Vitamin K Epoxide Reductase Complex (VKORC1) Electrochemical Genosensors: Towards the Identification of 1639 G>A Genetic Polymorphism
Abstract
1. Introduction
2. Materials and Methods
2.1. Apparatus and Electrodes
2.2. Reagents, Samples and Solutions
2.3. Electrochemical Genosensor Design
3. Results and Discussion
3.1. Selection of the VKORC1 SNP Probes
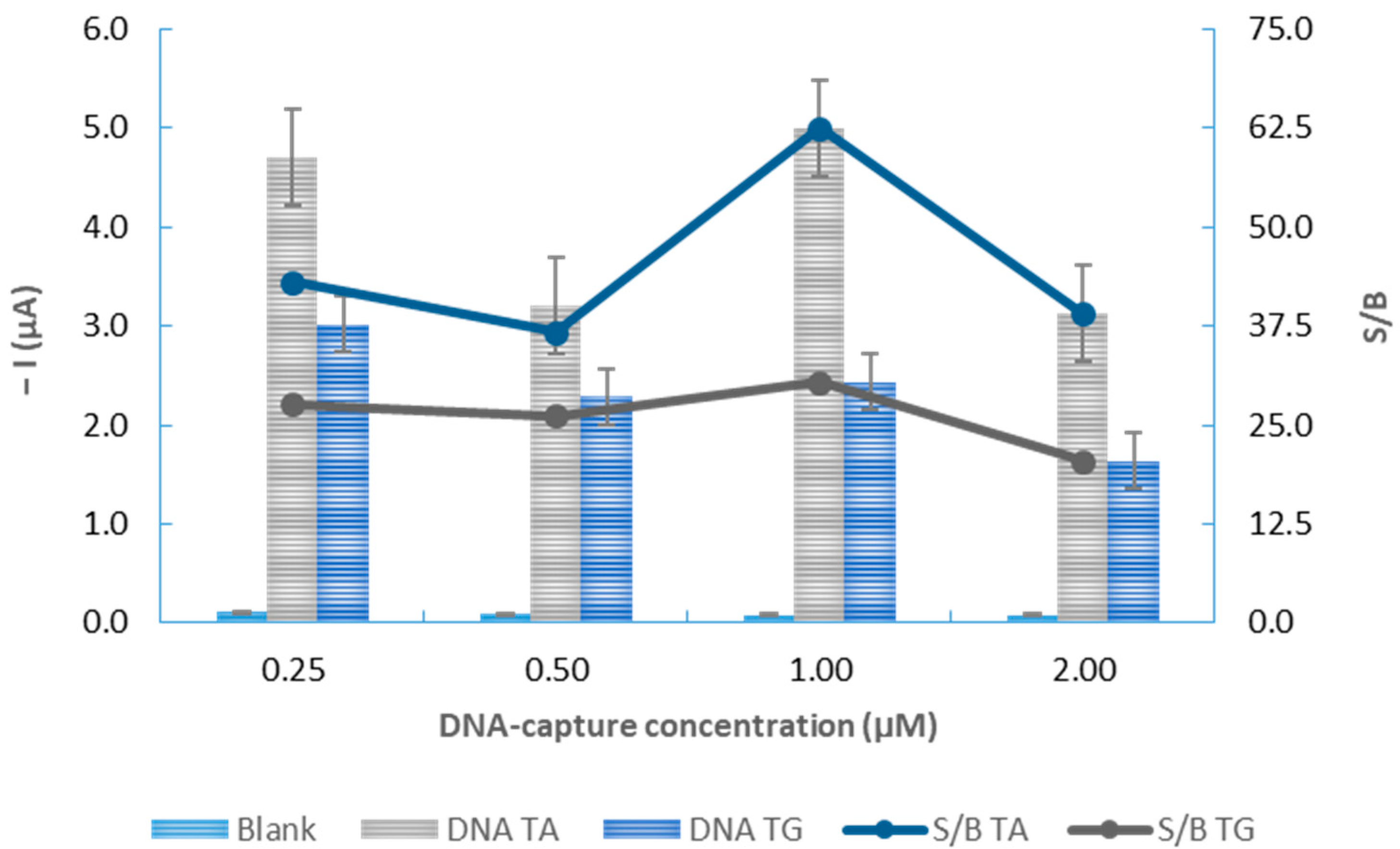
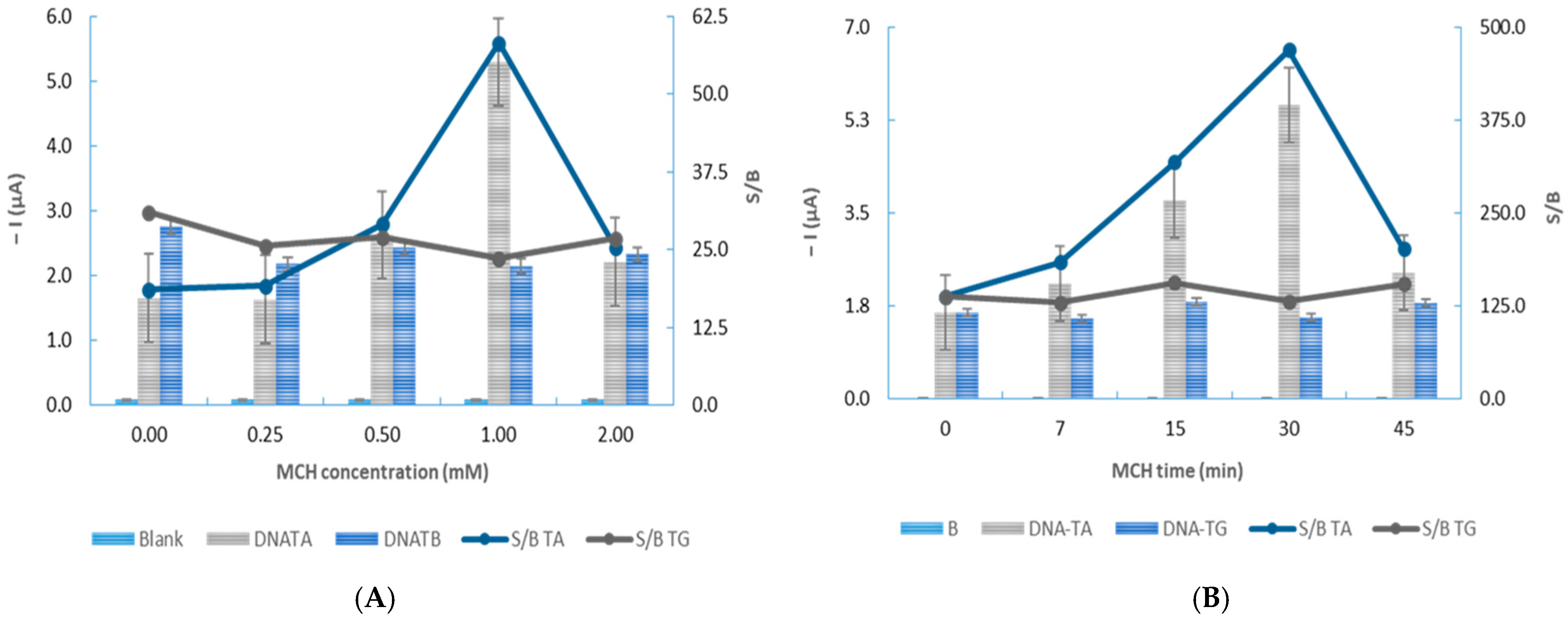
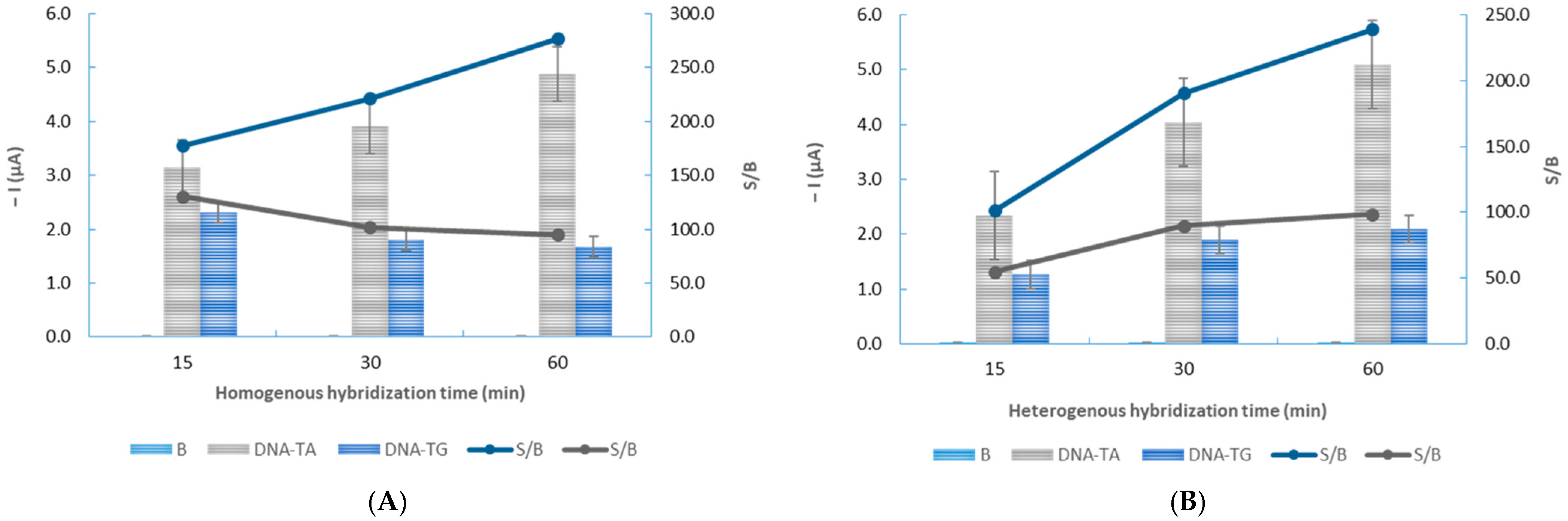
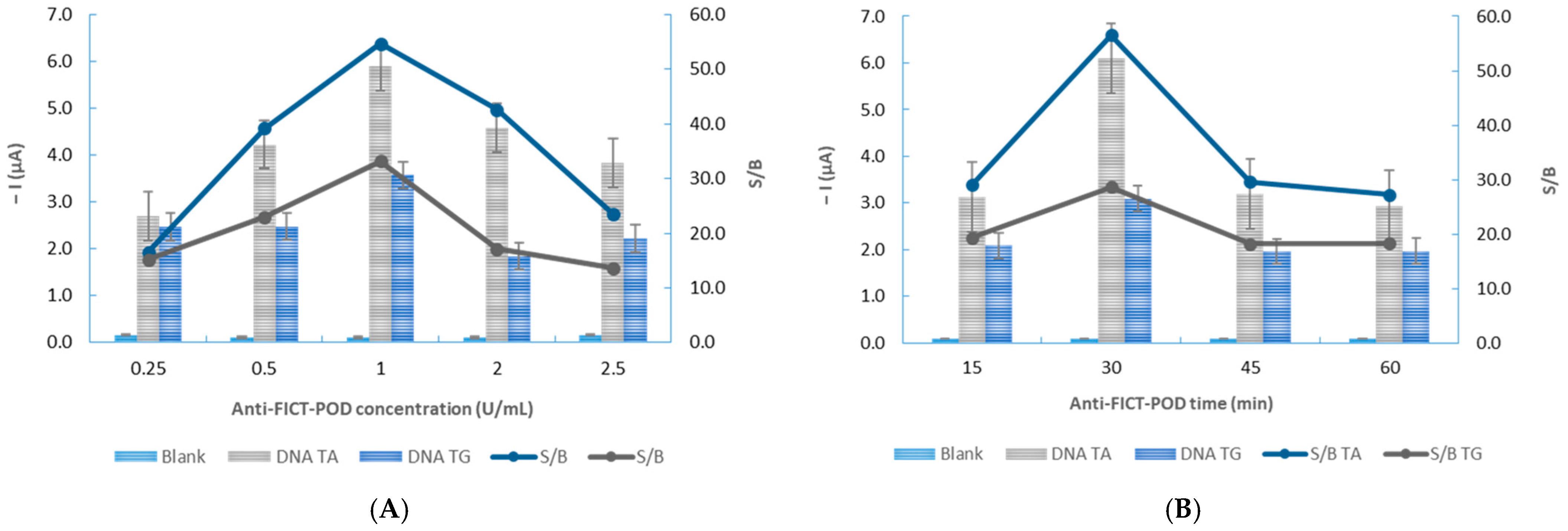
3.2. Optimization of the Experimental Variables
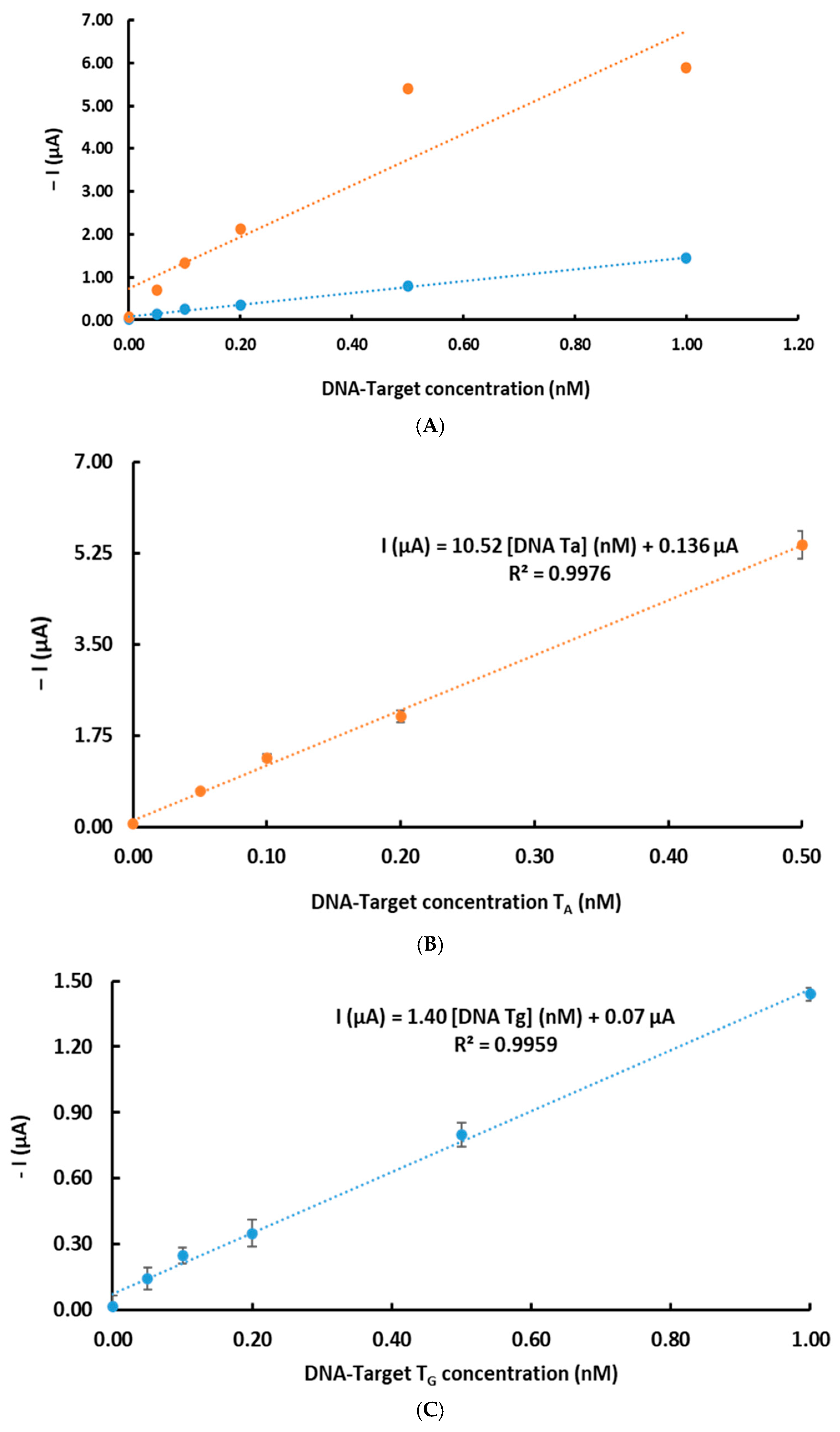
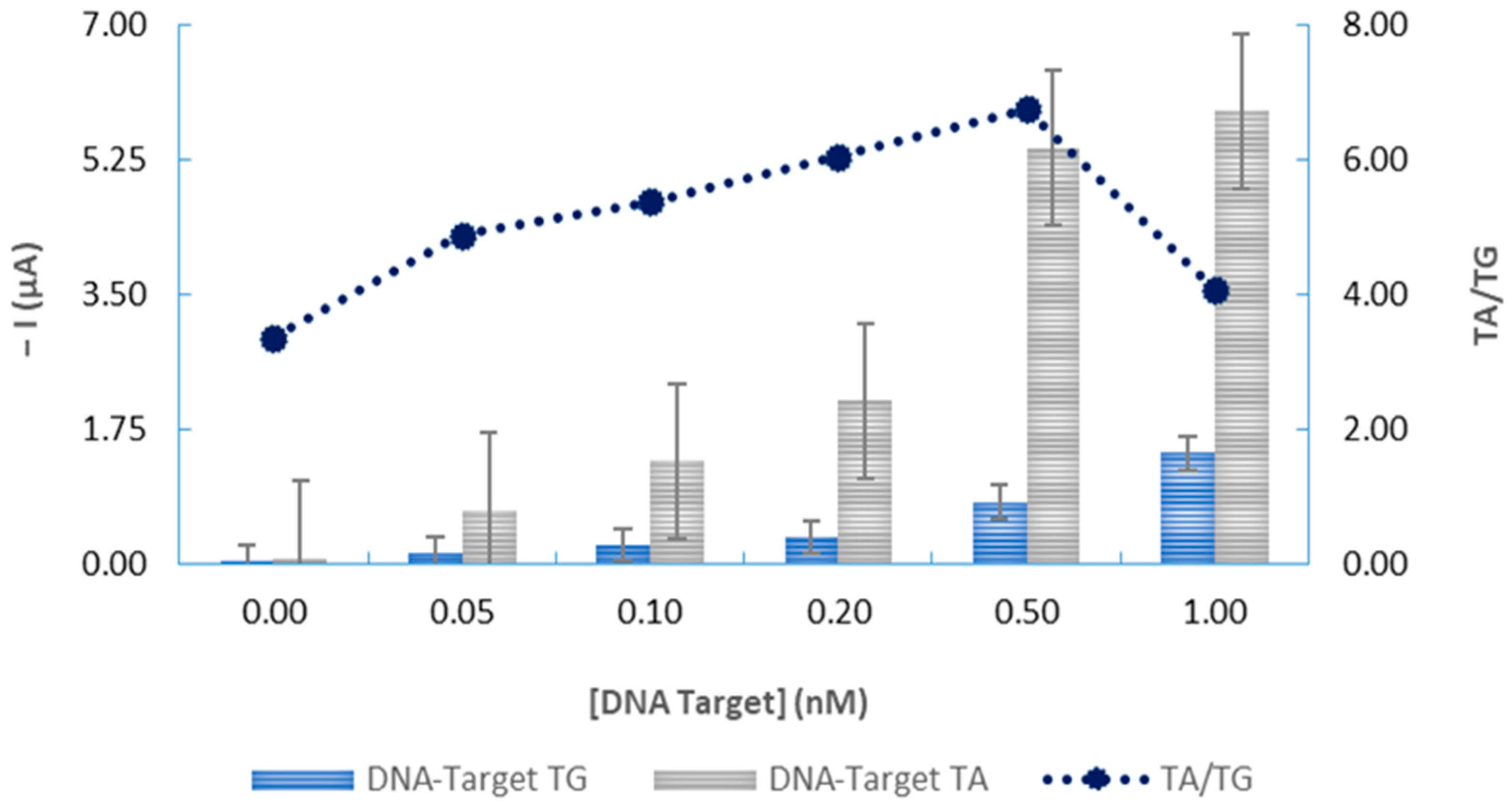
3.3. Analytical Characteristics and Genosensor Validation
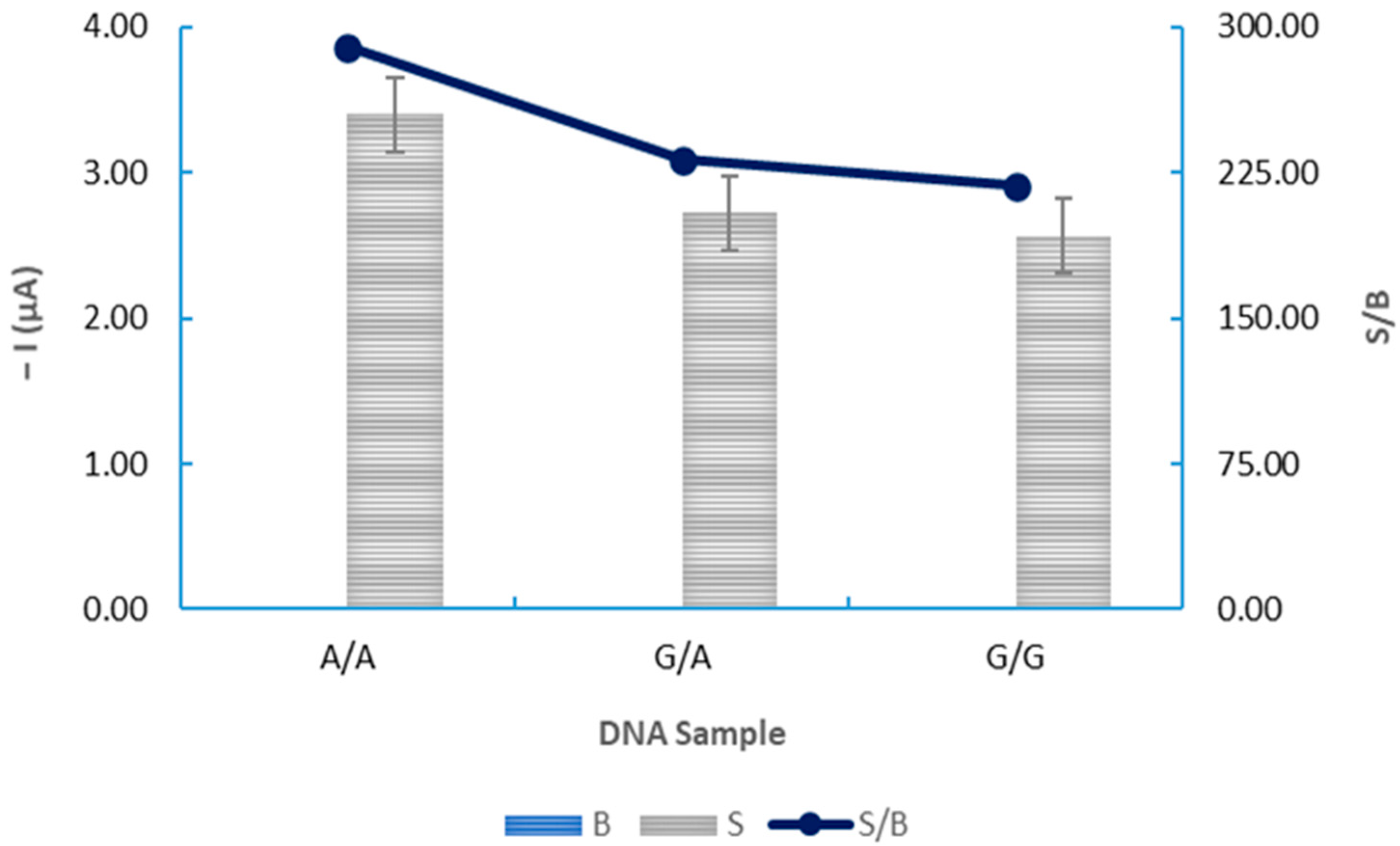
3.4. Application of the Electrochemical DNA-Based Biosensor to the Analysis of Biological DNA Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCR | Polymerase chain reaction |
| RT-PCR | Real time polymerase chain reaction |
| VKORC1 | Vitamin K epoxide reductase complex subunit 1 |
| SNP | Single nucleotide polymorphism |
