Advanced GC-MS Chemosensing Combined with Atomistic Modeling: A Synergistic Approach for Environmental Water Analysis
Abstract
1. Introduction
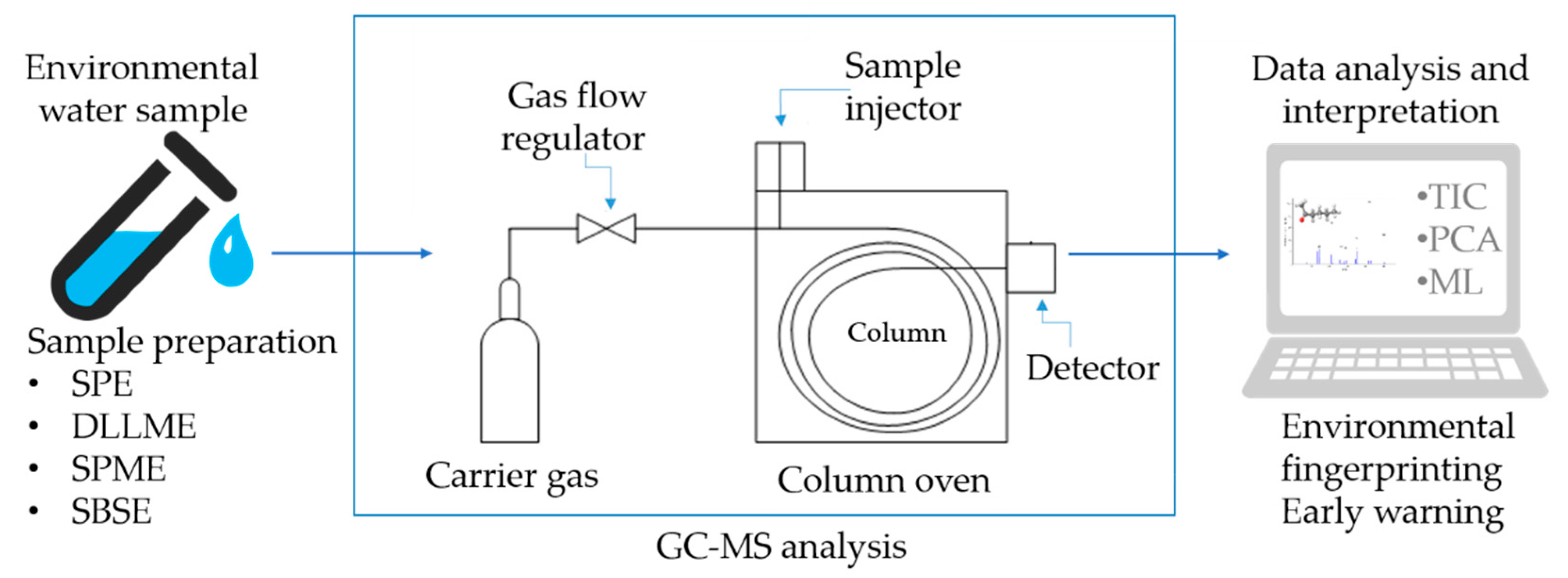
2. GC-MS as a Chemosensing Platform for Environmental Analysis
2.1. Principles and Evolution of Chemosensing in Environmental Applications
2.2. Transition from Qualitative to Quantitative Chemosensing
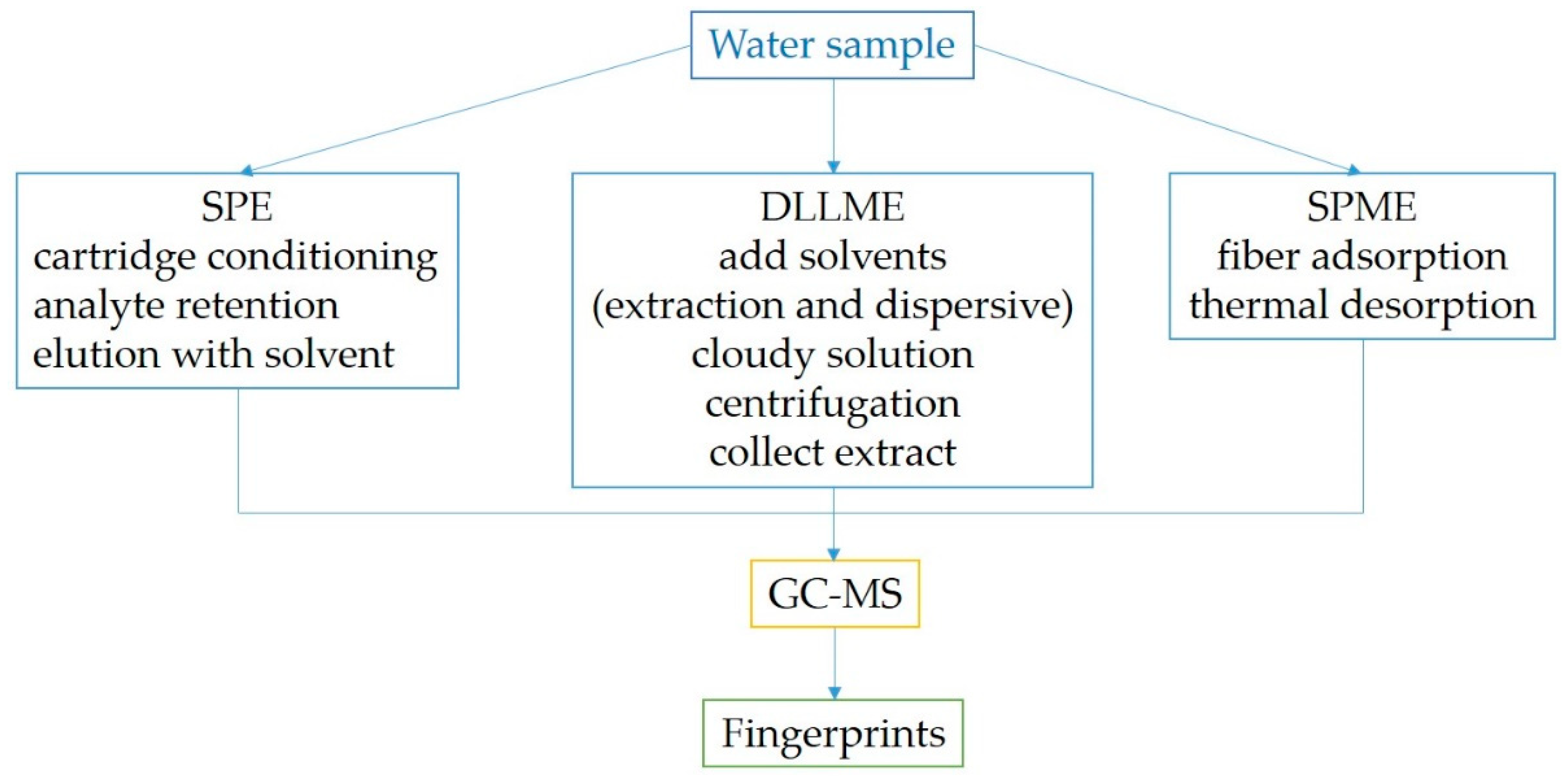
3. Sample Preparation Trends in GC-MS-Based Chemosensing
4. The Role of Atomistic Calculations in Complementing GC, MS, and GC-MS Analytical Methods
4.1. Introduction to Atomistic Calculations
4.2. Software Packages and Codes for Performing Atomistic Calculations
4.3. Atomistic Calculations as a Complement to GC, MS, and GC-MS
4.3.1. Supporting Interpretation of Fragmentation and Degradation
4.3.2. Local and Global Reactivity Descriptors
- Ionization potential (IP) and electron affinity (EA): These fundamental quantities reflect a molecule’s tendency to lose or gain electrons and can be directly linked to oxidation or reduction potential in environmental reactions.
- Chemical hardness and softness: Hard molecules are generally less reactive, while soft molecules are more prone to undergo chemical transformations. These indices are derived from frontier orbital energies and provide a theoretical basis for pollutant persistence.
- Electrophilicity index (): This parameter quantifies a molecule’s overall tendency to accept electrons and engage in reactions with nucleophiles. Molecules with high ω may react rapidly with biological nucleophiles or natural reductants in aquatic systems.
- Chemical potential (): Indicates the escaping tendency of electrons from a molecule, closely related to molecular stability and charge transfer behavior.
4.3.3. Molecular Dynamics for Environmental Behavior
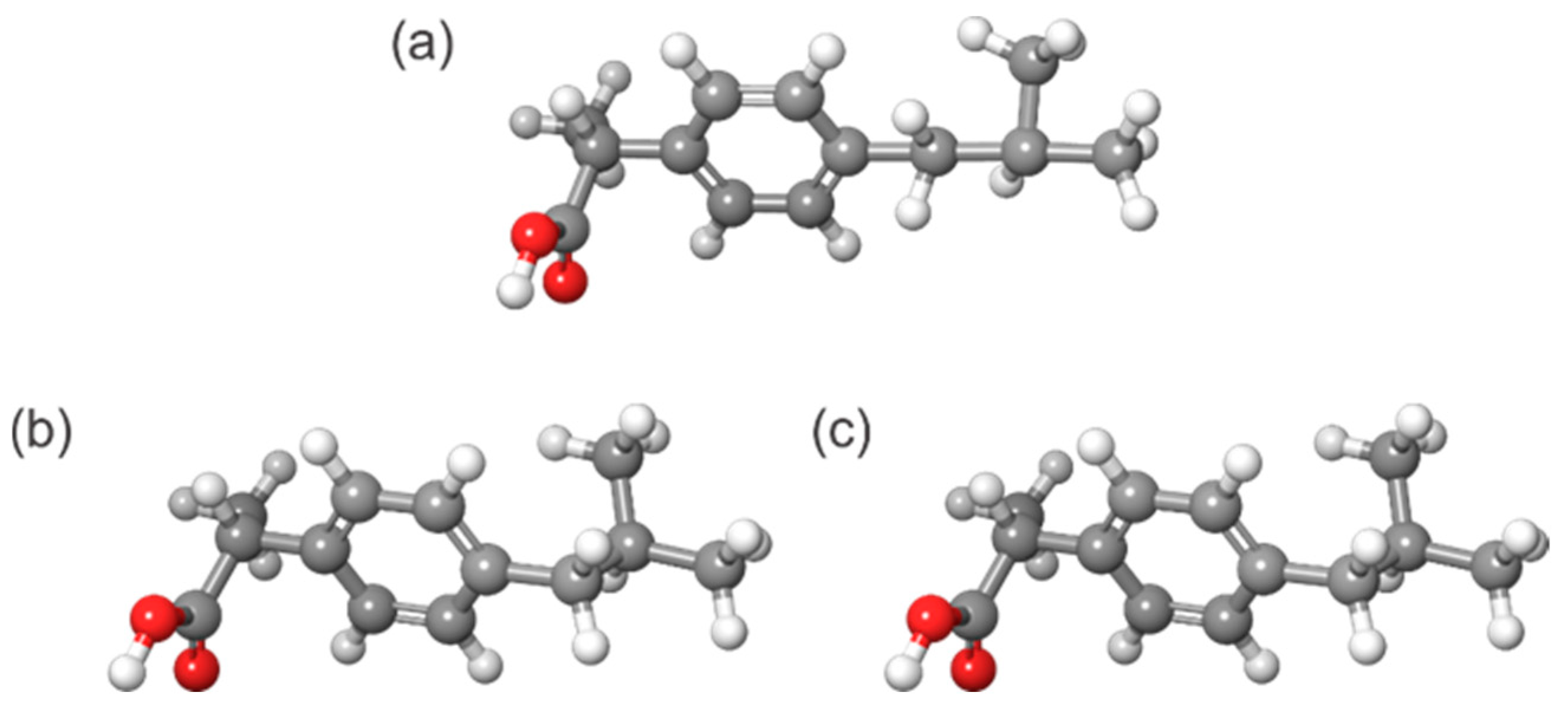
4.3.4. Searching Conformational Space
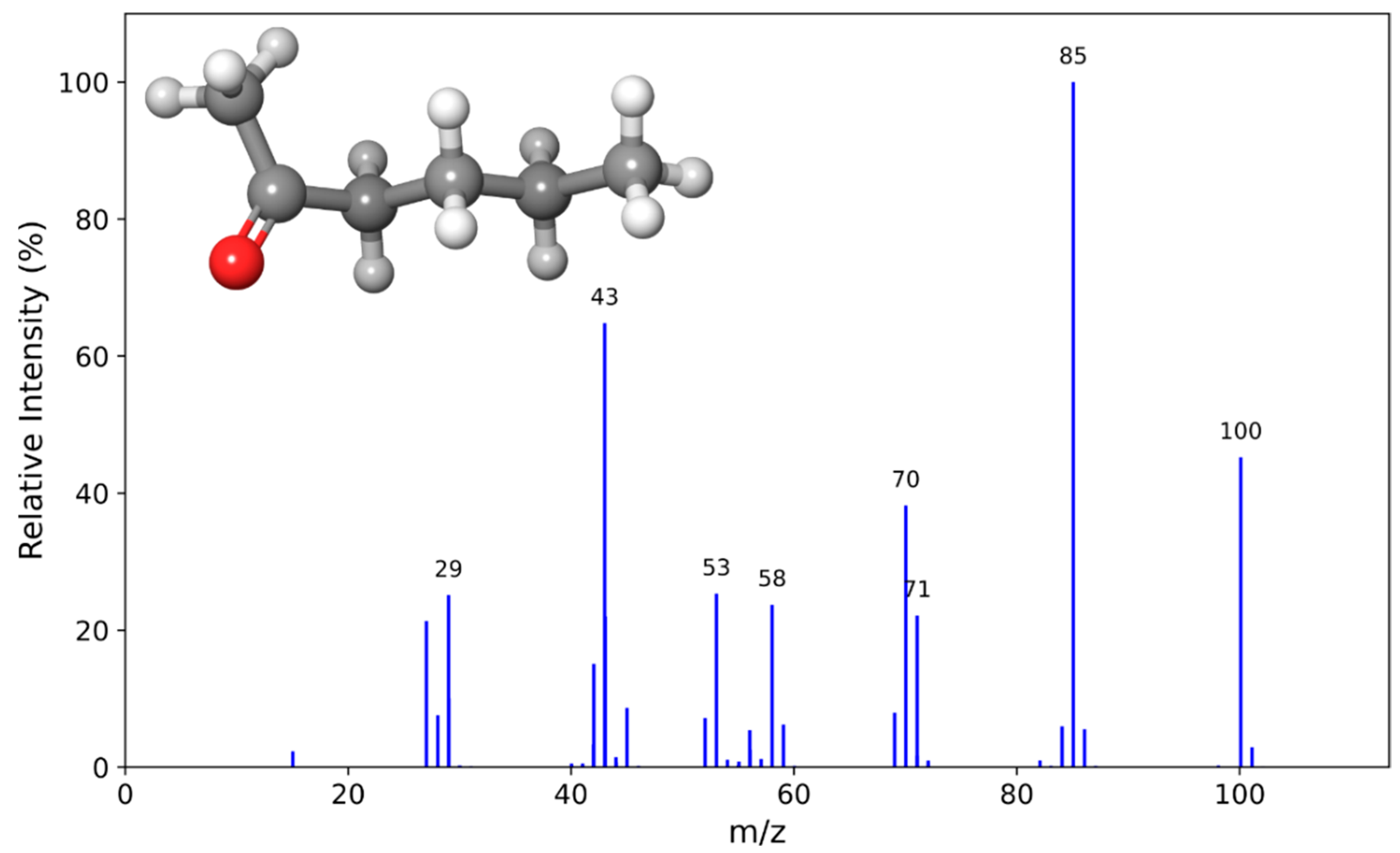
4.4. Simulation of MS Spectra Using QCxMS Codes
- EI: This mode is fully supported in both versions. It simulates ionization via an energetic (typically 70 eV) electron beam that produces an open-shell radical cation. The excess internal energy from ionization induces fragmentation and rearrangement events. QCxMS handles this through MD, while QCxMS2 employs a network-based approach to simulate possible reaction pathways following ionization.
- Dissociative Electron Attachment (DEA): In the DEA approach, a radical anion is generated. While this mode is technically supported in both QCxMS and QCxMS2, it requires DFT calculations and diffuse basis sets, significantly increasing computational resources necessary for calculations. Furthermore, the DEA in QCxMS2 remains experimental, and users are advised to proceed cautiously when applying it.
- CID: CID is widely used to fragment (de)protonated ions produced by electrospray ionization (ESI), offering a softer alternative to EI or DEA. Although QCxMS2 does not yet provide a dedicated CID mode, its EI mode can be tuned to approximate CID spectra by lowering internal energy distributions. However, these adjustments should be benchmarked against experiments, and for more robust CID predictions, the original QCxMS is currently recommended.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| Abbreviation | Meaning |
| ECs | Emerging contaminants |
| MS | Mass Spectrometry |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| VOCs | Volatile Organic Compounds |
| AI | Artificial Intelligence |
| POPs | Persistent Organic Pollutants |
| CECs | Contaminants of Emerging Concern |
| TIC | Total Ion Chromatogram |
| TIMS | Trapped Ion Mobility Spectrometry |
| ML | Machine Learning |
| HR–ToFMS | High-Resolution Time-of-Flight Mass Spectrometry |
| GC×GC | Two-Dimensional Gas Chromatography |
| EI | Electron Ionization |
| CI | Chemical Ionization |
| NTS | Non-Target Screening |
| PCA | Principal Component Analysis |
| PLS-DA | Partial Least Squares–Discriminant Analysis |
| HCA | Hierarchical Cluster Analysis |
| SVM | Support Vector Machine |
| RF | Random Forest |
| LLE | Liquid–Liquid Extraction |
| SPE | Solid Phase Extraction |
| SPME | Solid Phase Microextraction |
| SBSE | Stir Bar Sorptive Extraction |
| DLLME | Dispersive Liquid–Liquid Microextraction |
| MIPs | Molecularly Imprinted Polymers |
| SPME-GC | Solid Phase Microextraction coupled with Gas Chromatography |
| PFAS | Per- and Polyfluoroalkyl Substances |
| AISTI | All Ion Switching Tandem Ionization |
| HF | Hartree–Fock |
| CCSD | Coupled Cluster with Single and Double excitations |
| DFT | Density Functional Theory |
| PBE | Perdew–Burke–Ernzerhof (functional) |
| MNDO | Modified Neglect of Diatomic Overlap |
| AM1 | Austin Model 1 (semiempirical method) |
| PM3 and PM6 | Parametric Method 3 and 6 (semiempirical methods) |
| DFTB | Density Functional Tight Binding |
| GFN | Geometry, Frequency, Noncovalent |
| GFN2-xTB | Geometry, Frequency, Noncovalent extended Tight Binding |
| GFN-FF | Geometry, Frequency, Noncovalent–Force Field |
| SQM | Semiempirical Quantum Mechanics |
| MM | Molecular Mechanics |
| QM | Quantum Mechanics |
| ONIOM | Our Own N-layered Integrated molecular Orbital and molecular Mechanics |
| CAM | Coulomb-Attenuating Method |
| B3LYP/6-31+G(d,p) | Becke, 3-parameter, Lee–Yang–Parr functional with 6-31+G(d,p) basis set |
| LANL2DZ | Los Alamos National Laboratory 2 Double-Zeta basis set |
| BDEs | Bond Dissociation Energies |
| H-BDE | Hydrogen Bond Dissociation Energies |
| MEP | Molecular Electrostatic Potential |
| ALIE | Average Local Ionization Energy |
| HOMO-LUMO | Highest Occupied Molecular Orbital-Lowest Unoccupied Molecular Orbital |
| IP | Ionization Potential |
| EA | Electron Affinity |
| MD | Molecular Dynamics |
| HDX-MS | Hydrogen–Deuterium Exchange Mass Spectrometry |
| QCG | Quantum Cluster Growth |
| MSREACT | Mass Spectrometry Reaction (simulation tool) |
| QCxMS | Quantum Chemical Mass Spectrometry |
| BOMD | Born–Oppenheimer MD |
| DEA | Dissociative Electron Attachment |
References
- Vosough, M.; Schmidt, T.C.; Renner, G. Non-Target Screening in Water Analysis: Recent Trends of Data Evaluation, Quality Assurance, and Their Future Perspectives. Anal. Bioanal. Chem. 2024, 416, 2125–2136. [Google Scholar] [CrossRef]
- Houhou, R.; Bocklitz, T. Trends in Artificial Intelligence, Machine Learning, and Chemometrics Applied to Chemical Data. Anal. Sci. Adv. 2021, 2, 128–141. [Google Scholar] [CrossRef]
- Ganaie, M.I.; Jan, I.; Mayer, A.N.; Dar, A.A.; Mayer, I.A.; Ahmed, P.; Sofi, J.A. Health Risk Assessment of Pesticide Residues in Drinking Water of Upper Jhelum Region in Kashmir Valley-India by GC-MS/MS. Int. J. Anal. Chem. 2023, 2023, 6802782. [Google Scholar] [CrossRef]
- Moufid, M.; Hofmann, M.; El Bari, N.; Tiebe, C.; Bartholmai, M.; Bouchikhi, B. Wastewater Monitoring by Means of E-Nose, VE-Tongue, TD-GC-MS, and SPME-GC-MS. Talanta 2021, 221, 121450. [Google Scholar] [CrossRef]
- Guimarães, L.F.L.; da Silva, M.Z.F.; do Nascimento, R.F.; Alcântara, D.B. Method Validation and Determination of Ametryn Pesticide in Water Samples by QuEChERS-GC-MS. Chemosensors 2025, 13, 103. [Google Scholar] [CrossRef]
- Feng, W.; Deng, Y.; Yang, F.; Miao, Q.; Ngien, S.K. Systematic Review of Contaminants of Emerging Concern (CECs): Distribution, Risks, and Implications for Water Quality and Health. Water 2023, 15, 3922. [Google Scholar] [CrossRef]
- Lazofsky, A.; Buckley, B. Recent Trends in Multiclass Analysis of Emerging Endocrine Disrupting Contaminants (EDCs) in Drinking Water. Molecules 2022, 27, 8835. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xu, Y.; Xie, Y.; Yang, L.; Zhang, J. A Review of the Sources, Monitoring, Detection, and Removal of Typical Olfactory Substances Geosmin and 2-Methylisoborneol. Water 2025, 17, 1236. [Google Scholar] [CrossRef]
- Kumar, M.; Sridharan, S.; Sawarkar, A.D.; Shakeel, A.; Anerao, P.; Mannina, G.; Sharma, P.; Pandey, A. Current Research Trends on Emerging Contaminants Pharmaceutical and Personal Care Products (PPCPs): A Comprehensive Review. Sci. Total Environ. 2023, 859, 160031. [Google Scholar] [CrossRef]
- Schreiber, L.; Halko, R.; Santana-Viera, S.; Michalides, N.M.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Evaluation of European Watch List Contaminants in Environmental Matrices and Microplastics: Analytical Strategies, Mechanisms of Adsorption and Occurrence. Trends Environ. Anal. Chem. 2024, 44, e00245. [Google Scholar] [CrossRef]
- Ranjan Maji, S.; Roy, C.; Kumar Sinha, S. Gas Chromatography–Mass Spectrometry (GC-MS): A Comprehensive Review of Synergistic Combinations and Their Applications in the Past Two Decades. J. Anal. Sci. Appl. Biotechnol. 2023, 5, 72–85. [Google Scholar] [CrossRef]
- Santos, F.J.; Galceran, M.T. Modern Developments in Gas Chromatography–Mass Spectrometry-Based Environmental Analysis. J. Chromatogr. A 2003, 1000, 125–151. [Google Scholar] [CrossRef]
