Luminescent Oxygen Gas Sensors Based on Nanometer-Thick Hybrid Films of Iridium Complexes and Clay Minerals
Abstract
:1. Introduction
2. Preparation of the Thin Films of Clay Minerals by the Modified Langmuir-Blodgett (LB) Method
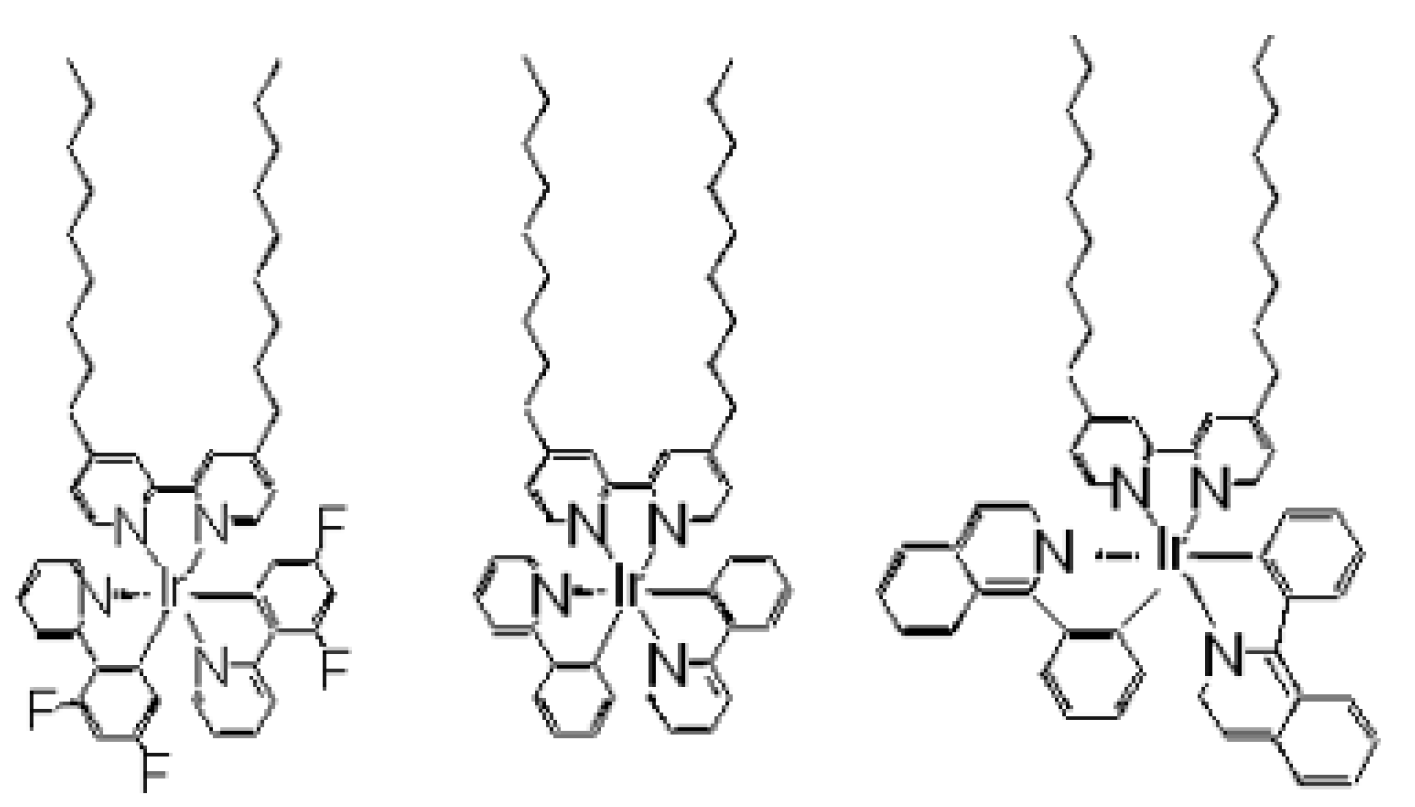
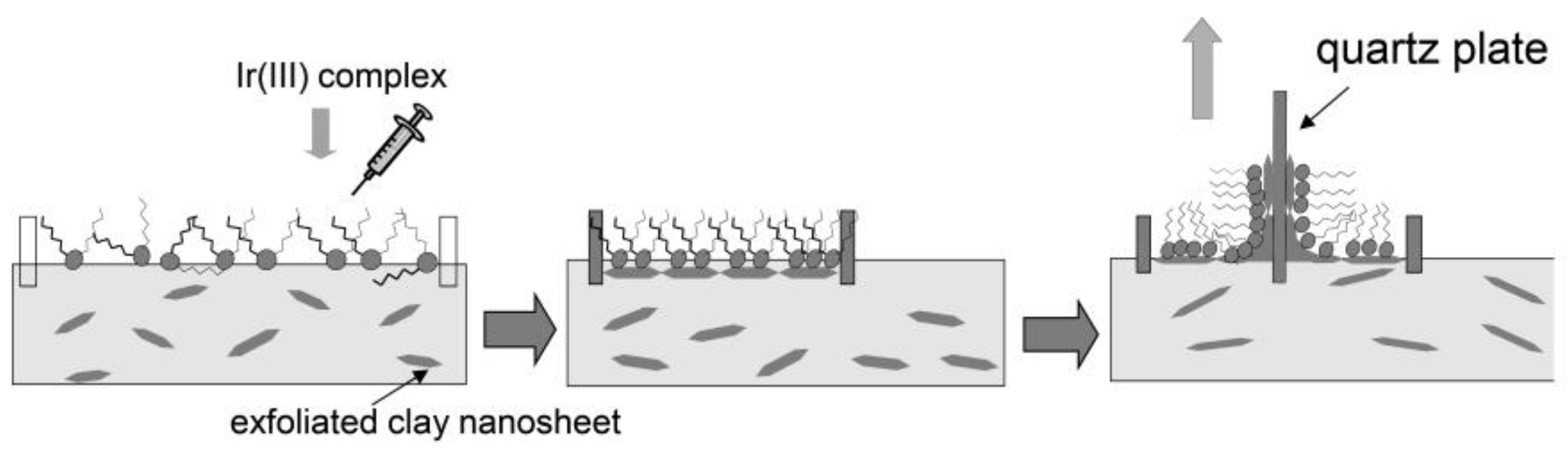
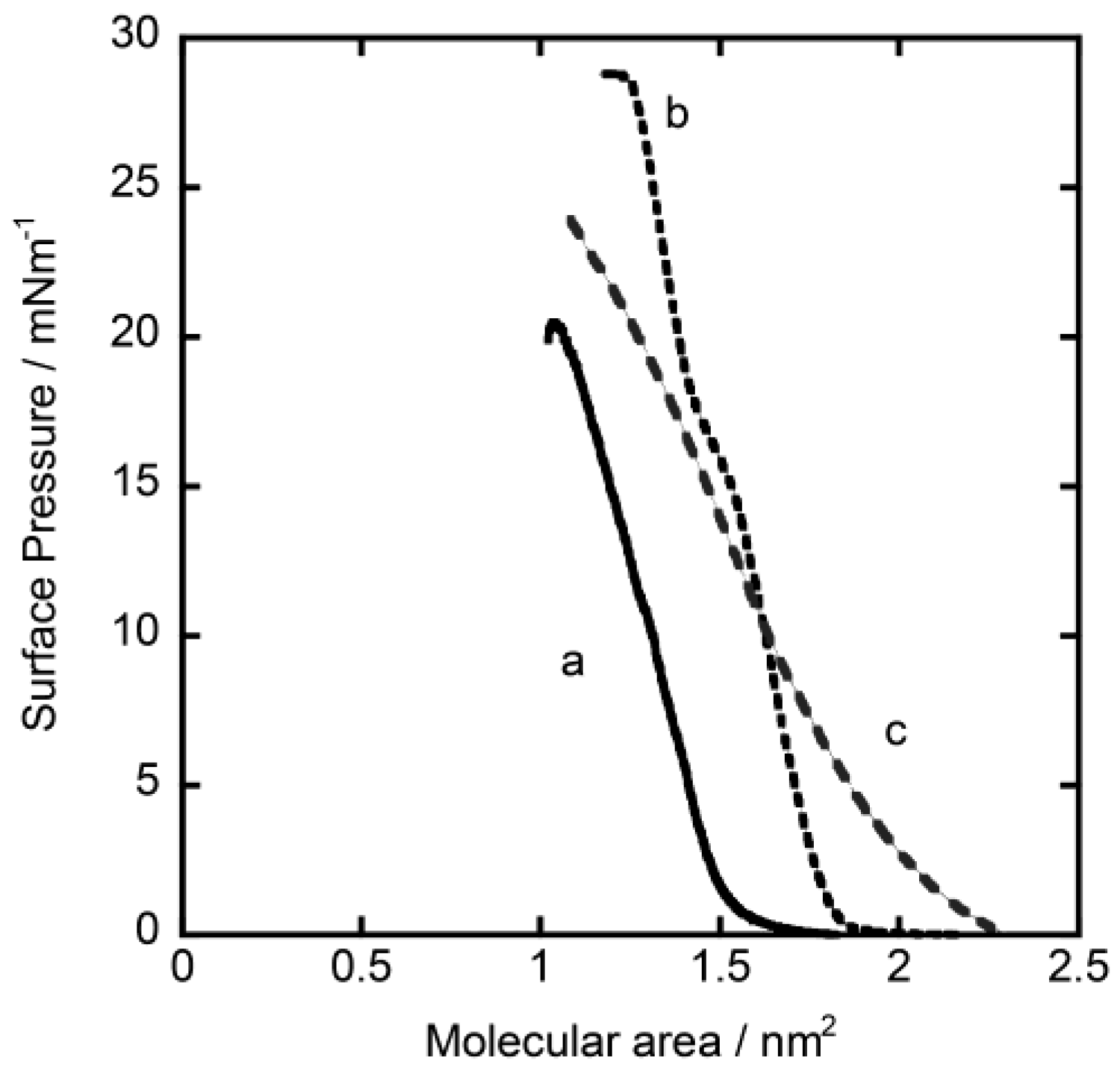
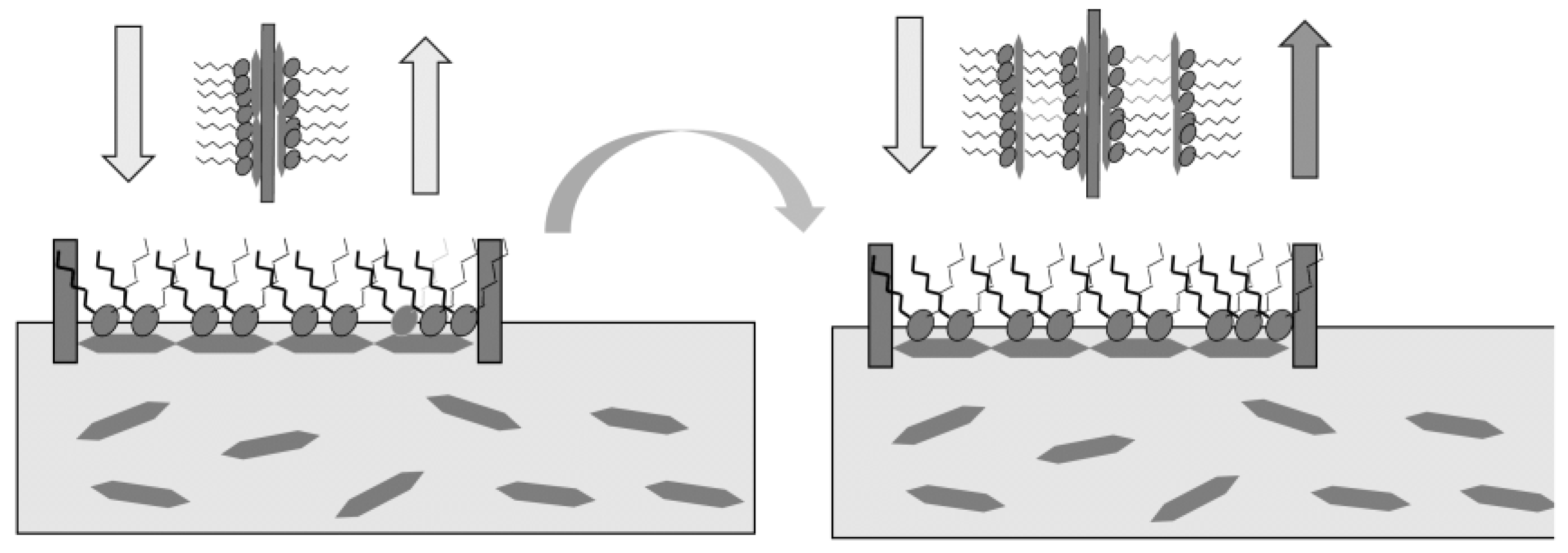
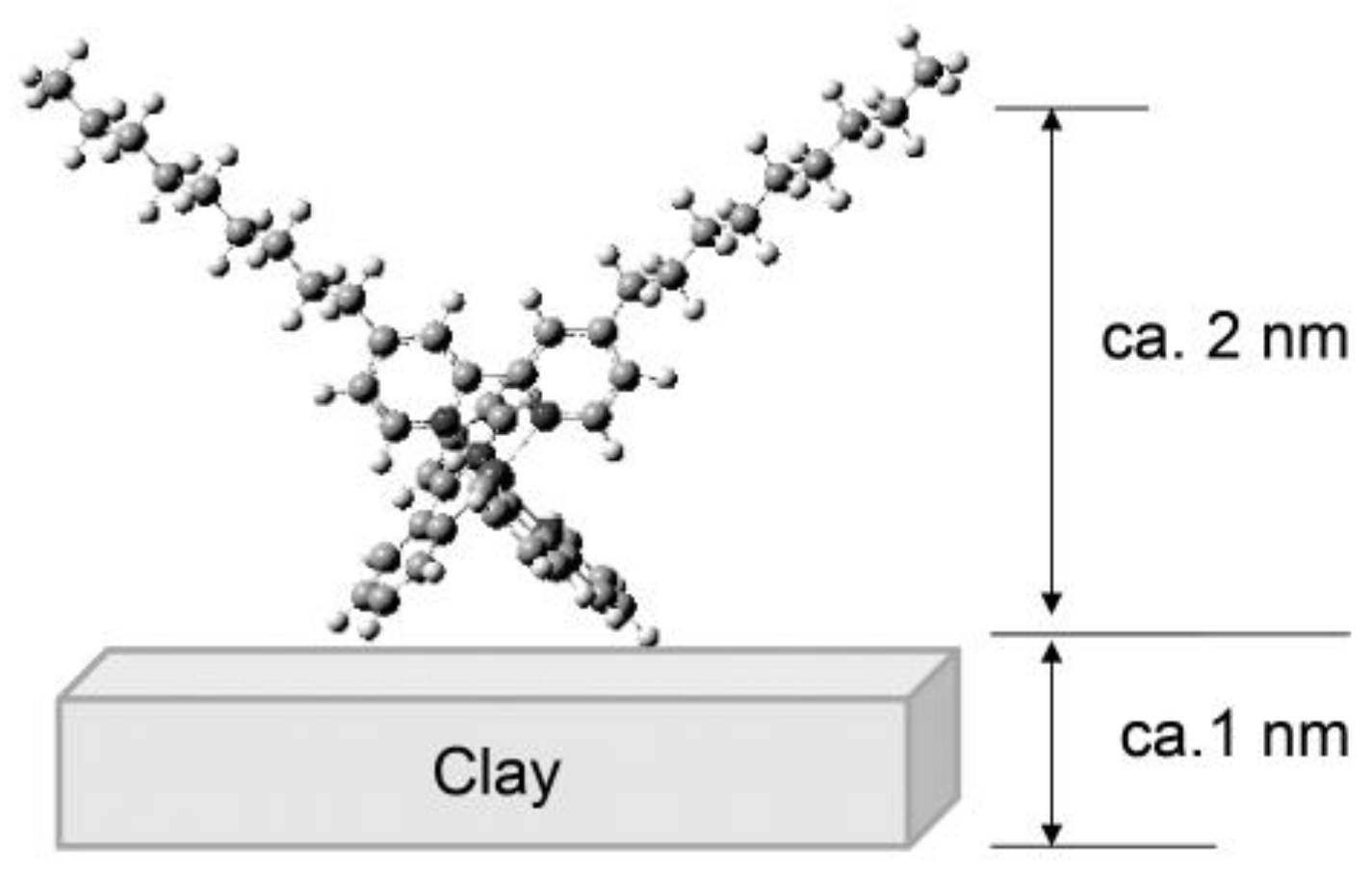
3. Photosensing of Gases by Hybrid LB Films
3.1. Oxygen Photosensing by Hybrid Monolayered Films
3.2. Dual-Emitting Properties of Double-Layered Films in Oxygen Sensing
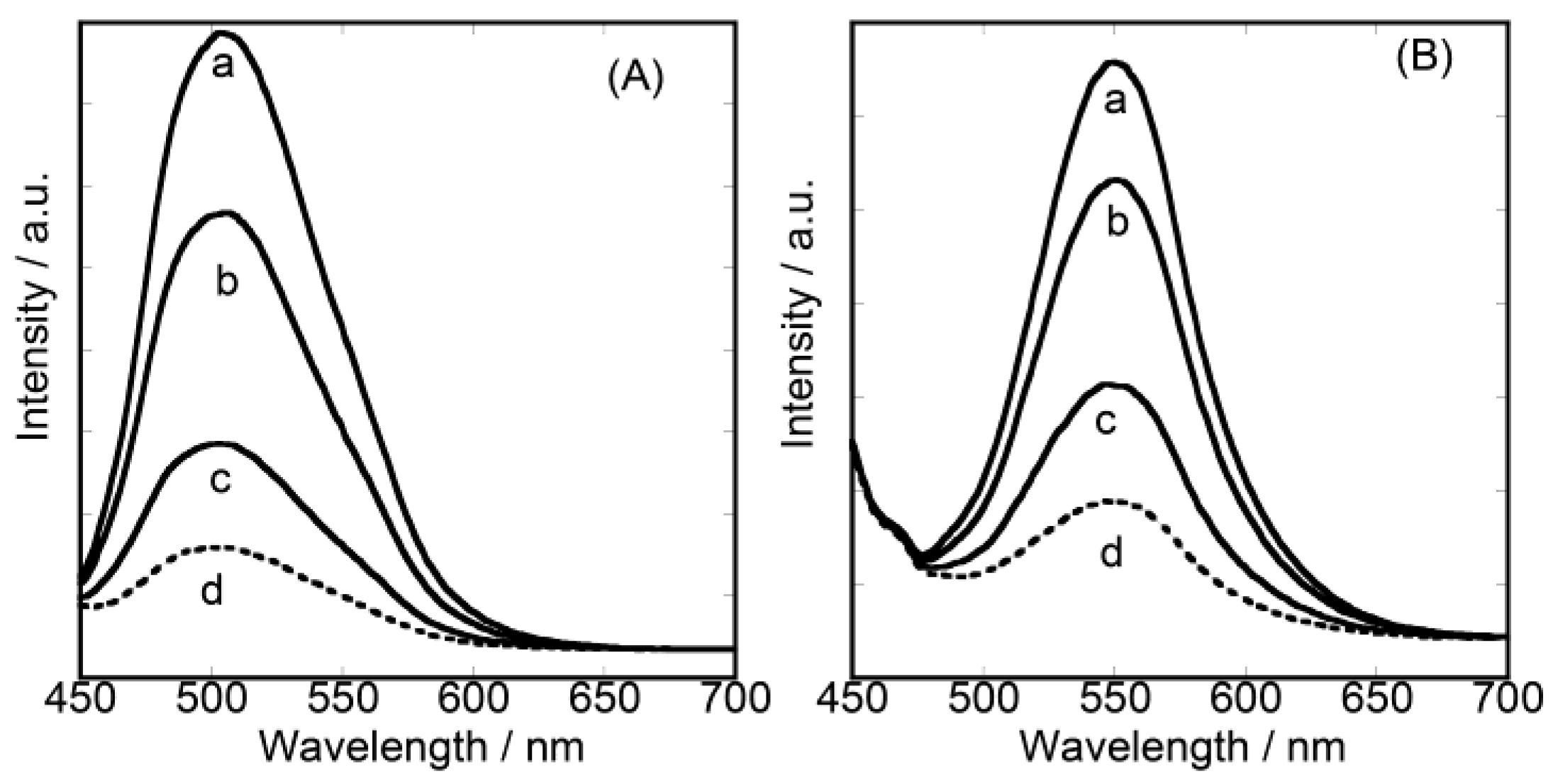
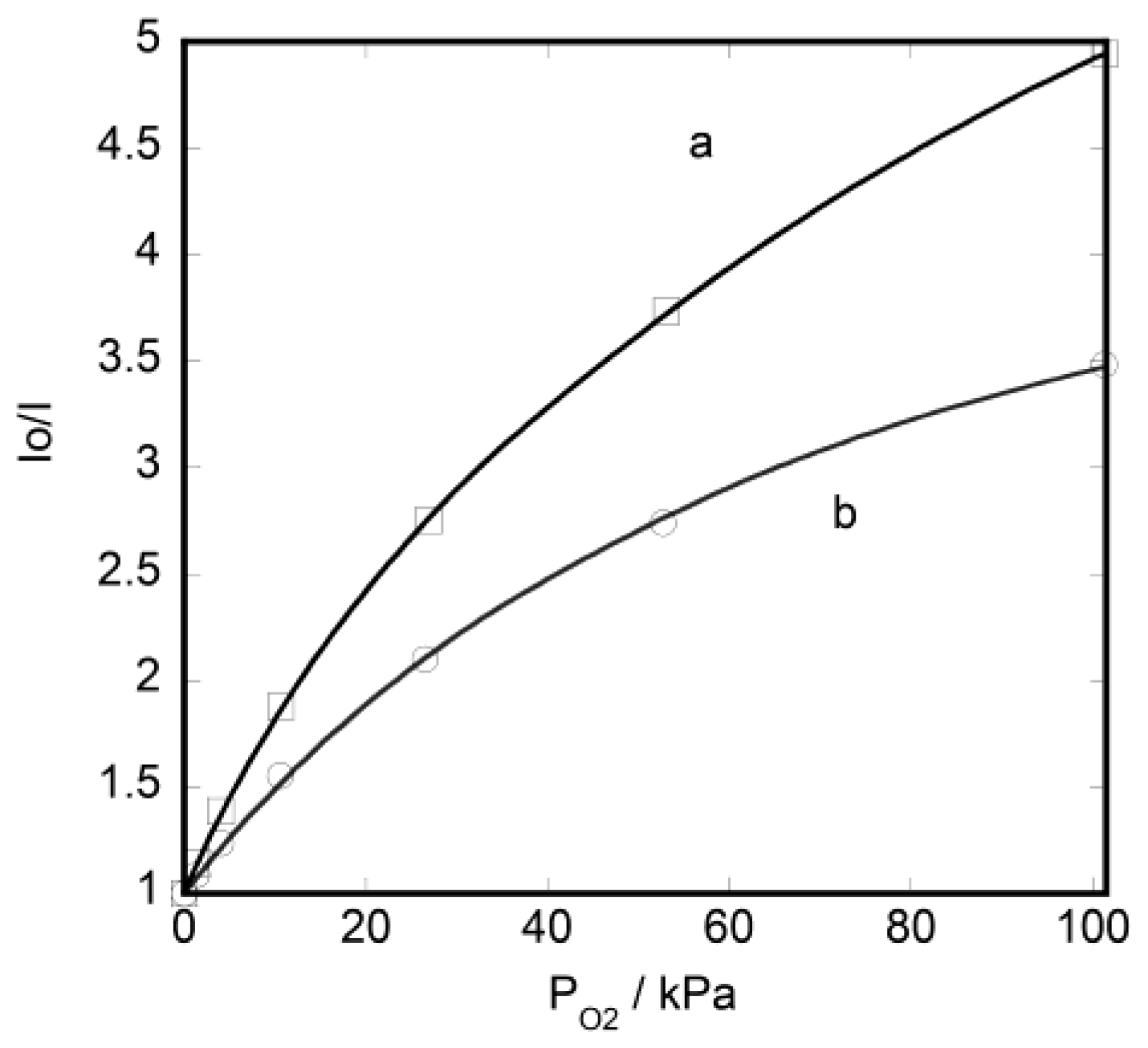
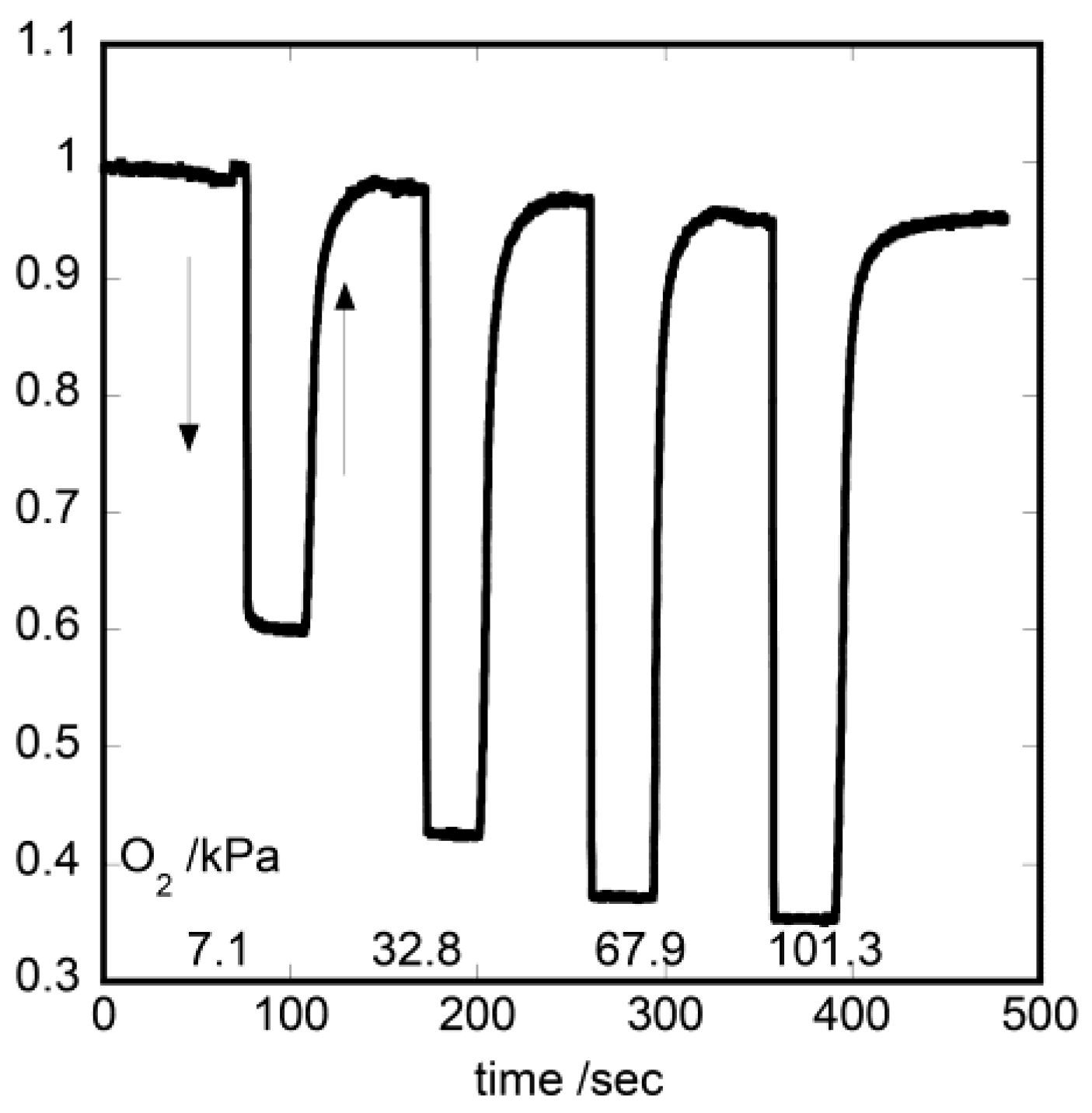
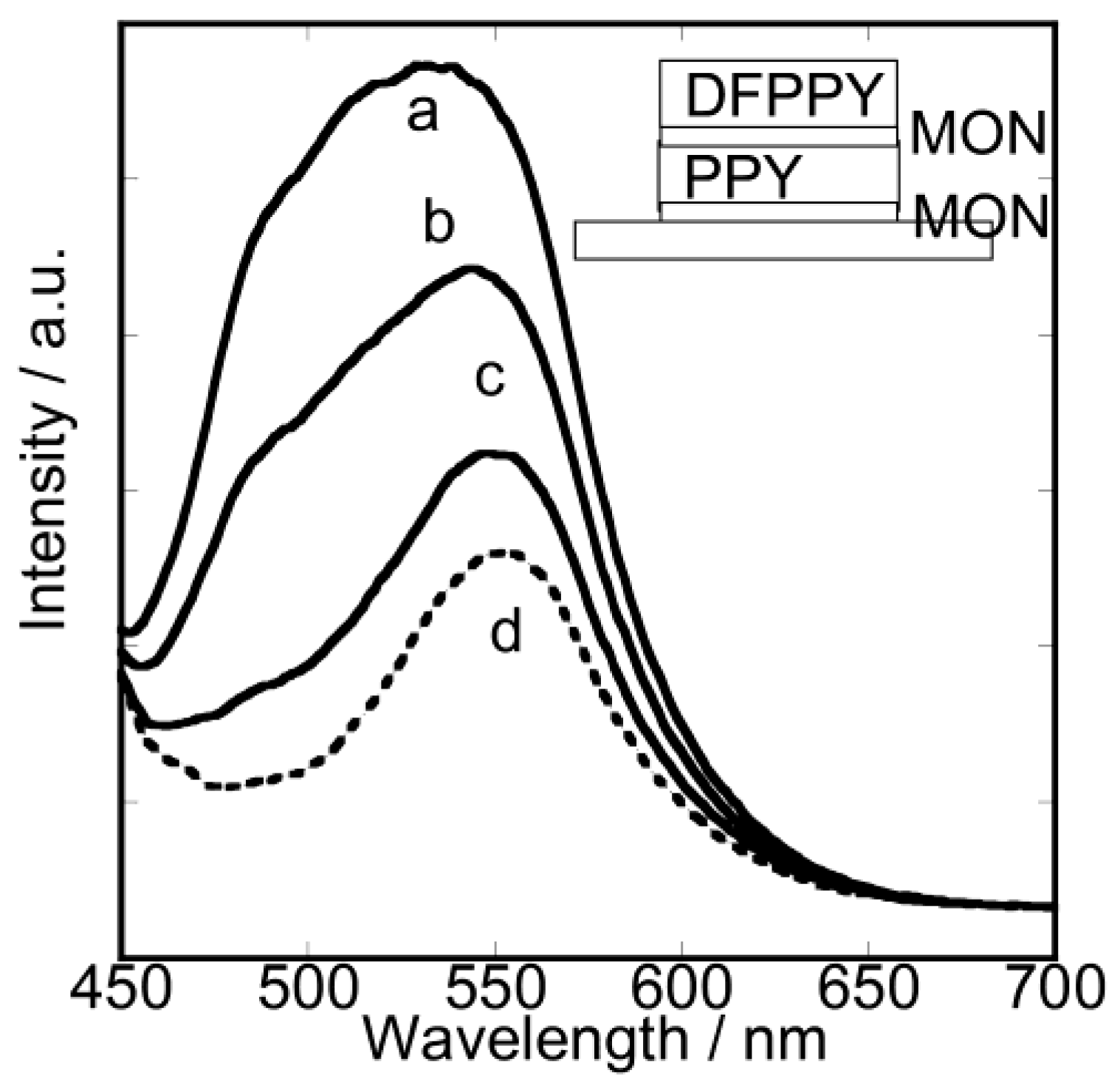
3.3. Unique Character of Triple-Layered Hybrid LB Films


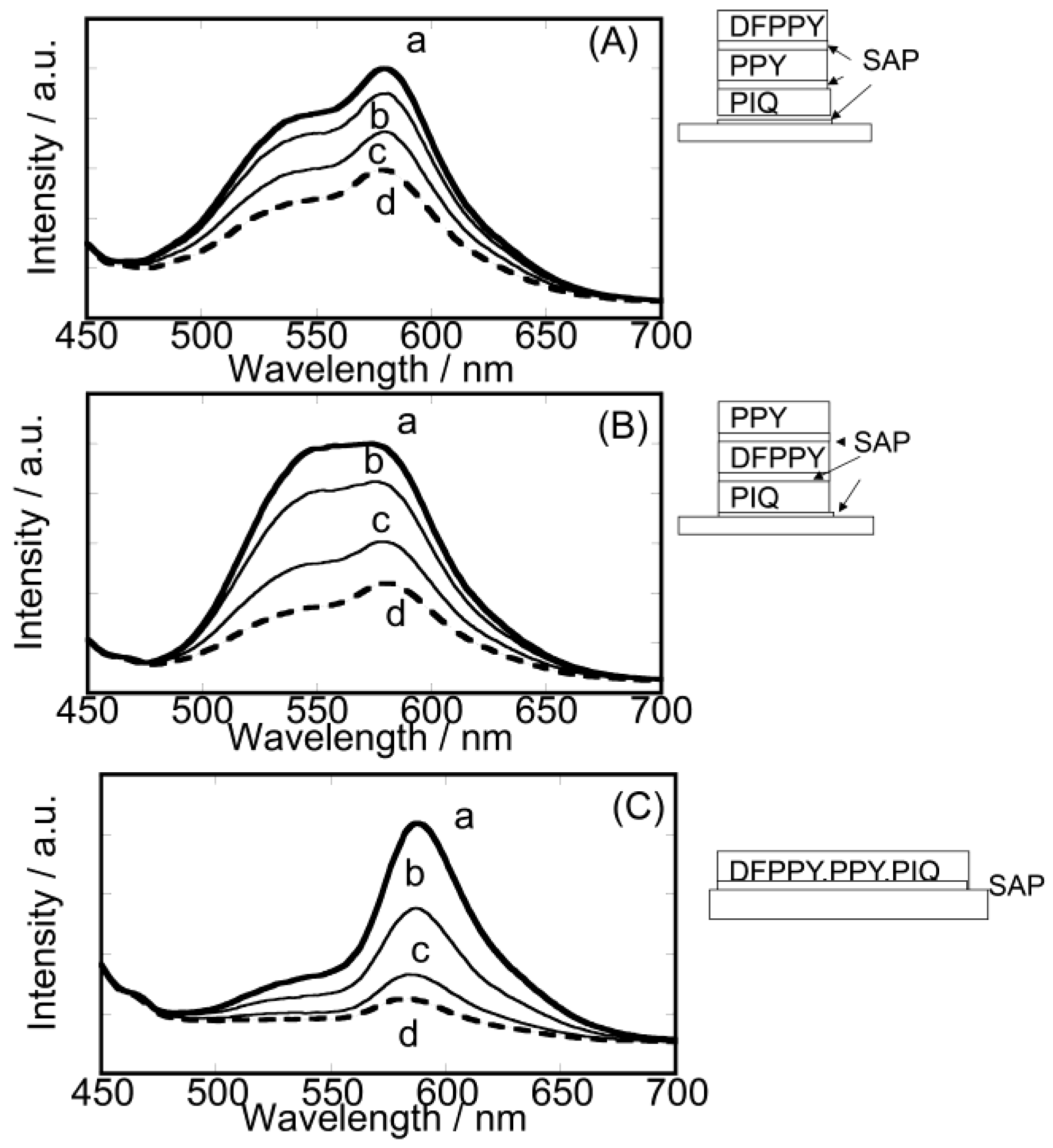
4. Perspective
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Balzani, V.; Juris, A.; Venturi, M.; Campagna, S.; Serroni, S. Luminescent and redox-active polynuclear transition metal complexes. Chem. Rev. 1996, 96, 759–833. [Google Scholar] [CrossRef]
- Wong, W.-Y.; Ho, C.-L. Heavy Metal organometallic electrophosphors derived from multi-component chromophores. Coord. Chem. Rev. 2009, 253, 1709–1968. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Nakagawa, T.; Kawai, T. Recent progress of luminescent metal complexes with photochromic units. Coord. Chem. Rev. 2010, 254, 2643–2651. [Google Scholar] [CrossRef]
- Chi, Y.; Chou, P.-T. Transition-metal phosphors with cyclometalating ligands: Fundamentals and applications. Chem. Soc. Rev. 2010, 39, 638–655. [Google Scholar] [CrossRef]
- Sato, H.; Yamagishi, A. Application of the ΔΛ isomerism of octahedral metal complexes as a chiral source in photochemistry. J. Photochem. Photobiol. C Photochem. Rev. 2007, 8, 67–84. [Google Scholar] [CrossRef]
- King, K.A.; Spellane, P.J.; Watts, R.J. Excited-state properties of a triply ortho-metalated Iridium(III) complex. J. Am. Chem. Soc. 1985, 107, 1431–1432. [Google Scholar]
- Tsuboyama, A.; Iwawaki, H.; Furugori, M.; Mukaide, T.; Kamatani, J.; Igawa, S.; Moriyama, T.; Miura, S.; Takiguchi, T.; Okada, S.; et al. Homoleptic Cyclometalated iridium complexes with highly dfficient red phosphorescence and application to organic light-emitting diode. J. Am. Chem. Soc. 2003, 125, 12971–12979. [Google Scholar] [CrossRef]