| DNA | Deoxyribonucleic acid |
References
- Zhang, B.; Schmidlin, T. Recent Advances in Cardiovascular Disease Research Driven by Metabolomics Technologies in the Context of Systems Biology. Npj. Metab. Health Dis. 2024, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A.; Loscalzo, J. Emerging Role of Precision Medicine in Cardiovascular Disease. Circ. Res. 2018, 122, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Krasi, G.; Precone, V.; Paolacci, S.; Stuppia, L.; Nodari, S.; Romeo, F.; Perrone, M.; Bushati, V.; Dautaj, A.; Bertelli, M. Genetics and Pharmacogenetics in the Diagnosis and Therapy of Cardiovascular Diseases. Acta Biomed. 2019, 90, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Auwerx, C.; Sadler, M.C.; Reymond, A.; Kutalik, Z. From Pharmacogenetics to Pharmaco-Omics: Milestones and Future Directions. Hum. Genet. Genom. Adv. 2022, 3, 100100. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, R.; Vasan, R.S. Biomarkers in Cardiovascular Disease: Statistical Assessment and Section on Key Novel Heart Failure Biomarkers. Trends Cardiovasc. Med. 2017, 27, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Shubbar, Q.; Alchakee, A.; Issa, K.W.; Adi, A.J.; Shorbagi, A.I.; Saber-Ayad, M. From Genes to Drugs: CYP2C19 and Pharmacogenetics in Clinical Practice. Front. Pharmacol. 2024, 15, 1326776. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Yang, L.; Sun, Z.; Zhan, X. Personalized Drug Therapy: Innovative Concept Guided With Proteoformics. Mol. Cell Proteom. 2024, 23, 100737. [Google Scholar] [CrossRef]
- Cross, B.; Turner, R.M.; Zhang, J.E.; Pirmohamed, M. Being Precise with Anticoagulation to Reduce Adverse Drug Reactions: Are We There Yet? Pharmacogenomics J. 2024, 24, 7. [Google Scholar] [CrossRef] [PubMed]
- Dean, L. Warfarin Therapy and VKORC1 and CYP Genotype. In Medical Genetics Summaries; Pratt, V., McLeod, H., Rubinstein, W., Dean, L., Kattman, B., Malheiro, A., Eds.; National Center for Biotechnology Information: Bethesda, MD, USA, 2012. [Google Scholar]
