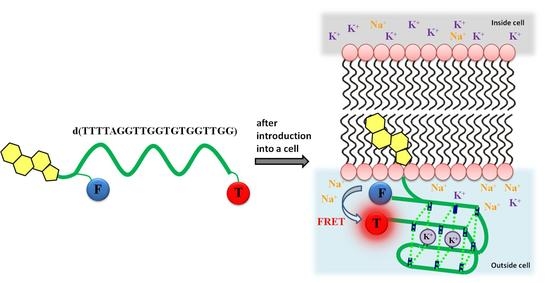
Effect of Cholesterol Anchoring Group on the Properties of G-Quadruplex-Based FRET Probes for Potassium Ion
Abstract
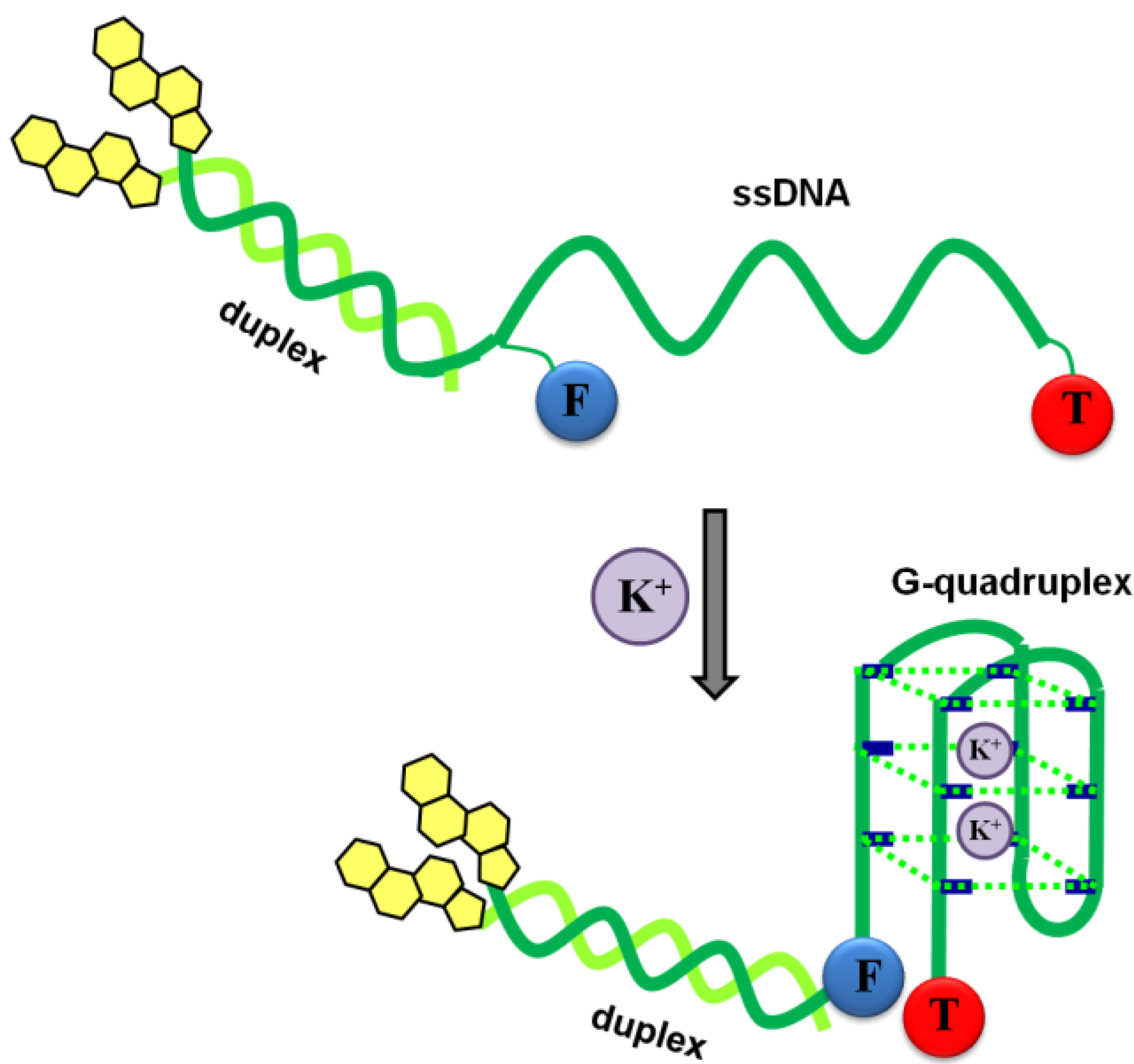
:1. Introduction
2. Experimental Section
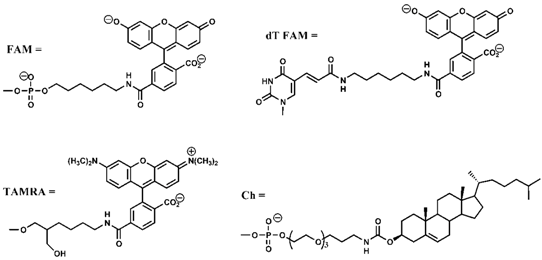
2.1. Materials
| Abbreviation Sequence and Structure | |
|---|---|
| F19T | FAM-5’GGTTGGTGTGGTTGGATTT3’-TAMRA |
| 16F19T | 5’CATCGAGCGTACGTTA (dT FAM) TTTAGGTTGGTGTGGTTGGATTT3’-TAMRA |
| ChF19T | Ch-5’(dT FAM)TTTAGGTTGGTGTGGTTGGATTT3’-TAMRA |
| Ch16F19T | Ch-5’CATCGAGCGTACGTTA(dT FAM) TTTAGGTTGGTGTGGTTGGATTT3’-TAMRA |
| Ch16’ | 5’TAACGTACGCTCGATG3’-Ch |
 | |
2.2. Methods
2.2.1. UV-Vis Absorption Studies
2.2.2. Fluorescence Studies
3. Results and Discussion
3.1. Spectral Properties of the Fluorescent Probes with Cholesterol Moiety
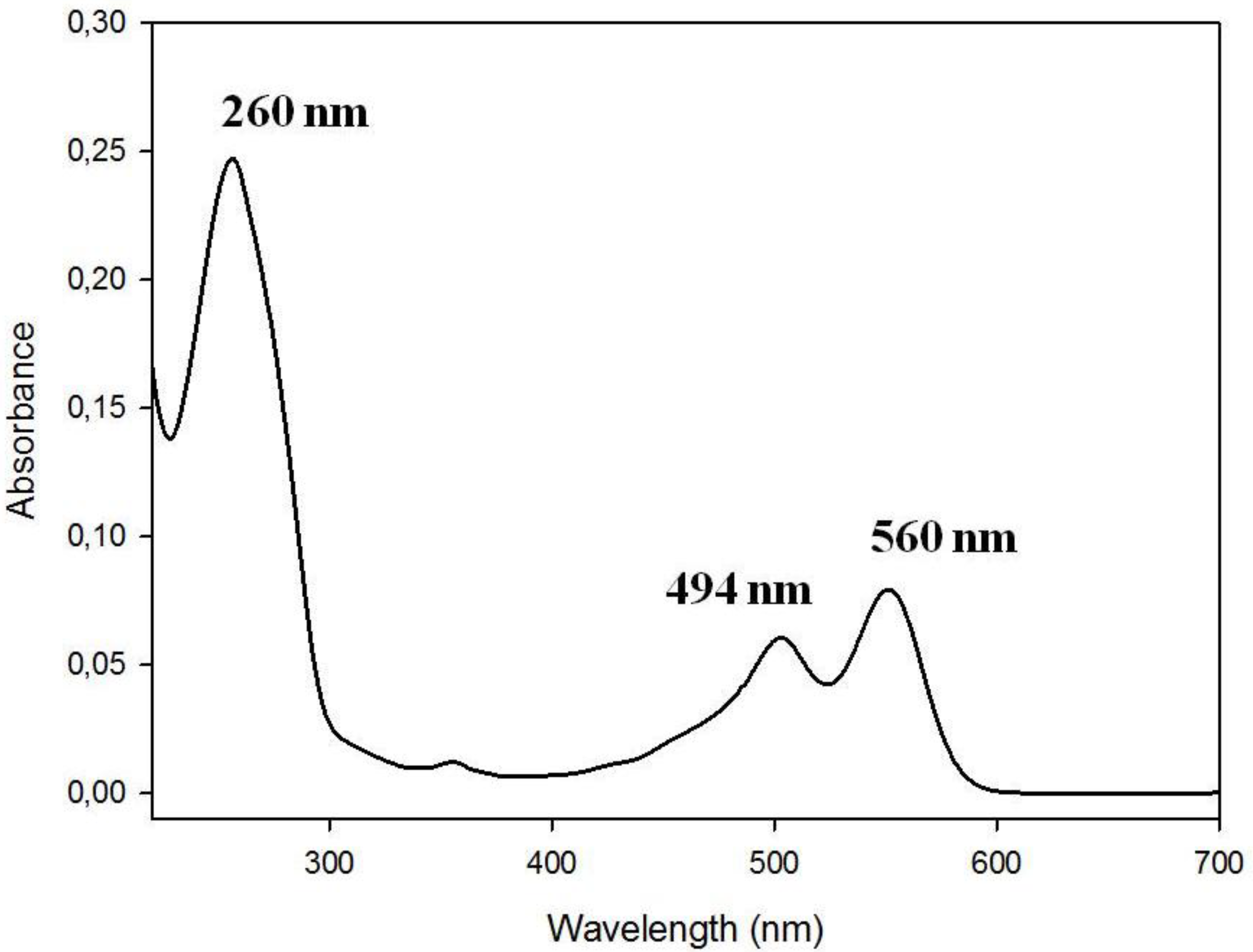
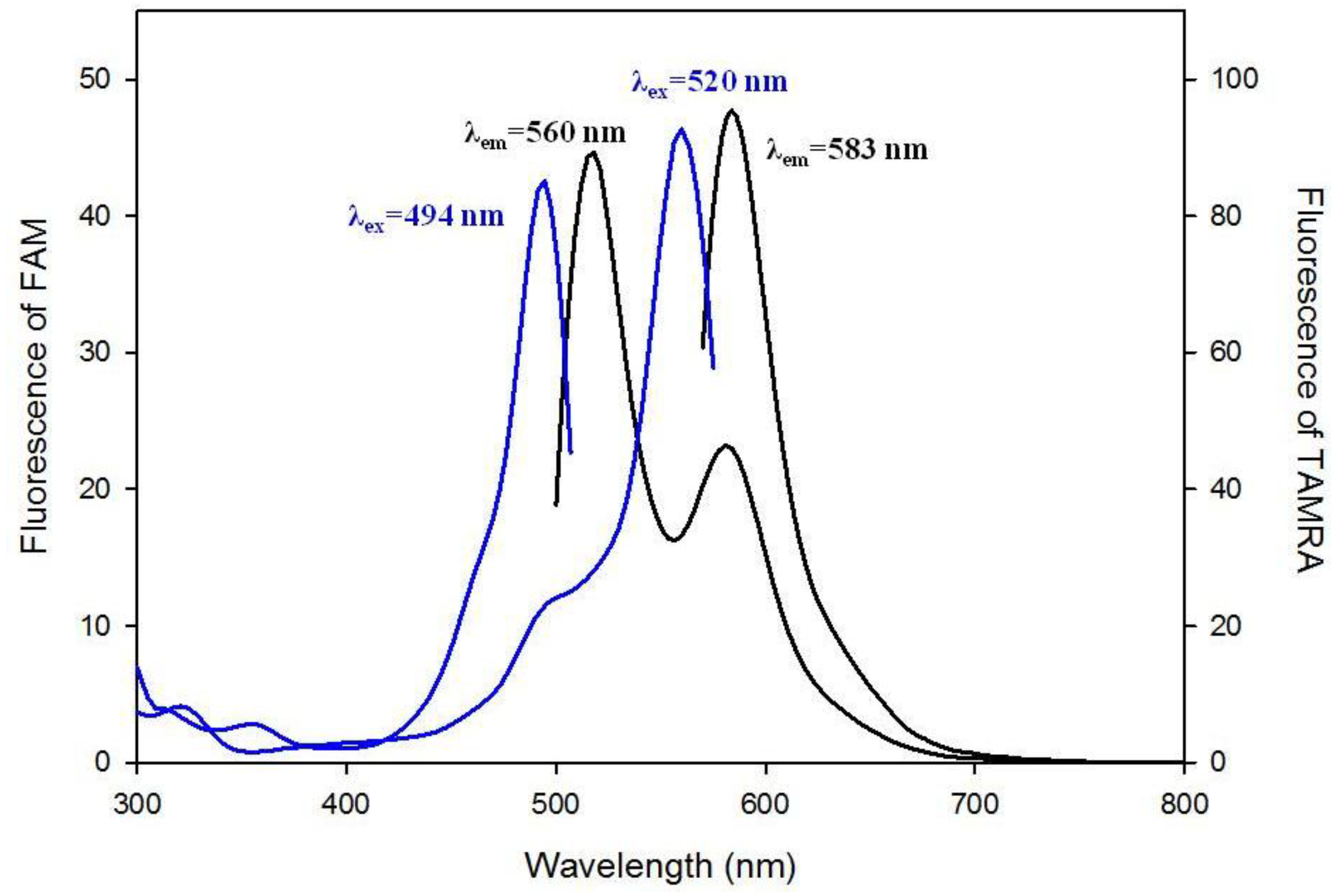
3.2. Stability of G-Quadruplex Structures
3.2.1. UV-Vis Melting Profiles
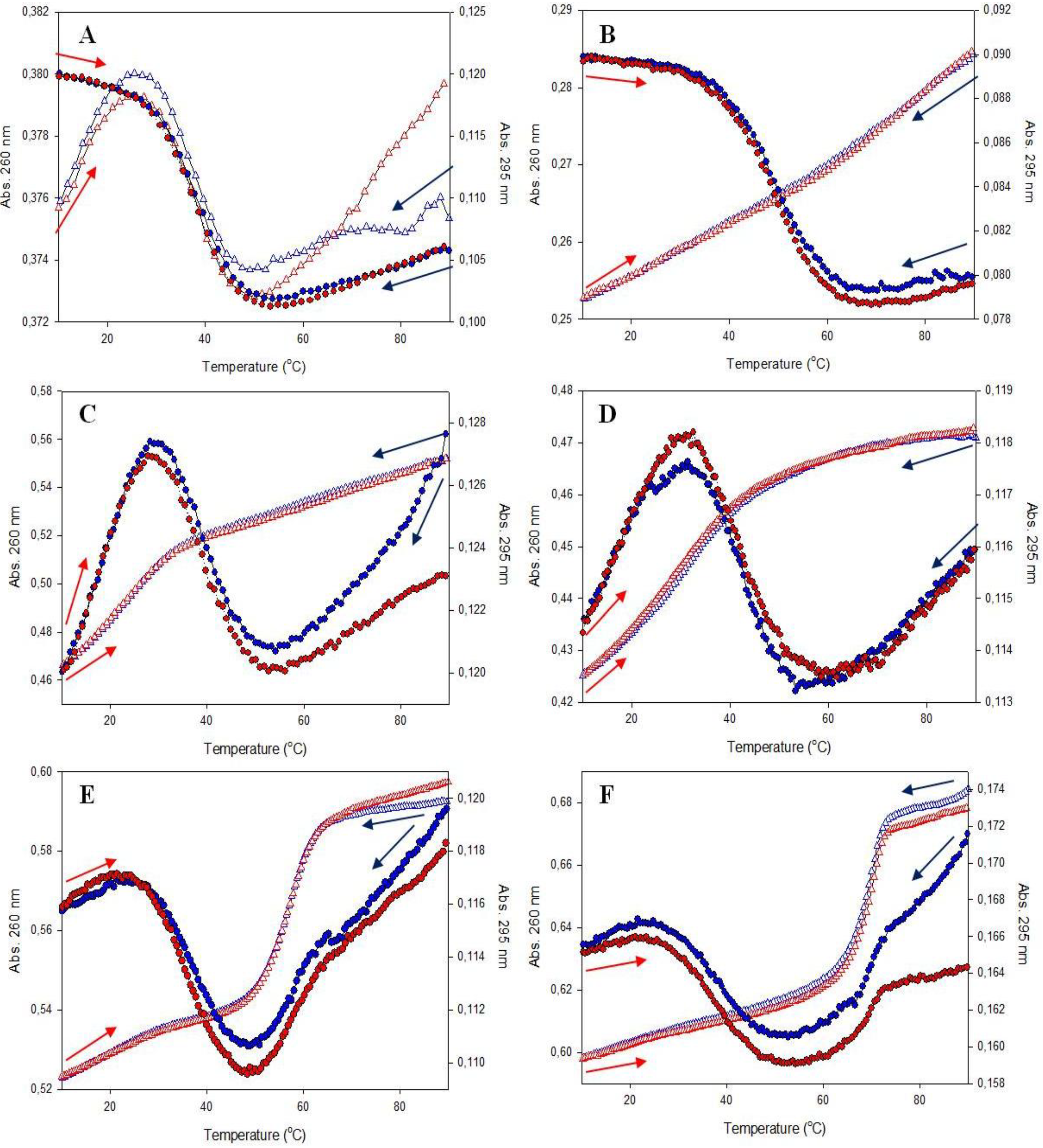
| Abbreviation of Probes | Tm, A at 260 nm (°C) | Tm, A at 295 nm (°C) | Tm, F at 520 nm (°C ) |
|---|---|---|---|
| F19T | 38.0 | 38.0 | 38.0 |
| 16F19T | 22.0 a | 38.0 | 37.5 |
| Ch16F19T | 30.5 a | 41.5 | 41.0 |
| ChF19T | - a | 48.0 | 47.0 |
| ChF19T/Ch16’ | 68.0 | 37.7 | 37.5 |
| 16F19T/Ch16’ | 56.5 | 37.0 | 37.0 |
3.2.2. Fluorescence Melting Profiles
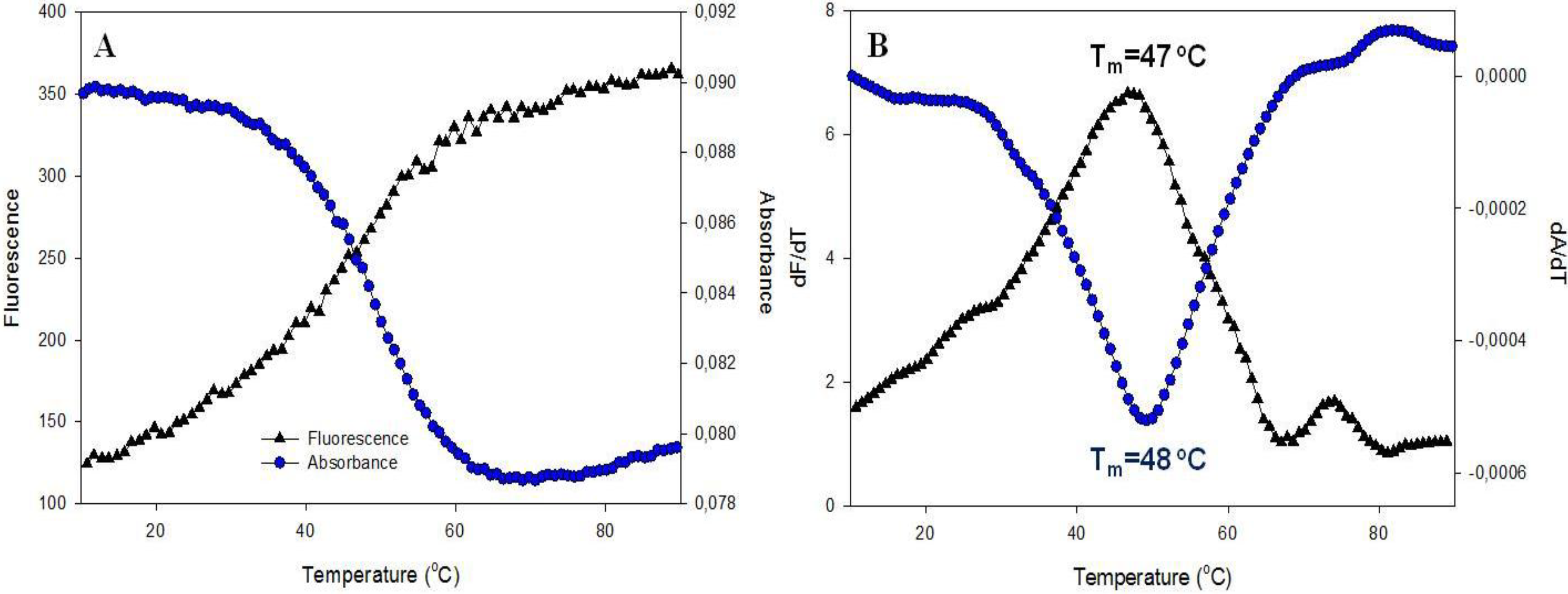