- Tamayo, A.B.; Alleyne, B.D.; Djurovich, P.I.; Lamansky, S.; Tsyba, I.; Ho, N.N.; Bau, R.; Thompson, M.E. Synthesis and characterization of facial and meridional tris-cyclometalated iridium(III) complexes. J. Am. Chem. Soc. 2003, 125, 7377–7387. [Google Scholar] [CrossRef]
- Tamayo, A.B.; Garon, S.; Sajoto, T.; Djurovich, P.I.; Tsyba, I.M.; Bau, R.; Thompson, M.E. Cationic bis-cyclometalated iridium(III) diimine complexes and their use in efficient blue, green, and red electroluminescent devices. Inorg. Chem. 2005, 44, 8723–8732. [Google Scholar] [CrossRef]
- Sajoto, T.; Djurovich, P.I.; Tamayo, A.B.; Oxgaard, J.; Goddard, W.A., III; Thompson, M.E. Temperature dependence of blue phosphorescent cyclometalated Ir(III) complexes. J. Am. Chem. Soc. 2009, 131, 9813–9822. [Google Scholar]
- Happ, B.; Winter, A.; Hager, M.D.; Schubert, U.S. Photogenerated avenues in macromolecules containing Re(I), Ru(II), Os(II), and Ir(III) metal complexes of pyridine-based ligands. Chem. Soc. Rev. 2012, 41, 2222–2255. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, S.; Wu, W.; Wu, W.; Guo, H.; Sun, J.; Sum, H.; Liu, Y.; Li, Q.; Huang, L. Transition metal complexes with strong absorption of visible light and long-lived triplet excited States: From molecular design to applications. RSC Adv. 2012, 2, 1712–1728. [Google Scholar] [CrossRef]
- Zhou, G.; Wong, W.Y.; Yang, X. New design tactics in OLEDs using functionalized 2-phenylpyridine-type cyclometalates of iridium(III) and platinum(II). Chem. Asian. J. 2011, 6, 1706–1727. [Google Scholar] [CrossRef]
- Ladouceur, S.; Fortin, D.; Zysman-Colman, E. Role of substitution on the photophysical properties of 5,5′-Diaryl-2,2′-bipyridine (bpy*) in [Ir(ppy)2(bpy*)]PF6 complexes: A combined experimental and theoretical study. Inorg. Chem. 2010, 49, 5625–5641. [Google Scholar] [CrossRef]
- Fischer, L.H.; Stich, M.I.J.; Wolfbeis, O.S.; Tian, N.; Holder, E.; Scäferling, M. Red- and green-emitting iridium(III) complexes for a dual barometric and temperature-sensitive paint. Chem. Eur. J. 2009, 15, 10857–10863. [Google Scholar] [CrossRef]
