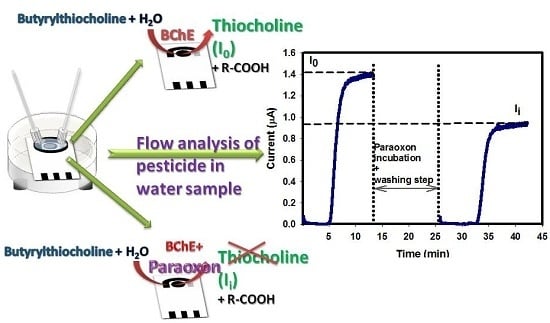
Automatable Flow System for Paraoxon Detection with an Embedded Screen-Printed Electrode Tailored with Butyrylcholinesterase and Prussian Blue Nanoparticles
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents
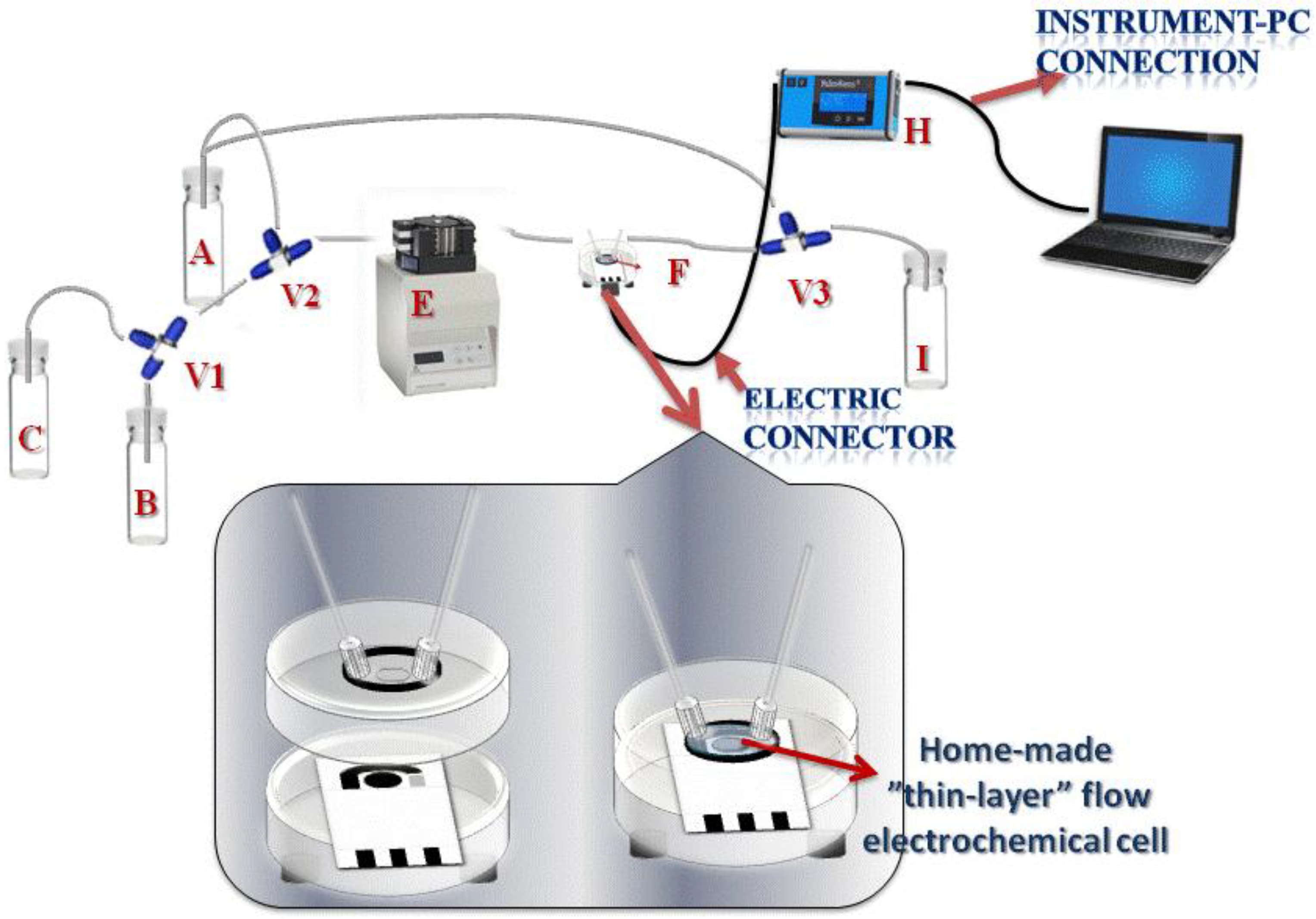
2.2. Apparatus
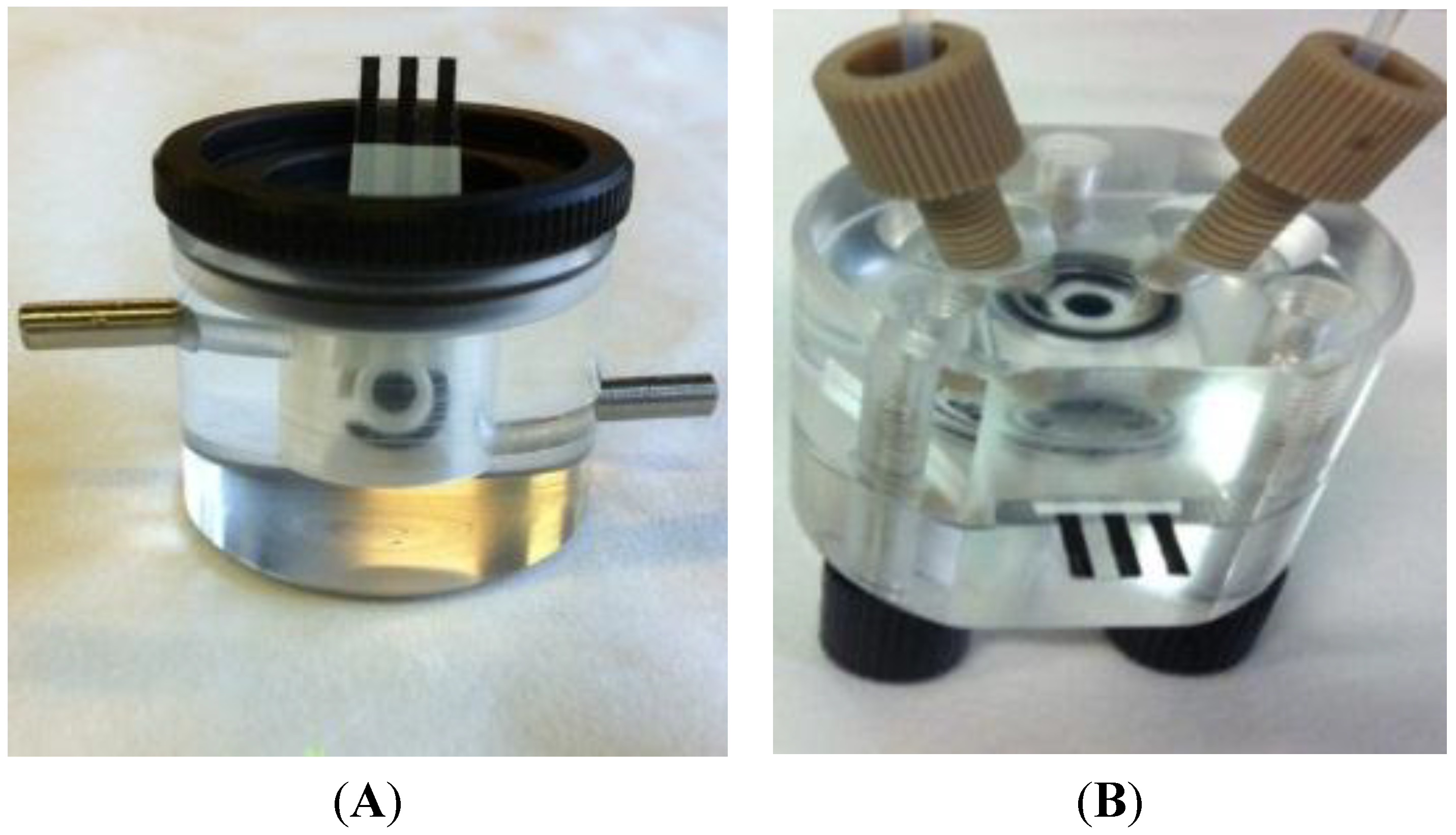
2.3. Electrodes
2.4. Preparation of Prussian Blue Nanoparticles (PBNPs) Modified SPEs
2.5. Preparation of BChE Biosensors Based on PBNPs Modified SPEs
2.6. Flow Measurements of Biosensor Enzymatic Activity
2.7. Organophosphate Flow Analysis
- Conditioning step. Applied potential +200 mV; valves V2 and V1 opened in position of BUFFER (B) and valve V3 in position of WASTE (I) for a time of 5 min; flow rate 0.12 mL/min.
- Enzymatic activity measurement before enzyme inhibition (I0). Applied potential +200 mV; valve V2 opened in position of SUBSTRATE (A) and valve V3 in position of SUBSTRATE (A) for a time of 10 min; flow rate 0.12 mL/min.
- Inhibition step. Valves V2 and V1 opened in position of PARAOXON (C) and valve V3 in position of WASTE (I) for a time of 10 min; flow rate 0.25 mL/min. Then, valves V2 and V1 opened in the position of BUFFER (B) and valve V3 in the position of WASTE (I) for a time of 2–3 min; flow rate 0.25 mL/min.
- Washing step. Valves V2 and V1 opened in position of BUFFER (B) and valve V3 in position of WASTE (I) for a time of 5 min; flow rate 0.12 mL/min.
- Enzymatic activity measurement after enzyme inhibition (Ii). Applied potential +200 mV; valve V2 opened in position of SUBSTRATE (A) and valve V3 in position of SUBSTRATE (A) for a time of 10 min; flow rate 0.12 mL/min.
