Abstract
We use a set of three resistive sensors based on undoped multi-walled carbon nanotubes, B-doped multi-walled carbon nanotubes, and N-doped multi-walled carbon nanotubes to study fungal infection in strawberries inoculated with Rhizopus sp. or with Aspergillus sp. section Nigri. We apply tristimulus analysis using the conductance variation of the sensors when exposed to the infected strawberries to distinguish between uninfected strawberries and strawberries infected with Rhizopus sp. or with Aspergillus sp. section Nigri, and to obtain a graphical representation providing a tool for the simple and fast detection and identification of the fungal infection.
1. Introduction
The strawberry fruit is pleasant to the eye and to taste. It has a characteristic flavor, a bright red color, and good consumer acceptance. It can be consumed in natura, or in the form of candies and beverages, having a large market. Strawberries are non-climacteric fruits [1,2], having a slow and steady decline in post-harvest respiratory rate. They only ripen on the plant and do not produce ethylene after harvest, constituting a highly perishable product. They present a limited post-harvest life, different from climacteric fruits that react in the presence of ethylene, maturing even after being separated from the plant and producing ethylene at high rates [3]. If strawberries are harvested in an advanced maturation state, they may be difficult to commercialize because of decomposition and rot; if they are harvested before maturation and without post-harvest ripening, they may show high acidity, astringency, and no flavor [4,5]. After harvesting, careful planning is also required during storage and transport to ensure that the fruits are not degraded by microorganisms [6,7,8].
Some factors that influence strawberry quality after harvest are related to physical, physiological, and pathological damage which may occur during transport [5,9] and storage in the markets, or in the hands of consumers [10,11,12]. Physical damage found in fruits can be the gateway to many pathogens (mainly fungi [13]), causing post-harvest rot [11,12]. Additionally, strawberries constitute an excellent substrate for the development of fungi [14] due to the low pH, high water content, as well as the nutritional composition of the fruit, which consists of sugars, acids, and vitamins. Fungi begin their colonization by feeding on these nutrients, causing the putrefaction of the fruit, which suffers intense metabolic activity as it matures [15,16,17]. Strawberries are commonly susceptible to fungi such as Botrytis sp., Penicillium sp., Phomopsis sp. [18], Aspergillus sp. section Nigri [19], and Rhizopus sp. [20]. Several tests and studies have been conducted on strawberries in order to expand and optimize the strawberry life cycle [21], because the appearance of fungal infection directly affects the commercial value of strawberries, constituting a major cause of fruit rejection and hence reducing the supply to the consumer [22,23].
It is common for fungi to produce volatile organic compounds (VOCs) during fruit infection [24]. These gaseous compounds are made up of various carbon-based mixtures [6,13,16]. Researchers have identified about 250 VOCs released due to fungi metabolism during colonization, when fungi feed on nutrients found in fruit [16,25]. The main VOCs found in this rotting process are iso-amylalcohol, 1-octen-3-ol, and numerous other eight-membered carbon ketones and alcohols [2,9,19,22,25,26].
The discovery of simple procedures to make a range of novel carbon nanostructures has led researchers to use these new materials to develop organic electronic devices [27,28,29], including chemical sensors [30] based on these carbons. The use of carbon nanostructure-based sensors [31,32] to target fruit VOCs constitutes an opportunity to develop strategies to detect strawberry fungi-related VOCs, and to perform real-time monitoring of the evolution of fungal infection in strawberries.
The objective of this work is to demonstrate a potentially inexpensive, fast, and highly-sensitive set of unspecific chemical sensors and subsequent related data analysis procedures that are able to detect the appearance of the fungus at an early stage in strawberry ripening. This may allow, for example, the withdrawal of batches of infected fruits before contamination dissemination, and thus the ability to timely determine anti-fungicidal applications and the prediction of the ideal harvesting time. The availability of such a technology may contribute to a reduction in post-harvest losses.
In this study, we investigate the chemical sensor response based on three different carbon nanostructures (CNSs) that can identify the possible appearance of fungus on the strawberry fruits. They are: multi-walled carbon nanotubes (MWCNTs), multi-walled carbon nanotubes doped with nitrogen (N-MWCNTs) [31], and multi-walled carbon nanotubes doped with boron (B-MWCNTs), [33]. We identified two genera of fungi: Aspergillus sp. section Nigri and Rhizopus sp., which in the study will be denoted simply as Aspergillus and Rhizopus, respectively. The mathematical method used to graphically present the data is based on tristimulus analysis [34].