- Papkovsky, D.B.; Dmitriec, R.I. Biological detection by optical oxygen sensing. Chem. Soc. Rev. 2013, 42, 8700–8732. [Google Scholar] [CrossRef]
- Schäferling, M. The Art of fluorescence imaging with chemical sensors. Angew. Chem. Int. Ed. 2012, 51, 3532–3554. [Google Scholar] [CrossRef]
- Quaranta, M.; Borisov, S.M.; Klimant, I. Indicatiors for optical oxygen sensors. Bioanal. Rev. 2012, 4, 115–157. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. Fiber-optic chemical sensors and biosensors. Anal. Chem. 2008, 80, 4269–4283. [Google Scholar] [CrossRef]
- Amao, Y. Probes and polymers for optical sensing of oxygen. Microchim. Acta 2003, 143, 1–12. [Google Scholar] [CrossRef]
- Sailor, M.J.; Wu, E.C. Photoluminescence-based sensing with porous silicon films, microparticles, and nanoparticles. Adv. Funct. Mater. 2009, 19, 3195–3208. [Google Scholar] [CrossRef]
- Ju, S.-Y.; Kopcha, W.P.; Papadimitrakopoulos, F. Brightly Fluorescent single-walled carbon nanotubes via an oxygen excluding surfactant organization. Science 2009, 323, 1319–1323. [Google Scholar] [CrossRef]
- Achatz, D.E.; Meier, R.J.; Fischer, L.H.; Wolfbeis, O.S. Luminescent sensing of oxygen using a quenchable probe and upconverting nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 260–263. [Google Scholar] [CrossRef]
- Chu, C.-S.; Lo, Y.-L.; Sung, T.-W. Review on recent developments of fluorescent oxygen and carbon dioxide optical fiber sensors. Photonic Sens. 2011, 1, 234–250. [Google Scholar] [CrossRef]
- Lopez-Ruiz, N.; Martinez-Olmos, A.; de Vargas-Sansalvador, I.M.P.; Fernandez-Ramos, M.D.; Carvajal, M.A.; Capitan-Vallvey, L.F.; Palma, A.J. Determination of O2 using colour sensing from image processing with mobile devices. Sens. Actuators B 2012, 171–172, 938–945. [Google Scholar] [CrossRef]
- Sotelo-Gonzalez, E.; Coto-Garcia, A.M.; Fernandez-Arguelles, M.T.; Costa-Fernandez, J.M.; Sanz-Medel, A. Immobilization of phosphorescent quantum dots in a sol–gel matrix for acetone sensing. Sens. Actuators B 2012, 174, 102–108. [Google Scholar]
- Rothe, C.; Chiang, C.-J.; Jankus, V.; Abdullah, K.; Zeng, X.; Jitchati, R.; Batsanov, A.; Bryce, M.R.; Monkman, A.P. Ionic iridium(III) complexes with bulky side groups for use in light emitting cells: Reduction of concentration quenching. Adv. Funct. Mater. 2009, 19, 2038–2044. [Google Scholar] [CrossRef]
