Abstract
NO2 emission is mostly related to combustion processes, where gas temperatures exceed far beyond 500 °C. The detection of NO2 in combustion and exhaust gases at elevated temperatures requires sensors with high NO2 selectivity. The thermodynamic equilibrium for NO2/NO ≥ 500 °C lies on the NO side. High temperature stability of TiO2 makes it a promising material for elevated temperature towards CO, H2, and NO2. The doping of TiO2 with Al3+ (Al:TiO2) increases the sensitivity and selectivity of sensors to NO2 and results in a relatively low cross-sensitivity towards CO. The results indicate that NO2 exposure results in a resistance decrease of the sensors with the single Al:TiO2 layers at 600 °C, with a resistance increase at 800 °C. This alteration in the sensor response in the temperature range of 600 °C and 800 °C may be due to the mentioned thermodynamic equilibrium changes between NO and NO2. This work investigates the NO2-sensing behavior of duplex layers consisting of Al:TiO2 and BaTi(1-x)RhxO3 catalysts in the temperature range of 600 °C and 900 °C. Al:TiO2 layers were deposited by reactive magnetron sputtering on interdigitated sensor platforms, while a catalytic layer, which was synthesized by wet chemistry in the form of BaTi(1-x)RhxO3 powders, were screen-printed as thick layers on the Al:TiO2-layers. The use of Rh-incorporated BaTiO3 perovskite (BaTi(1-x)RhxO3) as a catalytic filter stabilizes the sensor response of Al-doped TiO2 layers yielding more reliable sensor signal throughout the temperature range.
1. Introduction
As compared with other emission pollutants, the amount of NOx generated by combustion processes is very high in the atmosphere and the increase of NOx and derivatives damage the upper atmospheric ozone layer which provides a protective shield acting as a filter to UV radiation coming from the sun. Moreover, NOx may react with atmospheric water resulting in the formation of acid rain [1].
In case of complete combustion, the reactant burns in oxygen, producing a limited number of products. When a hydrocarbon burns in pure oxygen, the stoichiometric reaction will only yield carbon dioxide and water as well as nitrogen oxides (NOx). Incomplete fuel oxidation yields nitrogen oxides (NOx), mainly unburned hydrocarbons (HC), carbon monoxide (CO), CO2, and water [2,3]:
Nitrogen oxides need to be converted over noble metal-containing catalysts to harmless compounds such as N2, H2O, and CO2. Despite the presence of a catalyst, for instance a three-way-catalytic converter in automotive exhausts, the conversion is not always completed to fully eliminate NO and/or NO2. The resulting emission still contains toxic greenhouse gases.
NOx concentrations in diesel exhausts vary typically between 50 ppm up to 1000 ppm [4]. If concentrations are given in mass units, NOx is usually expressed as NO2 equivalent [4]. Catalytic converters reduce exhaust gas emission below a level foreseen by regulations in order to protect human life [5,6].
Although modern vehicles are getting cleaner due to improved engines and better emission controls, this holds only when engine and converter are in proper condition. When an engine is not at maximum efficiency, consequently performance is lost, fuel is wasted, and air pollutant emissions increase. Typically, in order to adjust the fuel-efficiency, excess air (or oxygen) is added in diesel engines during combustion. Additionally, emissions will increase drastically when defects occur in a catalytic converter for instance by engine misfire or due to the driving-related issues. These may remain unnoticed if the vehicles have no integrated emission control system. Onboard diagnostic (OBD), which is in vehicles since 1996, can detect emission problems of a car or truck. OBD systems are designed to alert the driver when a component in the engine management or exhaust system begins to deteriorate or malfunction. Early detection of minor problems can often prevent more costly damage to components, such as the catalytic converter or even the engine itself. Systems such as OBD rely on multiple gas sensors controlling the exhaust condition and emissions [7].
Generally, metal oxide semiconducting (MOx materials are employed for the detection of various oxidizing and reducing gases due their wide applicability and with simple and cheap fabrication [8]. Despite having many benefits, limited working temperature range and poor selectivity are two main drawbacks highlighted in the literature [9,10,11]. At lower temperatures (below 150 °C) and at temperatures above 400 °C, the sensing properties of the semiconducting materials are reduced drastically due to decreased conductivity and/or thermal instabilities [10,11,12,13].
A large amount of efforts are being spent by current research and development to address such issues in order to broaden the application spectrum of semiconducting oxides in high-tech industries. The most widely utilized semiconducting oxides are SnO2, WO3, ZnO, and TiO2 [10,11,12,13,14,15,16,17,18,19]. Among those, TiO2-based thin films and layers are reported to be more stable at higher temperatures and the stability can be further improved by bulk doping and by surface decorating with suitable cations [10,13,15,16,17,18,19,20]. In a previous study [10], we demonstrated that Al-doped TiO2 sensor layers can detect NO2 up to 800 °C in the concentration range of 50 ppm to 200 ppm, but the sensor response alters from the n-type at 600 °C to the p-type at 800 °C. Studying the reasons causing this change in sensor response, we demonstrated that this correlates well with the NO2/NO thermodynamic equilibrium change occurring at temperatures above 600 °C.
Typically, the MOx-containing resistive sensors yield signals in opposite directions toward NO and NO2. Thus, such changes in the high-temperature environmental condition are detrimental for diagnostic sensors and impair their long-term reliability. A common trend of improving the chemical stability and selectivity of this type of sensors is the use of a catalytic layer that acts as a catalyst, a sequestration barrier or a physical or chemical filter for undesired gases [21,22]. Hence, in order to maintain a reasonable sensitivity, better chemical stability and reliable sensor response with MOx sensor layers at elevated temperatures, the use of an additional catalytic filter or layer may be necessary and useful. Previously, it was reported that mesoporous and noble metal-containing catalytic filters can improve the sensing ability of semiconductors [21,22]. The use of SnO2/CeO2 duplex layers is shown to yield improved CO-response when the sensor is exposed to heavily-reducing gas mixtures (i.e., methane and/or other hydrocarbons) [23]. Hübner et al. [24] demonstrated later that a BaTi1-xRhxO3 catalytic layer on the surface of SnO2 sensor layer can eliminate the sensitivity towards hydrogen when exposed to high humidity CO + H2 gas mixture while maintaining the selectivity towards CO. Our recent report indicates that La-based complex perovskite (LaFeCoPdO3) layers deposited on sputtered TiO2 sensor layers enable the hold of their sensing ability despite aging in a harsh exhaust environment at 850 °C [25].
The current context follows this type of an approach where a BaTi0.95Rh0.05O3 perovskite layer is combined with the Al-doped TiO2 (Al:TiO2) sensing layer in order to achieve a sensor array that can be integrated in catalytic converter monoliths of vehicles and, thus, allowing reliable monitoring of NO2 emission in exhaust environments at temperatures exceeding 600 °C. Such sensor arrays will be placed within the catalytic converter of a vehicle and, therefore, must detect NO2 with long-term selectively over a threshold concentration by withstanding these harsh conditions (i.e., thermal aging caused by high gas flow rates and elevated temperatures). An adaptive and self-regenerative catalyst such as this can provide a thermal protection for the MOx-based sensing layer, as well as enabling a reliable sensor response toward NO2 at temperatures as high as 900 °C.
2. Experimental Section
2.1. Synthesis of Al-Doped TiO2 Layers by Sputtering
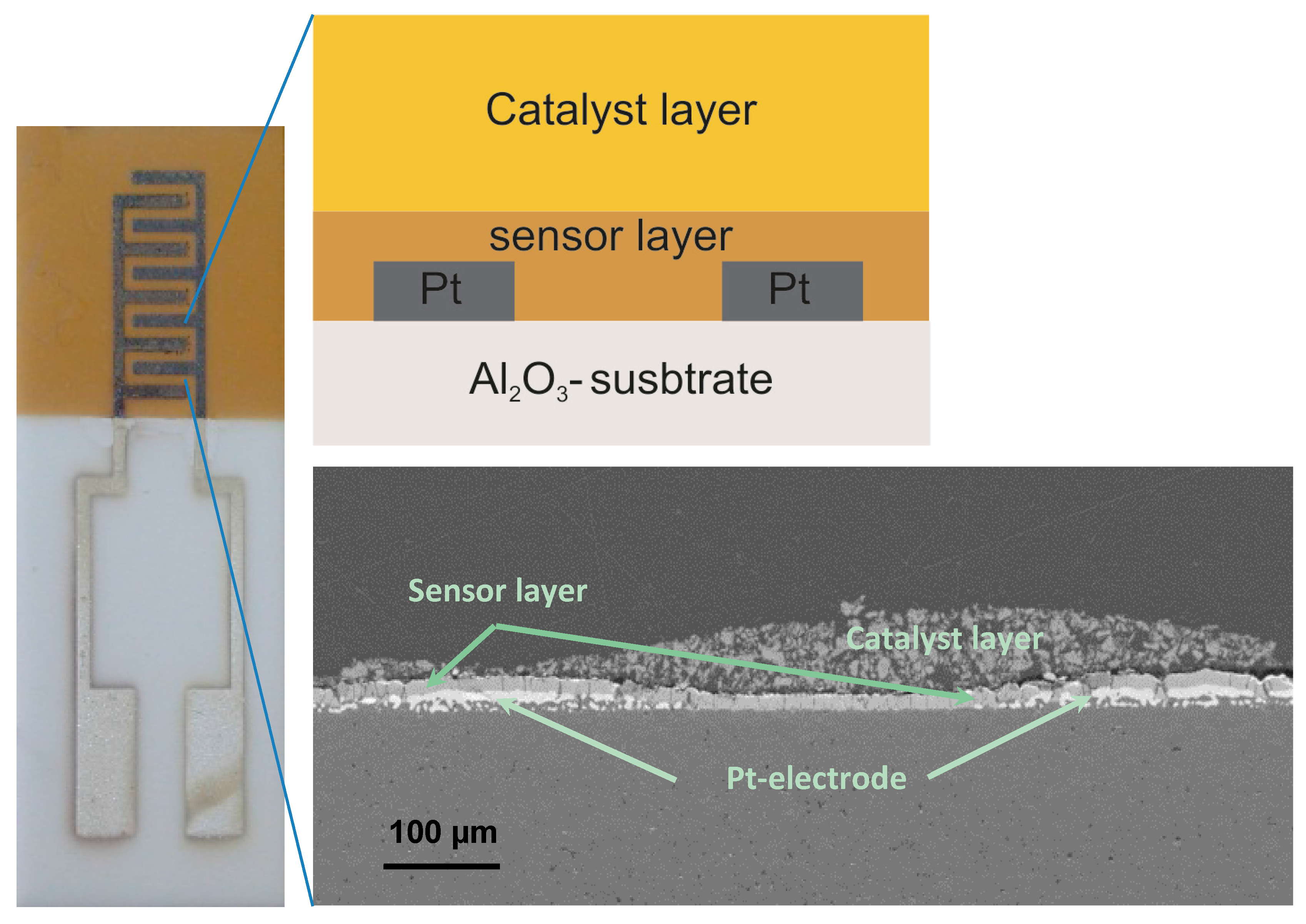
Al-doped TiO2 thin layers were deposited via a reactive magnetron radio frequency (RF) sputtering technique from metallic targets using oxygen as a reactive gas and without applying any heating of the substrate or system. Sputtering equipment (from von Ardenne GmbH, Dresden, Germany) with two sputter sources was used to deposit the coatings in an argon + oxygen gas mixture. Al-doped TiO2 thin layers were deposited on the polished Al2O3 sensor platforms, onto which the interdigitated Pt-circuits were previously deposited by screen-printing. For the deposition of Al-doped TiO2, two metallic targets, i.e., Ti (99.95% pure) and Al (99.99% pure) were used and were placed face to face in a plasma reactor chamber. During the coating process, the substrate holder was rotated at 13 rpm. This rotational movement enables the homogenous distribution of aluminum in the TiO2 matrix. A power of 80 W at the Al target and 500 W at the Ti target yielded 4.64 at.% Al in TiO2. The partial pressure of argon and oxygen during the coating was 27.4 and 5.7 sccm, respectively. After 12 h of sputtering process, an Al-doped TiO2 coating with a thickness of 1.2 µm was achieved. Pt-interdigitated electrodes (IDEs) are composed of two connection tracks having 300 µm-wide interlaces with bands/gaps of 300 µm from each other on alumina substrates (see Figure 1). The employed (IDE) sensor design requires no heater at the backside of the sensor platforms. Simultaneously, we deposited thin undoped and Al-doped TiO2 layers (ca. 1–1.5 µm) onto the sapphire single crystal substrates in order to enable XRD measurements without any interference from the polycrystalline alumina substrate material.
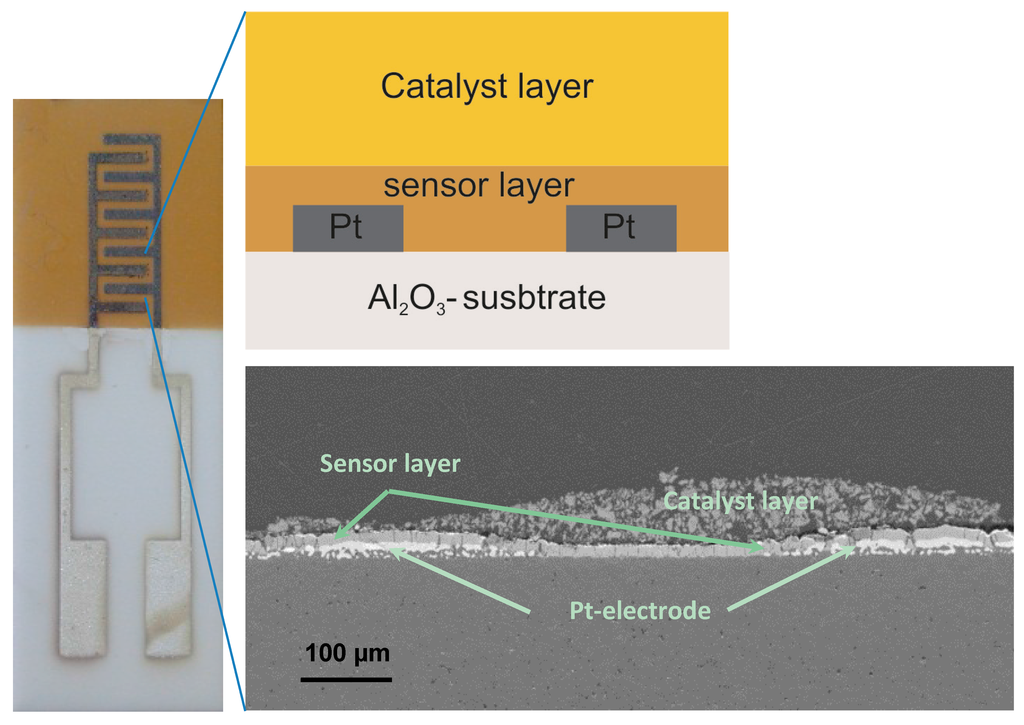
Figure 1.
Sensor platform consisting of interdigitated Pt-electrodes (IDE), a semiconducting oxide sensor layer, and a catalytic filter layer. The SEM micrograph shows these layers in the cross-section as above, schematically specified: top layer: BaTi0.9Rh0.1O3 catalytic layer, middle layer: Al-doped TiO2 sensor layer and bottom layer: Pt-electrode on alumina substrate.
2.2. Synthesis of the Catalysts
Ba-acetate (Ba(C2H3O2)2, 98% Chempur), Ti-isopropoxide (Ti{OCH(CH3)2}4, 99.999% Aldrich), and Rh-nitrate (Rh(H2O)(OH)3-y(NO3)y y=2-3) were employed as starting chemicals to prepare the BaTi(1-x)RhxO3 perovskite. Barium acetate was dissolved in acetic acid and Ti-isopropoxide was diluted with isopropanol and acetyl-acetone solution. Barium and Ti sols were homogenously mixed and then the Rh-nitrate solution was added to this mixture. The amount of the aqueous Rh-nitrate was calculated to obtain perovskite powders of the following composition BaTi0.9Rh0.1O3. The solvents were slowly evaporated under constant stirring at room temperature. The catalysts were finally calcined in a furnace under static air up to 1000 °C for 5 h with a heating rate of 10 °C min−1 [26].
The catalytic powder was then milled and mixed with some binder for screen printing on the interdigitated electrode sensor platforms and/or on the Al-doped TiO2 sensing layer, which was previously sputtered on Pt-interdigitated electrodes.
2.3. Sensor Array Layout
Sensor platforms were fabricated by deposition of sensing layer on interdigitated Pt-electrodes (IDE) which were deposited previously on Al2O3 substrates as shown in Figure 1, left. The catalytic filter was then brought on the sensor layer by means of screen printing as thick and porous layer (see Figure 1, bottom).