2. Materials and Methods
The MWCNTs, N-MWCNTs, and B-MWCNTs used in this study have an inner diameter of ~10 nm and an outer diameter of around 20–30 nm. Their synthesis and characterization procedures have been reported elsewhere [31,32,34,35].
The composites used in the sensors were prepared using one of the CNSs dispersed in a 1.3 × 105 Da poly(vinyl alcohol) (PVA) matrix. The dispersion was prepared by adding 6 mg/mL hexadecyltrimethylammonium bromide (CTAB) and 4 mg/mL of one of the CNSs in water [33], using a procedure similar to that reported elsewhere [35,36,37,38]. The dispersion was ultrasonicated for 30 min at room temperature and sequentially, for 60 min at 0 °C. It was then left for 4 days at ~0 °C, which is below the Kraft temperature (TK = 25 °C) of CTAB [39], allowing for the precipitation of the excess surfactant in the form of hydrated crystals. In the sequence, 50% of the total volume of the supernatant was removed and mixed with 6 mg/mL of PVA in water, giving a final CNS weight content in PVA of 87%. The mixture was then stirred for 2 h at a temperature below TK, thus avoiding the formation of CTAB micelles.
We used interdigitated ENIG (Electroless Nickel Immersion Gold) electrodes (nine pairs; 7.5 mm long, with a gap of 0.3 mm between electrode strips) patterned on a FR4 epoxy resin/fiber glass board, supplied by Micropress SA (see Figure 1a). Interdigitated electrodes were sequentially cleaned in acetone, isopropanol, and ultrapure water (20 min each) in an ultrasonic bath and dried in air stream.
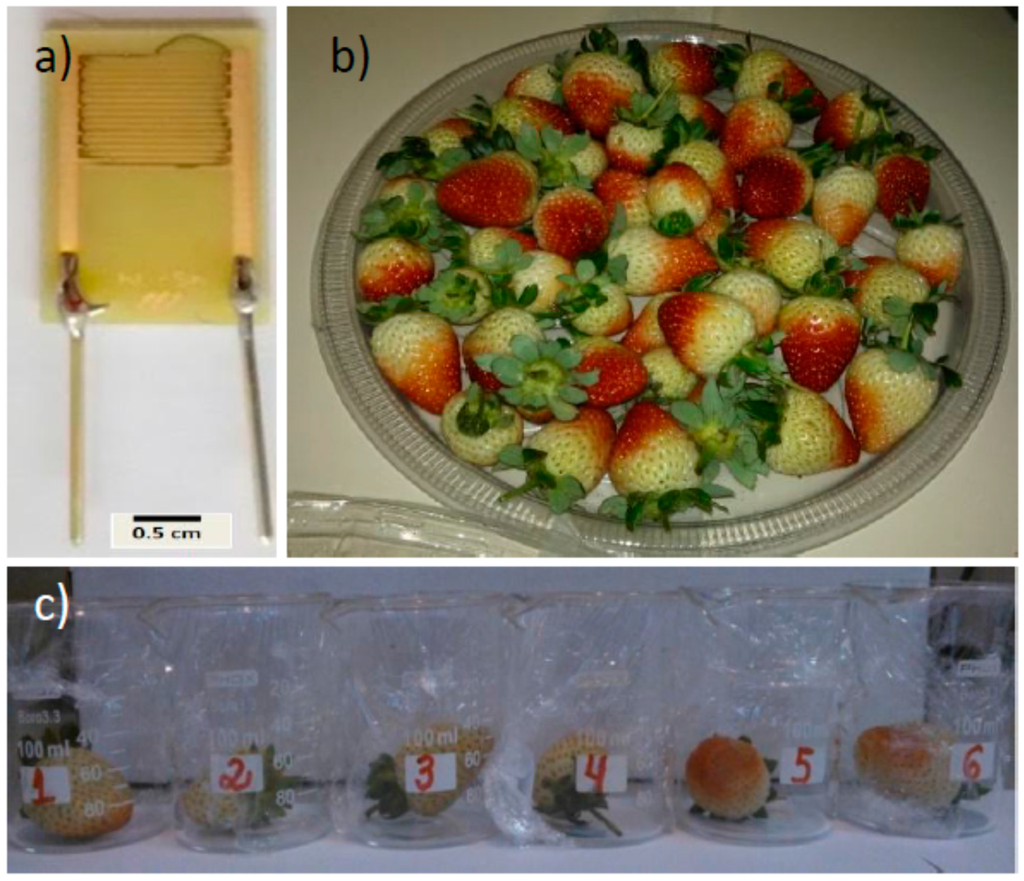
Figure 1.