- Nika, M.-C.; Alygizakis, N.; Arvaniti, O.S.; Thomaidis, N.S. Non-Target Screening of Emerging Contaminants in Landfills: A Review. Curr. Opin. Environ. Sci. Health 2023, 32, 100430. [Google Scholar] [CrossRef]
- Smith, D.; Španěl, P.; Demarais, N.; Langford, V.S.; McEwan, M.J. Recent Developments and Applications of Selected Ion Flow Tube Mass Spectrometry (SIFT-MS). Mass. Spectrom. Rev. 2025, 44, 101–134. [Google Scholar] [CrossRef]
- Jia, W.; Liu, H.; Ma, Y.; Huang, G.; Liu, Y.; Zhao, B.; Xie, D.; Huang, K.; Wang, R. Reproducibility in Nontarget Screening (NTS) of Environmental Emerging Contaminants: Assessing Different HLB SPE Cartridges and Instruments. Sci. Total Environ. 2024, 912, 168971. [Google Scholar] [CrossRef]
- Duff, D.; Lennard, C.; Li, Y.; Doyle, C.; Edge, K.J.; Holland, I.; Lothridge, K.; Johnstone, P.; Beylerian, P.; Spikmans, V. Portable Gas Chromatography–Mass Spectrometry Method for the in-Field Screening of Organic Pollutants in Soil and Water at Pollution Incidents. Environ. Sci. Pollut. Res. 2023, 30, 93088–93102. [Google Scholar] [CrossRef]
- Ieda, T.; Hashimoto, S. GC × GC and Computational Strategies for Detecting and Analyzing Environmental Contaminants. TrAC Trends Anal. Chem. 2023, 165, 117118. [Google Scholar] [CrossRef]
- Cairoli, M.; Van Den Doel, A.; Postma, B.; Offermans, T.; Zemmelink, H.; Stroomberg, G.; Buydens, L.; Van Kollenburg, G.; Jansen, J. Monitoring Pollution Pathways in River Water by Predictive Path Modelling Using Untargeted GC-MS Measurements. npj Clean. Water 2023, 6, 48. [Google Scholar] [CrossRef]
- Lübeck, J.S.; Alexandrino, G.L.; Christensen, J.H. GC × GC–HRMS Nontarget Fingerprinting of Organic Micropollutants in Urban Freshwater Sediments. Environ. Sci. Eur. 2020, 32, 78. [Google Scholar] [CrossRef]
- Zaid, A.; Hassan, N.H.; Marriott, P.J.; Wong, Y.F. Comprehensive Two-Dimensional Gas Chromatography as a Bioanalytical Platform for Drug Discovery and Analysis. Pharmaceutics 2023, 15, 1121. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Sharmin, R.S.; Fiutowski, J.; Mishra, Y.K.; De Oliveira Hansen, R. Sensing Volatile Organic Compounds in Aquatic Samples: A Review. Water Supply 2024, 24, 3314–3325. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, H.; Song, N.; Liu, S.; Wang, Y.; Ye, F.; Ju, Y.; Jiao, S.; Shi, L. New Insight into Fate and Transport of Organic Compounds from Pollution Sources to Aquatic Environment Using Non-Targeted Screening: A Wastewater Treatment Plant Case Study. Sci. Total Environ. 2023, 863, 161031. [Google Scholar] [CrossRef]
- Liberatore, N.; Felizzato, G.; Mengali, S.; Viola, R.; Romolo, F.S. A Novel Signal Processing Approach Enabled by Machine Learning for the Detection and Identification of Chemical Warfare Agent Simulants Using a GC-QEPAS System. Forensic Sci. Res. 2025, 10, owaf002. [Google Scholar] [CrossRef]
- Feizi, N.; Hashemi-Nasab, F.S.; Golpelichi, F.; Saburouh, N.; Parastar, H. Recent Trends in Application of Chemometric Methods for GC-MS and GC × GC-MS-Based Metabolomic Studies. TrAC Trends Anal. Chem. 2021, 138, 116239. [Google Scholar] [CrossRef]
- Huang, T.-Y.; Chung Yu, J.C. Assessment of Artificial Intelligence to Detect Gasoline in Fire Debris Using HS-SPME-GC/MS and Transfer Learning. J. Forensic Sci. 2024, 69, 1222–1234. [Google Scholar] [CrossRef]
- Baccolo, G.; Quintanilla-Casas, B.; Vichi, S.; Augustijn, D.; Bro, R. From Untargeted Chemical Profiling to Peak Tables—A Fully Automated AI Driven Approach to Untargeted GC-MS. TrAC Trends Anal. Chem. 2021, 145, 116451. [Google Scholar] [CrossRef]
- Niarchos, G.; Alygizakis, N.; Carere, M.; Dulio, V.; Engwall, M.; Hyötyläinen, T.; Kallenborn, R.; Karakitsios, S.; Karakoltzidis, A.; Kärrman, A.; et al. Pioneering an Effect-Based Early Warning System for Hazardous Chemicals in the Environment. TrAC Trends Anal. Chem. 2024, 180, 117901. [Google Scholar] [CrossRef]
- Catarro, G.; Pelixo, R.; Feijó, M.; Rosado, T.; Socorro, S.; Araújo, A.R.T.S.; Gallardo, E. Analytical Approaches Using GC-MS for the Detection of Pollutants in Wastewater Towards Environmental and Human Health Benefits: A Comprehensive Review. Chemosensors 2025, 13, 253. [Google Scholar] [CrossRef]
- Licen, S.; Muzic, E.; Briguglio, S.; Tolloi, A.; Barbieri, P.; Giungato, P. Derivatized Volatile Organic Compound Characterization of Friulano Wine from Collio (Italy–Slovenia) by HS-SPME-GC-MS and Discrimination from Other Varieties by Chemometrics. Br. Food J. 2021, 123, 2844–2855. [Google Scholar] [CrossRef]
- Wong, S.L.; Ng, L.T.; Tan, J.; Pan, J. Screening Unknown Novel Psychoactive Substances Using GC–MS Based Machine Learning. Forensic Chem. 2023, 34, 100499. [Google Scholar] [CrossRef]
- Nie, W.; Alimujiang, S.; Zhang, Y.; Zhang, S.; Li, W. A Multi-Omics Approach Combining GC-MS, LC-MS, and FT-NIR with Chemometrics and Machine Learning for Metabolites Systematic Profiling and Geographical Origin Tracing of Artemisia Argyi Folium. J. Chromatogr. A 2025, 1757, 466138. [Google Scholar] [CrossRef]
- Gan, Y.; Yang, T.; Gu, W.; Guo, L.; Qiu, R.; Wang, S.; Zhang, Y.; Tang, M.; Yang, Z. Using HS-GC-MS and Flash GC e-Nose in Combination with Chemometric Analysis and Machine Learning Algorithms to Identify the Varieties, Geographical Origins and Production Modes of Atractylodes lancea. Ind. Crops Prod. 2024, 209, 117955. [Google Scholar] [CrossRef]
- Herruzo-Ruiz, A.M.; Peralbo-Molina, Á.; López, C.-M.; Michán, C.; Alhama, J.; Chicano-Gálvez, E. Mass Spectrometry Imaging in Environmental Monitoring: From a Scarce Existing Past to a Promising Future. Trends Environ. Anal. Chem. 2024, 42, e00228. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Wang, Y.; Ren, X.; Liu, X.; Dong, Y.; Ma, J.; Song, R.; Wei, J.; Yu, A.; et al. Rapid Discrimination and Screening of Volatile Markers for Varietal Recognition of Curcumae radix Using ATR-FTIR and HS-GC-MS Combined with Chemometrics. J. Ethnopharmacol. 2021, 280, 114422. [Google Scholar] [CrossRef]
- Song, K.; Guo, S.; Gong, Y.; Lv, D.; Wan, Z.; Zhang, Y.; Fu, Z.; Hu, K.; Lu, S. Non-Target Scanning of Organics from Cooking Emissions Using Comprehensive Two-Dimensional Gas Chromatography-Mass Spectrometer (GC × GC-MS). Appl. Geochem. 2023, 151, 105601. [Google Scholar] [CrossRef]
- Petrick, L.M.; Shomron, N. AI/ML-Driven Advances in Untargeted Metabolomics and Exposomics for Biomedical Applications. Cell Rep. Phys. Sci. 2022, 3, 100978. [Google Scholar] [CrossRef]
- Tufariello, M.; Pati, S.; Palombi, L.; Grieco, F.; Losito, I. Use of Multivariate Statistics in the Processing of Data on Wine Volatile Compounds Obtained by HS-SPME-GC-MS. Foods 2022, 11, 910. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Rankin, J.G.; Bondra, A.; Trader, C.; Heeren, A.; Harrington, P. de B. Ignitable Liquid Identification Using Gas Chromatography/Mass Spectrometry Data by Projected Difference Resolution Mapping and Fuzzy Rule-Building Expert System Classification. Forensic Sci. Int. 2012, 220, 210–218. [Google Scholar] [CrossRef]
- Barea-Sepúlveda, M.; Duarte, H.; Aliaño-González, M.J.; Romano, A.; Medronho, B. Total Ion Chromatogram and Total Ion Mass Spectrum as Alternative Tools for Detection and Discrimination (A Review). Chemosensors 2022, 10, 465. [Google Scholar] [CrossRef]
- Diera, T.; Thomsen, A.H.; Tisler, S.; Karlby, L.T.; Christensen, P.; Rosshaug, P.S.; Albrechtsen, H.-J.; Christensen, J.H. A Non-Target Screening Study of High-Density Polyethylene Pipes Revealed Rubber Compounds as Main Contaminant in a Drinking Water Distribution System. Water Res. 2023, 229, 119480. [Google Scholar] [CrossRef]
- Mok, S.; Lee, S.; Choi, Y.; Jeon, J.; Kim, Y.H.; Moon, H.-B. Target and Non-Target Analyses of Neutral per- and Polyfluoroalkyl Substances from Fluorochemical Industries Using GC-MS/MS and GC-TOF: Insights on Their Environmental Fate. Environ. Int. 2023, 182, 108311. [Google Scholar] [CrossRef]
- Mazur, D.M.; Detenchuk, E.A.; Sosnova, A.A.; Artaev, V.B.; Lebedev, A.T. GC-HRMS with Complementary Ionization Techniques for Target and Non-Target Screening for Chemical Exposure: Expanding the Insights of the Air Pollution Markers in Moscow Snow. Sci. Total Environ. 2021, 761, 144506. [Google Scholar] [CrossRef]
- Dąbrowski, Ł. Non-Target Screening of Chemicals in Selected Cotton Products by GC/MS and Their Safety Assessment. Molecules 2024, 29, 3584. [Google Scholar] [CrossRef]
- Wojnowski, W.; Kalinowska, K.; Majchrzak, T.; Zabiegała, B. Real-Time Monitoring of the Emission of Volatile Organic Compounds from Polylactide 3D Printing Filaments. Sci. Total Environ. 2022, 805, 150181. [Google Scholar] [CrossRef]
- Meurs, J.; Sakkoula, E.; Cristescu, S.M. Real-Time Non-Invasive Monitoring of Short-Chain Fatty Acids in Exhaled Breath. Front. Chem. 2022, 10, 853541. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-L.; Wang, X.-C.; Zhang, J.-N.; Liu, J.-N.; Ma, M.-H.; Ma, F.-L.; Lv, Y.; Yu, Y.-J.; She, Y. A Study of Flavor Variations during the Flaxseed Roasting Procedure by Developed Real-Time SPME GC–MS Coupled with Chemometrics. Food Chem. 2023, 410, 135453. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, C.; Shao, H.; Zheng, Q. Non-Targeted Screening and Analysis of Volatile Organic Compounds in Drinking Water by DLLME with GC–MS. Sci. Total Environ. 2019, 694, 133494. [Google Scholar] [CrossRef]