2.4. Characterization Methods
Al-doped TiO2 sensing layers and BaTi0.9Rh0.1O3 catalytic powder layers are investigated by SEM/EDX for their compositions and by X-ray powder diffraction method (XRD) for the phase condition.
Microstructure and morphology analysis of the sensing electrodes in terms of porosity, grain size, and surface condition were done by means of FE-SEM (Carl Zeiss Microscopy, Ultra 55, Oberkochen, Germany). EDX analysis were carried out by GEMINI (Oxford Inst. GmbH, Wiesbaden, Germany). The XRD diffractograms of the catalysts were obtained in a diffractometer D5000 from SIEMENS with Cu Kα radiation (λ = 1.54178 Å). The reflections from the JCPDS (Joint Committee on Powder Diffraction Standards) database were assigned to the experimental diffractograms with the program EVA from BRUKER AXS. Scanning Electron Microscopic (SEM) analysis of the catalysts was carried out in a Zeiss Ultra 55 microscope equipped with an Energy Dispersive X-ray Spectrometer (EDS) from Oxford Instruments. For high-temperature XRD measurement of BaTi0.95Rh0.05O3 catalyst, the specimens were prepared by packing powder in Ø 25 mm alumina crucible. Data collection time was 30 s per scan. The test powders were initially calcined at 700 °C prior to in situ high-temperature X-ray diffraction (HT-XRD) measurements.
Sensing characterization was carried out in a specially constructed apparatus consisting of a tube furnace with cascade control and a custom-built quartz glass reactor providing a thermocouple directed at the specimen. The composition of the mixed gas was controlled by an eight-channel mass flow controller (MFC-647b from MKS Instruments GmbH). The gas flow rate was adjusted to 400 mL/min and the NO2 concentrations were varied between 50 and 200 ppm. Mostly argon was used as the carrier gas. In some of our experiments, synthetic air (80% N2 + 20% O2) was employed as the carrier gas indicated relevantly. The DC electrical measurements were carried out by using a Keithley 2635A Sourcemeter, with a computer-controlled LabView program. A constant current of 1 × 10−6 A was applied during all sensor measurements. Steady-state gas response measurements were started under a constant carrier gas flow. These sensors were initially exposed to a constant flow of carrier gas to equilibrate the operating conditions. After a stable baseline was reached, the target gas was introduced into the chamber, continuously flowing with a constant for 40 min at the end of which the NO2-gas flow was ceased and only carrier gas is let flown through. These two steps were repeated for 50, 100, and 200 ppm NO2 and/or CO gas concentrations.
3. Results and Discussion
3.1. Al-Doped TiO2 Sensor Layer
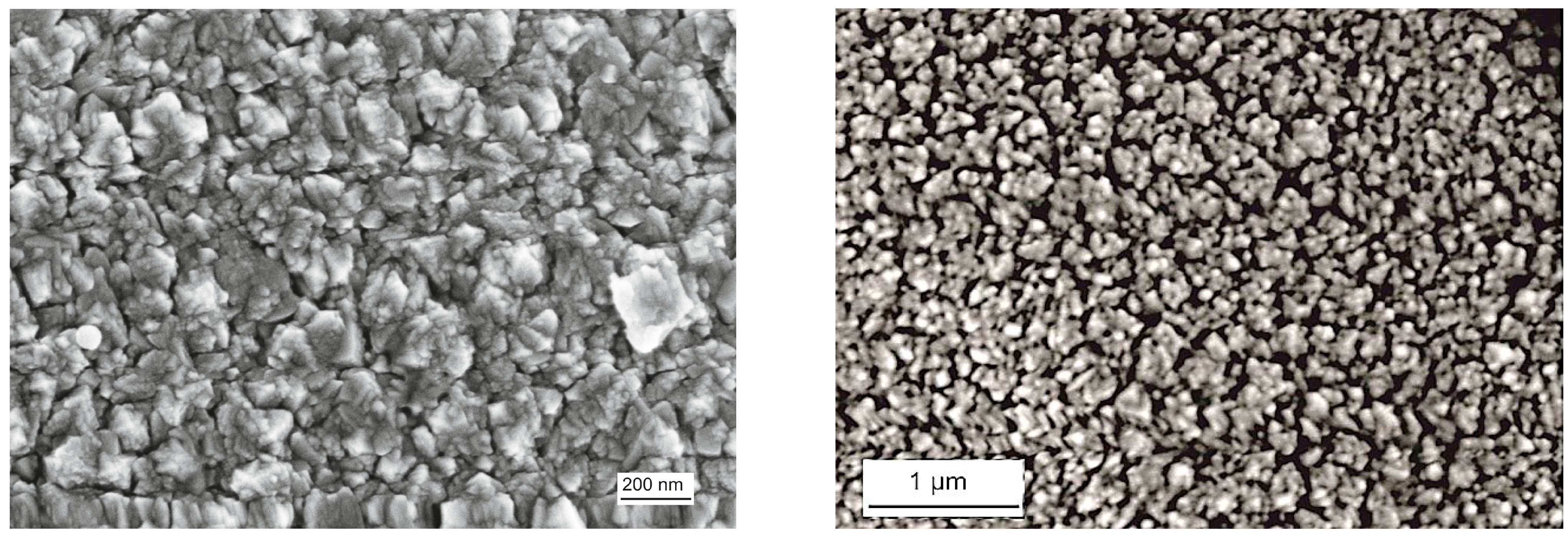
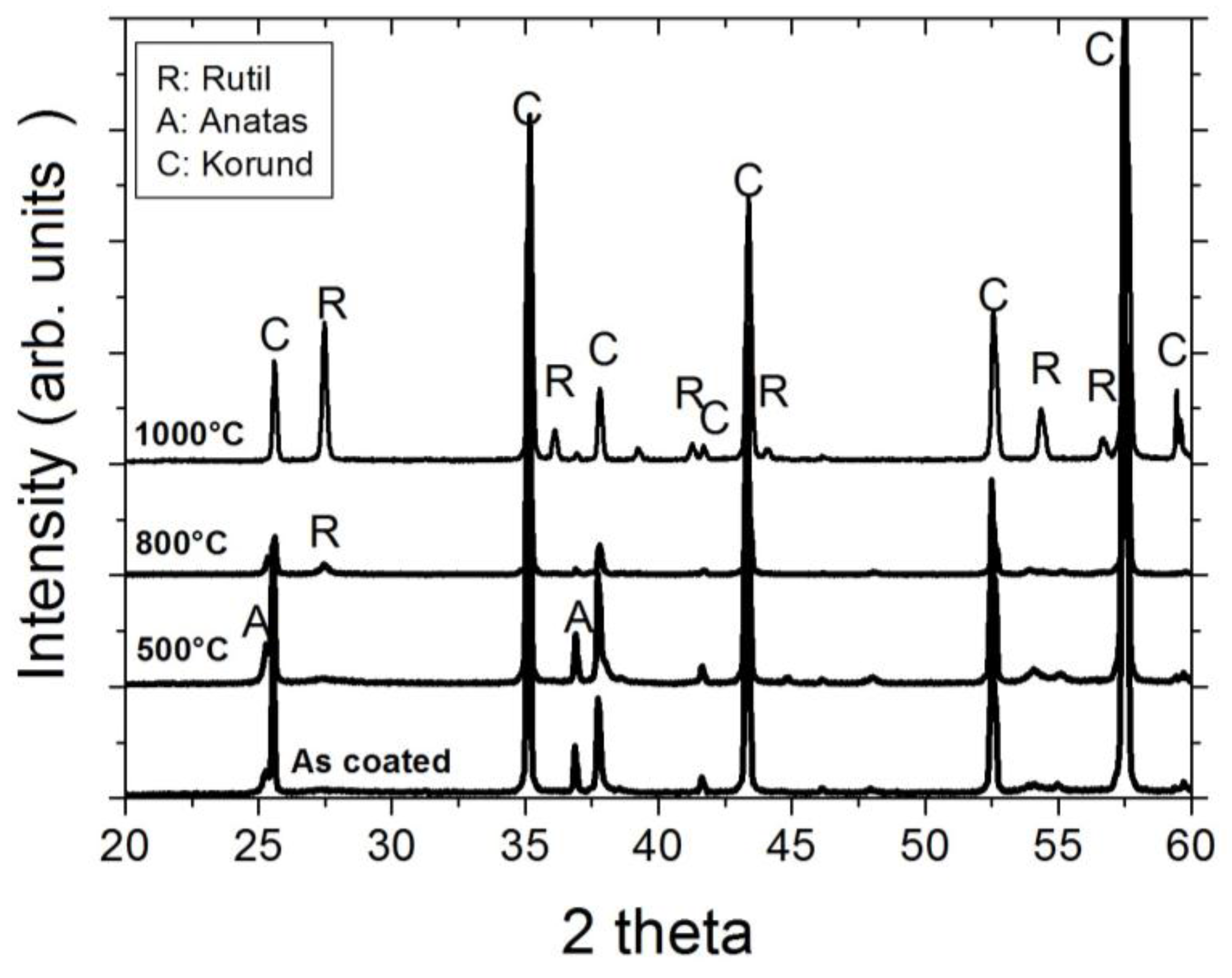
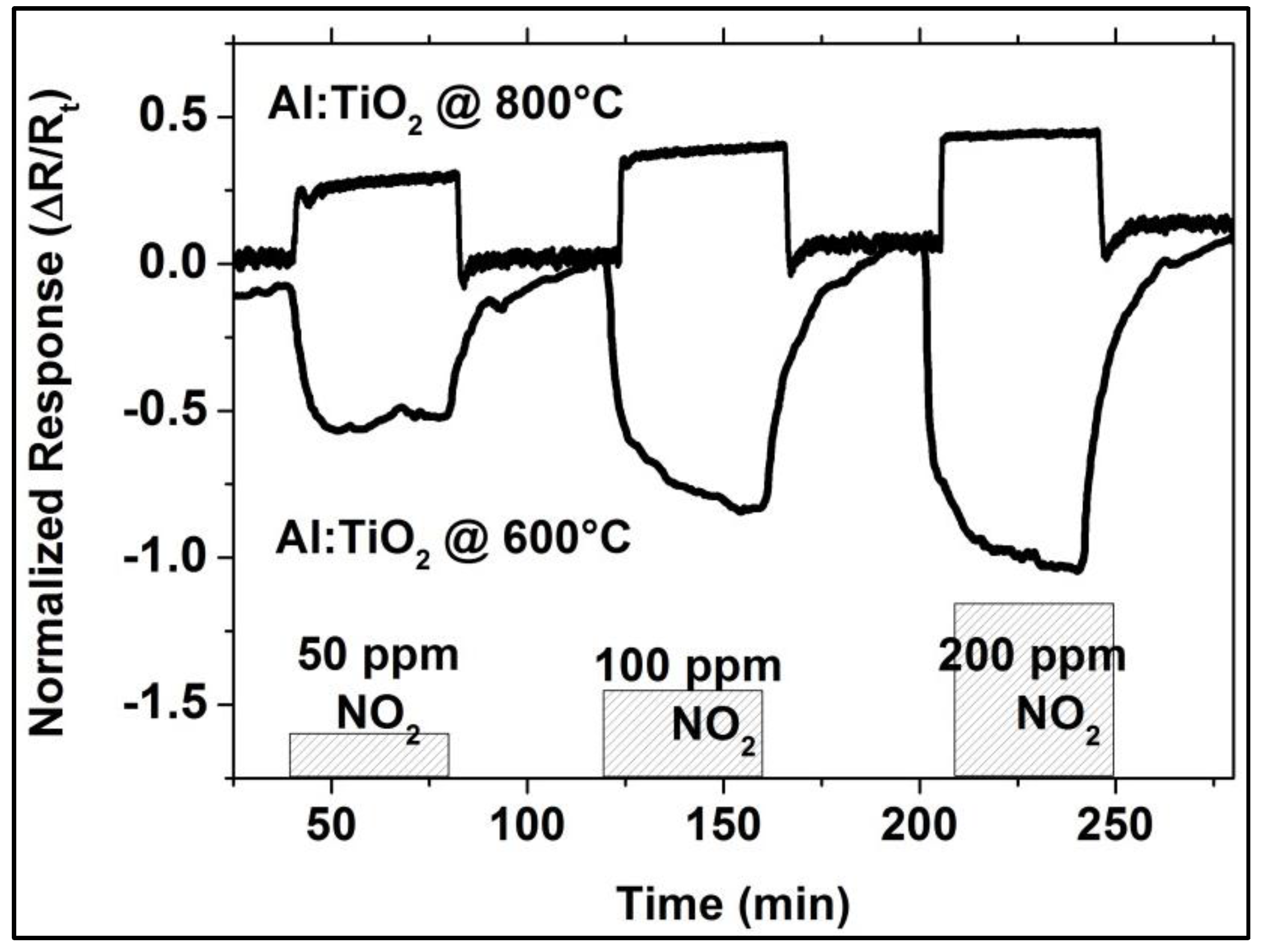
As the XRD investigation of the as-coated and ex situ at 500 °C, 800 °C, and 1000 °C heat-treated Al-doped TiO2 layers indicate, the layer is weakly crystalline and contains the anatase phase in the as-coated case. The top-view SEM image of the as-coated layer displays densely packed fine columnar morphology of the layer (Figure 2, left). Further heat-treatment increases the anatase crystallinity. At around 800 °C, the first indication of anatase-rutile transformation can be seen (Figure 3). After 3 h of heat-treatment at 1000 °C, full conversion to rutile takes place. The Al-doped TiO2 layers which were used for sensor testing were calcined at 800 °C and, thus, contained mainly anatase phase and some minor amounts of rutile. The SEM top-view micrograph of the 800 °C heat-treated Al-doped layer indicates the crystallization development yielding some porosity at the grain and column boundaries (Figure 2, right). The sensor tests of Al-doped TiO2 layers towards NO2 were published in details elsewhere [10]. The most striking observation with this high-temperature sensor material was the alteration of the sensor signal direction at 800 °C yielding a resistance decrease under NO2 exposure being opposite to the resistance increase observed at 600 °C (see Figure 4). No significant effect of humidity on the sensor response was observed at these temperatures.
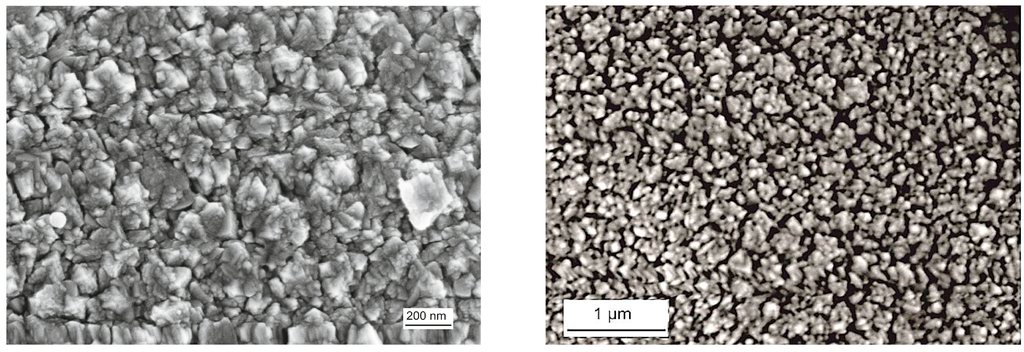
Figure 2.
Top-view SEM micrographs of the sputter-coated Al-doped TiO2 layer in the as-coated case (left), and after heat-treatment at 800 °C for 3 h in air (right).
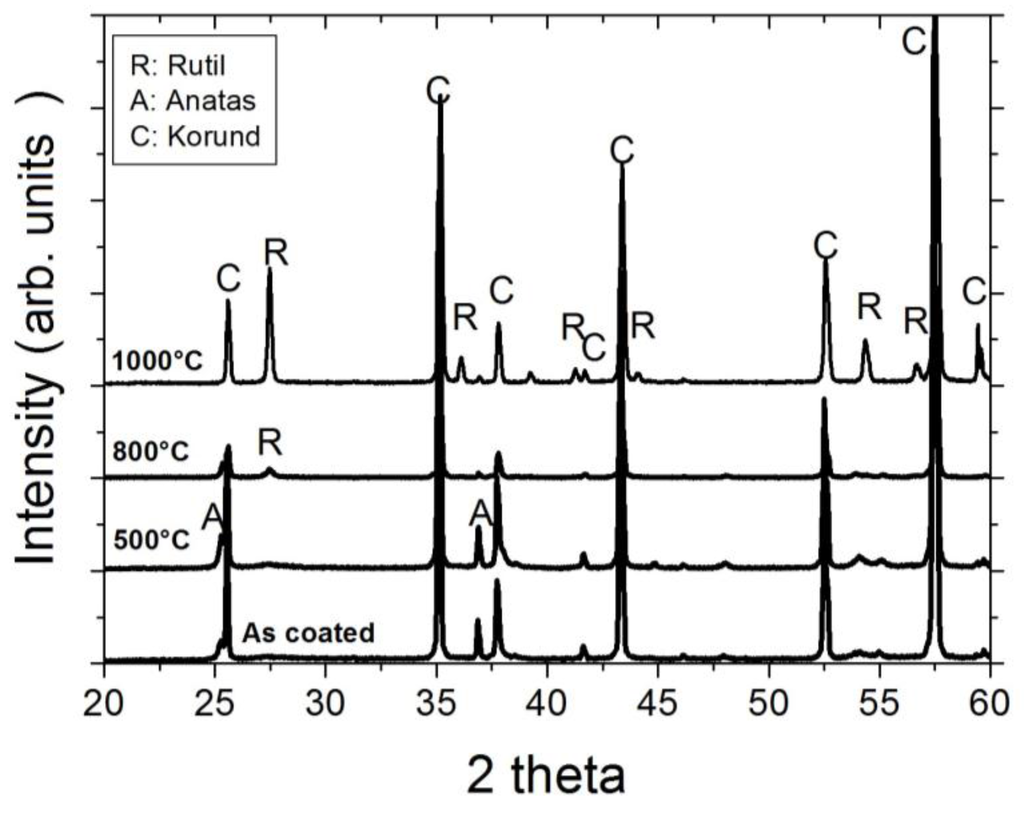
Figure 3.