2.8. Safety Conditions
3. Results and Discussion
3.1. System Configuration
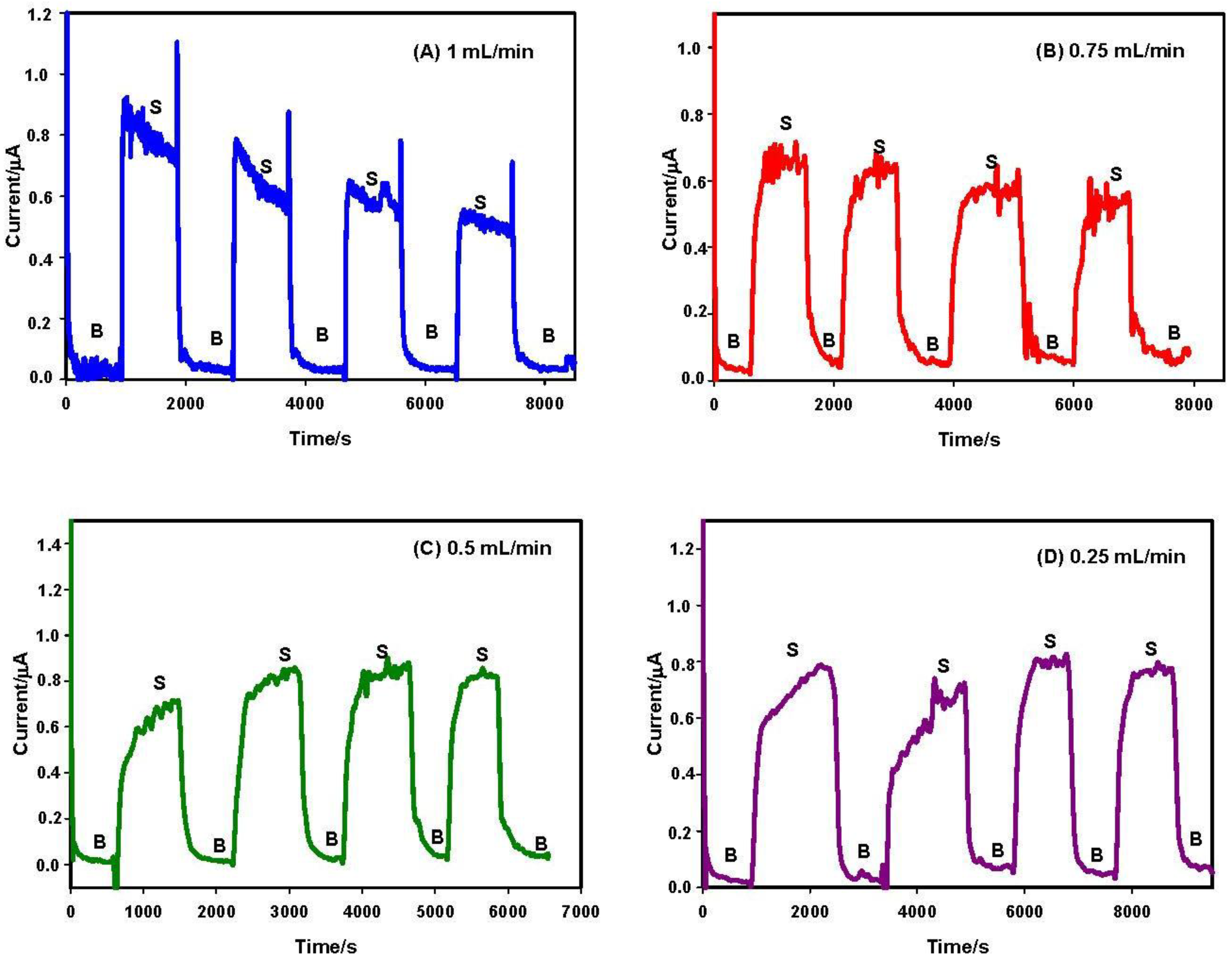
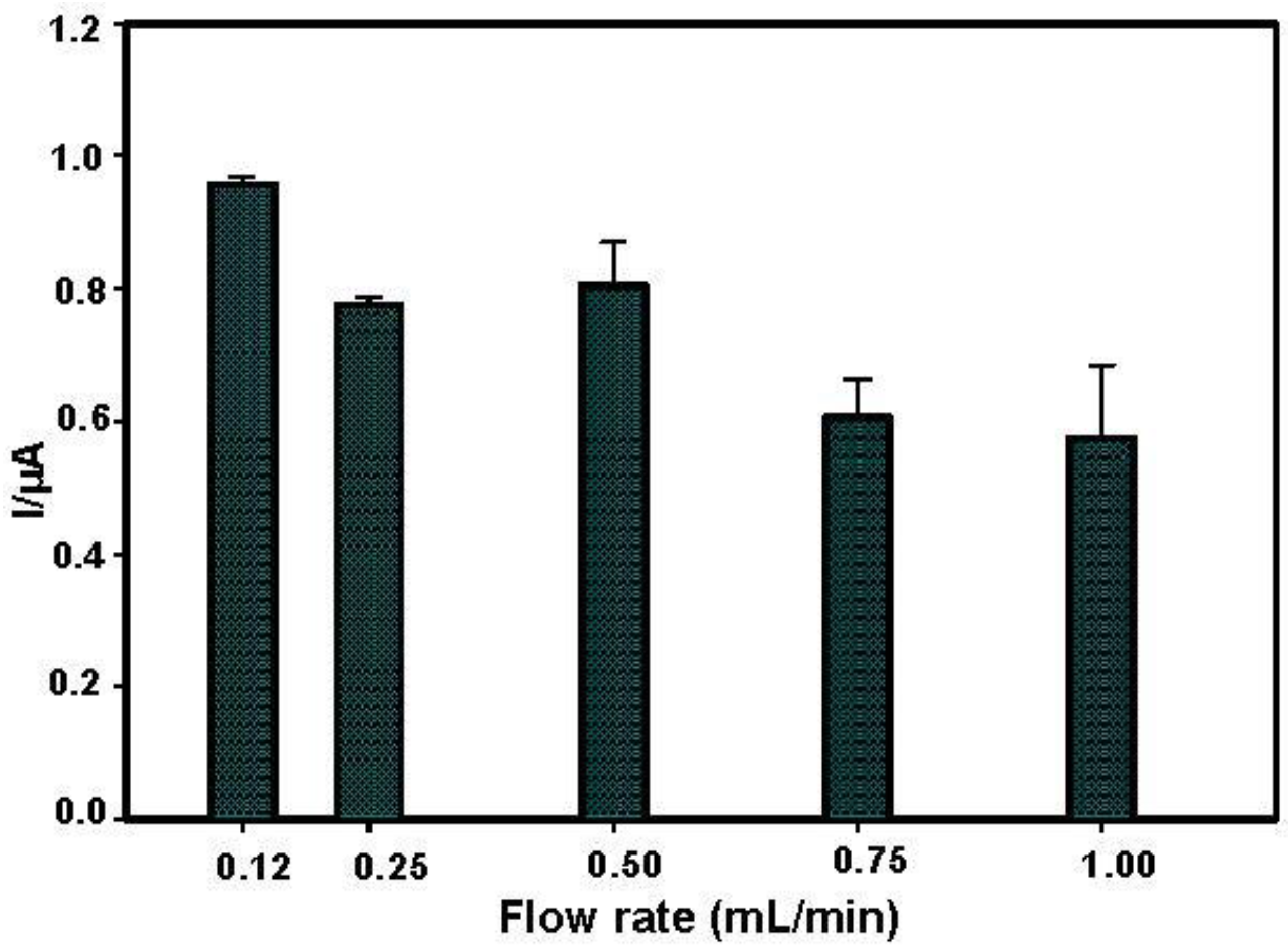
3.2. Flow Rate and Operational Stability Studies
- Stability of the enzymatic membrane. During the measurement at high flow rate, the membrane can be partially removed from the surface of the electrode. For this reason, a further test was performed measuring the enzymatic activity “in drop” with a biosensor previously tested in flow at 1 mL/min rate. We have observed (data not shown) a stable current signal, demonstrating that the enzymatic membrane steadily and actively persisted on the sensor surface.
- Onset of air bubbles. The formation of bubbles within the electrochemical cell occurred more frequently with increased flow rates. For this reason, a flow rate of 0.12 mL/min was finally selected to avoid the formation of air bubbles and to perform measurements for longer times. In Figure 4, current signals with the selected flow rate were reported. These signals were more stable but they increased during the first analyses, probably due to the increase of temperature in the laboratory that can influence the enzyme activity. To overcome this drawback, the use of thermostatic chamber to allocate the flow cell or a temperature correction should be considered. Despite these effects, the stability of the biosensor in working conditions was satisfactory for at least eight substrate measurements.

3.3. Intra-Electrode Repeatability
3.4. Paraoxon Detection

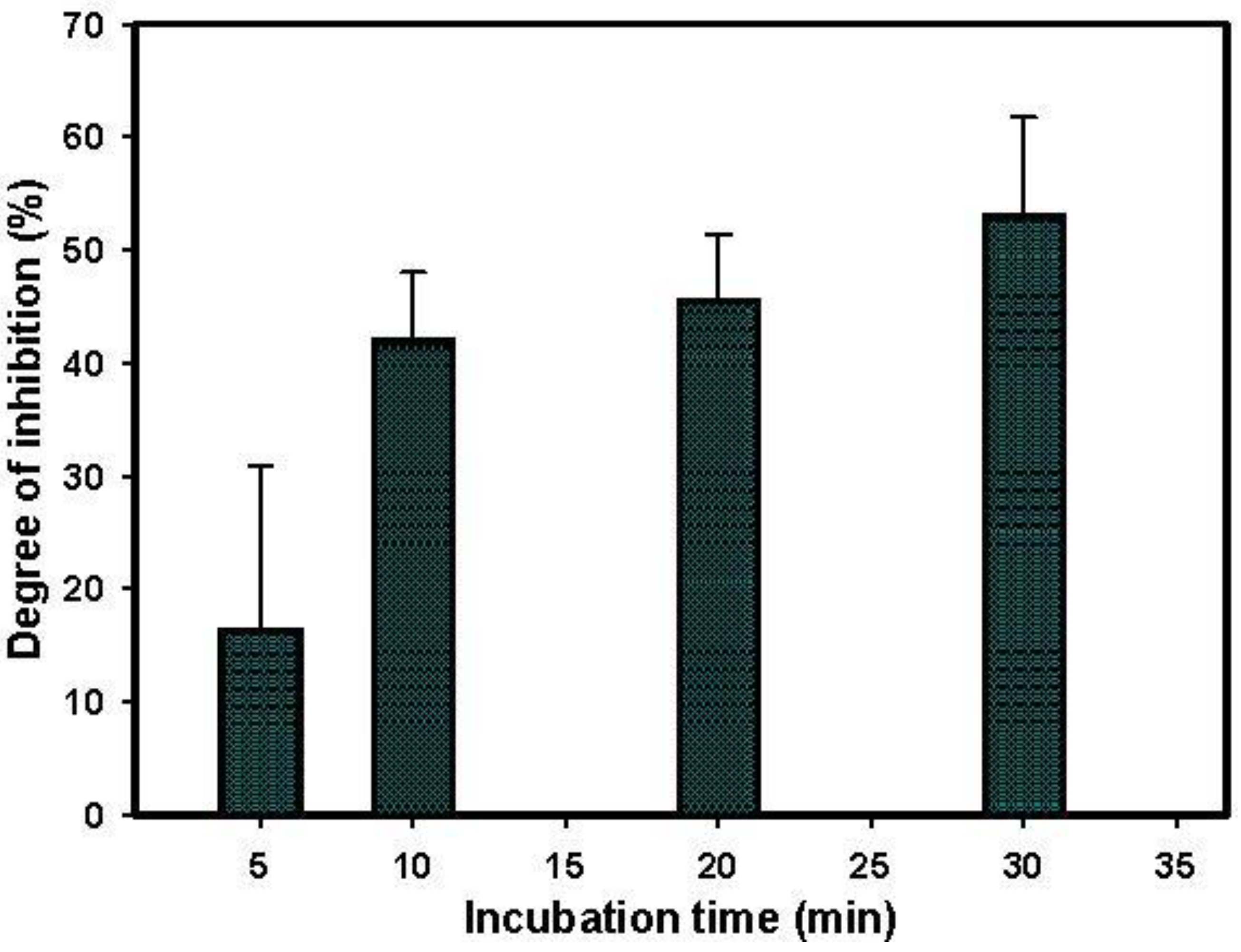
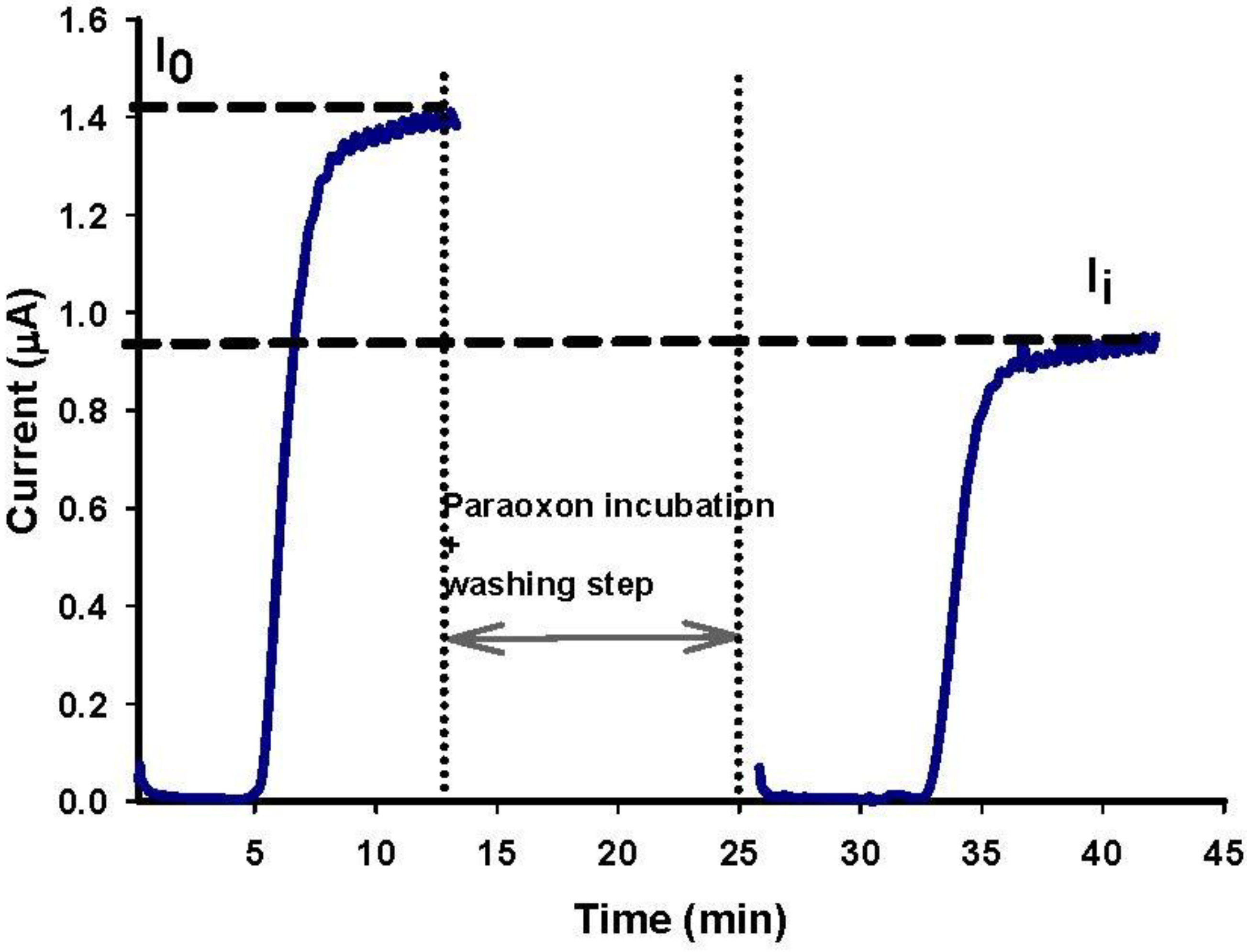
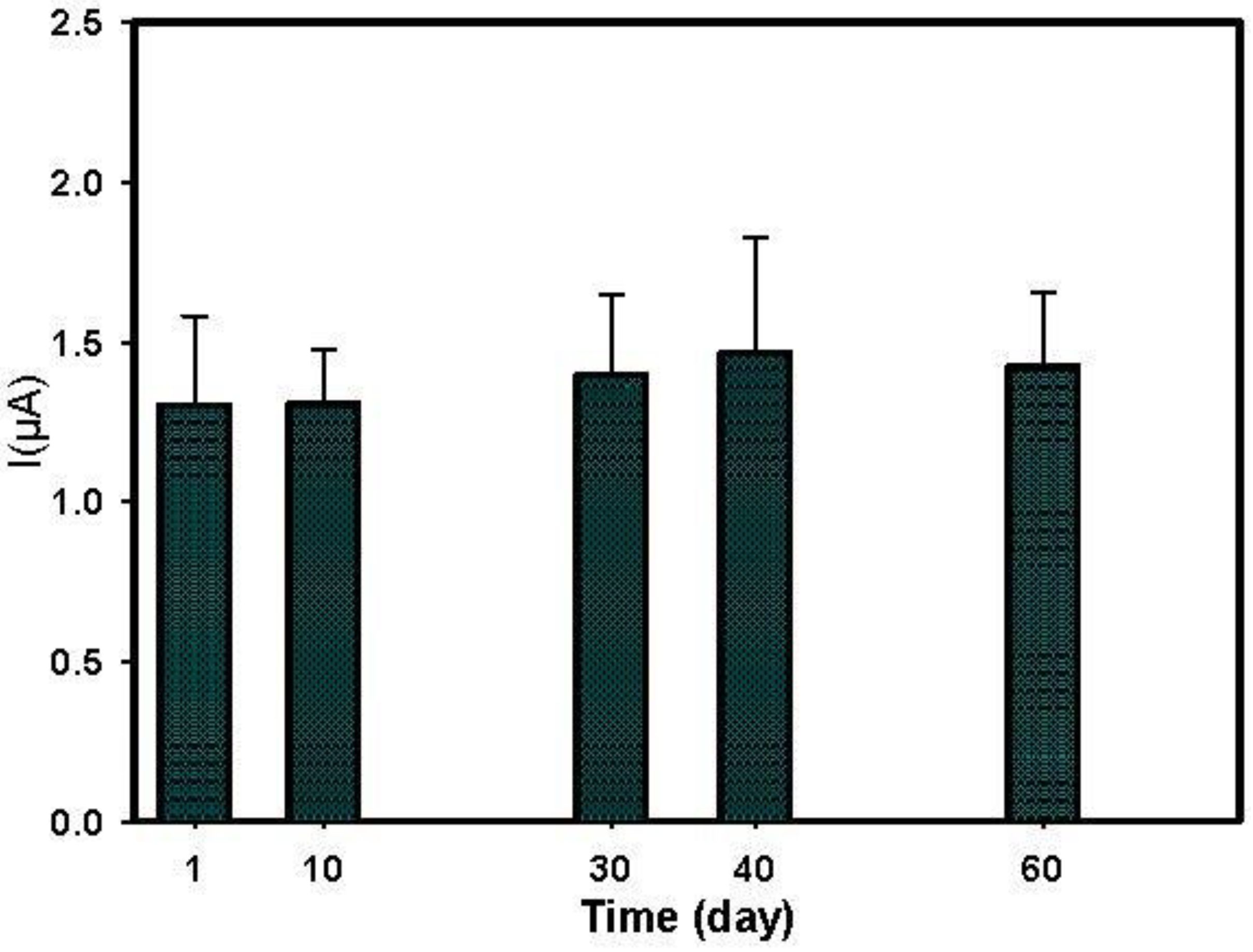
3.5. Paraoxon Detection in Water Sample
| Sample | Paraoxon Added (ppb) | Paraoxon Found ± σ (ppb) | Recovery ± σ (%) |
|---|---|---|---|
| Tap water | 25 | 22.5 ± 1.2 | 90 ± 5 |
| Albano Lake | 25 | 28.8 ± 3.1 | 115 ± 12 |
| Tiber River | 25 | 29.8 ± 2.3 | 119 ± 9 |
| Biosensor Type | Applied Potential | Flow Analysis Type | Analyte | Linear Range (M) | Detection Limit (M) | Storage Condition | Samples | Reference |
|---|---|---|---|---|---|---|---|---|