- Jirayupat, C.; Nagashima, K.; Hosomi, T.; Takahashi, T.; Tanaka, W.; Samransuksamer, B.; Zhang, G.; Liu, J.; Kanai, M.; Yanagida, T. Image Processing and Machine Learning for Automated Identification of Chemo-/Biomarkers in Chromatography–Mass Spectrometry. Anal. Chem. 2021, 93, 14708–14715. [Google Scholar] [CrossRef]
- Izquierdo-Sandoval, D.; Sancho, J.V.; Hernández, F.; Portoles, T. Approaches for GC-HRMS Screening of Organic Microcontaminants: GC-APCI-IMS-QTOF versus GC-EI-QOrbitrap. Environ. Sci. Technol. 2025, 59, 2436–2448. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Lee, J.; Lim, C.-U.; Ahn, J. Quantification of Pesticides in Food Crops Using QuEChERS Approaches and GC-MS/MS. Food Addit. Contam. Part A 2016, 33, 1803–1816. [Google Scholar] [CrossRef]
- Elmastas, A.; Umaz, A.; Pirinc, V.; Aydin, F. Quantitative Determination and Removal of Pesticide Residues in Fresh Vegetables and Fruit Products by LC–MS/MS and GC–MS/MS. Environ. Monit. Assess. 2023, 195, 277. [Google Scholar] [CrossRef]
- Yıldırım, İ.; Çiftçi, U. Monitoring of Pesticide Residues in Peppers from Çanakkale (Turkey) Public Market Using QuEChERS Method and LC–MS/MS and GC–MS/MS Detection. Environ. Monit. Assess. 2022, 194, 570. [Google Scholar] [CrossRef]
- Tsiantas, P.; Bempelou, E.; Doula, M.; Karasali, H. Validation and Simultaneous Monitoring of 311 Pesticide Residues in Loamy Sand Agricultural Soils by LC-MS/MS and GC-MS/MS, Combined with QuEChERS-Based Extraction. Molecules 2023, 28, 4268. [Google Scholar] [CrossRef]
- Hakami, R.A.; Aqel, A.; Ghfar, A.A.; ALOthman, Z.A.; Badjah-Hadj-Ahmed, A.-Y. Development of QuEChERS Extraction Method for the Determination of Pesticide Residues in Cereals Using DART-ToF-MS and GC-MS Techniques. Correlation and Quantification Study. J. Food Compos. Anal. 2021, 98, 103822. [Google Scholar] [CrossRef]
- Migowska, N.; Caban, M.; Stepnowski, P.; Kumirska, J. Simultaneous Analysis of Non-Steroidal Anti-Inflammatory Drugs and Estrogenic Hormones in Water and Wastewater Samples Using Gas Chromatography–Mass Spectrometry and Gas Chromatography with Electron Capture Detection. Sci. Total Environ. 2012, 441, 77–88. [Google Scholar] [CrossRef]
- Gumbi, B.P.; Moodley, B.; Birungi, G.; Ndungu, P.G. Detection and Quantification of Acidic Drug Residues in South African Surface Water Using Gas Chromatography-Mass Spectrometry. Chemosphere 2017, 168, 1042–1050. [Google Scholar] [CrossRef]
- Perin, M.; Dallegrave, A.; Suchecki Barnet, L.; Zanchetti Meneghini, L.; de Araújo Gomes, A.; Pizzolato, T.M. Pharmaceuticals, Pesticides and Metals/Metalloids in Lake Guaíba in Southern Brazil: Spatial and Temporal Evaluation and a Chemometrics Approach. Sci. Total Environ. 2021, 793, 148561. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.H.M.L.M.; Insa, S.; Arxé, M.; Buttiglieri, G.; Rodríguez-Mozaz, S.; Barceló, D. Analysis of Microplastics in the Environment: Identification and Quantification of Trace Levels of Common Types of Plastic Polymers Using Pyrolysis-GC/MS. MethodsX 2023, 10, 102143. [Google Scholar] [CrossRef] [PubMed]
- Ishimura, T.; Iwai, I.; Matsui, K.; Mattonai, M.; Watanabe, A.; Robberson, W.; Cook, A.-M.; Allen, H.L.; Pipkin, W.; Teramae, N.; et al. Qualitative and Quantitative Analysis of Mixtures of Microplastics in the Presence of Calcium Carbonate by Pyrolysis-GC/MS. J. Anal. Appl. Pyrolysis 2021, 157, 105188. [Google Scholar] [CrossRef]
- Garcia, M.A.; Liu, R.; Nihart, A.; El Hayek, E.; Castillo, E.; Barrozo, E.R.; Suter, M.A.; Bleske, B.; Scott, J.; Forsythe, K.; et al. Quantitation and Identification of Microplastics Accumulation in Human Placental Specimens Using Pyrolysis Gas Chromatography Mass Spectrometry. Toxicol. Sci. 2024, 199, 81–88. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Rauert, C.; Charlton, N.; Bagley, A.; Dunlop, S.A.; Symeonides, C.; Thomas, K.V. Assessing the Efficacy of Pyrolysis–Gas Chromatography–Mass Spectrometry for Nanoplastic and Microplastic Analysis in Human Blood. Environ. Sci. Technol. 2025, 59, 1984–1994. [Google Scholar] [CrossRef]
- Feng, X.; Wang, H.; Wang, Z.; Huang, P.; Kan, J. Discrimination and Characterization of the Volatile Organic Compounds in Eight Kinds of Huajiao with Geographical Indication of China Using Electronic Nose, HS-GC-IMS and HS-SPME-GC–MS. Food Chem. 2022, 375, 131671. [Google Scholar] [CrossRef]
- Nie, S.; Li, L.; Wang, Y.; Wu, Y.; Li, C.; Chen, S.; Zhao, Y.; Wang, D.; Xiang, H.; Wei, Y. Discrimination and Characterization of Volatile Organic Compound Fingerprints during Sea Bass (Lateolabrax japonicas) Fermentation by Combining GC-IMS and GC-MS. Food Biosci. 2022, 50, 102048. [Google Scholar] [CrossRef]
- Bajo-Fernández, M.; Souza-Silva, É.A.; Barbas, C.; Rey-Stolle, M.F.; García, A. GC-MS-Based Metabolomics of Volatile Organic Compounds in Exhaled Breath: Applications in Health and Disease. A Review. Front. Mol. Biosci. 2024, 10, 1295955. [Google Scholar] [CrossRef]
- Yuan, N.; Chi, X.; Ye, Q.; Liu, H.; Zheng, N. Analysis of Volatile Organic Compounds in Milk during Heat Treatment Based on E-Nose, E-Tongue and HS-SPME-GC-MS. Foods 2023, 12, 1071. [Google Scholar] [CrossRef]
- Schanzmann, H.; Gaar, S.; Keip, S.; Telgheder, U.; Sielemann, S. Comparison of the Quantification Performance of Thermal Desorption GC-IMS and GC-MS in VOC Analysis. Anal. Bioanal. Chem. 2025, 417, 4179–4198. [Google Scholar] [CrossRef] [PubMed]
- Vaye, O.; Ngumbu, R.S.; Xia, D. A Review of the Application of Comprehensive Two-Dimensional Gas Chromatography MS-Based Techniques for the Analysis of Persistent Organic Pollutants and Ultra-Trace Level of Organic Pollutants in Environmental Samples. Rev. Anal. Chem. 2022, 41, 63–73. [Google Scholar] [CrossRef]
- Maurin, N.; Sayen, S.; Guillon, E. Gas Chromatography–Mass Spectrometry Analysis of Organic Pollutants in French Soils Irrigated with Agro-Industrial Wastewater. Front. Environ. Sci. 2023, 11, 1125487. [Google Scholar] [CrossRef]
- Chowdhary, P.; Singh, A.; Chandra, R.; Kumar, P.S.; Raj, A.; Bharagava, R.N. Detection and Identification of Hazardous Organic Pollutants from Distillery Wastewater by GC-MS Analysis and Its Phytotoxicity and Genotoxicity Evaluation by Using Allium cepa and Cicer arietinum L. Chemosphere 2022, 297, 134123. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, B.; Dong, J.; Tian, Y.; Lin, Y.; Fang, G.; Wang, S. Identification of Mouldy Rice Using an Electronic Nose Combined with SPME-GC/MS. J. Stored Prod. Res. 2022, 95, 101921. [Google Scholar] [CrossRef]
- Chen, W.; Zou, Y.; Mo, W.; Di, D.; Wang, B.; Wu, M.; Huang, Z.; Hu, B. Onsite Identification and Spatial Distribution of Air Pollutants Using a Drone-Based Solid-Phase Microextraction Array Coupled with Portable Gas Chromatography-Mass Spectrometry via Continuous-Airflow Sampling. Environ. Sci. Technol. 2022, 56, 17100–17107. [Google Scholar] [CrossRef]
- Vu-Duc, N.; Phung Thi, L.A.; Le-Minh, T.; Nguyen, L.-A.; Nguyen-Thi, H.; Pham-Thi, L.-H.; Doan-Thi, V.-A.; Le-Quang, H.; Nguyen-Xuan, H.; Thi Nguyen, T.; et al. Analysis of Polycyclic Aromatic Hydrocarbon in Airborne Particulate Matter Samples by Gas Chromatography in Combination with Tandem Mass Spectrometry (GC-MS/MS). J. Anal. Methods Chem. 2021, 2021, 6641326. [Google Scholar] [CrossRef] [PubMed]
- Martinello, M.; Manzinello, C.; Dainese, N.; Giuliato, I.; Gallina, A.; Mutinelli, F. The Honey Bee: An Active Biosampler of Environmental Pollution and a Possible Warning Biomarker for Human Health. Appl. Sci. 2021, 11, 6481. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Owczarek, K.; Namieśnik, J. Modern Solutions in the Field of Microextraction Using Liquid as a Medium of Extraction. TrAC Trends Anal. Chem. 2016, 85, 46–64. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; De La Guardia, M.; Namieśnik, J. Modern Trends in Solid Phase Extraction: New Sorbent Media. TrAC Trends Anal. Chem. 2016, 77, 23–43. [Google Scholar] [CrossRef]
- Peñalver, R.; Ortiz, A.; Arroyo-Manzanares, N.; Campillo, N.; López-García, I.; Viñas, P. Non-Targeted Analysis by DLLME-GC-MS for the Monitoring of Pollutants in the Mar Menor Lagoon. Chemosphere 2022, 286, 131588. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Y.; Dong, K.; Zhao, B.; Tang, S.; Nie, X.; Yan, Y. Advances in Rapid Detection of Volatile Organic Compounds (VOCs): From Conventional Techniques to Surface-Enhanced Raman Spectroscopy. Results Chem. 2025, 16, 102329. [Google Scholar] [CrossRef]
- Pardina, D.; Santamaria, A.; Alonso, M.L.; Bartolomé, L.; Alonso, R.M.; Maña, J.A.; Bilbao, E.; Lombraña, J.I.; Bartolome, M.; Hernando, L.M. HS-SPME-GC/MS Method for the Simultaneous Determination of Trihalomethanes, Geosmin, and 2-Methylisoborneol in Water Samples. Chemosensors 2023, 11, 84. [Google Scholar] [CrossRef]
