Microfluidic Electronic Tongue Applied to Soil Analysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bindraban, P.S.; Stoorvogel, J.J.; Jansen, D.M.; Vlaming, J.; Groot, J.J.R. Land quality indicators for sustainable land management: Proposed method for yield gap and soil nutrient balance. Agric. Ecosyst. Environ. 2000, 81, 103–112. [Google Scholar]
- Roberts, D.C.; Brorsen, B.W.; Solie, J.B.; Raun, W.R. Is data needed from every field to determine in-season precision nitrogen recommendations in winter wheat? Precis. Agric. 2013, 14, 245–269. [Google Scholar] [CrossRef]
- Smolka, M.; Puchberger-Enengl, D.; Bipoun, M.; Klasa, A.; Kiczkajlo, M.; Śmiechowski, W.; Sowiński, P.; Krutzler, C.; Keplinger, F.; Vellekoop, M.J. A mobile lab-on-a-chip device for on-site soil nutrient analysis. Precis. Agric. 2017, 18, 152–168. [Google Scholar]
- Viscarra Rossel, R.A.; McBratney, A.B.; Minasny, B. Proximal Soil Sensing; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Soriano-Disla, J.M.; Janik, L.J.; Viscarra Rossel, R.A.; Macdonald, L.M.; McLaughlin, M.J. The performance of visible, near-, and mid-infrared reflectance spectroscopy for prediction of soil physical, chemical, and biological properties. Appl. Spectrosc. Rev. 2014, 49, 139–186. [Google Scholar]
