1. Introduction
Diffusion in polymers is a new application to be studied in the optical sensors field. Usually the diffusion in polymers is used in the field of food packaging. Diffusion of organic solvents in polymers is done in three main steps. Firstly, adsorption onto the polymer surface. Secondly, diffusion through the polymer molecules. Finally, desorption of the diffusant to another phase. The reason why the diffusion through polymers occurs is the random motion of the individual molecules. The force that drives the transport process is the concentration of the substance difference between the first and the second phase. The main task of the diffusion is to equalize the concentration of the diffusant into the polymer membrane [
1]. There are many factors that affect the diffusion into polymers depending on the nature of the polymer and the diffusant. Some of these factors are the molecular size, the state of the diffusant, the structure of the polymer, the solubility of the solvent within the polymer, the temperature that will lead to diffusant vapor pressure, and the surface energy [
2].
Micro-optical cavities have been used as optical sensors with high sensitivities due to their high quality factor
that can be exhibited, where
and
are the wavelength of the light (~1.3146 µm) and the resonance line-width respectively. They can also be used as sensing elements for different physical quantities such as pressure [
3], vibration [
4], temperature [
5], and magnetic field [
6]. Whispering gallery optical modes (WGM) are the main sensing phenomenon used in this paper. When the light is coupled to the cavity through the tapered section of the single-mode optical fiber, the WGM would be seen as sharp dips on the transmission spectrum. By tracking the WGM shifts (d