3.2.3. Fluorescence Spectra
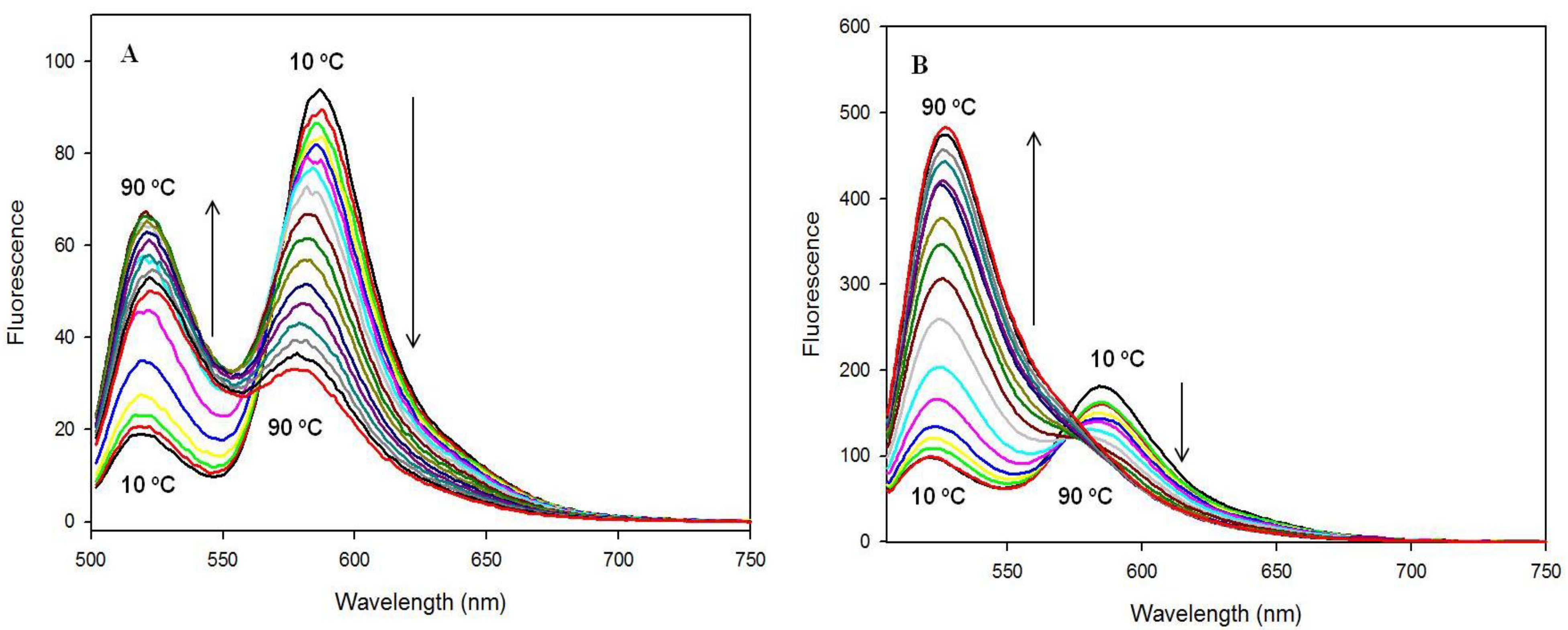
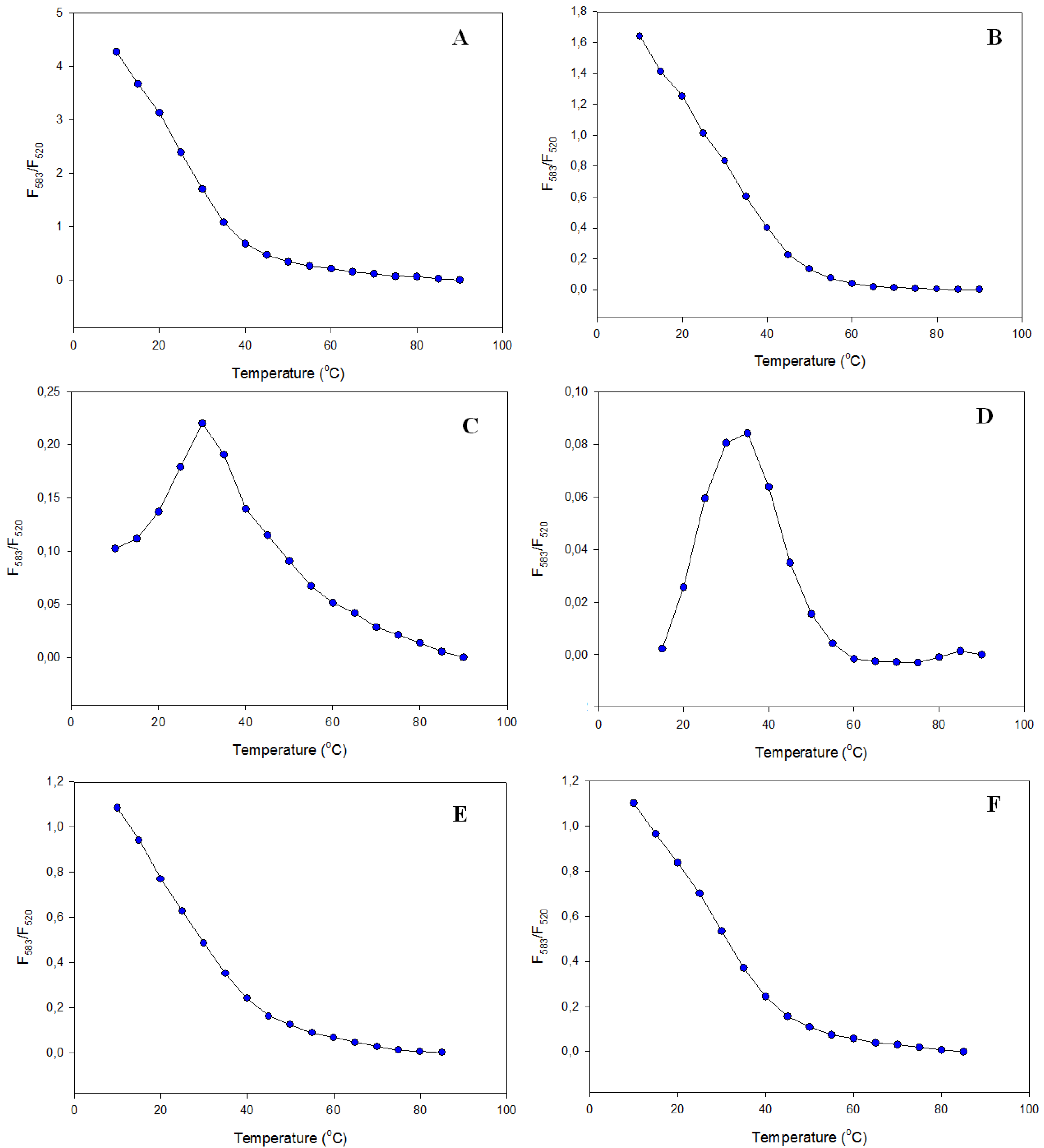
3.3. Potassium and Sodium Cation Selectivity of the FRET Probes
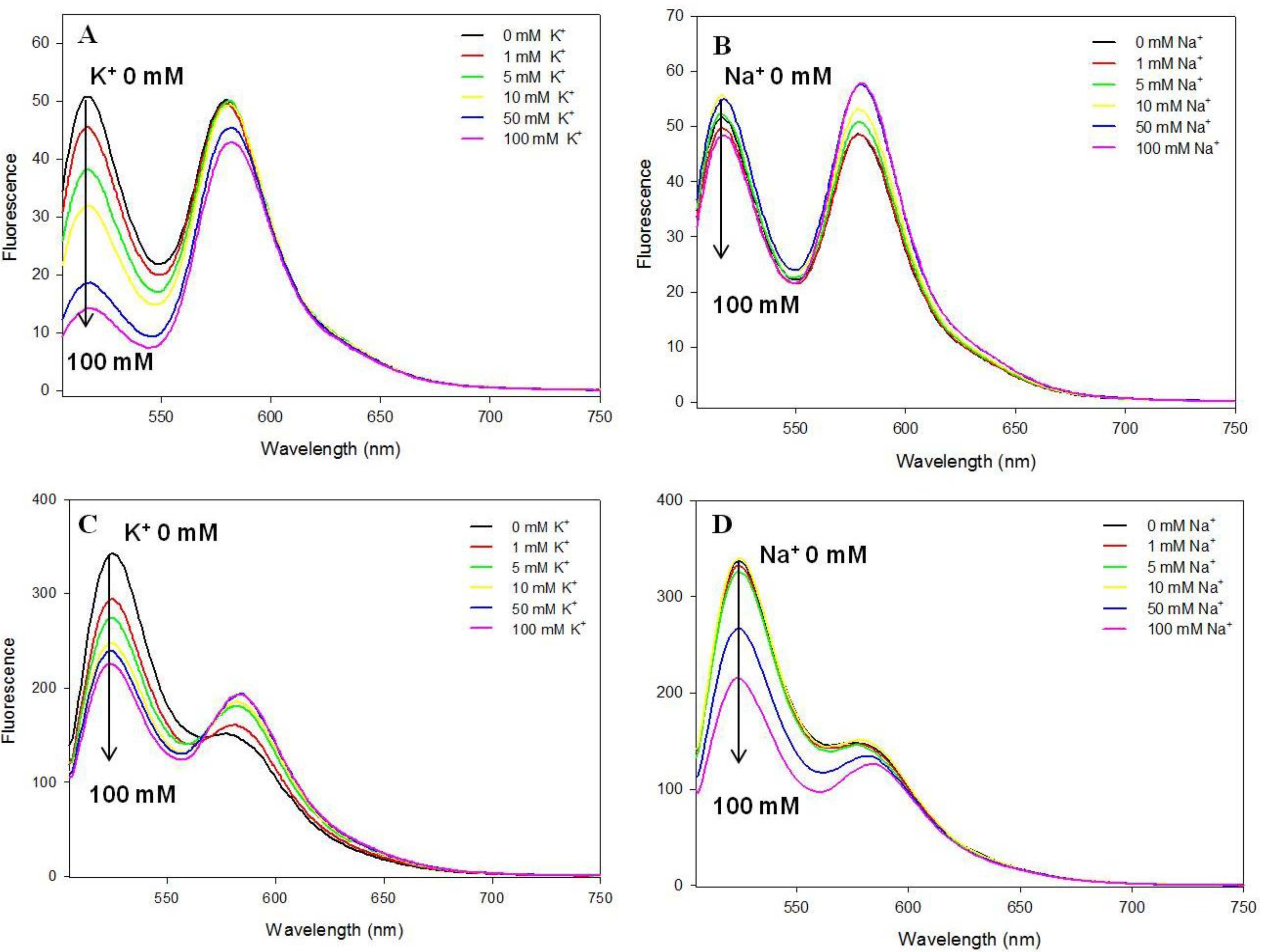
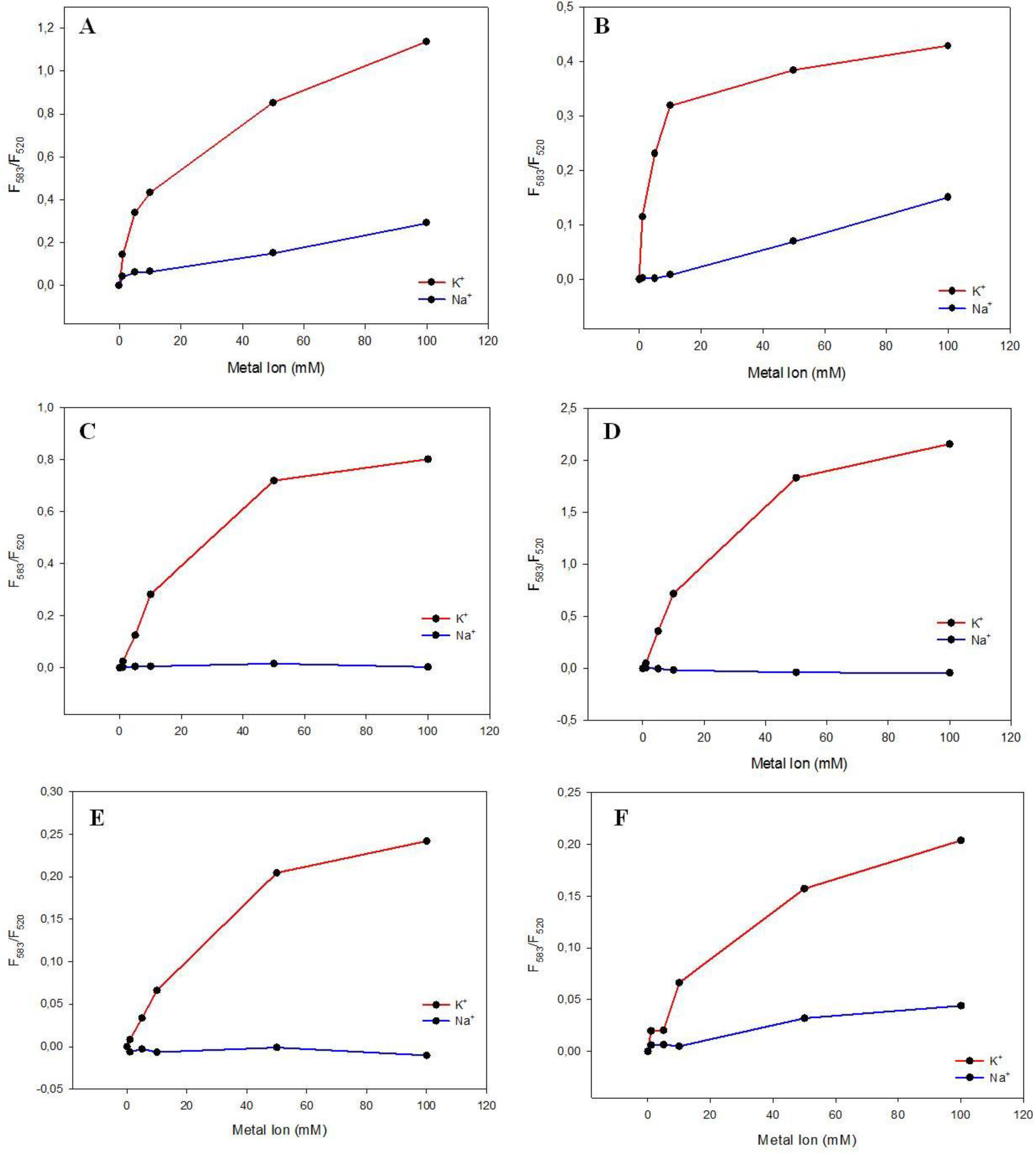
4. Conclusions
Supporting Information
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bang, I. Untersuchungen über die Guanylsäure. Biochem. Z 1910, 26, 293–311. [Google Scholar]
- Gellert, M.; Lipsett, M.N.; Davies, D.R. Helix formation by guanylic acid. Proc. Natl. Acad. Sci. USA 1962, 48, 2013–2018. [Google Scholar] [CrossRef] [PubMed]
- Bochman, M.L.; Paeschke, K.; Zakian, V.A. DNA secondary structures: Stability and function of G-quadruplex structures. Nat. Rev. Genet. 2012, 13, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhou, X. Targeting non-B-form DNA in living cells. Chem. Record 2013, 13, 371–384. [Google Scholar] [CrossRef]
- Williamson, J.R. G-tetrad structures in telomeric DNA. Ann. Rev. Biophys. Biomol. Struct. 1994, 23, 703–730. [Google Scholar] [CrossRef]
- Kelly, J.A.; Feigon, J.; Yeates, T.O. Reconciliation of the X-ray and NMR structures of the thrombin-binding aptamer d(GGTTGGTGTGGTTGG). J. Mol. Biol. 1996, 256, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Huppert, J.L.; Balasubramanian, S. G-quadruplexes in promoters throughout the human genome. Nucleic Acids Res. 2007, 35, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Brosh, R.M. G-quadruplex nucleic acids and human disease. FEBS J. 2010, 277, 3470–3488. [Google Scholar] [CrossRef] [PubMed]
