Abstract
Triethylamine (TEA), a volatile organic compound (VOC), has important applications in industrial production. However, TEA has an irritating odor and potential toxicity, making it necessary to develop sensitive TEA gas sensors with high efficiency. This study focused on preparing LaFeO3 nanoparticles modified by SnS2 nanosheets (SnS2/LaFeO3 composite) using a hydrothermal method together with sol–gel technique. According to the comparison results of the gas-sensing performance between pure LaFeO3 and SnS2/LaFeO3 composite with varying composition ratios, 5% SnS2/LaFeO3 sensor had a sensitivity for TEA that was 3.2 times higher than pure LaFeO3 sensor. The optimized sensor operates at 140 °C and demonstrates strong stability, selectivity, and long-term durability. Detailed analyses revealed that the SnS2 nanosheets enhanced oxygen vacancy (OV) content and carrier mobility through heterojunction formation with LaFeO3. This study provides insights into improving gas-sensing performance via p-n heterostructure design and proposes a novel LaFeO3-based material for TEA detection.
1. Introduction
The release and leakage of volatile organic compounds (VOCs) have severely affected human survival and safety over the last couple of decades [1,2,3]. Triethylamine (TEA) is a hazardous VOC primarily serving as a preservative, catalyst, and solvent in the chemical industry [4,5]. As a toxic gas, it is colorless, is transparent, and has an irritating odor. Being exposed to a TEA environment for a long term possibly induces many health issues of headaches, nausea, and skin and respiratory irritation, and in severe cases, death [6,7]. In food safety, TEA is the primary characteristic malodorous gas produced during the spoilage of aquatic products, such as fish. Its concentration directly correlates with fish freshness, making it a critical biomarker for assessing aquatic product quality and enabling food safety monitoring [8,9]. The Occupational Safety and Health Administration has confirmed an allowable exposure concentration for triethanolamine in air not to exceed 10 ppm [10,11]. Although conventional gas detection techniques are effective in accurately identifying toxic gases, they have several limitations. Therefore, efforts need to be made to develop a gas sensor with a relatively simple operation and good performance for industrial and daily life applications.
Metal oxide semiconductor (MOS)-based nanostructured materials have emerged as innovative solutions to overcome these challenges, finding extensive applications across multiple technological domains with notable implementation in catalytic processes and chemical detection systems [12,13,14]. MOS gas sensors are characterized by simple operation and low synthesis cost, and they are capable of detecting low concentrations of toxic gases [15,16]. Perovskite-type ABO3 compounds, characterized by their ternary oxide configuration comprising A- and B-site metal cations with distinct ionic radii, exhibit structurally robust crystalline frameworks that demonstrate considerable potential in gas sensor applications [17,18,19,20]. Recent advancements in LaFeO3-based sensing materials have demonstrated varied performance characteristics under different synthetic conditions [21]. Wang et al. fabricated hollow LaFeO3 microspheres with a yolk-shell structure that exhibited a gas response value of 25.5 to 100 ppm ethanol at 225 °C [22]. Li et al. fabricated Au-LaFeO3 nanocomposites with a simple wet-chemical method. The optimally proportioned Au-LaFeO3 sensor had a response value of 44 to 100 ppm ethanol at 200 °C, representing a 27-fold enhancement compared to the pristine LaFeO3 sensor, with a recovery time reduced to 8 s [23]. Xiao et al. successfully synthesized uniform porous LaFeO3 microspheres via a hydrothermal method. According to gas-sensing tests, the porous LaFeO3 microspheres had a response value of 29 to 100 ppm acetone at 260 °C [24].
Two-dimensional SnS2 with a nanosheet morphology exhibits a narrow band gap, excellent adsorption kinetics, and low gas adsorption energy, making it well applied in the gas-sensing field [25]. The SnS2 sensor developed by Yan et al. demonstrates a sensitivity of 13,000% toward 9 ppm NO2, a strong selectivity, and a low detection limit for NO2 [26]. Dong et al. adopted a hydrothermal treatment process for synthesizing α-MoO3@SnS2 nanosheets, which exhibited a response value of 114.9 to 100 ppm TEA at 175 °C. The foam-like structure, with its excellent permeability, facilitates enhanced gas-sensing performance [27]. Xu et al. applied a hydrothermal method for synthesizing SnS2/ZnS flower-like microspheres presenting a high specific surface area (SSA), achieving a response of ~11 toward 50 ppm TEA at 180 °C [28].
By reasonably building p-n heterojunctions in MOS nanomaterials to modulate the electron and hole concentration, gas-sensing performance can be effectively enhanced [29]. Shuai et al. successfully synthesized NiO/BiVO4 p-n heterojunction microspheres via hydrothermal treatment. The 10% NiO-BiVO4 sensor presented a gas response of 183.11 to 30 ppm TEA versus the pure BiVO4 sensor [30]. Wu et al. synthesized In2O3-decorated Mn2O3 nanorod-based multidimensional nanostructures via a hydrothermal method, which exhibited an enhanced SSA. The In2O3/Mn2O3 composite with an In:Mn ratio of 1:0.12 demonstrated a response value of 44.3 toward 100 ppm TEA at 180 °C [31].
The study successfully synthesized SnS2/LaFeO3 composite by combining the hydrothermal method with high-temperature annealing treatment. In the SnS2/LaFeO3 composite material, the combination of p-type LaFeO3 and n-type SnS2 forms a p-n heterojunction. This creates a built-in electric field at the interface, enabling more efficient carrier separation and transport. The introduction of SnS2 increases the specific surface area of the composite material, providing more reactive sites for the target gas and thereby enhancing gas-sensing performance. The 5% SnS2/LaFeO3 sensor demonstrates a response of 124.5 to 100 ppm TEA, along with excellent selectivity and long-term stability. Compared to similar sensors, the performance of our sensor significantly outperforms comparable counterparts. For example, Hao et al. fabricated a RuO2/LaFeO3 gas sensor exhibiting a response value of 60.4 towards 100 ppm TEA at 260 °C [32]. Ma et al. fabricated a WO3-SnWO4 nanorod-based gas sensor showing a response value of 80.2 towards 100 ppm TEA at 130 °C [33]. Zhao et al. fabricated a gas sensor based on WO3-modified ErVO4 nanoparticles. At its optimal operating temperature of 200 °C, the ErVO4/WO3 composite exhibited a response of 15.79 towards 100 ppm TEA [34]. The LaFeO3/ZnO composite prepared by Ni et al. exhibited a response value of 50 to 100 ppm TEA at 200 °C [35].
2. Experiment
2.1. Preparation of LaFeO3 Nanoparticles and SnS2 Nanosheets
The preparation of LaFeO3 nanoparticles was achieved by using the citric acid sol–gel method [36] by first dissolving Fe(NO3)3·9H2O and La(NO3)3·6H2O in 50 mL of deionized water (DW) to form an orange solution A and then dissolving citric acid (210.0 mg, 1 mmol) in 30 mL of DW to obtain a colorless transparent solution B. The two solutions were mixed under magnetic agitation, followed by 5 h of continuous stirring at 80 °C until obtaining a reddish-brown gel. After 12 h of drying treatment, the gel underwent 2 h of calcination at 350 °C and 4 h of calcination at 700 °C in succession to synthesize the LaFeO3.
A one-step hydrothermal method was applied for synthesizing SnS2 nanosheets [37]. Firstly, a colorless transparent solution was obtained by completely dissolving SnCl4·5H2O (17.5 mg, 0.05 mmol) and thiourea (22.8 mg, 0.3 mmol) in 50 mL of DW. The hydrothermal reaction proceeded for 12 h at 180 °C, followed by the collection of the generated yellow product.
2.2. Synthesis of SnS2/LaFeO3 Composite
LaFeO3 powder (100 mg) was dissolved in a 40 mL ethylene glycol (EG) solution using 30 min of ultrasonication. SnS2 powder with varying masses ultrasonically dispersed in 20 mL of EG solution was added dropwise into the LaFeO3 solution before half an hour of stirring. The mixture underwent 12 h of hydrothermal heating at 160 °C to obtain a powder sample. The powder sample was sequentially rinsed using DW and ethanol and dried again. The SnS2/LaFeO3 composite with different contents (5 wt%, 10 wt%, and 20 wt%) could be made. The resulting materials were designated as 5% SnS2/LaFeO3, 10% SnS2/LaFeO3, and 20% SnS2/LaFeO3, respectively.
2.3. Control Experiment: In Situ Synthesis of SnS2/LaFeO3 Composite
LaFeO3 powder (100 mg) was dissolved in a 40 mL ethylene glycol (EG) solution using 30 min of ultrasonication. Afterwards, specific quantities of SnCl4·5H2O (0.025, 0.05, and 0.075 mmol) and thiourea (0.15, 0.3, and 0.45 mmol) were completely dissolved in 20 mL of ethylene glycol and then added dropwise to the LaFeO3 solution under magnetic stirring. The mixture underwent 12 h of hydrothermal heating at 160 °C to obtain a powder sample. The powder sample was sequentially rinsed using DW and ethanol and dried again. The obtained SnS2/LaFeO3 composite with different molar ratios are designated as SnS2/LaFeO3-1, SnS2/LaFeO3-2, and SnS2/LaFeO3-3.