(a) Interdigitated electrodes with multi-walled carbon nanotubes (MWCNTs) film; (b) green strawberries; and (c) strawberries inoculated with Aspergillus.
Sensors were prepared by simply dropping 10 μL of a CNS–PVA dispersion in water on top of an interdigitated electrode. After deposition on top of the substrate, the dispersion was annealed for 30 min at 50 °C to completely evaporate the solvent.
In this study, we used chemical sensors to monitor six green strawberries (Fragaria x ananassa) (see Figure 1b), for the detection of Aspergillus and Rhizopus fungal infestation. The strawberries were harvested in organic strawberry fields, located in Pinhais, Paraná state, Brazil (25°23’S, 49°07′W). Strawberries were sequentially washed in water, ethanol (50%) for 1 min each, and 1% sodium hypochlorite solution in sterile water for 30 s to remove any microorganisms present during harvesting. We began monitoring the green strawberries on the same day as the harvesting occurred. After the measurements of the organic green strawberries (used as reference), they were transported to the microbiology laboratory to inoculate with the fungi (Aspergillus or Rhizopus, see Figure 1c).
For the inoculation process, the fungi were transferred and placed in glass Petri dishes measuring 9 cm in diameter, containing on average 20 mL of potato dextrose agar for spore growth. Subsequently, the plates were incubated for seven days in a BOD (biochemical oxygen demand) chamber and subjected to a temperature of 28 ± 2 °C at a relative humidity of 70% ± 10% and 14 h photophase. After these seven days, the mycelium was then scraped from the Petri dishes with the help of sterile material, and the fungal spore solution was prepared by adding 20 mL of sterile distilled water in a tube containing Tween 80® sterile solution. After the scraping process, the solution underwent a screening procedure using sterile gauze. Then, the number of spores of Rhizopus and Aspergillus were counted with the help of a Neubauer chamber, giving counts of 3.2 × 107, and 1.3 × 108 spores/mL−1, respectively. For the fungal inoculation, the fruits were fully immersed in the respective fungi spore solutions for 3 min. Immediately after drying, three fruits were inoculated with each fungus and placed in a beaker coated with polyethylene terephthalate (PET) film (Figure 1c). Micrographs of Rhizopus sp. and Aspergillus sp. section Nigri fungi can be seen in Figure 2.
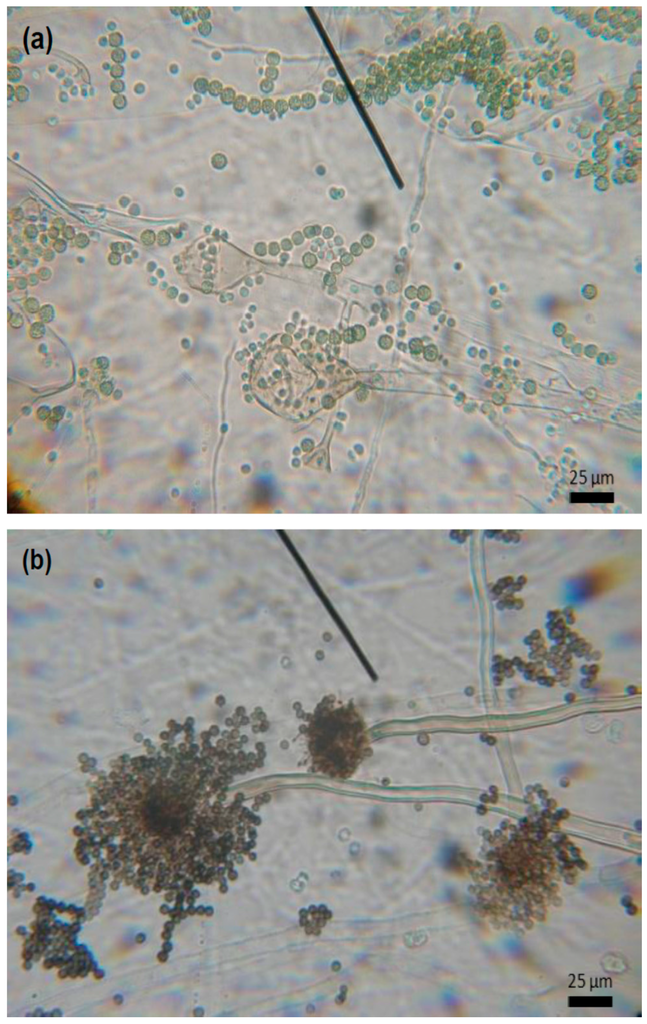
Figure 2.