- Chantot, J.; Guschlbauer, W. Physicochemical properties of nucleosides 3. Gel formation by 8-bromoguanosine. FEBS Lett. 1969, 4, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Guschlbauer, W.; Chantotand, J.F.; Thiele, D. Four-stranded nucleic acid structures 25 years later: From guanosine gels to telomer DNA. J. Biomol. Struct. Dyn. 1990, 8, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, H.; Takagi, M.; Takenaka, S. A novel potassium sensing in aqueous media with a synthetic oligonucleotide derivative. Fluorescence resonance energy transfer associated with Guanine quartet-potassium ion complex formation. S. J. Am. Chem. Soc. 2002, 124, 14286–14287. [Google Scholar] [CrossRef]
- Takenaka, S.; Ueyama, H.; Nojima, T.; Takagi, M. Comparison of potassium ion preference of potassium-sensing oligonucleotides, PSO-1 and PSO-2, carrying the human and Oxytricha telomeric sequence, respectively. Anal. Bioanal. Chem. 2003, 375, 1006–1010. [Google Scholar] [PubMed]
- Kumar, N.; Maiti, S. Quadruplex to Watson-Crick duplex transition of the thrombin binding aptamer: A fluorescence resonance energy transfer study. Biochem. Biophys. Res. Commun. 2004, 319, 759–767. [Google Scholar] [CrossRef]