2.4. Characterizations of Gas-Sensitive Materials
X-ray diffraction (XRD) was employed to analyze the composition, crystal type, and grain size of the samples. The model of the diffractometer used in this study was a SHIMADZU XRD-7000S (SHIMADZU, Kyoto, Japan). The scanning 2θ angle range during testing was 5–80°, with a scanning speed of 5°/min. Field-emission scanning electron microscopy (SEM) was used to observe the microscopic morphology of the synthetic material precursors and target samples. The model of the SEM used in this study was an FEI Nova NanoSEM450 (FEI, Hillsboro, OR, USA). Prior to testing, high-purity ethanol, clean silicon wafers, conductive adhesive, and other materials were prepared, and the accelerating voltage was set to 18 kV. To test the specific surface area, pore volume, and pore size distribution of the samples, a fully automated physical adsorption instrument produced by Beijing JWGB Technology Co., Ltd. (Beijing, China), model BK100C, was used to analyze the experimental materials. Before testing, cleaned BET tubes and a certain amount of liquid nitrogen were prepared. To determine the chemical composition, elemental valence states, and their percentages on the surface of the target samples, X-ray photoelectron spectroscopy (XPS) was performed using an X-ray photoelectron spectrometer manufactured and designed by Thermo Fisher Scientific (Waltham, MA, USA), model ESCALABTM 250Xi.
2.5. Fabrication of the Gas Sensors
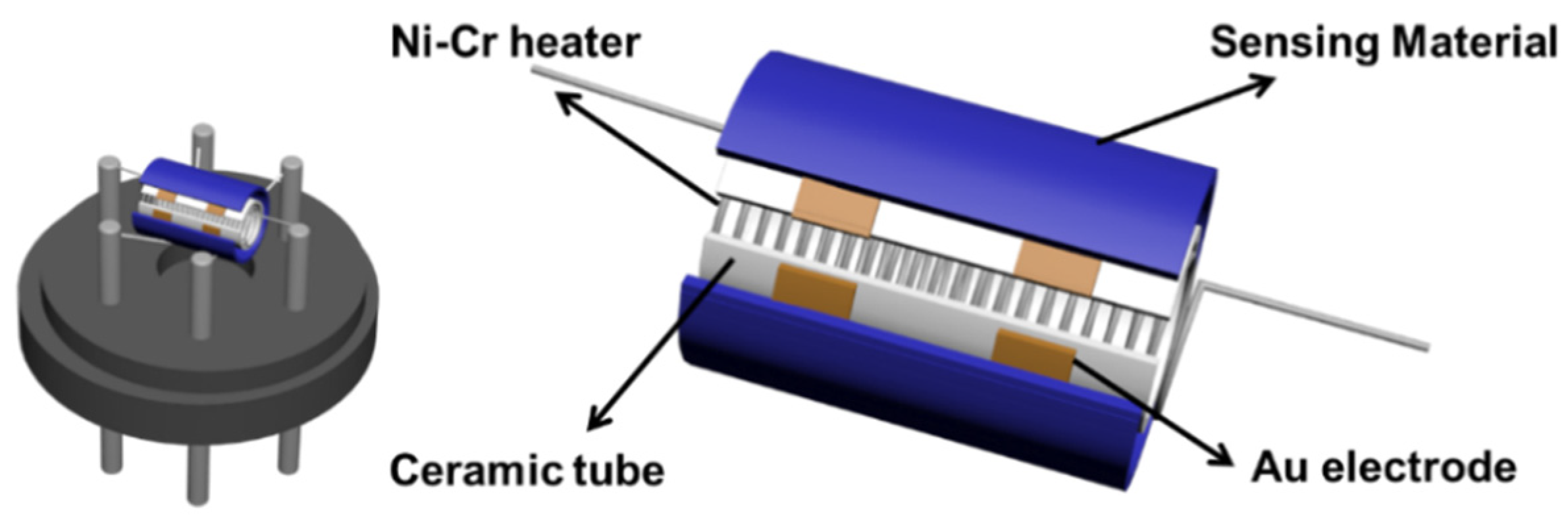
Gas sensors were constructed and measured following a previous description [38]. Initially, a specific amount of pure LaFeO3, pure SnS2, and SnS2/LaFeO3 composite was dispersed in DW. This suspension was then used to prepare a slurry with the appropriate viscosity for coating onto the ceramic tube for fabricating the sensors (Figure 1). A CGS-8 intelligent gas-sensitive analysis system (Beijing Elite Technology, Beijing, China) was employed for testing the sensor’s sensitivity (Ra/Rg or Rg/Ra). Ra and Rg denote the resistance values of the sensor in air and the target gas, respectively. The response and recovery time denote the duration necessary for the sensor to achieve 90% of the total resistance variation in the target gas and air.
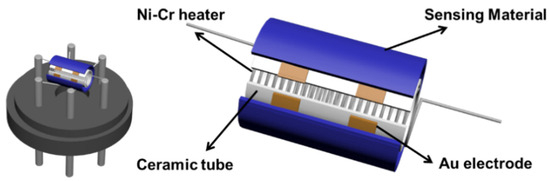
Figure 1.
The structure of the gas-sensing device.
3. Results and Discussion
3.1. Structural and Morphological Characteristics
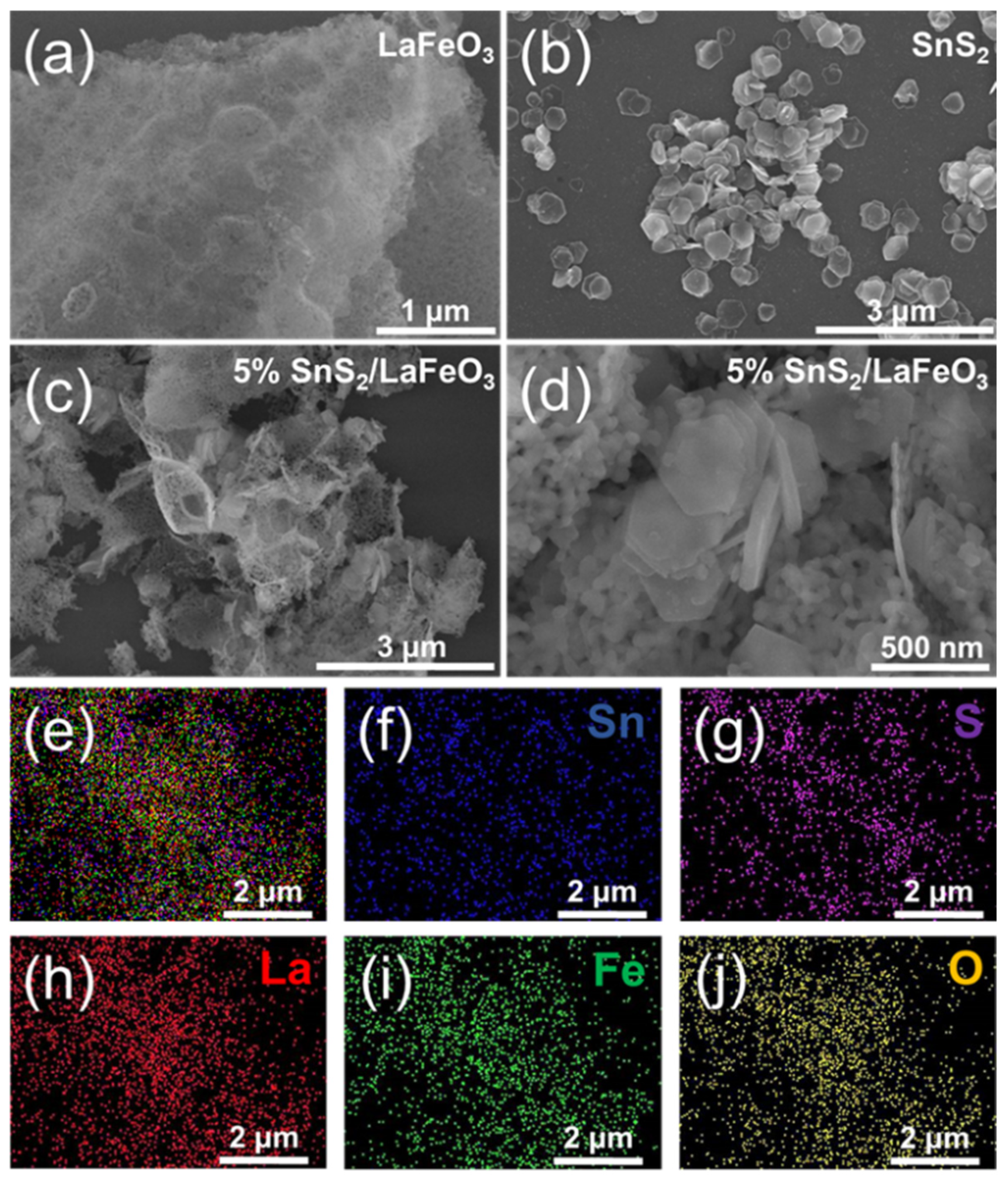
Figure 2 presents the microscopic morphology and elemental distribution of pure SnS2, pure LaFeO3, and the 5% SnS2/LaFeO3 composite. As shown in Figure 2a, LaFeO3 primarily consists of aggregated nanoparticle structures, while SnS2 in Figure 2b exhibits a well-defined hexagonal morphology. Figure 2c,d reveal that SnS2 nanosheets in the 5% SnS2/LaFeO3 composite are randomly and densely anchored onto the LaFeO3 nanoparticle framework, with no significant alterations in their original dimensions or morphology after composite formation. Elemental mapping images (Figure 2e–j) demonstrate a homogeneous spatial distribution of Sn, S, La, Fe, and O components, further confirming the successful synthesis of the SnS2/LaFeO3 composite.
