Comparison of the Sensing Properties of ZnO Nanowalls-Based Sensors toward Low Concentrations of CO and NO2
Abstract
:1. Introduction
2. Materials and Methods
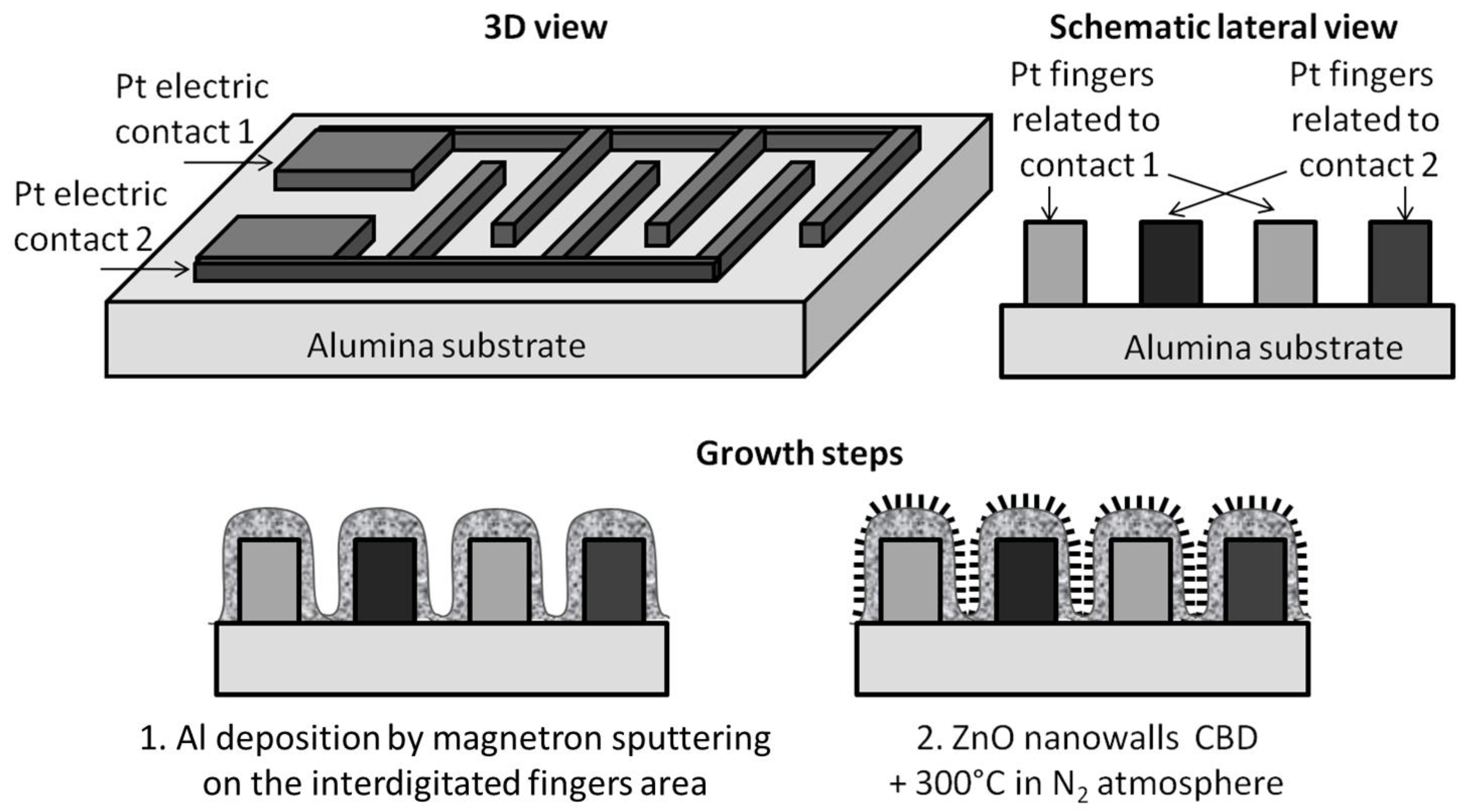
2.1. Sensor Fabrication
2.2. Characterization of the Sensing Layer
2.3. Sensing Tests
3. Results
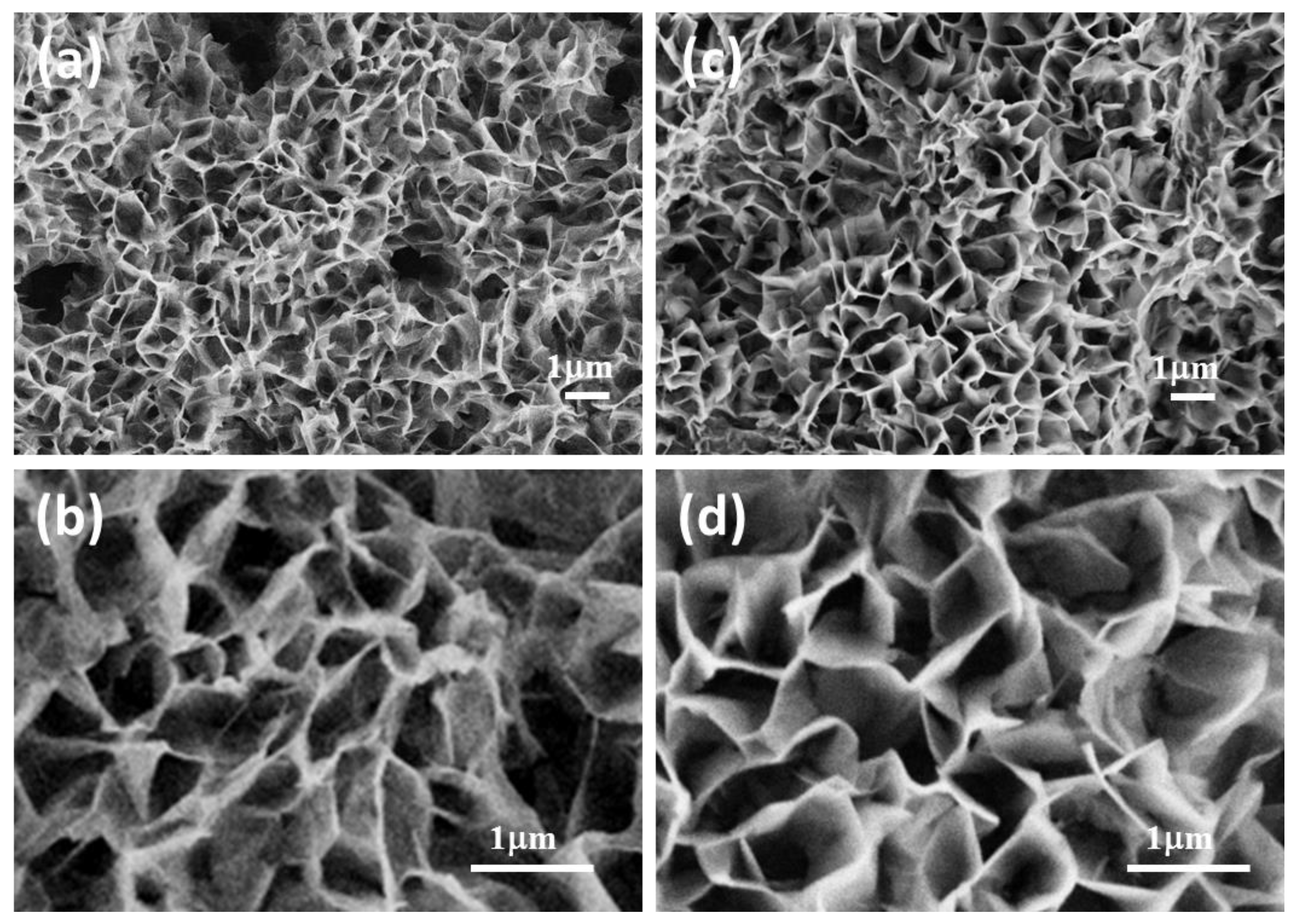
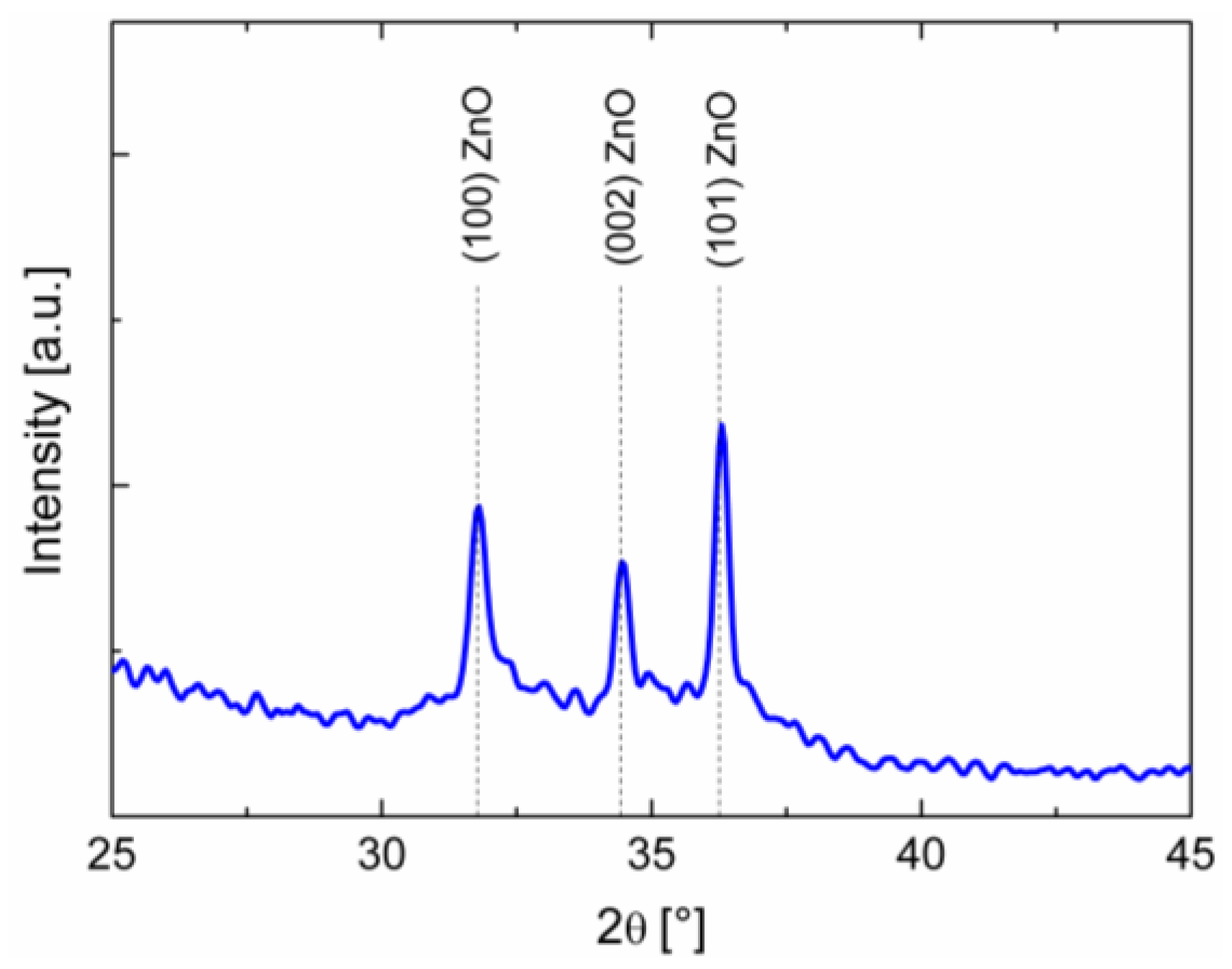
3.1. Sensing Layer Synthesis and Characterization
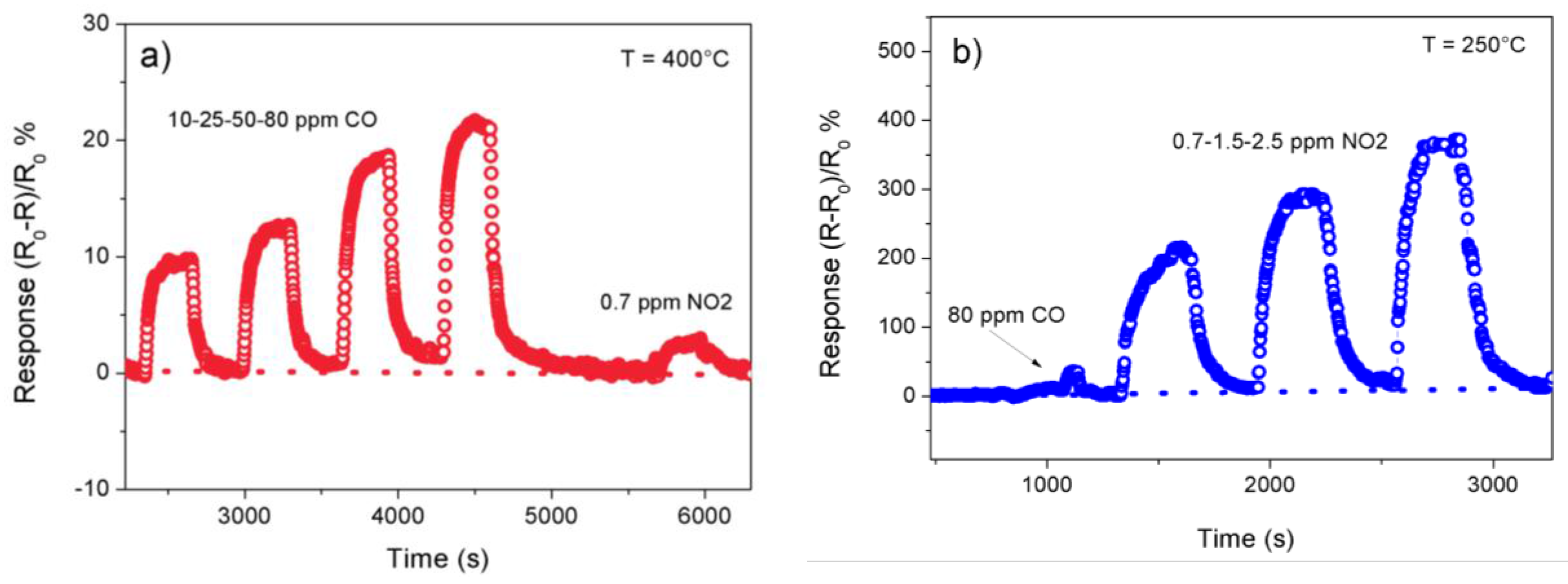
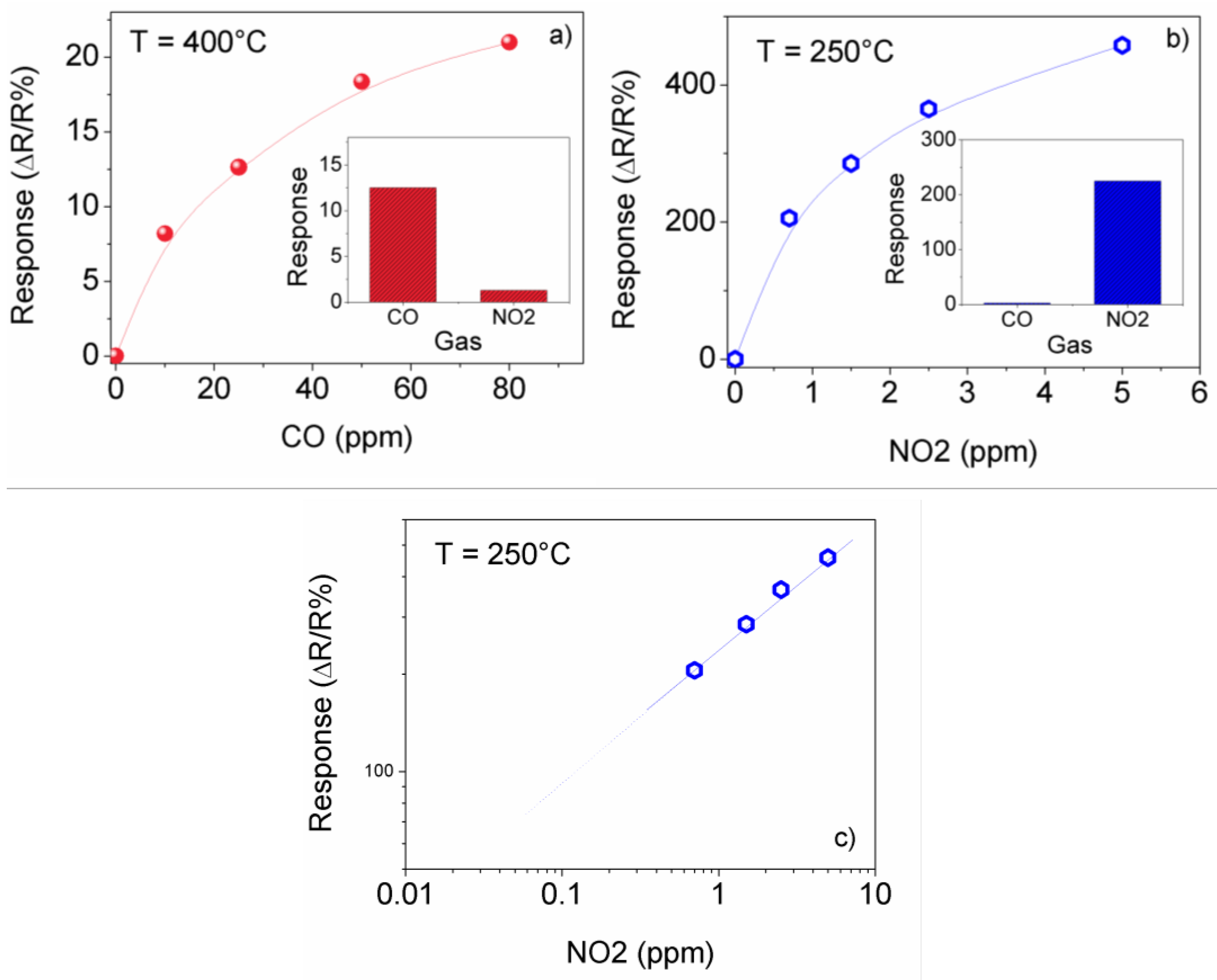
3.2. Sensing Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Neri, G. First fifty years of chemoresistive gas sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Ye, C.; Bando, Y.; Shen, G.; Golberg, D. Thickness-dependent photocatalytic performance of ZnO nanoplatelets. J. Phys. Chem. B 2006, 110, 15146–15151. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, Y.; Li, Z.; Song, J.; Wang, Z.L. Single-crystal mesoporous ZnO thin films composed of nanowalls. J. Phys. Chem. C 2009, 113, 1791–1794. [Google Scholar] [CrossRef]