- Hardin, C.C.; Perry, A.G.; White, K. Thermodynamic and kinetic characterization of the dissociation and assembly of quadruplex nucleic acids. Biopolymers 2000, 56, 147–194. [Google Scholar] [CrossRef] [PubMed]
- Bugaut, A.; Balasubramanian, S. A sequence-independent study of the influence of short loop lengths on the stability and topology of intramolecular DNA G-quadruplexes. Biochemistry 2008, 47, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Guedin, A.; Gros, J.; Alberti, P.; Mergny, J.L. How long is too long? Effects of loop size on G-quadruplex stability. Nucleic Acids Res. 2010, 38, 7858–7868. [Google Scholar] [CrossRef] [PubMed]
- Sen, D.; Gilbert, W. Formation of parallel four-stranded complexes by guanine-rich motifs in DNA and its implication for meiosis. Nature 1988, 334, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E. Structure and function of telomeres. Nature 1991, 350, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Sen, D.; Gilbert, W. A sodium-potassium switch in the formation of four-stranded G4-DNA. Nature 1990, 344, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Kofuji, P.; Newman, E.A. Potassium buffering in the central nervous system. Neuroscience 2004, 129, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.P.; Canzoniero, L.M.T.; Choi, D.W. Ion homeostasis and apoptosis. Curr. Opin. Cell Biol. 2001, 13, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.; Mammene, O.K.; Wilting, I.; Sachsh, G.S.; Ferrier, I.N.; Cassidy, F.; Beaulieul, S.; Yathamn, L.N.; Berk, M. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009, 11, 559–595. [Google Scholar] [CrossRef] [PubMed]
