Fluorescent Assay for Salmonella Detection Based on Triangle Multivalent Aptamer-Initiated Catalytic Hairpin Assembly
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Apparatus
2.2. Bacterial Culture
2.3. Preparation of Hairpin Probes
2.4. Gel Electrophoresis and Fluorescence Analysis
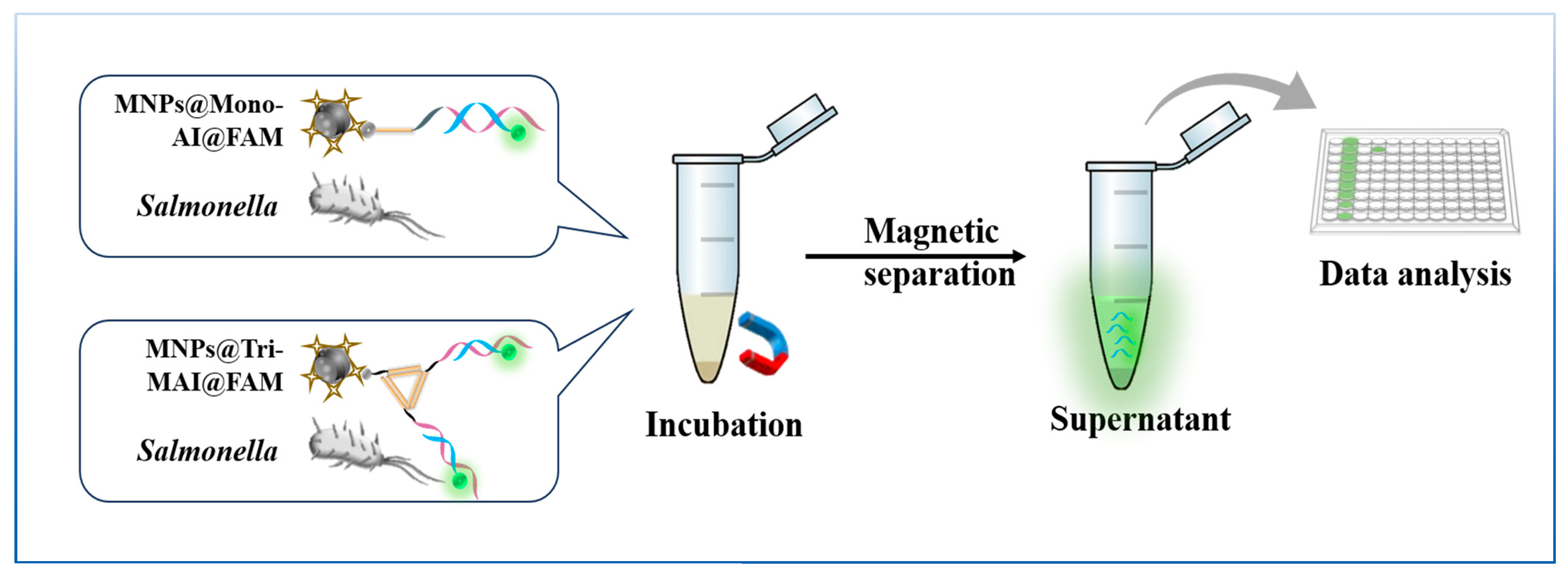
2.5. Modification and Detection of Monovalent Aptamer-Initiator (Mono-AI) on the Surface of Streptavidin Magnetic Beads (SA-MNPs)
2.6. Modification and Detection of Triangle Multivalent Aptamer-Initiator (Tri-MAI) on the Surface of Streptavidin Magnetic Beads (SA-MNPs)
2.7. Preparation of Spiked Samples
3. Results and Discussion
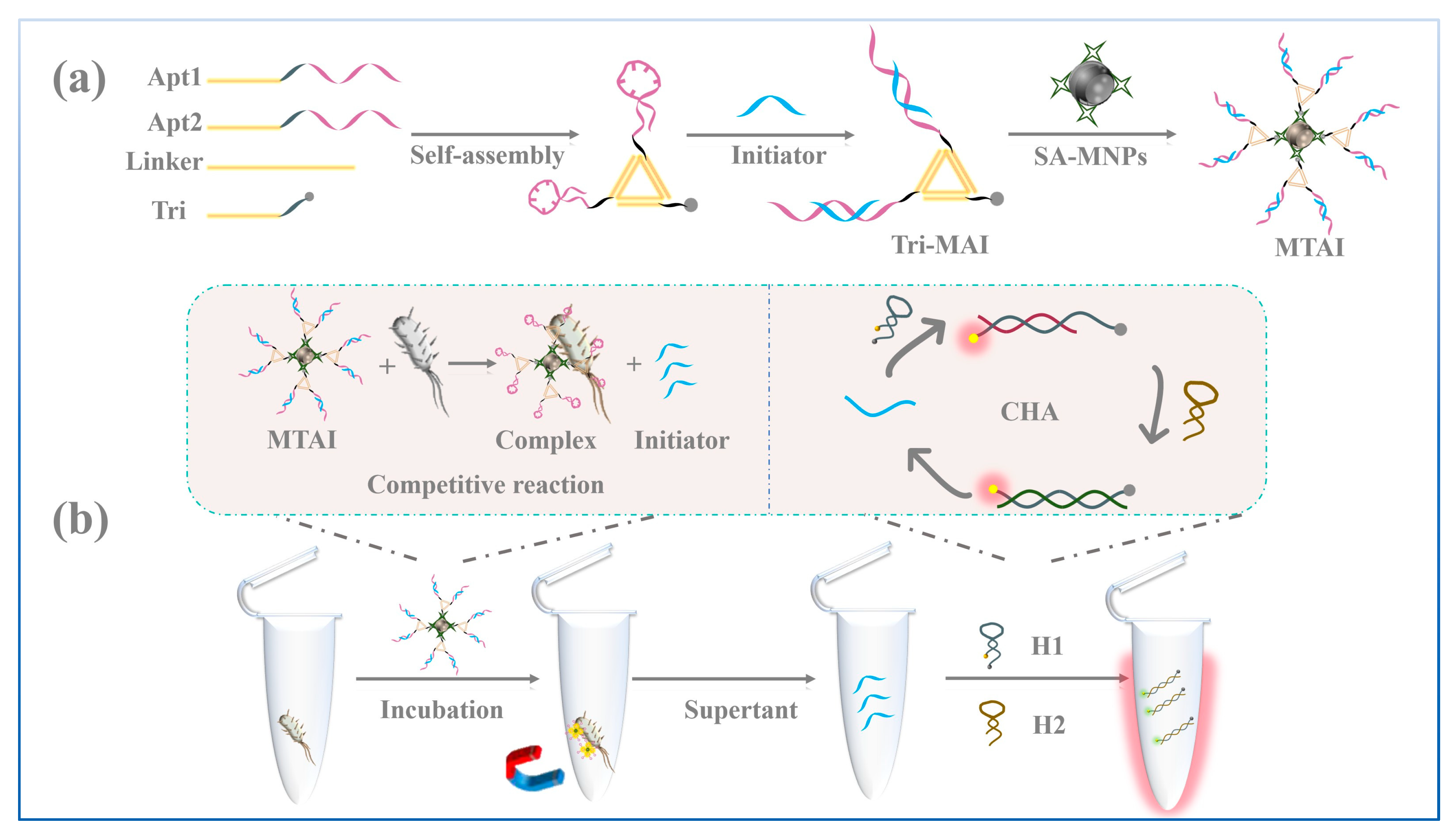
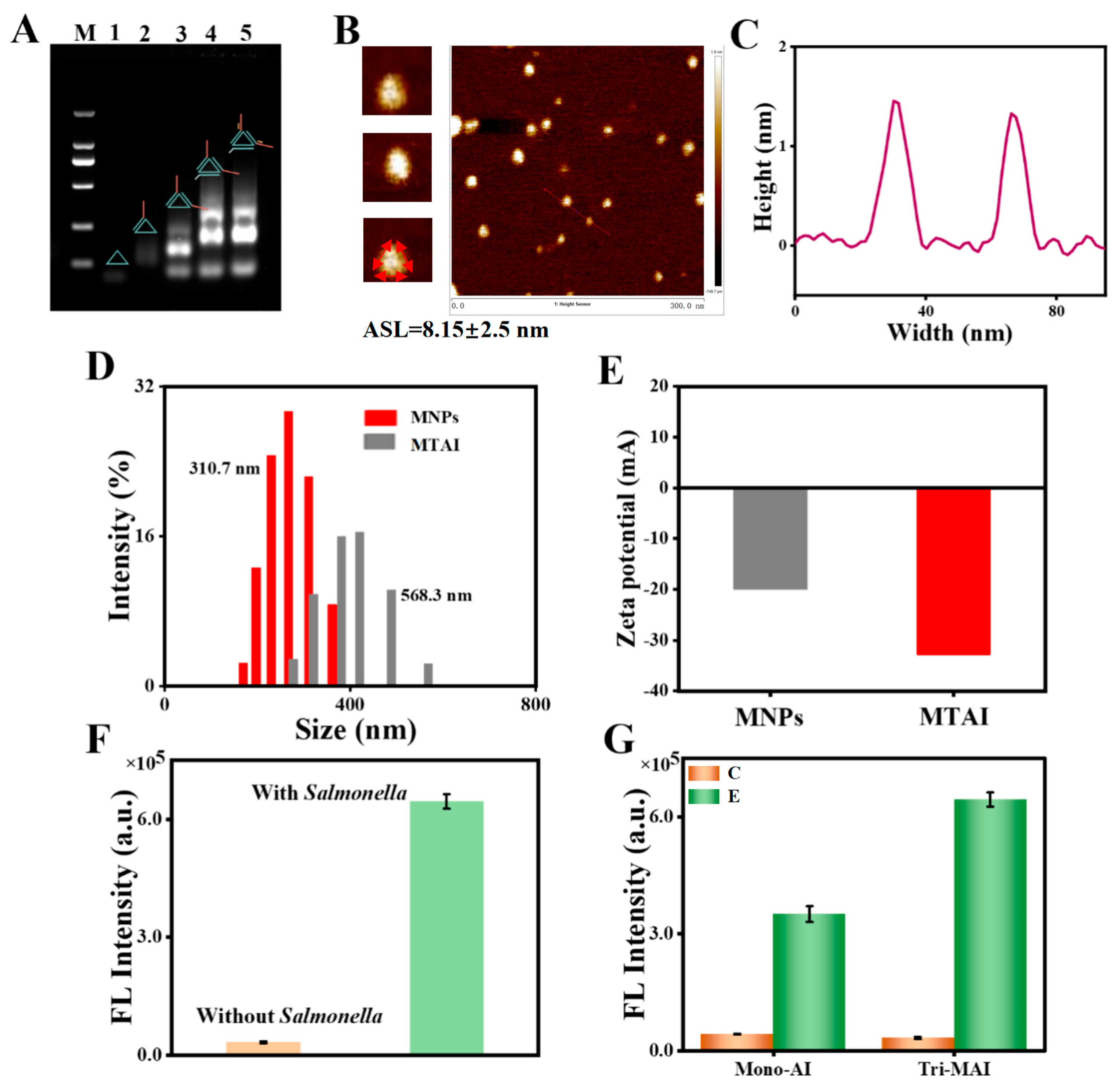
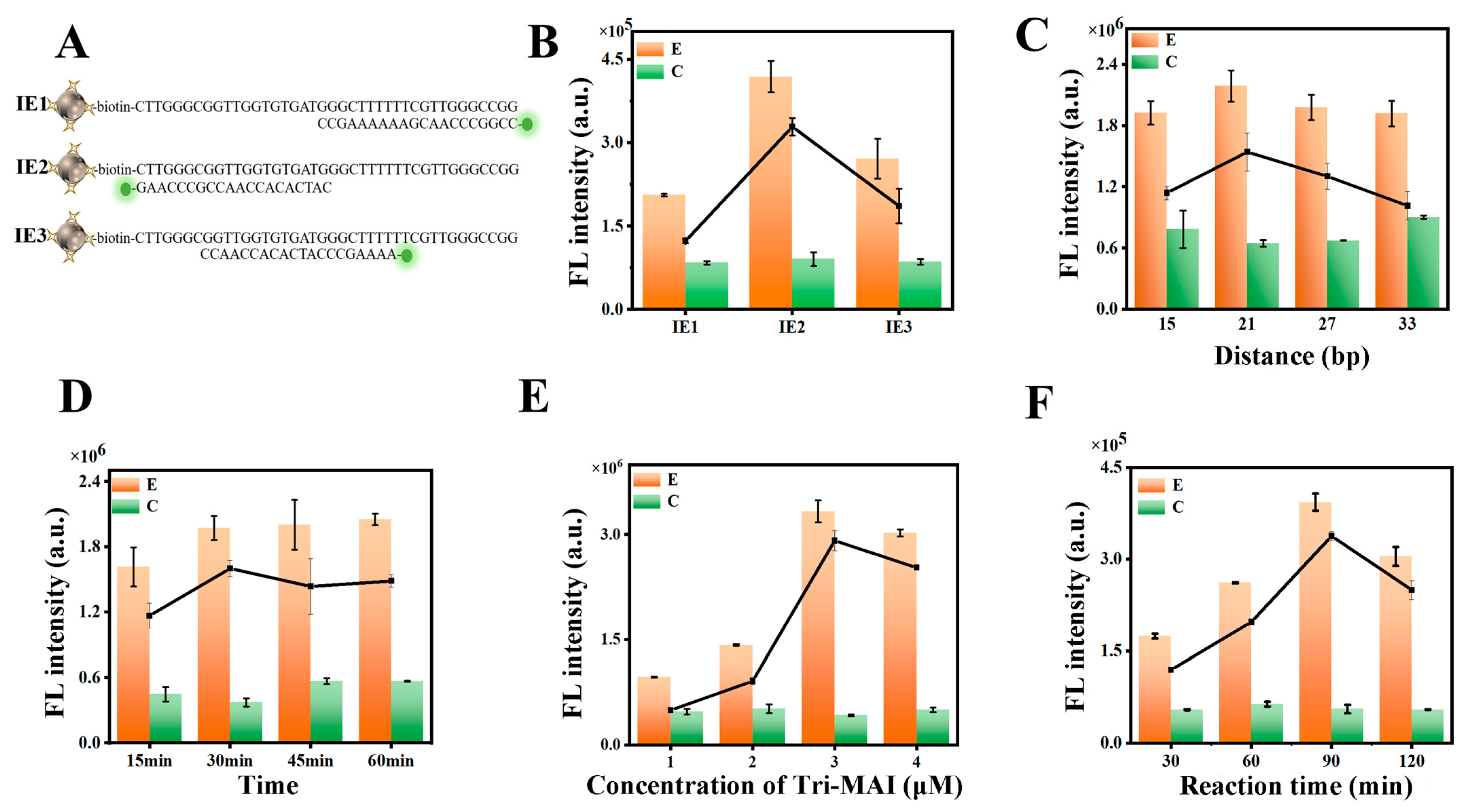
3.1. Construction and Characterization of the MTAI
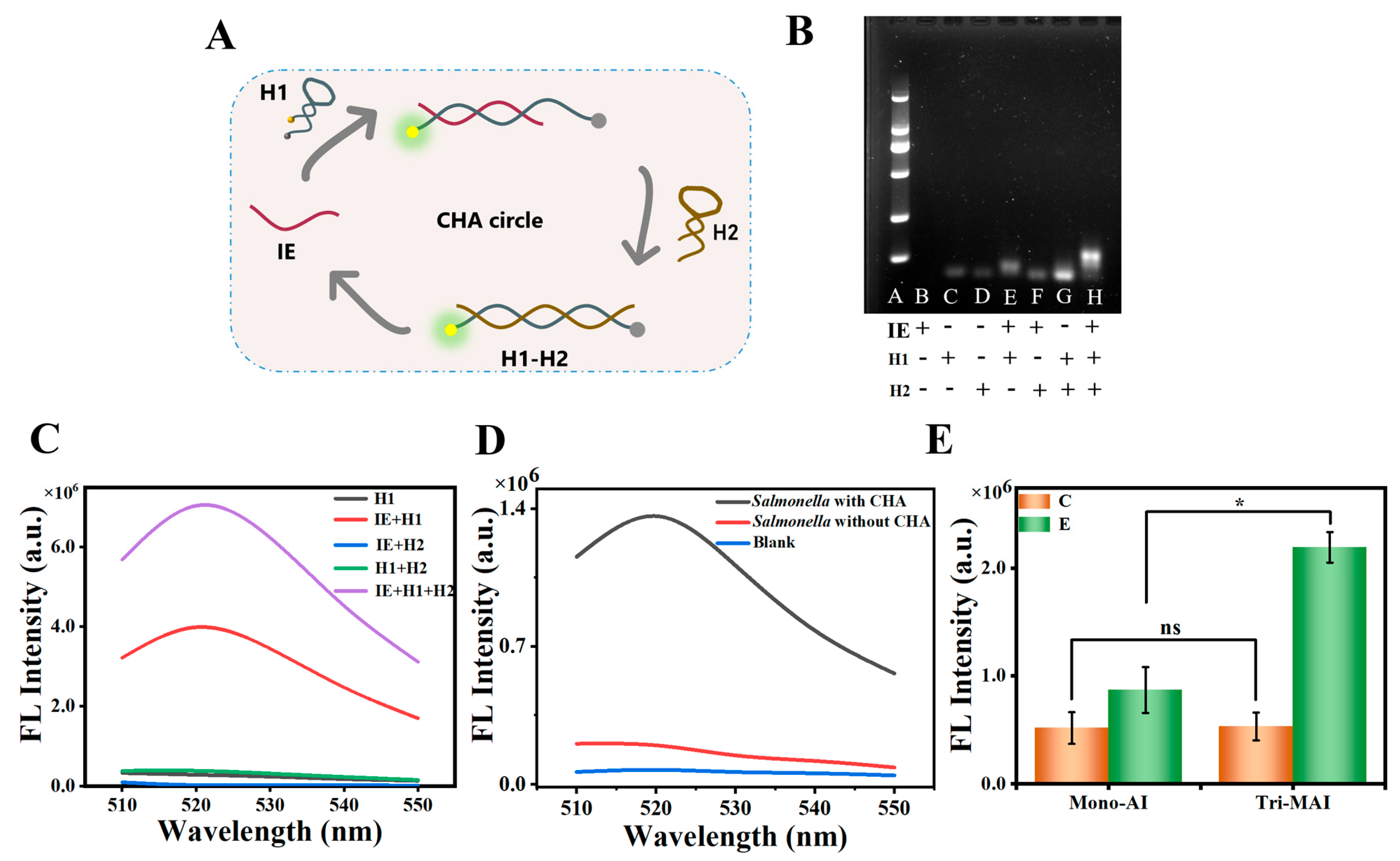
3.2. Validation of the CHA Performance
3.3. Optimization of Experimental Parameters
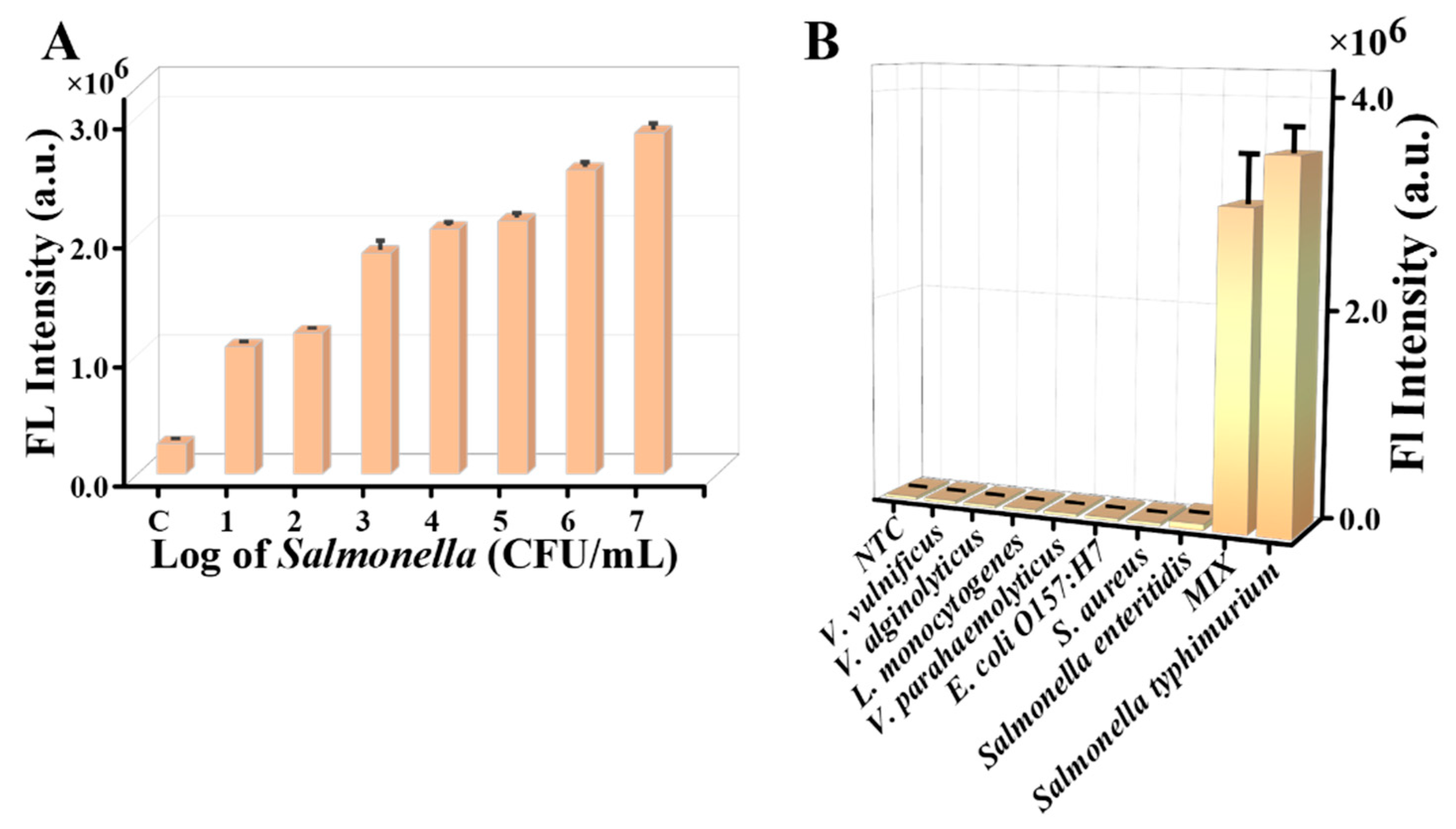
3.4. Sensitivity of the Assay
3.5. Specificity of the Assay
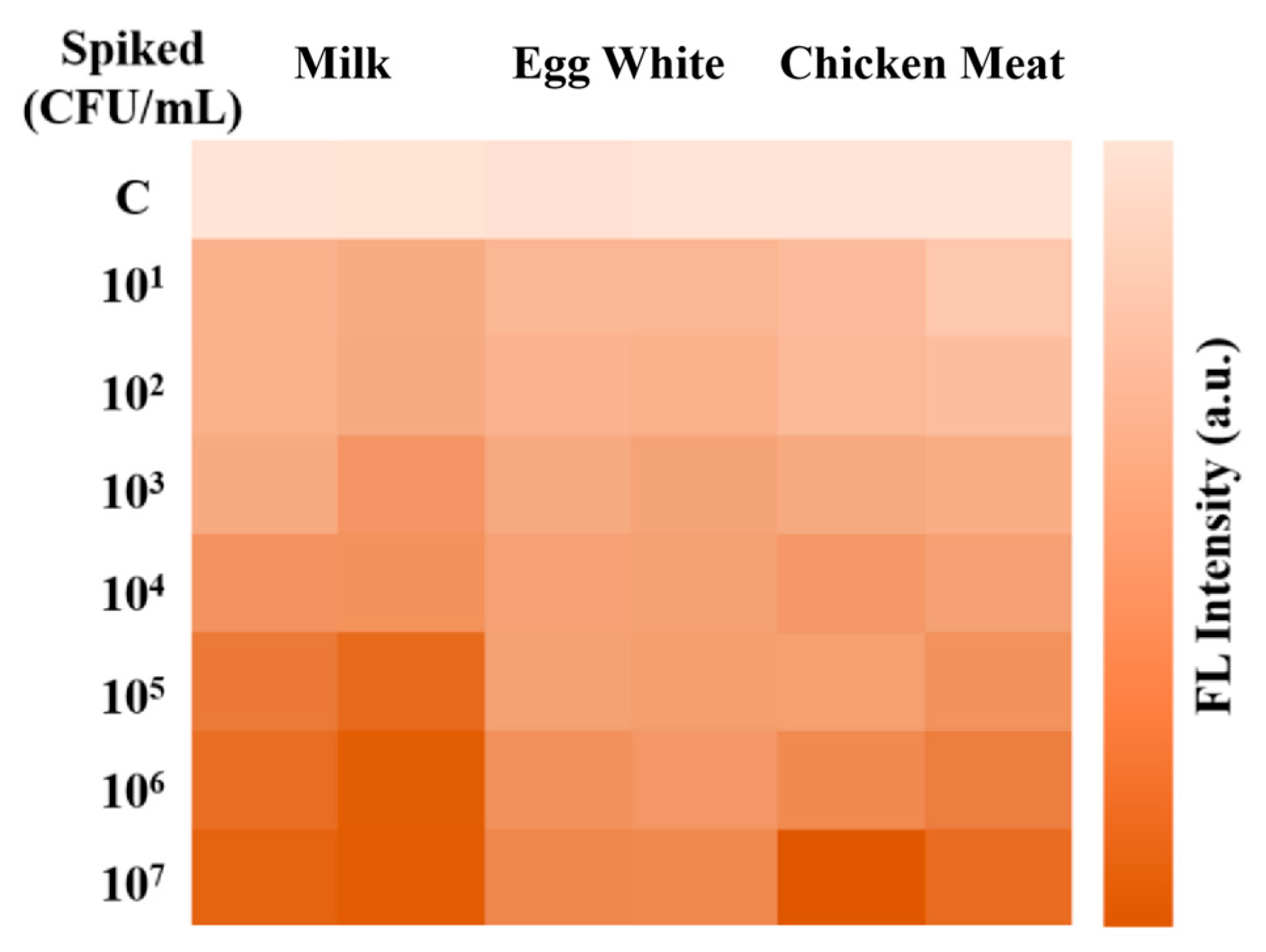
3.6. Analysis of Real Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teklemariam, A.D.; Al-Hindi, R.R.; Albiheyri, R.S.; Alharbi, M.G.; Alghamdi, M.A.; Filimban, A.A.R.; Al Mutiri, A.S.; Al-Alyani, A.M.; Alseghayer, M.S.; Almaneea, A.M.; et