- Ferracane, A.; Aloisi, I.; Galletta, M.; Zoccali, M.; Tranchida, P.Q.; Micalizzi, G.; Mondello, L. Automated Sample Preparation and Fast GC–MS Determination of Fatty Acids in Blood Samples and Dietary Supplements. Anal. Bioanal. Chem. 2022, 414, 8423–8435. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Xu, Z.; Zhang, H.; Liu, D.; Yang, Q.; Tao, Q.; Wen, M.; Kang, X.; Zhang, Z.; Lu, H. Fully Automatic Resolution of Untargeted GC-MS Data with Deep Learning Assistance. Talanta 2022, 244, 123415. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.L.; de la Mata, A.P.; Harynuk, J.J. Automated Screening and Filtering Scripts for GC × GC-TOFMS Metabolomics Data. Separations 2021, 8, 84. [Google Scholar] [CrossRef]
- Kumar, R.; Parashar, A. Atomistic Simulations of Pristine and Nanoparticle Reinforced Hydrogels: A Review. WIREs Comput. Mol. Sci. 2023, 13, e1655. [Google Scholar] [CrossRef]
- Bahraq, A.A.; Al-Osta, M.A.; Al-Amoudi, O.S.B.; Saleh, T.A.; Obot, I.B. Atomistic Simulation of Polymer-Cement Interactions: Progress and Research Challenges. Constr. Build. Mater. 2022, 327, 126881. [Google Scholar] [CrossRef]
- Ward, L.; Wolverton, C. Atomistic Calculations and Materials Informatics: A Review. Curr. Opin. Solid. State Mater. Sci. 2017, 21, 167–176. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Ross Kunz, M.; He, X.; Fushimi, R. Recent Progress toward Catalyst Properties, Performance, and Prediction with Data-Driven Methods. Curr. Opin. Chem. Eng. 2022, 37, 100843. [Google Scholar] [CrossRef]
- Shambhawi; Mohan, O.; Choksi, T.S.; Lapkin, A.A. The Design and Optimization of Heterogeneous Catalysts Using Computational Methods. Catal. Sci. Technol. 2024, 14, 515–532. [Google Scholar] [CrossRef]
- Thomas, R.; Mary, Y.S.; Resmi, K.S.; Narayana, B.; Sarojini, S.B.K.; Armaković, S.; Armaković, S.J.; Vijayakumar, G.; Alsenoy, C.V.; Mohan, B.J. Synthesis and Spectroscopic Study of Two New Pyrazole Derivatives with Detailed Computational Evaluation of Their Reactivity and Pharmaceutical Potential. J. Mol. Struct. 2019, 1181, 599–612. [Google Scholar] [CrossRef]
- Haruna, K.; Kumar, V.S.; Armaković, S.J.; Armaković, S.; Mary, Y.S.; Thomas, R.; Popoola, S.A.; Almohammedi, A.R.; Roxy, M.S.; Al-Saadi, A.A. Spectral Characterization, Thermochemical Studies, Periodic SAPT Calculations and Detailed Quantum Mechanical Profiling Various Physico-Chemical Properties of 3,4-Dichlorodiuron. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117580. [Google Scholar] [CrossRef] [PubMed]
- Bielenica, A.; Beegum, S.; Mary, Y.S.; Mary, Y.S.; Thomas, R.; Armaković, S.; Armaković, S.J.; Madeddu, S.; Struga, M.; Van Alsenoy, C. Experimental and Computational Analysis of 1-(4-Chloro-3-Nitrophenyl)-3-(3,4-Dichlorophenyl)Thiourea. J. Mol. Struct. 2020, 1205, 127587. [Google Scholar] [CrossRef]
- Aghdasi, P.; Yousefi, S.; Ansari, R. A DFT Investigation on the Mechanical and Structural Properties of Halogen- and Metal-Adsorbed Silicene Nanosheets. Mater. Chem. Phys. 2022, 283, 126029. [Google Scholar] [CrossRef]
- El-Sayed, D.S. Electronic Band Structure and Density of State Modulation of Amphetamine and ABW Type–Zeolite Adsorption System: DFT-CASTEP Analysis. J. Mol. Model. 2023, 29, 96. [Google Scholar] [CrossRef]
- Bai, Z.; Lan, M.; Yu, W.; Shen, S. Predicting Strain Effects on Adsorption Energy Based on Atomistic Structure and Density of States. Int. J. Mech. Sci. 2025, 294, 110234. [Google Scholar] [CrossRef]
- Yang, B.; Uphoff, M.; Zhang, Y.-Q.; Reichert, J.; Seitsonen, A.P.; Bauer, A.; Pfleiderer, C.; Barth, J.V. Atomistic Investigation of Surface Characteristics and Electronic Features at High-Purity FeSi(110) Presenting Interfacial Metallicity. Proc. Natl. Acad. Sci. USA 2021, 118, e2021203118. [Google Scholar] [CrossRef]
- Gomez, H.; Groves, M.N.; Neupane, M.R. Study of the Structural Phase Transition in Diamond (100) & (111) Surfaces. Carbon. Trends 2021, 3, 100033. [Google Scholar] [CrossRef]
- Stampelou, M.; Ladds, G.; Kolocouris, A. Computational Workflow for Refining AlphaFold Models in Drug Design Using Kinetic and Thermodynamic Binding Calculations: A Case Study for the Unresolved Inactive Human Adenosine A3 Receptor. J. Phys. Chem. B 2024, 128, 914–936. [Google Scholar] [CrossRef]
- Sellner, M.; Fischer, A.; Don, C.G.; Smieško, M. Conformational Landscape of Cytochrome P450 Reductase Interactions. Int. J. Mol. Sci. 2021, 22, 1023. [Google Scholar] [CrossRef]
- Friedman, R. Computational Studies of Protein–Drug Binding Affinity Changes upon Mutations in the Drug Target. WIREs Comput. Mol. Sci. 2022, 12, e1563. [Google Scholar] [CrossRef]
- Chu, W.-T.; Yan, Z.; Chu, X.; Zheng, X.; Liu, Z.; Xu, L.; Zhang, K.; Wang, J. Physics of Biomolecular Recognition and Conformational Dynamics. Rep. Prog. Phys. 2021, 84, 126601. [Google Scholar] [CrossRef]
- Mishra, S.B.; Marutheeswaran, S.; Roy, S.C.; Natarajan, V.; Rai, P.K.; Nanda, B.R.K. Adsorption and Degradation Mechanism of 2,4,6-Trinitrotoluene on TiO2 (110) Surface. Surf. Sci. 2021, 713, 121902. [Google Scholar] [CrossRef]
- Shen, L.; Yu, X.; Li, M.; Deng, S.; Cao, H. The In-Situ Generation of ClO• by Single- and Dual-Atom Catalysis of Chloride Ions to Degrade Sulfonamide Antibiotics: A DFT Study. Chem. Eng. J. 2024, 485, 149719. [Google Scholar] [CrossRef]
- Ma, Q.; Ming, J.; Sun, X.; Zhang, H.; An, G.; Kawazoe, N.; Chen, G.; Yang, Y. Photocatalytic Degradation of Multiple-Organic-Pollutant under Visible Light by Graphene Oxide Modified Composite: Degradation Pathway, DFT Calculation and Mechanism. J. Environ. Manag. 2023, 347, 119128. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yang, J.; Zhu, C.; Fang, Q.; Song, S.; Chen, B. Mechanistic Insights into the Atomic Distance Effect on Adsorption and Degradation of Aromatic Compounds. ACS Catal. 2023, 13, 8943–8954. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, C.; Wu, Y.; Li, X.; Zhang, J.; Liang, J.; Li, Y. Adsorption of Tetracycline by Polycationic Straw: Density Functional Theory Calculation for Mechanism and Machine Learning Prediction for Tetracyclines’ Remediation. Environ. Pollut. 2024, 340, 122869. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, J.; Zhang, W.; Yang, Y.; Guo, D.; Zhang, H.; Liu, L. Reveal the Main Factors and Adsorption Behavior Influencing the Adsorption of Pollutants on Natural Mineral Adsorbents: Based on Machine Learning Modeling and DFT Calculation. Sep. Purif. Technol. 2024, 331, 125706. [Google Scholar] [CrossRef]
- Lyshchuk, H.; Verkhovtsev, A.V.; Kočišek, J.; Fedor, J.; Solov’yov, A.V. Release of Neutrals in Electron-Induced Ligand Separation from MeCpPtMe3: Theory Meets Experiment. J. Phys. Chem. A 2025, 129, 2016–2023. [Google Scholar] [CrossRef]
- Kurzydym, I.; Błaziak, A.; Podgórniak, K.; Kułacz, K.; Błaziak, K. Mechanistic Insight into the Kinetic Fragmentation of Norpinonic Acid in the Gas Phase: An Experimental and Density Functional Theory (DFT) Study. Atmos. Chem. Phys. 2024, 24, 9309–9322. [Google Scholar] [CrossRef]
- Eskandari, M.; Faraz, S.M.; Hosseini, S.E.; Moradi, S.; Saeidian, H. Electron Ionization Mass Spectrometry Fragmentation Routes of Chemical Weapons Convention-Related Organoarsenic Compounds: Electron Ionization and Density Functional Theory Studies. Rapid Commun. Mass. Spectrom. 2023, 37, e9511. [Google Scholar] [CrossRef]
- Sandoval-Pauker, C.; Yin, S.; Castillo, A.; Ocuane, N.; Puerto-Diaz, D.; Villagrán, D. Computational Chemistry as Applied in Environmental Research: Opportunities and Challenges. ACS EST Eng. 2024, 4, 66–95. [Google Scholar] [CrossRef]
- Armaković, S.J.; Armaković, S.; Savanović, M.M. Photocatalytic Application of Polymers in Removing Pharmaceuticals from Water: A Comprehensive Review. Catalysts 2024, 14, 447. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate Spin-Dependent Electron Liquid Correlation Energies for Local Spin Density Calculations: A Critical Analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple [Phys. Rev. Lett. 77, 3865 (1996)]. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density Functionals with Broad Applicability in Chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef]
- Valero, R.; Costa, R.; Moreira, I.d.P.R.; Truhlar, D.G.; Illas, F. Performance of the M06 Family of Exchange-Correlation Functionals for Predicting Magnetic Coupling in Organic and Inorganic Molecules. J. Chem. Phys. 2008, 128, 114103. [Google Scholar] [CrossRef]