- Maeng, J.; Jo, G.; Choe, M.; Park, W.; Kwon, M.-K.; Park, S.-J.; Lee, T. Structural and photoluminescence characterization of ZnO nanowalls grown by metal organic chemical vapor deposition. Thin Solid Films 2009, 518, 865–869. [Google Scholar] [CrossRef]
- Cheng, J.P.; Zhang, X.B.; Luo, Z.Q. Oriented growth of ZnO nanostructures on Si and Al substrates. Surf. Coat. Technol. 2008, 202, 4681–4686. [Google Scholar] [CrossRef]
- Yao, L.; Zheng, M.; Li, C.; Ma, L.; Shen, W. Facile synthesis of superhydrophobic surface of ZnO nanoflakes: Chemical coating and UV-induced wettability conversion. Nanoscale Res. Lett. 2012, 7, 216. [Google Scholar] [PubMed]
- Gao, S.Y.; Li, H.D.; Yuan, J.J.; Li, Y.A.; Yang, X.X.; Liu, J.W. ZnO nanorods/plates on Si substrate grown by low-temperature hydrothermal reaction. Appl. Surf. Sci. 2010, 256, 2781–2785. [Google Scholar] [CrossRef]
- Iwu, K.O.; Strano, V.; Di Mauro, A.; Impellizzeri, G.; Mirabella, S. Enhanced quality, growth kinetics, and photocatalysis of ZnO nanowalls prepared by chemical bath deposition. Cryst. Growth Des. 2015, 15, 4206–4212. [Google Scholar] [CrossRef]