al. Human Salmonellosis: A Continuous Global Threat in the Farm-to-Fork Food Safety Continuum. Foods 2023, 12, 1756. [Google Scholar] [CrossRef]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M.; International Collaboration on Enteric Disease “Burden of Illness” Studies. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef]
- Salmonella (Non-Typhoidal). Available online: https://www.who.int/news-room/fact-sheets/detail/Salmonella-(non-typhoidal) (accessed on 4 October 2024).
- Rinn, N.; Müller, A.; Braun, A.S.; Greif, G.; Stiefel, D.; Kehrenberg, C. Food products confiscated from air passengers travelling from third countries into the European Union: Microbiological analyses and genomic characterization of zoonotic and multiresistant bacteria. Food Microbiol. 2025, 131, 104783. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.C.; Isaksson, M.; Nilsson, B.; Eppink, M.; Ottens, M. Optimization of multi-column chromatography for capture and polishing at high protein load. Biotechnol. Prog. 2025, e70047. [Google Scholar] [CrossRef]
- Zhou, C.; Li, W.; Zhao, Y.; Gu, K.; Liao, Z.; Guo, B.; Huang, Z.; Yang, M.; Wei, H.; Ma, P.; et al. Sensitive detection of viable salmonella bacteria based on tertiary cascade signal amplification via splintR ligase ligation-PCR amplification-CRISPR/Cas12a cleavage. Anal. Chim. Acta 2023, 1248, 340885. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, S.; Qi, L.; Zhang, X.; Yang, M.; Guo, Z.; Wang, Z.; Du, Y. Advancing Multiple Detection in RT-LAMP with a Specific Probe Assembled from Plural Three-Way-Junction Structures. Anal. Chem. 2023, 95, 17808–17817. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yan, H.; Zheng, Y.; Zu, Y.; Yang, S.; Hu, H.; Shi, S.; Liang, H.; Niu, X. Joint concanavalin A-aptamer enabled dual recognition for anti-interference visual detection of Salmonella typhimurium in complex food matrices. Food Chem. 2023, 426, 136581. [Google Scholar] [CrossRef]
- Wang, L.; Wang, R.; Chen, F.; Jiang, T.; Wang, H.; Slavik, M.; Wei, H.; Li, Y. QCM-based aptamer selection and detection of Salmonella typhimurium. Food Chem. 2017, 221, 776–782. [Google Scholar] [CrossRef]
- Lu, Y.; Xie, Q.; Chen, J.; Chu, Z.; Zhang, F.; Wang, Q. Aptamer-mediated double strand displacement amplification with microchip electrophoresis for ultrasensitive detection of Salmonella typhimurium. Talanta 2024, 273, 125875. [Google Scholar] [CrossRef]
- Li, H.; Xu, H.; Shi, X.; Zhao, C.; Li, J.; Wang, J. Colorimetry/fluorescence dual-mode detection of Salmonella typhimurium based on a “three-in-one” nanohybrid with high oxidase-like activity for AIEgen. Food Chem. 2024, 449, 139220. [Google Scholar] [CrossRef]