- Ulbricht, C.; Beyer, B.; Friebe, C.; Winter, A.; Schubert, U.S. Recent developments in the application of phosphorescent iridium(III) complex systems. Adv. Mater. 2009, 21, 4418–4441. [Google Scholar]
- Guerchais, V.; Fillaut, J.-L. Sensory luminescent iridium(III) and platinum(II) complexes for cation recognition. Coord. Chem. Rev. 2011, 255, 2448–2457. [Google Scholar] [CrossRef]
- Lowry, M.S.; Bernhard, S. Synthetically tailored excited states: Phosphorescent, cyclometalated iridium(III) complexes and their applications. Chem. Eur. J. 2006, 12, 7970–7977. [Google Scholar] [CrossRef]
- Tian, N.; Lenkeit, D.; Pelz, S.; Fischer, L.H.; Escudero, D.; Schiewek, R.; Klink, D.; Schmitz, O.J.; Gomzález, L.; Schäferling, M.; et al. Structure–property relationship of red- and rreen-emitting iridium(III) complexes with respect to their temperature and oxygen sensitivity. Eur. J. Inorg. Chem. 2010, 2010, 4875–4885. [Google Scholar] [CrossRef]
- Xie, Z.; Ma, L.; deKrafft, K.E.; Jin, A.; Lin, W. Porous phosphorescent coordination polymers for oxygen sensing. J. Am. Chem. Soc. 2010, 132, 922–923. [Google Scholar] [CrossRef]
- DeRosa, M.C.; Mosher, P.J.; Yap, G.P.A.; Focsaneanu, K.-S.; Crutchley, R.J.; Evans, C.E.B. Synthesis, characterization, and evaluation of [Ir(ppy)2(vpy)Cl] as a polymer-bound oxygen Sensor. Inorg. Chem. 2003, 42, 4864–4872. [Google Scholar] [CrossRef]
- DeRosa, M.C.; Hodgson, D.J.; Enright, G.D.; Dawson, B.; Evans, C.E.B.; Crutchley, R.J. Iridium luminophore complexes for unimolecular oxygen sensors. J. Am. Chem. Soc. 2004, 126, 7619–7626. [Google Scholar]
- Clemente-León, M.; Coronado, E.; López-Muñoz, Á.; Repetto, D.; Ito, T.; Konya, T.; Yamase, T.; Constable, E.C.; Housecroft, C.E.; Doyle, K.; et al. Dual-emissive photoluminescent Langmuir–Blodgett films of decatungstoeuropate and an amphiphilic iridium complex. Langmuir 2010, 26, 1316–1324. [Google Scholar] [CrossRef]
- Bolink, H.J.; Baranoff, E.; Clemente-León, M.; Coronado, E.; Lardiés, N.; López-Muñoz, A.; Repetto, D.; Nazeeruddin, M.K. Dual-emitting Langmuir–Blodgett film-based organic light-emitting diode. Langmuir 2010, 26, 11461–11468. [Google Scholar] [CrossRef]
