Lifetime and Fluorescence Quantum Yield of Two Fluorescein-Amino Acid-Based Compounds in Different Organic Solvents and Gold Colloidal Suspensions
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Burchak, O.N.; Mugherli, L.; Chatelain, F.; Balakirev, M.Y. Fluorescein-Based Amino Acids for Solid Phase Synthesis of Fluorogenic Protease Substrates. Bioorg. Med. Chem. 2006, 14, 2559–2568. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.; Bértolo, E.; Núñez, C.; Pilla, V.; Santos, H.M.; Fernández-Lodeiro, J.; Fernández-Lodeiro, A.; Djafari, J.; Capelo, J.L.; Lodeiro, C. Green and Red Fluorescent Dyes for Translational Applications in Imaging and Sensing Analytes: A Dual-Color Flag. ChemistryOpen 2018, 7, 9–52. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Song, F.; Wang, J.; Zhang, Y.; Xue, Y.; Sun, L.; Jiang, N.; Gao, P.; Tian, L.; Peng, X. Thermally Activated Delayed Fluorescence of Fluorescein Derivative for Time-Resolved and Confocal Fluorescence Imaging. J. Am. Chem. Soc. 2014, 136, 9590–9597. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhan, X.Q.; Bian, Q.N.; Zhang, X.J. Advances in Modifying Fluorescein and Rhodamine Fluorophores as Fluorescent Chemosensors. Chem. Commun. 2013, 49, 429–447. [Google Scholar] [CrossRef] [PubMed]
- Sjöback, R.; Nygren, J.; Kubista, M. Absorption and Fluorescence Properties of Fluorescein. Spectrochim. Acta A 1995, 51, L7–L21. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhang, J.; Liu, L. Fluorescence Properties of Twenty Fluorescein Derivatives: Lifetime, Quantum Yield, Absorption and Emission Spectra. J. Fluoresc. 2014, 24, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Barba-Bom, A.; Costero, A.M.; Gil, S.; Parra, M.; Soto, J.; Martínez-Máñez, R.; Sancenón, F. A New Selective Fluorogenic Probe for Trivalent Cations. Chem. Commun. 2012, 48, 3000–3002. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Zhang, J.; Zhang, M.; Shen, T.; Tang, J. Spectral Properties and Structure of Fluorescein and its Alkyl Derivatives in Micelles. Colloids Surf. A Physicochem. Eng. Asp. 2000, 167, 253–262. [Google Scholar] [CrossRef]
- Martin, M.M.; Lindqvist, L. The pH Dependence of Fluorescein Fluorescence. J. Lumin. 1975, 10, 381–390. [Google Scholar] [CrossRef]
- Martin, E.; Pardo, A.; Guijarro, M.S.; Fernandez-Alonso, J.I. Photophysics Properties of Fluorescein in Alcoholic Medium for Different pH. J. Mol. Struct. 1986, 142, 197–200. [Google Scholar] [CrossRef]
- Zhao, Z.G.; Shen, T.; Xu, H.J. The Absorption and Structure of Fluorescein and its Ethyl Derivatives in Various Solutions. Spectrochim. Acta A 1989, 45, 1113–1116. [Google Scholar] [CrossRef]
- Song, A.; Wu, T.; Chen, S.; Zhang, M.; Shen, T. Syntheses and Photophysical Properties of Amphiphilic Dyads of Fluorescein and Carbazole Linked with a Flexible or Semi-rigid Bridge. Dyes Pigments 1998, 39, 371–382. [Google Scholar] [CrossRef]
- Martin, M.M. Hydrogen Bond Effects on Radiationless Electronic Transitions in Xanthene Dyes. Chem. Phys. Lett. 1975, 35, 105–111. [Google Scholar] [CrossRef]
- Klonis, N.; Clayton, A.H.A.; Voss, E.W., Jr.; Sawyer, W.H. Spectral Properties of Fluorescein in Solvent-Water Mixtures: Applications as a Probe of Hydrogen Bonding Environments in Biological Systems. Photochem. Photobiol. 1998, 67, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Mchedlov-Petrossyan, N.O.; Ivanov, V.V. Effect of the Solvent on the Absorption Spectra and Protonation of Fluorescein Dye Anions. Russ. J. Phys. Chem. A 2007, 81, 112–115. [Google Scholar] [CrossRef]
- Homocianu, M.; Airinei, A.; Dorohoi, D.O. Solvent Effects on the Electronic Absorption and Fluorescence Spectra. J. Adv. Res. Phys. 2017, 2, 011105. Available online: http://stoner.phys.uaic.ro/jarp/index.php?journal=jarp&page=article&op=view&path%5B%5D=30 (accessed on 8 May 2018).
- Magde, D.; Rojas, G.E.; Seybold, P.G. Solvent Dependence of the Fluorescence Lifetimes of Xanthene Dyes. Photochem. Photobiol. 1999, 70, 737–744. [Google Scholar] [CrossRef]
- Gidwani, M.S.; Menon, S.K.; Agrawal, Y.K. Fluorescence and Lasing Characteristics of Fluorescein Calix[4]aryl Hydroxamic Acid. Indian J. Chem. Technol. 2003, 10, 519–524. Available online: http://nopr.niscair.res.in/handle/123456789/22786 (accessed on 8 May 2018).