- Jacquemin, D.; Perpète, E.A.; Ciofini, I.; Adamo, C.; Valero, R.; Zhao, Y.; Truhlar, D.G. On the Performances of the M06 Family of Density Functionals for Electronic Excitation Energies. J. Chem. Theory Comput. 2010, 6, 2071–2085. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Systematic Optimization of Long-Range Corrected Hybrid Density Functionals. J. Chem. Phys. 2008, 128, 084106. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-Range Corrected Hybrid Density Functionals with Damped Atom–Atom Dispersion Corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Bannwarth, C.; Caldeweyher, E.; Ehlert, S.; Hansen, A.; Pracht, P.; Seibert, J.; Spicher, S.; Grimme, S. Extended Tight-Binding Quantum Chemistry Methods. WIREs Comput. Mol. Sci. 2021, 11, e1493. [Google Scholar] [CrossRef]
- Dral, P.O.; Zubatiuk, T. Chapter 24—Improving Semiempirical Quantum Mechanical Methods with Machine Learning. In Quantum Chemistry in the Age of Machine Learning; Dral, P.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 559–575. ISBN 978-0-323-90049-2. [Google Scholar]
- Dral, P.O.; Řezáč, J. Chapter 3—Semiempirical Quantum Mechanical Methods. In Quantum Chemistry in the Age of Machine Learning; Dral, P.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 67–92. ISBN 978-0-323-90049-2. [Google Scholar]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods IV: Extension of MNDO, AM1, and PM3 to More Main Group Elements. J. Mol. Model. 2004, 10, 155–164. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods II. Applications. J. Comput. Chem. 1989, 10, 221–264. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods I. Method. J. Comput. Chem. 1989, 10, 209–220. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods VI: More Modifications to the NDDO Approximations and Re-Optimization of Parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef]
- Köhler, C.; Seifert, G.; Frauenheim, T. Density Functional Based Calculations for Fen (n ≤ 32). Chem. Phys. 2005, 309, 23–31. [Google Scholar] [CrossRef]
- Elstner, M.; Porezag, D.; Jungnickel, G.; Elsner, J.; Haugk, M.; Frauenheim, T.; Suhai, S.; Seifert, G. Self-Consistent-Charge Density-Functional Tight-Binding Method for Simulations of Complex Materials Properties. Phys. Rev. B 1998, 58, 7260–7268. [Google Scholar] [CrossRef]
- Seifert, G.; Porezag, D.; Frauenheim, T. Calculations of Molecules, Clusters, and Solids with a Simplified LCAO-DFT-LDA Scheme. Int. J. Quantum Chem. 1996, 58, 185–192. [Google Scholar] [CrossRef]
- Porezag, D.; Frauenheim, T.; Köhler, T.; Seifert, G.; Kaschner, R. Construction of Tight-Binding-like Potentials on the Basis of Density-Functional Theory: Application to Carbon. Phys. Rev. B 1995, 51, 12947–12957. [Google Scholar] [CrossRef]
- Ehlert, S.; Stahn, M.; Spicher, S.; Grimme, S. Robust and Efficient Implicit Solvation Model for Fast Semiempirical Methods. J. Chem. Theory Comput. 2021, 17, 4250–4261. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB—An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef]
- Grimme, S.; Bannwarth, C.; Shushkov, P. A Robust and Accurate Tight-Binding Quantum Chemical Method for Structures, Vibrational Frequencies, and Noncovalent Interactions of Large Molecular Systems Parametrized for All Spd-Block Elements (Z = 1–86). J. Chem. Theory Comput. 2017, 13, 1989–2009. [Google Scholar] [CrossRef] [PubMed]
- Spicher, S.; Grimme, S. Robust Atomistic Modeling of Materials, Organometallic, and Biochemical Systems. Angew. Chem. Int. Ed. 2020, 59, 15665–15673. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. Software Update: The ORCA Program System—Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Vreven, T.; Byun, K.S.; Komáromi, I.; Dapprich, S.; Montgomery, J.A., Jr.; Morokuma, K.; Frisch, M.J. Combining Quantum Mechanics Methods with Molecular Mechanics Methods in ONIOM. J. Chem. Theory Comput. 2006, 2, 815–826. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Poi, R.; Baskey Sen, M.; Mandal, S.; Hazra, D.K.; Karmakar, R. Efficient Fabrication of pH-Modified Graphene Nano-Adsorbent for Effective Determination and Monitoring of Multi-Class Pesticide Residues in Market-Fresh Vegetables by GC-MS. J. Food Compos. Anal. 2023, 118, 105153. [Google Scholar] [CrossRef]
- Riyaz, M.; Goel, N. A QM/MM Study to Investigate Selectivity of Nanoporous Graphene Membrane for Arsenate and Chromate Removal from Water. Chem. Phys. Lett. 2017, 685, 371–376. [Google Scholar] [CrossRef]
- Mollaamin, F.; Monajjemi, M. Graphene Embedded with Transition Metals for Capturing Carbon Dioxide: Gas Detection Study Using QM Methods. Clean. Technol. 2023, 5, 403–417. [Google Scholar] [CrossRef]
- Turney, J.M.; Simmonett, A.C.; Parrish, R.M.; Hohenstein, E.G.; Evangelista, F.A.; Fermann, J.T.; Mintz, B.J.; Burns, L.A.; Wilke, J.J.; Abrams, M.L.; et al. Psi4: An Open-Source Ab Initio Electronic Structure Program. WIREs Comput. Mol. Sci. 2012, 2, 556–565. [Google Scholar] [CrossRef]
- Parrish, R.M.; Burns, L.A.; Smith, D.G.A.; Simmonett, A.C.; DePrince, A.E.I.; Hohenstein, E.G.; Bozkaya, U.; Sokolov, A.Y.; Di Remigio, R.; Richard, R.M.; et al. Psi4 1.1: An Open-Source Electronic Structure Program Emphasizing Automation, Advanced Libraries, and Interoperability. J. Chem. Theory Comput. 2017, 13, 3185–3197. [Google Scholar] [CrossRef]
- Smith, D.G.A.; Burns, L.A.; Simmonett, A.C.; Parrish, R.M.; Schieber, M.C.; Galvelis, R.; Kraus, P.; Kruse, H.; Di Remigio, R.; Alenaizan, A.; et al. PSI4 1.4: Open-Source Software for High-Throughput Quantum Chemistry. J. Chem. Phys. 2020, 152, 184108. [Google Scholar] [CrossRef]
- Lu, T. A Comprehensive Electron Wavefunction Analysis Toolbox for Chemists, Multiwfn. J. Chem. Phys. 2024, 161, 082503. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, Q. Van Der Waals Potential: An Important Complement to Molecular Electrostatic Potential in Studying Intermolecular Interactions. J. Mol. Model. 2020, 26, 315. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Manzetti, S. Wavefunction and Reactivity Study of Benzo[a]Pyrene Diol Epoxide and Its Enantiomeric Forms. Struct Chem 2014, 25, 1521–1533. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Froitzheim, T.; Müller, M.; Hansen, A.; Grimme, S. G-xTB: A General-Purpose Extended Tight-Binding Electronic Structure Method For the Elements H to Lr (Z=1–103). ChemRxiv 2025. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; in ’t Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS—A Flexible Simulation Tool for Particle-Based Materials Modeling at the Atomic, Meso, and Continuum Scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Páll, S.; Abraham, M.J.; Kutzner, C.; Hess, B.; Lindahl, E. Tackling Exascale Software Challenges in Molecular Dynamics Simulations with GROMACS. In Proceedings of the Solving Software Challenges for Exascale, EASC 2014, Stockholm, Sweden, 2–3 April 2014; Markidis, S., Laure, E., Eds.; Springer: Cham, Switzerland, 2015; pp. 3–27. [Google Scholar]
- Bekker, H.; Berendsen, H.; Dijkstra, E.; Achterop, S.; Vondrumen, R.; Vanderspoel, D.; Sijbers, A.; Keegstra, H.; Renardus, M. Gromacs—A Parallel Computer for Molecular-Dynamics Simulations. In Proceedings of the 4th International Conference on Computational Physics (PC 92), Prague, Czech Republic, 24–28 August 1992; World Scientific Publishing: Singapore, 1993; pp. 252–256. [Google Scholar]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Pronk, S.; Páll, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; van der Spoel, D.; et al. GROMACS 4.5: A High-Throughput and Highly Parallel Open Source Molecular Simulation Toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef]
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, Flexible, and Free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Lindahl, E.; Hess, B.; Van Der Spoel, D. GROMACS 3.0: A Package for Molecular Simulation and Trajectory Analysis. J. Mol. Model. 2001, 7, 306–317. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; van der Spoel, D.; van Drunen, R. GROMACS: A Message-Passing Parallel Molecular Dynamics Implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Sohrabi, S.; Rahimi, P.; Khedri, M.; Heydari, R.; Mirzaei, M.; Bahrami, A.; Akhlaghian, F.; Taghipoor, M. Evaluation of Machine Learning and Molecular Dynamics Models for Photocatalytic Water Decontamination. Process Saf. Environ. Prot. 2025, 195, 106780. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA Quantum Chemistry Program Package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Neese, F. The ORCA Program System. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software Update: The ORCA Program System, Version 4.0. WIREs Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Hansen, A.; Becker, U. Efficient, Approximate and Parallel Hartree–Fock and Hybrid DFT Calculations. A ‘Chain-of-Spheres’ Algorithm for the Hartree–Fock Exchange. Chem. Phys. 2009, 356, 98–109. [Google Scholar] [CrossRef]
- Teale, A.M.; Helgaker, T.; Savin, A.; Adamo, C.; Aradi, B.; Arbuznikov, A.V.; Ayers, P.W.; Baerends, E.J.; Barone, V.; Calaminici, P.; et al. DFT Exchange: Sharing Perspectives on the Workhorse of Quantum Chemistry and Materials Science. Phys. Chem. Chem. Phys. 2022, 24, 28700–28781. [Google Scholar] [CrossRef]
