An Organophosphorus(III)-Selective Chemodosimeter for the Ratiometric Electrochemical Detection of Phosphines
Abstract
1. Introduction
2. Materials and Methods
3. Results
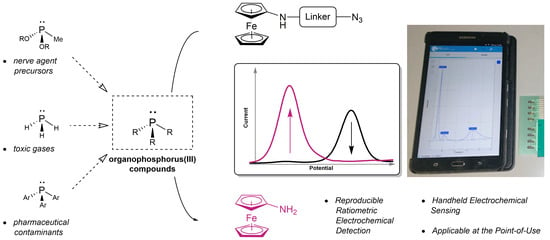
3.1. Concept
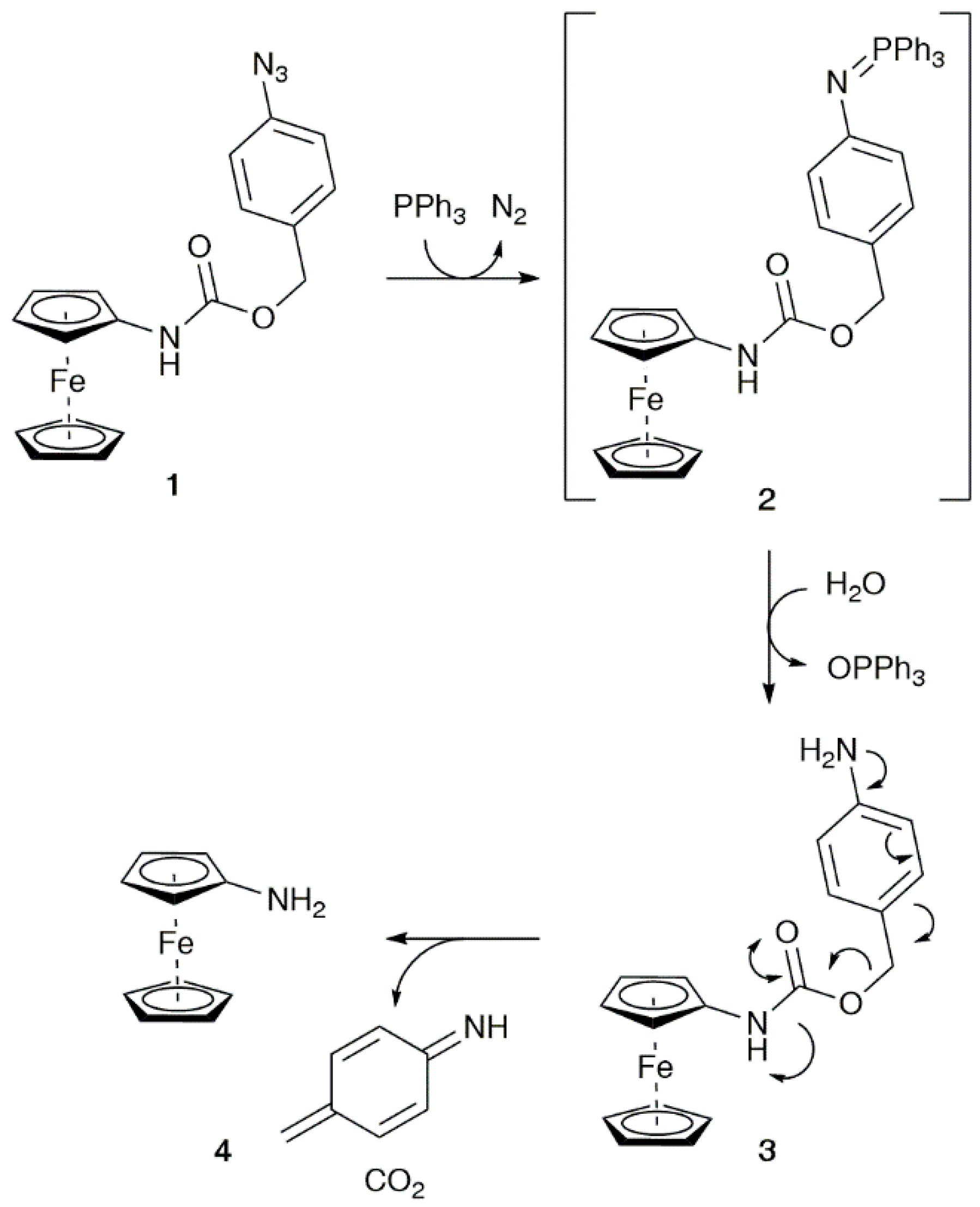
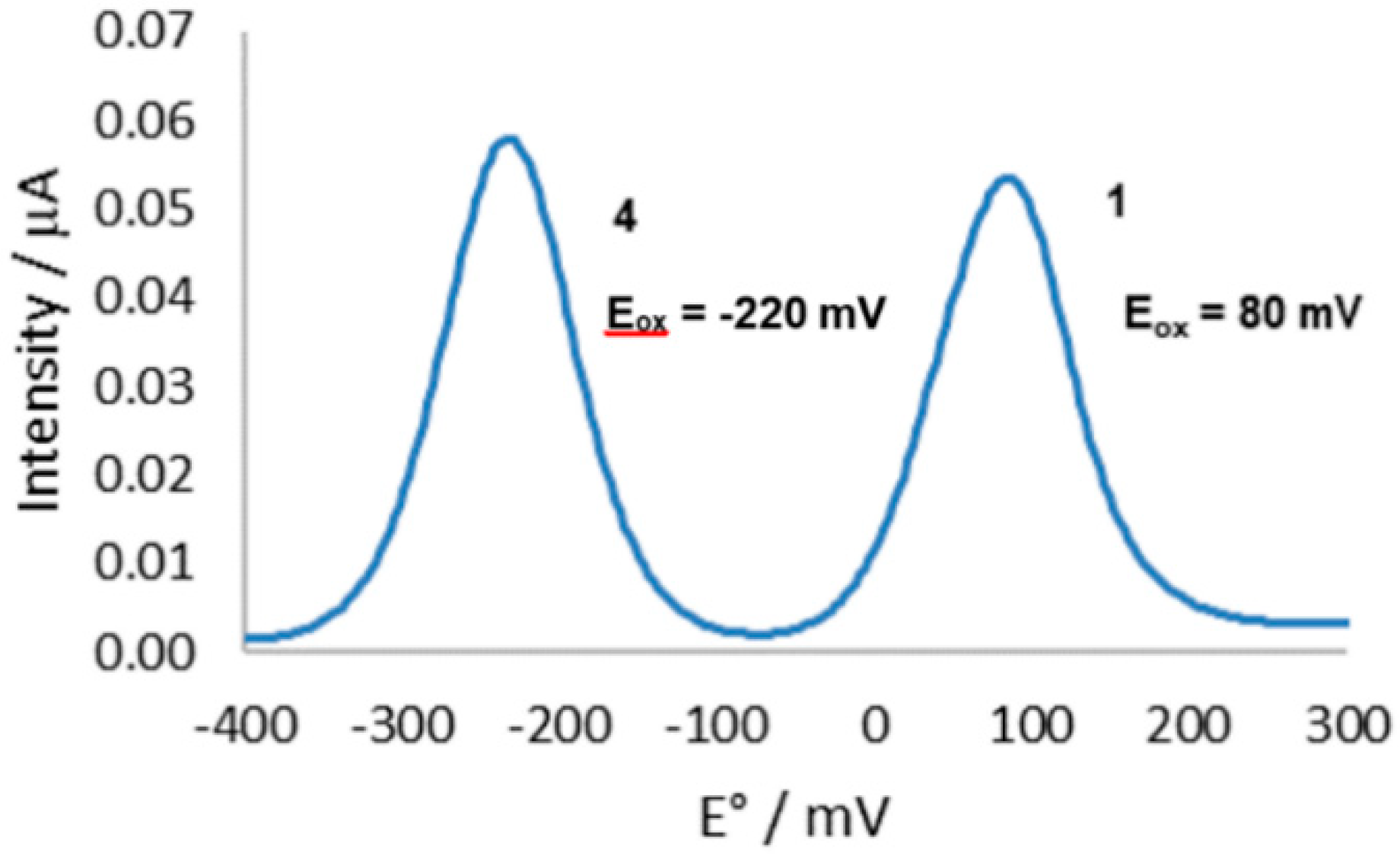
3.2. Synthesis, Stability, and Electrochemical Properties of 1
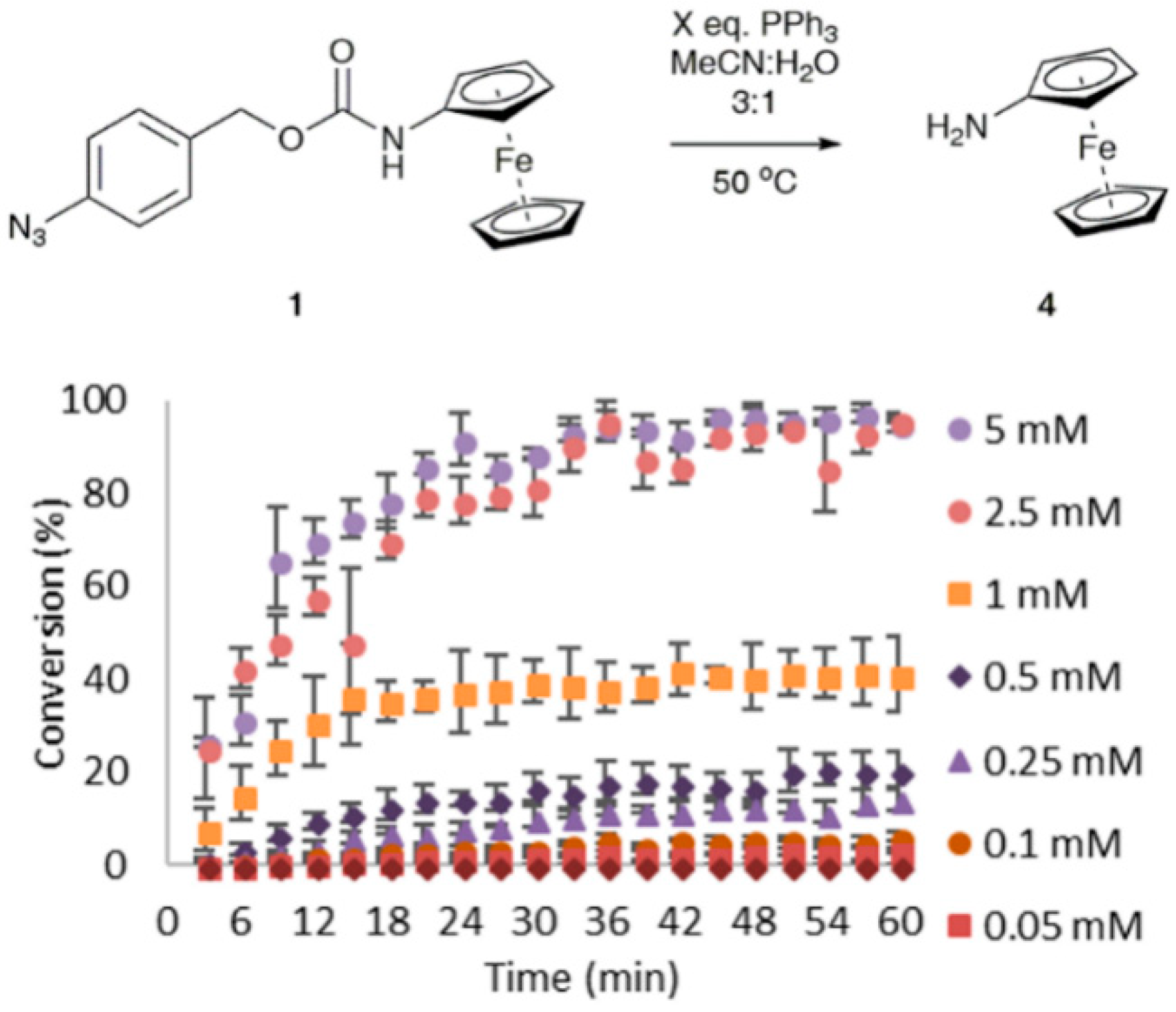
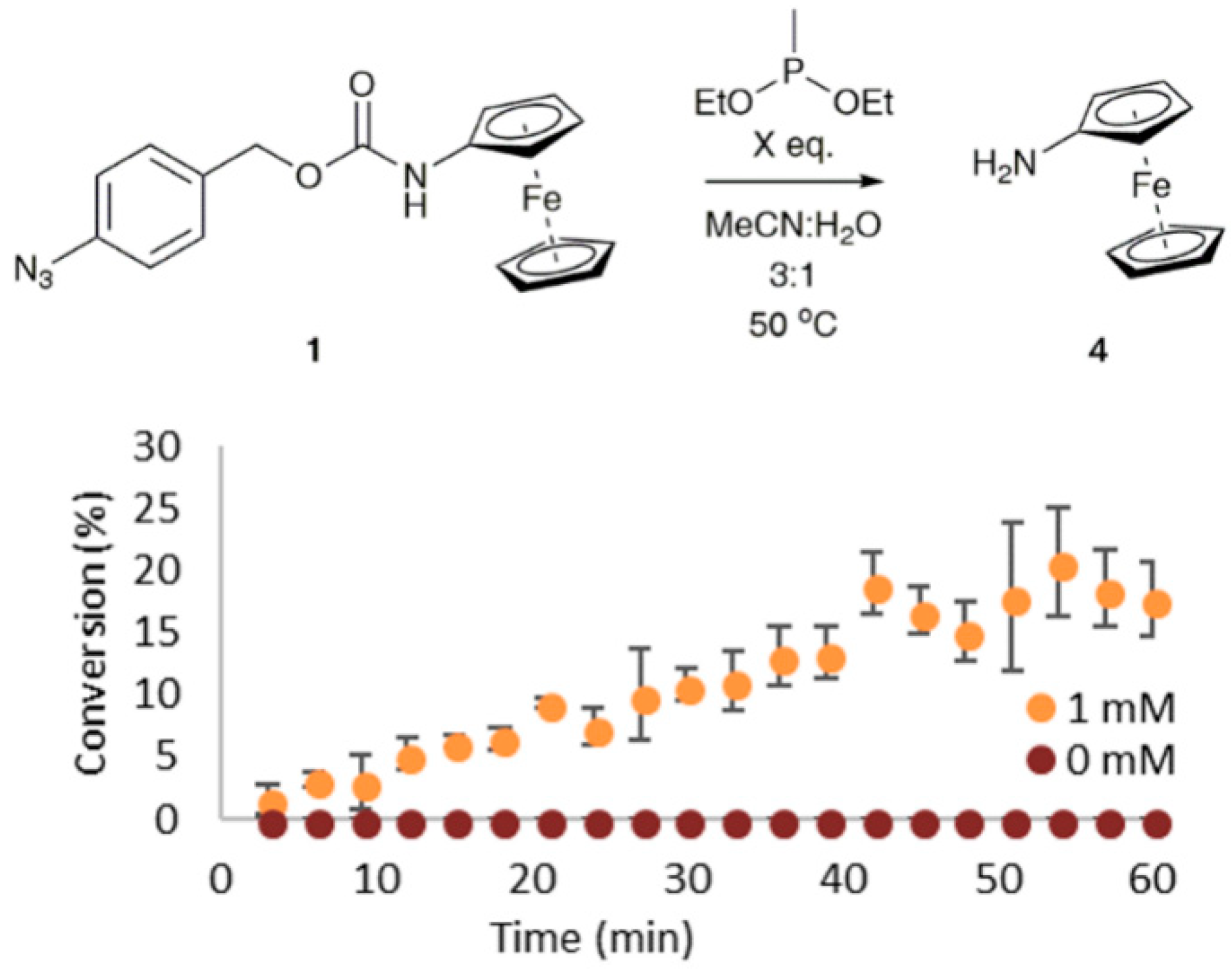
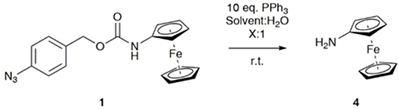
3.3. Sensitivity and Selectivity of 1 towards Organophosphorus(III) Compounds
3.4. Applications towards a Point-of-Use Assay with a Handheld Potentiostat
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chaudhry, M.Q. A Review of the Mechanisms Involved in the Action of Phosphine as an Insecticide and Phosphine Resistance in Stored-Product Insects. Pestic. Sci. 1997, 49, 213–228. [Google Scholar] [CrossRef]
- Nath, N.S.; Bhattacharya, I.; Tuck, A.G.; Schlipalius, D.I.; Ebert, P.R. Mechanisms of Phosphine Toxicity. J. Toxicol. 2011, 2011, 494168. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, M.O.; Macnicol, A.D.; Price, N.R. Alkylphosphines as pesticidal agents. U.S. Patent 6,096,330, 1 August 2000. [Google Scholar]
