Thin 2D: The New Dimensionality in Gas Sensing
Abstract
1. Introduction
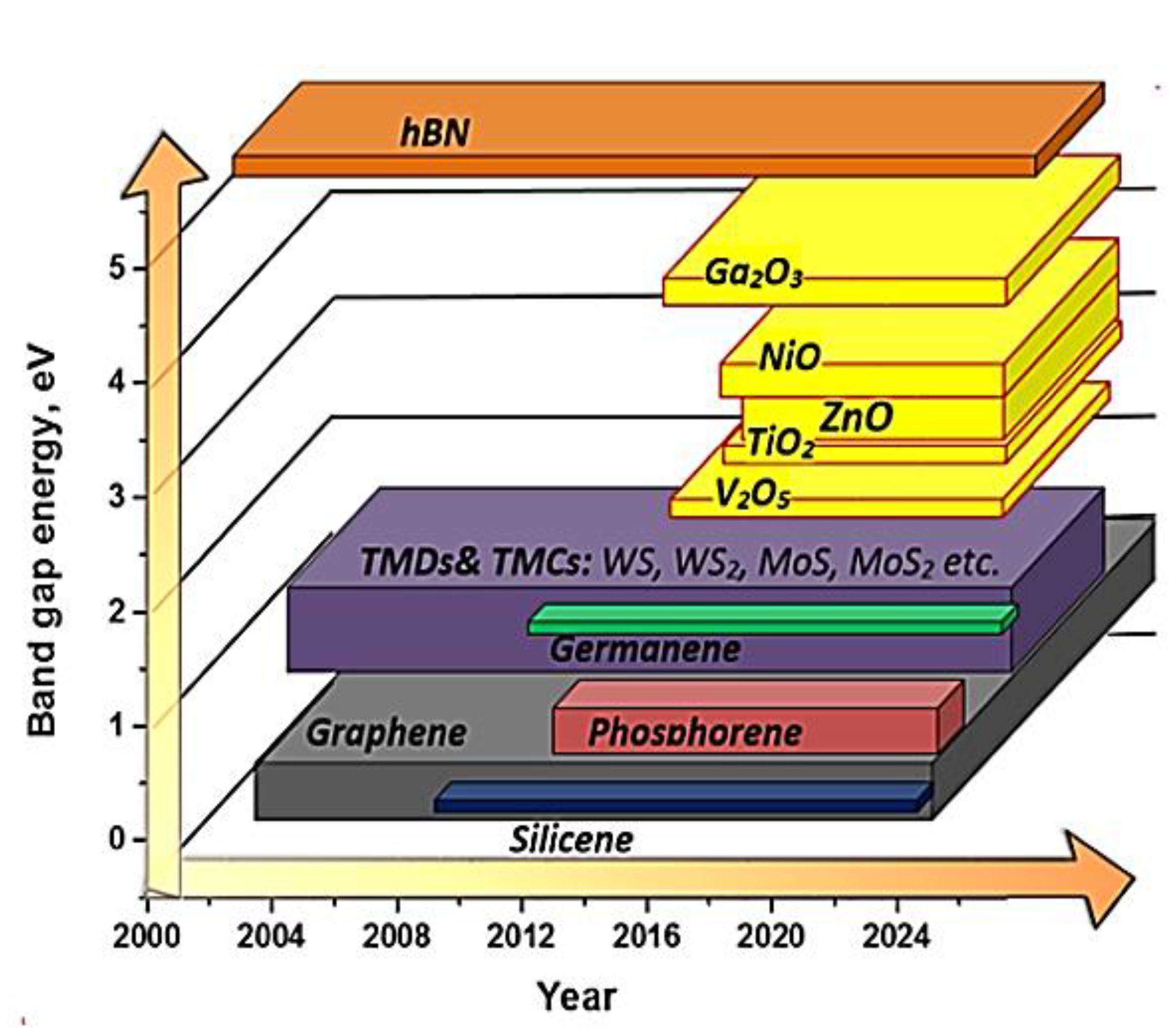
2. Factors Influencing the Gas Sensing Characteristics of Thin 2D Nanostructures
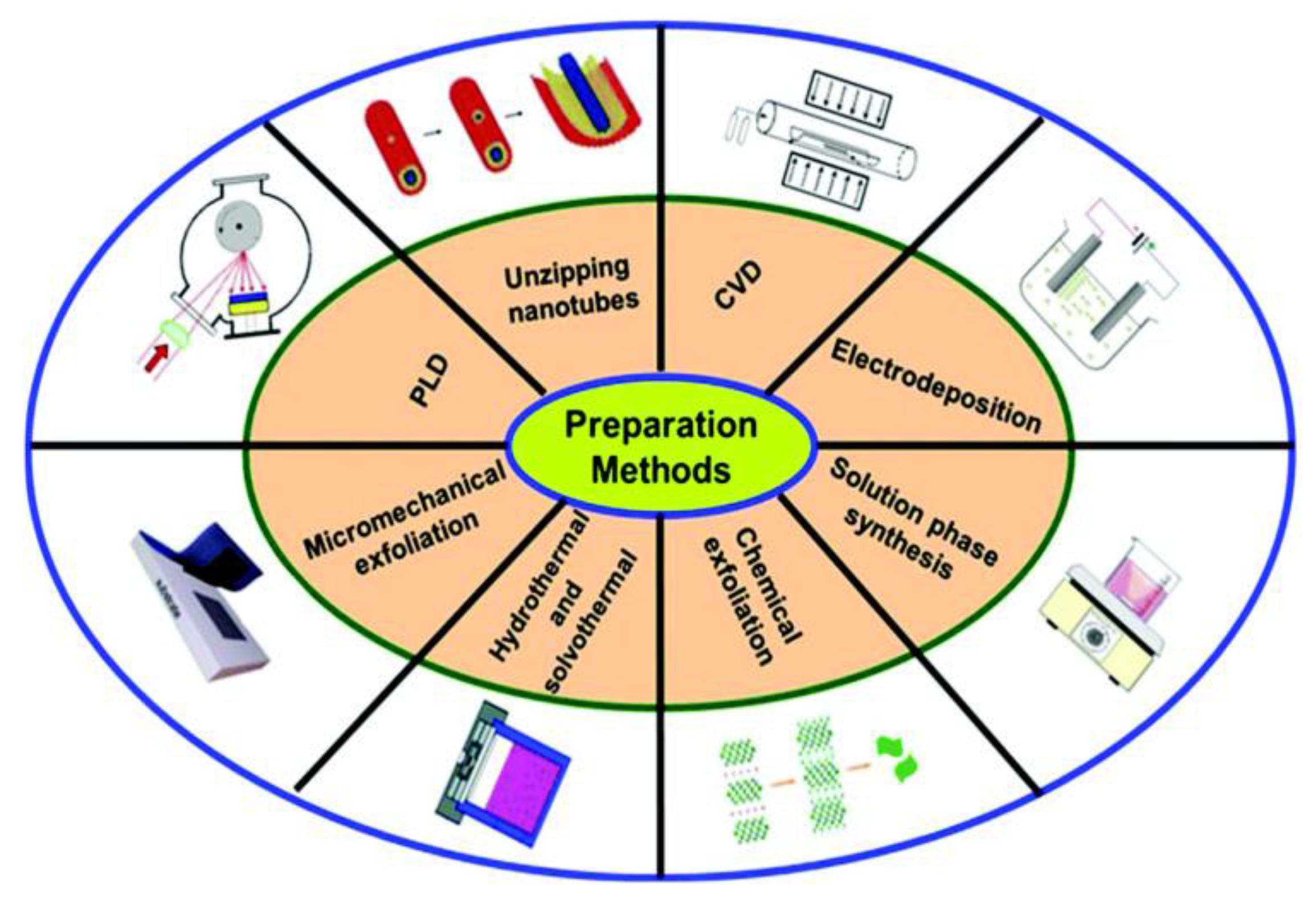
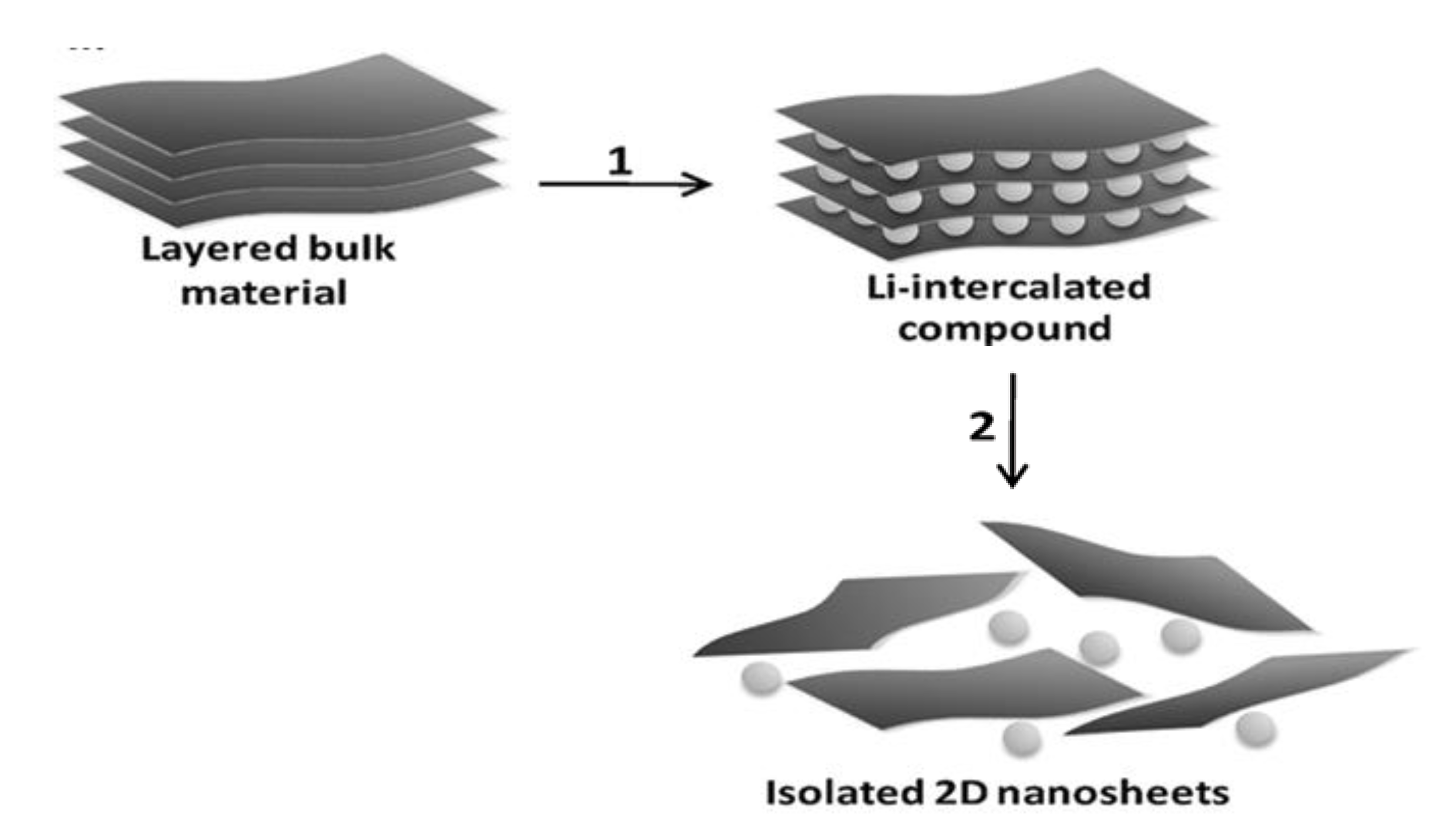
3. Synthesis Methods of Thin 2D Inorganic Materials
4. Gas Sensing Properties of Thin 2D Inorganic Materials
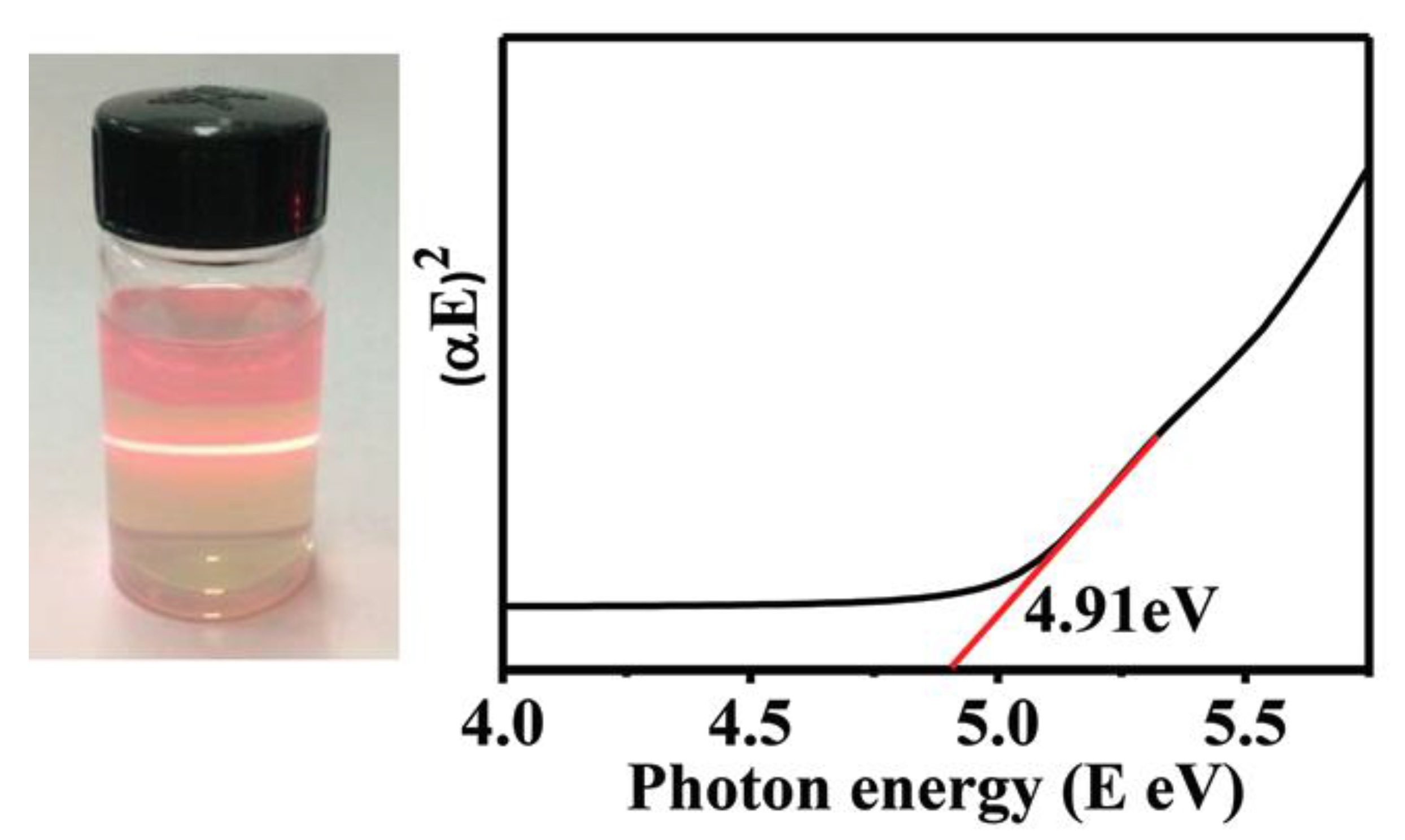
4.1. LHDs Sheets
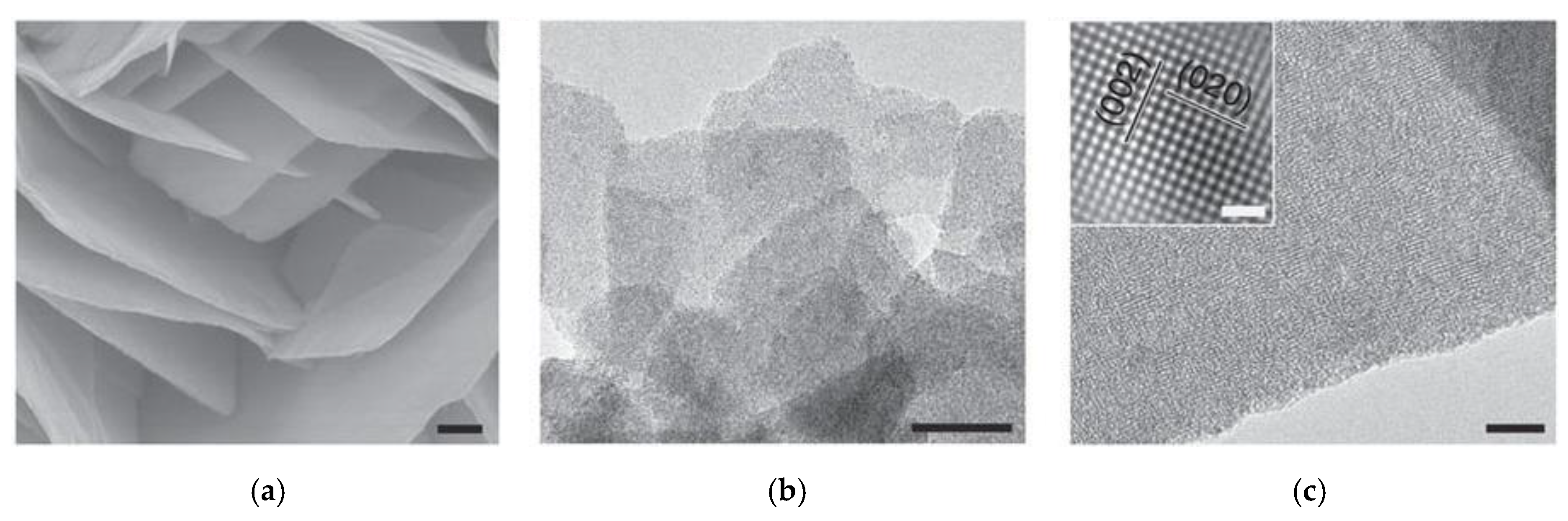
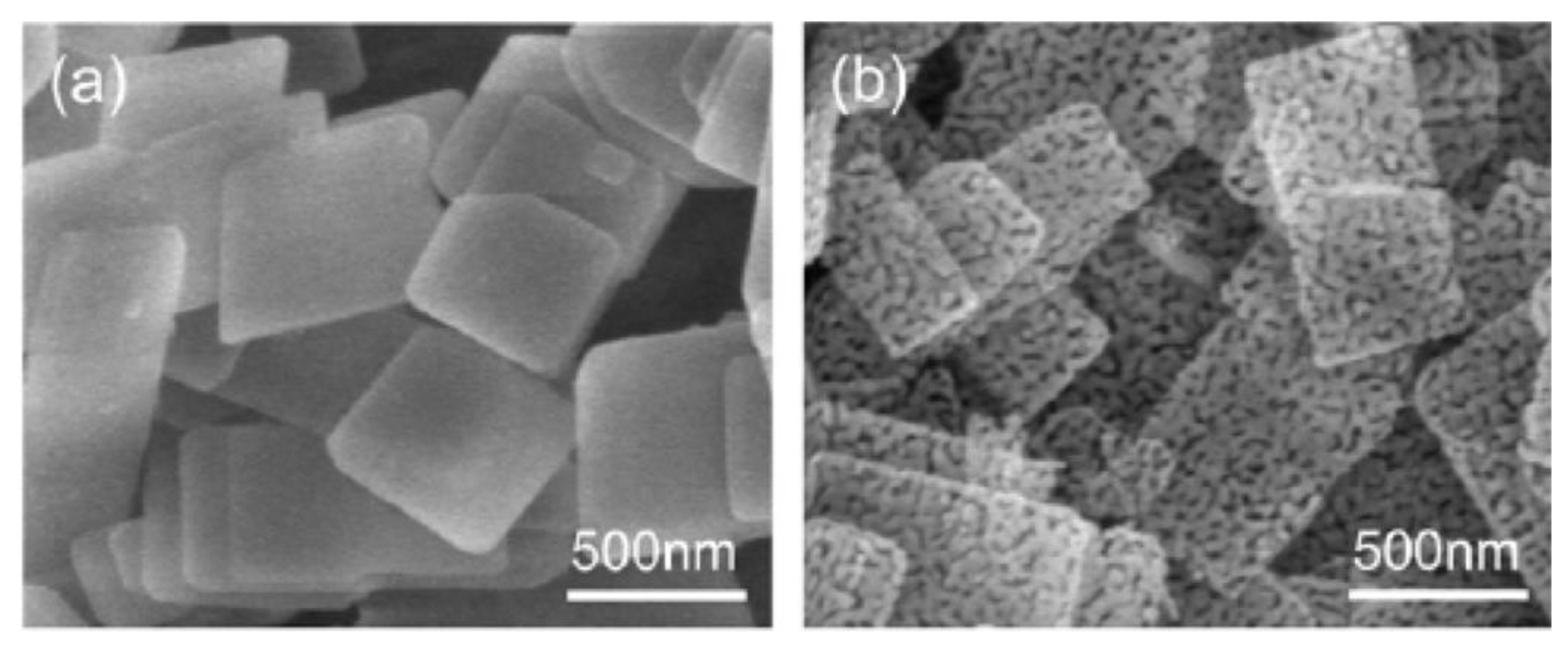
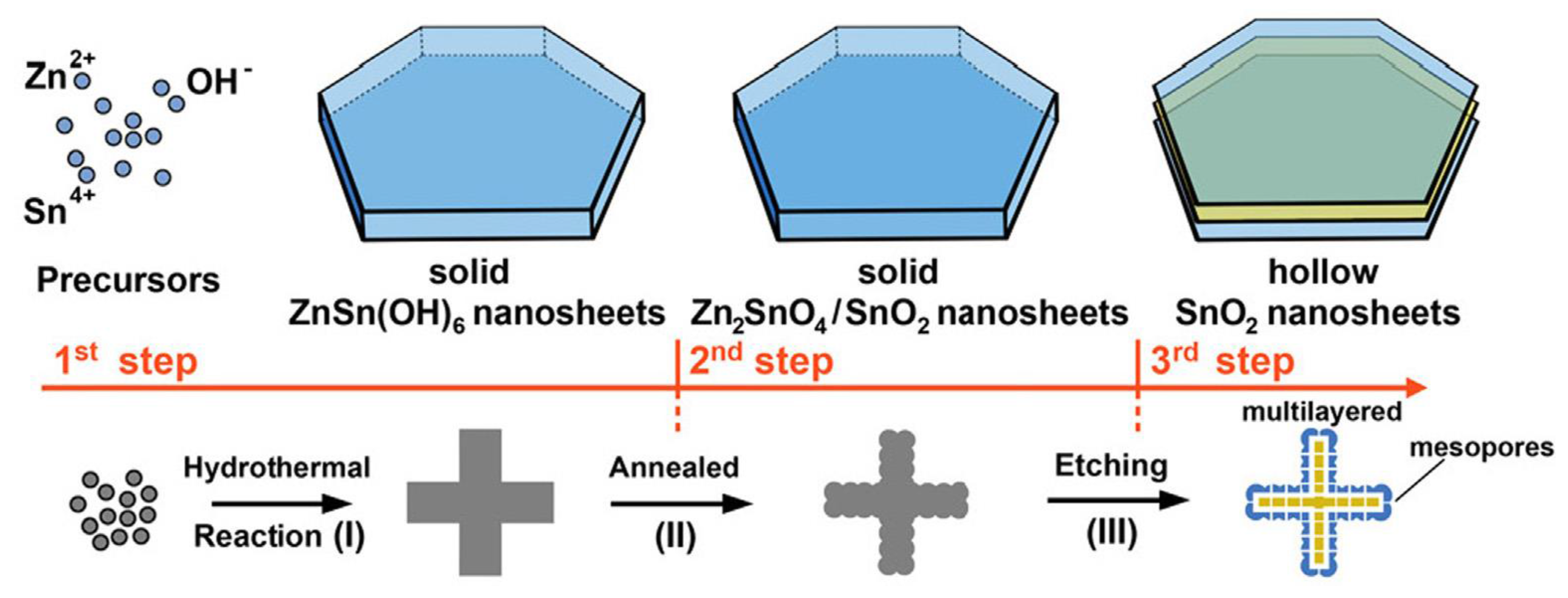
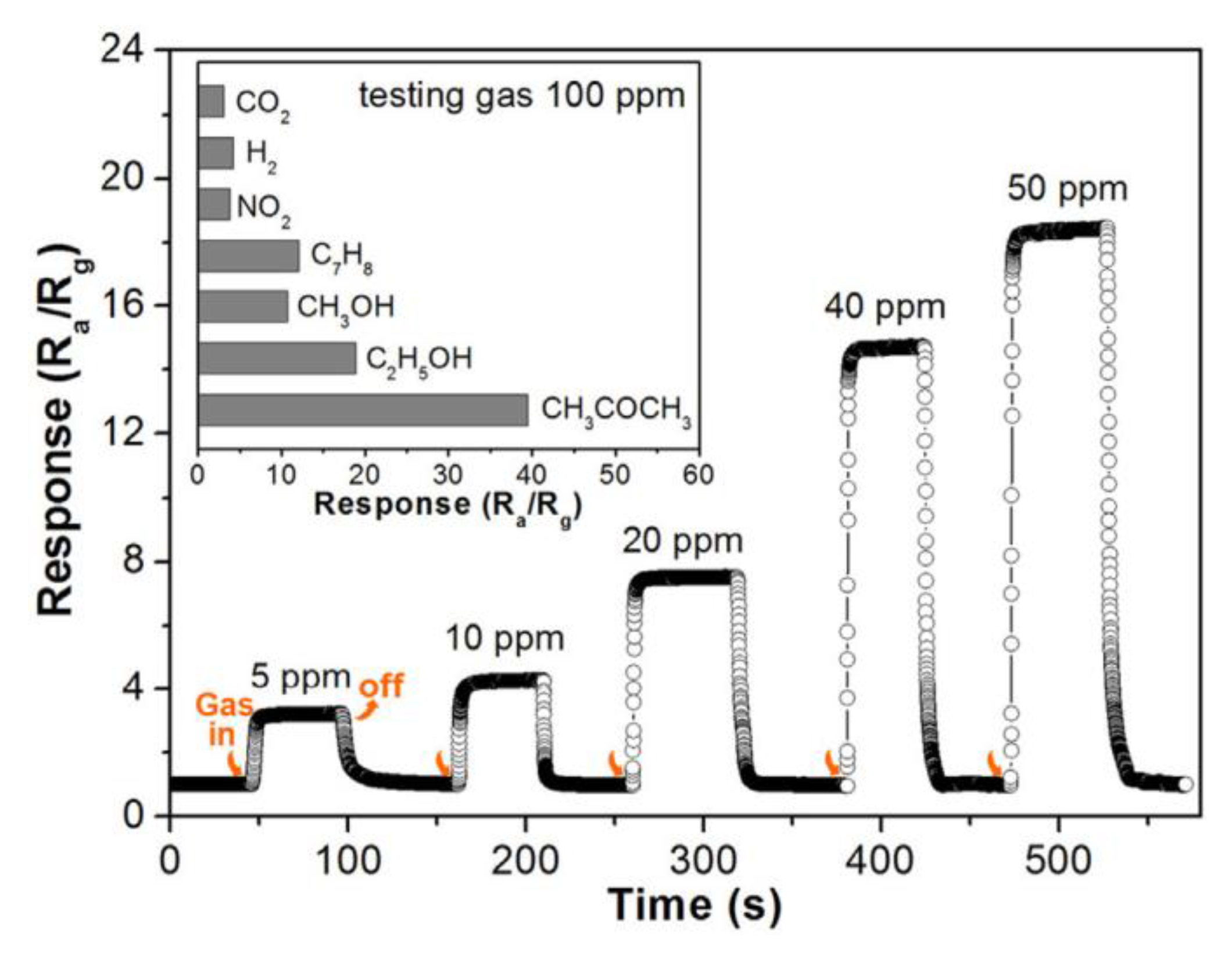
4.2. Thin 2D Metal Oxides
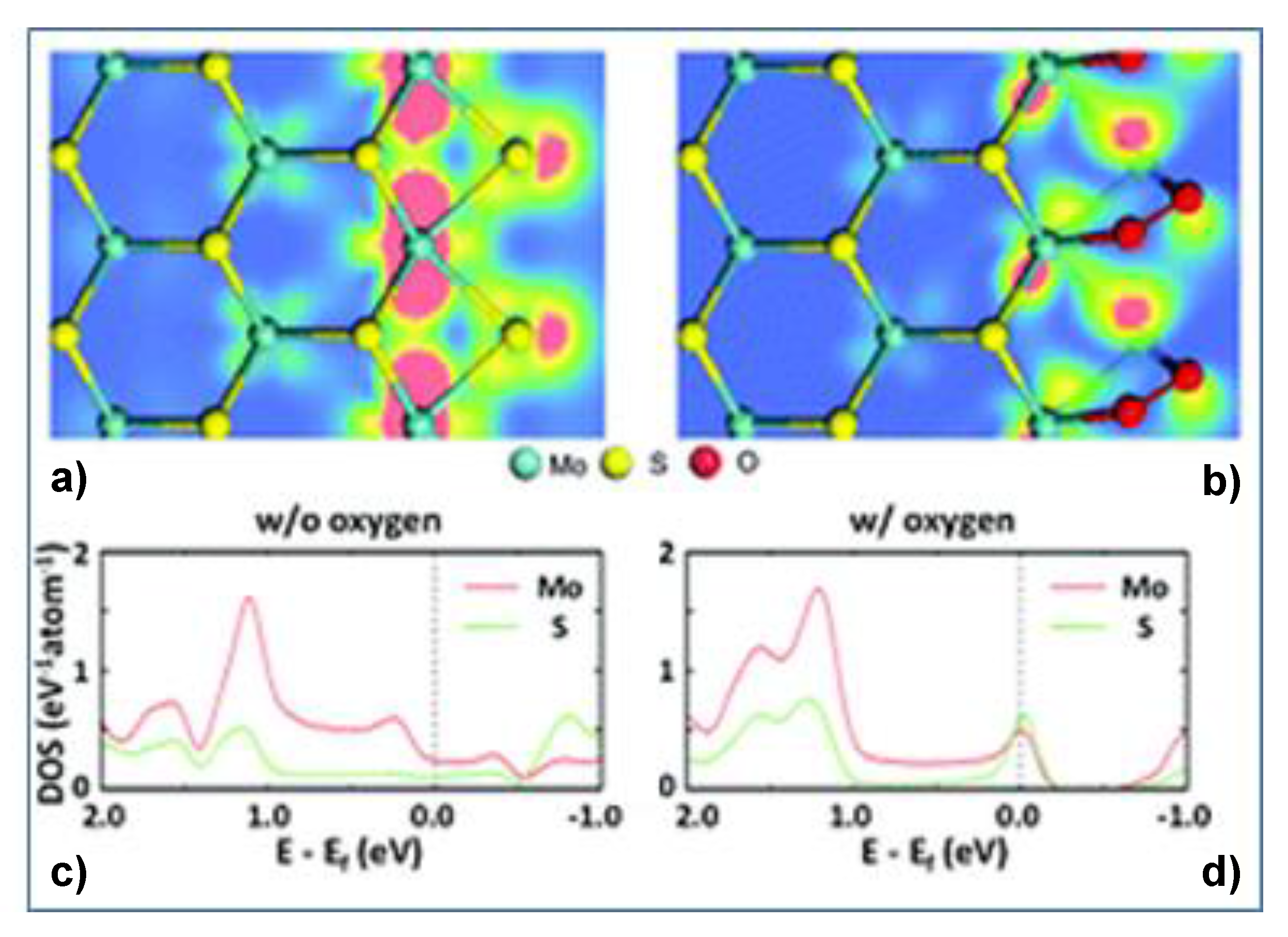
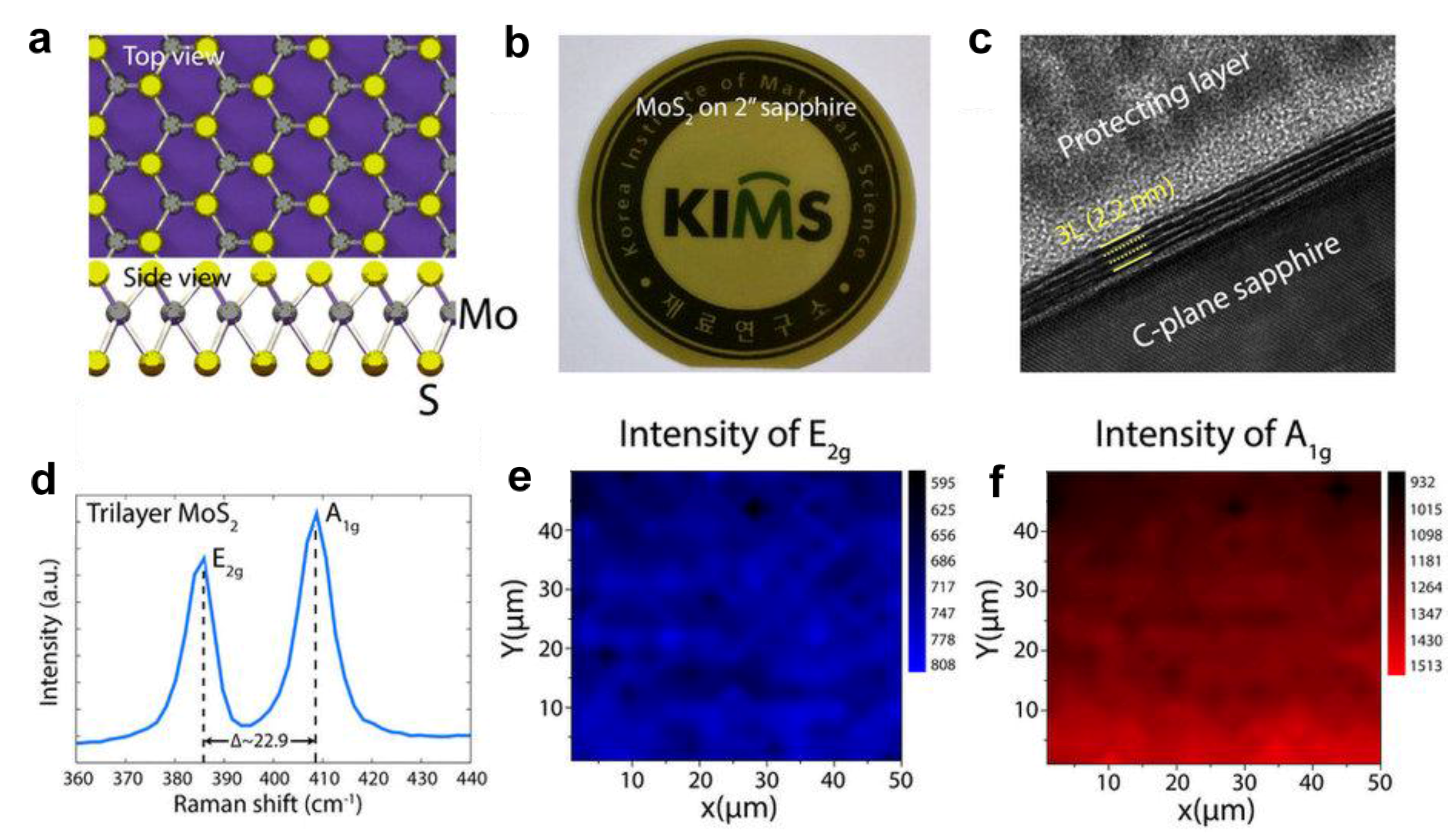
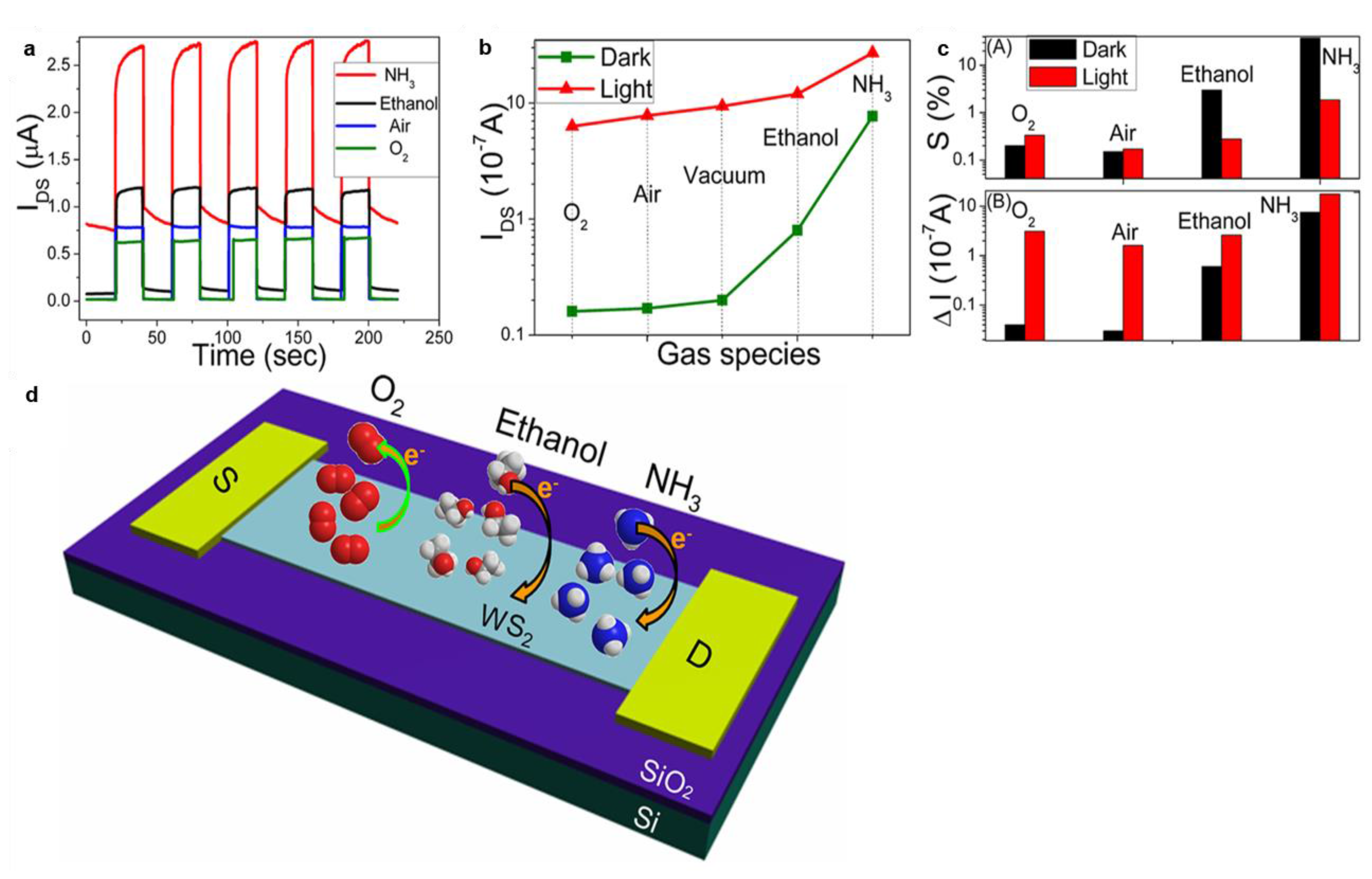
4.3. TMDs Layered Materials
5. Thin 2D Nanostructure and Heterostructure-Based Composites for Gas Sensing
5.1. Assembled Thin 2D Materials
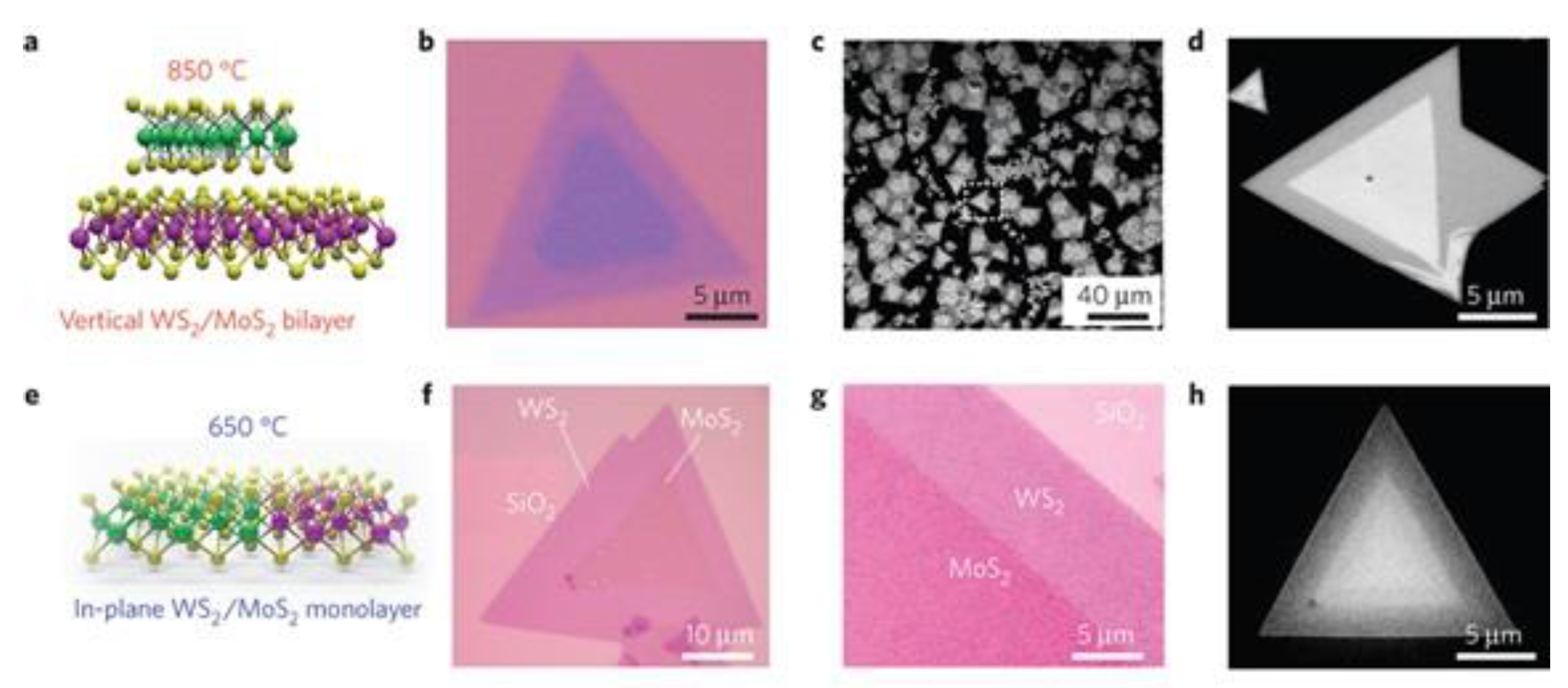
5.2. TMDs Heterostructures
5.3. Thin 2D Graphene/Inorganic Composites
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Huang, X.; Tan, C.; Yin, Z.; Zhang, H. 25th anniversary article: Hybrid nanostructures based on two-dimensional nanomaterials. Adv. Mater. 2014, 26, 2185–2204. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lei, F.; Gao, S.; Pan, B.; Zhou, J.; Xie, Y. Atomically thin tin dioxide sheets for efficient catalytic oxidation of carbon monoxide. Angew. Chem. 2013, 125, 10763–10766. [Google Scholar] [CrossRef]
- Late, D.J.; Liu, B.; Matte, H.S.S.R.; Dravid, V.P.; Rao, C.N.R. Hysteresis in single-layer MoS2 field effect transistors. ACS Nano 2012, 6, 5635. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sakthivel, T.; Seal, S. Recent development in 2D materials beyond graphene. Prog. Mater. Sci. 2015, 73, 44–126. [Google Scholar] [CrossRef]
- Mas-Ballesté, R.; Gómez-Navarro, C.; Gómez-Herrero, J.; Zamora, F. 2D materials: To graphene and beyond. Nanoscale 2011, 3, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Ultrathin two-dimensional nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; O’Hare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Polese, D.; Mattoccia, A.; Giorgi, F.; Pazzini, L.; Ferrone, A.; Di Giamberardino, L.; Maiolo, L.; Pecora, A.; Convertino, A.; Fortunato, G.; et al. Layered double hydroxides intercalated with chlorine used as low temperature gas sensors. Proc. Eng. 2015, 120, 1175–1178. [Google Scholar] [CrossRef]