- Poopak, B.; Rabieipoor, S.; Safari, N.; Naraghi, E.; Sheikhsofla, F.; Khosravipoor, G. Identification of CYP2C9 and VKORC1 Polymorphisms in Iranian Patients Who Are under Warfarin Therapy. Int. J. Hematol. Oncol. Stem Cell Res. 2015, 9, 185–192. [Google Scholar] [PubMed] [PubMed Central]
- Tamura, T.; Katsuda, N.; Hamajima, N. A PCR Method for VKORC1 G-1639A and CYP2C9 A1075C Genotyping Useful to Warfarin Therapy among Japanese. Springerplus 2014, 3, 499. [Google Scholar] [CrossRef] [PubMed]
- Maass, K.F.; Barfield, M.D.; Ito, M.; James, C.A.; Kavetska, O.; Kozinn, M.; Kumar, P.; Lepak, M.; Leuthold, L.A.; Li, W.; et al. Leveraging Patient-Centric Sampling for Clinical Drug Development and Decentralized Clinical Trials: Promise to Reality. Clin. Transl. Sci. 2022, 15, 2785–2795. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.L.; Barros, P.; Santos, M.; Delerue-Matos, C.; Gomes, A.C.; Fátima Barroso, M. Electrochemical Genosensor for the Detection of Alexandrium Minutum Dinoflagellates. Talanta 2021, 222, 121416. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, A.; Yamanaka, E.S.; Maquieira, Á.; Tortajada-Genaro, L.A. Allele-Specific Ligation and Recombinase Polymerase Amplification for the Detection of Single Nucleotide Polymorphisms. Sens. Actuators B Chem. 2019, 298, 126877. [Google Scholar] [CrossRef]
- Huang, T.-S.; Zhang, L.; He, Q.; Li, Y.-B.; Dai, Z.-L.; Zheng, J.-R.; Cheng, P.-Q.; He, Y.-S. DNA Sensors to Assess the Effect of VKORC1 and CYP2C9 Gene Polymorphisms on Warfarin Dose Requirement in Chinese Patients with Atrial Fibrillation. Australas Phys. Eng. Sci. Med. 2017, 40, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Guo, X.; Wang, J.; Li, H.; Ma, X. Real-Time and Label-Free Detection of VKORC1 Genes Based on a Magnetoelastic Biosensor for Warfarin Therapy. J. Mater. Chem. B. 2020, 8, 6271–6276. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.R.; Santos, S.; Ribeiro-dos-Santos, Â.; Resque, R.L.; Pinto, G.R.; Yoshioka, F.K.N. Population Data of the 46 Insertion–Deletion (INDEL) Loci in Population in Piauí State, Northeastern Brazil. Forensic Sci. Int. Genet. 2014, 9, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Zuker, M. Mfold Web Server for Nucleic Acid Folding and Hybridization Prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef] [PubMed]