- Waritz, R.S.; Brown, R.M. Acute and Subacute Inhalation Toxicities of Phosphine, Phenylphosphine and Triphenylphosphine. Am. Ind. Hyg. Assoc. J. 1975, 36, 452–458. [Google Scholar] [CrossRef]
- Bercu, J.P.; Galloway, S.M.; Parris, P.; Teasdale, A.; Masuda-Herrera, M.; Dobo, K.; Heard, P.; Kenyon, M.; Nicolette, J.; Vock, E.; et al. Potential impurities in drug substances: Compound-specific toxicology limits for 20 synthetic reagents and by-products, and a class-specific toxicology limit for alkyl bromides. Regul. Toxicol. Pharmacol. 2018, 94, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.G.; Boström, J. Analysis of Past and Present Synthetic Methodologies on Medicinal Chemistry: Where Have All the New Reactions Gone? J. Med. Chem. 2016, 59, 4443–4458. [Google Scholar] [CrossRef]
- King, A.O.; Yasuda, N. Organometallics in Process Chemistry; Springer: Berlin/Heidelberg, Germany, 2004; pp. 205–245. ISBN 978-3-540-01603-8. [Google Scholar]
- Chasteen, T.G.; Fall, R.; Birks, J.W.; Martin, H.R.; Glinski, R.J. Fluorine-induced chemiluminescence detection of phosphine, alkyl phosphines and monophosphinate esters. Chromatographia 1991, 31, 342–346. [Google Scholar] [CrossRef]