λ), any minute perturbation in the environment surrounding the cavity can be determined. The optical resonance is the condition that we have to reach to start using these micro-cavities as sensors. For the first-order approximation, once the round trip of the light is equal to a multiple integer of the light wavelength, the optical resonance is satisfied. Equation 1 attempts to describe that phenomenon.
where
no and
R are the refractive index and radius of the micro-cavity, respectively, and
l is a multiple integer indicating the circumferential mode number. Any infinitesimal change in the microsphere size or the index of refraction will lead to a change in the wavelength, as seen in the equation below.
2. Proposed Sensor Configuration, Fabrication, and Opto-Electronic Setup
Studying the effect of the driving force of diffusion on these sensing elements using a high-quality factor resonator (with
Q ≈ 10
7) is the main concern of this paper. We prepared the cavity for that function; the evanescent field outside the fiber needs to be extended in order to achieve efficient optical coupling between the micro-optical cavity and the fiber, as reported in [
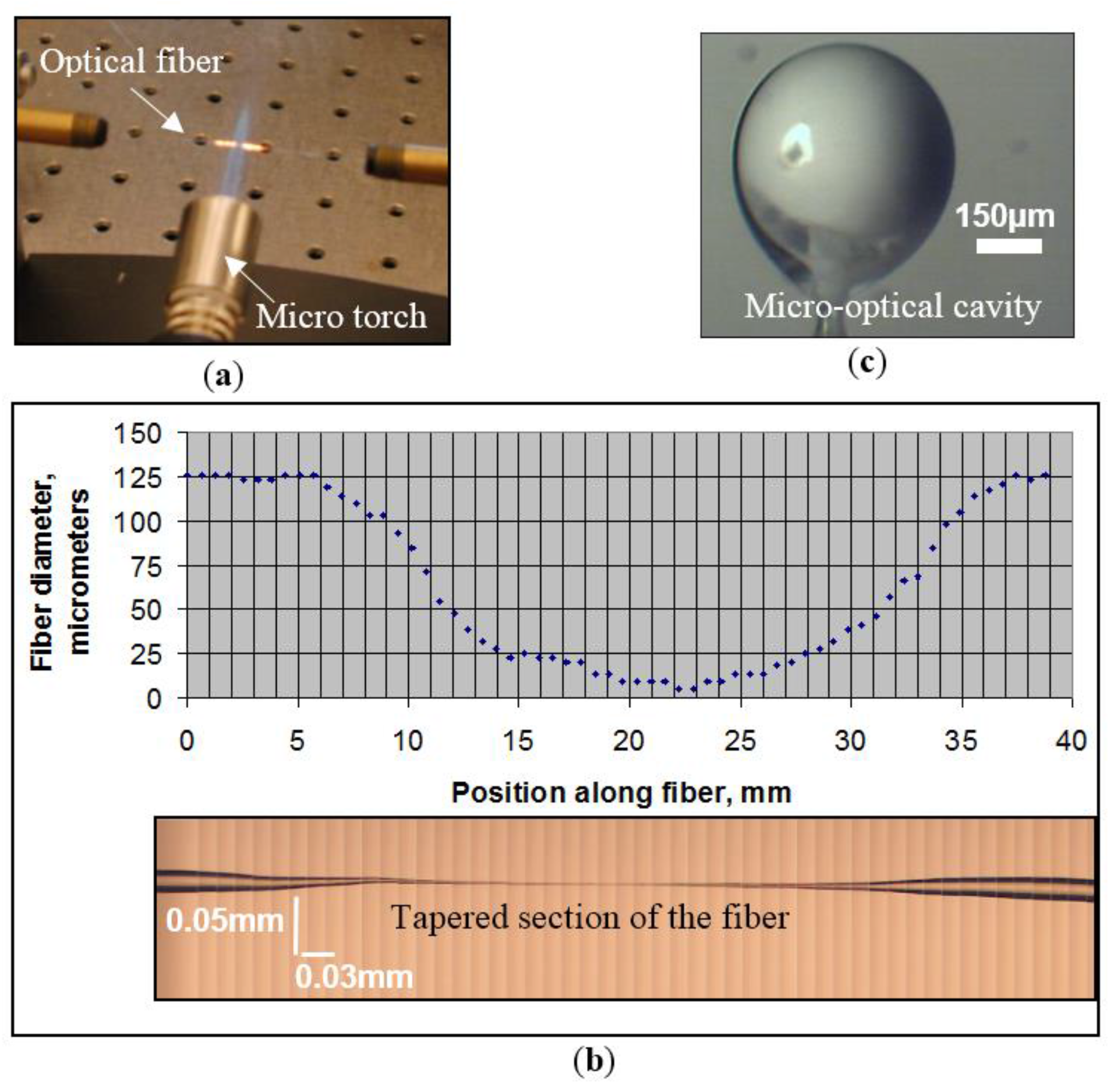
7]. This can be achieved by first removing the outermost protective buffer layer and then heating and stretching a portion of the fiber (with both the cladding and core) to create a tapered section with a minimum waist of 10 to 20 μm, as seen in
Figure 1a,b, respectively. A micro-torch filled with butane is used in the present work to prepare a tapered section of the fiber.
The optical cavity is manufactured using a ~5 cm long piece of optical fiber which is first stripped of its plastic cladding using an optical stripper and then heated and stretched to provide a stem end (with a tip diameter of ~60 μm). The tip of this silica fiber is then dipped into a 60:1 polydimethylsiloxane (PDMS) polymer pool. Surface tension and the weight of the mixture allow for the formation of a cavity at the end of the stem, as shown in
Figure 1c. The micro-cavity/stem assembly is placed in an oven at a temperature of ~100 °C for four hours to allow for proper curing of the polymer material.