- Vlasov, Y.; Legin, A.; Rudnitskaya, A.; Natale, C.D.; D’Amico, A. Nonspecific sensor arrays (“electronic tongue”) for chemical analysis of liquids. Pure Appl. Chem. 2005, 77, 1965–1983. [Google Scholar] [CrossRef]
- Riul, A., Jr.; Dantas, C.A.R.; Miyazaki, C.M.; Oliveira, O.N., Jr. Recent advances in electronic tongues. Analyst 2010, 135, 2481–2495. [Google Scholar] [CrossRef] [PubMed]
- Toko, K. Taste sensor with global selectivity. Mater. Sci. Eng. C 1996, 4, 69–82. [Google Scholar] [CrossRef]
- Legin, A.; Rudnitskaya, A.; Vlasov, Y.; Di Natale, C.; Mazzone, E.; D’Amico, A. Application of electronic tongue for quantitative analysis of mineral water and wine. Electroanalysis 1999, 11, 814–820. [Google Scholar]
- Krantz-Rülcker, C.; Stenberg, M.; Winquist, F.; Lundström, I. Electronic tongues for environmental monitoring based on sensor arrays and pattern recognition: A review. Anal. Chim. Acta 2001, 426, 217–226. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Alegret, S.; Cáceres, R.; Casadesús, J.; Marfà, O.; Del Valle, M. Nutrient solution monitoring in greenhouse cultivation employing a potentiometric electronic tongue. J. Agric. Food Chem. 2008, 56, 1810–1817. [Google Scholar] [PubMed]