- Norman, K.N.T.; Leonard, K. Gas Chromatography−Mass Spectrometry Determination of Phosphine Residues in Stored Products and Processed Foods. J. Agric. Food Chem. 2000, 48, 4066–4070. [Google Scholar] [CrossRef] [PubMed]
- Fahrenholtz, S.; Hühnerfuss, H.; Baur, X.; Budnik, L.T. Determination of phosphine and other fumigants in air samples by thermal desorption and 2D heart-cutting gas chromatography with synchronous SIM/Scan mass spectrometry and flame photometric detection. J. Chromatogr. A 2010, 1217, 8298–8307. [Google Scholar] [CrossRef]
- Sun, X.; Dahlhauser, S.D.; Anslyn, E.V. New Autoinductive Cascade for the Optical Sensing of Fluoride: Application in the Detection of Phosphoryl Fluoride Nerve Agents. J. Am. Chem. Soc. 2017, 139, 4635–4638. [Google Scholar] [CrossRef]
- Sun, X.; Boulgakov, A.A.; Smith, L.N.; Metola, P.; Marcotte, E.M.; Anslyn, E.V. Photography Coupled with Self-Propagating Chemical Cascades: Differentiation and Quantitation of G- and V-Nerve Agent Mimics via Chromaticity. ACS Cent. Sci. 2018, 4, 854–861. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical biosensors: Towards point-of-care cancer diagnostics. Biosens. Bioelectron. 2006, 21, 1887–1892. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef]
