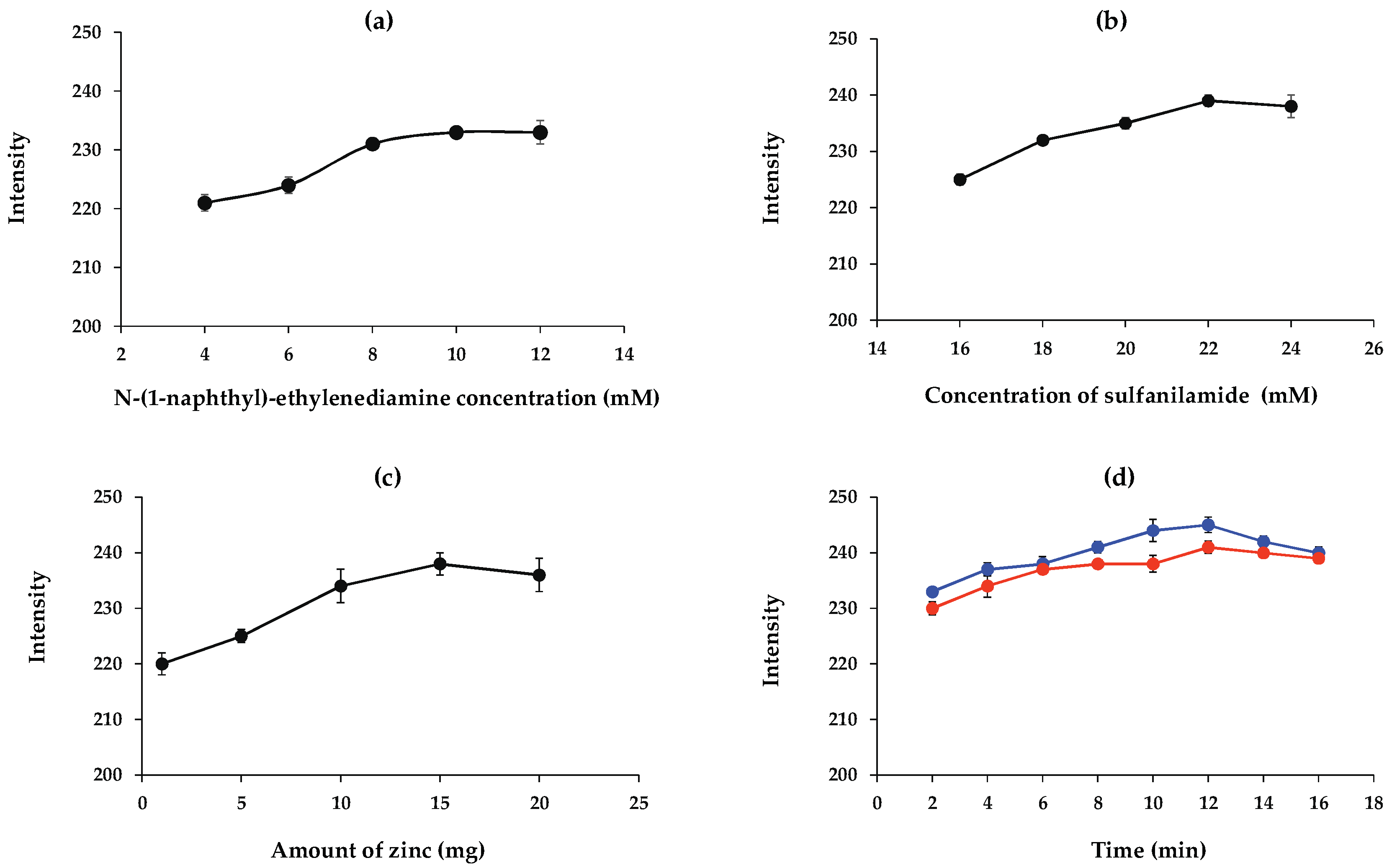
3.3. Optimization of the Polylactic Acid Concentration
3.4. Application for Nitrite and Nitrate Detection
3.5. Optimization of the Nitrite and Nitrate Assays
3.6. Method Validation
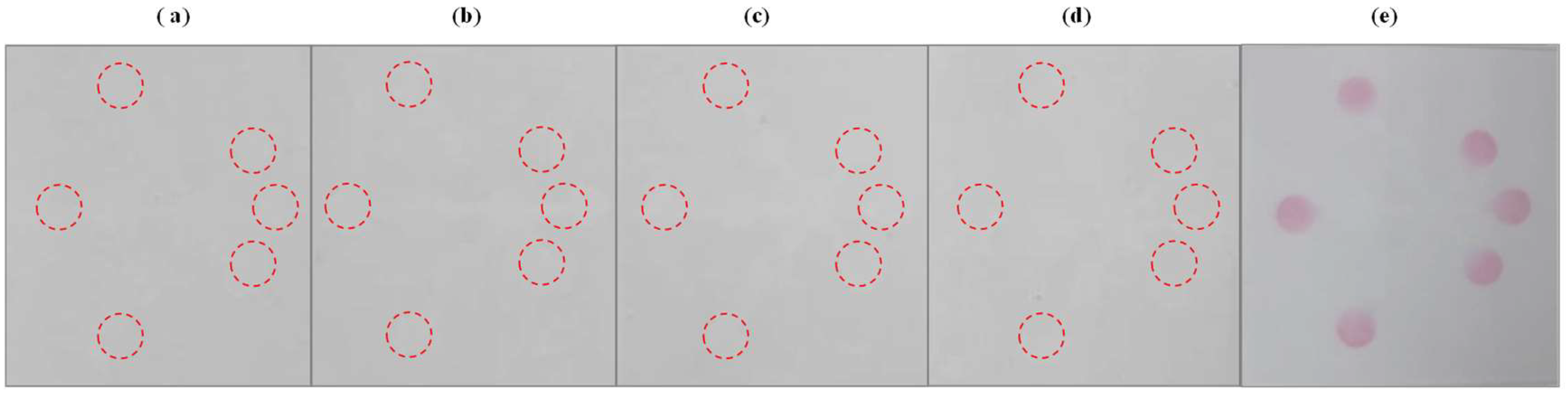
3.7. Selectivity of the µPADs
3.8. Analysis of Nitrite and Nitrate in Food Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Almeida, M.I.G.S.; Jayawardane, B.M.; Kolev, S.D.; McKelvie, I.D. Developments of microfluidic paper-based analytical devices (μPADs) for water analysis: A review. Talanta 2018, 177, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Akyazi, T.; Basabe-Desmonts, L.; Benito-Lopez, F. Review on microfluidic paper-based analytical devices towards commercialization. Anal. Chim. Acta 2018, 1001, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.M.; Wang, Y.N. Detection methods and applications of microfluidic paper-based analytical devices. Trends Anal. Chem. 2018, 107, 196–211. [Google Scholar] [CrossRef]
- Morbioli, G.G.; Mazzu-Nascimento, T.; Stockton, A.M.; Carrilho, E. Technical aspects and challenges of colorimetric detection with microfluidic paper-based analytical devices (μPADs)—A review. Anal. Chim. Acta 2017, 970, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Gritsenko, D.; Feng, S.; The, Y.C.; Lu, X.; Xu, J. Detection of heavy metal by paper-based microfluidics. Biosens. Bioelectron. 2016, 83, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Ruecha, N.; Shin, K.; Chailapakul, O.; Rodthongkum, N. Label-free paper-based electrochemical impedance immunosensor for human interferon gamma detection. Sens. Actuators B Chem. 2019, 279, 298–304. [Google Scholar] [CrossRef]
- Liu, C.; Gomez, F.A.; Miao, A.Y.; Cui, P.; Lee, W. A colorimetric assay system for dopamine using microfluidic paper-based analytical devices. Talanta 2019, 194, 171–176. [Google Scholar] [CrossRef]
- Yang, R.J.; Tseng, C.C.; Ju, W.J.; Fu, L.M.; Syu, M.P. Integrated microfluidic paper-based system for determination of whole blood albumin. Sens. Actuators B Chem. 2018, 273, 1091–1097. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Liu, L.; Wu, X.; Kuang, H.; Xu, C.; Xu, L. A colorimetric paper-based sensor for toltrazuril and its metabolites in feed, chicken, and egg samples. Food Chem. 2019, 276, 707–713. [Google Scholar] [CrossRef]