- Maiolo, L.; Mirabella, S.; Maita, F.; Alberti, A.; Minotti, A.; Strano, V.; Pecora, A.; Shacham-Diamand, Y.; Fortunato, G. Flexible pH sensors based on polysilicon thin film transistors and ZnO nanowalls. Appl. Phys. Lett. 2014, 105, 093501. [Google Scholar] [CrossRef]
- Yu, L.-M.; Guo, F.; Liu, Z.-Y.; Liu, S.; Yang, B.; Yin, M.-L.; Fan, X.-H. Facile synthesis of three dimensional porous ZnO films with mesoporous walls and gas sensing properties. Mater. Charact. 2016, 112, 224–228. [Google Scholar] [CrossRef]
- Yu, L.; Guo, F.; Liu, S.; Yang, B.; Jiang, Y.; Qi, L.; Fan, X. Both oxygen vacancies defects and porosity facilitated NO2 gas sensing response in 2D ZnO nanowalls at room temperature. J. Alloys Compd. 2016, 682, 352–356. [Google Scholar] [CrossRef]
- Chang, S.-P.; Wen, C.-H.; Chang, S.-J. Two-dimensional ZnO nanowalls for gas sensor and photoelectrochemical applications. Electron. Mater. Lett. 2014, 10, 693–697. [Google Scholar] [CrossRef]
- Chen, T.P.; Chang, S.P.; Chang, S.J. Fabrication of ZnO nanowall-based hydrogen gas nanosensor. Adv. Mater. Res. 2013, 684, 21–25. [Google Scholar] [CrossRef]