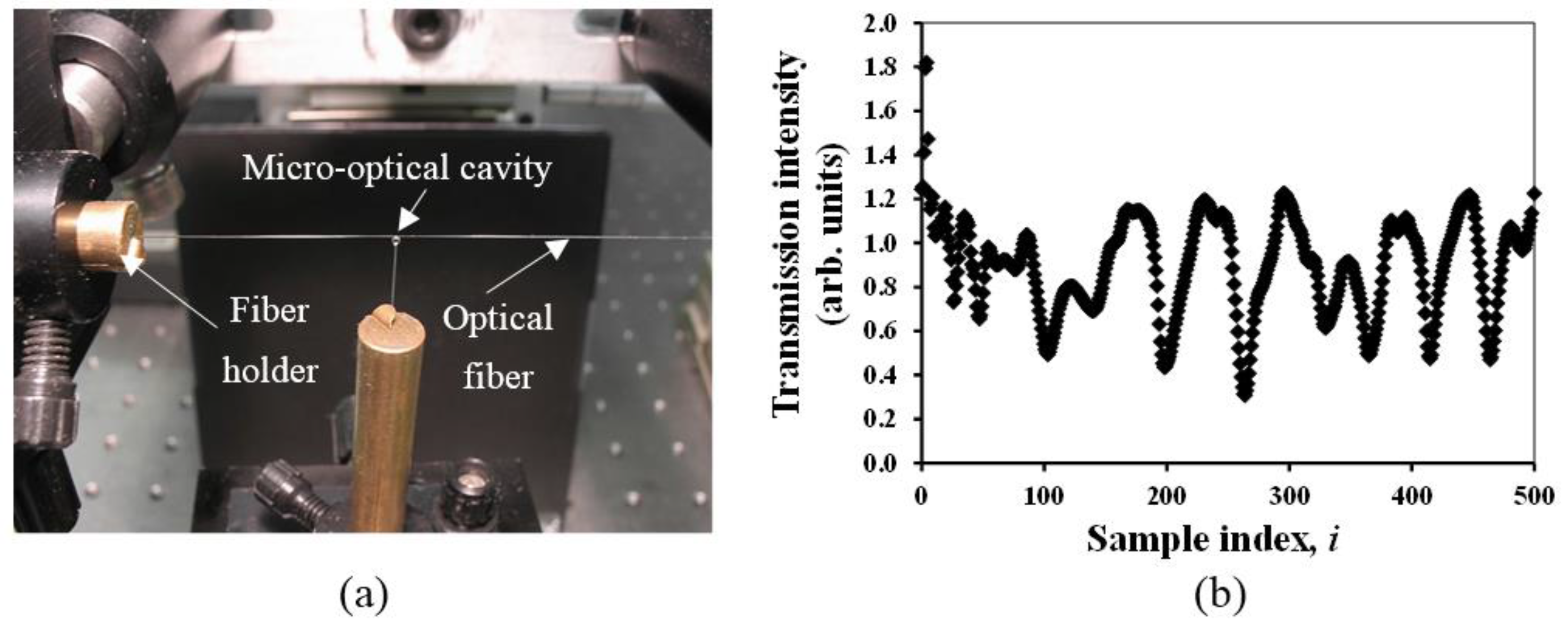
Figure 2 shows laser light coupled to the cavity using the tapered fiber.
Figure 3 shows a block diagram and a photograph of the opto-electronic setup for the proposed sensor design. The function generator supplies the tuning waveform to the laser controller, which thusly presents the same waveform in the electrical current to the laser to modulate its wavelength. The laser output is coupled directly into a single mode optical fiber which is cut up into two parts: one side is coupled to the sensor resonator (with 90% of the total intensity of the light) supplying the transmission spectra, and the second side is used as a reference signal (with ~10% of the total intensity). Every fiber is ended into a photograph diode as demonstrated. Both the transmission spectra and the reference signal are sampled utilizing a 16-bit analog-to-digital converter and processed by a host machine. The reference is utilized to normalize the transmission spectra. A software module, created in-house, investigates the normalized transmission spectra to monitor the WGM shifts.
The driving force of diffusion is acting on the outer surface of the sphere leading the vapor molecules to fill the gaps between the polymer molecules, in turn increasing the mass of the sphere, causing an increase in the sphere diameter and change in the optical cavity and its WGM.
Figure 2 shows a tapered optical fiber used to couple the IR tunable laser where the WGM would be seen as sharp dips in the transmission spectrum (see
Figure 2b).
3. Analysis and Mathematical Background
In 1855, Fick proposed a mathematical technique to represent the law of mass diffusion by adopting the mathematical equation of heat conduction derived earlier by Fourier 1822 [
8]. The differential equation representing the one-dimensional diffusion is
where
J is the flux or the rate of transfer per unit area of section,
D is the diffusion coefficient,
C is the concentration, and
x is the direction of diffusion.