- Wang, L.; Huo, X.; Qi, W.; Xia, Z.; Li, Y.; Lin, J. Rapid and sensitive detection of Salmonella Typhimurium using nickel nanowire bridge for electrochemical impedance amplification. Talanta 2020, 211, 120715. [Google Scholar] [CrossRef]
- Zhang, P.; Song, M.; Dou, L.; Xiao, Y.; Li, K.; Shen, G.; Ying, B.; Geng, J.; Yang, D.; Wu, Z. Development of a fluorescent DNA nanomachine for ultrasensitive detection of Salmonella enteritidis without labeling and enzymes. Mikrochim. Acta 2020, 187, 376. [Google Scholar] [CrossRef]
- Ooi, H.W.; Kocken, J.M.M.; Morgan, F.L.C.; Malheiro, A.; Zoetebier, B.; Karperien, M.; Wieringa, P.A.; Dijkstra, P.J.; Moroni, L.; Baker, M.B. Multivalency Enables Dynamic Supramolecular Host–Guest Hydrogel Formation. Biomacromolecules 2020, 21, 2208–2217. [Google Scholar] [CrossRef]
- Dong, N.; Liu, Z.; He, H.; Lu, Y.; Qi, J.; Wu, W. “Hook&Loop” multivalent interactions based on disk-shaped nanoparticles strengthen active targeting. J. Contr. Release 2023, 354, 279–293. [Google Scholar]
- Guo, L.; Zhang, Y.; Wang, Y.; Xie, M.; Dai, J.; Qu, Z.; Zhou, M.; Cao, S.; Shi, J.; Wang, L.; et al. Directing Multivalent Aptamer-Receptor Binding on the Cell Surface with Programmable Atom-Like Nanoparticles. Angew. Chem. Int. Ed. Engl. 2022, 