- Roldán-Carmona, C.; González-Delgado, A.M.; Guerrero-Martínez, A.; de Cola, L.; Giner-Casares, J.J.; Pérez-Morales, M.; Martín-Romero, M.T.; Camacho, L. Molecular organization and effective energy transfer in iridium metallosurfactant–porphyrin assemblies embedded in Langmuir–Schaefer films. Phys. Chem. Chem. Phys. 2011, 13, 2834–2841. [Google Scholar]
- Sato, H.; Tamura, K.; Taniguchi, M.; Yamagishi, A. Highly luminescent Langmuir–Blodgett films of amphiphilic Ir(III) complexes for application in gas sensing. New J. Chem. 2010, 34, 617–622. [Google Scholar]
- Sato, H.; Tamura, K.; Ohara, K.; Nagaoka, S.; Yamagishi, A. Hybridization of clay minerals with the floating film of a cationic Ir(III) complex at an air–water interface. New J. Chem. 2011, 35, 394–399. [Google Scholar] [CrossRef]
- Borisov, S.M.; Klimant, I. Ultrabright Oxygen optodes based on cyclometalated iridium(III) coumarin complexes. Anal. Chem. 2007, 79, 7501–7509. [Google Scholar] [CrossRef]
- Mak, C.S.K.; Pentlehner, D.; Stich, M.; Wolfbeis, O.S.; Chan, W.K.; Yersin, H. Exceptional oxygen sensing capabilities and triplet state properties of Ir(ppy-NPh2)3. Chem. Mater. 2009, 21, 2173–2175. [Google Scholar]
- Toro, M.M.-S.; Ferández-Sánchez, J.F.; Baranoff, E.; Nazeeruddin, M.K.; Graetzela, M.; Ferandez-Gutierrez, A. Novel luminescent Ir(III) dyes for developing highly sensitive oxygen sensing films. Talanta 2010, 82, 620–626. [Google Scholar] [CrossRef]
- Medina-Castillo, A.L.; Ferández-Sánchez, J.F.; Klein, C.; Nazeeruddin, M.K.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Graetzel, M.; Spichinger-Keller, U.E. Engineering of efficient phosphorescent iridium cationic complex for developing oxygen-sensitive polymeric and nanostructured films. Analyst 2007, 132, 929–936. [Google Scholar] [CrossRef]
- Marín-Suárez, M.; Curchod, B.F.E.; Tavernelli, I.; Rothlisberger, U.; Scopelliti, R.; Jung, I.; Di Censo, D.; Grätzel, M.; Fernández-Sánchez, J.F.; Fernándex-Gutiérrez, A.; et al. Nanocomposites containing neutral blue emitting cyclometalated iridium(III) emitters for oxygen sensing. Chem. Mater. 2012, 24, 2330–2338. [Google Scholar] [CrossRef]