- Dimeski, G.; Badrick, T.; John, A.S. Ion Selective Electrodes (ISEs) and interferences-a review. Clin. Chim. Acta 2010, 411, 309–317. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Tang, Y.; Wang, S.; Li, Y.; Zhu, D. Fluorescent amplifying recognition for DNA G-quadruplex folding with a cationic conjugated polymer: A platform for homogeneous potassium detection. J. Am. Chem. Soc. 2005, 127, 12343–12346. [Google Scholar] [CrossRef] [PubMed]
- Nagatoishi, S.; Nojima, T.; Galezowska, E.; Gluszynska, A.; Juskowiak, B.; Takenaka, S. Fluorescence energy transfer probes based on the guanine quadruplex formation for the fluorometric detection of potassium ion. Anal. Chim. Acta 2007, 581, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Nagatoishi, S.; Nojima, T.; Juskowiak, B.; Takenaka, S. Pyrene-Labeled G-Quadruplex oligonucleotide as a fluorescent probe for potassium ion detection in biological applications. Angew. Chem. Int. 2005, 44, 5067–5070. [Google Scholar] [CrossRef]
- Patolsky, F.; Katz, E.; Bardea, A.; Willner, I. Enzyme-Linked amplified electrochemical sensing of oligonucleotide-dna interactions by means of impedance spectroscopy. Langmuir 1999, 15, 3703–3706. [Google Scholar] [CrossRef]