| Genetically-modified AChE immobilized on CoPc-SPE | +100 mV | Automated flow system using a modular syringe pump | Paraoxon | 5 × 10−7–3 × 10−8 | 7.5 × 10−9 | The biosensor retained full enzymatic activity after storage for up to 1 month in PBS at 4 °C | Milk | [22] |
| AChE immobilized on platinum electrode modified with MWCNTs | +630 mV | Flow-injection system | Paraoxon | 1 × 10−11–1 × 10−8 | 9 × 10−13 | The biosensor retained 80% of enzymatic activity after storage for 30 days in PBS at 4 °C | - | [21] |
| AChE immobilized on glassy carbon electrode modified with MWCNTs | +150 mV | Flow-injection system (stop and flow) | Paraoxon | 1 × 10−12–1 × 10−8 | 4 × 10−13 | The biosensor retained 94% of enzymatic activity after storage for 1 month in PBS at 4 °C | - | [32] |
| BChE immobilized on PBNPs-SPE | +200 mV | Flow system using T valves (continuous flow) | Paraoxon | 7 × 10−9–4 × 10−8 | 4 × 10−9 | The biosensor retained full enzymatic activity after storage for 60 days dry at RT | Tap, lake and river water | [this work] |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bajgar, J. Organophosphates/nerve agent poisoning: Mechanism of action, diagnosis, prophylaxis and treatment. In Advances in Clinical Chemistry; Makowsky, G.M., Ed.; Elsevier Academic Press: San Diego, CA, USA, 2004; Volume 38, pp. 151–216. [Google Scholar]
- Harel, M.; Sonoda, L.K.; Silman, I.; Sussman, J.L.; Rosenberry, T.L. Crystal structure of thioflavin T bound to the peripheral site of Torpedo californica acetylcholinesterase reveals how thioflavin T acts as a sensitive fluorescent reporter of ligand binding to the acylation site. J. Am. Chem. Soc. 2008, 130, 7856–7861. [Google Scholar] [CrossRef] [PubMed]
- Millard, C.B.; Koellner, G.; Ordentlich, A.; Shafferman, A.; Silman, I.; Sussman, J.L. Reaction products of acetylcholinesterase and VX reveal a mobile histidine in the catalytic triad. J. Am. Chem. Soc. 1999, 121, 9883–9884. [Google Scholar] [CrossRef]