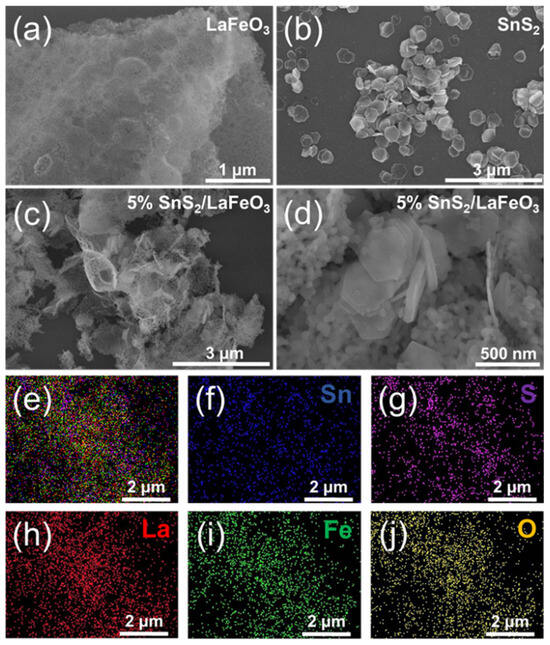
Figure 2.
SEM images of (a) LaFeO3 nanoparticles, (b) SnS2 nanosheets, and (c,d) 5% SnS2/LaFeO3 composite. EDS spectrum of (e–j) for the 5% SnS2/LaFeO3 composite.
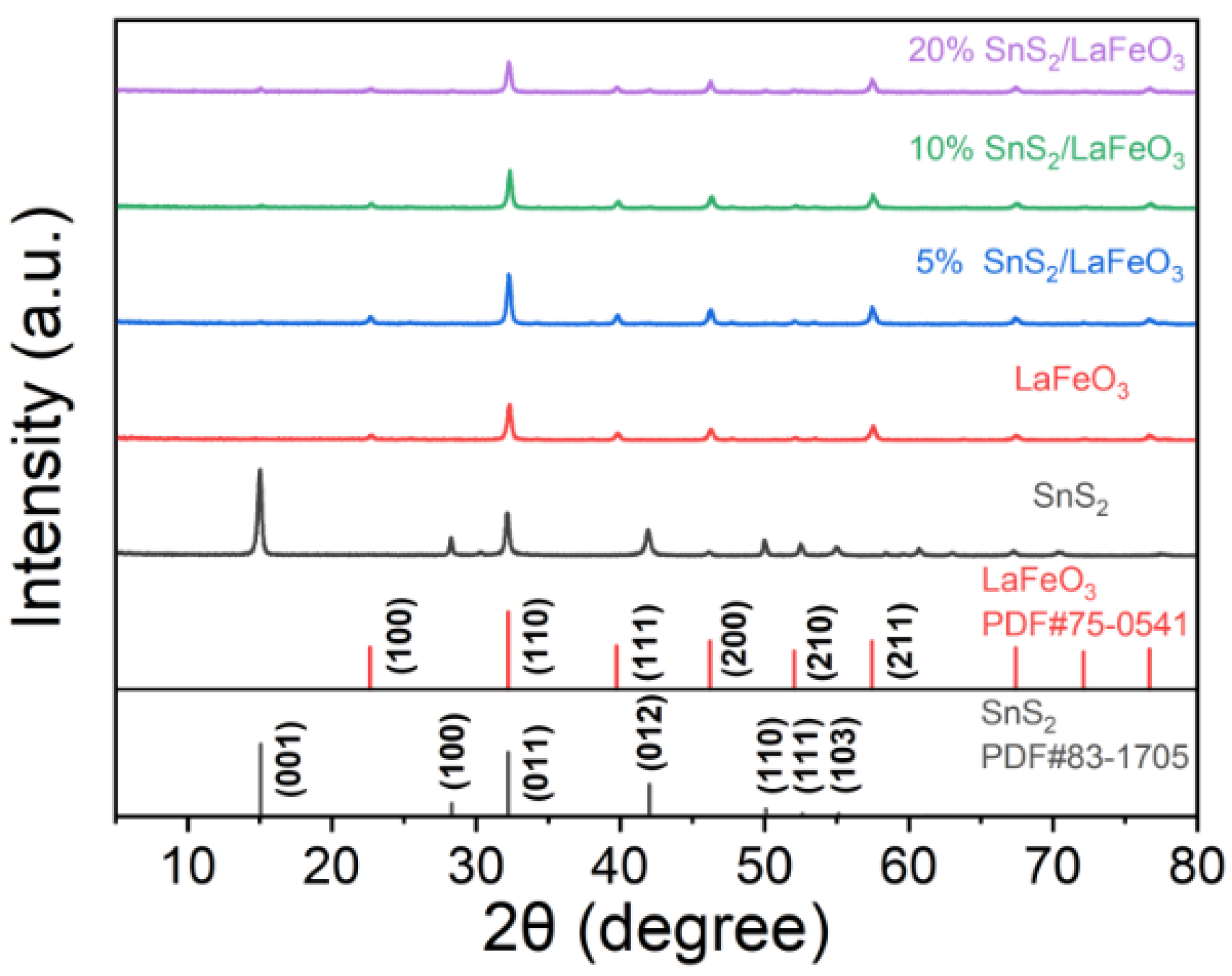
According to Figure 3, the SnS2/LaFeO3 composite with varying content ratios has diffraction peaks corresponding to the pure LaFeO3 crystal phase (JCPDS Card No. 75-0541) [39]. With rising SnS2 content, the intensities of the diffraction peaks at 15.02° and 41.87° in the SnS2/LaFeO3 composite gradually become more pronounced, matching those of pure SnS2 crystals (JCPDS Card No. 83-1705) [40]. The SnS2/LaFeO3 composite is successfully synthesized, as evidenced by the impurity peaks not being detected in the patterns.
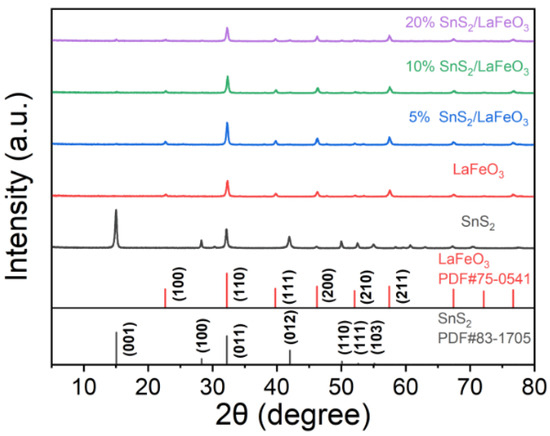
Figure 3.
XRD patterns of pure LaFeO3, pure SnS2, and SnS2/LaFeO3 composite.
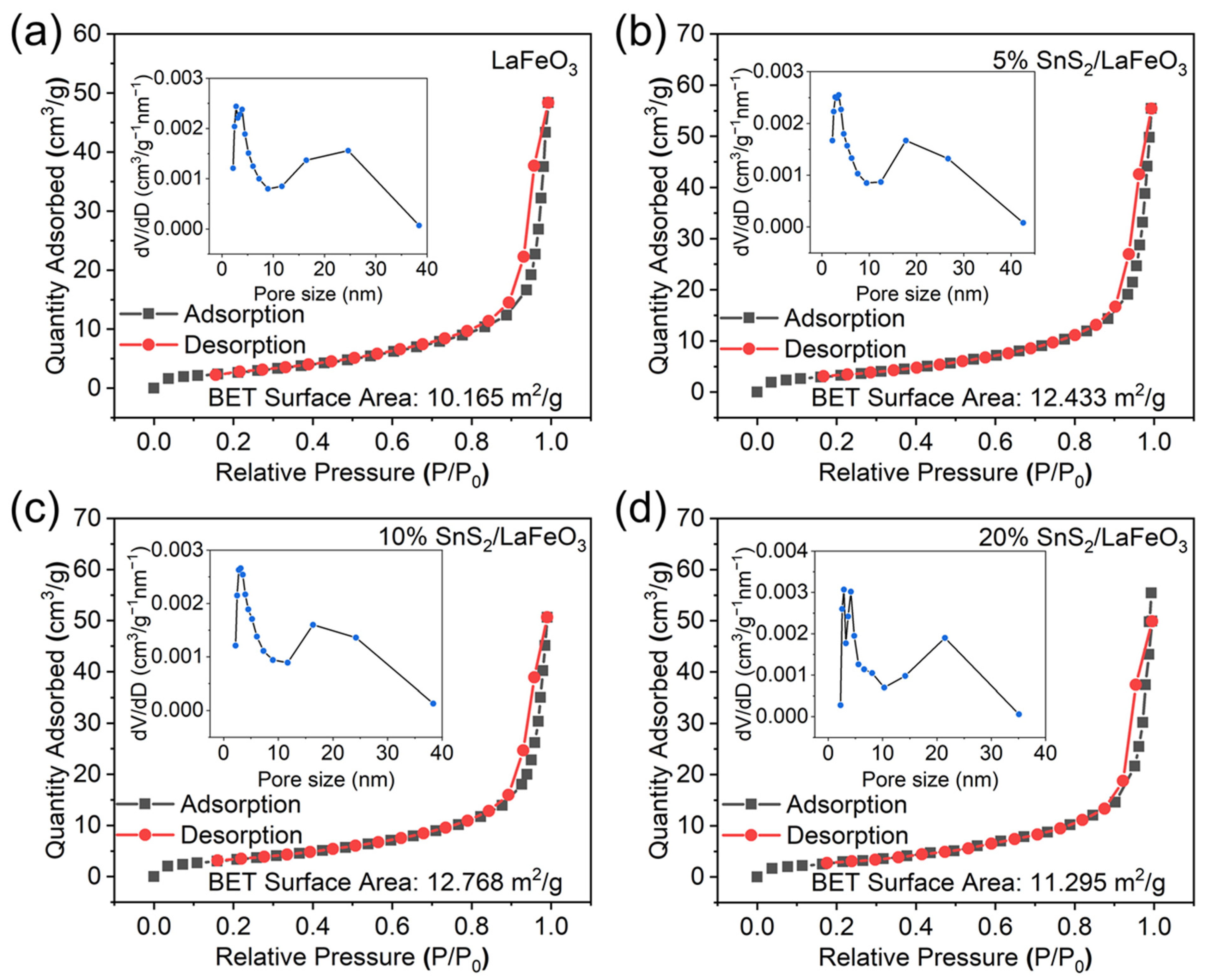
The results from Figure 4 and Table 1 show that specific surface areas (SSAs) of LaFeO3 and 5% SnS2/LaFeO3 are 10.165 m2/g and 12.433 m2/g, respectively. Incorporating SnS2 elevates the material’s SSA. A larger SSA offers more reactive sites, contributing to improved gas-sensing performance. Although the sample of 10% SnS2/LaFeO3 has the largest SSA, there are still other key factors that affect the gas-sensing performance, such as the sensors’ baseline resistances in air, which is discussed later.