61, e202117168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Peng, Y.; Yao, L.; Shang, H.; Zheng, Z.; Chen, W.; Xu, J. Self-Assembly of Multivalent Aptamer-Tethered DNA Monolayers Dedicated to a Fluorescence Polarization-Responsive Circular Isothermal Strand Displacement Amplification for Salmonella Assay. Anal. Chem. 2023, 95, 2570–2578. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, T.; Mo, F.; Yu, W.; Shen, Y.; Jiang, Q.; Wang, F.; Liu, X. Programmable Assembly of Multivalent DNA-Protein Superstructures for Tumor Imaging and Targeted Therapy. Angew. Chem. Int. Ed. Engl. 2022, 61, e202211505. [Google Scholar] [CrossRef]
- Li, H.; Cheng, S.; Zhang, Q.; Zhou, T.; Zhang, T.; Liu, S.; Peng, Y.; Yu, J.; Xu, J.; Wang, Q.; et al. Dual-Multivalent Aptamer-Based Drug Delivery Platform for Targeted SRC Silencing to Enhance Doxorubicin Sensitivity in Endometrial Cancer. Int. J. Biol. Sci. 2024, 20, 5812–5830. [Google Scholar] [CrossRef]
- Qiao, Z.; Xue, L.; Sun, M.; Ma, N.; Shi, H.; Yang, W.; Cheong, L.Z.; Huang, X.; Xiong, Y. Dual-Functional Tetrahedron Multivalent Aptamer Assisted Amplification-Free CRISPR/Cas12a Assay for Sensitive Detection of Salmonella. J. Agric. Food Chem. 2024, 72, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.S.; Bhadra, S.; Li, B.; Ellington, A.D. Mismatches improve the performance of strand-displacement nucleic Acid circuits. Angew. Chem. Int. Ed. Engl. 2014, 53, 1845–1848. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, S.; Ellington, A.D. Design and application of cotranscriptional non-enzymatic RNA circuits and signal transducers. Nucleic Acids Res. 2014, 42, e58. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, X.; Yuan, R.; Chai, Y. An “off-on” electrochemiluminescent biosensor based on DNAzyme-assisted target recycling and rolling circle amplifications for ultrasensitive detection of microRNA. Anal. Chem. 2015, 87, 3202–3207. [Google Scholar] [CrossRef]
- Tang, Y.; Lin, Y.; Yang, X.; Wang, Z.; Le, X.C.; Li, F. Universal strategy to engineer catalytic DNA hairpin assemblies for protein analysis. Anal. Chem. 2015, 87, 8063–8066. [Google Scholar] [CrossRef]
- Qing, Z.; Hu, J.; Xu, J.; Zou, Z.; Lei, Y.; Qing, T.; Yang, R. An intramolecular catalytic hairpin assembly on a DNA tetrahedron for mRNA imaging in living cells: Improving reaction kinetics and signal stability. Chem. Sci. 2019, 11, 1985–1990. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lv, W.; Yang, F.; Zhen, S.; Huang, C. Simultaneous Imaging of Dual microRNAs in Cancer Cells through Catalytic Hairpin Assembly on a DNA Tetrahedron. ACS Appl. Mater. Interfaces 2022, 14, 12059–12067. [Google Scholar] [CrossRef] [PubMed]