XRD diffractograms of the Al-doped TiO2 layers deposited by a sputtering process in the as-coated state and ex situ, heat-treated at 500 °C, 800 °C, and 1000 °C.
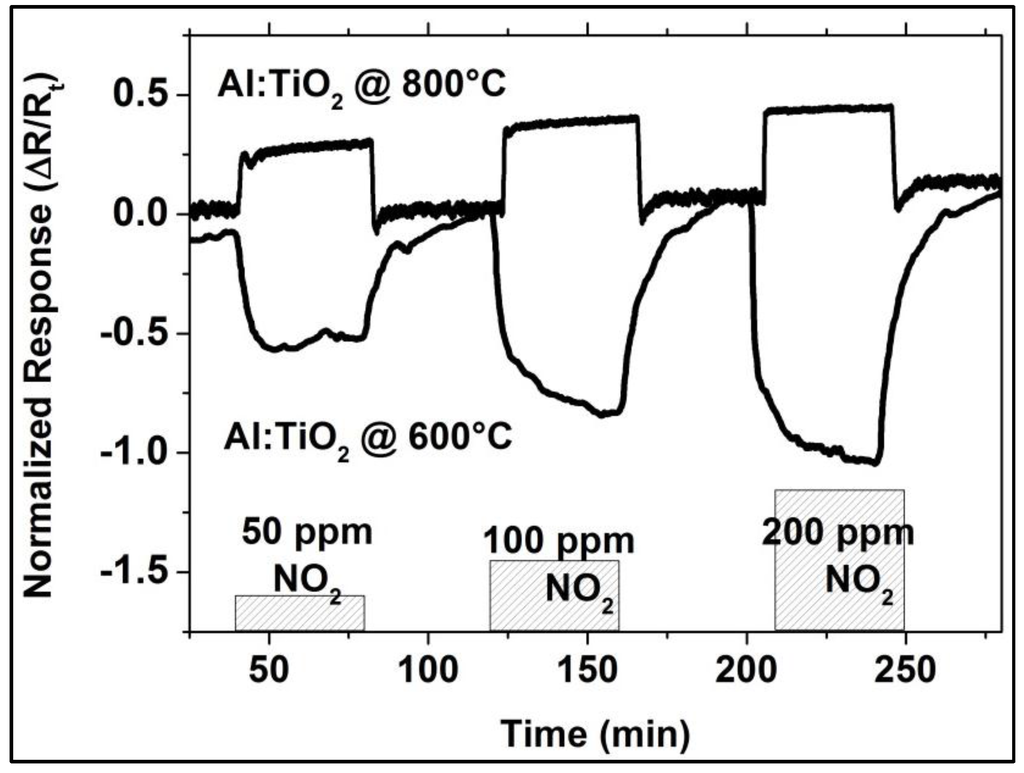
Figure 4.
NO2 response of Al-doped TiO2 in dry argon environment at 600 °C and 800 °C.
This behavior can be assumed to be due to the conversion of NO2 to NO because of the above-mentioned and the Figure 5 illustrated thermodynamic equilibrium conditions which may result in the formation of ionized oxygen species (e.g., O2−-ion) taking over the control of sensing mechanism through surface adsorption (see Equation (2) and [10]):
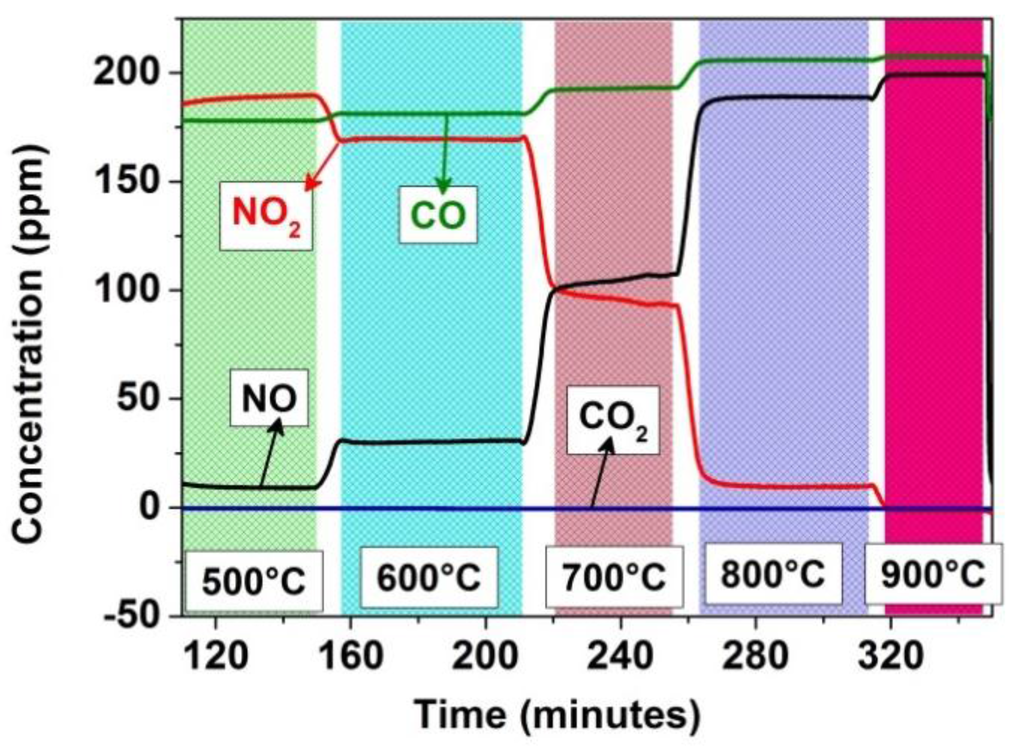
Figure 5.
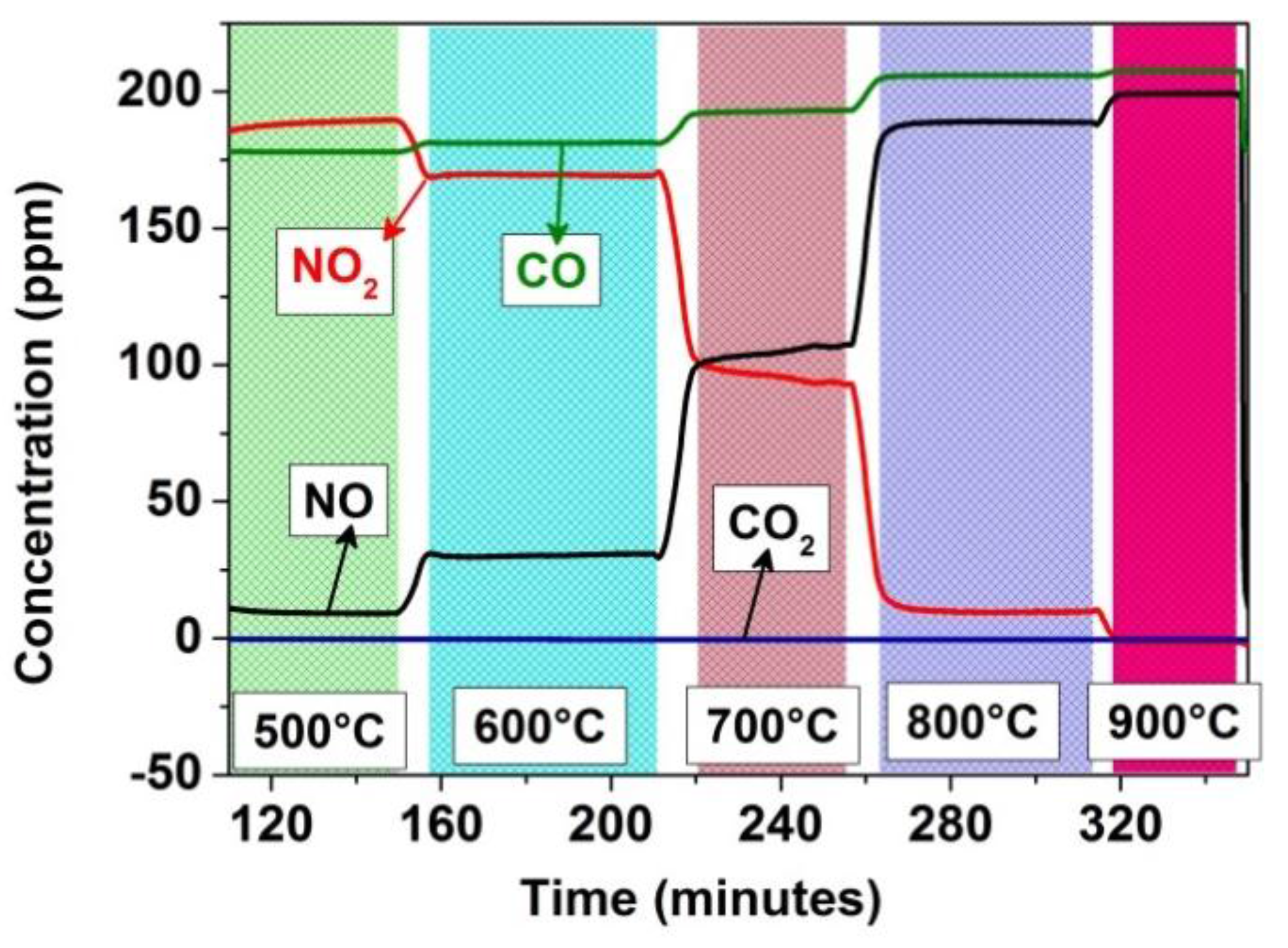
Temperature-dependent conversion of NO2 to NO in the empty sensor test chamber, with no visible conversion of CO to CO2.
This behavior of the Al-doped TiO2 layer indicates poor NO2-sensing capability at temperatures above 600 °C. On the other hand, Al-doped TiO2 shows negligible sensitivity towards CO concentrations varying from 50 to 200 ppm in the given temperature range. Moreover, these layers are more sensitive to NO2 when NO2 and CO are simultaneously present in the test environment, indicating a minimized cross-sensitivity towards CO [9].
In order to overcome the NO2 sensing deficit of the Al-doped TiO2 layer, a perovskite layer as a catalytic filter is employed. This Rh-incorporating perovskite has a self-regenerative property which results in the segregation of Rh nanoparticles on the reduction and re-incorporation into the lattice upon oxidation [25]. Relying on the NO oxidizing capability of Rh, the effects caused on a sensor due to the high-temperature deviations in NO2 containing gas environments can be eliminated.
3.2. Catalytic BaTi0.95Rh0.05O3 Powder
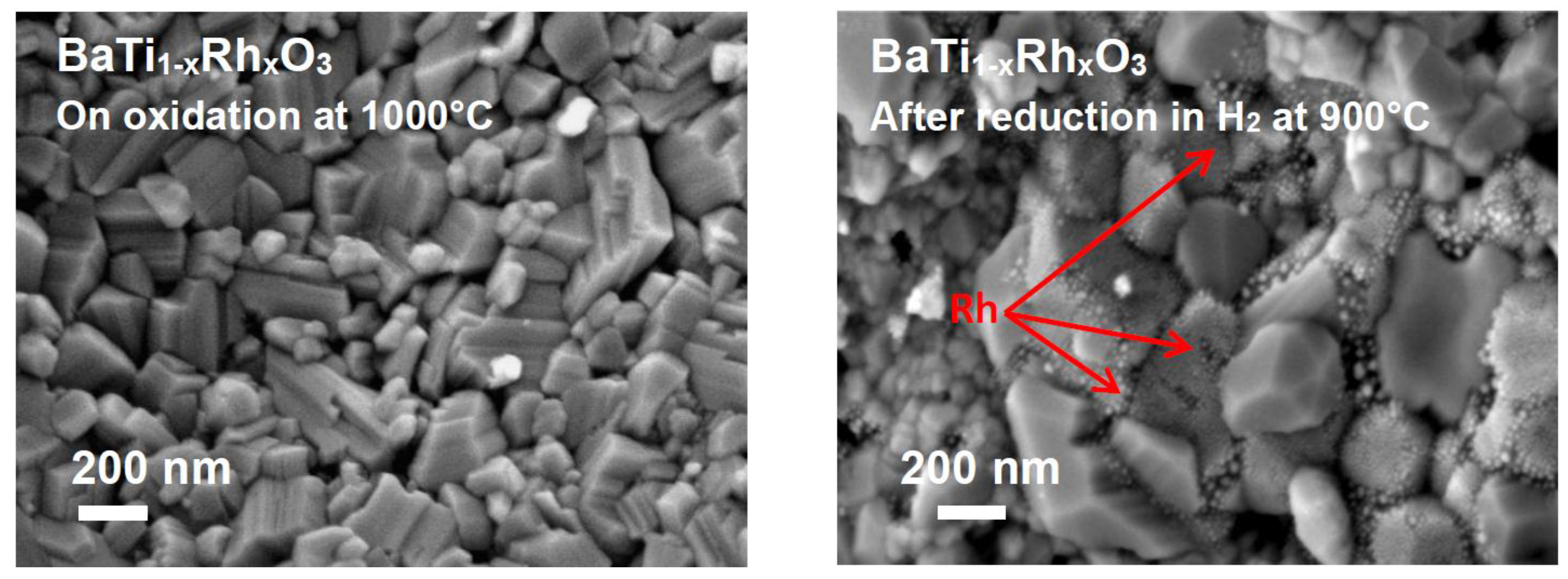
The SEM observations of the BaTi0.95Rh0.05O3 catalytic material exhibited that under oxidized conditions (e.g., after calcining at 1000 °C in air), large prismatic grains of perovskite were formed. In turn, on heat-treatment of the oxidized powder, this time under reduced conditions (e.g., 900 °C in 2.5 vol.% H2), Rh metallic particles segregates at the grain boundaries of the perovskite (Figure 6, left and right, respectively).
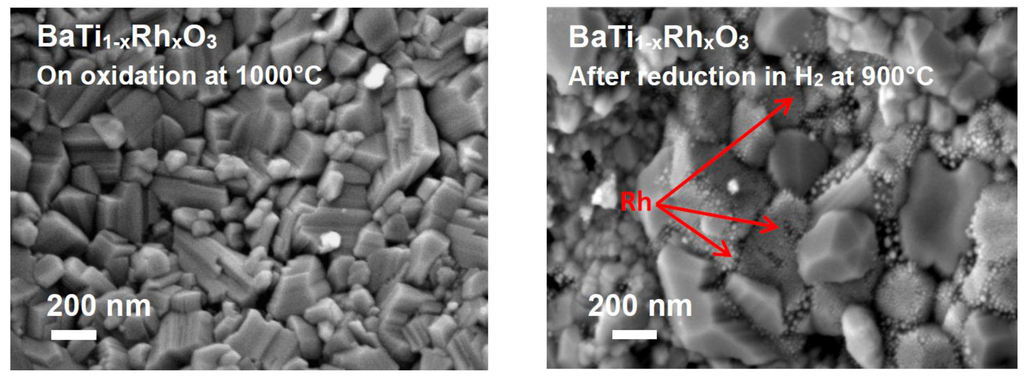
Figure 6.
(Left) SE micrograph of the BaTi0.95Rh0.05O3 catalytic filter after calcining at 1000 °C in air and (Right) after reducing treatment at 900 °C under 2.5 vol.% H2 in argon, displaying the segregation of Rh nanoparticles.