- Medina-Rodríguez, S.; Marín-Suárez, M.; Fernández-Sánchez, J.-F.; de la Torre-Vega, Á.; Baranoff, E.; Fernández-Gutiérrez, A. High performance optical sensing nanocomposites for low and ultra-low oxygen concentrations using phase-shift measurements. Analyst 2013, 138, 4607–4617. [Google Scholar] [CrossRef]
- Aiello, D.; Talarico, A.M.; Teocoli, F.; Szerb, E.I.; Aiello, I.; Testa, F.; Ghedini, M. Self-incorporation of a luminescent neutral iridium(III) complex in different mesoporous micelle-templated silicas. New J. Chem. 2011, 35, 141–148. [Google Scholar]
- Mori, K.; Tottori, M.; Watanabe, K.; Che, M.; Yamashita, H. Photoinduced aerobic oxidation driven by phosphorescence Ir(III) complex anchored to mesoporous silica. J. Phys. Chem. C 2011, 115, 21358–21362. [Google Scholar] [CrossRef]
- Waki, M.; Mizoshita, N.; Tani, T.; Inagaki, S. Periodic mesoporous organosilica derivatives bearing a high density of metal complexes on pore surfaces. Angew. Chem. Int. Ed. 2011, 50, 11667–11671. [Google Scholar] [CrossRef]
- Machizuki, D.; Sugiyama, M.; Maitani, M.M.; Wada, Y. Rigidochromic phosphorescence of [Ir(2-phenylpyridine)2(2,2_-bipyridine)]+ in C16TMA+: Layered silicate and its Förster resonance energy transfer. Eur. J. Inorg. Chem. 2013, 2013, 2324–2329. [Google Scholar]
- Sato, H.; Tamura, K.; Taniguchi, M.; Yamagishi, A. Metal ion sensing by luminescence from an ion-exchange adduct of clay and cationic cyclometalated iridium(III) complex. Chem. Lett. 2009, 38, 14–15. [Google Scholar] [CrossRef]
- Sato, H.; Tamura, K.; Aoki, R.; Kato, M.; Yamagishi, A. Enantioselective sensing by luminescence from cyclometalated iridium(III) complexes adsorbed on a colloidal saponite clay. Chem. Lett. 2011, 40, 63–65. [Google Scholar] [CrossRef]
- Hussain, S.A.; Chakraborty, S.; Bhattacharjee, D.; Schoonheydt, R.A. Fluorescence resonance energy transfer between organic dyes adsorbed onto nano-clay and Langmuir–Blodgett (LB) films. Spectrochim. Acta Part A 2010, 75, 664–667. [Google Scholar] [CrossRef]
- Shichi, T.; Takagi, K. Clay minerals as photochemical reaction fields. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 113–130. [Google Scholar] [CrossRef]
- Ogawa, M.; Kuroda, K. Photofunctions of intercalation compounds. Chem. Rev. 1995, 95, 399–438. [Google Scholar] [CrossRef]