- Kolovskaya, O.S.; Savitskaya, A.G.; Zamay, T.N.; Reshetneva, I.T.; Zamay, G.S.; Erkaev, E.N.; Wang, X.; Wehbe, M.; Salmina, A.B.; Perianova, O.V.; et al. Development of bacteriostatic DNA aptamers for salmonella. J. Med. Chem. 2013, 56, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Kurouski, D. Elucidation of Tip-Broadening Effect in Tip-Enhanced Raman Spectroscopy (TERS): A Cause of Artifacts or Potential for 3D TERS. J. Phys. Chem. C 2018, 122, 24334–24340. [Google Scholar] [CrossRef]
- Johansson, M.K.; Fidder, H.; Dick, D.; Cook, R.M. Intramolecular dimers: A new strategy to fluorescence quenching in dual-labeled oligonucleotide probes. J. Am. Chem. Soc. 2022, 124, 6856–6950. [Google Scholar] [CrossRef]
- Jia, W.; Xie, D.; Li, F.; Wu, X.; Wang, R.; Yang, L.; Liu, L.; Yin, W.; Chang, S. Evaluation the effect of nanoparticles on the structure of aptamers by analyzing the recognition dynamics of aptamer functionalized nanoparticles. Anal. Chim. Acta 2021, 1183, 338976. [Google Scholar] [CrossRef]
| Name | Sequence (5′–3′) |
|---|---|
| Apt1(linker1-apt). | CTATCATGGCAAGGTCCTACGCAAAACTCCTCTGACTGTAACCACGGTGGTTTGATCACTATTGGGCCTTTGTGATGTCGGTAGT |
| Apt2(linker2-apt) | CTACGTCAGCGTTAGACTGAACAAAACTCCTCTGACTGTAACCACGGTGGTTTGATCACTATTGGGCCTTTGTGATGTCGGTAGT |
| Tri | CGAGTCATCTGTCTACTGAGCCAAAAAAAAA-biotin |