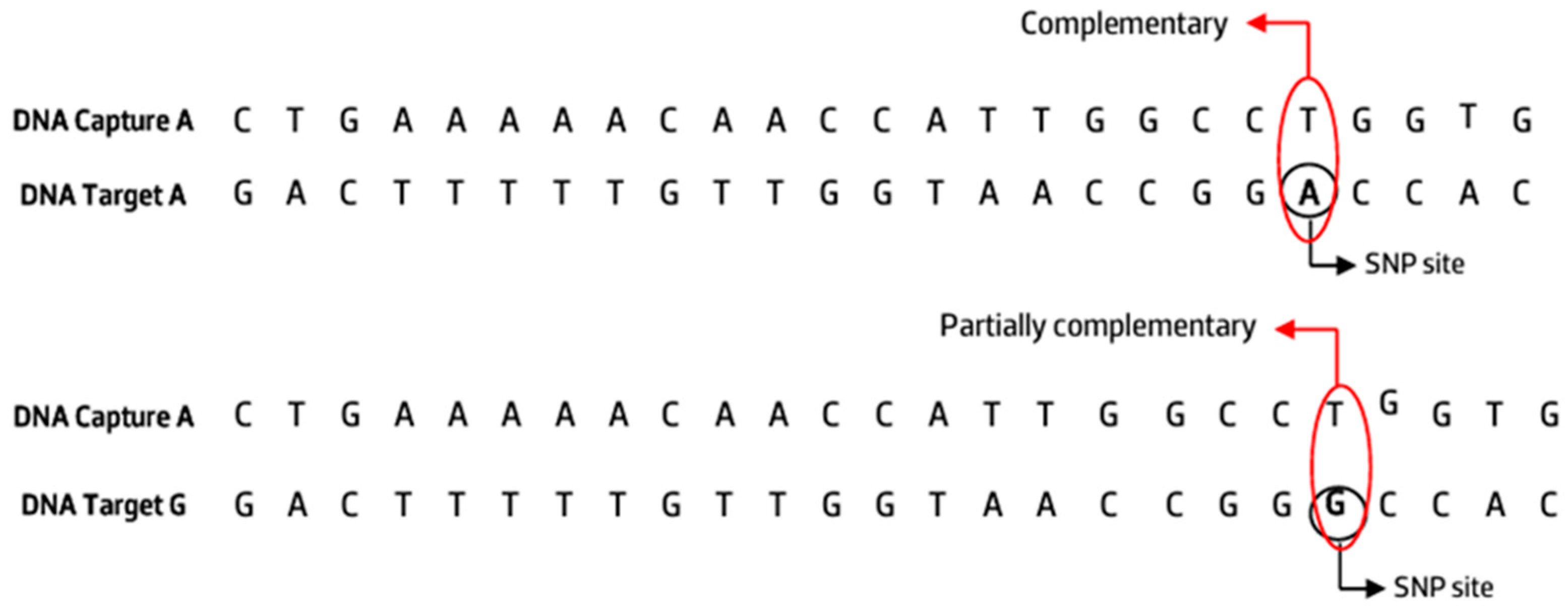
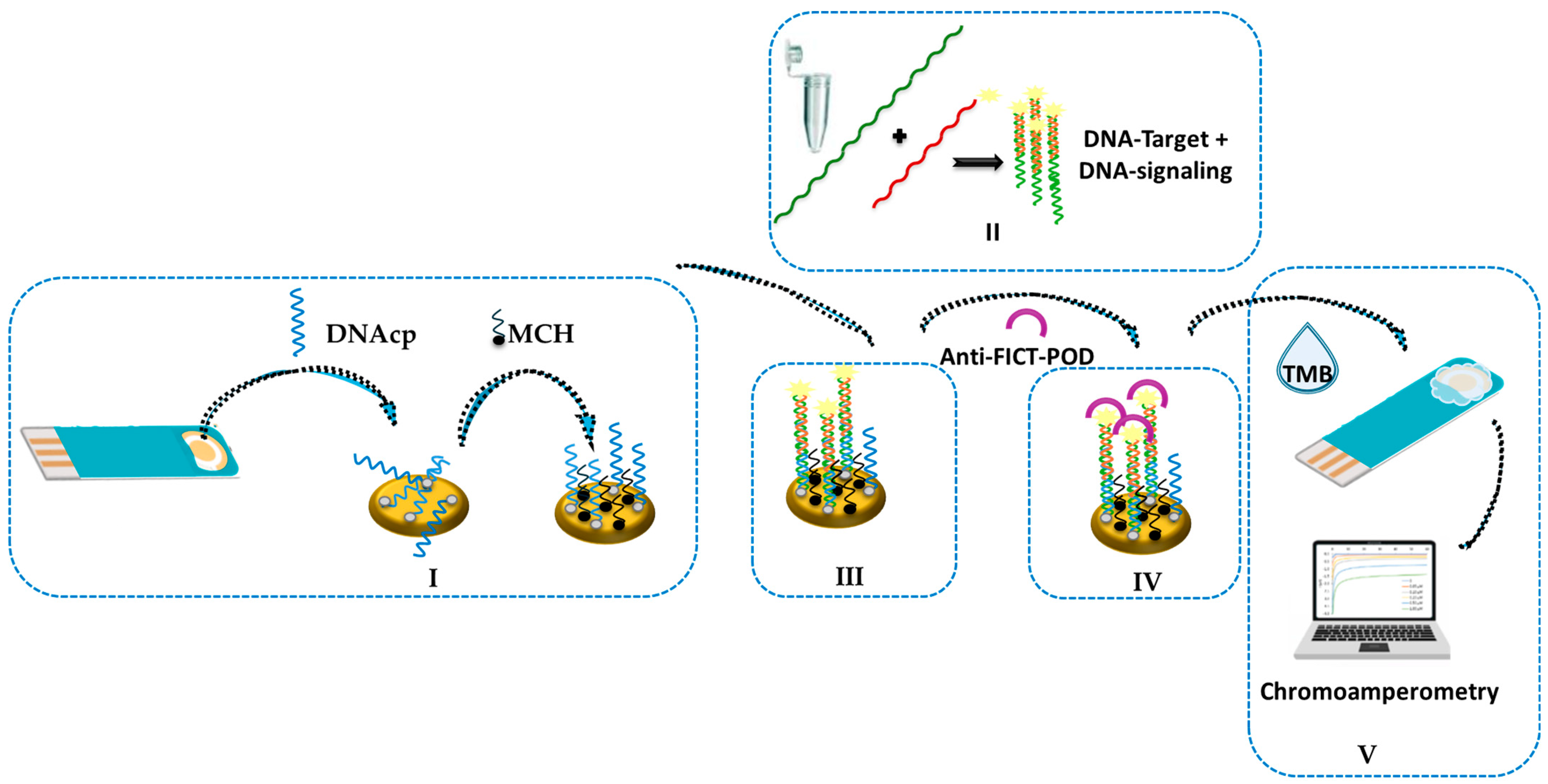
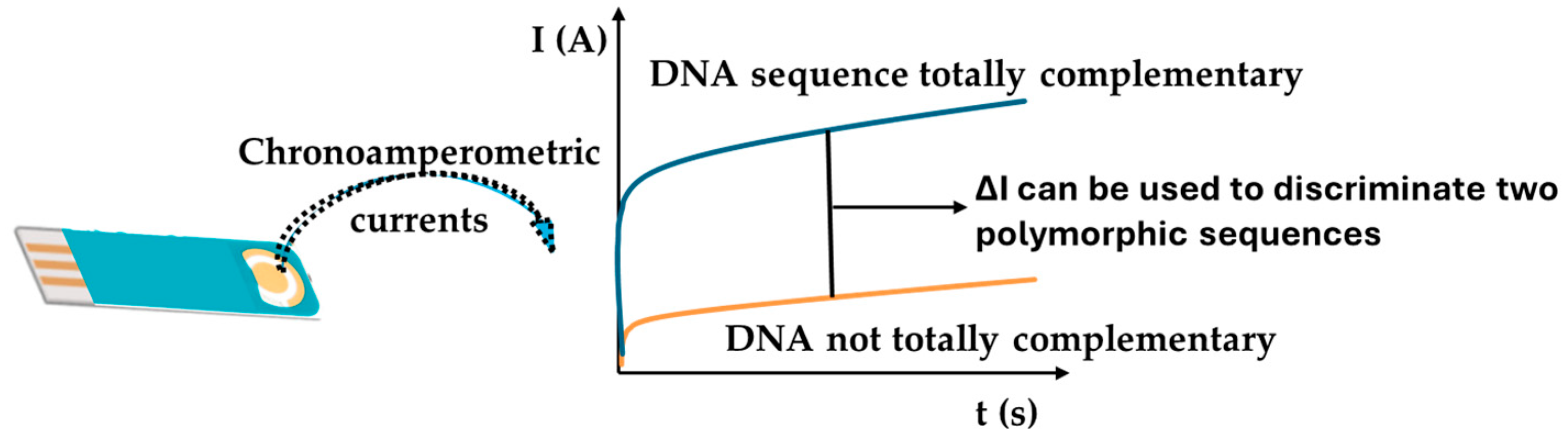
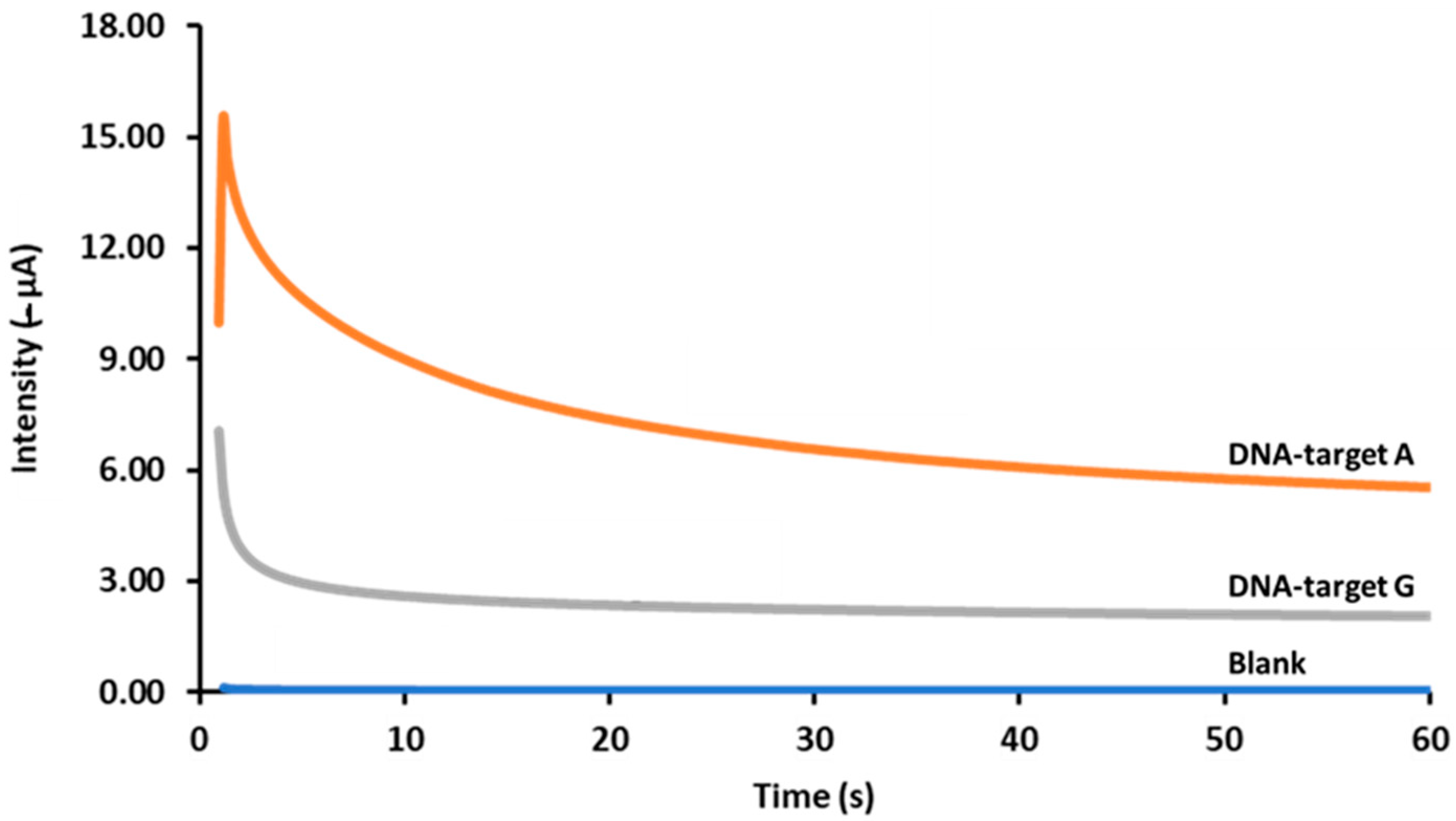

| Oligonucleotide | Sequence 5′ → 3′ | Bp |
|---|---|---|
| DNA-Capture probe | SHC6OH–CTGAAAAACAACCATTGGCCTGGTG | 25 |
| DNA-Signalling probe | CGGTGGCTCACGCCTATAATCCTAGCA- FITC | 27 |
| DNA-Target A (TA) | TGCTAGGATTATAGGCGTGAGCCACCGCACCAGGCCAATGGTTGTTTTTCAG | 52 |
| DNA-Target G (TG) | TGCTAGGATTATAGGCGTGAGCCACCGCACCGGGCCAATGGTTGTTTTTCAG | 52 |
| TA | ||
|---|---|---|
| Variables | Tested Range | Selected Value |
| DNA-capture probe concentration (µM) | 0.25–2.00 | 1.00 |
| MCH concentration (mM) | 0.50–2.00 | 1.00 |
| MCH incubation time (min) | 0–45 | 30 |
| Homogeneous hybridization incubation time (min) | 30–60 | 60 |
| Antibody incubation time (min) | 15–60 | 30 |
| Heterogeneous hybridization incubation time (min) | 15–60 | 60 |
| DNA-signalling concentration (µM) | 0.15–0.50 | 0.50 |