- Du, Y.; Lim, B.J.; Li, B.; Jiang, Y.S.; Sessler, J.L.; Ellington, A.D. Reagentless, Ratiometric Electrochemical DNA Sensors with Improved Robustness and Reproducibility. Anal. Chem. 2014, 86, 8010–8016. [Google Scholar] [CrossRef]
- Jin, H.; Gui, R.; Yu, J.; Lv, W.; Wang, Z. Fabrication strategies, sensing modes and analytical applications of ratiometric electrochemical biosensors. Biosens. Bioelectron. 2017, 91, 523–537. [Google Scholar] [CrossRef]
- Beer, P.D. Redox responsive macrocyclic receptor molecules containing transition metal redox centres. Chem. Soc. Rev. 1989, 18, 409–450. [Google Scholar] [CrossRef]
- Beer, P.D.; Gale, P.A.; Chen, G.Z. Mechanisms of electrochemical recognition of cations, anions and neutral guest species by redox-active receptor molecules. Coord. Chem. Rev. 1999, 185, 3–36. [Google Scholar] [CrossRef]
- Kang, D.; Ricci, F.; White, R.J.; Plaxco, K.W. Survey of Redox-Active Moieties for Application in Multiplexed Electrochemical Biosensors. Anal. Chem. 2016, 88, 10452–10458. [Google Scholar] [CrossRef]
- van Staveren, D.R.; Metzler-Nolte, N. Bioorganometallic Chemistry of Ferrocene. Chem. Rev. 2004, 104, 5931–5986. [Google Scholar] [CrossRef]