Micrographs of (a) Rhizopus sp.; and (b) Aspergillus sp. section Nigri fungi.
Electrical measurements were made using an Agilent 4284 LCR meter. All conductance measurements were made at a frequency of 27 kHz and a voltage amplitude of 500 mV.
Temperature and relative humidity were monitored with a Minipa MT-241 sensor [34,35]. For the electrical measurements, nine sensors (three prepared with each of the CNSs) were fixed in the screw cap of a glass recipient (see Figure 3) and exposed to air for 60 s for the stabilization of the chemical sensor response and determination of the conductance base line (G0). Then, the sensors were exposed for 240 s to volatile organic compounds exhaled by the three strawberry fruits inoculated with Rhizopus and placed inside the 3200 mL glass chamber. The procedure was repeated for the three strawberry fruits inoculated with Aspergillus. Similar measurements were also made once using chambers containing only the fungal colonies. In these experiments, the screw cap was fixed on the glass, exposing the sensor to the inner environment containing the fruits or fungal colonies until the conductance saturated (corresponding to Gmax). After 240 s, the screw cap was removed, exposing the sensor to air for 60 s to allow sensor recovery and further confirmation of the baseline. The conductance data were collected in the complete sequence of measurements, as exemplified in Figure 4.

Figure 3.
Array of sensors placed in the cap.
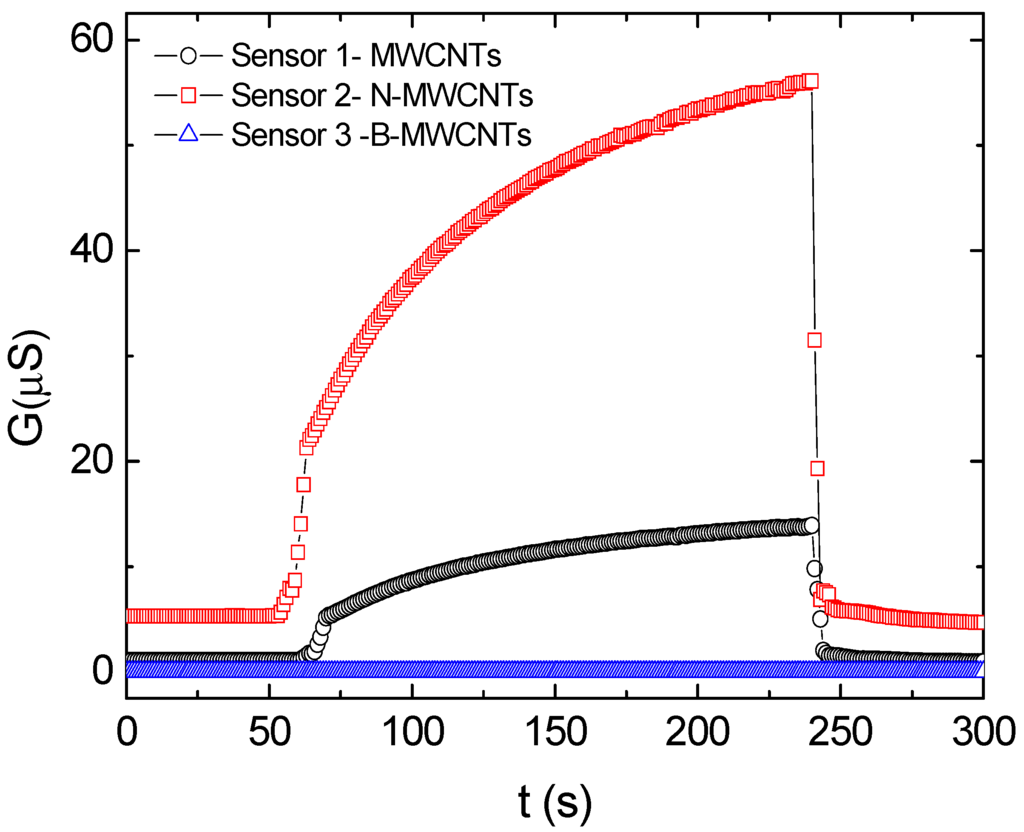
Figure 4.
G(t) response of the composite-based chemical sensors. At t = 60 s, the sensor is exposed to the recipient environment containing the fruits. At t = 240 s, the sensor is exposed again to air. MWCNT: multi-walled carbon nanotubes; B-MWCNT: multi-walled carbon nanotubes doped with boron; N-MWCNT: multi-walled carbon nanotubes doped with nitrogen.