- Sato, H.; Hiroe, Y.; Tamura, K.; Yamagishi, A. Orientation tuning of a polypyridyl Ru(II) complex immobilized on a clay surface toward chiral discrimination. J. Phys. Chem. B 2005, 109, 18935–18941. [Google Scholar] [CrossRef]
- Sasai, R.; Itoh, T.; Ohmori, W.; Itoh, H.; Kusunoki, M. Preparation and characterization of Rhodamine 6G/alkyltrimethylammonium/laponite hybrid solid materials with higher emission quantum yield. J. Phys. Chem. C 2009, 113, 415–421. [Google Scholar] [CrossRef]
- Umemura, Y.; Yamagishi, A.; Schoonhedyt, R.; Persoons, A.; de Schryver, F. Langmuir−Blodgett films of a clay mineral and Ruthenium(II) complexes with a noncentrosymmetric structure. J. Am. Chem. Soc. 2002, 124, 992–997. [Google Scholar] [CrossRef]
- Shimada, T.; Yamada, H.; Umemura, Y. Surface potential studies on adsorption processes of clay nanosheets onto a floating molecular film of an amphiphilic alkylammonium cation. J. Phys. Chem. B 2012, 116, 4484–4491. [Google Scholar] [CrossRef]
- Ishida, Y.; Shimada, T.; Masui, D.; Tachibana, H.; Inoue, H.; Takagi, S. Efficient excited energy transfer reaction in clay/porphyrin complex toward an artificial light-harvesting system. J. Am. Chem. Soc. 2011, 133, 14280–14286. [Google Scholar]
- Ishida, Y.; Masui, D.; Tachibana, H.; Inoue, H.; Shimada, T.; Takagi, S. Controlling the microadsorption structure of porphyrin dye assembly on clay surfaces using the “Size-Matching Rule” for constructing an efficient energy transfer system. ACS Appl. Mater. Interfaces 2012, 4, 811–816. [Google Scholar] [CrossRef]
- Ishida, Y.; Masui, D.; Shimada, T.; Tachibana, H.; Inoue, H.; Takagi, S. The mechanism of the porphyrin spectral shift on inorganic nanosheets: The molecular flattening induced by the strong host–guest interaction due to the “Size-Matching Rule”. J. Phys. Chem. C 2012, 116, 7879–7885. [Google Scholar]
- Ishida, Y.; Shimada, T.; Tachibana, H.; Inoue, H.; Takagi, S. Regulation of the collisional self-quenching of fluorescence in clay/porphyrin complex by strong host–guest interaction. J. Phys. Chem. A 2012, 116, 12065–12072. [Google Scholar] [CrossRef]
- Takagi, S.; Tryk, D.A.; Inoue, H. Photochemical energy transfer of cationic porphyrin complexes on clay surface. J. Phys. Chem. B 2002, 106, 5455–5460. [Google Scholar]