- Morandi, S.; Prinetto, F.; Di Martino, M.; Ghiotti, G.; Lorret, O.; Tichit, D.; Malagù, C.; Vendemiati, B.; Carotta, M.C. Synthesis and characterisation of gas sensor materials obtained from Pt/Zn/Al layered double hydroxides. Sens. Actuators B Chem. 2006, 118, 215–220. [Google Scholar] [CrossRef]
- Yin, H.; Tang, Z. Ultrathin two-dimensional layered metal hydroxides: An emerging platform for advanced catalysis, energy conversion and storage. Chem. Soc. Rev. 2016, 45, 4873–4891. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.H.; Xu, D.M.; Guan, M.Y.; Guo, Y.; Qi, Q.; Li, G.D. ZnO/Al2O3/CeO2 composite with enhanced gas sensing performance. Sens. Actuators B Chem. 2013, 177, 1134–1141. [Google Scholar] [CrossRef]
- Neri, G. First fifty years of chemoresistive gas sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Sun, Z.; Liao, T.; Dou, Y.; Hwang, S.M.; Park, M.-S.; Jiang, L.; Kim, J.H.; Dou, S.X. Generalized self-assembly of scalable two-dimensional transition metal oxide nanosheets. Nat. Commun. 2014, 5, 3813. [Google Scholar] [CrossRef] [PubMed]
- Radisavljevic, B.; Radenovic, A.; Brivio, V.; Giacometti, J.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 2011, 331, 568. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, Y.H.; Park, S.Y.; Kim, S.Y.; Jang, H.W. Two-Dimensional Transition Metal Disulfides for Chemoresistive Gas Sensing: Perspective and Challenges. Chemosensors 2017, 5, 15. [Google Scholar] [CrossRef]
- Liu, C.; Lu, H.; Zhang, J.; Yang, Z.; Zhu, G.; Yin, F.; Gao, J.; Chen, C.; Xin, X. Abnormal p-type sensing response of TiO2 nanosheets with exposed {001} facets. J. Alloy. Compd. 2017, 705, 112–117. [Google Scholar] [CrossRef]
- Shavanova, K.; Bakakina, Y.; Burkova, I.; Shtepliuk, I.; Viter, R.; Ubelis, A.; Beni, V.; Starodub, N.; Yakimova, R.; Khranovskyy, V. Application of 2D non-graphene materials and 2D oxide nanostructures for biosensing technology. Sensors 2016, 16, 223. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, K.Y.; Choi, Y.R.; Shim, Y.-S.; Jeon, J.-M.; Lee, J.-H.; Kim, S.Y.; Han, S.; Jang, H.W. Ultrasensitive reversible oxygen sensing by using liquid-exfoliated MoS2 nanoparticles. J. Mater. Chem. A 2016, 4, 6070–6076. [Google Scholar] [CrossRef]