Each measurement was repeated three times in sequence for the nine sensors, and the average value for each type of sensor was recorded. This procedure was repeated over 20 days, during the progress of the strawberry fungi infection. The fruits were maintained inside the closed chamber for the duration of the 20 days, except during measurement, when the screw cap was changed.
Control measurements with interdigitated electrodes without deposited CNSs were also made, to exclude the possibility of response occurrence in the absence of CNSs.
3. Results
Variation of the conductance G as a function of time t was used to calculate the response ∆G/G0, where ∆G = (Gmax − G0)/G0, the subscript “0” indicates the initial value of the conductance G, and Gmax indicates the maximum conductance value during the period of the measurement (see Figure 4). The response data for the three types of sensors based either on the MWCNTs, N-MWCNTs, or B-MWCNTs were used as input to the mathematical analysis applying the tristimulus methodology [34], employed to facilitate the visualization of data.
Briefly, we define XG ≡ [∆G/G0]MWCNTs, YG ≡ [∆G/G0]N-MWCNTs, and ZG ≡ [∆G/G0]B-MWCNTs (where the subscript indicates the type of CNSs used in the sensor), that were used to construct the three components of the tristimulus vector XG + YG + ZG. We then calculated the coordinates (xG, yG, zG) at the point where the tristimulus vector crossed a unitary plane, defined as xG + yG + zG = 1. These coordinates are given by xG = XG/(XG + YG + ZG), yG = YG/(XG + YG + ZG), and zG = ZG/(XG + YG + ZG). Since xG + yG + zG = 1, two of the coordinates are sufficient to univocally determine the unitary plane crossing the point (xG, yG, zG) and the projection of the point on one of the planes xy, yz, or zx (see Reference [34] for a more detailed presentation of the method). Figure 5 shows the plots corresponding to the three possible projections.
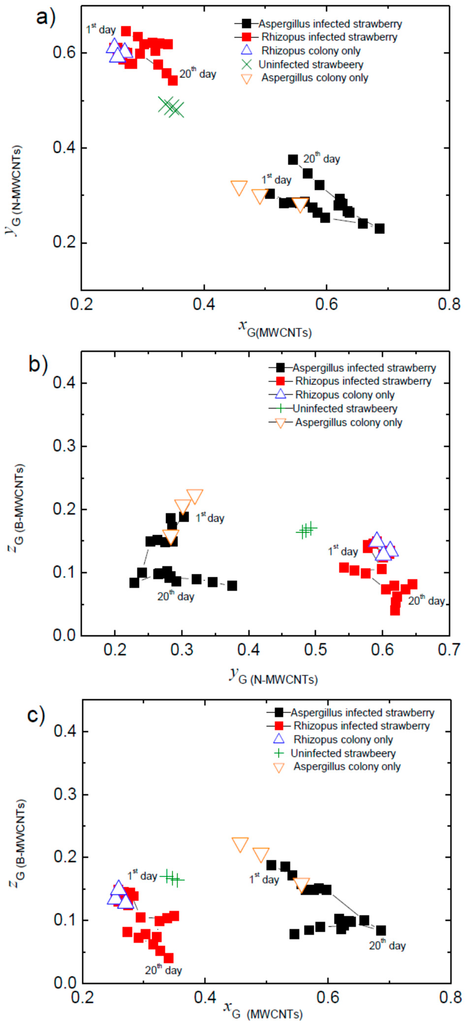
Figure 5.
∆G/G0 responses plotted using tristimulus representation graphs (in the three possible projections) for strawberry deterioration over 20 days following a fungal inoculation.
4. Discussion
Strawberries exhale a large quantity of volatiles, and the response of each sensor is due to the contribution of all of these volatiles. No attempt has been made at this stage to investigate the specific sensor–VOC interactions. In the case of a constant sensor sensitivity, variations in the concentration of these compounds will only modify the magnitude of the tristimulus vector, so that the region of intersection between the vector and the unit plane will remain the same. The intersection region will change only when the sensors are concomitantly exposed to the compounds exhaled by the fungi and the volatile compounds exhaled by the strawberries. This characteristic is useful for practical applications, as it can distinguish between healthy strawberries and those infected with fungus, and can also distinguish between Rhizopus and Aspergillus infections.
As can be seen in Figure 5, the tristimulus coordinates of the strawberry infected with Aspergillus are separated from those infected with Rhizopus over the full 20 day measurement period. The data tend to be grouped in a region that corresponds only to the coordinates of the corresponding fungal colony. Additionally, both groups of coordinates are separated from the coordinates of uninfected strawberries, independent of the plane used for the representation, xy, yz, or zx. This separation constitutes an advantage that makes this set of sensors and the applied procedure useful as a screening tool to identify infected strawberry batches, easily allowing distinction between both types of fungal infection. In strawberry production sites, this identification may provide for the adoption of prevention strategies or even to a specific fungal species-oriented agrochemical fungicide selection.