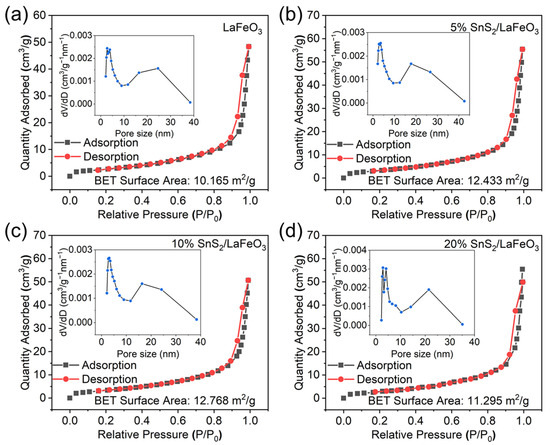
Figure 4.
N2 adsorption/desorption curves: (a) LaFeO3; (b–d) 5%, 10%, and 20% SnS2/LaFeO3.

Table 1.
Samples’ textural parameters from BET analysis.
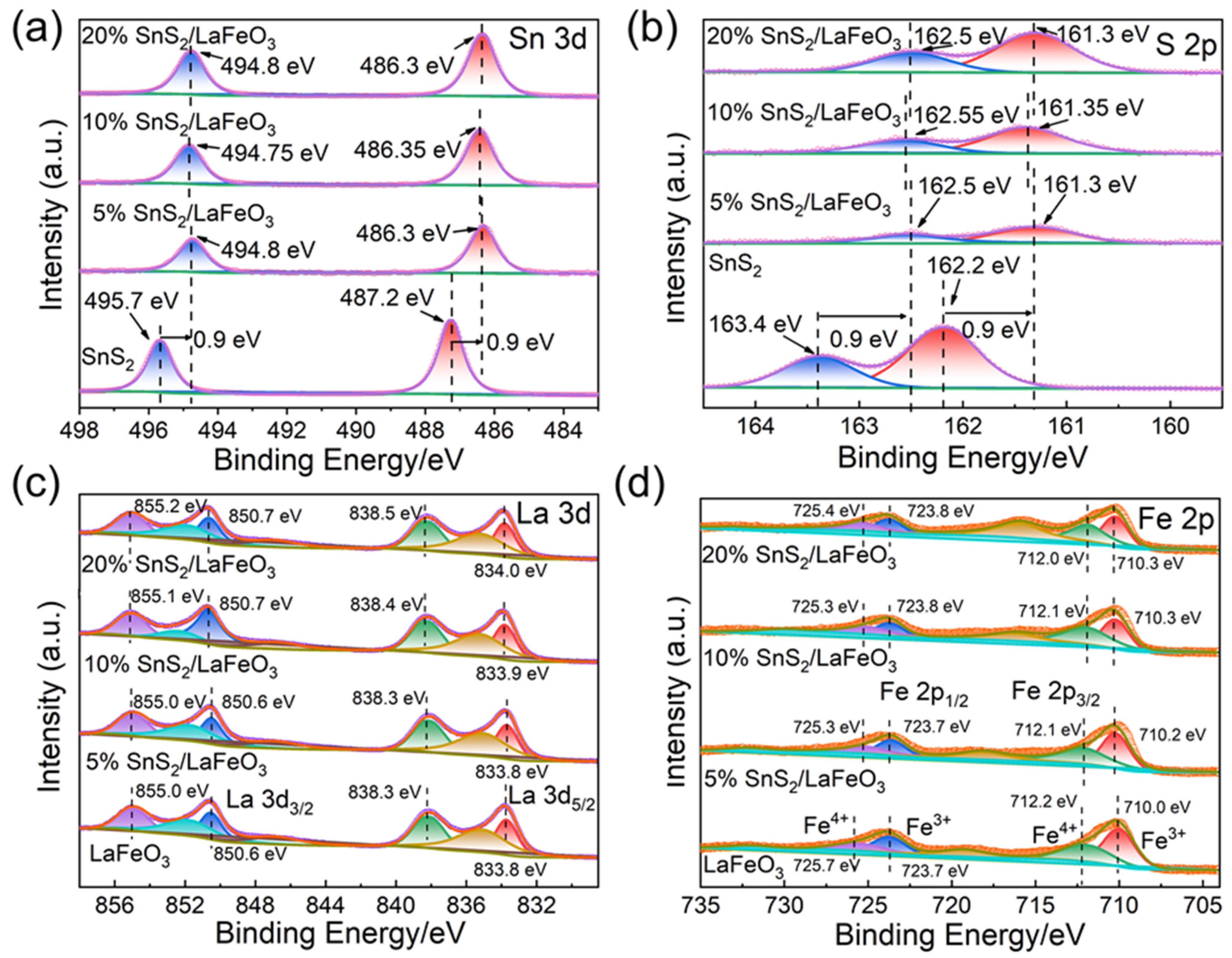
The XPS analysis results revealed the chemical states of various elements in pure SnS2, pure LaFeO3, and SnS2/LaFeO3 composite with different ratios. As shown in Figure 5a, the doublet peaks at 487.2 and 495.7 eV are assigned to the Sn4+ 3d5/2 and Sn4+ 3d3/2 orbitals of SnS2 [41]. Compared with pure SnS2, the Sn4+ 3d5/2 and Sn4+ 3d3/2 peaks in the SnS2/LaFeO3 composite exhibited a 0.9 eV shift to lower binding energies. Similarly, the doublet peaks at 162.2 and 163.4 eV in Figure 5b are assigned to the S2− 2p3/2 and S2− 2p1/2 orbitals of SnS2, respectively [41]. These S2− 2p peaks in the SnS2/LaFeO3 composite also showed a 0.9 eV negative shift relative to those in pure SnS2. The observed binding energy shifts of the elements demonstrate a strong electronic interaction between SnS2 and LaFeO3, ultimately promoting SnS2/LaFeO3 heterojunctions to be formed.
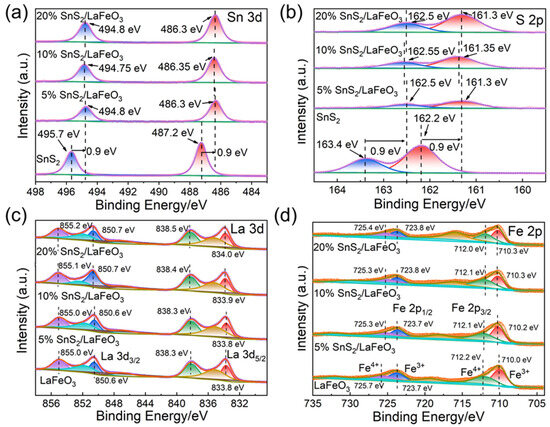
Figure 5.
The high-resolution XPS spectra of Sn 3d (a), S 2p (b), La 3d (c), and Fe 2p (d) for all samples.
In Figure 5c, the peaks located at 833.7, 838.2, 850.6, and 855.0 eV are assigned to the La3+ 3d5/2 and La3+ 3d3/2 orbitals in pure LaFeO3, accompanied by three satellite peaks. Compared to pure LaFeO3, the La 3d peaks in the SnS2/LaFeO3 composite exhibit a shift toward higher binding energy by approximately 0.1–0.2 eV [42]. In Figure 5d, the peaks at 710.0 and 723.7 eV target the Fe3+ 2p3/2 and 2p1/2 orbitals, while those at 712.2 and 725.5 eV target the Fe4+ 2p3/2 and 2p1/2 orbitals [43,44]. Binding energy presents a slight shift resulting from the heterostructure effect at the interface of the SnS2/LaFeO3 composite.