- Liu, C.C.; Wang, Y.N.; Fu, L.M.; Chen, K.L. Microfluidic paper-based chip platform for benzoic acid detection in food. Food Chem. 2018, 249, 162–167. [Google Scholar] [CrossRef]
- Ma, L.; Nilghaz, A.; Choi, J.R.; Liu, X.; Lu, X. Rapid detection of clenbuterol in milk using microfluidic paper-based ELISA. Food Chem. 2018, 246, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Saraji, M.; Bagheri, N. Paper-based headspace extraction combined with digital image analysis for trace determination of cyanide in water samples. Sens. Actuators B Chem. 2018, 270, 28–34. [Google Scholar] [CrossRef]
- Kumar, S.B.; Shinde, A.H.; Meht, R.; Bhattachary, A. Simple, one-step dye-based kit for bacterial contamination detection in a range of water sources. Sens. Actuators B Chem. 2018, 276, 121–127. [Google Scholar] [CrossRef]
- Devadhasan, J.P.; Kim, J. A chemically functionalized paper-based microfluidic platform for multiplex heavy metal detection. Sens. Actuators B Chem. 2018, 273, 18–24. [Google Scholar] [CrossRef]
- Asano, H.; Shiraishi, Y. Development of paper-based microfluidic analytical device for iron assay using photomask printed with 3D printer for fabrication of hydrophilic and hydrophobic zones on paper by photolithography. Anal. Chim. Acta 2015, 883, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Fang, C.; Zeng, R.; Zhao, X.; Jiang, Y.; Chen, Z. Paper-based microfluidic devices for electrochemical immunofiltration analysis of human chorionic gonadotropin. Biosens. Bioelectron. 2017, 92, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Bretel, G.; Rull-Barrull, J.; Nongbe, M.C.; Terrier, J.P.; Grognec, E.L.; Felpin, F.X. Hydrophobic Covalent Patterns on Cellulose Paper through Photothiol-X Ligations. ACS Omega 2018, 3, 9155–9159. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, H.; Liu, W.; Guo, Y.; Chu, W. Plasma treatment of paper for protein immobilization on paper-based chemiluminescence immunodevice. Biosens. Bioelectron. 2016, 79, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Yang, Y.; Henry, C.S. Laminated and infused Parafilm®–paper for paper-based analytical devices. Sens. Actuators B Chem. 2018, 255, 3654–3661. [Google Scholar] [CrossRef]
