Development and Validation of an HPLC-MS/MS Method for Quantifying Deoxynivalenol and Zearalenone Biomarkers in Dried Porcine Blood Spots
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Sample Collection
2.3. Dried Blood Spots Preparation and Extraction Procedure
2.4. Extracting Dried Blood Spots
2.5. HPLC-MS/MS Performance Parameters
2.6. Method Validation
2.6.1. Limit of Detection and Quantification
2.6.2. Analytical Curve and Linearity
2.6.3. Matrix Effect and Selectivity
2.6.4. Recovery, Repeatability, and Intermediate Precision
2.7. Real Samples Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DON | Deoxynivalenol |
| ZEN | Zearalenone |
| α-ZAL | α-zearalanol |
| β-ZAL | β-zearalanol |
| α-ZEL | α-zearalenol |
| β-ZEL | β-zearalenol |
| ZAN | Zearalanone |
| DOM-1 | Deepoxy-DON |
| 3-ADON | 3-acetyl-DON |
| 15-ADON | 15-acetyl-DON |
| HPLC-MS/MS | High-performance liquid chromatography coupled with tandem mass spectrometry |
| AMAs | Anti-mycotoxin additives |
| DBSs | Dried blood spots |
| MeOH | Methanol |
| ACN | Acetonitrile |
| FA | Formic acid |
| AmAc | Ammonium acetate |
| SS | Standard solution |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| RSD | Relative standard deviation |
| Re | Mean recovery |
References
- ABPA—Associação Brasileira de Proteína Animal. Annual Report 2025; ABPA: São Paulo, Brazil, 2025; Available online: https://abpa-br.org/abpa-relatorio-anual/ (accessed on 19 May 2025).
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of Mycotoxin on Immune Response and Consequences for Pig Health. Anim. Nutr. 2016, 2, 63–68. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide Contamination of Food-Crops with Mycotoxins: Validity of the Widely Cited ‘FAO Estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Feng, N.; Wang, Y.; Noll, L.; Xu, S.; Liu, X.; Lu, N.; Zou, H.; Gu, J.; Yuan, Y.; et al. Effects of Zearalenone and Its Derivatives on the Synthesis and Secretion of Mammalian Sex Steroid Hormones: A Review. Food Chem. Toxicol. 2019, 126, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Pack, E.; Stewart, J.; Rhoads, M.; Knight, J.; De Vita, R.; Clark-Deener, S.; Schmale, D.G. Quantification of Zearalenone and α-Zearalenol in Swine Liver and Reproductive Tissues Using GC-MS. Toxicon X 2020, 8, 100058. [Google Scholar] [CrossRef] [PubMed]
- Serviento, A.M.; Brossard, L.; Renaudeau, D. An Acute Challenge with a Deoxynivalenol-Contaminated Diet Has Short- and Long-Term Effects on Performance and Feeding Behavior in Finishing Pigs. J. Anim. Sci. 2018, 96, 5209–5221. [Google Scholar] [CrossRef]