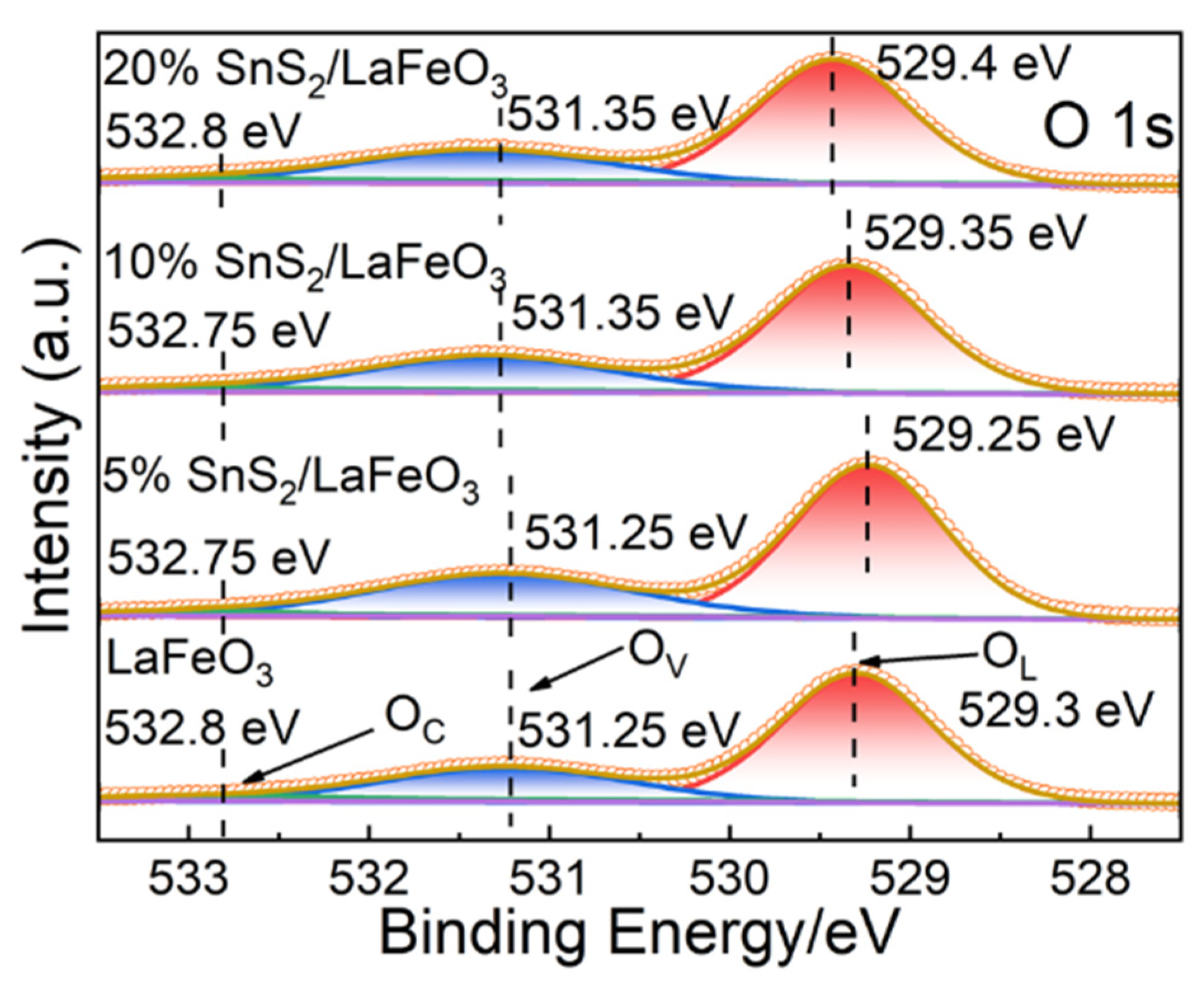
In Figure 6, the O 1s binding energy peak is deconvoluted into three peaks located at 529.3, 531.25, and 532.8 eV, targeting lattice oxygen (OL), oxygen vacancies (OV), and chemisorbed oxygen (OC), respectively [45,46], with relevant peak area ratios displayed in Table 2. The 5% SnS2/LaFeO3 composite exhibit a higher OV content than pure LaFeO3. Oxygen vacancies (OV) are active sites for surface oxygen adsorption, capable of accelerating surface redox reactions [47]. Generally, a higher OV content more remarkably strengthens gas-sensing performance.
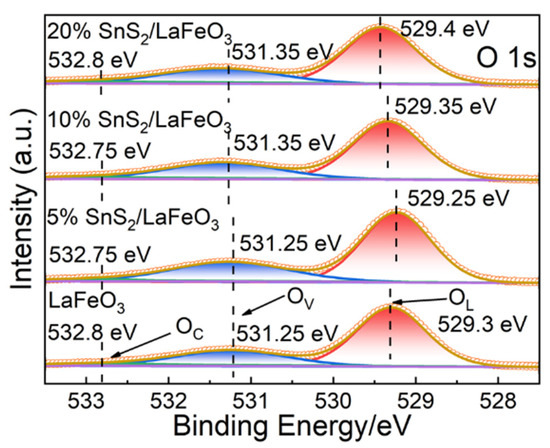
Figure 6.
The high-resolution XPS spectra of O 1s for all samples.

Table 2.
XPS O1s peak area ratios of varying oxygen species.
According to the analyses from XRD, SEM, EDS, and XPS, the synthesis of the SnS2/LaFeO3 composite was successfully accomplished.
3.2. Gas-Sensing Properties of the Nanomaterials
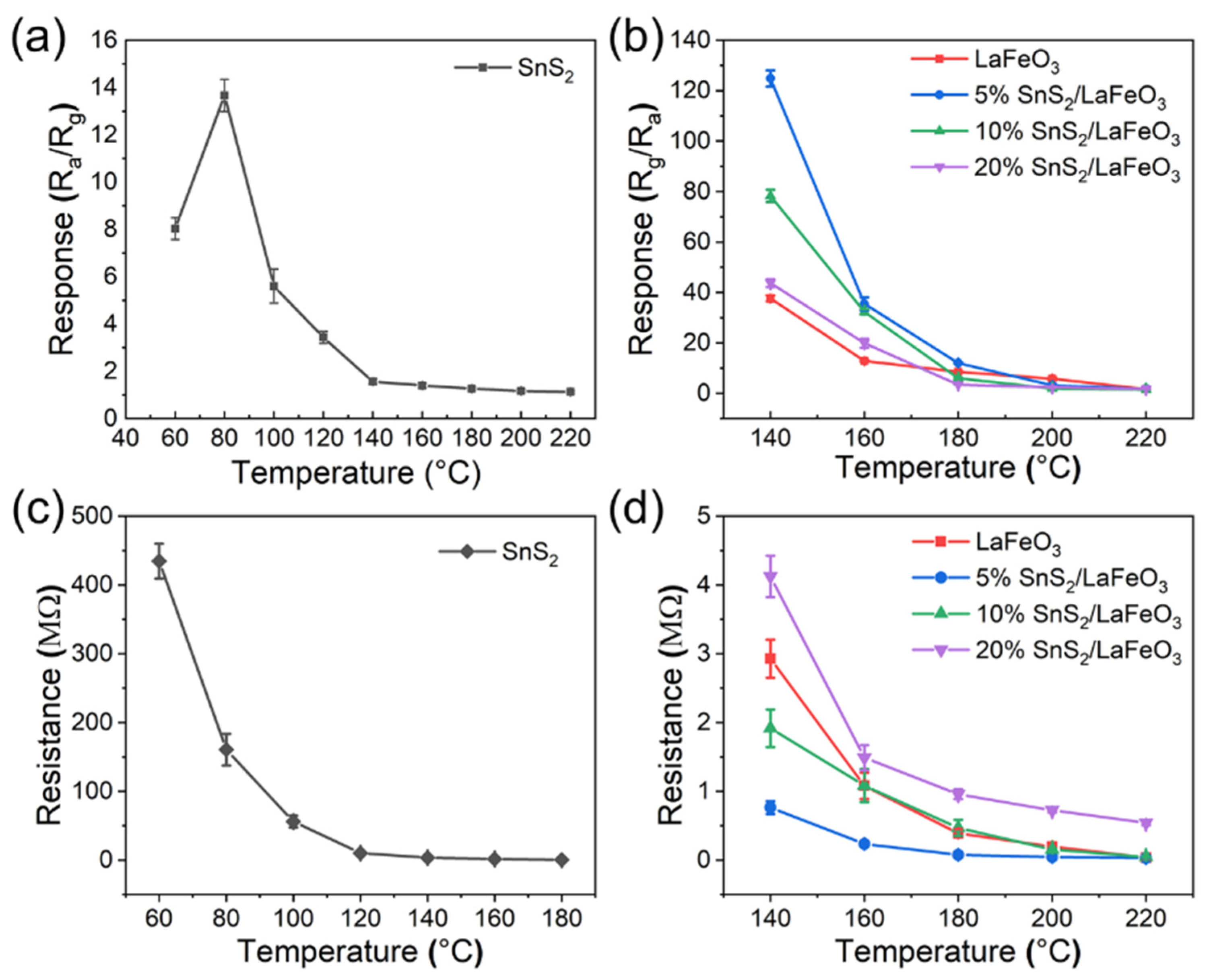
The optimal operating temperature is a critical indicator in gas sensor testing. According to Figure 7a,b, these sensors exhibit two distinct optimal operating temperatures and demonstrate significantly divergent response behaviors to TEA gas. The pure SnS2-based sensor achieves optimal performance at 80 °C, with a response value of 14.2 to 100 ppm TEA. Comparatively, LaFeO3-based sensors reach their optimal working temperature at 140 °C. Notably, the 5% SnS2/LaFeO3 sensor delivers a response value of 124.5 to 100 ppm TEA, representing an approximately three-fold enhancement compared to the pure LaFeO3 sensor. According to Figure 7c,d, as temperature increases, the semiconductor’s thermal excitation effect dominates, inducing a monotonic decline in baseline resistance with rising temperature. With the increased addition of SnS2, the baseline resistance of the SnS2/LaFeO3 composite starts to increase. Meanwhile, the difference in Rg/Ra gradually decreases, thus reducing the sensitivity.