From a visual inspection of the strawberries, it was observed that from the first to the third day, there was no significant change that could indicate the development of fungus on the inoculated strawberries; on the fourth day, however, there was a sign of mycelium development on the strawberry surface, indicating deterioration by fungi. We observed that the fungus Rhizopus showed a grayish color with a dense and smooth growth on the surface, whereas Aspergillus showed a black appearance in the fruit.
To test the reproducibility, all measurements were made with a second batch of strawberries, over 12 days, confirming the results obtained in the first batch.
5. Conclusions
In this study, we have applied a tristimulus analysis to the set of responses of chemical sensors based on carbon nanostructures to identify fungal microorganisms (Aspergillus sp. section Nigri and Rhizopus sp.) that promote the degradation of strawberry fruits in the post-harvest stages, during storage, transportation, and sale. We have demonstrated by using tristimulus projection plots that it is possible to distinguish between strawberries infected with Aspergillus and those infected with Rhizopus. The use of the chemical sensors for the detection of early deterioration may be used as a tool to help prevent losses of stored strawberry and the further contamination of other strawberries by infected strawberries.
Acknowledgments
the authors would like to thank CNPq and PNPD/CAPES(Brazil); and NRF (South Africa) for research grants.
Author Contributions
These authors contributed equally to this work.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| B-MWCNTs | B-doped multiwalled carbon nanotubes |
| BOD | Biochemical oxygen demand |
| CNSs | Carbon nanostructures |
| CTAB | Hexadecyltrimethylammonium bromide |
| ENIG | Electroless nickel immersion gold |
| MWCNTs | Multiwalled carbon nanotubes |
| N-MWCNTs | N-doped multiwalled carbon nanotubes |
| PET | Polyethylene terephthalate |
| PVA | Poly(vinyl alcohol) |
| VOC | Volatile organic compounds |
References
- Siqueira, H.H.; Vilas Boas, B.M.; Silva, J.D.; Nunes, E.E.; Lima, L.C.O.; Santana, M.T.A. Armazenamento de morango sob atmosfera modificada e refrigeração. Ciênc. Agrotec. 2009, 33, 1712–1715. [Google Scholar]
- Pombo, M.A.; Rosli, H.G.; Martínez, G.A.; Civello, P.M. UV-C treatment affects the expression and activity of defense genes in strawberry fruit (Fragaria × ananassa, Duch.). Postharvest Biol. Technol. 2011, 59, 94–102. [Google Scholar] [CrossRef]
- Ghaouth, A.; Arul, J.; Grenier, J.; Asselin, A. Antifungal activity of chitosan on two postharvest pathogens of strawberry fruits. Phytopathology 1992, 82, 398–402. [Google Scholar] [CrossRef]
- Brackmann, A.; Giehl, R.F.H.; Eisermann, A.C.; Weber, A.; Heldwein, A.B. Inibição da ação do etileno e temperatura de armazenamento no padrão de amadurecimento de tomates. Ciênc. Rural 2009, 39, 1688–1694. [Google Scholar] [CrossRef]
- Cunha, L.C., Jr.; Jacomino, A.P.; Ogassavara, F.O.; Trevisan, M.J.; Parisi, M.C. Armazenamento refrigerado de morango submetido a altas concentrações de CO2. Hortic. Bras. 2012, 30, 688–694. [Google Scholar] [CrossRef]
- Kopjar, M.; Piližota, V.; Hribar, J.; Simčič, M.; Zlatič, E.; Tiban, N.N. Influence of trehalose addition and storage conditions on the quality of strawberry cream filling. J. Food Eng. 2008, 87, 341–350. [Google Scholar] [CrossRef]
- Mohsen, F.; Hossein, K.; Rohollah, S.; Mojtaba, R.; Javad, H. Chemical composition and antifungal effects of three species of Satureja (S. hortensis, S. spicigera, and S. khuzistanica) essential oils on the main pathogens of strawberry fruit. Postharvest Biol. Technol. 2015, 109, 145–151. [Google Scholar]