- Nah, J.; Kumar, S.B.; Fang, H.; Chen, Y.-Z.; Plis, E.; Chueh, Y.-L.; Krishna, S.; Guo, J.; Javey, A. Quantum size effects on the chemical sensing performance of two-dimensional semiconductors. J. Phys. Chem. C 2012, 116, 9750–9754. [Google Scholar] [CrossRef]
- Gurlo, A.; Riedel, R. In situ and operando spectroscopy for assessing mechanisms of gas sensing. Angew. Chem. 2007, 46, 3826–3848. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.L.; Chen, L.; Liu, G.; Abbas, A.N.; Fathi, M.; Zhou, C.W. High-performance chemical sensing using Schottky-contacted chemical vapor deposition grown monolayer MoS2 transistors. ACS Nano 2014, 8, 5304–5314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured materials for room-temperature gas sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.Z.; Ge, W.; Carey, B.; Daeneke, T.; Rotbart, A.; Shan, W.; Wang, Y.; Fu, Z.; Chrimes, A.F.; Wlodarski, W.; et al. Physisorption-based charge transfer in two-dimensional SnS2 for selective and reversible NO2 gas sensing. ACS Nano 2015, 9, 10313–10323. [Google Scholar] [CrossRef] [PubMed]
- Kannan, P.K.; Late, D.J.; Morgan, H.; Rout, C.S. Recent developments in 2D layered inorganic nanomaterials for sensing. Nanoscale 2015, 7, 13293–13312. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, G.; Li, L. Synthesis Strategies about 2D Materials in Two-Dimensional Materials—Synthesis, Characterization and Potential Applications; Nayak, P.K., Ed.; Intech: Rijeka, Croatia, 2016. [Google Scholar]
- Sun, Y.F.; Cheng, H.; Gao, S.; Liu, Q.H.; Sun, Z.H.; Xiao, C.; Wu, C.Z.; Wei, S.Q.; Xie, Y. Atomically thick bismuth selenide freestanding single layers achieving enhanced thermoelectric energy harvesting. J. Am. Chem. Soc. 2012, 134, 20294. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, J.; Yin, Z.Y.; Zhang, H. Preparation and applications of mechanically exfoliated single and multi-layer MoS2 and WSe2 nanosheets. Acc. Chem. Res. 2014, 47, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lu, G.; Wang, Y.; Yin, Z.; Cong, C.; He, Q.; Wang, L.; Ding, F.; Yu, T.; Zhang, H. Mechanical exfoliation and characterization of single and few-layer nanosheets of WSe2, TaS2, and TaSe2. Small 2013, 9, 1974–1981. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-G.; Ko, Y.-D.; Choi, K.J.; Park, J.-G.; Kim, D.-W. Fabrication of tin monosulfide nanosheet arrays using laser ablation. Appl. Phys. A 2011, 103, 505–510. [Google Scholar] [CrossRef]