- Wellington, M.O.; Bosompem, M.A.; Rodrigues, L.A.; Columbus, D.A. Effect of Long-Term Feeding of Graded Levels of Deoxynivalenol on Performance, Nutrient Utilization, and Organ Health of Grower-Finisher Pigs (35 to 120 kg). J. Anim. Sci. 2021, 99, skab109. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Jedziniak, P.; Zielonka, Ł.; Dąbrowski, M.; Ochodzki, P.; Rudawska, A. Biomarkers of Deoxynivalenol, Citrinin, Ochratoxin A and Zearalenone in Pigs after Exposure to Naturally Contaminated Feed Close to Guidance Values. Toxins 2021, 13, 750. [Google Scholar] [CrossRef] [PubMed]
- Al-Jaal, B.A.; Jaganjac, M.; Barcaru, A.; Horvatovich, P.; Zomer, P.; Horvatovich, M. Aflatoxin, Fumonisin, Ochratoxin, Zearalenone and Deoxynivalenol Biomarkers in Human Biological Fluids: A Systematic Literature Review, 2001–2018. Food Chem. Toxicol. 2019, 129, 211–228. [Google Scholar] [CrossRef]
- Payros, D.; Alassane-Kpembi, I.; Pierron, A.; Loiseau, N.; Pinton, P.; Oswald, I.P. Toxicology of Deoxynivalenol and Its Acetylated and Modified Forms. Arch. Toxicol. 2016, 90, 2931–2957. [Google Scholar] [CrossRef]
- Llorens, P.; Herrera, M.; Juan-García, A.; Payá, J.J.; Moltó, J.C.; Ariño, A.; Juan, C. Biomarkers of Exposure to Zearalenone in In Vivo and In Vitro Studies. Toxins 2022, 14, 291. [Google Scholar] [CrossRef]
- Tonini, C.; Oliveira, M.S.; Parmeggiani, E.B.; Sturza, D.A.F.; Mallmann, A.O.; Rubin, M.I.B.; Mallmann, C.A. Serological Biomarkers of Zearalenone Exposure in Beef Heifers Receiving Anti-Mycotoxin Additive. World Mycotoxin J. 2021, 14, 357–365. [Google Scholar] [CrossRef]
- Muñoz-Solano, B.; Lizarraga Pérez, E.; González-Peñas, E. Monitoring Mycotoxin Exposure in Food-Producing Animals (Cattle, Pig, Poultry, and Sheep). Toxins 2024, 16, 218. [Google Scholar] [CrossRef] [PubMed]
- Gambacorta, L.; Olsen, M.; Solfrizzo, M. Pig Urinary Concentration of Mycotoxins and Metabolites Reflects Regional Differences, Mycotoxin Intake and Feed Contaminations. Toxins 2019, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Povedano, M.d.M.; Maris, E.; Kellner, N.; Mulisa, G.; Gámiz-Gracia, L.; García-Campaña, A.M.; De Boevre, M.; De Saeger, S.; Pero-Gascon, R. Liquid Chromatography–Tandem Mass Spectrometry for the Determination of Multiple Mycotoxins in Serum through Suspect Screening and Targeted Approaches: Advancing Human Mycotoxin Biomonitoring. Microchem. J. 2025, 208, 112562. [Google Scholar] [CrossRef]
- Chen, M.; Liu, X.; Yang, S.; Chen, Z.; Di, B.; Liu, W.; Yan, H. HPLC–MS/MS Method for the Simultaneous Determination of Aflatoxins in Blood: Toxicokinetics of Aflatoxin B1 and Aflatoxin M1 in Rats. J. Anal. Sci. Technol. 2022, 13, 27. [Google Scholar] [CrossRef]
- Panisson, J.C.; Wellington, M.O.; Bosompem, M.A.; Nagl, V.; Schwartz-Zimmermann, H.E.; Columbus, D.A. Urinary and Serum Concentration of Deoxynivalenol (DON) and DON Metabolites as an Indicator of DON Contamination in Swine Diets. Toxins 2023, 15, 120. [Google Scholar] [CrossRef]
- Guthrie, R.; Susi, A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics 1963, 32, 338–343. [Google Scholar] [CrossRef]