| Linker | GATGACTCGTTTCAGTCTAACGCTGACGTAGTCGTAGGACCTTGCCATGATAGTGCTCAGTAGACA |
| H1 | FAM-GGTTACAGTTATGTGTACCACTGTAACCACGGTG-BHQ1 |
| H2 | TATGTGTACCTGGTTACAGTGGTACACATAACTGTAACC |
| Initiator | CACCGTGGTTACAGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Wang, Z.; Lu, W.; Peng, X.; Wang, C.; Qiao, Z.; Hua, X. Fluorescent Assay for Salmonella Detection Based on Triangle Multivalent Aptamer-Initiated Catalytic Hairpin Assembly. Chemosensors 2025, 13, 334. https://doi.org/10.3390/chemosensors13090334
Chen S, Wang Z, Lu W, Peng X, Wang C, Qiao Z, Hua X. Fluorescent Assay for Salmonella Detection Based on Triangle Multivalent Aptamer-Initiated Catalytic Hairpin Assembly. Chemosensors. 2025; 13(9):334. https://doi.org/10.3390/chemosensors13090334
Chicago/Turabian StyleChen, Shu, Zhen Wang, Wen Lu, Xingxing Peng, Chuanpi Wang, Zhaohui Qiao, and Xiude Hua. 2025. "Fluorescent Assay for Salmonella Detection Based on Triangle Multivalent Aptamer-Initiated Catalytic Hairpin Assembly" Chemosensors 13, no. 9: 334. https://doi.org/10.3390/chemosensors13090334
APA StyleChen, S., Wang, Z., Lu, W., Peng, X., Wang, C., Qiao, Z., & Hua, X. (2025). Fluorescent Assay for Salmonella Detection Based on Triangle Multivalent Aptamer-Initiated Catalytic Hairpin Assembly. Chemosensors, 13(9), 334. https://doi.org/10.3390/chemosensors13090334