- Oliveira, O.N., Jr.; Pavinatto, F.J.; Constantino, C.J.L.; Paulovich, F.V.; de Oliveira, M.C.F. Information visualization to enhance sensitivity and selectivity in biosensing. Biointerphases 2012, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mimendia, A.; Gutiérrez, J.M.; Alcañiz, J.M.; del Valle, M. Discrimination of soils and assessment of soil fertility using information from an ion selective electrodes array and artificial neural networks. Clean Soil Air Water 2014, 42, 1808–1815. [Google Scholar] [CrossRef]
- Jacesko, S.; Abraham, J.K.; Ji, T.; Varadan, V.K.; Cole, M.; Gardner, J.W. Investigations on an electronic tongue with polymer microfluidic cell for liquid sensing and identification. Smart Mater. Struct. 2005, 14, 1010–1016. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Daikuzono, C.M.; Dantas, C.A.R.; Volpati, D.; Constantino, C.J.L.; Piazzetta, M.H.O.; Gobbi, A.L.; Taylor, D.M.; Oliveira, O.N., Jr.; Riul, A., Jr. Microfluidic electronic tongue. Sens. Actuators B Chem. 2015, 207, 1129–1135. [Google Scholar] [CrossRef]
- Riul, A., Jr.; dos Santos, D.S., Jr.; Wohnrath, K.; Di Tommazo, R.; Carvalho, A.C.P.L.F.; Fonseca, F.J.; Oliveira, O.N., Jr.; Taylor, D.M.; Mattoso, L.H.C. Artificial taste sensor: Efficient combination of sensors made from Langmuir-Blodgett films of conducting polymers and a ruthenium complex and self-assembled films of an azobenzene-containing polymer. Langmuir 2002, 18, 239–245. [Google Scholar] [CrossRef]