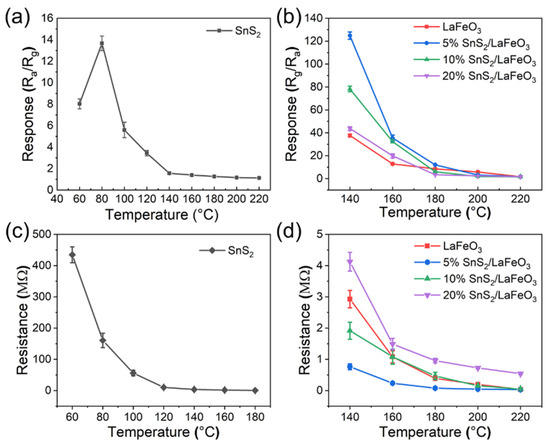
Figure 7.
(a,b) Responses of pure SnS2, pure LaFeO3, and SnS2/LaFeO3 composite to 100 ppm TEA at 60–220 °C; (c,d) the sensors’ baseline resistances in air at different temperatures.
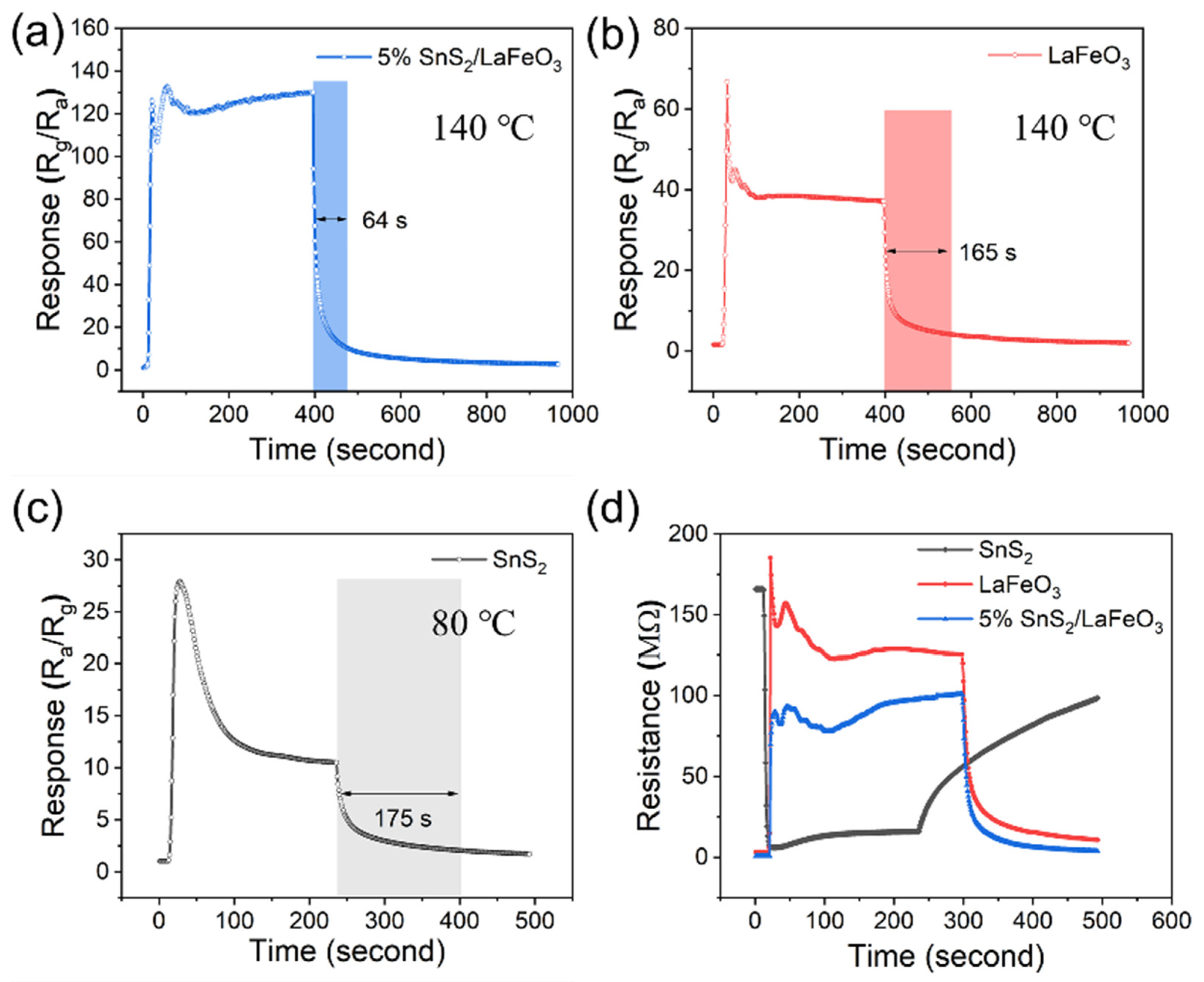
Figure 8a–c shows the gas sensors’ time-varying response–recovery curves to 100 ppm TEA concentration. In terms of response time, the 5% SnS2/LaFeO3 sensor does not show a significant response advantage, and with regard to the recovery time, the pure LaFeO3 and pure SnS2 sensor have a recovery time of 165 and 175 s, respectively, while the 5% SnS2/LaFeO3 sensor has a recovery time elevated to 64 s, which is significantly faster than that of the two single material sensors. Figure 8d illustrates the resistance response variation curves of different sensors to 100 ppm TEA at the optimal working temperature.
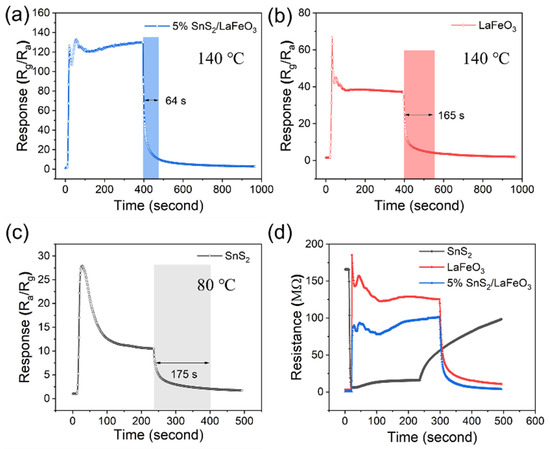
Figure 8.
(a–c) Time-varying response curves of 3 sensors to 100 ppm TEA. (d) Resistance response variation curves of 3 sensors at the optimal operating temperature.
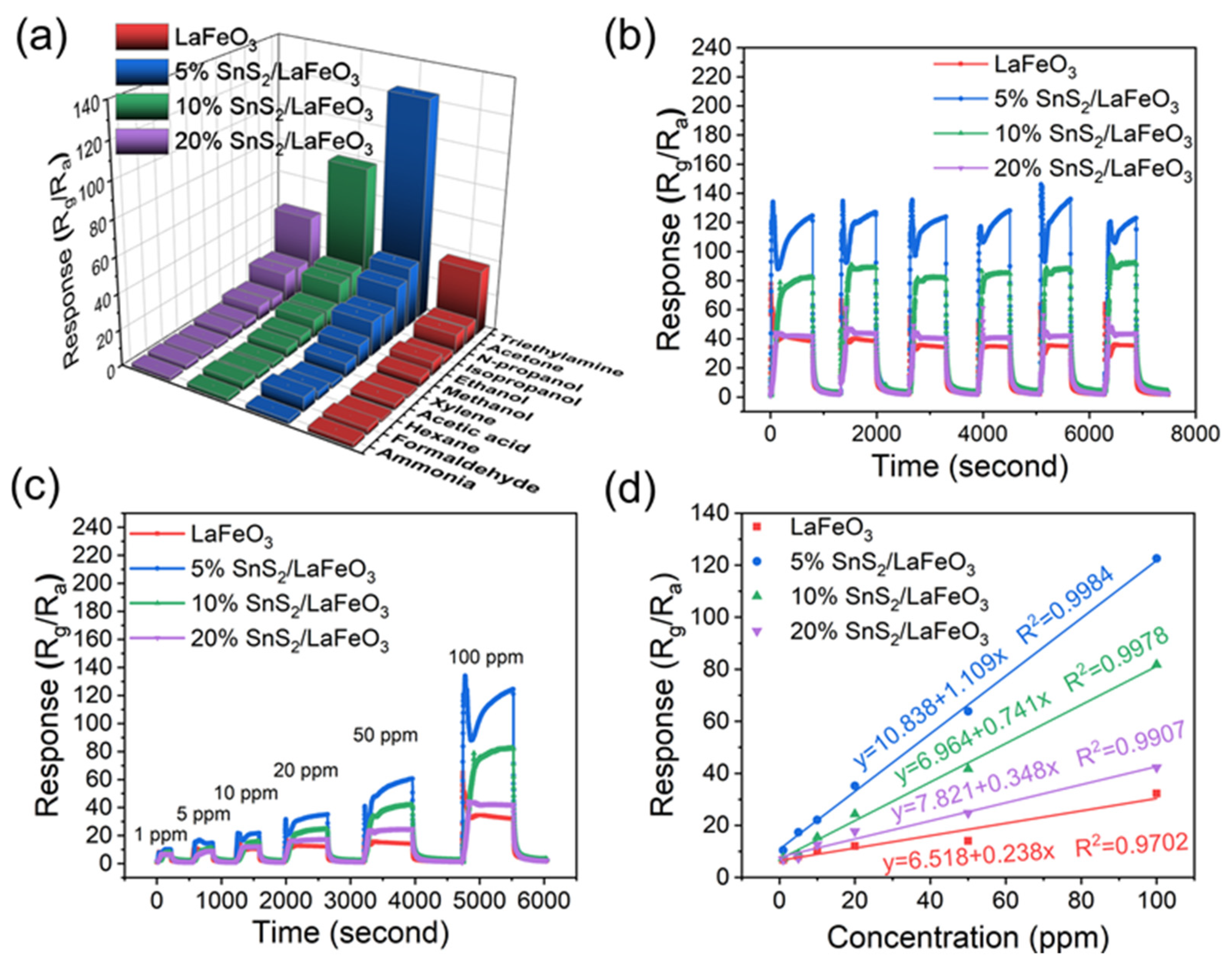
Selectivity is also a fundamental criterion for gas sensors. The sensors were used to test the selectivity at 140 °C to 100 ppm of acetone, ethanol, n-propanol, isopropanol, methanol, xylene, acetic acid, hexane, formaldehyde, ammonia, and other gases (Figure 9a). Compared to other tested gases (such as acetone with a C=O bond energy of 728 kJ/mol and methanol with an O-H bond energy of 458.8 kJ/mol), the 5% SnS2/LaFeO3 sensor exhibits significantly enhanced response characteristics toward TEA. This superiority primarily stems from the lower bond energy (305 kJ/mol) of the C-N bond in TEA molecules. The disparity in bond energy facilitates easier surface interfacial reactions between TEA and the gas-sensitive material, thereby enhancing the gas selectivity.