- Neese, F. The SHARK Integral Generation and Digestion System. J. Comput. Chem. 2022, 44, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Riplinger, C.; Liakos, D.G.; Becker, U.; Saitow, M.; Neese, F. Linear Scaling Perturbative Triples Correction Approximations for Open-Shell Domain-Based Local Pair Natural Orbital Coupled Cluster Singles and Doubles Theory [DLPNO-CCSD(T0/T)]. J. Chem. Phys. 2020, 152, 024116. [Google Scholar] [CrossRef] [PubMed]
- Liakos, D.G.; Guo, Y.; Neese, F. Comprehensive Benchmark Results for the Domain Based Local Pair Natural Orbital Coupled Cluster Method (DLPNO-CCSD(T)) for Closed- and Open-Shell Systems. J. Phys. Chem. A 2020, 124, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Armaković, S.; Armaković, S.J. Predicting Properties of Imidazolium-Based Ionic Liquids via Atomistica Online: Machine Learning Models and Web Tools. Computation 2025, 13, 216. [Google Scholar] [CrossRef]
- Armaković, S.; Armaković, S.J. Atomistica.Online—Web Application for Generating Input Files for ORCA Molecular Modelling Package Made with the Anvil Platform. Mol. Simul. 2023, 49, 117–123. [Google Scholar] [CrossRef]
- Armaković, S.; Armaković, S.J. Online and Desktop Graphical User Interfaces for Xtb Programme from Atomistica.Online Platform. Mol. Simul. 2024, 50, 560–570. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Shermo: A General Code for Calculating Molecular Thermochemistry Properties. Comput. Theor. Chem. 2021, 1200, 113249. [Google Scholar] [CrossRef]
- Landrum, G.; Tosco, P.; Kelley, B.; Rodriguez, R.; Cosgrove, D.; Vianello, R.; Sriniker; Gedeck, P.; Jones, G.; Kawashima, E.; et al. Rdkit/Rdkit: 2025_03_6 (Q1 2025) Release 2025. Available online: https://zenodo.org/records/15605628 (accessed on 1 August 2025).
- Lienard, P.; Gavartin, J.; Boccardi, G.; Meunier, M. Predicting Drug Substances Autoxidation. Pharm. Res. 2015, 32, 300–310. [Google Scholar] [CrossRef]
- Andersson, T.; Broo, A.; Evertsson, E. Prediction of Drug Candidates’ Sensitivity Toward Autoxidation: Computational Estimation of C-H Dissociation Energies of Carbon-Centered Radicals. J. Pharm. Sci. 2014, 103, 1949–1955. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.S.; Muthu, S.; Prasana, J.C.; Armaković, S.J.; Armaković, S.; Rizwana, F.; Ben Geoffrey, A.S. Spectroscopic Profiling (FT-IR, FT-Raman, NMR and UV-Vis), Autoxidation Mechanism (H-BDE) and Molecular Docking Investigation of 3-(4-Chlorophenyl)-N,N-Dimethyl-3-Pyridin-2-Ylpropan-1-Amine by DFT/TD-DFT and Molecular Dynamics: A Potential SSRI Drug. Comput. Biol. Chem. 2018, 77, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Khemalapure, S.S.; Katti, V.S.; Hiremath, C.S.; Basanagouda, M.; Hiremath, S.M.; Armaković, S.J.; Armaković, S. Molecular Structure, Optoelectronic Properties, Spectroscopic (FT-IR, FT-Raman and UV–Vis), H-BDE, NBO and Drug Likeness Investigations on 7, 8-Benzocoumarin-4-Acetic Acid (7BAA). J. Mol. Struct. 2019, 1195, 815–826. [Google Scholar] [CrossRef]
- Shree Sowndarya, S.V.; Kim, Y.; Kim, S.; St. John, P.C.; Paton, R.S. Expansion of Bond Dissociation Prediction with Machine Learning to Medicinally and Environmentally Relevant Chemical Space. Digit. Discov. 2023, 2, 1900–1910. [Google Scholar] [CrossRef]
- Wada, O.Z.; Olawade, D.B. Recent Occurrence of Pharmaceuticals in Freshwater, Emerging Treatment Technologies, and Future Considerations: A Review. Chemosphere 2025, 374, 144153. [Google Scholar] [CrossRef]
- Tufail, A.; Price, W.E.; Mohseni, M.; Pramanik, B.K.; Hai, F.I. A Critical Review of Advanced Oxidation Processes for Emerging Trace Organic Contaminant Degradation: Mechanisms, Factors, Degradation Products, and Effluent Toxicity. J. Water Process Eng. 2021, 40, 101778. [Google Scholar] [CrossRef]
- Rossomme, E.; Hart-Cooper, W.M.; Orts, W.J.; McMahan, C.M.; Head-Gordon, M. Computational Studies of Rubber Ozonation Explain the Effectiveness of 6PPD as an Antidegradant and the Mechanism of Its Quinone Formation. Environ. Sci. Technol. 2023, 57, 5216–5230. [Google Scholar] [CrossRef]
- Li, T.; Song, Y.; Zhang, Z. DFT Study on the Mechanism of As(III) Oxidation in the Presence of Fe(II) and O2. J. Phys. Chem. A 2024, 128, 10143–10150. [Google Scholar] [CrossRef]
- Jiang, Y.-X.; Chen, Y.; Zhang, Y.-T. Modeling Study of OH Radical-Dominated H-Abstraction Reaction for Understanding Nucleotides Oxidation Induced by Cold Atmospheric Plasmas. Plasma 2024, 7, 498–509. [Google Scholar] [CrossRef]
- Zarrouk, T.; Ibragimova, R.; Bartók, A.P.; Caro, M.A. Experiment-Driven Atomistic Materials Modeling: A Case Study Combining X-Ray Photoelectron Spectroscopy and Machine Learning Potentials to Infer the Structure of Oxygen-Rich Amorphous Carbon. J. Am. Chem. Soc. 2024, 146, 14645–14659. [Google Scholar] [CrossRef] [PubMed]
- Kubicki, J.D.; Ohno, T. Integrating Density Functional Theory Modeling with Experimental Data to Understand and Predict Sorption Reactions: Exchange of Salicylate for Phosphate on Goethite. Soil Syst. 2020, 4, 27. [Google Scholar] [CrossRef]
- Lowe, B.; Cardona, A.L.; Salas, J.; Bodi, A.; Mayer, P.M.; Burgos Paci, M.A. Probing the Pyrolysis of Ethyl Formate in the Dilute Gas Phase by Synchrotron Radiation and Theory. J. Mass. Spectrom. 2023, 58, e4901. [Google Scholar] [CrossRef]
- Rossi, E.; Ciancaleoni, G. Theoretical Insights into the Reversible CO2 Absorption by Ethylene Glycol/KOH/Boric Acid Low Temperature Transition Mixture. J. Mol. Liq. 2023, 381, 121843. [Google Scholar] [CrossRef]
- Chandrakumar, K.R.S.; Pal, S. The Concept of Density Functional Theory Based Descriptors and Its Relation with the Reactivity of Molecular Systems: A Semi-Quantitative Study. Int. J. Mol. Sci. 2002, 3, 324–337. [Google Scholar] [CrossRef]
- Tabti, K.; Sbai, A.; Maghat, H.; Lakhlifi, T.; Bouachrine, M. Computational Assessment of the Reactivity and Pharmaceutical Potential of Novel Triazole Derivatives: An Approach Combining DFT Calculations, Molecular Dynamics Simulations, and Molecular Docking. Arab. J. Chem. 2024, 17, 105376. [Google Scholar] [CrossRef]
- Guan, H.; Sun, H.; Zhao, X. Application of Density Functional Theory to Molecular Engineering of Pharmaceutical Formulations. Int. J. Mol. Sci. 2025, 26, 3262. [Google Scholar] [CrossRef] [PubMed]
- Gusarov, S. Advances in Computational Methods for Modeling Photocatalytic Reactions: A Review of Recent Developments. Materials 2024, 17, 2119. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S. Molecular Electrostatic Potentials and Chemical Reactivity. In Reviews in Computational Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1991; pp. 273–312. ISBN 978-0-470-12579-3. [Google Scholar]
- Politzer, P.; Murray, J.S.; Peralta-Inga, Z. Molecular Surface Electrostatic Potentials in Relation to Noncovalent Interactions in Biological Systems. Int. J. Quantum Chem. 2001, 85, 676–684. [Google Scholar] [CrossRef]
- Sjoberg, P.; Politzer, P. Use of the Electrostatic Potential at the Molecular Surface to Interpret and Predict Nucleophilic Processes. J. Phys. Chem. 1990, 94, 3959–3961. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. The Fundamental Nature and Role of the Electrostatic Potential in Atoms and Molecules. Theor. Chem. Acc. 2002, 108, 134–142. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. Molecular Electrostatic Potentials: Significance and Applications. In Chemical Reactivity in Confined Systems; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 113–134. ISBN 978-1-119-68335-3. [Google Scholar]
- Murray, J.S.; Politzer, P. The Electrostatic Potential: An Overview. WIREs Comput. Mol. Sci. 2011, 1, 153–163. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P. Molecular Electrostatic Potentials and Noncovalent Interactions. WIREs Comput. Mol. Sci. 2017, 7, e1326. [Google Scholar] [CrossRef]
- Politzer, P.; Jin, P.; Murray, J.S. Atomic Polarizability, Volume and Ionization Energy. J. Chem. Phys. 2002, 117, 8197–8202. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Grice, M.E.; Brinck, T.; Ranganathan, S. Radial Behavior of the Average Local Ionization Energies of Atoms. J. Chem. Phys. 1991, 95, 6699–6704. [Google Scholar] [CrossRef]
- Politzer, P.; Peralta-Inga Shields, Z.; Bulat, F.A.; Murray, J.S. Average Local Ionization Energies as a Route to Intrinsic Atomic Electronegativities. J. Chem. Theory Comput. 2011, 7, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S. Chapter 8 The Average Local Ionization Energy: Concepts and Applications. In Theoretical and Computational Chemistry; Toro-Labbé, A., Ed.; Theoretical Aspects of Chemical Reactivity; Elsevier: Amsterdam, The Netherlands, 2007; Volume 19, pp. 119–137. [Google Scholar]
- Politzer, P.; Murray, J.S.; Bulat, F.A. Average Local Ionization Energy: A Review. J. Mol. Model. 2010, 16, 1731–1742. [Google Scholar] [CrossRef]
- Kanagavalli, A.; Jayachitra, R.; Thilagavathi, G.; Elangovan, N.; Sowrirajan, S.; Thomas, R. Synthesis, Characterization, Computational, Excited State Properties, Wave Function, and Molecular Docking Studies of (E)-4-((2-Hydroxybenzylidene)Amino)N-(Thiazol-2-Yl) Benzenesulfonamide. J. Indian. Chem. Soc. 2023, 100, 100885. [Google Scholar] [CrossRef]