- Yoo, D.; Kim, M.; Jeong, S.; Han, J.; Cheon, J. Chemical synthetic strategy for single-layer transition-metal chalcogenides. J. Am. Chem. Soc. 2014, 136, 14670–14673. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.P.; Yin, Z.Y.; Zhu, J.X.; Huang, X.; Wu, X.J.; Zeng, Z.Y.; Yan, Q.Y.; Zhang, H. A general method for the large-scale synthesis of uniform ultrathin metal sulphide nanocrystals. Nat. Commun. 2012, 3, 1177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Single-layer semiconducting nanosheets: High-yield preparation and device fabrication. Angew. Chem. 2011, 50, 11093–11097. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; McCreary, A.; Briggs, N.; Subramanian, S.; Zhang, K.; Sun, Y.; Li, X.; Borys, N.J.; Yuan, H.; Fullerton-Shirey, S.K.; et al. 2D materials advances: From large scale synthesis and controlled heterostructures to improved characterization techniques, defects and applications. 2D Mater. 2016, 3, 042001. [Google Scholar] [CrossRef]
- Lee, K.; Gatensby, R.; McEvoy, N.; Hallam, T.; Duesberg, G.S. High-performance sensors based on molybdenum disulfide thin films. Adv. Mater. 2013, 25, 6699–6702. [Google Scholar] [CrossRef] [PubMed]
- Di Francia, G.; Alfano, B.; La Ferrara, V. Conductometric gas nanosensors. J. Sens. 2009, 2009, 659275:1–659275:19. [Google Scholar] [CrossRef]
- Zhang, L.; Li, F.; Evans, D.; Duan, X. Structure and surface characteristics of Cu-based composite metal oxides derived from layered double hydroxides. Mater. Chem. Phys. 2004, 87, 402–410. [Google Scholar] [CrossRef]
- Osada, M.; Sasaki, T. Exfoliated oxide nanosheets: New solution to nanoelectronics. J. Mater. Chem. 2009, 19, 2503–2511. [Google Scholar] [CrossRef]
- Osada, M.; Sasaki, T. Two-Dimensional Dielectric Nanosheets: Novel Nanoelectronics From Nanocrystal Building Blocks. Adv. Mater. 2012, 24, 210. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.J.; Patil, A.V.; Dighavkar, C.G.; Thakare, K.S.; Borase, R.Y.; Nandre, S.J.; Deshpande, N.G.; Ahire, R.R. Semiconductor metal oxide compounds based gas sensors: A literature review. Front. Mater. Sci. 2015, 9, 14–37. [Google Scholar] [CrossRef]