- Marsh, B.J.; Hampton, L.; Goggins, S.; Frost, C.G. Fine-tuning of ferrocene redox potentials towards multiplex DNA detection. New J. Chem. 2014, 38, 5260–5263. [Google Scholar] [CrossRef]
- Lim, B.J.; Hwang, I.; Ellington, A.D.; Sessler, J.L. Synthesis of Ferrocene Derivatives Allowing Linear Free Energy Studies of Redox Potentials. Helv. Chim. Acta 2018, 102, e1800186. [Google Scholar] [CrossRef]
- Amir, R.J.; Pessah, N.; Shamis, M.; Shabat, D. “Cascade-Release Dendrimers” Liberate All End Groups upon a Single Triggering Event in the Dendritic Core. Angew. Chem. Int. Ed. 2003, 42, 4494–4499. [Google Scholar] [CrossRef]
- Sella, E.; Shabat, D. Dendritic Chain Reaction. J. Am. Chem. Soc. 2009, 131, 9934–9936. [Google Scholar] [CrossRef]
- Spring, S.A.; Goggins, S.; Frost, C.G. Ratiometric electrochemical detection of β-galactosidase. Org. Biomol. Chem. 2017, 15, 7122–7126. [Google Scholar] [CrossRef]
- Goggins, S.; Naz, C.; Marsh, B.J.; Frost, C.G. Ratiometric electrochemical detection of alkaline phosphatase. Chem. Commun. 2015, 51, 561–564. [Google Scholar] [CrossRef]
- Manibalan, K.; Mani, V.; Huang, S.T. A switchable electrochemical redox ratiometric substrate based on ferrocene for highly selective and sensitive fluoride detection. RSC Adv. 2016, 6, 71727–71732. [Google Scholar] [CrossRef]