- Takagi, S.; Eguchi, M.; Tryk, D.A.; Inoue, H. Light-harvesting energy transfer and subsequent electron transfer of cationic porphyrin complexes on clay surfaces. Langmuir 2006, 22, 1406–1408. [Google Scholar] [CrossRef]
- eklovský, A.; Takagi, S. Oxygen sensing materials based on clay/metalloporphyrin hybrid systems. Org. Eur. J. Chem. 2013, 11, 1132–1135. [Google Scholar]
- Morimoto, K.; Nakae, T.; Ohara, K.; Tamura, K.; Nagaoka, S.; Sato, H. Dual emitting Langmuir—Blodgett films of cationic iridium complexes and montmorillonite clay for oxygen sensing. New. J. Chem. 2012, 36, 2467–2471. [Google Scholar] [CrossRef]
- Sato, H.; Tamura, K.; Ohara, K.; Nagaoka, S. Multi-emitting properties of hybrid Langmuir-Blodgett films of amphiphilic iridium complexes and the exfoliated nanosheets of saponite clay. New. J. Chem. 2013, 38. [Google Scholar] [CrossRef]
- Sato, H.; Tamura, A.; Yamagishi, A. Application of Clay Mineral-Iridium(III) Complexes Hybrid Langmuir-Blodgett Films for Photosensing. In Clay Minerals in Nature, 1st ed.; Valášková, M., Martynková, G.S., Eds.; Intechopen. Com.: Rijeka, Croatia, 2012; pp. 259–272. [Google Scholar]
- Inukai, K.; Hotta, Y.; Taniguchi, M.; Tomura, S.; Yamagishi, A. Formation of a clay monolayer at an air-water interface. J. Chem. Soc. Chem. Commun. 1994. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sato, H.; Tamura, K.; Yamagishi, A. Luminescent Oxygen Gas Sensors Based on Nanometer-Thick Hybrid Films of Iridium Complexes and Clay Minerals. Chemosensors 2014, 2, 41-55. https://doi.org/10.3390/chemosensors2010041
Sato H, Tamura K, Yamagishi A. Luminescent Oxygen Gas Sensors Based on Nanometer-Thick Hybrid Films of Iridium Complexes and Clay Minerals. Chemosensors. 2014; 2(1):41-55. https://doi.org/10.3390/chemosensors2010041
Chicago/Turabian StyleSato, Hisako, Kenji Tamura, and Akihiko Yamagishi. 2014. "Luminescent Oxygen Gas Sensors Based on Nanometer-Thick Hybrid Films of Iridium Complexes and Clay Minerals" Chemosensors 2, no. 1: 41-55. https://doi.org/10.3390/chemosensors2010041
APA StyleSato, H., Tamura, K., & Yamagishi, A. (2014). Luminescent Oxygen Gas Sensors Based on Nanometer-Thick Hybrid Films of Iridium Complexes and Clay Minerals. Chemosensors, 2(1), 41-55. https://doi.org/10.3390/chemosensors2010041