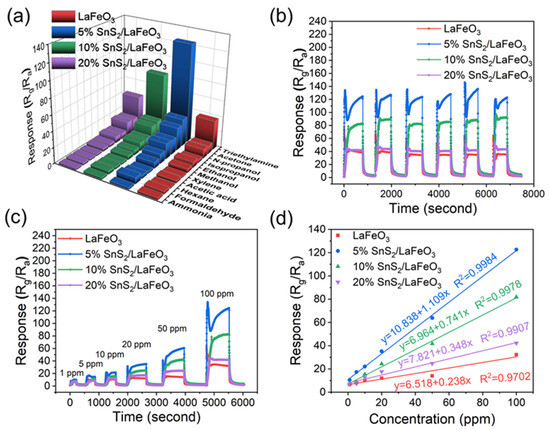
Figure 9.
(a) Selectivity of 4 sensors to 100 ppm of varying VOC gases at 140 °C. (b,c) Cyclic stability and dynamic response curve of sensors. (d) Fit curve of the 5% SnS2/LaFeO3 sensor responses.
These sensors present excellent stability and reproducibility (Figure 9b). Notably, the 5% SnS2/LaFeO3 gas sensor exhibits more prominent response and recovery characteristics at 1 ppm TEA, achieving a response value of 10.2 (Figure 9c). This result indicates that the 5% SnS2/LaFeO3 gas sensor possesses a lower detection limit. In addition, in Figure 9d, a linear fitting was conducted for all sensors in 1–100 ppm. The 5% SnS2/LaFeO3 sensor exhibited the fitting equation of (R2 = 0.998), demonstrating a strong linear relationship.
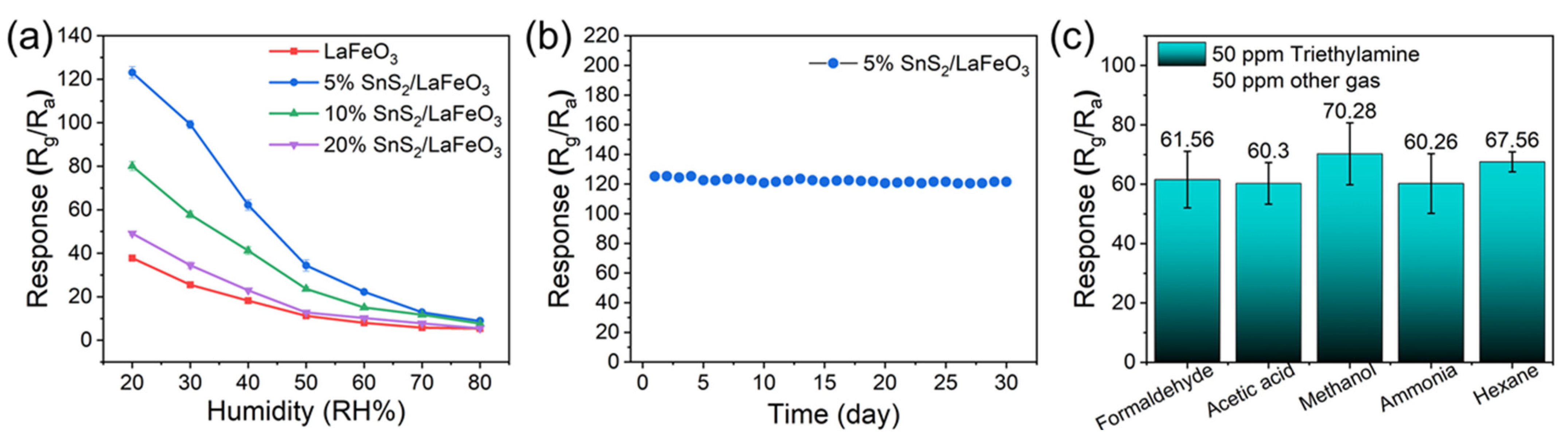
Relative humidity (RH) also critically affects the sensor performance. Figure 10a illustrates the humidity resistance tests of four gas sensors toward 100 ppm TEA. With the RH rising from 20% to 80%, the 5% SnS2/LaFeO3 sensor has a response declining from 123.4 to 9.6. This decline arises due to water molecules in the air competing with the target gas for active sites on the material surface, impeding the formation of adsorbed oxygen species. Consequently, the baseline resistance increases, leading to reduced gas-sensing performance [48]. Figure 10b shows a continuous 30-day gas sensitivity test of the 5% SnS2/LaFeO3 gas sensor, which maintains a stable response value to 100 ppm TEA at 140 °C, providing excellent long-term stability. Additionally, anti-interference tests were conducted using mixed gases containing 50 ppm TEA and 50 ppm interfering gases (formaldehyde, acetic acid, methanol, ammonia, and hexane), as shown in Figure 10c. The 5% SnS2/LaFeO3 sensor displays minimal response variations to the mixed gases, confirming its superior anti-interference capability.
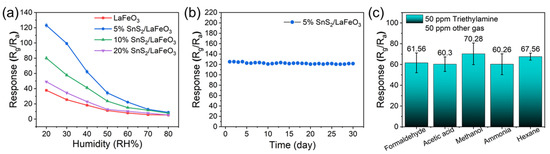
Figure 10.
(a) Humidity resistance tests and (b) long-term stability of four gas sensors; (c) the responses of the 5% SnS2/LaFeO3 gas sensor in the different gas mixtures.
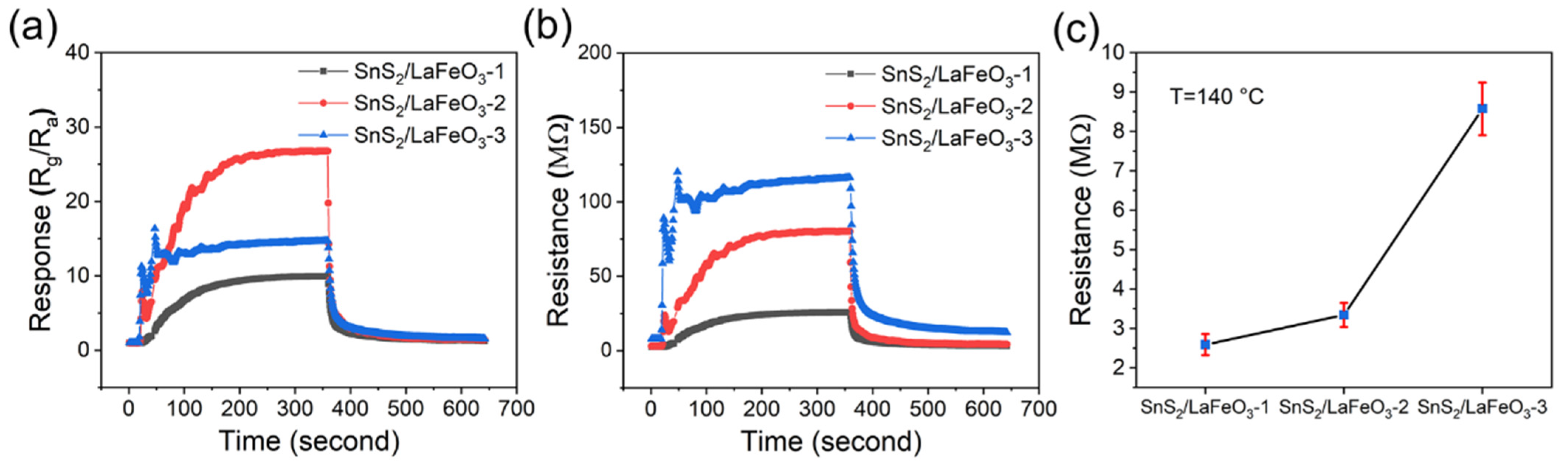
To demonstrate that the hydrothermal method of combining SnS2 and LaFeO3 materials is simple and that the synthesized SnS2/LaFeO3 composites have excellent properties, we conducted a control experiment of the in situ synthetic method. Figure 11a presents a control experiment on the sensing properties of in situ-grown SnS2 on LaFeO3. The SnS2/LaFeO3-2 composite shows a response of 26.2 to 100 ppm TEA at 140 °C and 40% RH. Compared to pure LaFeO3, there is no significant improvement in gas-sensing performance. Figure 11b shows the resistance variation curves of the three sensors. As the SnS2 loading content increases, the baseline resistance demonstrates an upward trend (Figure 11c).