- Rajan, M.S.; Thomas, R. Surface-Enhanced Raman Spectroscopic Sensing of the Herbicide Alachlor Using Au16 Nanocluster. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 338, 126132. [Google Scholar] [CrossRef]
- Geethapriya, J.; Shanthidevi, A.; Arivazhagan, M.; Elangovan, N.; Sowrirajan, S.; Manivel, S.; Thomas, R. Synthesis, Characterization, Computational, Excited State Properties, Wave Function and Molecular Docking Studies of (E)-1-(Perfluorophenyl)-N-(p-Tolyl) Methanimine. J. Indian. Chem. Soc. 2022, 99, 100785. [Google Scholar] [CrossRef]
- Isravel, A.D.; Jeyaraj, J.K.; Thangasamy, S.; John, W.J. DFT, NBO, HOMO-LUMO, NCI, Stability, Fukui Function and Hole—Electron Analyses of Tolcapone. Comput. Theor. Chem. 2021, 1202, 113296. [Google Scholar] [CrossRef]
- Bultinck, P.; Winter, H.D.; Langenaeker, W.; Tollenare, J.P. Computational Medicinal Chemistry for Drug Discovery; CRC Press: Boca Raton, FL, USA, 2003; ISBN 978-0-203-91339-0. [Google Scholar]
- Pal, R.; Chattaraj, P.K. Chemical Reactivity from a Conceptual Density Functional Theory Perspective. J. Indian. Chem. Soc. 2021, 98, 100008. [Google Scholar] [CrossRef]
- Rincón, L.; Rodríguez, W.M.; Mora, J.R.; Zambrano, C.; Seijas, L.E.; Reyes, A.; Torres, F.J. A Redefinition of Global Conceptual Density Functional Theory Reactivity Indexes by Means of the Cubic Expansions of the Energy. Phys. Chem. Chem. Phys. 2025, 27, 8174–8185. [Google Scholar] [CrossRef]
- Bhatia, M. An Overview of Conceptual-DFT Based Insights into Global Chemical Reactivity of Volatile Sulfur Compounds (VSCs). Comput. Toxicol. 2024, 29, 100295. [Google Scholar] [CrossRef]
- Ramírez-Martínez, C.; Zárate-Hernández, L.A.; Camacho-Mendoza, R.L.; González-Montiel, S.; Meneses-Viveros, A.; Cruz-Borbolla, J. The Use of Global and Local Reactivity Descriptors of Conceptual DFT to Describe Toxicity of Benzoic Acid Derivatives. Comput. Theor. Chem. 2023, 1226, 114211. [Google Scholar] [CrossRef]
- Wang, S.; Hao, C.; Gao, Z.; Chen, J.; Qiu, J. Theoretical Investigation on Photodechlorination Mechanism of Polychlorinated Biphenyls. Chemosphere 2014, 95, 200–205. [Google Scholar] [CrossRef]
- Armaković, S.; Armaković, S.J.; Abramović, B.F. Theoretical Investigation of Loratadine Reactivity in Order to Understand Its Degradation Properties: DFT and MD Study. J. Mol. Model. 2016, 22, 240. [Google Scholar] [CrossRef] [PubMed]
- Case, D.A.; Aktulga, H.M.; Belfon, K.; Cerutti, D.S.; Cisneros, G.A.; Cruzeiro, V.W.D.; Forouzesh, N.; Giese, T.J.; Götz, A.W.; Gohlke, H.; et al. AmberTools. J. Chem. Inf. Model. 2023, 63, 6183–6191. [Google Scholar] [CrossRef]
- MacKerell, A.D., Jr.; Brooks, B.; Brooks, C.L., III; Nilsson, L.; Roux, B.; Won, Y.; Karplus, M. CHARMM: The Energy Function and Its Parameterization. In Encyclopedia of Computational Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2002; ISBN 978-0-470-84501-1. [Google Scholar]
- Brooks, B.R.; Bruccoleri, R.E.; Olafson, B.D.; States, D.J.; Swaminathan, S.; Karplus, M. CHARMM: A Program for Macromolecular Energy, Minimization, and Dynamics Calculations. J. Comput. Chem. 1983, 4, 187–217. [Google Scholar] [CrossRef]
- Brooks, B.R.; Brooks, C.L.; MacKerell, A.D.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The Biomolecular Simulation Program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Tirado-Rives, J. The OPLS [Optimized Potentials for Liquid Simulations] Potential Functions for Proteins, Energy Minimizations for Crystals of Cyclic Peptides and Crambin. J. Am. Chem. Soc. 1988, 110, 1657–1666. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Shivakumar, D.; Williams, J.; Wu, Y.; Damm, W.; Shelley, J.; Sherman, W. Prediction of Absolute Solvation Free Energies Using Molecular Dynamics Free Energy Perturbation and the OPLS Force Field. J. Chem. Theory Comput. 2010, 6, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Harder, E.; Damm, W.; Maple, J.; Wu, C.; Reboul, M.; Xiang, J.Y.; Wang, L.; Lupyan, D.; Dahlgren, M.K.; Knight, J.L.; et al. OPLS3: A Force Field Providing Broad Coverage of Drug-like Small Molecules and Proteins. J. Chem. Theory Comput. 2016, 12, 281–296. [Google Scholar] [CrossRef]
- Roos, K.; Wu, C.; Damm, W.; Reboul, M.; Stevenson, J.M.; Lu, C.; Dahlgren, M.K.; Mondal, S.; Chen, W.; Wang, L.; et al. OPLS3e: Extending Force Field Coverage for Drug-Like Small Molecules. J. Chem. Theory Comput. 2019, 15, 1863–1874. [Google Scholar] [CrossRef]
- Lu, C.; Wu, C.; Ghoreishi, D.; Chen, W.; Wang, L.; Damm, W.; Ross, G.A.; Dahlgren, M.K.; Russell, E.; Von Bargen, C.D.; et al. OPLS4: Improving Force Field Accuracy on Challenging Regimes of Chemical Space. J. Chem. Theory Comput. 2021, 17, 4291–4300. [Google Scholar] [CrossRef]
- Dixon, T.; MacPherson, D.; Mostofian, B.; Dauzhenka, T.; Lotz, S.; McGee, D.; Shechter, S.; Shrestha, U.R.; Wiewiora, R.; McDargh, Z.A.; et al. Predicting the Structural Basis of Targeted Protein Degradation by Integrating Molecular Dynamics Simulations with Structural Mass Spectrometry. Nat. Commun. 2022, 13, 5884. [Google Scholar] [CrossRef]
- Wales, D.J.; Doye, J.P.K. Global Optimization by Basin-Hopping and the Lowest Energy Structures of Lennard-Jones Clusters Containing up to 110 Atoms. J. Phys. Chem. A 1997, 101, 5111–5116. [Google Scholar] [CrossRef]
- Goedecker, S. Minima Hopping: An Efficient Search Method for the Global Minimum of the Potential Energy Surface of Complex Molecular Systems. J. Chem. Phys. 2004, 120, 9911–9917. [Google Scholar] [CrossRef]
- de Souza, B. GOAT: A Global Optimization Algorithm for Molecules and Atomic Clusters. Angew. Chem. Int. Ed. 2025, 64, e202500393. [Google Scholar] [CrossRef]
- Wesołowski, P.A.; Wales, D.J.; Pracht, P. Multilevel Framework for Analysis of Protein Folding Involving Disulfide Bond Formation. J. Phys. Chem. B 2024, 128, 3145–3156. [Google Scholar] [CrossRef] [PubMed]
- Pracht, P.; Bannwarth, C. Fast Screening of Minimum Energy Crossing Points with Semiempirical Tight-Binding Methods. J. Chem. Theory Comput. 2022, 18, 6370–6385. [Google Scholar] [CrossRef]
- Spicher, S.; Plett, C.; Pracht, P.; Hansen, A.; Grimme, S. Automated Molecular Cluster Growing for Explicit Solvation by Efficient Force Field and Tight Binding Methods. J. Chem. Theory Comput. 2022, 18, 3174–3189. [Google Scholar] [CrossRef]
- Pracht, P.; Bauer, C.A.; Grimme, S. Automated and Efficient Quantum Chemical Determination and Energetic Ranking of Molecular Protonation Sites. J. Comput. Chem. 2017, 38, 2618–2631. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Exploration of Chemical Compound, Conformer, and Reaction Space with Meta-Dynamics Simulations Based on Tight-Binding Quantum Chemical Calculations. J. Chem. Theory Comput. 2019, 15, 2847–2862. [Google Scholar] [CrossRef]
- Pracht, P.; Grimme, S. Calculation of Absolute Molecular Entropies and Heat Capacities Made Simple. Chem. Sci. 2021, 12, 6551–6568. [Google Scholar] [CrossRef]
- Pracht, P.; Grimme, S.; Bannwarth, C.; Bohle, F.; Ehlert, S.; Feldmann, G.; Gorges, J.; Müller, M.; Neudecker, T.; Plett, C.; et al. CREST—A Program for the Exploration of Low-Energy Molecular Chemical Space. J. Chem. Phys. 2024, 160, 114110. [Google Scholar] [CrossRef]
- Koopman, J.; Grimme, S. Calculation of Mass Spectra with the QC × MS Method for Negatively and Multiply Charged Molecules. J. Am. Soc. Mass Spectrom. 2022, 33, 2226–2242. [Google Scholar] [CrossRef]
- Schnegotzki, R.; Koopman, J.; Grimme, S.; Süssmuth, R.D. Quantum Chemistry-Based Molecular Dynamics Simulations as a Tool for the Assignment of ESI-MS/MS Spectra of Drug Molecules. Chem.—A Eur. J. 2022, 28, e202200318. [Google Scholar] [CrossRef]
- Schreckenbach, S.A.; Anderson, J.S.M.; Koopman, J.; Grimme, S.; Simpson, M.J.; Jobst, K.J. Predicting the Mass Spectra of Environmental Pollutants Using Computational Chemistry: A Case Study and Critical Evaluation. J. Am. Soc. Mass. Spectrom. 2021, 32, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Koopman, J.; Grimme, S. From QCEIMS to QCxMS: A Tool to Routinely Calculate CID Mass Spectra Using Molecular Dynamics. J. Am. Soc. Mass. Spectrom. 2021, 32, 1735–1751. [Google Scholar] [CrossRef] [PubMed]
- Ásgeirsson, V.; Bauer, C.A.; Grimme, S. Unimolecular Decomposition Pathways of Negatively Charged Nitriles by Ab Initio Molecular Dynamics. Phys. Chem. Chem. Phys. 2016, 18, 31017–31026. [Google Scholar] [CrossRef] [PubMed]
- Koopman, J.; Grimme, S. Calculation of Electron Ionization Mass Spectra with Semiempirical GFNn-xTB Methods. ACS Omega 2019, 4, 15120–15133. [Google Scholar] [CrossRef] [PubMed]
- Ásgeirsson, V.; Bauer, C.A.; Grimme, S. Quantum Chemical Calculation of Electron Ionization Mass Spectra for General Organic and Inorganic Molecules. Chem. Sci. 2017, 8, 4879–4895. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.A.; Grimme, S. How to Compute Electron Ionization Mass Spectra from First Principles. J. Phys. Chem. A 2016, 120, 3755–3766. [Google Scholar] [CrossRef]