- Sussman, J.L.; Harel, M.; Frolow, F.; Oefner, C.; Goldman, A.; Toker, L.; Silman, I. Atomic structure of acetylcholinesterase from Torpedo californica: A prototypic acetylcholine-binding protein. Science 1991, 253, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Bajgar, J.; Fusek, J.; Kuca, K.; Bartosova, L.; Jun, D. Treatment of organophosphate intoxication using cholinesterase reactivators: Facts and fiction. Mini Rev. Med. Chem. 2007, 7, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Bergh, C.; Torgrip, R.; Ostman, C. Simultaneous selective detection of organophosphate and phthalate esters using gas chromatography with positive ion chemical ionization tandem mass spectrometry and its application to indoor air and dust. Rapid Commun. Mass Spectrom. 2010, 24, 2859–2867. [Google Scholar] [CrossRef] [PubMed]
- De Freitas Ventura, F.; de Oliveira, J., Jr.; dos Reis Pedreira Filho, W.; Gerardo Ribeiro, M. GC-MS quantification of organophosphorous pesticides extracted from XAD-2 sorbent tube and foam patch matrices. Anal. Methods 2012, 4, 3666–3673. [Google Scholar] [CrossRef]
- Arduini, F.; Amine, A.; Moscone, D.; Palleschi, G. Biosensors based on cholinesterase inhibition for insecticides, nerve agents and aflatoxin B1 detection. Microchim. Acta 2010, 170, 193–214. [Google Scholar] [CrossRef]
- Andreescu, S.; Marty, J.L. Twenty years research in cholinesterase biosensors: From basic research to practical applications. Biomol. Eng. 2006, 23, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M.; Musilek, K.; Kuca, K. Progress of biosensors based on cholinesterase inhibition. Curr. Med. Chem. 2009, 16, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, A.P.; Umasankar, Y.; Chen, S.M. Nanomaterials—Acetylcholinesterase Enzyme Matrices for Organophosphorus Pesticides Electrochemical Sensors: A Review. Sensors 2009, 9, 4034–4055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Asiri, A.M.; Liu, D.; Du, D.; Lin, Y. Nanomaterial-based biosensors for environmental and biological monitoring of organophosphorus pesticides and nerve agents. Trends Anal. Chem. 2014, 54, 1–10. [Google Scholar] [CrossRef]
- Arduini, F.; Guidone, S.; Amine, A.; Palleschi, G.; Moscone, D. Acetylcholinesterase biosensor based on self-assembled monolayer-modified gold-screen printed electrodes for organophosphorus insecticide detection. Sens. Actuators B Chem. 2013, 179, 201–208. [Google Scholar] [CrossRef]