- Chen, T.-P.; Chang, S.-P.; Hung, F.-Y.; Chang, S.-J.; Hu, Z.-S.; Chen, K.-J. Simple fabrication process for 2D ZnO nanowalls and their potential application as a methane sensor. Sensors 2013, 13, 3941. [Google Scholar] [CrossRef] [PubMed]
- Hjiri, M.; El Mir, L.; Leonardi, S.G.; Donato, N.; Neri, G. CO and NO2 selective monitoring by ZnO-based sensors. Nanomaterials 2013, 3, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Raj, S.; Ko, K.-J.; Park, K.-K.; Yu, Y.-T. Synthesis of flower-like ZnO microstructures for gas sensor applications. Sens. Actuators B Chem. 2013, 178, 107–112. [Google Scholar] [CrossRef]
- Major, S.; Banerjee, A.; Chopra, K.L. Annealing studies of undoped and indium-doped films of zinc oxide. Thin Solid Films 1984, 122, 31–43. [Google Scholar] [CrossRef]
- Kobrinsky, V.; Fradkin, E.; Lumelsky, V.; Rothschild, A.; Komem, Y.; Lifshitz, Y. Tunable gas sensing properties of p- and n-doped ZnO thin films. Sens. Actuators B 2010, 148, 379–387. [Google Scholar] [CrossRef]
- Dai, Z.; Lee, C.-S.; Tian, Y.; Kim, I.-D.; Lee, J.-H. Highly reversible switching from P- to N-type NO2 sensing in a monolayer Fe2O3 inverse opal film and the associated P–N transition phase diagram. J. Mater. Chem. A 2015, 3, 3372–3381. [Google Scholar] [CrossRef]