- Pfeiffer, I.; Höök, F. Bivalent cholesterol-based coupling of oligonucletides to lipid membrane assemblies. J. Am. Chem. Soc. 2004, 126, 10224–10225. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, I.; Höök, F. Quantification of oligonucleotide modifications of small unilamellar lipid vesicles. Anal. Chem. 2006, 78, 7493–7498. [Google Scholar] [CrossRef] [PubMed]
- Jaumot, J.; Gargallo, R. Experimental methods for studying the interactions between Gquadruplex structures and ligands. Curr. Pharm. Des. 2012, 18, 1900–1916. [Google Scholar] [CrossRef] [PubMed]
- Simonsson, T.; Sjoback, R. DNA tetraplex formation studied with fluorescence resonance energy transfer. J. Biol. Chem. 1999, 274, 17379–17383. [Google Scholar] [CrossRef] [PubMed]
- Juskowiak, B. Analytical potential of the quadruplex DNA-based FRET probes. Anal. Chim. Acta 2006, 568, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.M.; Galliardt, H.; Schneider, J.; Barisas, B.G.; Seidel, T. Quantification of Förster resonance energy transfer by monitoring sensitized emission in living plant cells. Front. Plant. Sci. 2013, 4, 413–433. [Google Scholar] [PubMed]
- Juskowiak, B.; Galezowska, E.; Zawadzka, A.; Takenaka, S. Fluorescence anisotropy and FRET studies of G-quadruplex formation in presence of different cations. Spectrochim. Acta A 2006, 64, 835–843. [Google Scholar] [CrossRef]