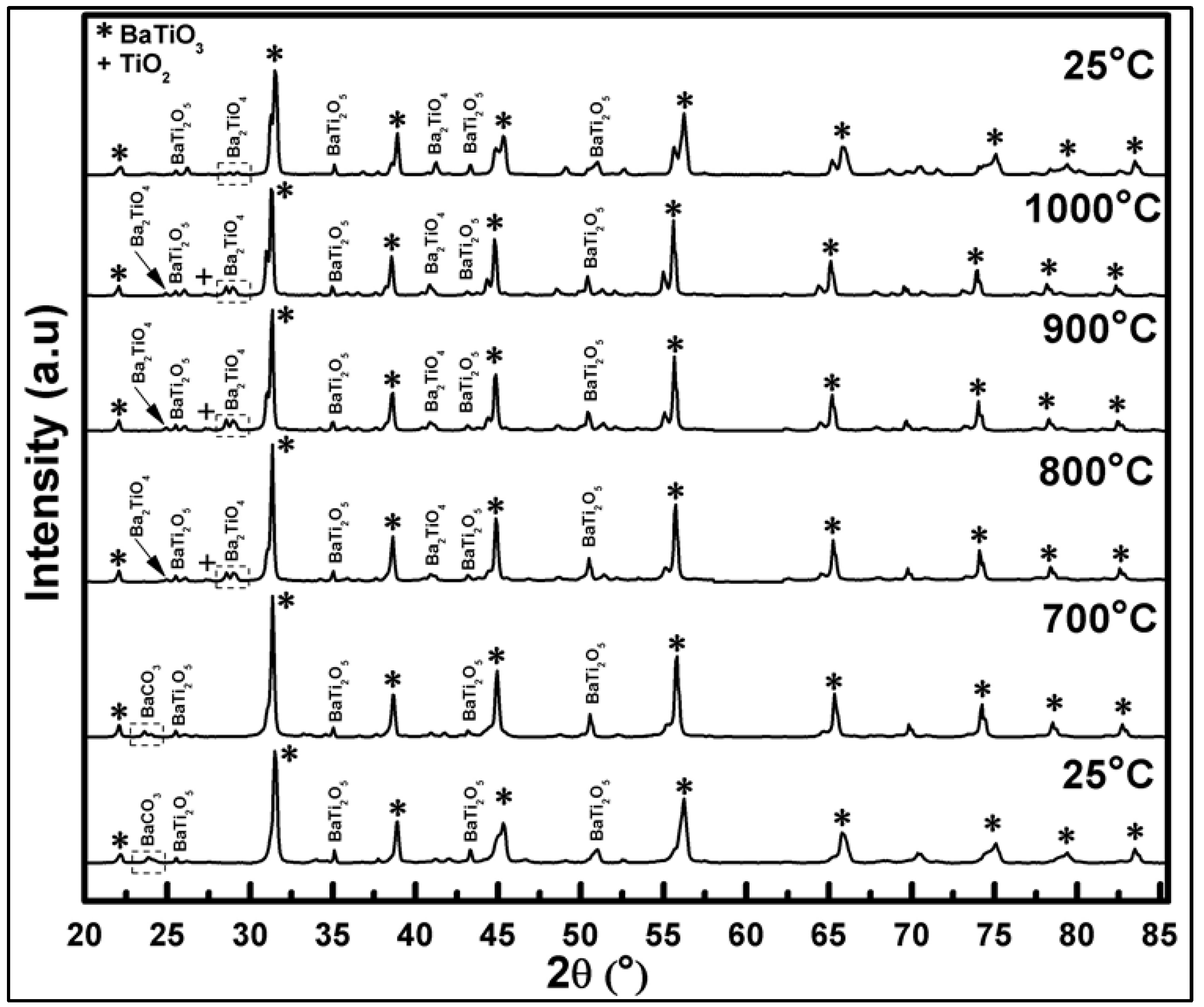
In situ high-temperature XRD analysis of pre-calcined powder in the range of RT-1000 °C-RT showed that the increase of temperature results in shifting of all X-ray diffraction peak positions of BaTi0.95Rh0.05O3 to lower Bragg angles (2θ) due to expansion of the lattice (see Figure 7). After heating to 700 °C in air, the BaTi0.95Rh0.05O3 powder yields the BaTiO3 perovskite, BaTi2O5, and BaCO3 phases.
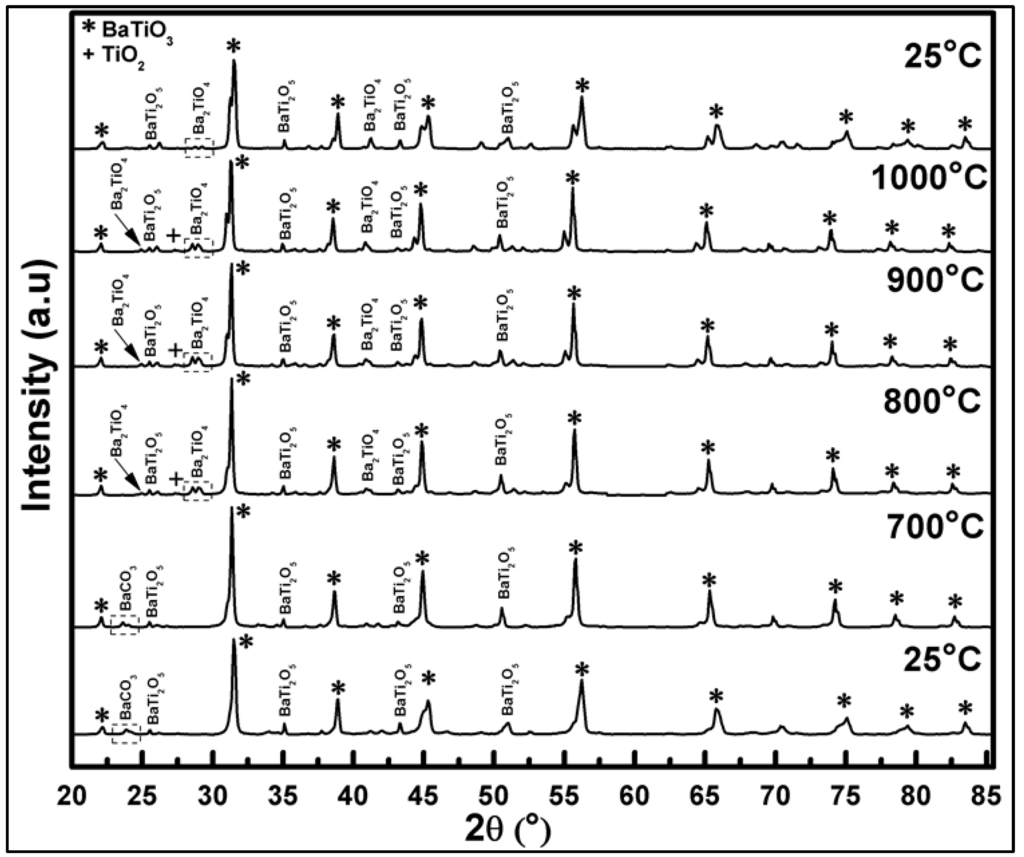
Figure 7.
In-situ HT-XRD diffractograms of BaTi0.95Rh0.05O3 powder collected over a temperature range varying from 25 °C to 1000 °C and cooling back to 25 °C.
BaCO3 disappears at around 800 °C, probably due to the low melting point of the compound. In turn, at this temperature, Ba2TiO4 phase appears to form. On heating to 1000 °C, next to the well-crystallized BaTiO3, the BaTi2O5, and Ba2TiO4 phases at lower intensity, and a trace TiO2 phase are detected. At this stage, the presence of some trace amounts of RhO2 cannot be ruled out even though the matching peak positions were not identified due to the overlapping. After cooling down to RT, the in situ to 1000 °C heated powder contains BaTiO3, BaTi2O5, and the (111) plane of Ba2TiO4 phases.
In summary, it can be concluded that heating to 1000 °C and cooling down to RT in air of the BaTi0.95Rh0.05O3 catalytic powder results in full decomposition of BaCO3 and the formation of BaTi2O5 and Ba2TiO4 through the irreversible phase conversion. These phases may contain partially-incorporated Rh. No clear identification of the presence of metallic Rh or RhO2 was possible during in situ X-ray of this in air heat-treated powder.
After reduction treatment in H2, BaTi0.95Rh0.05O3 perovskite powder displays fine agglomerates which are associated with the presence of metallic Rh (Figure 6, right). The presence of cubic Rh with the 2θ position of 47.8° in the X-ray diffraction pattern of the reduced perovskite powder indicates that the hexagonal perovskite decreased upon reduction in H2. The sensor tests were carried out with a BaTi0.95Rh0.05O3 powder which was calcined in air at 1000 °C.
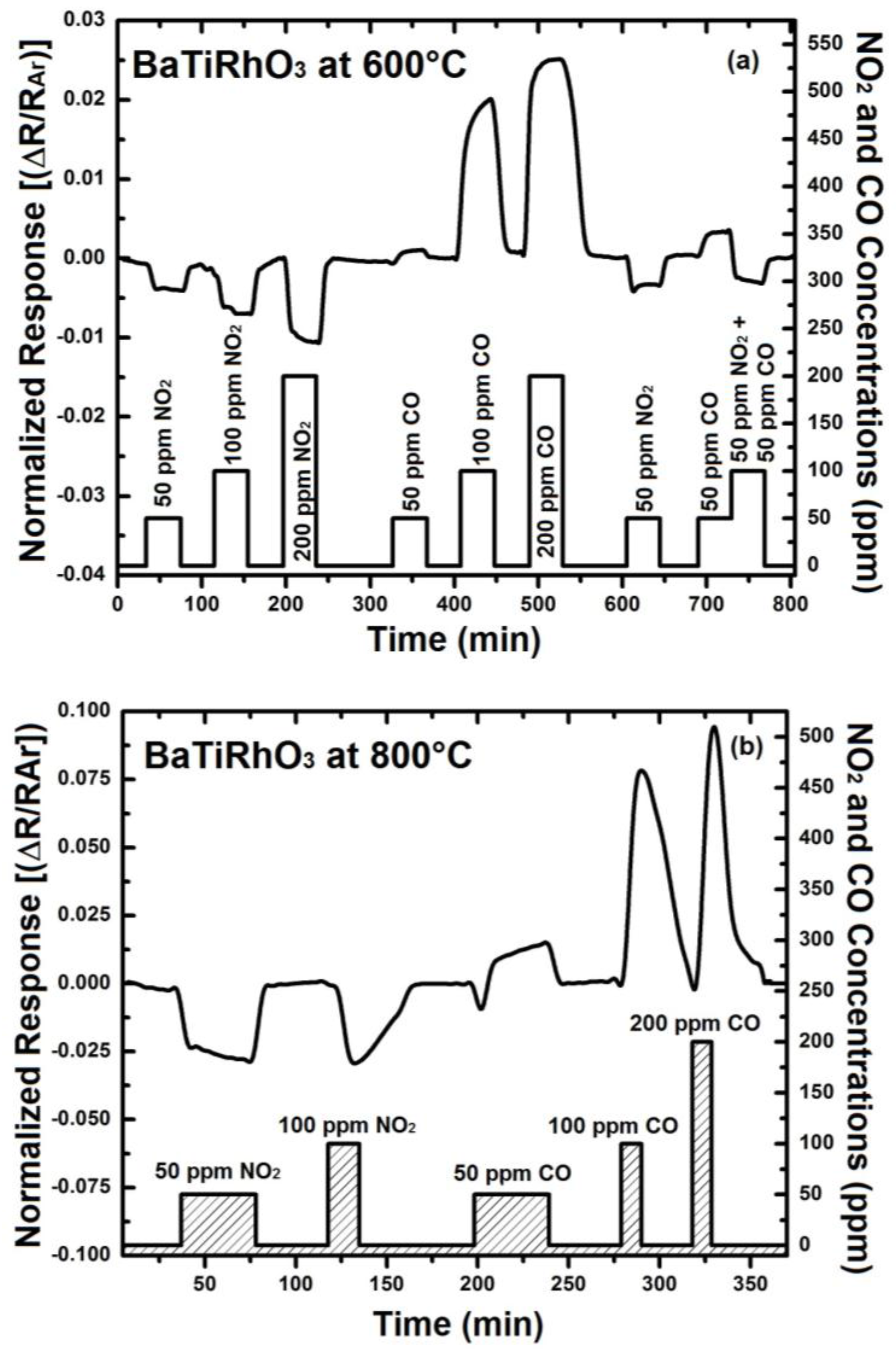
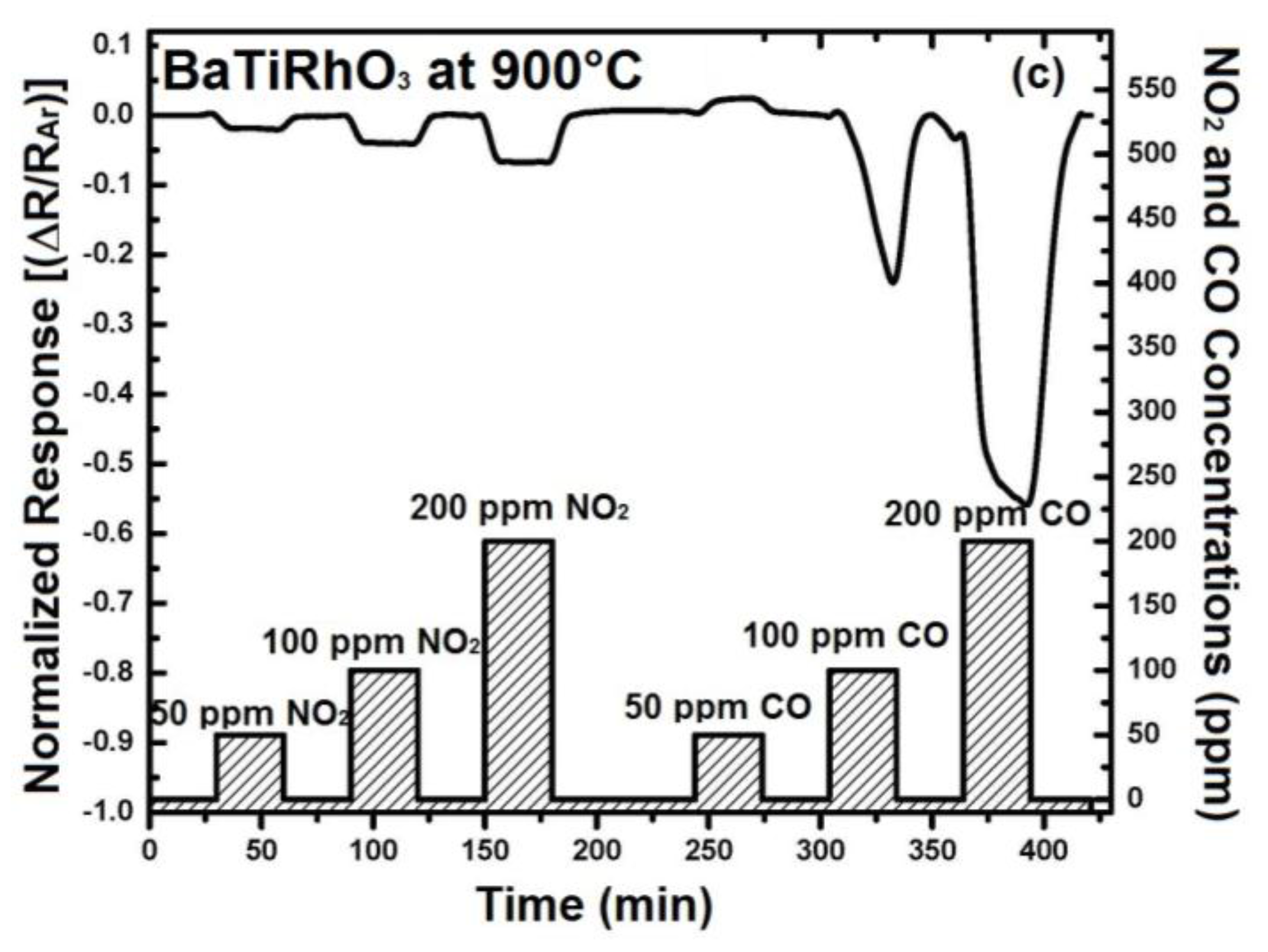
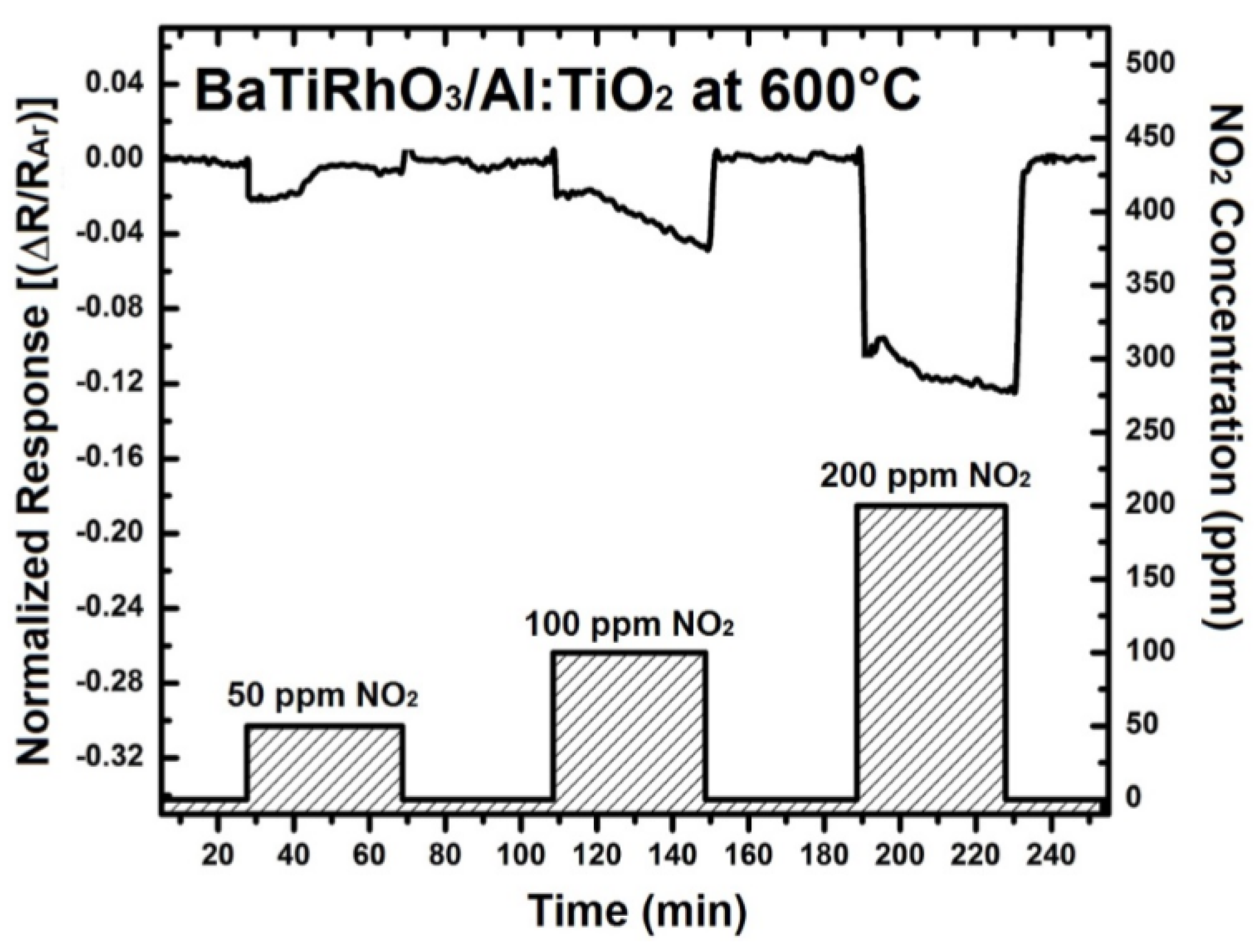
In order to understand the contribution of BaTi0.95Rh0.05O3 perovskite to the sensor response, initially the sensor response of this perovskite has been recorded. For that, some interdigital sensor platforms were coated by screen-printing with a thick BaTi0.95Rh0.05O3 perovskite powder. These sensors were tested towards NO2 and CO in the form of single gas exposures having different concentrations at 600 °C to 900 °C in a dry argon environment (see Figure 8).