- Moretti, M.; Freni, F.; Tomaciello, I.; Vignali, C.; Groppi, A.; Visonà, S.D.; Tajana, L.; Osculati, A.M.M.; Morini, L. Determination of Benzodiazepines in Blood and in Dried Blood Spots Collected from Post-Mortem Samples and Evaluation of the Stability over a Three-Month Period. Drug Test. Anal. 2019, 11, 1403–1411. [Google Scholar] [CrossRef]
- Gupta, K.; Mahajan, R. Applications and Diagnostic Potential of Dried Blood Spots. Int. J. Appl. Basic. Med. Res. 2018, 8, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Malsagova, K.; Kopylov, A.; Stepanov, A.; Butkova, T.; Izotov, A.; Kaysheva, A. Dried Blood Spot in Laboratory: Directions and Prospects. Diagnostics 2020, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Lauwers, M.; Croubels, S.; De Baere, S.; Sevastiyanova, M.; Romera Sierra, E.M.; Letor, B.; Gougoulias, C.; Devreese, M. Assessment of Dried Blood Spots for Multi-Mycotoxin Biomarker Analysis in Pigs and Broiler Chickens. Toxins 2019, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Agricultura, Pecuária e Abastecimento. Portaria n° 1.304, de 7 de agosto de 2018. In Normas técnicas de instalações e equipamentos para abate e industrialização de suínos; Ministério da Agricultura, Pecuária e Abastecimento: Brasília, DF, Brasil, 2018. [Google Scholar]
- Cramer, B.; Osteresch, B.; Muñoz, K.A.; Hillmann, H.; Sibrowski, W.; Humpf, H.-U. Biomonitoring Using Dried Blood Spots: Detection of Ochratoxin A and Its Degradation Product 2’R-Ochratoxin A in Blood from Coffee Drinkers*. Mol. Nutr. Food Res. 2015, 59, 1837–1843. [Google Scholar] [CrossRef]
- Osteresch, B.; Viegas, S.; Cramer, B.; Humpf, H.-U. Multi-Mycotoxin Analysis Using Dried Blood Spots and Dried Serum Spots. Anal. Bioanal. Chem. 2017, 409, 3369–3382. [Google Scholar] [CrossRef]
- Commission Regulation (EC). No. 401/2006 of 23 February 2006 Laying down the Methods of Sampling and Analysis for the Official Control of the Levels of Mycotoxins in Foodstuffs (Text with EEA Relevance). Off. J. Eur. Union. 2006, L70, 12–34. [Google Scholar]
- Eurachem. The Fitness for Purpose of Analytical Methods: A Laboratory Guide to Method Validation and Related Topics, 2nd ed.; Eurachem: Teddington, UK, 2014; ISBN 978-91-87461-59-0. [Google Scholar]
- Brezina, U.; Rempe, I.; Kersten, S.; Valenta, H.; Humpf, H.-U.; Dänicke, S. Diagnosis of Intoxications of Piglets Fed with Fusarium Toxin-Contaminated Maize by the Analysis of Mycotoxin Residues in Serum, Liquor and Urine with LC-MS/MS. Arch. Anim. Nutr. 2014, 68, 425–447. [Google Scholar] [CrossRef] [PubMed]
- Catteuw, A.; Broekaert, N.; De Baere, S.; Lauwers, M.; Gasthuys, E.; Huybrechts, B.; Callebaut, A.; Ivanova, L.; Uhlig, S.; De Boevre, M.; et al. Insights into In Vivo Absolute Oral Bioavailability, Biotransformation, and Toxicokinetics of Zearalenone, α-Zearalenol, β-Zearalenol, Zearalenone-14-Glucoside, and Zearalenone-14-Sulfate in Pigs. J. Agric. Food Chem. 2019, 67, 3448–3458. [Google Scholar] [CrossRef]