- Arduini, F.; Forchielli, M.; Amine, A.; Neagu, D.; Cacciotti, I.; Nanni, F.; Moscone, D.; Palleschi, G. Screen-printed biosensor modified with carbon black nanoparticles for the determination of paraoxon based on the inhibition of butyrylcholinesterase. Microchim. Acta 2015, 182, 643–651. [Google Scholar] [CrossRef]
- Arduini, F.; Ricci, F.; Tuta, C.S.; Moscone, D.; Amine, A.; Palleschi, G. Detection of carbamic and organophosphorous pesticides in water samples using a cholinesterase biosensor based on Prussian Blue-modified screen-printed electrode. Anal. Chim. Acta 2006, 580, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Alonso, G.A.; Istamboulie, G.; Noguer, T.; Marty, J.L.; Muñoz, R. Rapid determination of pesticide mixtures using disposable biosensors based on genetically modified enzymes and artificial neural networks. Sens. Actuators B Chem. 2012, 164, 22–28. [Google Scholar] [CrossRef]
- Ivanov, A.N.; Younusov, R.R.; Evtugyn, G.A.; Arduini, F.; Moscone, D.; Palleschi, G. Acetylcholinesterase biosensor based on single-walled carbon nanotubes—Co phtalocyanine for organophosphorus pesticides detection. Talanta 2011, 85, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Oujji, N.B.; Bakas, I.; Istamboulié, G.; Ait-Ichou, I.; Ait-Addi, E.; Rouillon, R.; Noguer, T. Sol-gel immobilization of acetylcholinesterase for the determination of organophosphate pesticides in olive oil with biosensors. Food Control 2013, 30, 657–661. [Google Scholar] [CrossRef]
- Di Tuoro, D.; Portaccio, M.; Lepore, M.; Arduini, F.; Moscone, D.; Bencivenga, U.; Mita, D.G. An acetylcholinesterase biosensor for determination of low concentrations of Paraoxon and Dichlorvos. New Biotechnol. 2011, 29, 132–138. [Google Scholar] [CrossRef]
- Liang, H.; Song, D.; Gong, J. Signal-on electrochemiluminescence of biofunctional CdTe quantum dots for biosensing of organophosphate pesticides. Biosens. Bioelectron. 2014, 53, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, Y.; Marinov, I.; Portaccio, M.; Lepore, M.; Mita, D.G.; Godjevargova, T. Flow-injection system with site-specific immobilization of acetylcholinesterase biosensor for amperometric detection of organophosphate pesticides. Biotechnol. Biotechnol. Equip. 2012, 26, 3044–3053. [Google Scholar] [CrossRef]