- Garcia, P.T.; Cardoso, T.M.G.; Garcia, C.D.; Carrilho, E.; Coltro, W.K.T. A handheld stamping process to fabricate microfluidic paper-based analytical devices with chemically modified surface for clinical assays. RSC Adv. 2014, 4, 37637–37644. [Google Scholar] [CrossRef]
- Liang, W.H.; Chu, C.H.; Yang, R.J. Bio-sample detection on paper-based devices with inkjet printer-sprayed reagents. Talanta 2015, 145, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Henares, T.G.; Suzuki, K.; Citterio, D. Paper-Based Inkjet-Printed Microfluidic Analytical Devices. Angew. Chem. Int. Ed. 2015, 54, 5294–5310. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.K.; Kurniawan, A.; Kaoa, C.Y.; Wang, M.J. Single step and mask-free 3D wax printing of microfluidic paper-based analytical devices for glucose and nitrite assays. Talanta 2019, 194, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ge, L.; Song, X.; Yu, J.; Ge, S.; Huang, J.; Zeng, F. Paper-based chemiluminescence ELISA: Lab-on-paper based on chitosan modified paper device and wax-screen-printing. Biosens. Bioelectron. 2012, 31, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Lamas-Ardisana, P.J.; Martínez-Paredes, G.; Añorga, L.; Grande, H.J. Glucose biosensor based on disposable electrochemical paper-basedtransducers fully fabricated by screen-printing. Biosens. Bioelectron. 2018, 109, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Maeki, M.; Mohamadi, R.M.; Ishidkihiko, A.; Tani, H.; Tokeshi, M. An instrument-free, screen-printed paper microfluidic device that enables bio and chemical sensing. Analyst 2015, 140, 6493–6499. [Google Scholar] [CrossRef]
- Konarsu, T.; Maeki, M.; Ishida, A.; Tani, H.; Tokeshi, M. Characteristics of microfluidic paper-based analytical devices fabricated by four different method. Anal. Sci. 2018, 34, 39–44. [Google Scholar]
- Sameenoi, Y.; Nongka, P.N.; Nouanthavong, S.; Henry, C.S.; Nacapricha, D. One-step polymer screen-printing for microfluidic paper-based analytical device (µPAD) fabrication. Analyst 2014, 139, 6580–6588. [Google Scholar] [CrossRef]
- Bruzewicz, D.A.; Reches, M.; Whitesides, G.M. Low-Cost Printing of Poly(dimethylsiloxane) Barriers to Define Microchannels in Paper. Anal. Chem. 2008, 80, 3387–3392. [Google Scholar] [CrossRef]