- Goggins, S.; Apsey, E.A.; Mahon, M.F.; Frost, C.G. Ratiometric electrochemical detection of hydrogen peroxide and glucose. Org. Biomol. Chem. 2017, 15, 2459–2466. [Google Scholar] [CrossRef]
- Kinski, E.; Marzenell, P.; Hofer, W.; Hagen, H.; Raskatov, J.A.; Knaup, K.X.; Zolnhofer, E.M.; Meyer, K.; Mokhir, A. 4-Azidobenzyl ferrocenylcarbamate as an anticancer prodrug activated under reductive conditions. J. Inorg. Biochem. 2016, 160, 218–224. [Google Scholar] [CrossRef]
- Dou, X.; Chu, X.; Kong, W.; Luo, J.; Yang, M. A gold-based nanobeacon probe for fluorescence sensing of organophosphorus pesticides. Anal. Chim. Acta 2015, 891, 291–297. [Google Scholar] [CrossRef]
- Leffler, J.E.; Temple, R.D. Staudinger reaction between triarylphosphines and azides. Mechanism. J. Am. Chem. Soc. 1967, 89, 5235–5246. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.; Li, H.; Pei, Z.; Sun, S.; Xu, Y. A simple excited-state intramolecular proton transfer probe based on a new strategy of thiol–azide reaction for the selective sensing of cysteine and glutathione. Chem. Commun. 2016, 52, 749–752. [Google Scholar] [CrossRef]
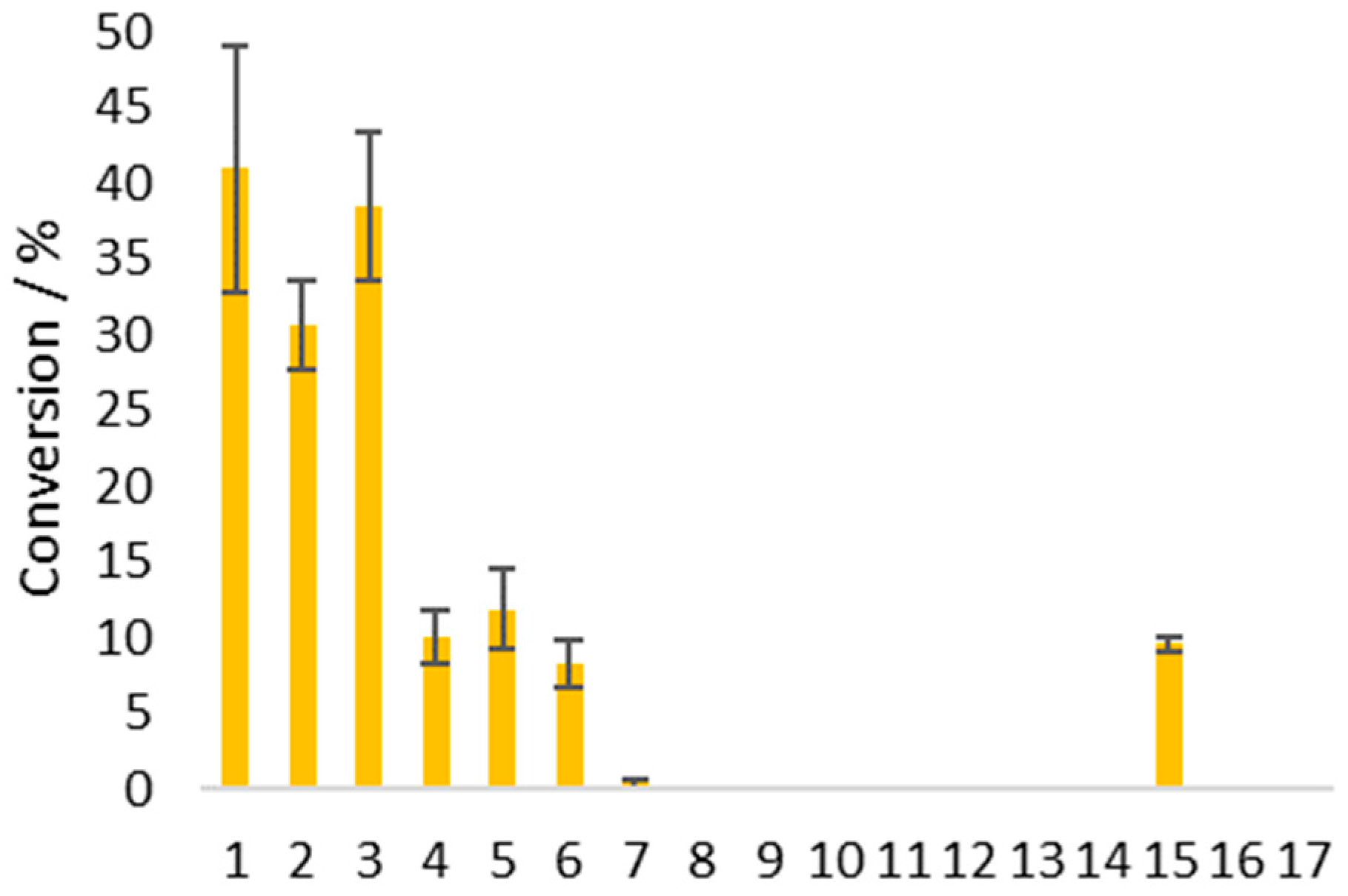
| Exp. No. | Solvent (1 M) | Solvent:Water Ratio | Conversion 1 |