- Mishra, R.K.; Dominguez, R.B.; Bhand, S.; Muñoz, R.; Marty, J.L. A novel automated flow-based biosensor for the determination of organophosphate pesticides in milk. Biosens. Bioelectron. 2012, 32, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Guan, Z.; Song, D. Biosensor based on acetylcholinesterase immobilized onto layered double hydroxides for flow injection/amperometric detection of organophosphate pesticides. Biosens. Bioelectron. 2013, 39, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Alonso, G.A.; Istamboulie, G.; Bhand, S.; Marty, J.L. Automated flow based biosensor for quantification of binary organophosphates mixture in milk using artificial neural network. Sens. Actuators B Chem. 2015, 208, 228–237. [Google Scholar] [CrossRef]
- Dominguez, R.B.; Alonso, G.A.; Muñoz, R.; Hayat, A.; Marty, J.L. Design of a novel magnetic particles based electrochemical biosensor for organophosphate insecticide detection in flow injection analysis. Sens. Actuators B Chem. 2015, 208, 491–496. [Google Scholar] [CrossRef]
- Alonso, G.A.; Dominguez, R.B.; Marty, J.L.; Muñoz, R. An Approach to an Inhibition Electronic Tongue to Detect On-Line Organophosphorus Insecticides Using a Computer Controlled Multi-Commuted Flow System. Sensors 2011, 11, 3791–3802. [Google Scholar] [CrossRef] [PubMed]
- Mascini, M.; Mazzei, F. Amperometric sensor for pyruvate with immobilized pyruvate oxidase. Anal. Chim. Acta 1987, 192, 9–16. [Google Scholar] [CrossRef]
- Arduini, F.; Neagu, D.; Dall’Oglio, S.; Moscone, D.; Palleschi, G. Towards a Portable Prototype Based on Electrochemical Cholinesterase Biosensor to be Assembled to Soldier Overall of Nerve Agent Detection. Electroanalysis 2012, 24, 581–590. [Google Scholar] [CrossRef]
- Cinti, S.; Arduini, F.; Vellucci, G.; Cacciottti, I.; Nanni, F.; Moscone, D. Carbon black assisted tailoring of Prussian Blue nanoparticles to tune sensitivity and detection limit towards H2O2 by using screen-printed electrode. Electrochem. Commun. 2014, 47, 63–66. [Google Scholar] [CrossRef]