- Ji, F.; Ren, X.; Zheng, X.; Liu, Y.; Pang, L.; Jiang, J.; Liu, S. 2D-MoO3 nanosheets for superior gas sensors. Nanoscale 2016, 8, 8696. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, T.; Zhao, R.; Xiao, B.; Li, Z.; Zhang, M. Fast formaldehyde gas sensing response properties of ultrathin SnO2 nanosheets. RSC Adv. 2015, 5, 104574–104581. [Google Scholar] [CrossRef]
- Leonardi, S.G. Two-Dimensional Zinc Oxide Nanostructures for Gas Sensor Applications. Chemosensors 2017, 5, 17. [Google Scholar] [CrossRef]
- Liu, J.Y.; Guo, Z.; Meng, F.L.; Luo, T.; Li, M.Q.; Liu, J.H. Novel porous single-crystalline ZnO nanosheets fabricated by annealing ZnS(en)(0.5) (en = ethylenediamine) precursor. Application in a gas sensor for indoor air contaminant detection. Nanotechnology 2009, 20, 125501. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.H.; Li, Z.; Liu, G.; Xing, J.; Sun, C.H.; Yang, H.G.; Li, C.Z. Ultra-thin anatase TiO2 nanosheets dominated with {001} facets: Thickness-controlled synthesis, growth mechanism, and water-splitting properties. CrystEngComm 2011, 13, 1378–1383. [Google Scholar] [CrossRef]
- Cho, B.; Hahm, M.G.; Choi, M.; Yoon, J.; Kim, A.R.; Lee, Y.-J.; Park, S.-G.; Kwon, J.-D.; Kim, C.S.; Song, M.; et al. Charge-transfer-based gas sensing using atomic-layer MoS2. Sci. Rep. 2015, 5, 8052. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yin, Z.; He, Q.; Li, H.; Huang, X.; Lu, G.; Fam, D.W.H.; Tok, A.I.Y.; Zhang, Q.; Zhang, H. Fabrication of single- and multilayer MoS2 film-based field-effect transistors for sensing NO at room temperature. Small 2012, 8, 63–67. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zeng, Z.; Yin, Z.; Li, H.; Wu, S.; Huang, X.; Zhang, H. Fabrication of flexible MoS2 thin-film transistor arrays for practical gas-sensing applications. Small 2012, 8, 2994–2999. [Google Scholar] [CrossRef] [PubMed]
- Late, D.J.; Huang, Y.-K.; Liu, B.; Acharya, J.; Shirodkar, S.N.; Luo, J.; Yan, A.; Charles, D.; Waghmare, U.V.; Dravid, V.; et al. Sensing behavior of atomically thin-layered MoS2 transistors. ACS Nano 2013, 7, 4879–4891. [Google Scholar] [CrossRef] [PubMed]
- Perkins, F.K.; Friedman, A.L.; Cobas, E.; Campbell, P.M.; Jernigan, G.G.; Jonker, B.T. Chemical vapor sensing with monolayer MoS2. Nano Lett. 2013, 13, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Donarelli, M.; Prezioso, S.; Perrozzi, F.; Bisti, F.; Nardone, M.; Giancaterini, L.; Cantalini, C.; Ottaviano, L. Response to NO2 and other gases of resistive chemically exfoliated MoS2-based gas sensors. Sens. Actuators B Chem. 2015, 207, 602–613. [Google Scholar] [CrossRef]