In polymeric and non-homogeneous systems, the diffusion coefficient depends on the concentration and varies from point to point. In most applications, diffusion is restricted to one direction, so a gradient of concentration is present and diffusion only occurs along the
x-axis [
9]. The differential equation representing the two-dimensional diffusion is given by
In our case where the diffusion is radial, the diffusion equation for a constant diffusion coefficient takes the form.
where
R is the radius of the optical cavity and
r is the distance from the center of the sphere and any point inside it as shown in
Figure 4.
We can assume a function
U where
r is the only variable
and C is constant referring to the concentration, then Equation (5) can be represented as
In this paper, we are dealing with the non-steady-state case with constant surface concentration and initial distribution
f(
r) this method is called “time lag method assuming a constant diffusion coefficient”. This means that
U can be expressed as
where
C0 is the concentration of the diffusant on the surface of the polymer.
If the initial concentration of the diffusant in the sphere is uniform,
C1 and the surface concentration is maintained,
C0 and the concentration at the center is given by the limit as
, then the solution of the differential Equation becomes
Equation (8) can be represented in terms of the diffusant mass instead of the concentrations. The total amount of diffusing substance entering or leaving the sphere is given by
where
Mt is the diffusant mass entering the sphere while time goes until it reaches
M∞, which is the equilibrium or the saturation mass.
This equation can be accurately replaced for
, as indicated by Reference [
10].
and can be approximated with very little error for
by
By substituting in Equations (10) and (11), we can estimate the diffusant mass entering the sphere at time t which acts at the external force acting on the optical sphere until the sphere reaches the equilibrium state, where the refractive and swelling forces are equal. Where the refractive force is a gradient force on the particles are (generally) attracted towards high intensity regions, which we refer to the effect on the interface between two different mediums. Because the resonator is made from polymeric material submersed in vapor medium, this kind of force will be generated.
Equations (10) and (11) are in terms of two main constants, which are the equilibrium mass
M∞ and the diffusion constant
D. To calculate
M∞, Equation (12) can be used assuming that the surface concentration is approximately equal to the concentration of the organic solvent vapor in the atmosphere.
The diffusion coefficient can be approximately calculated with an error of about 0.001% [
8] by observing the time of adsorption process experimentally for a system in which the diffusion coefficient is constant [
11,
12,
13]. The value of this constant can be determined from Equation (13).
where
t1/2 is the half time of an adsorption process and R is the sphere radius.
Micro-Cavity Sensing Analysis
In order to study the effect of the driving force of diffusion on the micro-optical cavity, the cavity is modeled as a spring with stiffness
Ks and the driving force of diffusion is acting as an external force exerted on it. The diffusion external force is the weight of the diffusant mass entering the sphere. It can be expressed as
So, the equation governing the cavity deformation is given by
such that
δ is the change in the cavity radius and
g is the gravitational acceleration.
A numerical model was conducted to show results for the driving force of diffusion of two different solvents: ethyl alcohol (ethanol, C
2H
6O) and methyl alcohol (methanol, CH
3OH). The numerical models were applied assuming that the solvents were at room temperature (fixed at 25 °C).