- Wang, J.X.; Sun, X.W.; Yang, Y.; Wu, C.M. N–P transition sensing behaviors of ZnO nanotubes exposed to NO2 gas. Nanotechnology 2009, 20, 465501. [Google Scholar] [CrossRef] [PubMed]
- Ahlers, S.; Müller, G.; Doll, T. A rate equation approach to the gas sensitivity of thin film metal oxide materials. Sens. Actuators B 2005, 107, 587–599. [Google Scholar] [CrossRef]
- Shishiyanu, S.T.; Shishiyanu, T.S.; Lupan, O. Sensing characteristics of tin-doped ZnO thin films as NO2 gas sensor. Sens. Actuators B Chem. 2005, 107, 379–386. [Google Scholar] [CrossRef]
- Lupan, O.; Shishiyanu, S.; Chow, L.; Shishiyanu, T. Nanostructured zinc oxide gas sensors by successive ionic layer adsorption and reaction method and rapid photothermal processing. Thin Solid Films 2008, 516, 3338–3345. [Google Scholar] [CrossRef]
- Dinesh, V.P.; Sukhananazerin, A.; Biji, P. An emphatic study on role of spill-over sensitization and surface defects on NO2 gas sensor properties of ultralong ZnO@Au heterojunction NRs. J. Alloys Compd. 2017, 712, 811–821. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, E.; Strano, V.; Mirabella, S.; Donato, N.; Leonardi, S.G.; Neri, G. Comparison of the Sensing Properties of ZnO Nanowalls-Based Sensors toward Low Concentrations of CO and NO2. Chemosensors 2017, 5, 20. https://doi.org/10.3390/chemosensors5030020
Bruno E, Strano V, Mirabella S, Donato N, Leonardi SG, Neri G. Comparison of the Sensing Properties of ZnO Nanowalls-Based Sensors toward Low Concentrations of CO and NO2. Chemosensors. 2017; 5(3):20. https://doi.org/10.3390/chemosensors5030020
Chicago/Turabian StyleBruno, Elena, Vincenzina Strano, Salvo Mirabella, Nicola Donato, Salvatore Gianluca Leonardi, and Giovanni Neri. 2017. "Comparison of the Sensing Properties of ZnO Nanowalls-Based Sensors toward Low Concentrations of CO and NO2" Chemosensors 5, no. 3: 20. https://doi.org/10.3390/chemosensors5030020
APA StyleBruno, E., Strano, V., Mirabella, S., Donato, N., Leonardi, S. G., & Neri, G. (2017). Comparison of the Sensing Properties of ZnO Nanowalls-Based Sensors toward Low Concentrations of CO and NO2. Chemosensors, 5(3), 20. https://doi.org/10.3390/chemosensors5030020