- Fleck, S.C.; Churchwell, M.I.; Doerge, D.R. Metabolism and Pharmacokinetics of Zearalenone Following Oral and Intravenous Administration in Juvenile Female Pigs. Food Chem. Toxicol. 2017, 106, 193–201. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Durjava, M.; Dusemund, B.; Kouba, M.; López-Alonso, M.; López Puente, S.; et al. Guidance on the Assessment of the Efficacy of Feed Additives. EFSA J. 2024, 22, e8856. [Google Scholar] [CrossRef]
- Osteresch, B.; Cramer, B.; Humpf, H.-U. Analysis of Ochratoxin A in Dried Blood Spots—Correlation between Venous and Finger-prick Blood, the Influence of Hematocrit and Spotted Volume. J. Chromatogr. B 2016, 1020, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Mallmann, C.A.; Tonial Simões, C.; Kobs Vidal, J.; Rosa da Silva, C.; de Lima Schlösser, L.M.; Araújo de Almeida, C.A. Occurrence and Concentration of Mycotoxins in Maize Dried Distillers’ Grains Produced in Brazil. World Mycotoxin J. 2021, 14, 259–268. [Google Scholar] [CrossRef]
- Duarte, V.; Mallmann, A.O.; Tonini, C.; Liberalesso, D.; da Silva, C.R.; Simões, C.T.; Gressler, L.T.; Bracarense, A.P.F.R.L.; Mallmann, C.A. Ex Vivo and in Vitro Poultry Intestinal Models to Evaluate Antimycotoxins Additives. Cienc. Rural. 2021, 52, e20210277. [Google Scholar] [CrossRef]
- Alves Sarturi, J.; Tonial Simões, C.; Rosa da Silva, C.; Fabris Laber, I.; de Lima Schlösser, L.M.; Sturza, D.F.; Mallmann, C.A. An Investigation of Different Antimycotoxin Additives in Swine Intestinal Explants Challenged with Aflatoxin and Fumonisin: Ex Vivo and In Vitro Models. World Mycotoxin J. 2023, 16, 337–348. [Google Scholar] [CrossRef]
- Warth, B.; Sulyok, M.; Krska, R. LC-MS/MS-Based Multibiomarker Approaches for the Assessment of Human Exposure to Mycotoxins. Anal. Bioanal. Chem. 2013, 405, 5687–5695. [Google Scholar] [CrossRef] [PubMed]
- Rosa da Silva, C.; Tonial Simões, C.; Kobs Vidal, J.; Reghelin, M.A.; Araújo de Almeida, C.A.; Mallmann, C.A. Development and Validation of an Extraction Method Using Liquid Chromatography-Tandem Mass Spectrometry to Determine Patulin in Apple Juice. Food Chem. 2022, 366, 130654. [Google Scholar] [CrossRef] [PubMed]
- Velghe, S.; De Troyer, R.; Stove, C. Dried Blood Spots in Therapeutic Drug Monitoring and Toxicology. Expert. Opin. Drug Metab. Toxicol. 2018, 14, 1–3. [Google Scholar] [CrossRef] [PubMed]
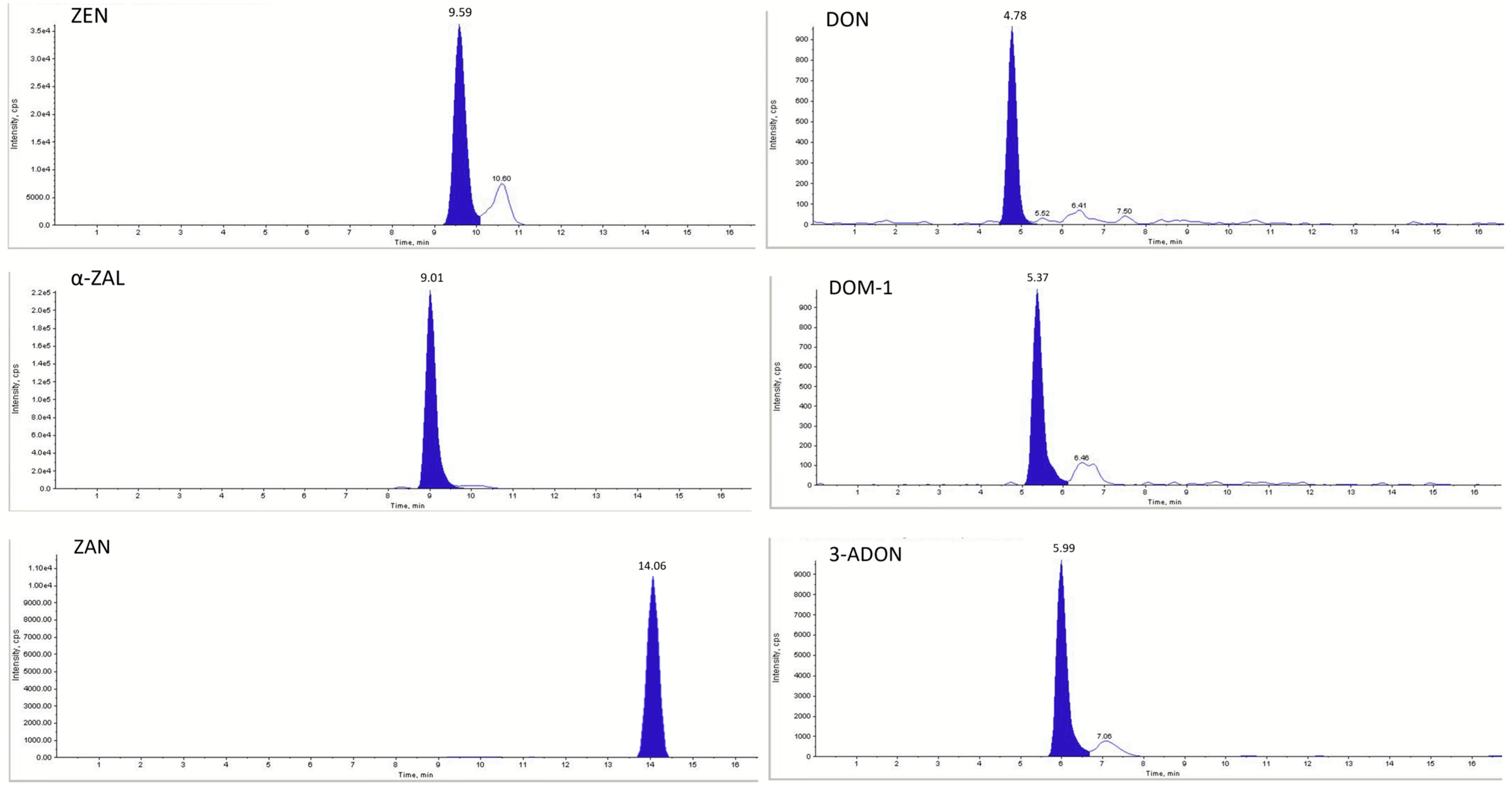
| Analyte 1 | Precursor Ion (m/z) | Product Ions (m/z) | Retention Time (min) | Declustering Potential (V) | Collision Energy (V) | Collision Cell Exit Potential (V) | |
|---|---|---|---|---|---|---|---|
| Quantification | Confirmation | ||||||
| ZEN | 317.1 | 175.0 | 130.0 | 9.59 | −175 | −35 | −17 |