- Juskowiak, B.; Takenaka, S. FRET in the studies of guanine-quadruplexes. In Fluorescent Energy Transfer Nucleic Acid Probes: Methods and Protocols; Didenko, V.V., Ed.; Humana Press: Totowa, NJ, USA, 2006; pp. 311–342. [Google Scholar]
- Takenaka, S.; Juskowiak, B. Fluorescence detection of potassium ion using G-quadruplex structure. Anal. Sci. 2011, 27, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Venner, H. Molekülverbindungen einiger Sterine und Sterinester mit Nitroaromaten. Chem. Ber. 1956, 89, 1634–1641. [Google Scholar] [CrossRef]
- Scaria, P.V.; Shire, S.J.; Shafer, R.H. Quadruplex structure of d(G3T4G3) stabilized by K+ or Na+ is an asymmetric hairpin dimer. Proc. Natl. Acad. Sci. USA 1992, 89, 10336–10340. [Google Scholar] [CrossRef] [PubMed]
- Mergny, J.L.; de Cian, A.; Ghelab, A.; Sacca, B.; Lacroix, L. Kinetics of tetramolecular quadruplexes. Nucleic Acids Res. 2005, 33, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Miyoshi, D.; Nakano, S.; Sugimoto, N. Structural competition involving G-quadruplex DNA and its complement. Biochemistry 2003, 42, 11736–11744. [Google Scholar] [CrossRef] [PubMed]
- Green, J.J.; Ying, L.M.; Klenerman, D.; Balasubramanian, S. Kinetics of unfolding the human telomeric DNA quadruplex using a PNA trap. J. Am. Chem. Soc. 2003, 125, 3763–3767. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.N.; Chaires, J.B.; Gray, R.D.; Trent, J.O. Stability and kinetics of G-quadruplex structures. Nucleic Acids Res. Oct. 2008, 36, 5482–5515. [Google Scholar] [CrossRef]