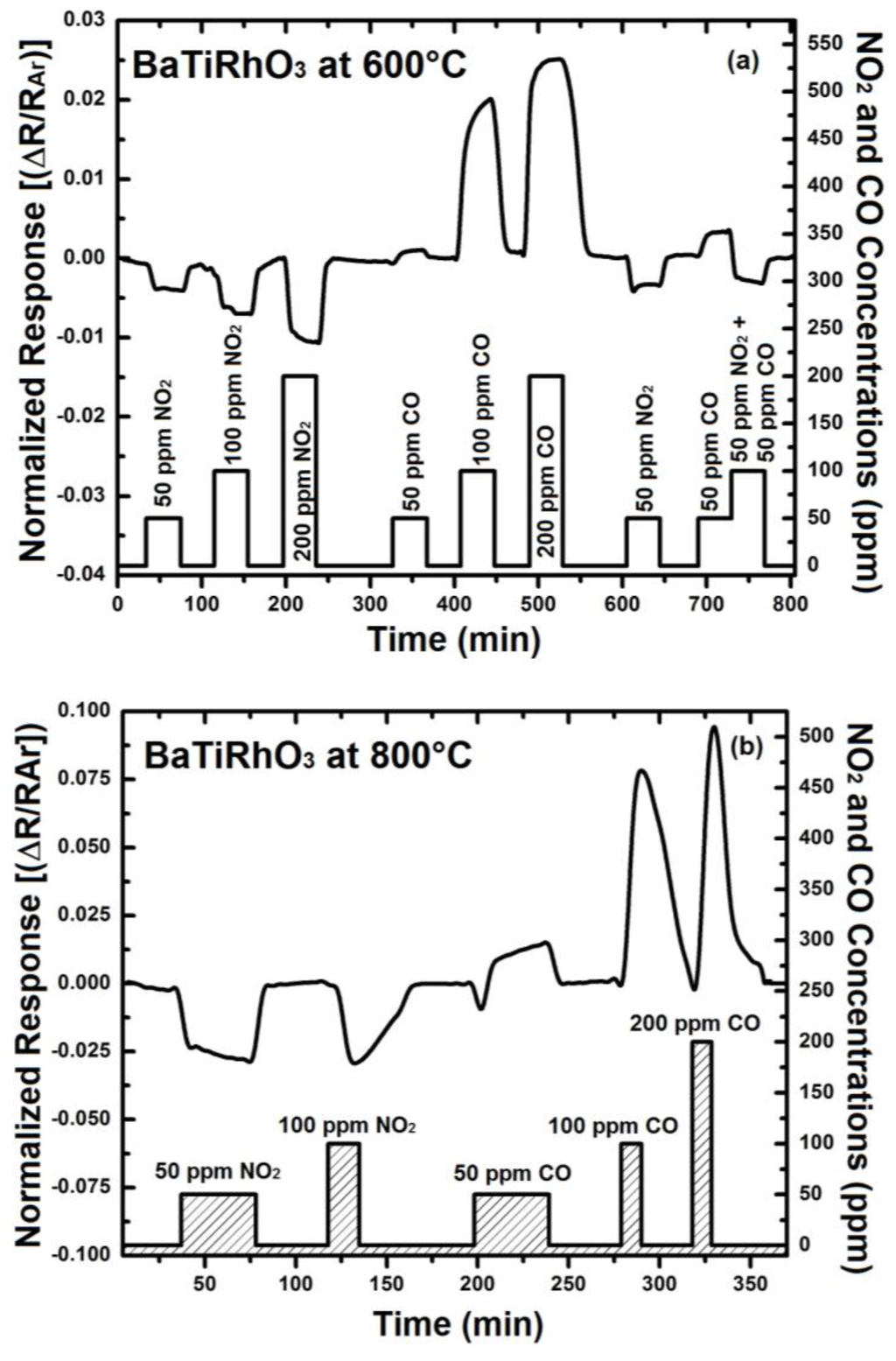
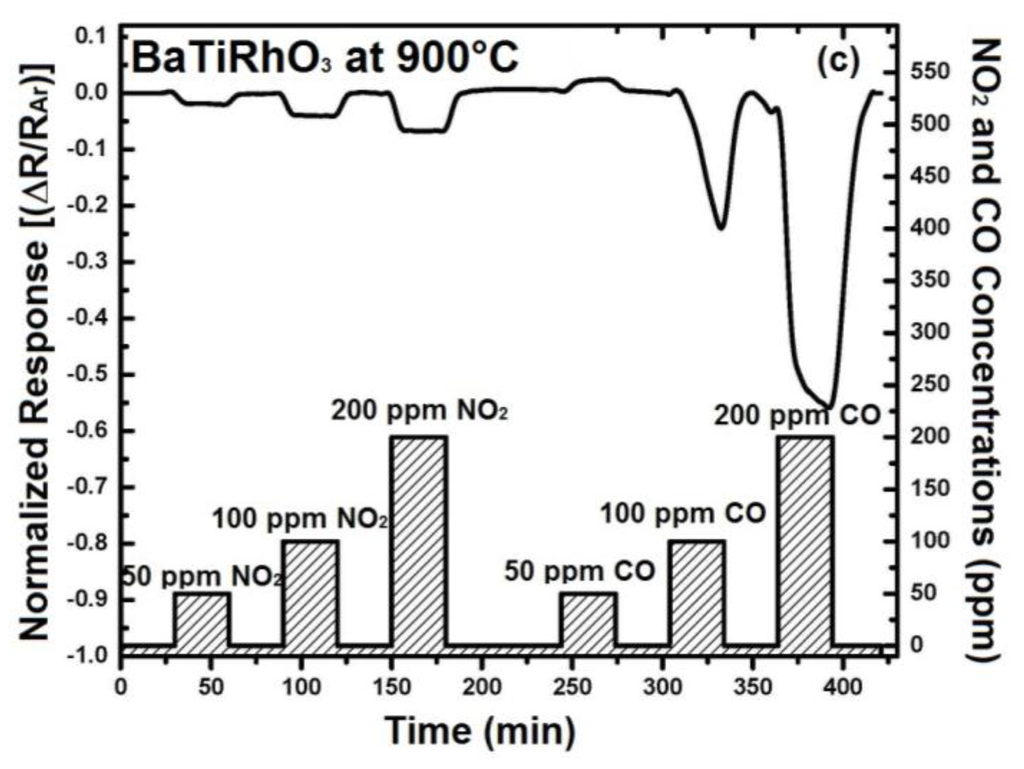
Figure 8.
Sensor response of the BaTi0.95Rh0.05O3 catalyst layer towards NO2 and CO at 600 °C (a) 800 °C (b), and at 900 °C (c) in dry argon.
Thick layers of BaTi0.95Rh0.05O3 perovskite catalyst yield remarkable response towards NO2 at the temperatures of 600 °C to 900 °C and in dry argon, but also a high sensor response towards CO (50 to 200 ppm). As the sensor response towards NO2 and CO improves with the temperature, the CO response alters to the opposite direction at 900 °C (see Figure 8). This behavior of the catalytic layer may be due to the CO-oxidation capability. Our previous unpublished tests carried out with this catalytic perovskite by using 1% CO, 5000 ppm O2 diluted in He indicated a very low light-off temperature for CO oxidation (below 210 °C). While under less oxygen-containing conditions, i.e., by exposing the perovskite to 1000 ppm CO and 1000 ppm NO in He, it was observed that CO conversion occurs at significantly higher temperatures.
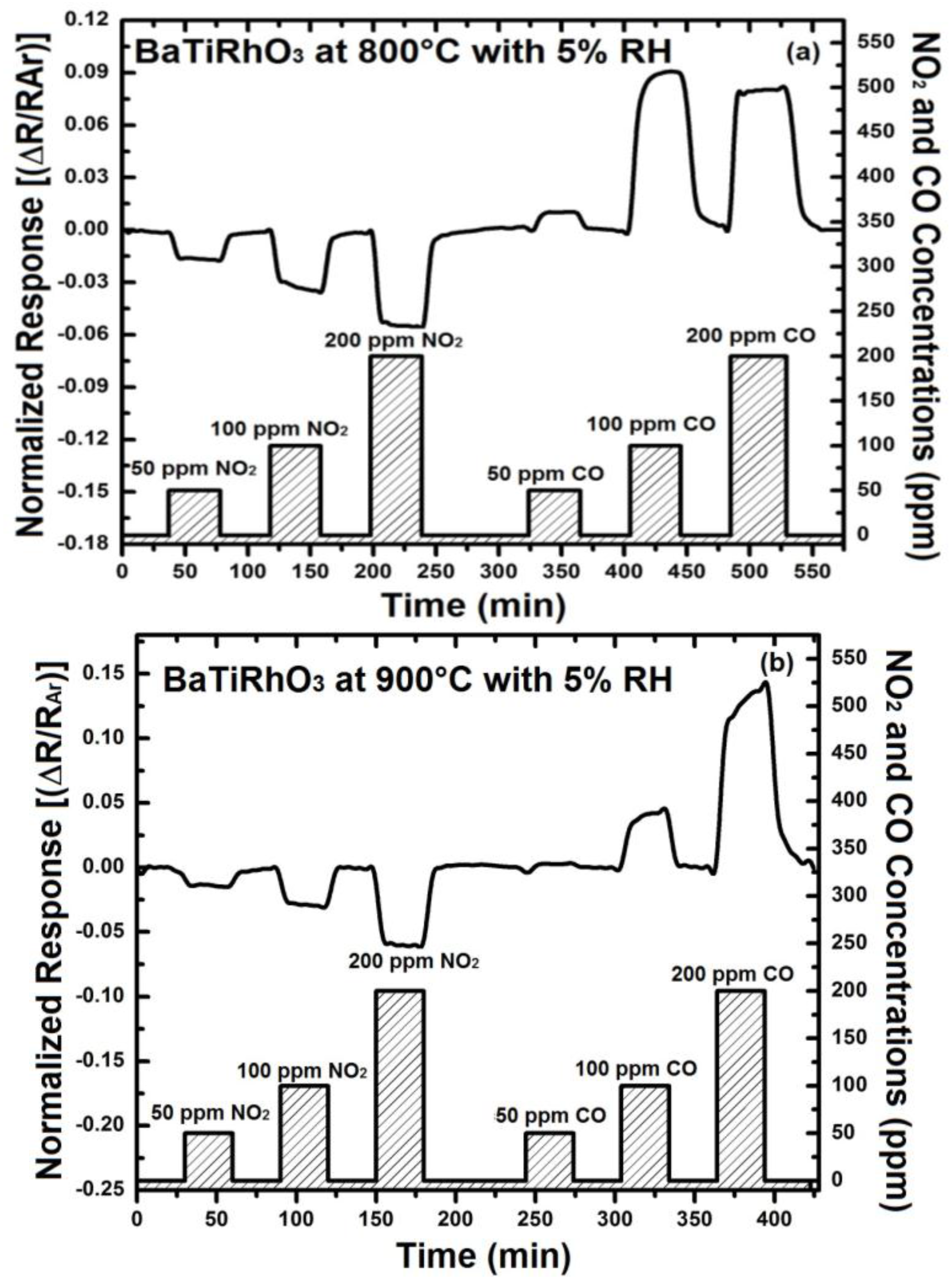
The addition of 5% humidity to the argon environment results in an improvement of the NO2 response of BaTi0.95Rh0.05O3 perovskite by a factor of two at 800 °C (e.g., 0.02 vs. 0.04 for 100 ppm at 800 °C) and somewhat less at 900 °C (see Figure 9). Similarly, CO response in the presence of humidity yields resistance increase at 900 °C, as observed under dry conditions, indicating an adsorption promotion effect of OH−δ ions (see [14]). The discrepancy, concerning CO response at 900 °C under dry conditions, can be explained with the facts that water vapor or OH-ions at this temperature may either react with CO during adsorption on the catalyst surfaces or the BaTi0.95Rh0.05O3 influences catalytically the adsorption mechanism of CO under oxidizing conditions. All sensor responses with BaTi0.95Rh0.05O3 were p-type at all test temperatures with the exception of CO exposure at 900 °C. Nevertheless, the sensor response towards CO is significant. Thus, considering this fact, it is expected that the BaTi0.95Rh0.05O3 layers as a gas sensing material may display a high CO cross-interference under exposure to CO concentrations higher than 50 ppm at high temperatures.