- Richardson, J.J.; Björnmalm, M.; Caruso, F. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348, 2491. [Google Scholar] [CrossRef] [PubMed]
- Riul, A., Jr.; Soto, A.M.G.; Mello, S.V.; Bone, S.; Taylor, D.M.; Mattoso, L.H.C. An electronic tongue using polypyrrole and polyaniline. Synth. Met. 2003, 132, 109–116. [Google Scholar] [CrossRef]
- Rencher, A.C. Methods of Multivariate Analysis, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2002. [Google Scholar]
- Paulovich, F.V.; Moraes, M.L.; Maki, R.M.; Ferreira, M.; Oliveira, O.N., Jr.; de Oliveira, M.C.F. Information visualization techniques for sensing and biosensing. Analyst 2011, 136, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Pang-Ning, T.; Steinbach, M.; Kumar, V. Introduction to Data Mining; Pearson Addison-Wesley: Essex, UK, 2006; p. 165. [Google Scholar]
- Rousseeuw, P.J. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef]
- Kato, C.; Kuroda, K.; Takahara, H. Preparation and electrical properties of quaternary ammonium montmorillonite-polystyrene complexes. Clays Clay Miner. 1981, 29, 294–298. [Google Scholar] [CrossRef]
- Bao, Z.; Lovinger, A.J.; Dodabalapur, A. Organic field-effect transistors with high mobility based on copper phthalocyanine. Appl. Phys. Lett. 1996, 69, 3066–3068. [Google Scholar] [CrossRef]