- Macaya, R.F.; Schultze, P.; Smith, F.W.; Roe, J.A.; Feigon, J. Thrombin-binding DNA aptamer forms a unimolecular quadruplex structure in solution. J. A. Roe J. Feigon Proc. Natl. Acad. Sci. USA 1993, 90, 3745–3749. [Google Scholar] [CrossRef]
- Schultze, P.; Macaya, R.F.; Feigon, J. Three-Dimensional solution structure of the thrombin-binding DNA aptamer d(GGTTGGTGTGGTTGG). J. Mol. Biol. 1994, 235, 1532–1547. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swiatkowska, A.; Juskowiak, B. Effect of Cholesterol Anchoring Group on the Properties of G-Quadruplex-Based FRET Probes for Potassium Ion. Chemosensors 2014, 2, 267-286. https://doi.org/10.3390/chemosensors2040267
Swiatkowska A, Juskowiak B. Effect of Cholesterol Anchoring Group on the Properties of G-Quadruplex-Based FRET Probes for Potassium Ion. Chemosensors. 2014; 2(4):267-286. https://doi.org/10.3390/chemosensors2040267
Chicago/Turabian StyleSwiatkowska, Angelika, and Bernard Juskowiak. 2014. "Effect of Cholesterol Anchoring Group on the Properties of G-Quadruplex-Based FRET Probes for Potassium Ion" Chemosensors 2, no. 4: 267-286. https://doi.org/10.3390/chemosensors2040267
APA StyleSwiatkowska, A., & Juskowiak, B. (2014). Effect of Cholesterol Anchoring Group on the Properties of G-Quadruplex-Based FRET Probes for Potassium Ion. Chemosensors, 2(4), 267-286. https://doi.org/10.3390/chemosensors2040267