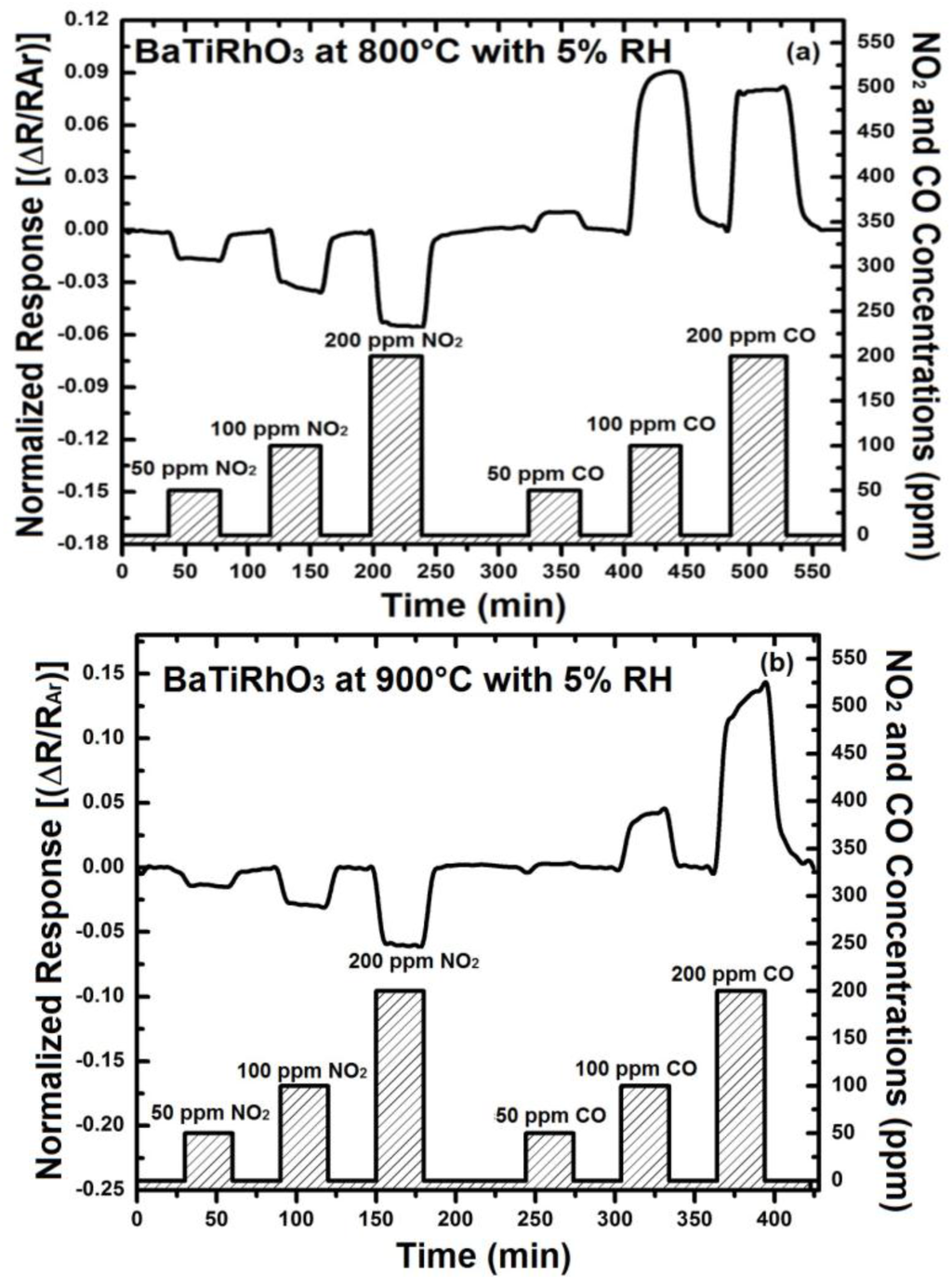
Figure 9.
(a) Sensor response of only BaTi0.95Rh0.05O3 thick catalytic layer towards NO2 at 800 °C, and (b) at 900 °C in the presence of 5% humidity.
3.3. NO2 Response of BaTi0.9Rh0.1O3 Catalyst + Al-Doped TiO2 Duplex Layers
According to the concept presented above in Section 2.1, duplex sensor layers having catalytic BaTi0.95Rh0.05O3 perovskite and Al-doped TiO2 are tested for their NO2-response in the temperature range of 600 °C to 900 °C.
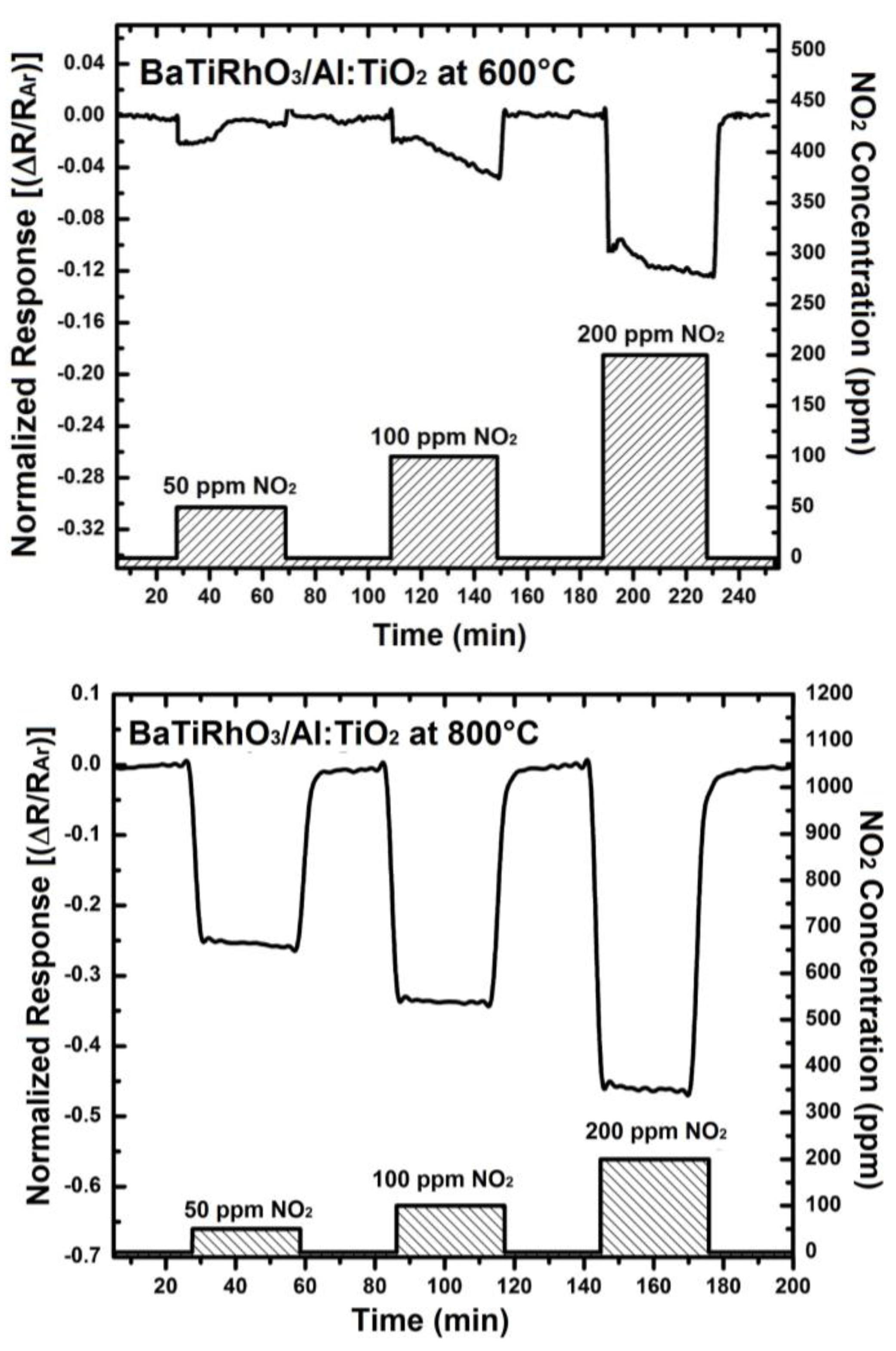
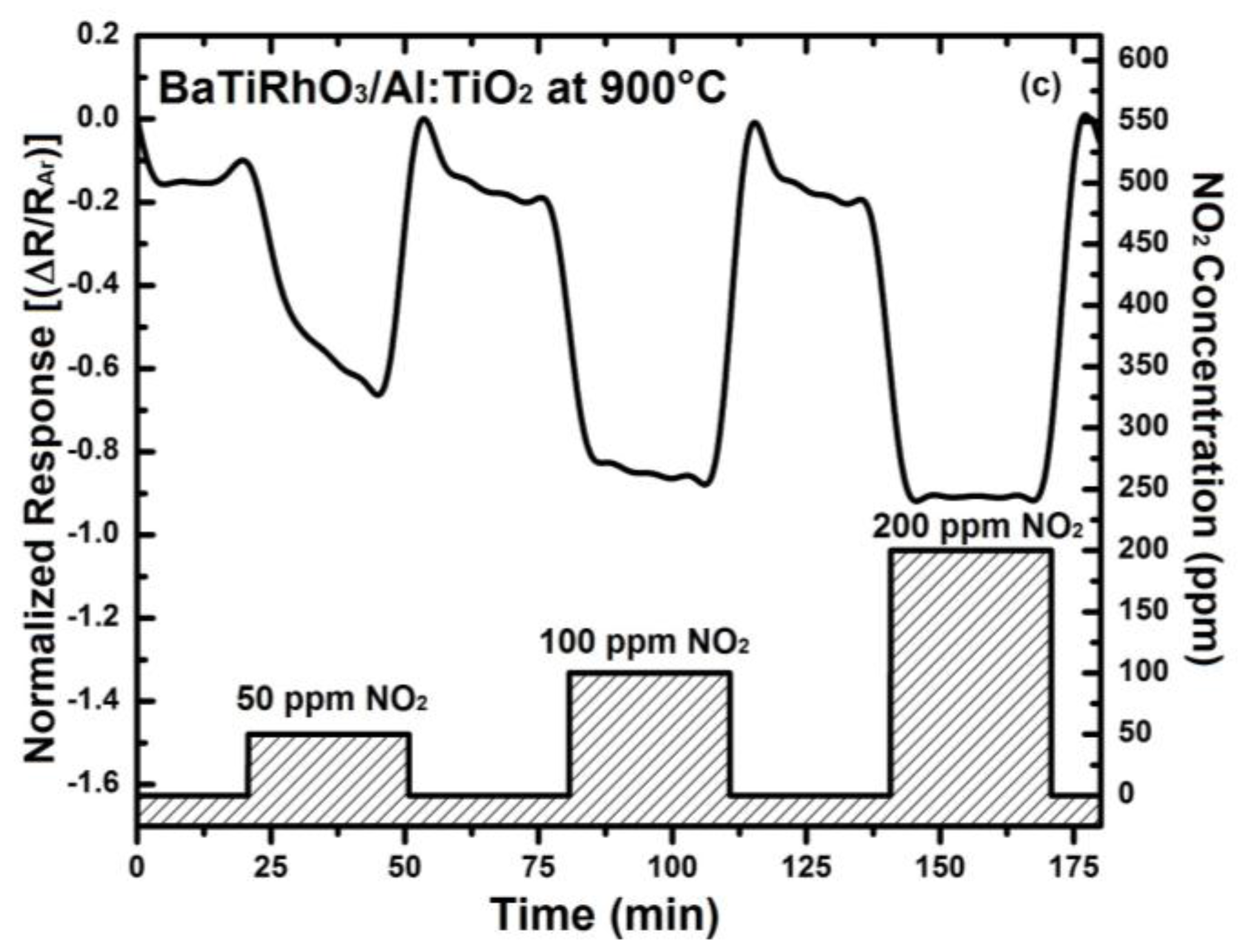
The NO2 sensor response obtained with these duplex layers display remarkable sensitivity values at 600 °C, 800 °C, and 900 °C despite high gas temperatures (e.g., 0.12 at 600 °C, 0.46 at 800 °C, and 0.92 at 900 °C, upon exposure to 200 ppm), (see Figure 10). Moreover, the change in signal direction which was observed with the Al-doped TiO2 layer as indicated with the sensor responses given in Figure 4 does not occur when duplex sensing layers are employed.
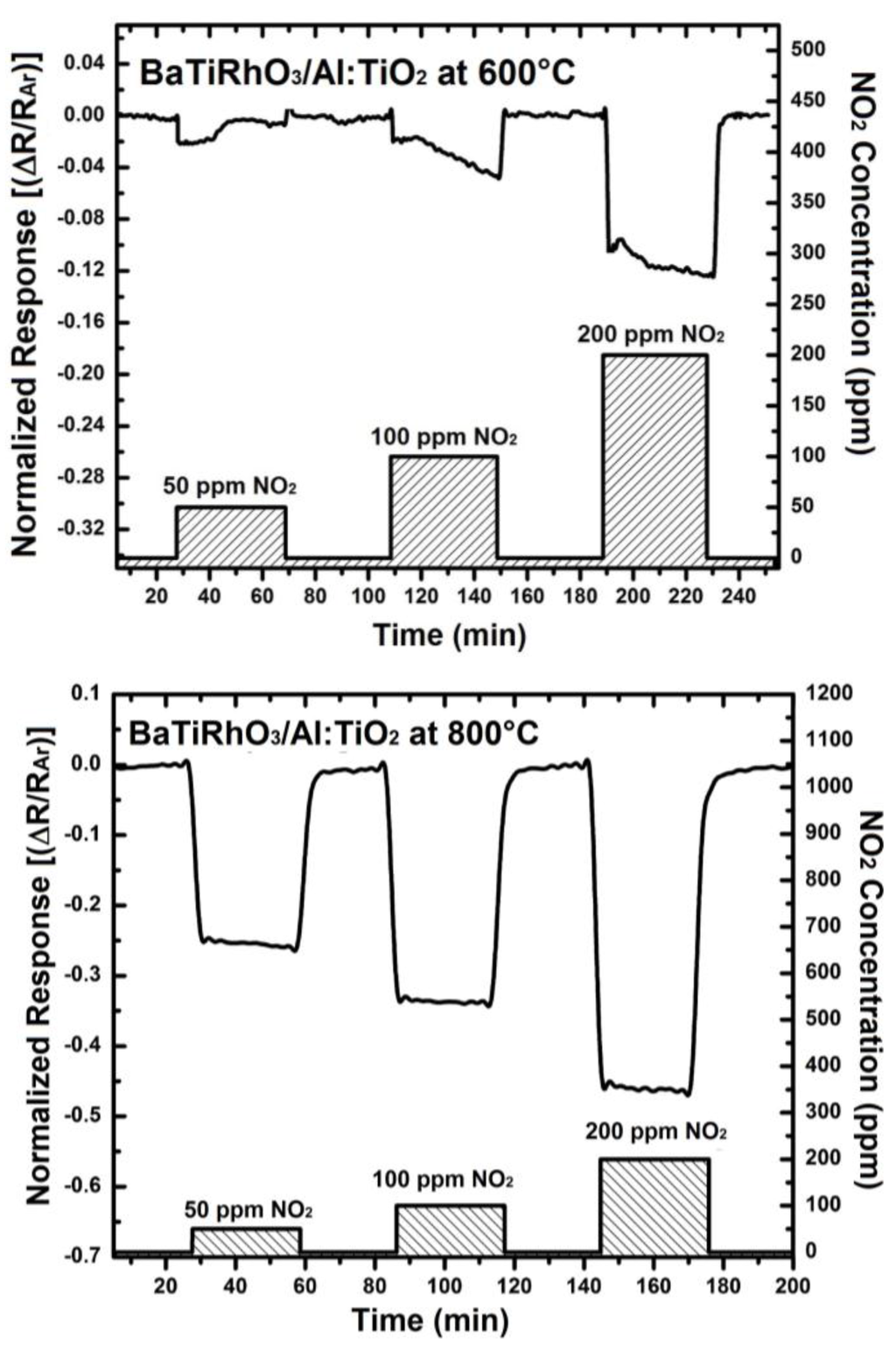
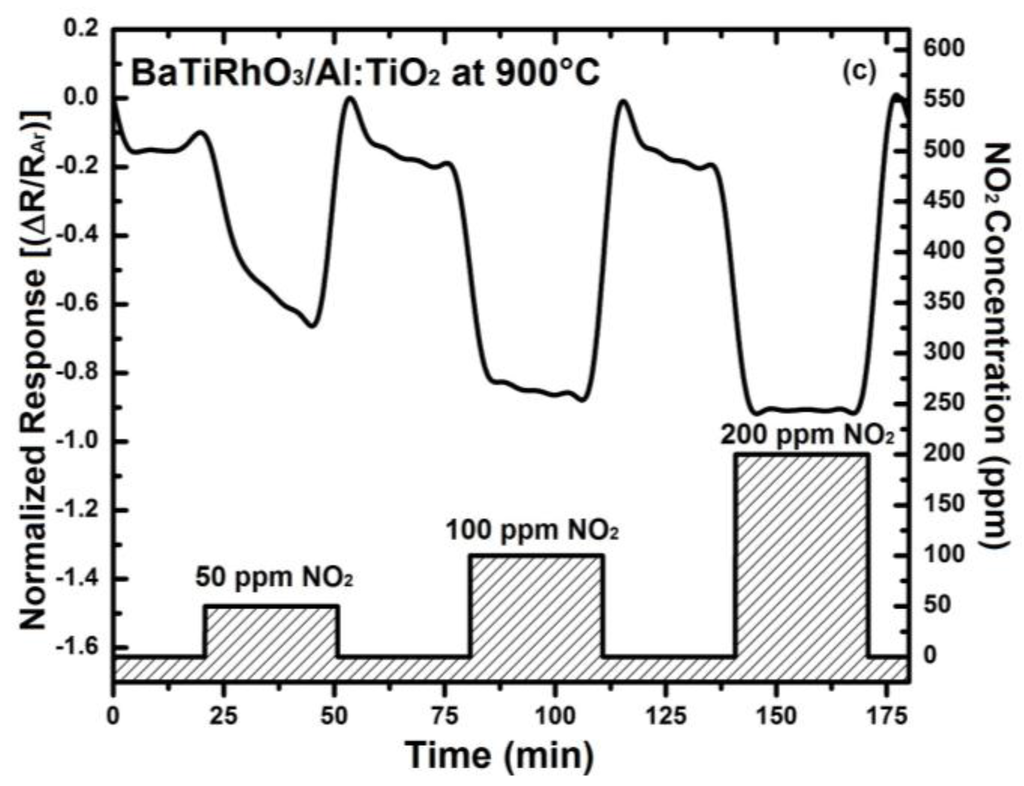
Figure 10.