- Bauer, C.A.; Grimme, S. Automated Quantum Chemistry Based Molecular Dynamics Simulations of Electron Ionization Induced Fragmentations of the Nucleobases Uracil, Thymine, Cytosine, and Guanine. Eur. J. Mass Spectrom. (Chichester) 2015, 21, 125–140. [Google Scholar] [CrossRef]
- Bauer, C.A.; Grimme, S. Elucidation of Electron Ionization Induced Fragmentations of Adenine by Semiempirical and Density Functional Molecular Dynamics. J. Phys. Chem. A 2014, 118, 11479–11484. [Google Scholar] [CrossRef]
- Bauer, C.A.; Grimme, S. First Principles Calculation of Electron Ionization Mass Spectra for Selected Organic Drug Molecules. Org. Biomol. Chem. 2014, 12, 8737–8744. [Google Scholar] [CrossRef]
- Grimme, S. Towards First Principles Calculation of Electron Impact Mass Spectra of Molecules. Angew. Chem. Int. Ed. 2013, 52, 6306–6312. [Google Scholar] [CrossRef]
- Gorges, J.; Grimme, S. QC × MS2—A Program for the Calculation of Electron Ionization Mass Spectra via Automated Reaction Network Discovery. Phys. Chem. Chem. Phys. 2025, 27, 6899–6911. [Google Scholar] [CrossRef]

| Concept | Key Features | Functional Role | Ref. |
|---|---|---|---|
| Signal recognition and processing | Detection of molecular-level responses (e.g., retention time shifts, mass spectra changes) | Enables identification of known/unknown analytes and structural elucidation | [1,12,34,35] |
| Pattern recognition | Use of PCA, PLS–DA, clustering, and AI/ML to recognize trends | Discriminates between sample types, pollution sources, and contamination events | [24,36,37] |
| Environmental fingerprinting | Total ion chromatograms and full-scan spectra representing the whole sample | Provides a holistic chemical profile for classification, source tracking, and pollution assessment | [38,39] |
| Non-Target Screening (NTS) | Data-rich acquisition without predefined target compounds | Discovery of novel and emerging contaminants | [40,41,42,43] |
| Real-time monitoring | Miniaturized GC-MS devices, sensor integration, and remote sensing | Field-based, rapid decision support for environmental safety | [44,45,46] |
| Application Area | Description | Ref. |
|---|---|---|
| Pesticides and Pharmaceuticals | Analysis of 113 pesticides in brown rice, red pepper and orange | [50] |
| Analysis of 222 pesticides in seven different vegetables and fruits | [51] | |
| Analysis for 283 pesticides in pepper samples of Çanakkale province (Turkey) | [52] | |
| Analysis of 311 pesticides in Loamy sand agricultural soils | [53] | |
| Analysis of 19 pesticides in 30 types of cereals | [54] | |
| Analysis of non-steroidal anti-inflammatory drugs and estrogenic hormones in water | [55] | |
| Quantification of salicylic acid, acetylsalicylic acid, nalidixic acid, ibuprofen, phenacetin, naproxen, ketoprofen, meclofenamic acid and diclofenac in South African surface water | [56] | |
| Quantification of 41 pharmaceuticals in the geological depression of Rio Grande do Sul State, Brazil | [57] | |
| Microplastics and additives | Qualitative and quantitative analysis 12 of the most common plastic polymers in environmental samples | [58] |
| Quantitative analysis of 12 common plastic polymers applying CaCO3 as a catalyst in pyrolytic behavior of polymers | [59] | |
| Quantitative analysis of plastics in samples of human tissues | [60] | |
| Quantification of plastic particles (diameter ≥ 700 nm) in human whole blood from 22 healthy volunteers | [61] | |
| Evaluation of Py-GC–MS for quantification of micro- and nanoplastics in human blood with a pilot study of the Australian population | [62] | |
| Volatile organic compounds | Analysis of volatile organic compounds in eight kinds of red and green huajiao spice from different regions of China | [63] |
| Identification and quantification of 104 volatile organic compounds in fermented sea bass | [64] | |
| Review of GC–MS analysis of volatile organic compounds in exhaled breath as biomarkers for cancer, pulmonary and infectious diseases | [65] | |
| Identification and quantification of 43 volatile organic compounds in raw, 65 °C- and 135 °C-treated milk | [66] | |
| Evaluation of a thermal desorption-gas chromatography-mass spectrometry-ion mobility spectrometry system for standardized quantification of volatile organic compounds | [67] | |
| Industrial and agricultural pollutants (e.g., nitrophenols, pesticides) | Review of comprehensive two-dimensional gas chromatography–mass spectrometry methodologies for analysis of ultra-trace organic pollutants in environmental matrices | [68] |
| Quantification of 19 organic pollutants (PAHs, BTEX, alkylphenols, tributyltin and diethylphthalate) in soils irrigated with agro-industrial wastewater | [69] | |
| Identification of organic pollutants in distillery wastewater and assessment of phytotoxic, cytotoxic and genotoxic effects | [70] | |
| Early warning systems | Identification of mildew markers (1-octen-3-ol and 3-octanone) in japonica rice | [71] |
| Remote sampling and identification of hazardous air pollutants using drone-mounted solid-phase microextraction coupled with portable gas chromatography–mass spectrometry | [72] | |
| Analysis of 16 polycyclic aromatic hydrocarbons in particulate matter (PM2.5 and PM10) from Hanoi, Vietnam using gas chromatography–tandem mass spectrometry | [73] | |
| Quantification of pesticide residues in honey bees, pollen, honey, vegetables and other matrices in Italy | [74] |
| Category | Principle and Typical Use | How It Complements GC–MS | Key Output | Ref. |
|---|---|---|---|---|
| Methods QM calculations: ab initio, DFT, semiempirical, hybrid MD simulations: Force field or ab initio | QM: DFT for accuracy-cost balance; semiempirical (PM7, GFN2-xTB, GFN-FF) for speed; hybrid QM/MM for local accuracy in large systems, post-HF for benchmarks MD: Classical: (AMBER/CHARMM/OPLS), Semiempirical: (GFN-xTB/FF), Ab initio (density functional) | QM: Mechanistic insights into fragmentation, stability, and transformation pathways MD: Model solvation, diffusion, aggregation, and stability relevant to aqueous analysis | QM: BDEs, activation barriers, reaction thermodynamics MD: RDFs, solvation shells, diffusion coefficients, conformational dynamics | [134,136,138,167] |
| Descriptors (local/global reactivity) | Local (MEP, ALIE, HOMO/LUMO topology) and global (IP, EA, hardness/softness, electrophilicity) indices from electronic structure | Pinpoint reactive sites, degradation hotspots, and fragmentation-prone bonds; prioritize pollutants | Mapped surfaces and scalar indices linked to fragmentation/persistence | [198,202] |
| Conformational search space (CREST, GOAT) | Systematic exploration of low-energy conformers/rotamers using GFN-xTB/GFN-FF; ensemble-aware modeling | Ensures reliable reactivity/BDE predictions; explains variability in retention and fragmentation | Conformer ensembles; global minima; ensemble-averaged properties | [229,232,234] |
| Engines/Modeling codes | ORCA (DFT/post-HF), xTB (GFN family), MOPAC (PM methods), PSI4, Multiwfn; MD engines (GROMACS, LAMMPS); QCxMS/QCxMS2 for MS simulation | Backends for structure, reactivity, dynamics, and spectrum simulation | Optimized structures, reactivity maps, simulated spectra, MD trajectories | [134,136,138,167] |
| Simulation of MS spectra (QCxMS/QCxMS2) | Automated EI/CID/DEA fragmentation modeling (xtb/ORCA backends); MD- or network-based | Predicts fragmentation routes and theoretical spectra; valdates/augments experimental assignments | Simulated spectra; mechanistic pathways and fragment identities | [240,245,248,249] |
| Online tools | atomistica.online integrating xTB, MOPAC, SHERMO, Multiwfn, browser-based workflows | Low-barrier access to semiempirical calculations, conformer search, RDG plots, and QCxMS input prep | Web workflows; inputs/outputs ready for GC-MS support | [170,171,172,173,174,175,176,177,178,179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armaković, S.J.; Armaković, S. Advanced GC-MS Chemosensing Combined with Atomistic Modeling: A Synergistic Approach for Environmental Water Analysis. Chemosensors 2025, 13, 353. https://doi.org/10.3390/chemosensors13090353
Armaković SJ, Armaković S. Advanced GC-MS Chemosensing Combined with Atomistic Modeling: A Synergistic Approach for Environmental Water Analysis. Chemosensors. 2025; 13(9):353. https://doi.org/10.3390/chemosensors13090353
Chicago/Turabian StyleArmaković, Sanja J., and Stevan Armaković. 2025. "Advanced GC-MS Chemosensing Combined with Atomistic Modeling: A Synergistic Approach for Environmental Water Analysis" Chemosensors 13, no. 9: 353. https://doi.org/10.3390/chemosensors13090353
APA StyleArmaković, S. J., & Armaković, S. (2025). Advanced GC-MS Chemosensing Combined with Atomistic Modeling: A Synergistic Approach for Environmental Water Analysis. Chemosensors, 13(9), 353. https://doi.org/10.3390/chemosensors13090353