|---|---|---|---|
| 1 | N,N-dimethylformamide (DMF) | 1:1 | 37% |
| 2 | 1,4-dioxane | 1:1 | 78% |
| 3 | acetonitrile (MeCN) | 1:1 | 79% |
| 4 | MeCN | 3:1 | 79% |
| 5 2 | MeCN | 3:1 | 43% |
| 6 3 | MeCN | 3:1 | 96% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spring, S.A.; Goggins, S.; Frost, C.G. An Organophosphorus(III)-Selective Chemodosimeter for the Ratiometric Electrochemical Detection of Phosphines. Chemosensors 2019, 7, 19. https://doi.org/10.3390/chemosensors7020019
Spring SA, Goggins S, Frost CG. An Organophosphorus(III)-Selective Chemodosimeter for the Ratiometric Electrochemical Detection of Phosphines. Chemosensors. 2019; 7(2):19. https://doi.org/10.3390/chemosensors7020019
Chicago/Turabian StyleSpring, Sam A., Sean Goggins, and Christopher G. Frost. 2019. "An Organophosphorus(III)-Selective Chemodosimeter for the Ratiometric Electrochemical Detection of Phosphines" Chemosensors 7, no. 2: 19. https://doi.org/10.3390/chemosensors7020019
APA StyleSpring, S. A., Goggins, S., & Frost, C. G. (2019). An Organophosphorus(III)-Selective Chemodosimeter for the Ratiometric Electrochemical Detection of Phosphines. Chemosensors, 7(2), 19. https://doi.org/10.3390/chemosensors7020019