- Bindhu, C.V.; Harilal, S.S.; Nampoori, V.P.N.; Vallabhan, C.P.G. Solvent Effect on Absolute Fluorescence Quantum Yield of Rhodamine 6G Determined Using Transient Thermal Lens Technique. Mod. Phys. Lett. B 1999, 13, 563–576. [Google Scholar] [CrossRef]
- Sinha, S.; Ray, A.; Dasgupta, K. Solvent Dependent Nonlinear Refraction in Organic Dye Solution. J. Appl. Phys. 2000, 87, 3222–3226. [Google Scholar] [CrossRef]
- Magde, D.; Wong, R.; Seybold, P.G. Fluorescence Quantum Yields and Their Relation to Lifetimes of Rhodamine 6G and Fluorescein in Nine Solvents: Improved Absolute Standards for Quantum Yields. Photochem. Photobiol. 2002, 75, 327–334. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Pilla, V.; Oliveira, E.; Santos, S.M.; Capelo, J.L.; Dos Santos, A.A.; Lodeiro, C. The Interaction of Hg2+ and Trivalent Ions with Two New Fluorescein Bio-inspired Dual Colorimetric/Fluorimetric Probes. Dalton Trans. 2016, 45, 9513–9522. [Google Scholar] [CrossRef] [PubMed]
- Akshath, U.S.; Bhatt, P. Gold Nanoparticle Synthesis Coupled to Fluorescence Turn-on for Sensitive Detection of Formaldehyde using Formaldehyde Dehydrogenase. RSC Adv. 2016, 6, 54777–54784. [Google Scholar] [CrossRef]
- Yang, G.; Xiang, S.; Zhang, K.; Gao, D.; Zeng, C.; Zhao, F. Breast Cancer Imaging with Fluoresce in Isothiocyanate-Modified Gold Nanoparticles In-Vidro and In-Vivo. Int. J. Clin. Exp. Med. 2016, 9, 753–759. Available online: http://www.ijcem.com/V9_No2.html (accessed on 8 May 2018).
- Tao, H.; Liao, X.; Xu, M.; Xie, X.; Zhong, F.; Yi, Z. Detection of Immunoglobulin G based on Nanoparticle Surface Energy Transfers from Fluorescein Isothiocyanate to Gold Nanoparticles. Anal. Methods 2014, 6, 2560–2565. [Google Scholar] [CrossRef]
- Barnoy, E.A.; Fixler, D.; Popovtzer, R.; Nayhoz, T.; Ray, K. An Ultra-Sensitive Dual-Mode Imaging System Using Metal-Enhanced Fluorescence in Solid Phantoms. Nano Res. 2015, 8, 3912–3921. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, D.; Sun, J.; Yang, X. A Dual-Mode Signaling Response of a AuNP-Fluorescein Based Probe for Specific Detection of Thiourea. Analyst 2016, 141, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Sironi, L.; Freddi, S.; D’Alfonso, L.; Collini, M.; Gorletta, T.; Soddu, S.; Chirico, G. p53 Detection by Fluorescence Lifetime on a Hybrid Fluorescein Isothiocyanate Gold Nanosensor. J. Biomed. Nanotechnol. 2009, 5, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.K.; Li, K.; Qin, H.H.; Zhou, Q.; Qian, C.H.; Liu, Y.H.; Yu, X.Q. Construction of pH-Sensitive “Submarine” Based on Gold Nanoparticles with Double Insurance for Intracellular pH Mapping, Quantifying of Whole Cells and in Vivo Applications. ACS Appl. Mater. Interfaces 2016, 8, 22839–22848. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lodeiro, J.; Núñez, C.; Oliveira, E.; Capelo, J.L.; Lodeiro, C. 1D Chain Fluorescein-Functionalized Gold and Silver Nanoparticles as New Optical Mercury Chemosensor in Aqueous Media. J. Nanopart. Res. 2013, 15, 1828. [Google Scholar] [CrossRef]
- Hoop, M.; Mushtaq, F.; Hurter, C.; Chen, X.Z.; Nelson, B.J.; Pané, S. A Smart Multifunctional Drug Delivery Nanoplatform for Targeting Cancer Cells. Nanoscale 2016, 8, 12723–12728. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lodeiro, J.; Rodríguez-González, B.; Santos, H.M.; Bértolo, E.; Capelo, J.L.; Dos Santos, A.A.; Lodeiro, C. Unraveling the Organotellurium Chemistry Applied to the Synthesis of Gold Nanomaterials. ACS Omega 2016, 1, 1314–1325. [Google Scholar] [CrossRef]
- Montalti, M.; Credi, A.; Prodi, L.; Gandolfi, M.T. Handbook of Photochemistry, 3rd ed.; Taylor & Francis: Boca Raton, FL, USA, 2006; ISBN 9780824723774. [Google Scholar]
- Wypych, G. Handbook of Solvents; ChemTec Publishing and William Andrew Inc.: Toronto, ON, Canada; New York, NY, USA, 2001; ISBN 1-895198-24-0. [Google Scholar]
- Mata, R.A.; Costa Cabral, B.J. Structural, Energetic, and Electronic Properties of (CH3CN)2-8 Clusters by Density Functional Theory. J. Mol. Struct. (THEOCHEM) 2004, 673, 155–164. [Google Scholar] [CrossRef]
- Aaron, J.J.; Maafi, M.; Párkányi, C.; Boniface, C. Quantitative Treatment of the Solvent Effects on the Electronic Absorption and Fluorescence Spectra of Acridines and Phenazines. The Ground and First Excited Singlet-State Dipole Moments. Spectrochim. Acta 1995, 51, 603–615. [Google Scholar] [CrossRef]
- Ali, M.; Dutta, P.; Pandey, S. Effect of Ionic Liquid on Prototropic and Solvatochromic Behavior of Fluorescein. J. Phys. Chem. B 2010, 114, 15042–15051. [Google Scholar] [CrossRef] [PubMed]
- Al-Tememee, N.A.A.; Al-Ani, S.K.J.; AbdAlfahdaw, A.A. Effect of Solvents on the Dipole Moments and Fluorescence Quantum Yield of Rhodamine Dyes. ISESCO J. Sci. Technol. 2013, 9, 34–42. Available online: https://www.isesco.org.ma/ISESCO_Technology_Vision/NUM16/doc/5.pdf (accessed on 8 May 2018).