| Antibody concentration (U/mL) | 0.25–2.5 | 1.00 |
| Parameters | DNA-Target A | DNA-Target G |
|---|---|---|
| Linearity (nM) | 0.05–0.50 | 0.05–1.00 |
| Slope (µA/nM) | 10.52 | 1.39 |
| Interception (µA) | 0.41 | 0.07 |
| Correlation (R) | 0.998 | 0.996 |
| Slope standard deviation (µA/nM) | 0.06 | 0.04 |
| Interception standard deviation (µA/nM) | 0.03 | 0.005 |
| LD (pM) | 20.0 | 22.4 |
| LQ (pM) | 90.3 | 86.4 |
| Repeatability | 6.54 | 5.21 |
| Reproducibility (%) | 5.41 | 4.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, T.; Morais, S.L.; Carvalho, R.; Magalhães, J.M.C.S.; Domingues, V.F.; Delerue-Matos, C.; Ferreira-Fernandes, H.; Pinto, G.R.; Santos, M.; Barroso, M.F. Vitamin K Epoxide Reductase Complex (VKORC1) Electrochemical Genosensors: Towards the Identification of 1639 G>A Genetic Polymorphism. Chemosensors 2025, 13, 248. https://doi.org/10.3390/chemosensors13070248
Barbosa T, Morais SL, Carvalho R, Magalhães JMCS, Domingues VF, Delerue-Matos C, Ferreira-Fernandes H, Pinto GR, Santos M, Barroso MF. Vitamin K Epoxide Reductase Complex (VKORC1) Electrochemical Genosensors: Towards the Identification of 1639 G>A Genetic Polymorphism. Chemosensors. 2025; 13(7):248. https://doi.org/10.3390/chemosensors13070248
Chicago/Turabian StyleBarbosa, Tiago, Stephanie L. Morais, Renato Carvalho, Júlia M. C. S. Magalhães, Valentina F. Domingues, Cristina Delerue-Matos, Hygor Ferreira-Fernandes, Giovanny R. Pinto, Marlene Santos, and Maria Fátima Barroso. 2025. "Vitamin K Epoxide Reductase Complex (VKORC1) Electrochemical Genosensors: Towards the Identification of 1639 G>A Genetic Polymorphism" Chemosensors 13, no. 7: 248. https://doi.org/10.3390/chemosensors13070248
APA StyleBarbosa, T., Morais, S. L., Carvalho, R., Magalhães, J. M. C. S., Domingues, V. F., Delerue-Matos, C., Ferreira-Fernandes, H., Pinto, G. R., Santos, M., & Barroso, M. F. (2025). Vitamin K Epoxide Reductase Complex (VKORC1) Electrochemical Genosensors: Towards the Identification of 1639 G>A Genetic Polymorphism. Chemosensors, 13(7), 248. https://doi.org/10.3390/chemosensors13070248