| α-ZAL | 321.1 | 277.0 | 258.0 | 9.01 | −95 | −34 | −11 |
| ZAN | 321.1 | 277.0 | 258.0 | 14.06 | −175 | −45 | −17 |
| DON | 295.0 | 265.0 | 137.8 | 4.78 | −60 | −16 | −7 |
| DOM-1 | 278.9 | 260.9 | 248.8 | 5.37 | −45 | −14 | −13 |
| 3-ADON | 397.1 | 384.2 | 307.1 | 5.99 | −40 | −18 | −14 |
| Analyte 1 | Repeatability and Intra-Day Recovery (n = 7) | Intermediate Precision and Inter-Day Recovery (n = 21) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 μg/L | 40 μg/L | 60 μg/L | 20 μg/L | 40 μg/L | 60 μg/L | |||||||
| RSDr a (%) | Re b (%) | RSDr a (%) | Re b (%) | RSDr a (%) | Re b (%) | RSDR c (%) | Re d (%) | RSDR c (%) | Re d (%) | RSDR c (%) | Re d (%) | |
| ZEN | 13.81 | 91.21 | 7.20 | 91.21 | 10.56 | 78.74 | 19.18 | 90.42 | 7.64 | 87.48 | 15.07 | 89.40 |
| α-ZAL | 14.81 | 106.64 | 10.11 | 62.11 | 10.88 | 66.43 | 25.44 | 87.65 | 16.68 | 57.86 | 15.16 | 67.25 |
| ZAN | 16.63 | 79.57 | 11.05 | 78.93 | 9.87 | 81.05 | 15.40 | 80.00 | 10.30 | 78.42 | 9.99 | 80.97 |
| DON | 9.35 | 112.43 | 9.90 | 95.18 | 6.57 | 110.29 | 19.98 | 103.33 | 16.96 | 94.23 | 8.76 | 106.94 |
| DOM-1 | 25.61 | 92.43 | 8.47 | 86.64 | 7.21 | 99.17 | 33.59 | 83.48 | 20.89 | 84.24 | 26.23 | 92.22 |
| 3-ADON | 13.22 | 101.00 | 12.83 | 58.96 | 11.13 | 55.60 | 19.78 | 93.18 | 17.94 | 62.26 | 28.04 | 59.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laber, I.F.; Simões, C.T.; da Silva, C.R.; Schlösser, L.M.d.L.; Sarturi, J.A.; Leal, L.M.C.; Theobald, R.V.; Mallmann, C.A. Development and Validation of an HPLC-MS/MS Method for Quantifying Deoxynivalenol and Zearalenone Biomarkers in Dried Porcine Blood Spots. Chemosensors 2025, 13, 296. https://doi.org/10.3390/chemosensors13080296
Laber IF, Simões CT, da Silva CR, Schlösser LMdL, Sarturi JA, Leal LMC, Theobald RV, Mallmann CA. Development and Validation of an HPLC-MS/MS Method for Quantifying Deoxynivalenol and Zearalenone Biomarkers in Dried Porcine Blood Spots. Chemosensors. 2025; 13(8):296. https://doi.org/10.3390/chemosensors13080296
Chicago/Turabian StyleLaber, Isadora Fabris, Cristina Tonial Simões, Cristiane Rosa da Silva, Luara Medianeira de Lima Schlösser, Janine Alves Sarturi, Luriane Medianeira Carossi Leal, Renê Valmor Theobald, and Carlos Augusto Mallmann. 2025. "Development and Validation of an HPLC-MS/MS Method for Quantifying Deoxynivalenol and Zearalenone Biomarkers in Dried Porcine Blood Spots" Chemosensors 13, no. 8: 296. https://doi.org/10.3390/chemosensors13080296
APA StyleLaber, I. F., Simões, C. T., da Silva, C. R., Schlösser, L. M. d. L., Sarturi, J. A., Leal, L. M. C., Theobald, R. V., & Mallmann, C. A. (2025). Development and Validation of an HPLC-MS/MS Method for Quantifying Deoxynivalenol and Zearalenone Biomarkers in Dried Porcine Blood Spots. Chemosensors, 13(8), 296. https://doi.org/10.3390/chemosensors13080296