- Cho, B.; Kim, A.R.; Park, Y.; Yoon, J.; Lee, Y.-J.; Lee, S.; Yoo, T.J.; Kang, C.G.; Lee, B.H.; Ko, H.C.; Kim, D.-H.; Hahm M., G. Bifunctional sensing characteristics of chemical vapor deposition synthesized atomic-layered MoS2. ACS Appl. Mater. Interfaces 2015, 7, 2952–2959. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Tolentino, L.; Yang, Z.; Song, X.; Zhang, W.; Chen, Y.; Wong, C. High-concentration aqueous dispersions of MoS2. Adv. Funct. Mater. 2013, 23, 3577. [Google Scholar] [CrossRef]
- Brousse, T.; Lee, S.M.; Pasquereau, L.; Defives, D.; Schleich, D.M. Composite negative electrodes for lithium ion cells. Solid State Ionics 1998, 115, 51–56. [Google Scholar] [CrossRef]
- Chang, H.J.; In, E.; Kong, K.J.; Lee, J.O.; Choi, Y.M.; Ryu, B.H. First-principles studies of SnS2 nanotubes: A potential semiconductor nanowire. J. Phys. Chem. B 2005, 109, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Huo, L.; Wang, H.; Zhang, H.; Yang, J.; Wei, P. Hydrothermal growth and gas sensing property of flower-shaped SnS2 nanostructures. Nanotechnology 2006, 17, 2918–2924. [Google Scholar] [CrossRef]
- Bharatula, L.D.; Erande, M.B.; Mulla, I.S.; Rout, C.S.; Late, D.J. SnS2 nanoflakes for efficient humidity and alcohol sensing at room temperature. RSC Adv. 2016, 6, 105421–105427. [Google Scholar] [CrossRef]
- Li, Y.; Leonardi, S.G.; Bonavita, A.; Neri, G.; Wlodarski, W. Two-dimensional (2D) SnS2-based oxygen sensor. Procedia Eng. 2016, 168, 1102–1105. [Google Scholar] [CrossRef]
- Lan, C.; Li, C.; Yin, Y.; Liu, Y. Large-area synthesis of monolayer WS2 and its ambient-sensitive photo-detecting performance. Nanoscale 2015, 7, 5974–5980. [Google Scholar] [CrossRef] [PubMed]
- Huo, N.; Yang, S.; Wei, Z.; Li, S.-S.; Xia, J.-B.; Li, J. Photoresponsive and gas sensing field-effect transistors based on multilayer WS2 nanoflakes. Sci. Rep. 2014, 4, 5209. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.; Li, Z.; Wang, J.; Zhang, J. WS2 nanoflakes based selective ammonia sensors at room temperature. Sens. Actuators B Chem. 2017, 240, 273–277. [Google Scholar] [CrossRef]