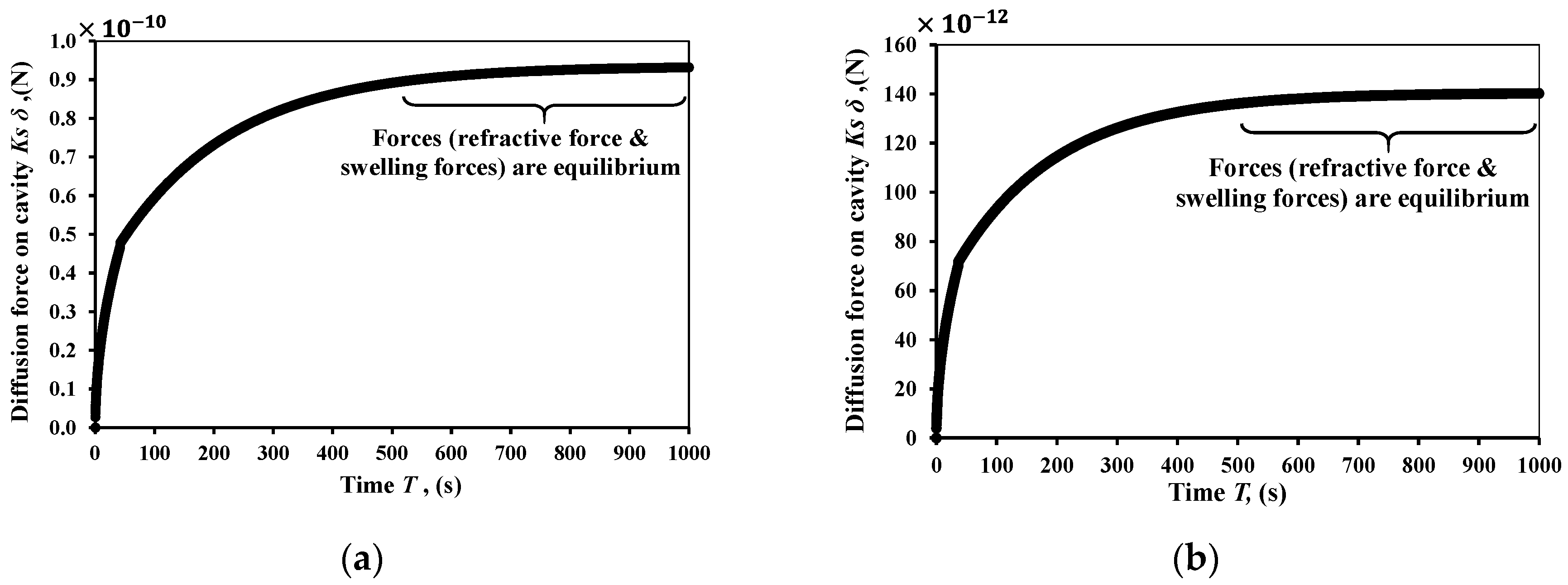
Figure 5 shows the driving force of diffusion numerically of the ethanol solvent and the methanol solvent, respectively. The resulting refractive force operates in opposition to the swelling force. There is a maximum degree of swelling shown in
Figure 5, at which point these two forces are at equilibrium. It is also clear that the maximum degree of swelling for the ethanol is higher than methanol.
A parametric study was carried out to show the effect of the micro-cavity stiffness Ks on the change of WGM shifts (dλ). The study shows that the lower stiffness for the cavity could experience more shifts in its WGM shifts. For the ethanol case, the cavities with Ks = 0.00049 N/m and 0.49 N/m can have WGM shifts when they reach the maximum degree of swelling up to 1000 pm and 1 pm, respectively. This means a three orders of magnitude enhancement of the sensing element just by selecting a proper stiffness or size of the cavity.