- Yew, C.H.T.; Azari, P.; Choi, J.R.; Li, F.; Pingguan-Murphy, B. Electrospin-coating of nitrocellulose membrane enhances sensitivity in nucleic acid-based lateral flow assay. Anal. Chim. Acta 2018, 1009, 81–88. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Ayyoob, M.; Joo, J.; Deri, F. Polylactic acid blends: The future of green, light and tough. Prog. Polym. Sci. 2018, 85, 83–127. [Google Scholar] [CrossRef]
- Hussain, T.; Tausif, M.; Ashraf, M. Review: A review of progress in the dyeing of eco-friendly aliphatic polyester-based polylactic acid fabrics. J. Clean. Prod. 2015, 108, 476–483. [Google Scholar] [CrossRef]
- Cammack, R.; Joannou, C.L.; Cui, X.Y.; Martinez, C.T.; Maraj, S.R.; Hughes, M.N. Nitrite and nitrosyl compounds in food preservation. Biochim. Biophys. Acta 1999, 1411, 475–488. [Google Scholar] [CrossRef]
- Morton, I.D. Nitrate and nitrite in food. Nutr. Food Sci. 1975, 75, 8–9. [Google Scholar] [CrossRef]
- Hospital, X.; Hierro, F.; Fernández, M. Effect of reducing nitrate and nitrite added to dry fermented sausages on the survival of Salmonella Typhimurium. Food Res. Int. 2014, 62, 410–415. [Google Scholar] [CrossRef]
- Azanza, P.V.; Rustia, A.S. Residual nitrite levels in Philippine sweet bacon. Food Control 2004, 15, 385–389. [Google Scholar] [CrossRef]
- Suzuki, H.; Iijima, K.; Moriya, A.; McElroy, K.; Scobie, G.; Fyfe, V.; McColl, K. Conditions for acid catalysed luminal nitrosation are maximal at the gastric cardia. Gut 2003, 52, 1059–1101. [Google Scholar] [CrossRef]
- Jayawardane, M.B.; Wei, S.; McKelvie, I.D.; Kolev, S.D. Microfluidic paper-based analytical device for the determination of nitrite and nitrate. Anal. Chem. 2014, 86, 7274–7279. [Google Scholar] [CrossRef]
- Iverson, N.M.; Hofferber, E.M.; Stapleton, J.A. Nitric Oxide Sensors for Biological Applications. Chemosensors 2018, 6, 8. [Google Scholar] [CrossRef]
- Ellis, P.S.; Shabani, A.M.H.; Gentle, B.S.; McKelvie, I.D. Field measurement of nitrate in marine and estuarine waters with a flow analysis system utilizing on-line zinc reduction. Talanta 2011, 84, 98–103. [Google Scholar] [CrossRef]
- Ridnour, L.A.; Sim, J.E.; Hayward, M.A.; Wink, D.A.; Martin, S.M.; Buettner, G.R.; Spitz, D.R. A Spectrophotometric Method for the Direct Detection and Quantitation of Nitric Oxide, Nitrite, and Nitrate in Cell Culture Media. Anal. Biochem. 2000, 281, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Law, K.Y. Definitions for Hydrophilicity, Hydrophobicity, and Superhydrophobicity: Getting the Basics Right. J. Phys. Chem. Lett. 2014, 54, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Kunduru, K.R.; Basu, A.; Tsah, T.; Domb, A.J. Polymer with pendant diazo-coupling functionality for colorimetric detection of nitrates. Sens. Actuators B Chem. 2017, 251, 21–26. [Google Scholar] [CrossRef]