- Jha, R.K.; Guha, P.K. Liquid exfoliated pristine WS2 nanosheets for ultrasensitive and highly stable chemiresistive humidity sensors. Nanotechnology 2016, 27, 475503. [Google Scholar] [CrossRef] [PubMed]
- Kuru, C.; Choi, D.; Kargar, A.; Liu, C.H.; Yavuz, S.; Choi, C.; Jin, S.; Bandaru, P.R. High-performance flexible hydrogen sensor made of WS2 nanosheet–Pd nanoparticle composite film. Nanotechnology 2016, 27, 195501. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Peng, L.; Wu, C.; Sun, X.; Hu, S.; Lin, C.; Dai, J.; Yang, J.; Xie, Y. Ultrathin nanosheets: Giant moisture responsiveness of VS2 ultrathin nanosheets for novel touchless positioning interface. Adv. Mater. 2012, 24, 1969–1974. [Google Scholar] [CrossRef] [PubMed]
- Late, D.J.; Doneux, T.; Bougouma, M. Single-layer MoSe2 based NH3 gas sensor. Appl. Phys. Lett. 2014, 105, 233103. [Google Scholar] [CrossRef]
- Feng, Z.; Xie, Y.; Chen, J.; Yu, Y.; Zheng, S.; Zhang, R.; Li, Q.; Chen, X.; Sun, C.; Zhang, H. Highly sensitive MoTe2 chemical sensor with fast recovery rate through gate biasing. 2D Mater. 2017, 4, 025018. [Google Scholar] [CrossRef]
- Sajjad, M.; Montes, E.; Singh, N.; Schwingenschlög, U. Superior gas sensing properties of monolayer PtSe2. Adv. Mater. Interfaces 2017, 4, 1600911. [Google Scholar] [CrossRef]
- Yim, C.; Lee, K.; McEvoy, N.; O’Brien, M.; Riazimehr, S.; Berner, N.C.; Cullen, C.P.; Kotakoski, J.; Meyer, J.C.; Lemme, M.C.; et al. High-performance hybrid electronic devices from layered PtSe2 films grown at low temperature. ACS Nano 2016, 10, 9550–9558. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wang, Y.; Qiao, L.; Bing, Y.; Zou, B.; Zheng, W. Synthesis and the improved sensing properties of hierarchical SnO2 hollow nanosheets with mesoporous and multilayered interiors. Sens. Actuators B Chem. 2016, 222, 354–361. [Google Scholar] [CrossRef]
- Tan, J.; Dun, M.; Li, L.; Zhao, J.; Tan, W.; Lin, Z.; Huang, X. Synthesis of hollow and hollowed-out Co3O4 microspheres assembled by porous ultrathin nanosheets for ethanol gas sensors: Responding and recovering in one second. Sens. Actuators B Chem. 2017, 249, 44–52. [Google Scholar] [CrossRef]
- Tan, J.; Huang, X. Ultra-thin nanosheets-assembled hollowed-out hierarchical α-Fe2O3 nanorods: Synthesis via an interface reaction route and its superior gas sensing properties. Sens. Actuators B Chem. 2016, 237, 159–166. [Google Scholar] [CrossRef]