- Alvarez-Pez, J.M.; Ballesteros, L.; Talavera, E.; Yguerabide, J. Fluorescein Excited-State Proton Exchange Reactions: Nanosecond Emission Kinetics and Correlation with Steady-State Fluorescence Intensity. J. Phys. Chem. A 2001, 105, 6320–6332. [Google Scholar] [CrossRef]
- Ueno, T.; Urano, Y.; Setsukinai, K.; Takakusa, H.; Kojima, H.; Kikuchi, K.; Ohkubo, K.; Fukuzumi, S.; Nagano, T. Rational Principles for Modulating Fluorescence Properties of Fluorescein. J. Am. Chem. Soc. 2004, 126, 14079–14085. [Google Scholar] [CrossRef] [PubMed]
- Strickler, S.J.; Berg, R.A. Relationship Between Absorption Intensity and Fluorescence Lifetime of Molecules. J. Chem. Phys. 1962, 37, 814–822. [Google Scholar] [CrossRef]
- Zhang, X.F. The Effect of Phenyl Substitution on the Fluorescence Characteristics of Fluorescein Derivatives via Intramolecular Photoinduced Electron Transfer. Photochem. Photobiol. Sci. 2010, 9, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Tamulis, A.; Tamuliene, J.; Balevicius, M.L.; Rinkevicius, Z.; Tamulis, V. Quantum