- Moscone, D.; D’Ottavi, D.; Compagnone, D.; Palleschi, G.; Amine, A. Construction and Analytical Characterization of Prussian Blue-Based Carbon Paste Electrodes and Their Assembly as Oxidase Enzyme Sensors. Anal. Chem. 2001, 73, 2529–2535. [Google Scholar] [CrossRef] [PubMed]
- DL 152/2006, 3 April 2006 “Norme in Materia Ambientale”. Available online: http://www.gazzettaufficiale.it/eli/id/2006/04/14/006G0171/sg (accessed on 24 April 2015).
- Liu, G.; Lin, Y. Biosensor Based on Self-Assembling Acetylcholinesterase on Carbon Nanotubes for Flow Injection/Amperometric Detection of Organophosphate Pesticides and Nerve Agents. Anal. Chem. 2006, 78, 835–843. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arduini, F.; Neagu, D.; Scognamiglio, V.; Patarino, S.; Moscone, D.; Palleschi, G. Automatable Flow System for Paraoxon Detection with an Embedded Screen-Printed Electrode Tailored with Butyrylcholinesterase and Prussian Blue Nanoparticles. Chemosensors 2015, 3, 129-145. https://doi.org/10.3390/chemosensors3020129
Arduini F, Neagu D, Scognamiglio V, Patarino S, Moscone D, Palleschi G. Automatable Flow System for Paraoxon Detection with an Embedded Screen-Printed Electrode Tailored with Butyrylcholinesterase and Prussian Blue Nanoparticles. Chemosensors. 2015; 3(2):129-145. https://doi.org/10.3390/chemosensors3020129
Chicago/Turabian StyleArduini, Fabiana, Daniela Neagu, Viviana Scognamiglio, Sabrina Patarino, Danila Moscone, and Giuseppe Palleschi. 2015. "Automatable Flow System for Paraoxon Detection with an Embedded Screen-Printed Electrode Tailored with Butyrylcholinesterase and Prussian Blue Nanoparticles" Chemosensors 3, no. 2: 129-145. https://doi.org/10.3390/chemosensors3020129
APA StyleArduini, F., Neagu, D., Scognamiglio, V., Patarino, S., Moscone, D., & Palleschi, G. (2015). Automatable Flow System for Paraoxon Detection with an Embedded Screen-Printed Electrode Tailored with Butyrylcholinesterase and Prussian Blue Nanoparticles. Chemosensors, 3(2), 129-145. https://doi.org/10.3390/chemosensors3020129