- Vestberg, M.; Kukkonen, S.; Saari, K.; Parikkab, P.; Huttunen, J.; Tainio, L.; Devos, N.; Weekers, F.; Kevers, C.; Thonart, P.; et al. Microbial inoculation for improving the growth and health of micropropagated strawberry. Appl. Soil Ecol. 2004, 27, 243–258. [Google Scholar] [CrossRef]
- Amiri, A.; Chai, W.; Schnabel, G. Effect of nutrient status, pH, temperature and water potential on germination and growth of Rhizopus Stolonifer and Gilbertella Persicaria. J. Plant Pathol. 2011, 93, 603–612. [Google Scholar]
- Borges, C.D.; Mendonça, C.R.B.; Zambiazi, R.C.; Silva, E.M.P.; Paiva, F.F. Conservação de morangos com revestimentos à base de goma xantana e óleo essencial de sálvia = Strawberries conservation with coatings based on xanthan gum and sage essential. Biosci. J. 2013, 29, 1071–1083. [Google Scholar]
- Romanazzi, G.; Feliziani, E.; Santini, M.; Landi, L. Effectiveness of postharvest treatment with chitosan and other resistance inducers in the control of storage decay of strawberry. Postharvest Biol. Technol. 2013, 75, 24–27. [Google Scholar] [CrossRef]
- Jensen, B.; Knudsen, I.M.B.; Andersen, B.; Nielsen, K.F.; Thrane, U.; Jensen, D.F.; Larsen, J. Characterization of microbial communities and fungal metabolites on field grown strawberries from organic and conventional production. Int. J. Food Microbiol. 2013, 160, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Duru, M.; Cakir, A.; Kordali, S.; Zengin, H.; Harmandar, M.; Izumi, S.; Hirata, T. Chemical composition and antifungal properties of essential oils of three Pistacia species. Fitoterapia 2003, 74, 170–176. [Google Scholar] [CrossRef]
- Nemec, S. Response of Three Root Rot Fungi to Strawberry Phenolics and the Relation of Phenolics to Disease Resistance. Mycopathologia 1976, 59, 37–40. [Google Scholar] [CrossRef]
- Niemi, M.; Vestberg, M. Inoculation of commercially grown strawberry with VA mycorrhizal fungi. Plant Soil 1992, 144, 133–142. [Google Scholar] [CrossRef]
- Morath, S.U.; Hung, R.; Bennett, J.W. Fungal volatile organic compounds: A review with emphasis on their biotechnological potential. Fungal Biol. Rev. 2012, 26, 73–83. [Google Scholar] [CrossRef]
- Bangerth, F.K.; Song, J.; Streif, J. Physiological Impacts of Fruit Ripening and Storage Conditions on Aroma Volatile Formation in Apple and Strawberry Fruit: A Review. Hortscience 2012, 47, 4–10. [Google Scholar]
- Reddy Bhaskara, M.V.; Angers, P.; Gosselin, J.A. Characterization and use of essential oil from Thymus vulgaris against Botrytis cinerea and Rhizopus stolonifer in strawberry fruits. Phytochemistry 1998, 47, 1515–1520. [Google Scholar] [CrossRef]
- Rosado-May, F.J.; Werner, M.R.; Gliessman, S.R.; Webb, R. Incidence of strawberry root fungi in conventional and organic production systems. Appl. Soil Ecol. 1994, 1, 261–267. [Google Scholar] [CrossRef]
- Siefkes-Boer, H.J.; Boyd-Wilson, K.S.; Petley, M.; Walter, M. Influence of Cold-Storage Temperatures on Strawberry Leak Caused by Rhizopus Spp. Plant Dis. 2009, 62, 243–249. [Google Scholar]
- Ayala-Zavala, J.F.; Wang, S.Y.; Wang, C.Y.; González-Aguilar, G. Effect of storage temperatures on antioxidant capacity and aroma compounds in strawberry fruit. LWT Food Sci. Technol. 2004, 37, 687–695. [Google Scholar] [CrossRef]
- Han, C.; Lederer, C.; McDaniel, M.; Zhao, Y. Sensory evaluation of fresh strawberries (Fragaria ananassa) coated with chitosan-based edible coatings. J. Food Sci. 2005, 70, S172–S178. [Google Scholar] [CrossRef]
- Calegaro, J.M.; Pezzi, E.; Bender, R.J. Utilização de atmosfera modificada na conservação de morangos em pós-colheita. Pesqui. Agropecu. Bras. 2002, 37, 1049–1055. [Google Scholar] [CrossRef]