Mechanical Studies of Intensity in Electronic Spectra of Fluorescein Dianion and Monoanion Forms. Struct. Chem. 2003, 14, 643–648. [Google Scholar] [CrossRef]
- Batistela, V.R.; da Costa Cedran, J.; de Oliveira, H.P.M.; Scarminio, I.S.; Ueno, L.T.; da Hora Machado, A.E.; Hioka, N. Protolytic Fluorescein Species Evaluated Using Chemometry and DFT Studies. Dyes Pigments 2010, 86, 15–24. [Google Scholar] [CrossRef]
- Togashi, D.M.; Szczupak, B.; Ryder, A.G.; Calvet, A.; O’Loughlin, M. Investigating Tryptophan Quenching of Fluorescein Fluorescence under Protolytic Equilibrium. J. Phys. Chem. A 2009, 113, 2757–2767. [Google Scholar] [CrossRef] [PubMed]
- Boens, N.; Qin, W.; Basarić, N.; Hofkens, J.; Ameloot, M.; Pouget, J.; Lefèvre, J.P.; Valeur, B.; Gratton, E.; VandeVen, M.; et al. Fluorescence Lifetime Standards for Time and Frequency Domain Fluorescence Spectroscopy. Anal. Chem. 2007, 79, 2137–2149. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lodeiro, J.; Nuñez, C.; Fernández-Lodeiro, A.; Oliveira, E.; Rodríguez-González, B.; Dos Santos, A.A.; Capelo, J.L.; Lodeiro, C. New-coated Fluorescent Silver Nanoparticles with a Fluorescein Thiol Esther Derivative: Fluorescent Enhancement upon Interaction with Heavy Metal Ions. J. Nanopart. Res. 2014, 16, 2315. [Google Scholar] [CrossRef]
- Kumar, A.; De, A.; Saxena, A.; Mozumdar, S. Environmentally Benign Synthesis of Positively Charged, Ultra-Low Sized Colloidal Gold in Universal Solvent. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 025017. [Google Scholar] [CrossRef]
- Lu, G.W.; Gao, P. Handbook of Non-Invasive Drug Delivery Systems; Emulsions and Microemulsions for Topical and Transdermal Drug Delivery, 1st ed.; William Andrew: Norwich, NY, USA, 2010; pp. 59–94. ISBN 9780815520252. [Google Scholar]
- Guo, J.; Armstrong, M.J.; O’Driscoll, C.M.; Holmes, J.D.; Rahme, K. Positively Charged, Surfactant-Free Gold Nanoparticles for Nuclei Acid Delivery. RSC Adv. 2015, 5, 17862–17871. [Google Scholar] [CrossRef]