| Parameter | Nitrite Detection | Nitrate Detection |
|---|---|---|
| Intra-day precision (% RSD) | 0.3 | 0.6 |
| Inter-day precision (% RSD) | 0.7 | 0.9 |
| Linear range | 2–10 mg L−1 | 10–50 mg L−1 |
| LOD | 1.2 mg L−1 | 3.6 mg L−1 |
| LOQ | 4 mg L−1 | 12 mg L−1 |
| Analysis time | 12 min | 12 min |
| Food Samples | Nitrite Concentration (mg kg−1) | Nitrate Concentration (mg kg−1) | ||
|---|---|---|---|---|
| µPAD | Spectrophotometric Method | µPAD | Spectrophotometric Method | |
| Smoked sausage | 13 ± 0.1 | 13 ± 0.6 | 63 ± 0.1 | 63 ± 0.5 |
| Chicken sausage | 12 ± 0.2 | 11 ± 0.4 | 91 ± 0.1 | 92 ± 0.6 |
| Fish ball | 23 ± 0.2 | 22 ± 0.7 | 76 ± 0.6 | 75 ± 0.8 |
| Meatballs | 26 ± 0.3 | 27 ± 0.2 | 78 ± 0.1 | 76 ± 0.4 |
| Sour pork | 11 ± 0.5 | 11 ± 0.8 | 83 ± 0.8 | 83 ± 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teepoo, S.; Arsawiset, S.; Chanayota, P. One-Step Polylactic Acid Screen-Printing Microfluidic Paper-Based Analytical Device: Application for Simultaneous Detection of Nitrite and Nitrate in Food Samples. Chemosensors 2019, 7, 44. https://doi.org/10.3390/chemosensors7030044
Teepoo S, Arsawiset S, Chanayota P. One-Step Polylactic Acid Screen-Printing Microfluidic Paper-Based Analytical Device: Application for Simultaneous Detection of Nitrite and Nitrate in Food Samples. Chemosensors. 2019; 7(3):44. https://doi.org/10.3390/chemosensors7030044
Chicago/Turabian StyleTeepoo, Siriwan, Supattra Arsawiset, and Pitchayatida Chanayota. 2019. "One-Step Polylactic Acid Screen-Printing Microfluidic Paper-Based Analytical Device: Application for Simultaneous Detection of Nitrite and Nitrate in Food Samples" Chemosensors 7, no. 3: 44. https://doi.org/10.3390/chemosensors7030044
APA StyleTeepoo, S., Arsawiset, S., & Chanayota, P. (2019). One-Step Polylactic Acid Screen-Printing Microfluidic Paper-Based Analytical Device: Application for Simultaneous Detection of Nitrite and Nitrate in Food Samples. Chemosensors, 7(3), 44. https://doi.org/10.3390/chemosensors7030044