- Schleibinger, H.; Laussmann, D.; Bornehag, C.G.; Eis, D.; Rueden, H. Microbial volatile organic compounds in the air of moldy and mold-free indoor environments. Indoor Air 2008, 18, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Kurze, S.; Bahl, H.; Dahl, R.; Berg, G. Biological Control of Fungal Strawberry Diseases by Serratia plymuthica HRO-C48. Plant Dis. 2001, 85, 529–534. [Google Scholar] [CrossRef]
- Sunesson, A.; Vaes, W.; Nilsson, C.; Blomquist, G.; Andersson, B.; Carlson, R. Identification of volatile metabolites from five fungal species cultivated on two media. Appl. Environ. Microbiol. 1995, 61, 2911–2918. [Google Scholar] [PubMed]
- Wilson, A.D.; Baietto, M. Applications and advances in electronic-nose technologies. Sensor 2009, 9, 5099–5148. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Da, H. Nanotube Molecular Wires as Chemical Sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Kuske, M.; Romain, A.; Nicolas, J. Microbial volatile organic compounds as indicators of fungi—Can an electronic nose detect fungi in indoor environments. Build. Environ. 2005, 40, 824–831. [Google Scholar] [CrossRef]
- Greenshields, M.W.; Meruvia, M.S.; Hümmelgen, I.A.; Coville, N.J.; Mhlanga, S.D.; Ceragioli, H.J.; Quispe, J.C.; Baranauskas, V. AC-conductance and capacitance measurements for ethanol vapor detection using carbon nanotube-polyvinyl alcohol composite based devices. J. Nanosci. Nanotechnol. 2011, 11, 2384–2388. [Google Scholar] [CrossRef] [PubMed]
- Greenshields, M.W.; Mamo, M.A.; Coville, N.J.; Spina, A.P.; Rosso, D.F.; Latocheski, E.C.; Destro, J.G.; Pimentel, I.C.; Hümmelgen, I.A. Electronic Detection of Drechslera sp. Fungi in Charentais Melon (Cucumis melo Naudin) Using Carbon-Nanostructure-Based Sensors. J. Agric. Food Chem. 2012, 60, 10420–10425. [Google Scholar] [CrossRef] [PubMed]
- Greenshields, M.W.; Hümmelgen, I.A.; Mamo, M.A.; Saikjee, A.; Mhalanga, S.D.; Otterlo van, W.A.; Coville, N.J. Composites of polyvinyl alcohol and carbon (coils, undoped and nitrogen doped multiwalled carbon nanotubes) as ethanol, methanol and toluene vapor sensors. J. Nanosci. Nanotechnol. 2011, 11, 10211–10218. [Google Scholar] [CrossRef] [PubMed]
- Greenshields, M.W.; Mamo, M.A.; Coville, N.J.; Pimentel, I.C.; Destro, J.G.; Porsani, M.V.; Bozza, A.; Hümmelgen, I.A. Tristimulus mathematical treatment application for monitoring fungi infestation evolution in melon using the electrical response of carbon nanostructure-polymer composite based sensors. Sens. Actuators B Chem. 2013, 188, 378–384. [Google Scholar] [CrossRef]
- Cunha, B.B.; Greenshields, M.W.; Mamo, M.; Coville, N.J.; Hümmelgen, I.A. A surfactant dispersed N-doped carbon sphere-poly(vinyl alcohol) composite as relative humidity sensor. J. Mater. Sci. Mater. Electron. 2015, 26, 198–4201. [Google Scholar] [CrossRef]
- Vaisman, L.; Wagner, H.D.; Marom, G. The role of surfactants in dispersion of carbon nanotubes. Adv. Colloid Interface Sci. 2006, 128–130, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.; Mamo, M.A.; Coville, N.J.; Hümmelgen, I.A. Hydrostatic pressure sensors based on carbon spheres dispersed in polyvinyl alcohol prepared using hexadecyltrimethylammonium bromide as surfactant and water as solvent. Mater. Res. Express 2014, 1. [Google Scholar] [CrossRef]
- Duan, W.H.; Wang, Q.; Collins, F. Dispersion of carbon nanotubes with SDS surfactants: A study from a binding energy perspective. Chem. Sci. 2011, 2, 1407–1413. [Google Scholar] [CrossRef]
- Howard, W. Dispersing carbon nanotubes using surfactants. Curr. Opin. Colloid Interface Sci. 2009, 14, 364–371. [Google Scholar]
- Dölle, S.; Lechner, B.D.; Park, J.H.; Shymura, S.; Lagerwall, J.P.F.; Scalia, G. Utilizing the Krafft Phenomenon to Generate Ideal Micelle-Free Surfactant-Stabilized Nanoparticle Suspensions. Angew. Chem. Int. Ed. 2012, 51, 32547. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).