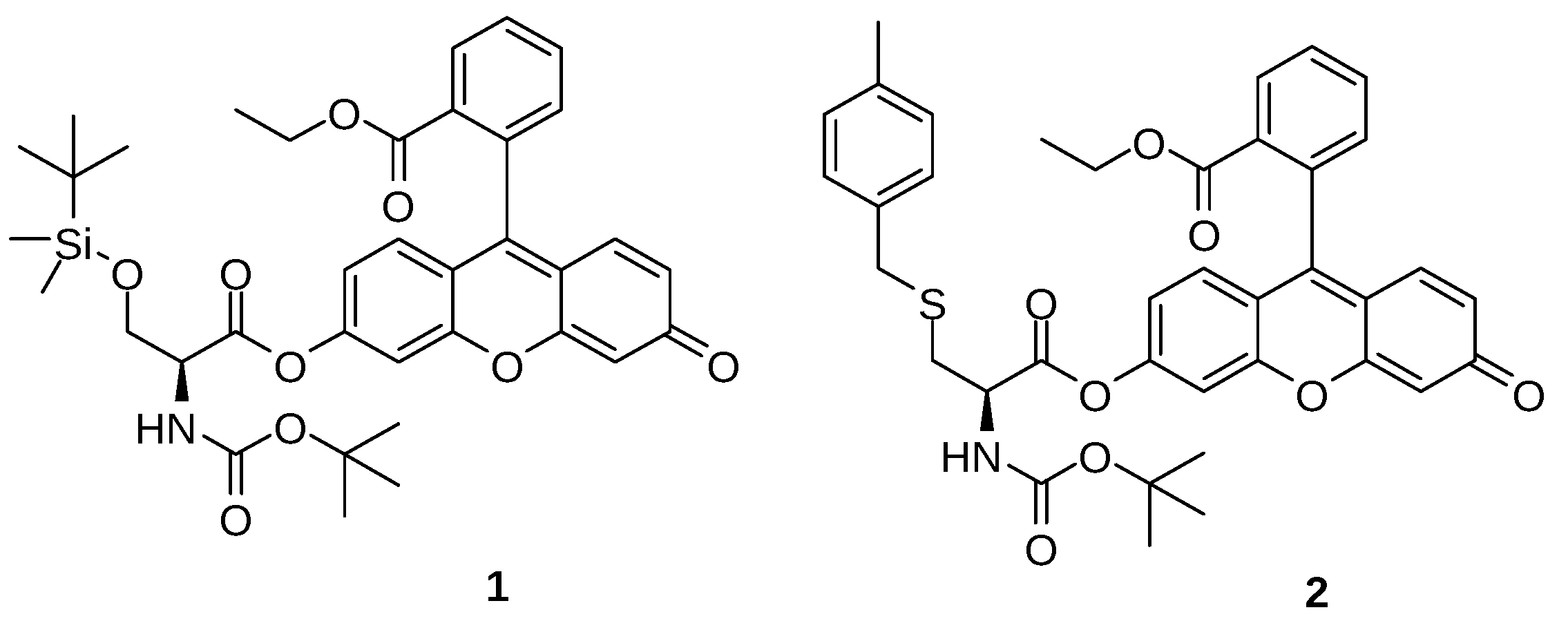
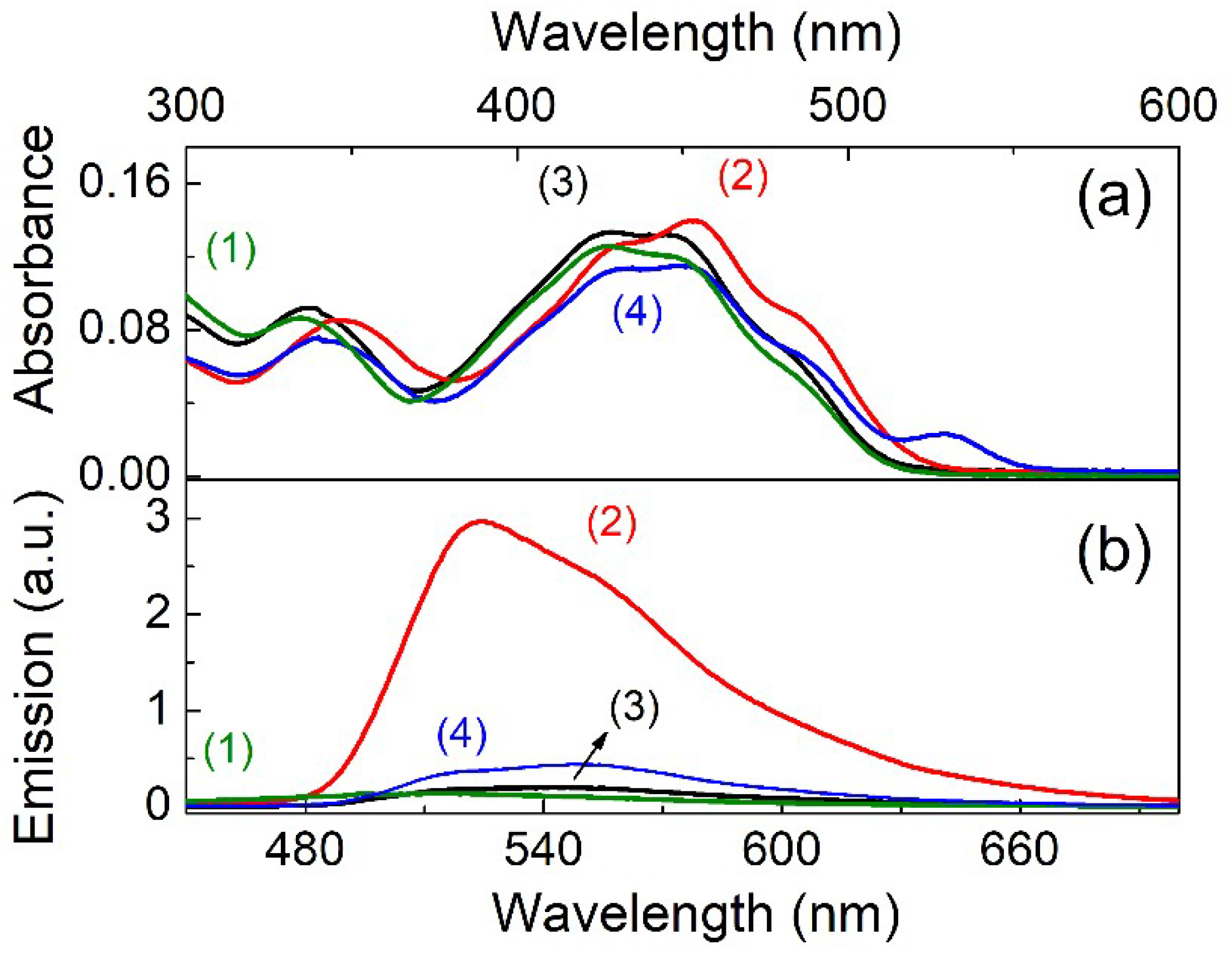
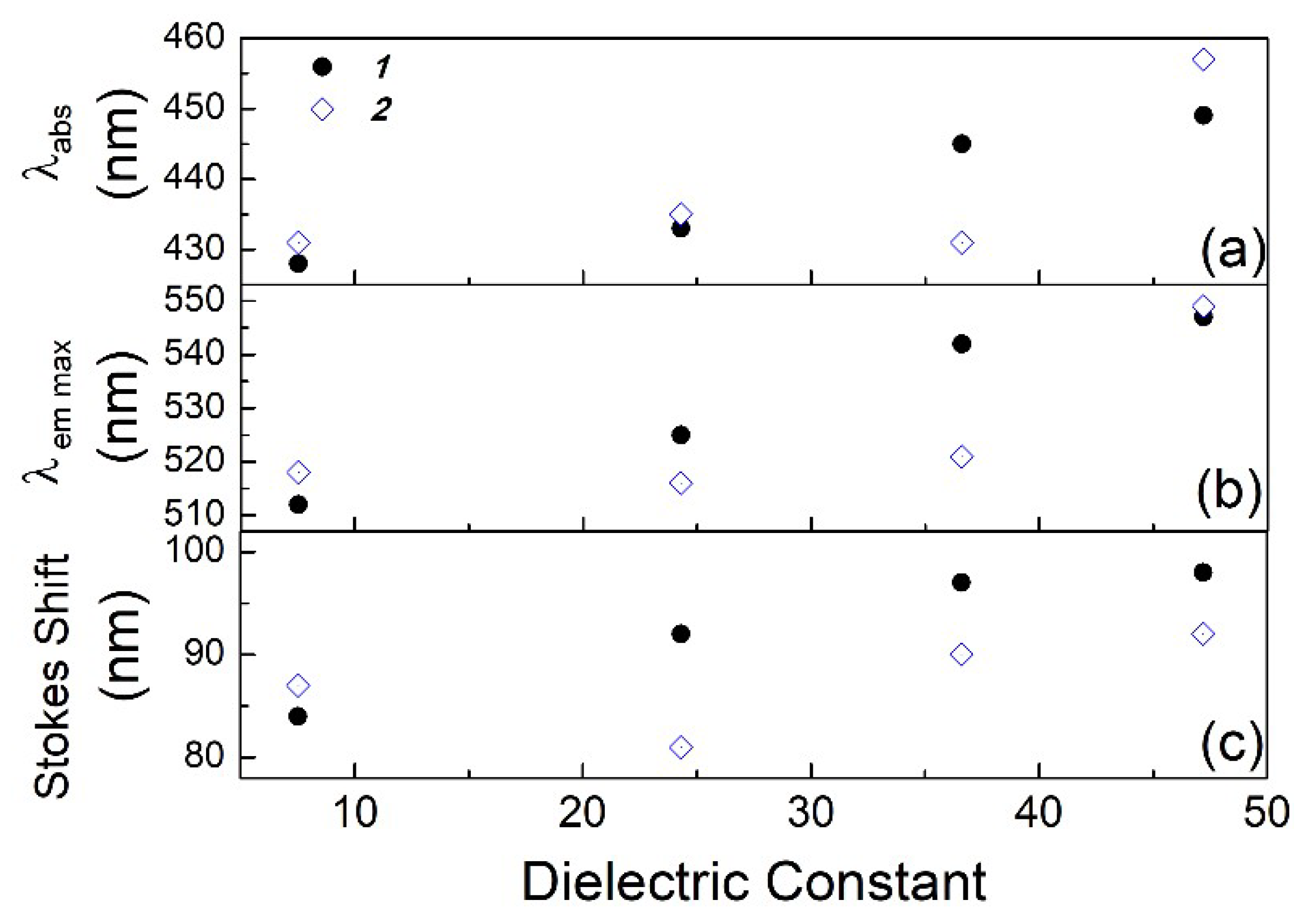
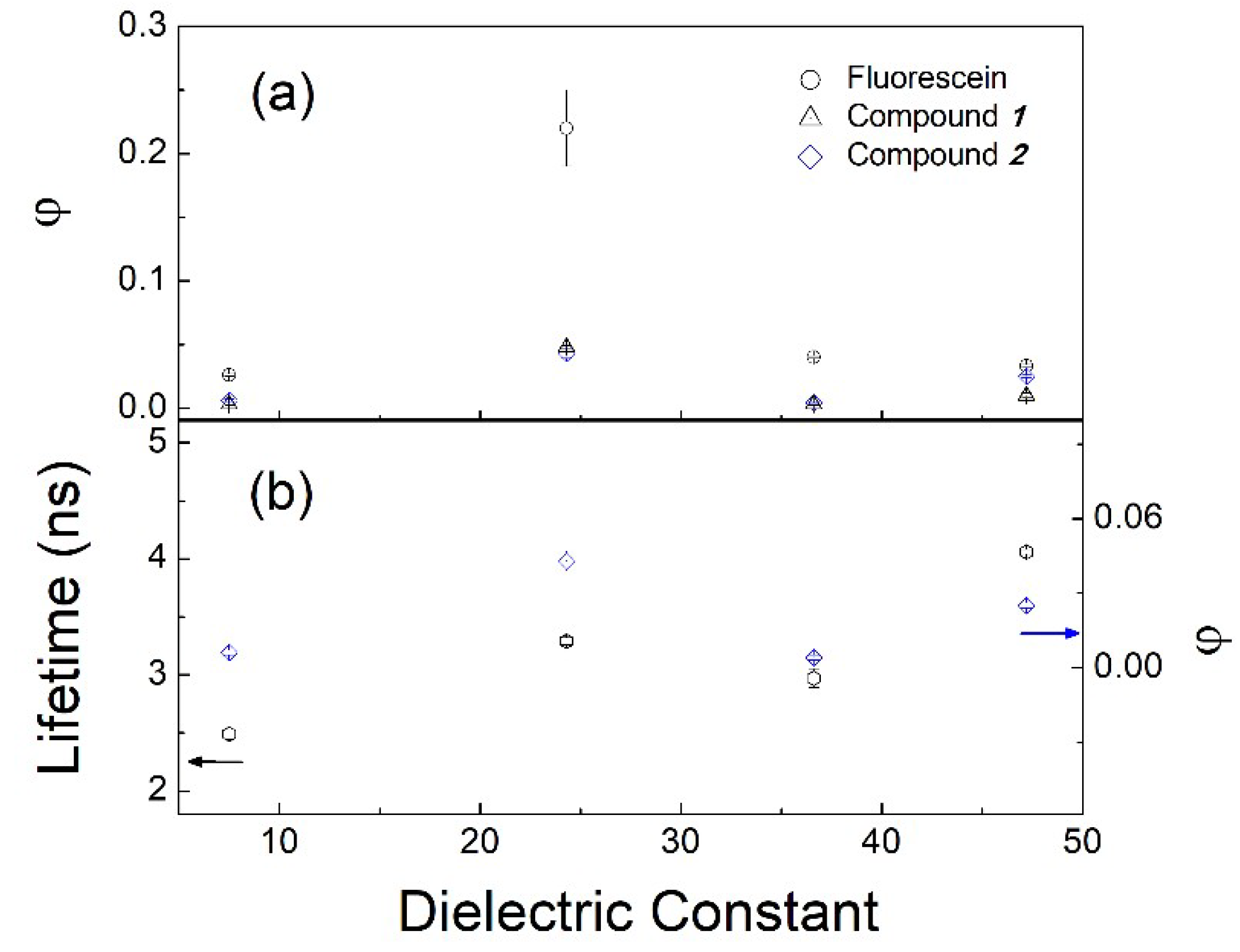
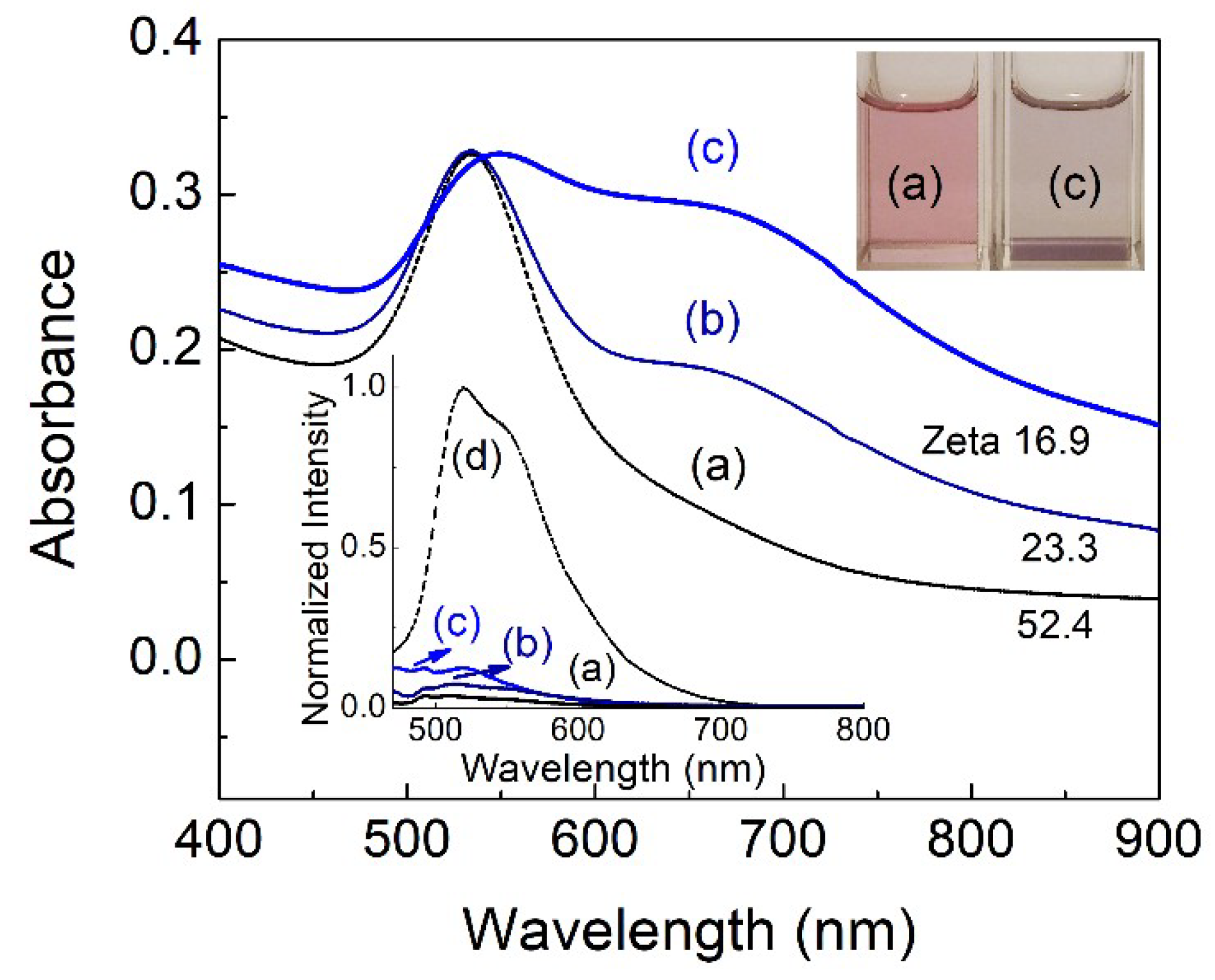
| Solvent | UV–vis λabs (nm) | Log ε | Fluorescence λem max (nm) | Stokes shift (nm) | Quantum Yield φ | Lifetime τ1 (ns) | Dielectric Constant (εr) | |
|---|---|---|---|---|---|---|---|---|
| 1 | THF | 428 | 4.10 | 512 | 84 | 0.003 | 2.3 ± 0.2 | 7.52 |
| Ethanol | 433 | 4.10 | 525 | 92 | 0.048 | 3.65 ± 0.02 | 24.3 | |
| Acetonitrile | 445 | 4.12 | 542 | 97 | 0.003 | 2.7 ± 0.1 | 36.6 | |
| DMSO | 449 | 4.06 | 547 | 98 | 0.010 | 4.03 ± 0.02 | 47.2 | |
| 2 | THF | 431 | 4.01 | 518 | 87 | 0.006 | 2.49 ± 0.05 | 7.52 |
| Ethanol | 435 | 3.89 | 516 | 81 | 0.043 | 3.29 ± 0.03 | 24.3 | |
| Acetonitrile | 431 | 4.01 | 521 | 90 | 0.004 | 2.97 ± 0.08 | 36.6 | |
| DMSO | 457 | 4.04 | 549 | 92 | 0.025 | 4.06 ± 0.02 | 47.2 |