Sensor response of BaTi0.95Rh0.05O3 catalyst + Al-doped TiO2 sensor duplex layers towards NO2 in a dry argon environment at 600 °C (a), at 800 °C (b), and at 900 °C (c).
In order to clarify whether this response at such elevated temperatures is from NO2 or from NO, the sensor response of this duplex layer towards NO was recorded in dry synthetic air (see Figure 11). Normally as shown with Figure 5, above 600 °C under dry conditions, a temperature-dependent conversion of NO2 to NO occurs.
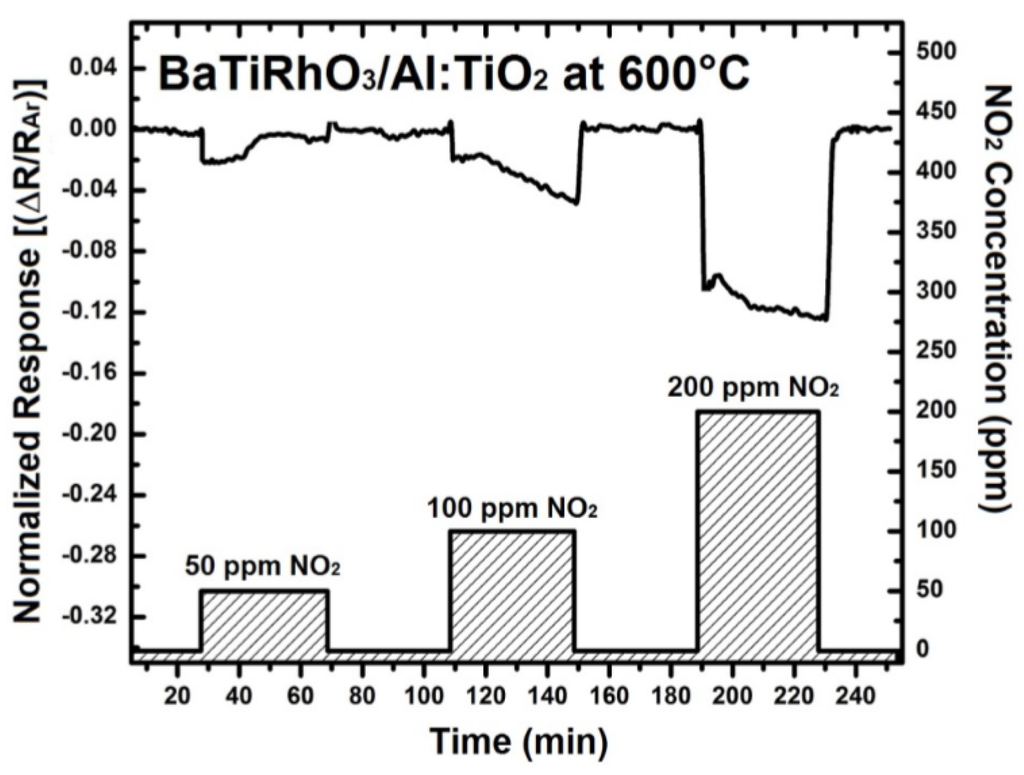
Figure 11.
Sensor response of BaTi0.95Rh0.05O3 catalytic + Al-doped TiO2 sensor duplex layers at 600 °C toward NO in a dry synthetic air environment.
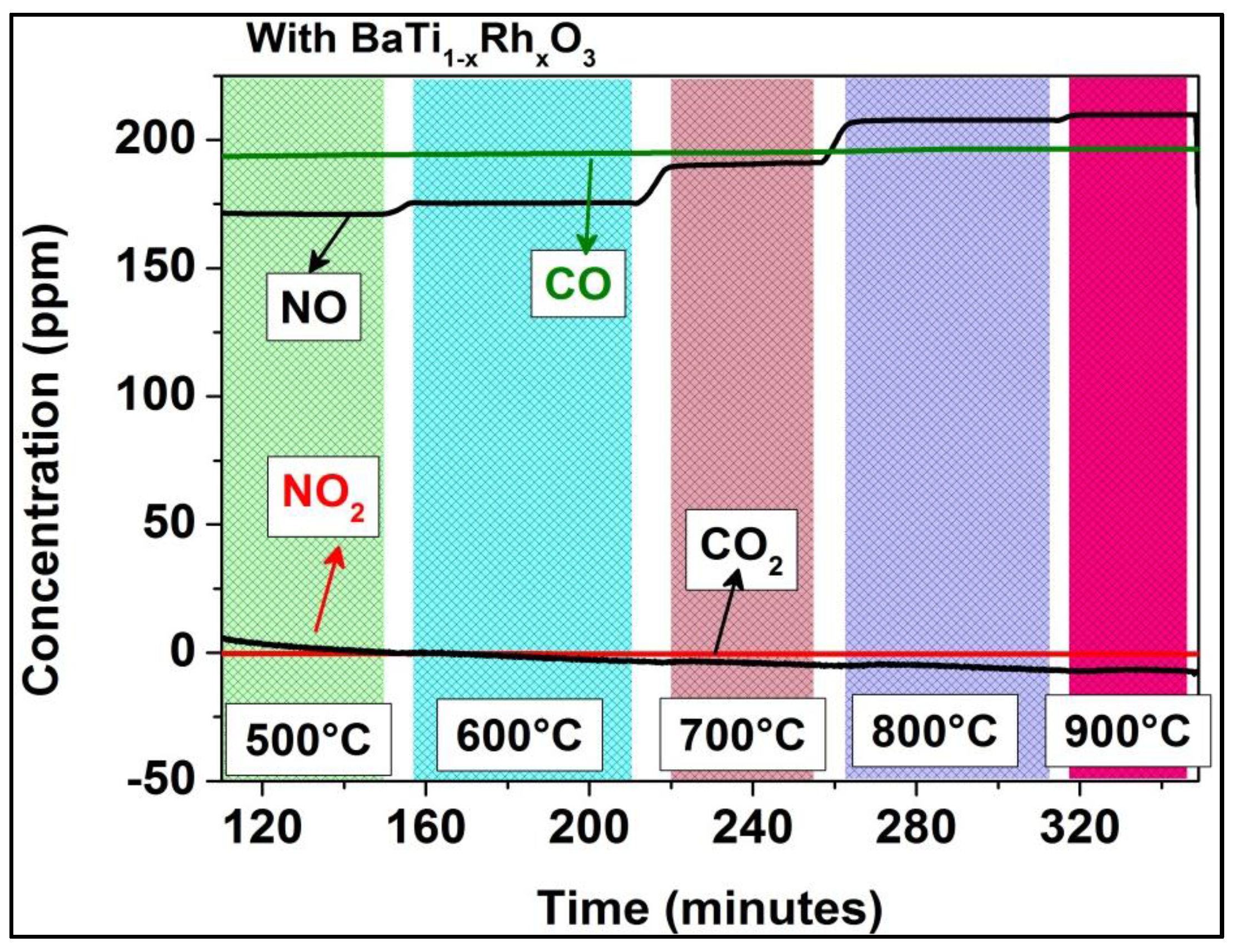
The sensor tests of the BaTi0.95Rh0.05O3 perovskite and Al-doped TiO2 duplex layer carried out toward NO in a dry synthetic air environment at 600 °C exhibited significantly lower sensitivity values (e.g., 0.018 and 0.023 under exposure to 50 ppm and 200 ppm, respectively). Further analyses, which were carried out to observe the effect of catalytic perovskite powder on NO2/NO conversion at high temperatures, indicated that full NO2 to NO conversion already occurs at 300 °C if BaTi0.95Rh0.05O3 catalytic powder was exposed to 200 ppm NO2 in the sensor chamber (see Figure 12). In turn, when the chamber is empty, i.e., without the perovskite catalyst, the NO2-to-NO conversion develops gradually, starting from 500 °C, and is completed above 800 °C as, at that temperature, no more NO2 can be measured in the system’s output (see Figure 5). Thus, it is reasonable to assume that the BaTi0.95Rh0.05O3 catalytic top layer conditions the test environment to have NO+O2− at all test temperatures and catalytically oxidizes NO to NO2 at the interface of the perovskite and the Al-doped TiO2 layer [10]. Thus it can be concluded that exposure of the sensor to NO2 at higher temperatures yields a sensor signal which cannot be correlated to NO and to molecular oxygen from the synthetic air, but to NO2.
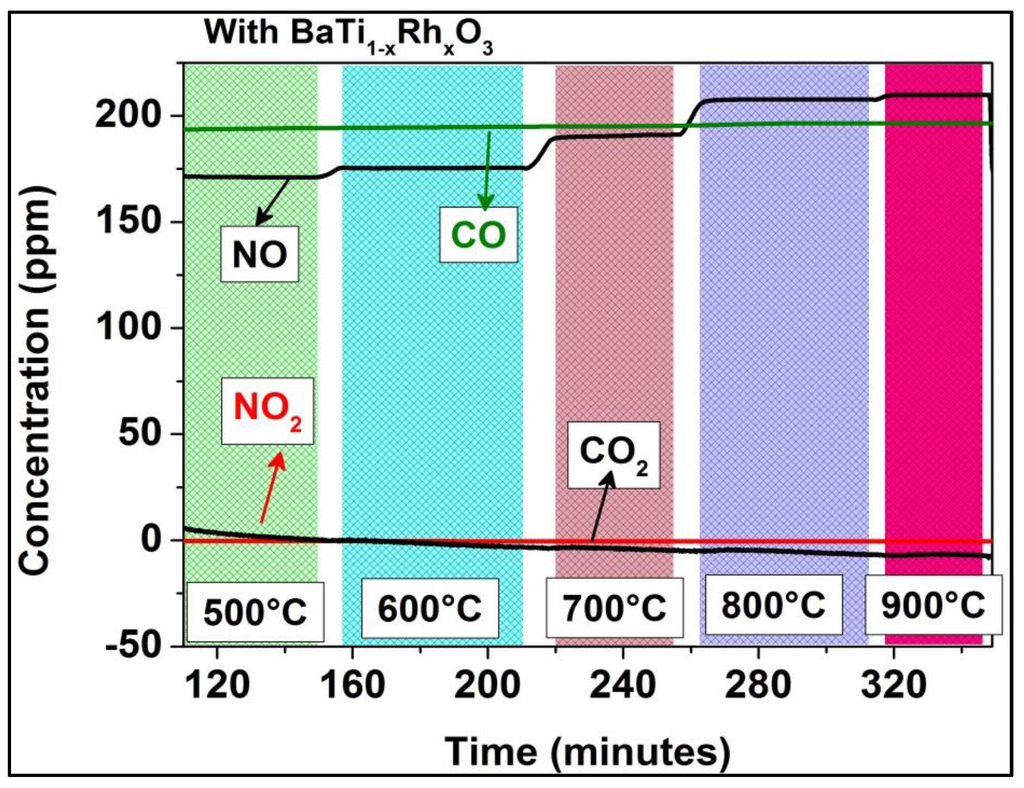
Figure 12.
Temperature-dependent conversion of NO2 and CO with BaTi0.95Rh0.05O3 catalytic powder present in the test chamber.
Table 1 summarizes a selection of sensor properties of single and duplex sensor layers investigated in this study. For comparison purposes, only the responses toward 200 ppm NO2 are considered. Sensitivities of Al:TiO2 single layer and BaTiRhO3/Al:TiO2 duplex layer are relatively high at 600 °C (0.70 and 0.92, respectively), but the recovery of sensor response with the single Al:TiO2 layer requires extremely longer times. At higher temperatures, and in a humid environment, this appears to be shortened. The sensor with the single BaTiRhO3 layer exhibits, in turn, the lowest sensitivities (0.11 and 0.05) at all test temperatures (600–900 °C). No further NO2-sensing measurement is carried out at 900 °C with the single Al:TiO2 layer because of the lack of reliability of its sensor signal.

Table 1.
Sensor properties of various sensor layers in the temperature range of 600–900 °C in dry or in 5% humid argon environment.
The sensitivity of the sensor with the duplex layer reduces down to 0.16 at 800 °C, and further to 0.06 at 900 °C. Although the sensitivity of the duplex layer sensor (i.e., BaTiRhO3/Al:TiO2) is lower than that of the Al:TiO2 single layer, the reaction and recovery times of the sensor with the duplex layer is partly to half as short than those of the Al:TiO2 single layer sensor (reaction times = 2.0 min vs. 2.5 min at 600 °C and 1.6 min vs. 3.8 min at 800 °C, recovery times = 4.3 min vs. 17 min at 600 °C and 2.5 min vs. 4.2 min at 800 °C, respectively). The sensitivities and the reaction and recovery times of the BaTiRhO3/Al:TiO2 duplex layer sensor remain in the same range at all test temperatures, indicating a maintained reliability with this sensor.
Humidity shows no significant effect on sensitivity, although some influence on reaction and recovery times are observed favoring humidity at 800 °C, and oppositely at 600 °C. Therefore, it is not possible to identify any specific trend regarding the humidity influence. Although it is obvious that the BaTiRhO3 catalytic layer intervenes in the thermodynamic equilibrium condition of NO2/NO environment by lowering the conversion temperature significantly, as a single sensor layer, the sensor with the BaTiRhO3 single layer does not yield good performance for NO2-sensing.
The literature indicates that perovskites with different formulations give great opportunity to detect gas mixtures by means of sensor arrays. Doping elements either in the lattice of perovskite or on the surface of perovskite could help to improve sensing performance of perovskite oxide and to decrease the sensing temperature [27,28]. La-based, Ce and Pd-doped perovskites are proven to yield good sensing behavior toward CO and CH4, respectively. No other sensor study is known to date with the BaTiRhO3 catalytic layer to confirm its behavior. However, Figure 9 displays that the sensor with a single BaTiRhO3 layer yields greater signals toward CO than NO2.