- Hong, D.; Zhang, J.; Rehman, A.U.; Gong, L.; Zhou, J.; Kan, K.; Li, L.; Shi, K. One-step synthesis of hierarchical Ni–Fe–Al layered double hydroxide with excellent sensing properties for NOx at room temperature. RSC Adv. 2016, 6, 103192–103198. [Google Scholar] [CrossRef]
- Kang, J.; Tongay, S.; Zhou, J.; Li, J.; Wu, J. Band offsets and heterostructures of two-dimensional semiconductors. Appl. Phys. Lett. 2013, 102, 012111. [Google Scholar] [CrossRef]
- Gong, Y.; Lin, J.; Wang, X.; Shi, G.; Lei, S.; Lin, Z.; Zou, X.; Ye, G.; Vajtai, R.; Yakobson, B.I.; et al. Vertical and in-plane heterostructures from WS2/MoS2 monolayers. Nat. Mater. 2014, 13, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Lin, N.; Ren, H.; Tang, C.; Yang, L.; Zhao, X. Gas adsorption on MoS2/WS2 in-plane heterojunctions and the I–V response: A first principles study. RSC Adv. 2016, 6, 17494. [Google Scholar] [CrossRef]
- Chua, C.K.; Loo, A.H.; Pumera, M. Nanostructured MoS2 nanorose/graphene nanoplatelet hybrids for electrocatalysis. Chem. Eur. J. 2016, 22, 5969. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.A.; Dar, M.A.; Gul, R.; Baek, J.-B. Graphene and molybdenum disulfide hybrids: Synthesis and applications. Mater. Today 2015, 18, 286–298. [Google Scholar] [CrossRef]
- Mohan, P.; Yang, J.; Jena, A.; Shin, H.S. VS2/rGO hybrid nanosheets prepared by annealing of VS4/rGO. J. Solid State Chem. 2015, 224, 82–87. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011, 133, 7296. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.; Yoon, J.; Lim, S.K.; Kim, A.R.; Kim, D.-H.; Park, S.-G.; Kwon, J.-D.; Lee, Y.-J.; Lee, K.-H.; Lee, B.H.; et al. Chemical sensing of 2D graphene/MoS2 heterostructure device. ACS Appl. Mater. Interfaces 2015, 7, 16775–16780. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neri, G. Thin 2D: The New Dimensionality in Gas Sensing. Chemosensors 2017, 5, 21. https://doi.org/10.3390/chemosensors5030021
Neri G. Thin 2D: The New Dimensionality in Gas Sensing. Chemosensors. 2017; 5(3):21. https://doi.org/10.3390/chemosensors5030021
Chicago/Turabian StyleNeri, Giovanni. 2017. "Thin 2D: The New Dimensionality in Gas Sensing" Chemosensors 5, no. 3: 21. https://doi.org/10.3390/chemosensors5030021
APA StyleNeri, G. (2017). Thin 2D: The New Dimensionality in Gas Sensing. Chemosensors, 5(3), 21. https://doi.org/10.3390/chemosensors5030021