| Compound 1 | Compound 2 | |||||
|---|---|---|---|---|---|---|
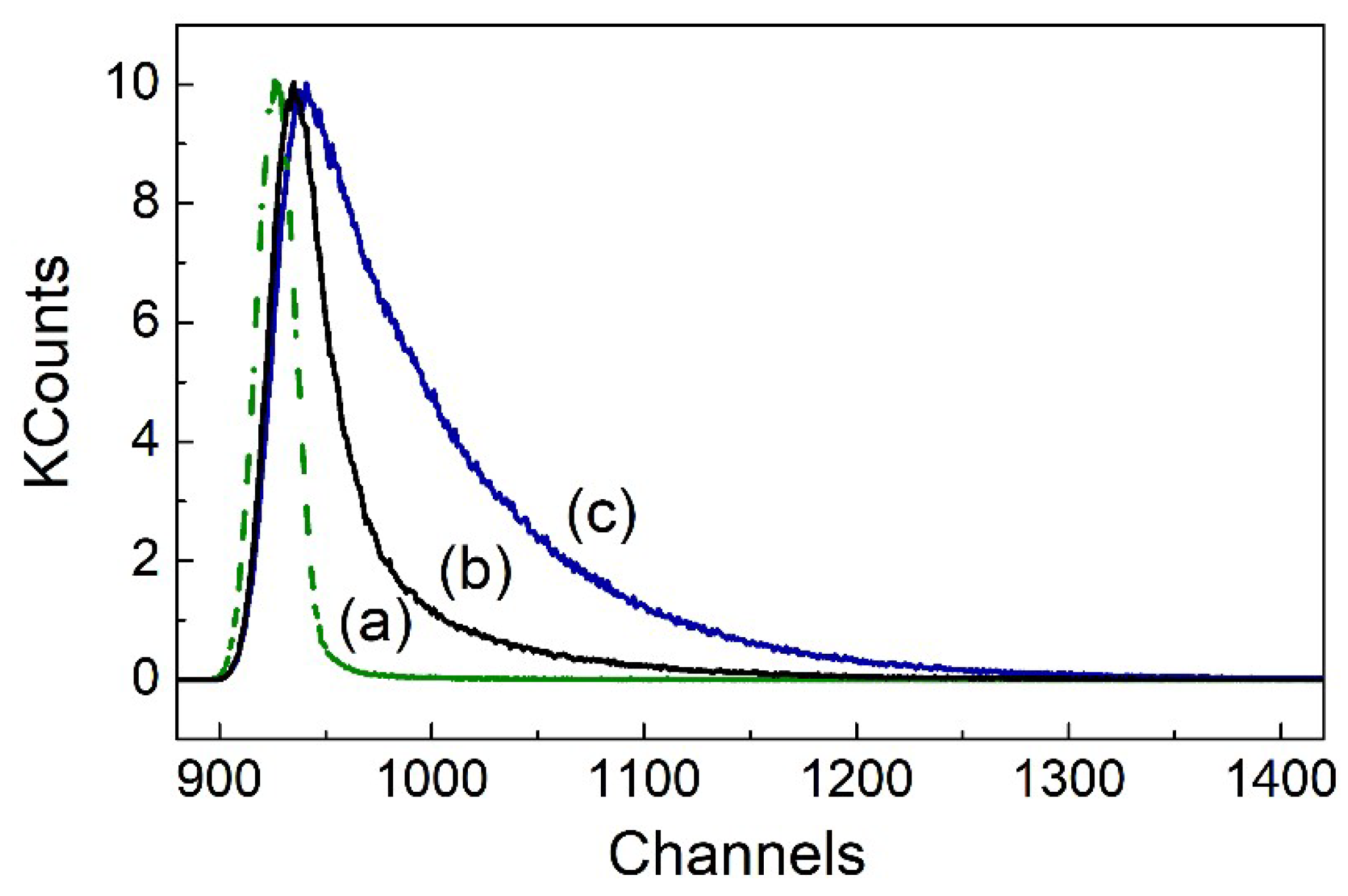
| τ1 (ns) | τ2 (ns) | χ2 | τ1 (ns) | τ2 (ns) | χ2 | |
| B1 (Rel. Amp.%) | B2 (Rel. Amp.%) | B1 (Rel. Amp.%) | B2 (Rel. Amp.%) | |||
| THF | 2.3 ± 0.2 | 0.08 ± 0.02 | 0.98 ± 0.09 | 2.49 ± 0.05 | 0.110 ± 0.005 | 1.01 ± 0.09 |
| (C4H8O) | 1.9 | 98.1 | 3.3 | 96.7 | ||
| Ethanol | 3.65 ± 0.02 | 0.788 ± 0.009 | 1.01 ± 0.04 | 3.29 ± 0.03 | 0.90 ± 0.06 | 1.1 ± 0.1 |
| (C2H6O) | 40 | 60 | 93 | 7 | ||
| Acetonitrile | 2.70 ± 0.06 | 0.231 ± 0.006 | 1.03 ± 0.08 | 2.97 ± 0.08 | 0.357 ± 0.003 | 1.0 ± 0.1 |
| (CH3CN) | 5.6 | 94.4 | 6 | 94 | ||
| DMSO | 4.03 ± 0.02 | 0.13 ± 0.08 | 1.09 ± 0.07 | 4.06 ± 0.02 | 0.65 ± 0.02 | 1.02 ± 0.07 |
| ((CH3)2SO) | 79 | 21 | 48 | 52 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilla, V.; Gonçalves, A.C.; Dos Santos, A.A.; Lodeiro, C. Lifetime and Fluorescence Quantum Yield of Two Fluorescein-Amino Acid-Based Compounds in Different Organic Solvents and Gold Colloidal Suspensions. Chemosensors 2018, 6, 26. https://doi.org/10.3390/chemosensors6030026
Pilla V, Gonçalves AC, Dos Santos AA, Lodeiro C. Lifetime and Fluorescence Quantum Yield of Two Fluorescein-Amino Acid-Based Compounds in Different Organic Solvents and Gold Colloidal Suspensions. Chemosensors. 2018; 6(3):26. https://doi.org/10.3390/chemosensors6030026
Chicago/Turabian StylePilla, Viviane, Augusto C. Gonçalves, Alcindo A. Dos Santos, and Carlos Lodeiro. 2018. "Lifetime and Fluorescence Quantum Yield of Two Fluorescein-Amino Acid-Based Compounds in Different Organic Solvents and Gold Colloidal Suspensions" Chemosensors 6, no. 3: 26. https://doi.org/10.3390/chemosensors6030026
APA StylePilla, V., Gonçalves, A. C., Dos Santos, A. A., & Lodeiro, C. (2018). Lifetime and Fluorescence Quantum Yield of Two Fluorescein-Amino Acid-Based Compounds in Different Organic Solvents and Gold Colloidal Suspensions. Chemosensors, 6(3), 26. https://doi.org/10.3390/chemosensors6030026