- Nardes, A.M.; Kemerink, M.; Janssen, R.A.J.; Bastiaansen, J.A.M.; Kiggen, N.M.M.; Langeveld, B.M.W.; van Breemen, A.J.J.M.; de Kok, M.M. Microscopic understanding of the anisotropic conductivity of PEDOT:PSS thin films. Adv. Mater. 2007, 19, 1196–1200. [Google Scholar] [CrossRef]
- Electroanalytical Methods—Guide to Experiments and Applications, 2nd ed.; Scholz, F. (Ed.) Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Soares, J.C.; Soares, A.C.; Pereira, P.A.R.; Rodrigues, V.D.C.; Shimizu, F.M.; Melendez, M.E.; Scapulatempo Neto, C.; Carvalho, A.L.; Leite, F.L.; Machado, S.A.S.; et al. Adsorption according to the Langmuir–Freundlich model is the detection mechanism of the antigen p53 for early diagnosis of cancer. Phys. Chem. Chem. Phys. 2016, 18, 8412–8418. [Google Scholar] [CrossRef] [PubMed]
- Olivati, C.A.; Riul, A., Jr.; Balogh, D.T.; Oliveira, O.N., Jr.; Ferreira, M. Detection of phenolic compounds using impedance spectroscopy measurements. Bioprocess Biosyst. Eng. 2009, 32, 41–46. [Google Scholar] [CrossRef] [PubMed]
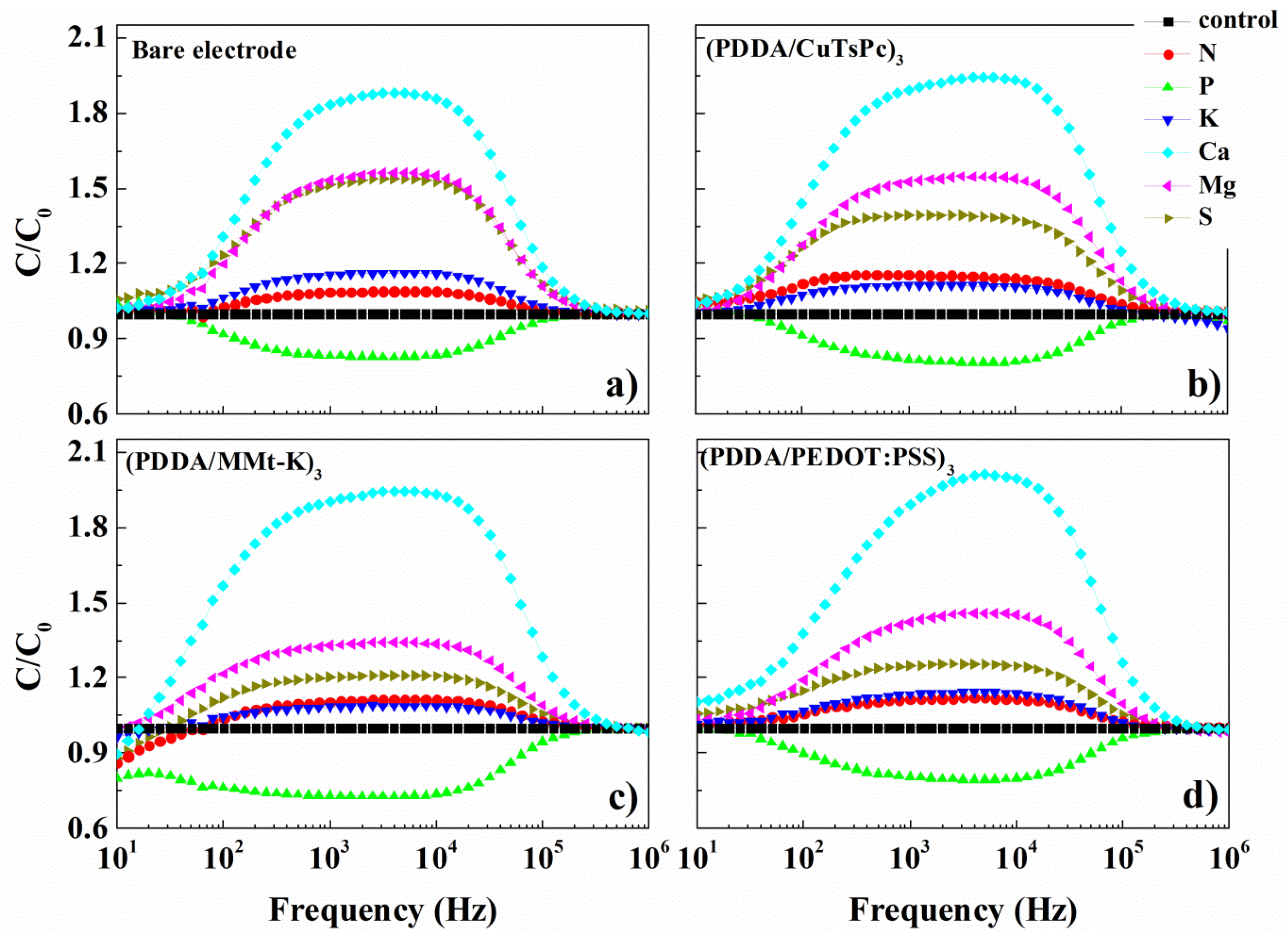

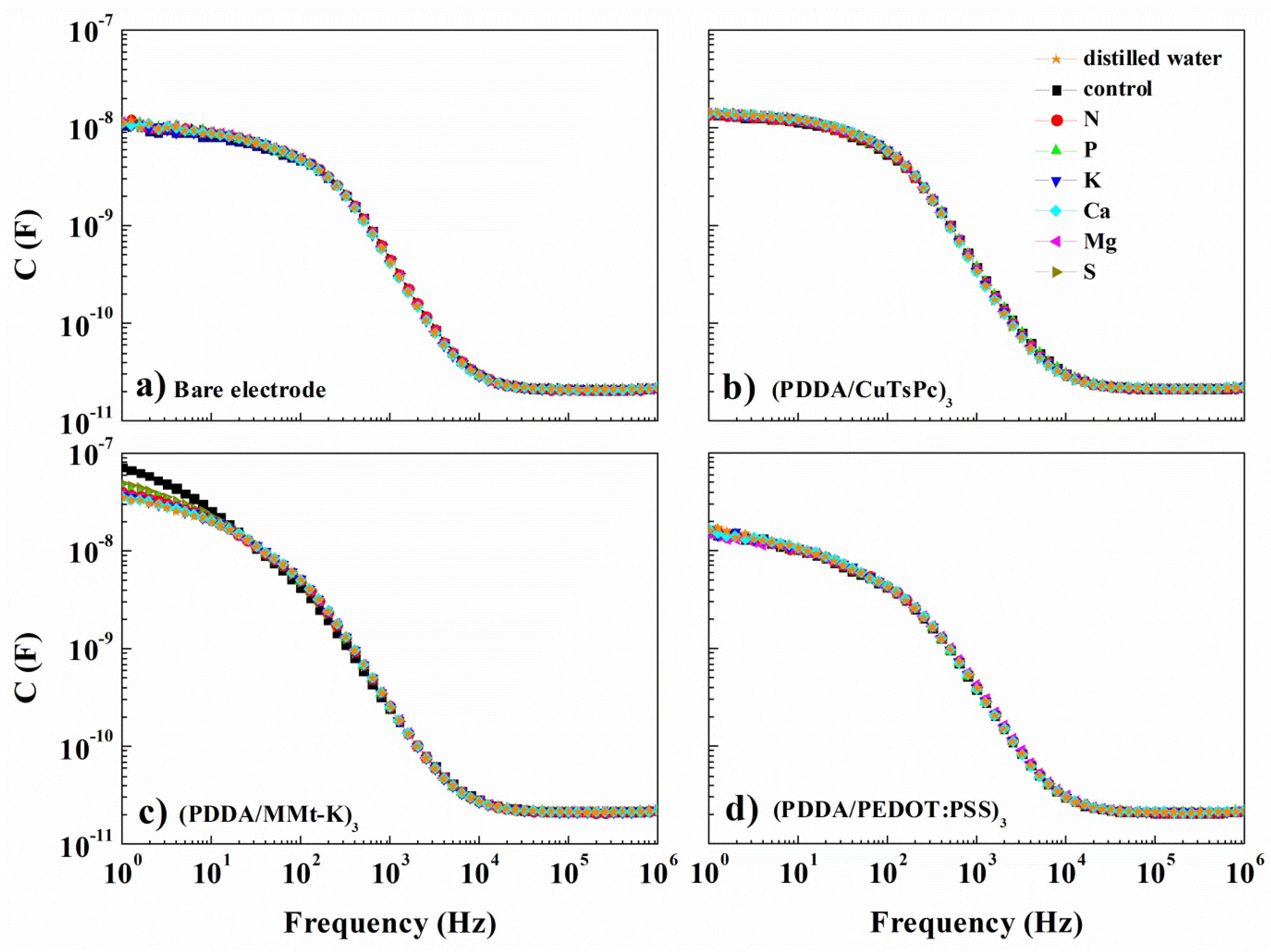
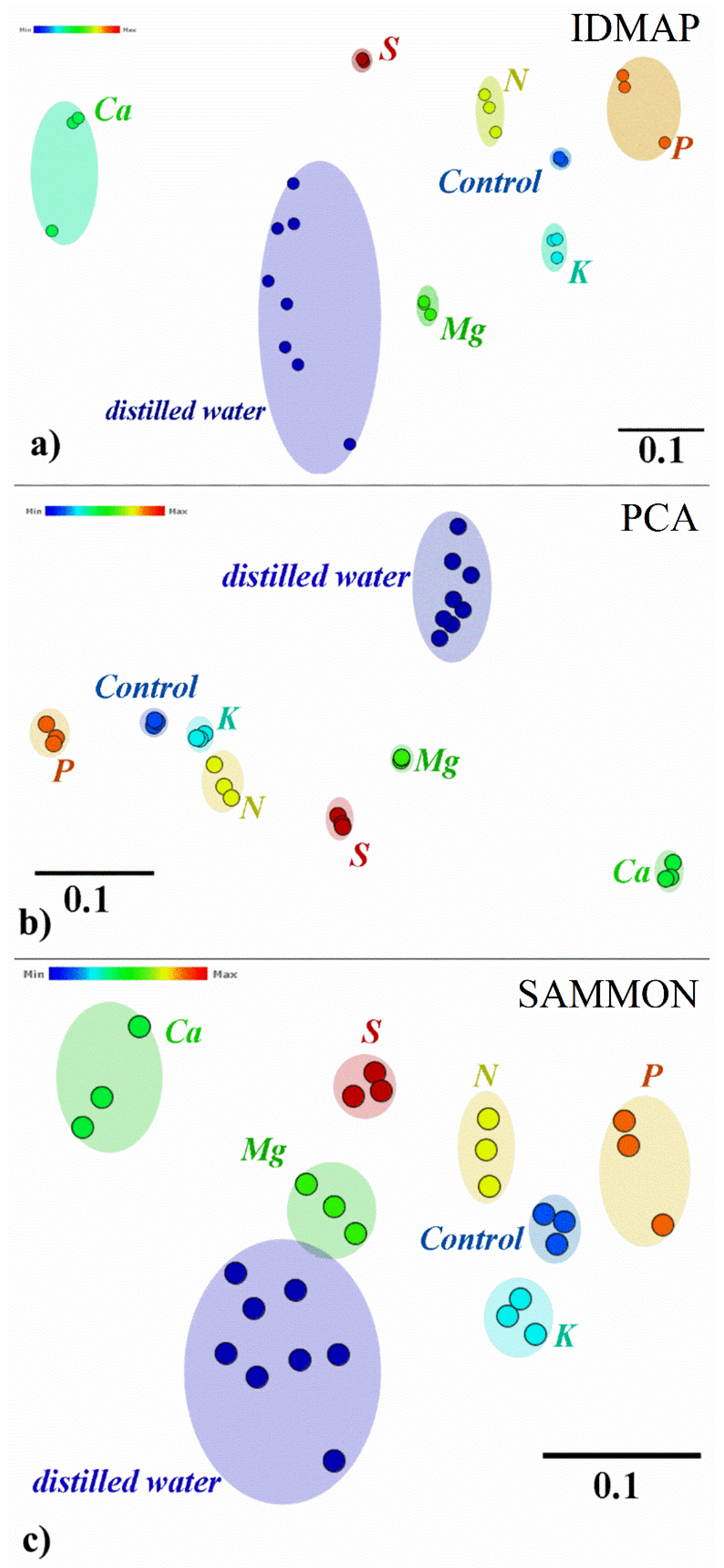

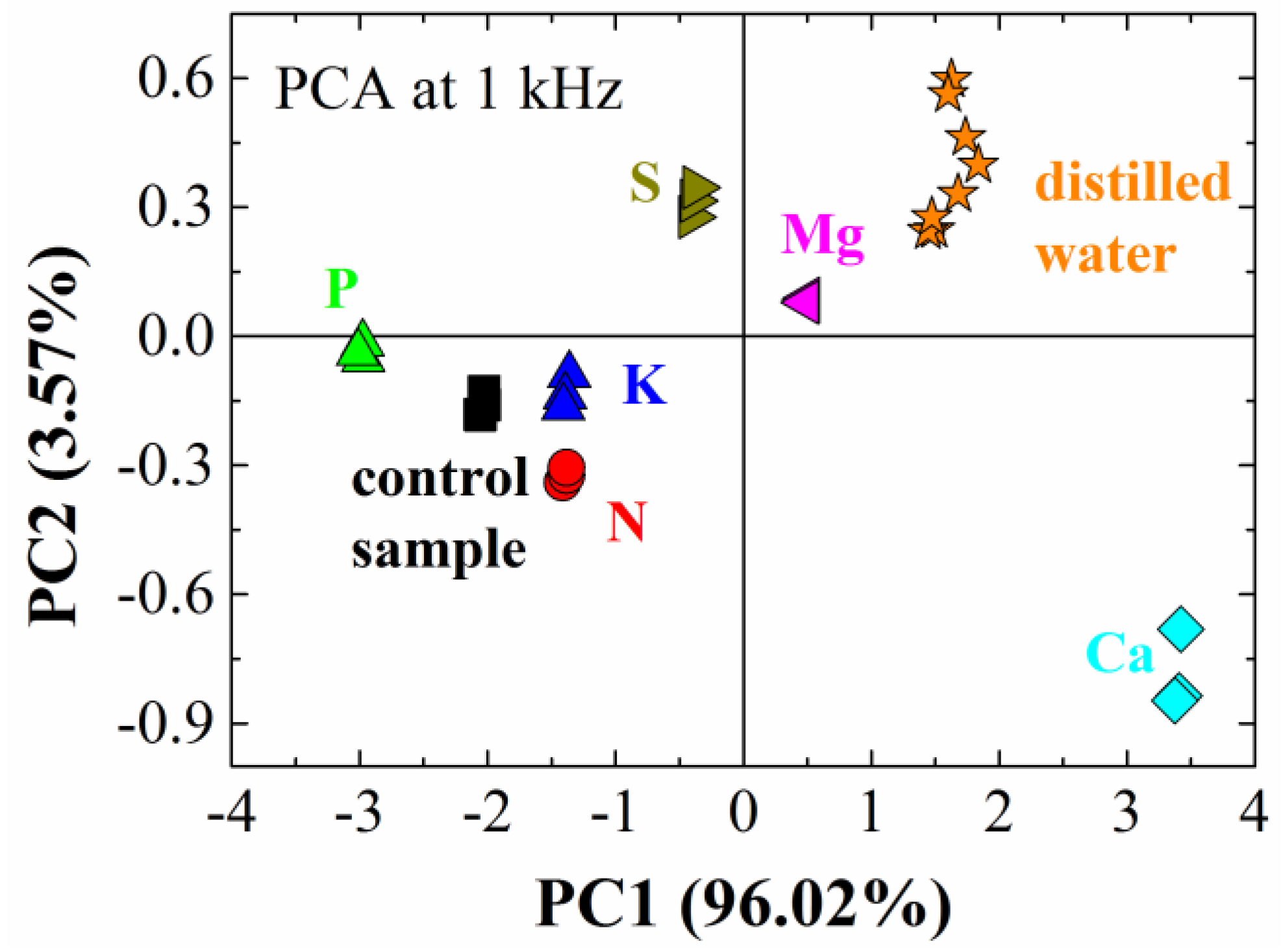
| Silhouette Coefficient Values | |||
|---|---|---|---|
| All Frequencies | Selected Frequencies | Increment (%) | |
| IDMAP | 0.730 | 0.910 | 24.65 |