5. Results and Discussion
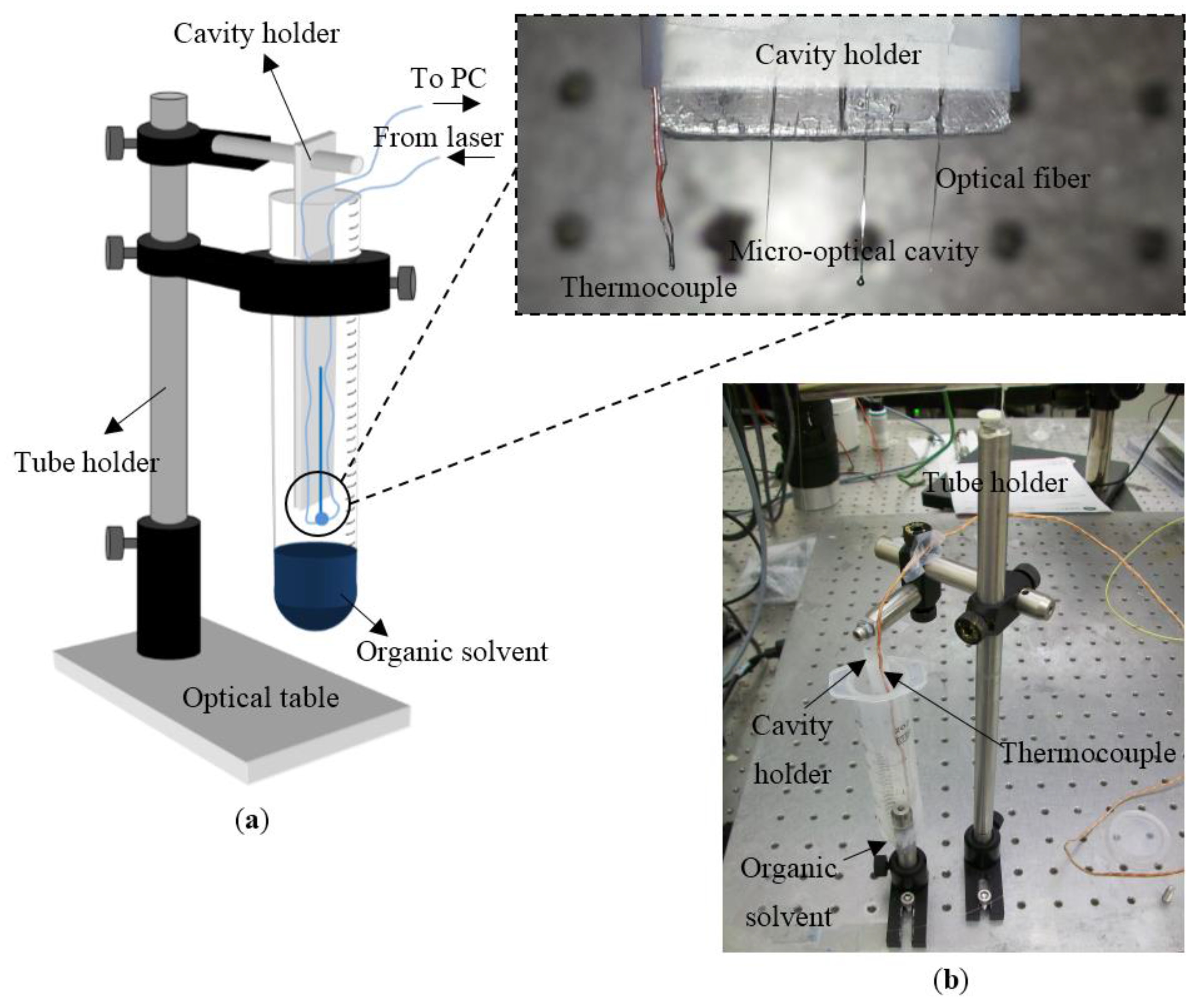
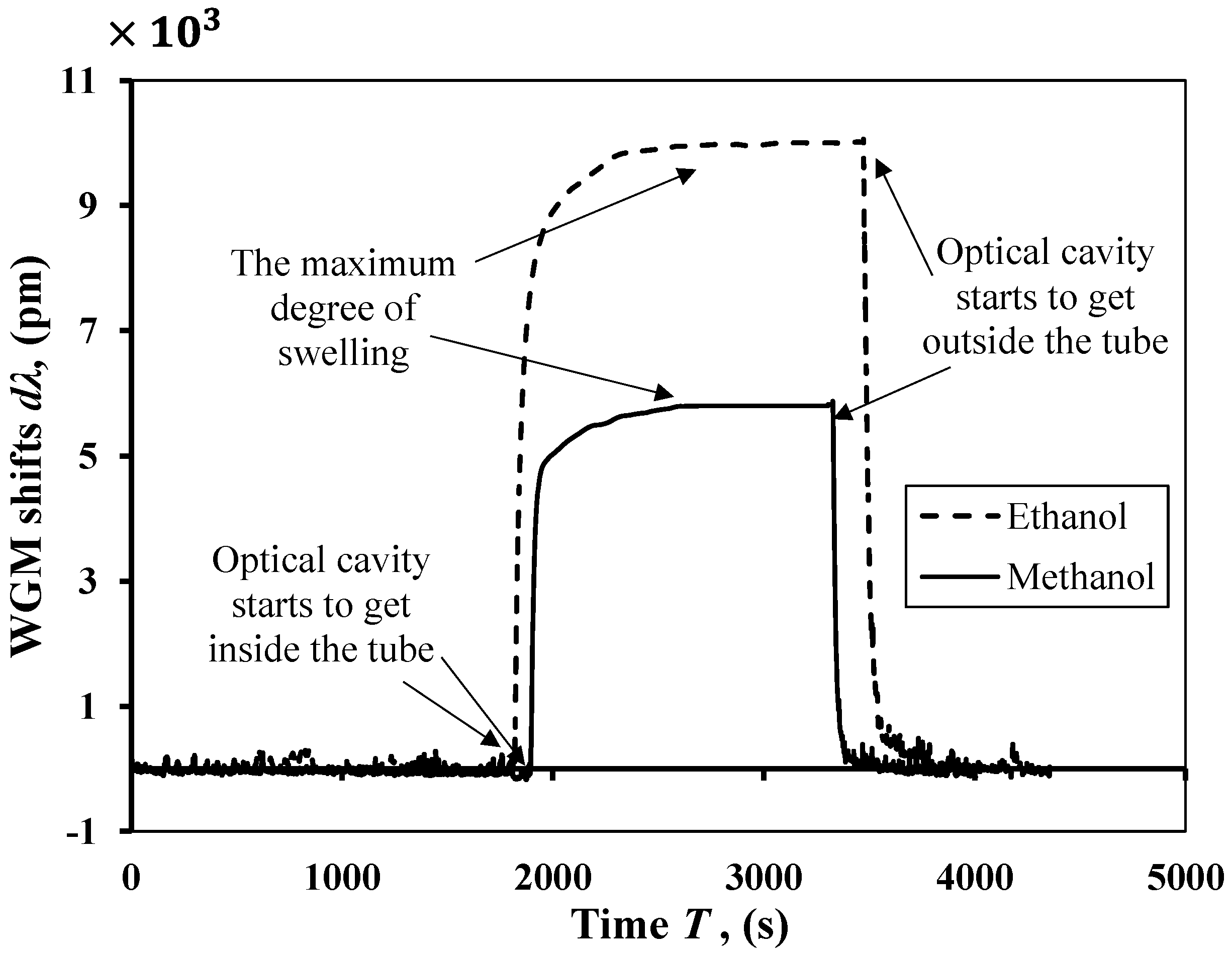
Figure 7 shows the experimental change in WGM shifts due to the driving force of diffusion of ethanol and methanol. The experimental data shows that the sensing element is subjected to the driving force of diffusion at time ~1900 s when the sensing element gets inside the tube filled with solvent. The sensing element starts to experience an increase in its size with respect to the time because the diffusion of solvents fills the network chains of the polymers. After ~2500 s, the cavity reaches the maximum degree of swelling, at which point these two forces are at equilibrium. When the cavity starts to get outside the tube at ~3500 s, it returns to its original size when the experiment was started. From the experimental results in
Figure 7 and by substituting in Equation (13), we can conclude that the diffusion coefficients for the ethanol and methanol solvents are 3.2667 × 10
11 and 3.7692 × 10
11, respectively. As shown in
Figure 8, the experimental results agree reasonably well with the analytical results of the change in the WGM shifts for each solvent.
The corresponding sensitivity plot is presented in
Figure 9. The best fit of the data using least squares method was used, and indicates a sensitivity of d
λ/d
Ksδ = 9.405 × 10
13 pm/N for ethanol and a sensitivity was d
λ/d
Ksδ = 2.3916 × 10
13 pm/N for methanol. The standard deviation of the data in the case of using ethanol was
σλ = 0.34 pm, and in the case of using methanol it was
σλ = 0.22 pm. The results indicate a swelling force resolution of (d
Ksδ/d
λ)
σλ ≈ 3.61 fN when using ethanol as an organic solvent and (d
Ksδ/d
λ)
σλ ≈ 9.19 fN for methanol.