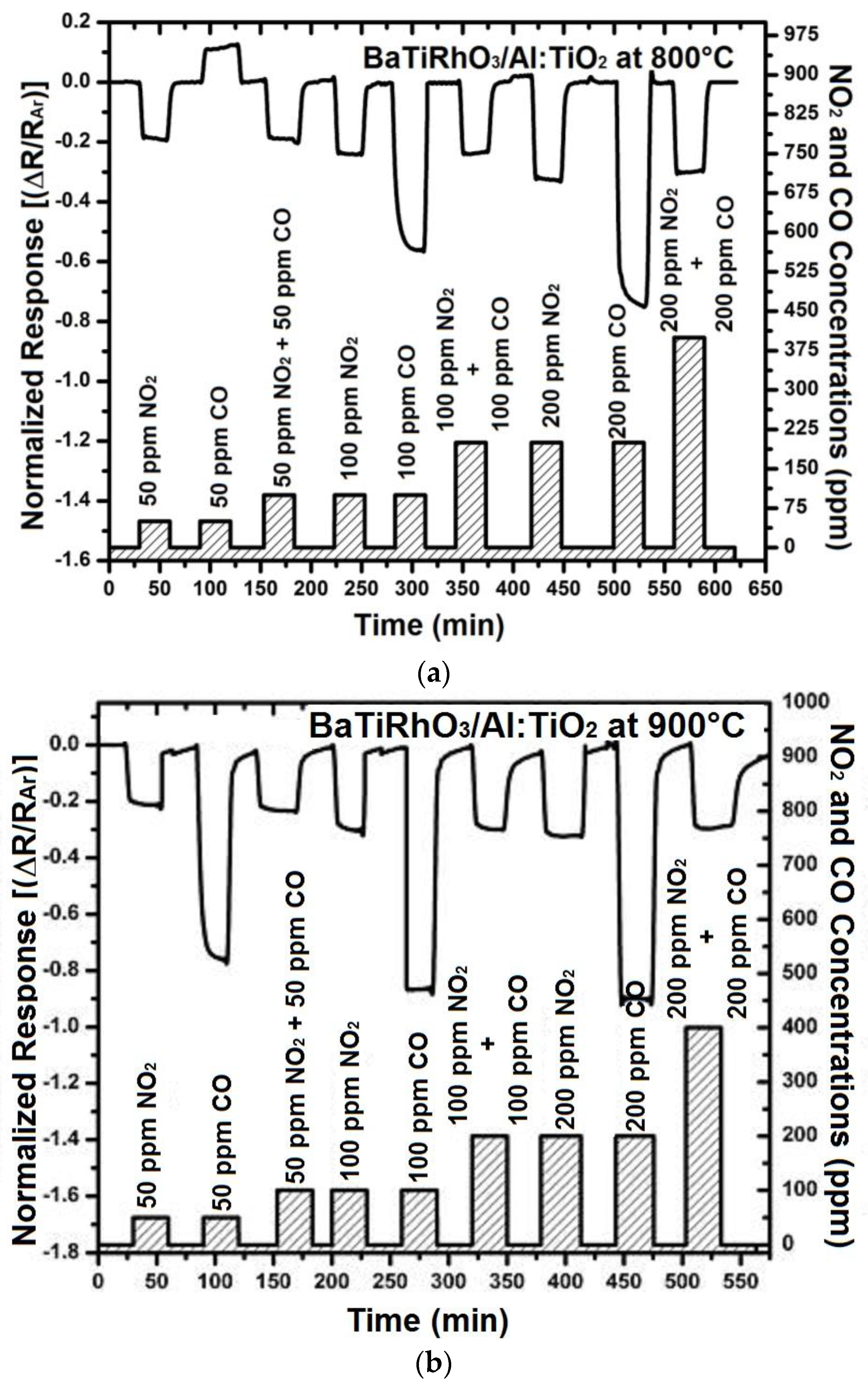
The sensors with the BaTiRhO3/Al:TiO2 duplex layers, in turn, exhibit a NO2-selective sensing as exposed to CO + NO2 mixed gas environments in the temperature range of 600 °C and 900 °C. Although stronger signals are observed by exposure only to CO, it appears that these signals are in the same direction as those toward NO2 (Figure 13). Usually, the semiconducting sensors yield signals in opposite directions when exposed to oxidizing (e.g., NO2, O2) and reducing gases (e.g., CO, CH4).
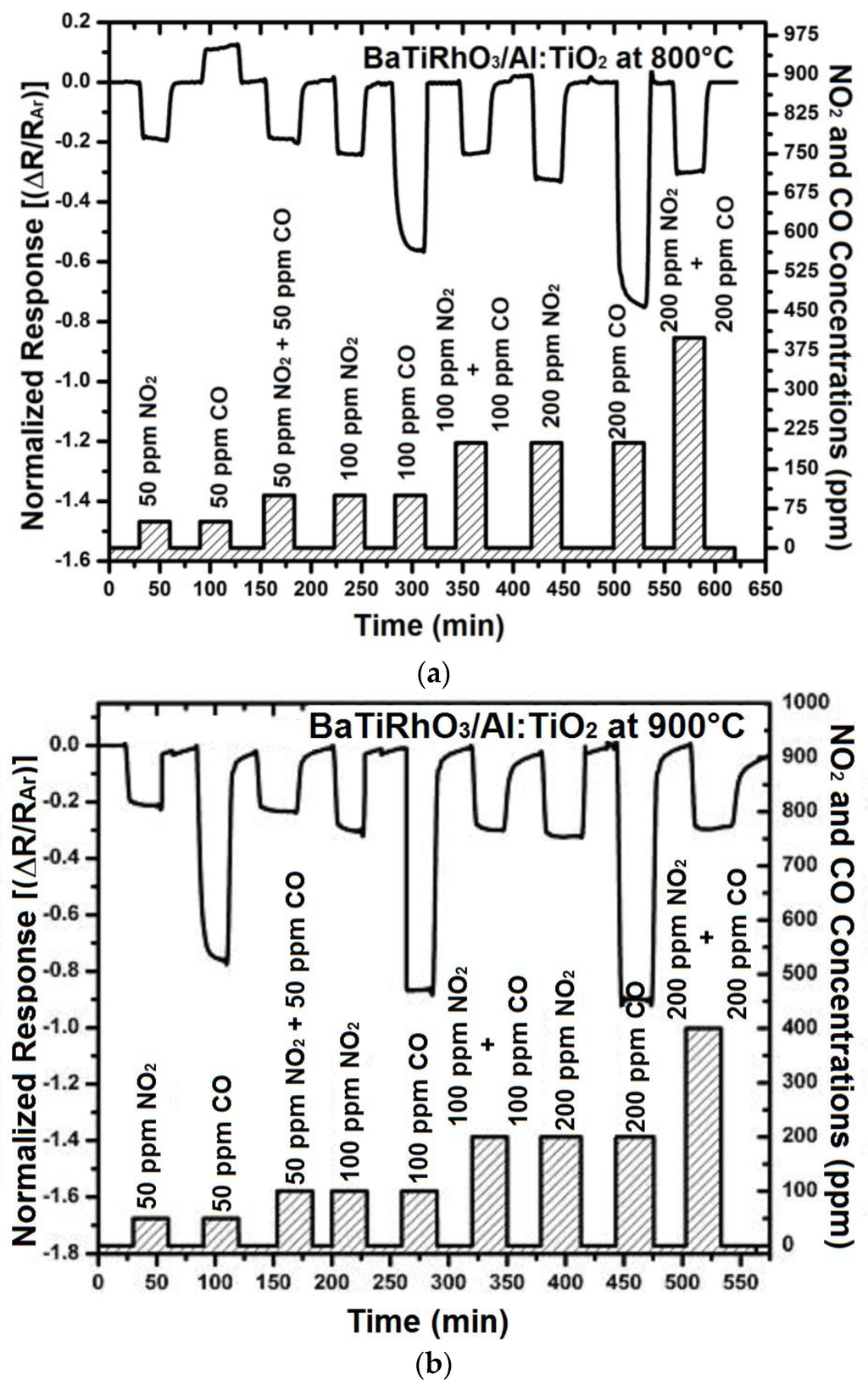
Figure 13.
Sensor response of BaTi0.95Rh0.05O3 catalyst + Al-doped TiO2 duplex layers at 800 °C (a) and 900 °C (b) in dry argon environment on exposure to NO2 and CO single and/or NO2 + CO gas mixtures.
Previous studies which used XPS to determine the nature of CO adsorption on the surface of doped LaCoO3 perovskite formulations as the sensor material have revealed that CO preferentially adsorbs on the lattice oxygen in the form of mono-dentate carbonate on the surface of perovskite-type oxide. Ce (e.g., La0.9Ce0.1CoO3) doping changed not only the total amount of adsorbed CO but also the nature of adsorption phenomena. By adding Ce, in addition to the mono-dentate form, the bi-dentate carbonate form was also appeared on the surface resulting in an increase of the total adsorbed CO [27]. In the case of Pd-doped LaFeO3, the noble metal gives an active site on the metal oxide to adsorb methane species. Pd is well known for its H dissolving capacity, which could help to create PdH pair in methane adsorption phenomena [28].
In our case, it is likely that under single CO gas exposure in dry argon, CO adsorption rapidly consumes the surface-adsorbed oxygen of the sensing layer forming CO2 and excess electrons according to Equation (3):
Hence, it is probable that the sensors with the duplex layer exhibit CO cross-interference. In order to test this, the sensor response of the BaTi0.95Rh0.05O3 catalyst + Al-doped TiO2 duplex layers was recorded under NO2 and CO single-gas exposure, followed by NO2 and CO mixed gas exposure at 800 °C and 900 °C (Figure 13 left and right). These tests displayed that the duplex layer is more sensitive to NO2 in the presence of CO, while under CO single-gas exposure, relatively high sensor sensitivity is recorded. The resistance decrease obtained on exposure to gas mixtures of 50 ppm NO2 + 50 ppm CO, 100 ppm NO2 + 100 ppm CO, and 200 ppm NO2 + 200 ppm CO equals to that obtained by exposure only to NO2 at concentrations of 50, 100, and 200 ppm, respectively. In turn, under NO2 + CO gas mixture exposure, NO2 is converted to NO + O2− at temperatures above 300 °C through the BaTi0.95Rh0.05O3 catalytic layer and this ionized oxygen may readily react with CO without being in interaction with the sensor surface and is removed as CO2, while the converted NO reacts with the oxygen adsorbed on the surface of Al-doped TiO2 sensing layer. Thus, at temperatures as high as 800 °C and 900 °C, the sensor with the BaTiRhO3/Al:TiO2-duplex layer gives signals preferentially corresponding to NO2, despite being exposed to CO + NO2 gas mixtures at relatively higher concentrations (Figure 13, left and right).
4. Conclusions
Trivalent doping of TiO2 enables sensor response towards NO2 at temperatures up to 800 °C. However, above 600 °C, a change in the direction of the sensor signal is observed. This behavior of TiO2 sensors can be eliminated by coating the sensor layers with a BaTi0.95Rh0.05O3 catalytic filter layer to obtain a reliable sensor response at temperatures as high as 900 °C. The BaTi0.95Rh0.05O3 layer catalytically converts NO2 to NO already above 300 °C and, thus, conditions the sensor environment by formation of ionized oxygen allowing a sensor response with descent sensitivity. Moreover, CO interference is eliminated by using BaTi0.95Rh0.05O3 as a catalytic filter for sensing NO2 in the CO + NO2-mixed gas environments. However, it is not clear if solely the precious metal, Rh, which is present in the BaTi0.95Rh0.05O3 perovskite is responsible for this conditioning or an interaction with the perovskite lattice occurs. In order to clarify this, the tests must be carried out using a catalytic filter made of pure BaTiO3 perovskite on the Al-doped TiO2 layer. Conventional TWC catalyst cannot be used for this purpose (i.e., as a catalytic filter) due to the lack of necessary electrical conductivity.
Acknowledgments
The authors acknowledge the financial support of DFG under the contract number SA 1343/5. The valuable scientific comments of W. Grünert of the Ruhr-University-Bochum are gratefully acknowledged.
Author Contributions
B.S., A.Y. and G.C.M.R. conceived and designed the experiments; A.Y., G.C.M.R and A.A.H. performed the experiments; B.S., A.A.H and E.C. analyzed the data; E.C. contributed XRD analysis; B.S. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baukal, C. Everything you need to know about NOx: Controlling and minimizing pollutant emissions is critical for meeting air quality regulations. Metal. Finish. 2005, 103, 18–24. [Google Scholar] [CrossRef]
- Glassman, I.; Yetter, R.A. Combustion, 4th ed.; Academic Press: Burlington, MA, USA, 2007. [Google Scholar]
- Global Digital Central, Thermal Fluidspedia. Available online: https://www.thermalfluidscentral.org/encyclopedia/index.php/Basics (Combustion) (accessed on 31 March 2016).
- DieselNet Technology Guide, Gasous Emissions. Available online: https://www.dieselnet.com/tech/emi_gas.php (accessed on 31 March 2016).
- European Commision, Environment. Available online: http://ec.europa.eu/environment/air/quality/standards.htm (accessed on 31 March 2016).
- United States Department of Labor. Available online: https://www.osha.gov/dts/chemicalsampling/data/CH_257400.html (accessed on 31 March 2016).
- The State of Nevada, Department of Motor Vehicles. Available online: http://dmvnv.com/emission_obd.htm (accessed on 31 March 2016).
- Yamazoe, N.; Shimanoe, K. Theory of power laws for semiconductor gas sensors. Sens. Actuators B: Chem. 2008, 128, 566–573. [Google Scholar] [CrossRef]
- Gardon, M.; Guilemany, J.M. A review on fabrication, sensing mechanisms and performance of metal oxide gas sensors. J. Mater. Sci.: Mater. Electron. 2013, 24, 1410–1421. [Google Scholar] [CrossRef]
- Saruhan, B.; Yüce, A.; Gönüllü, Y.; Kelm, K. Effect of Al doping on NO2 gas sensing of TiO2 at elevated temperatures. Sens. Actuators B: Chem. 2013, 187, 586–597. [Google Scholar] [CrossRef]
- Barsan, N.; Koziej, D.; Weimer, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B: Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Savage, N.O.; Akbar, S.A.; Dutta, P.K. Titanium dioxide based high temperature carbon monoxide selective sensor. Sens. Actuators B: Chem. 2001, 72, 239–248. [Google Scholar] [CrossRef]
- Kim, B.; Lu, Y.; Hannon, A.; Meyyappan, M.; Li, J. Low temperature Pd/SnO2 sensors for CO detection. Sens. Actuators B: Chem. 2013, 177, 770–775. [Google Scholar] [CrossRef]
- Haidry, A.A.; Kind, N.; Saruhan, B. Investigating the influence of Al-doping and background humidity on NO2 sensing characteristics of magnetron sputtered SnO2 sensors. J. Sens. Syst. 2015, 4, 271–280. [Google Scholar] [CrossRef]
- Gönüllü, Y.; Haidry, A.A.; Saruhan, B. Nanotubular Cr-doped TiO2 for use as high-temperature NO2-selective gas sensor. Sens. Actuators B: Chem. 2015, 217, 78–87. [Google Scholar] [CrossRef]
- Gönüllü, Y.; Kelm, K.; Mathur, S.; Saruhan, B. Equivalent Circuit Analysis of Impedance Response for Cr-doped TiO2-NTs towards NO2 Gas. Chemosensors 2014, 2, 69–84. [Google Scholar] [CrossRef]
- Gönüllü, Y.; Mondragón Rodríguez, C.G.; Saruhan, B.; Ürgen, M. Improvement of gas sensing performance of TiO2 towards NO2 by nano-tubular structuring. Sens. Actuators B: Chem. 2012, 169, 151–160. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Faglia, G.; Sberveglieri, G. TiO2 Nanotubes: Recent Advances in Synthesis and Gas Sensing Properties. Sensors 2013, 13, 14813–14838. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.J.S. Gas sensing applications of 1D-nanostructured zinc oxide: Insights from density functional theory calculations. Prog. Mater. Sci. 2012, 57, 437–486. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Park, S.; Lee, W.; Lee, C. Effects of functionalization of TiO2 nanotube array sensors with Pd nanoparticles on their selectivity. Sensors 2014, 14, 15849–15860. [Google Scholar] [CrossRef] [PubMed]
- Rzyhikov, A.; Latheau, M.; Gaskov, A. Al2O3 (M=Pt, Ru) catalytic membranes for selective semiconductor gas sensors. Sens. Actuators B: Chem. 2005, 109, 91–96. [Google Scholar] [CrossRef]
- Cabot, A.; Arbiol, J.; Cornet, A.; Morante, J.R.; Chen, F.; Liu, M. Mesoporous catalytic filters for semiconductor gas sensors. Thin Solid Films 2003, 436, 64–69. [Google Scholar] [CrossRef]
- Khodadadi, A.; Mohajerzadeh, S.S.; Mortazavi, Y.; Miri, A.M. Cerium oxide/SnO2-based semiconductor gas sensors with improved sensitivity to CO. Sens. Actuators B: Chem. 2001, 80, 267–271. [Google Scholar] [CrossRef]
- Hübner, M.; Yüce, A.; Mondragón-Rodríguez, C.G.; Saruhan, B.; Barsan, N.; Weimar, U. BaTi0,95Rh0,05O3 catalytic filter layer—A promising candidate for the selective detection of CO in the presence of H2. Procedia Eng. 2010, 5, 107–110. [Google Scholar] [CrossRef] [Green Version]
- Saruhan, B.; Mondragón Rodríguez, C.G.; Yüce, A.; Haidry, A.A.; Heikens, S.; Grünert, W. Integrated performance monitoring of three-way catalytic convertors by self-regenerative and adaptive high temperature catalyst and sensors. Adv. Eng. Mater. 2015. [Google Scholar] [CrossRef]
- Mondragón Rodríguez, G.C.; Gönüllü, Y.; Ferri, D.; Eyssler, A.; Otal, E.; Saruhan, B. Phase transitions of BaTi0.9Rh0.1O3 perovskite-type oxides under reducing environments. Mater. Res. Bull. 2014, 61, 130–135. [Google Scholar] [CrossRef]
- Ghasdi, M.; Alamdari, H.; Royer, S.; Adnot, A. Electrical and CO gas sensing properties of nanostructured La1−xCexCoO3 perovskite prepared by activated reactive synthesis. Sens. Actuators B: Chem. 2001, 156, 147–155. [Google Scholar] [CrossRef]
- Ghasdi, M.; Alamdari, H. CO sensitive nanocrystalline LaCoO3 perovskite sensor prepared by high energy ball milling. Sens. Actuators B: Chem. 2010, 148, 478–485. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).