| PCA | 0.857 | 0.884 | 3.15 |
| SAMMON | 0.667 | 0.848 | 27.13 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braunger, M.L.; Shimizu, F.M.; Jimenez, M.J.M.; Amaral, L.R.; Piazzetta, M.H.d.O.; Gobbi, Â.L.; Magalhães, P.S.G.; Rodrigues, V.; Oliveira, O.N.; Riul, A. Microfluidic Electronic Tongue Applied to Soil Analysis. Chemosensors 2017, 5, 14. https://doi.org/10.3390/chemosensors5020014
Braunger ML, Shimizu FM, Jimenez MJM, Amaral LR, Piazzetta MHdO, Gobbi ÂL, Magalhães PSG, Rodrigues V, Oliveira ON, Riul A. Microfluidic Electronic Tongue Applied to Soil Analysis. Chemosensors. 2017; 5(2):14. https://doi.org/10.3390/chemosensors5020014
Chicago/Turabian StyleBraunger, Maria L., Flávio M. Shimizu, Mawin J. M. Jimenez, Lucas R. Amaral, Maria H. de Oliveira Piazzetta, Ângelo L. Gobbi, Paulo S. G. Magalhães, Varlei Rodrigues, Osvaldo N. Oliveira, and Antonio Riul. 2017. "Microfluidic Electronic Tongue Applied to Soil Analysis" Chemosensors 5, no. 2: 14. https://doi.org/10.3390/chemosensors5020014
APA StyleBraunger, M. L., Shimizu, F. M., Jimenez, M. J. M., Amaral, L. R., Piazzetta, M. H. d. O., Gobbi, Â. L., Magalhães, P. S. G., Rodrigues, V., Oliveira, O. N., & Riul, A. (2017). Microfluidic Electronic Tongue Applied to Soil Analysis. Chemosensors, 5(2), 14. https://doi.org/10.3390/chemosensors5020014