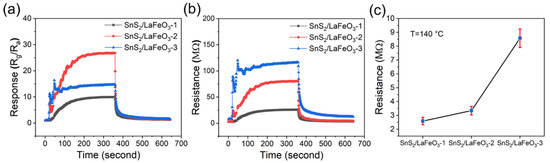
Figure 11.
(a,b) Time-varying response and resistance curves of the in situ SnS2/LaFeO3-1, -2, and -3 sensors to 100 ppm TEA at 140 °C and 40% RH; (c) baseline resistance values of the three sensors at 140 °C.
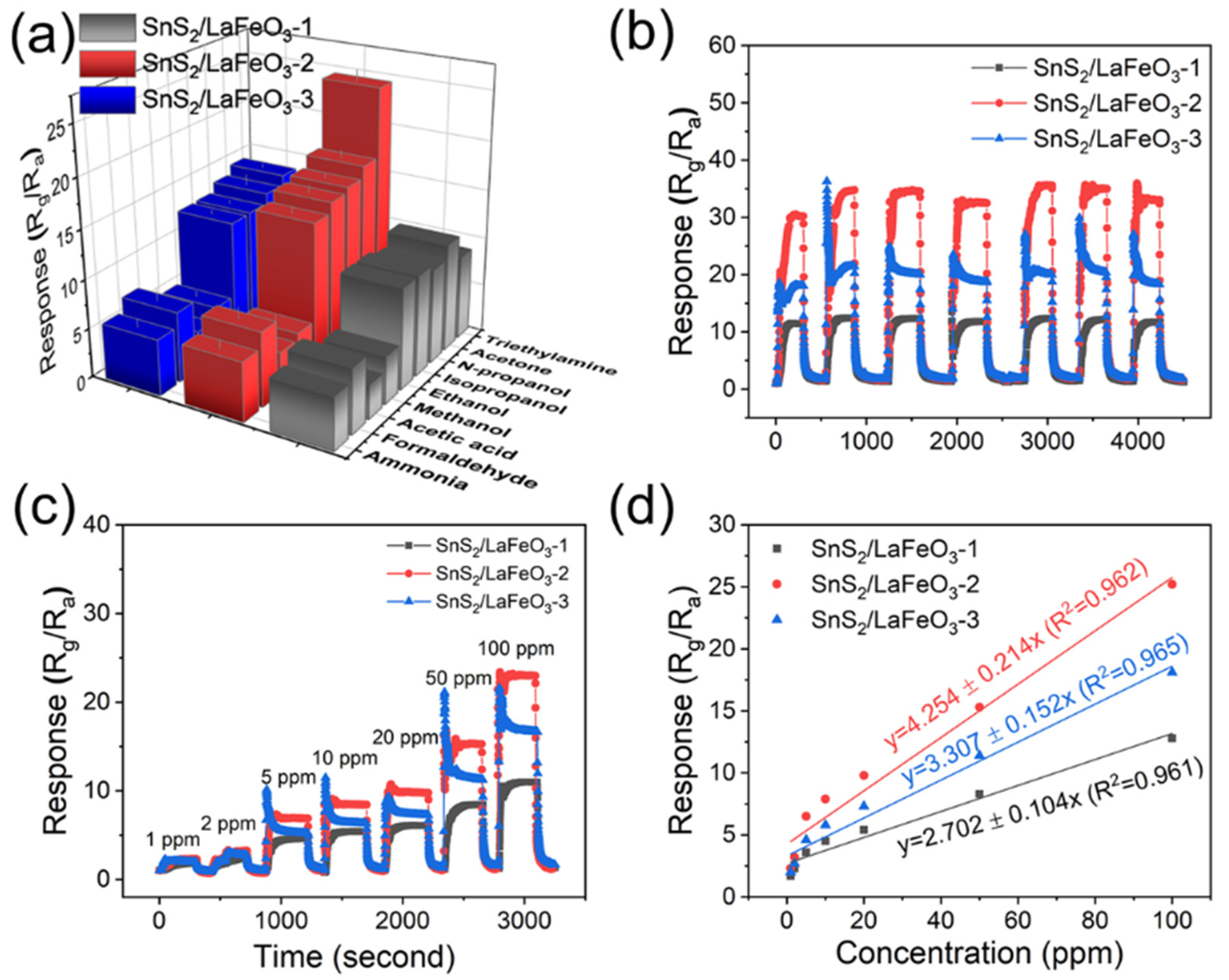
Figure 12a presents the selectivity tests of the three sensors based on the in situ synthesized samples toward eight different gases. The SnS2/LaFeO3-2 sensor exhibits relatively high responses to gases such as acetone, n-propanol, and iso-propanol, but shows no significant selective advantage for the target analyte. Figure 12b displays the cyclic stability test results, where the response values remain essentially unchanged over multiple cycles. Notably, the sensing performance improved during testing due to a decrease in ambient humidity to approximately 35% RH. Figure 12c,d show the concentration-dependent response curves of the three sensors to TEA from 1 ppm to 100 ppm. The SnS2/LaFeO3-2 composite demonstrates superior gas response values, with its concentration–response relationship following the linear fitting equation (y = 4.254 + 0.214x, R2 = 0.962).
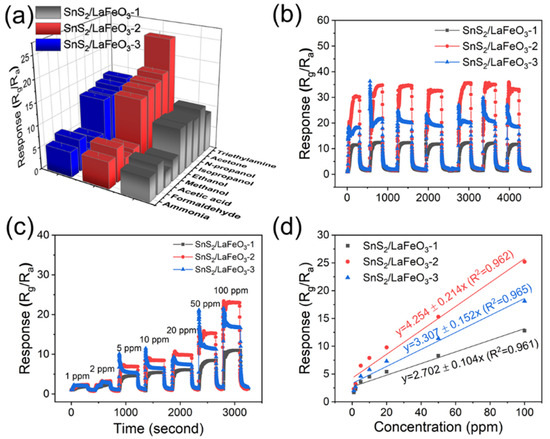
Figure 12.
(a) Selectivity tests of the three sensors toward different gases; (b) cyclic stability test; (c,d) response curves to increasing TEA concentrations (1–100 ppm) and corresponding fitting curves for the three sensors.
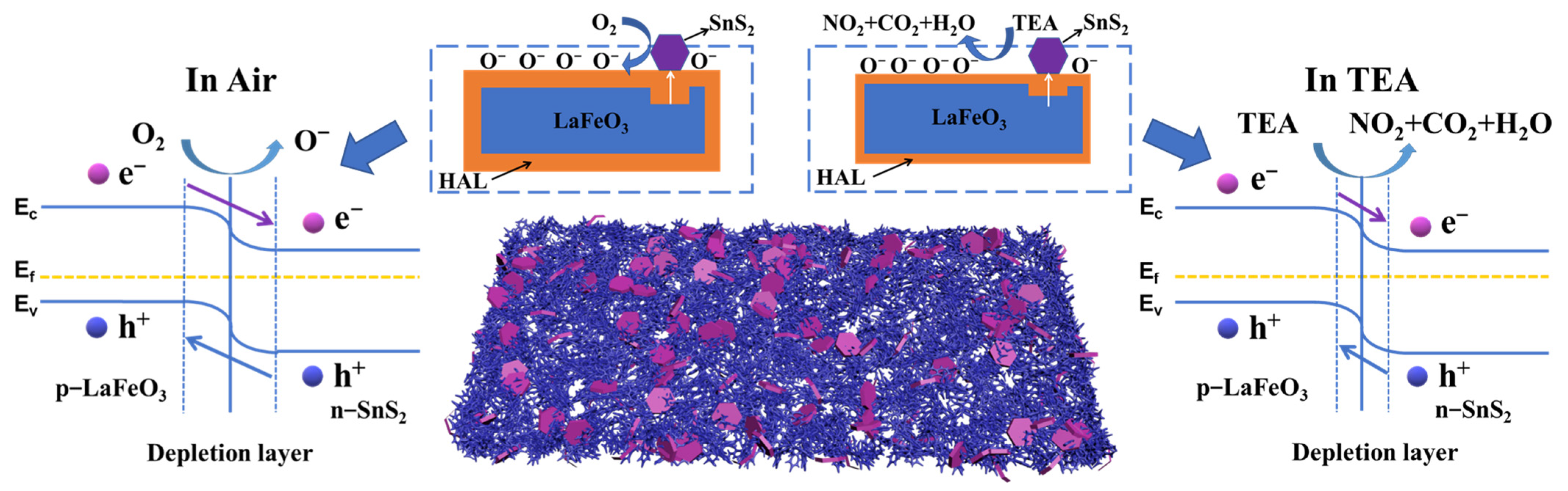
3.3. Gas-Sensing Mechanism
During the high-temperature annealing treatment of the LaFeO3 sol–gel precursor in air, cation vacancies (La and Fe) generated under high-temperature conditions induce hole formation, triggering a p-type LaFeO3 semiconductor. The equations below interpret the corresponding defect reaction [49,50]:
It is allowed to explain the sensing mechanism by examining the reversible conductivity variation of the sensing layer attributed to the process through which the sensing material interacts with adsorbed gas molecules [51]. The equations below interpret the overall steps:
Upon the exposure of the LaFeO3 to atmospheric air, oxygen molecules (O2) adsorbed onto the surface are converted to and through capturing electrons from the conduction band, forming a hole accumulation layer (HAL) on the LaFeO3 surface and reducing the electronic resistance of LaFeO3. Upon the exposure to TEA gas, the reaction between the TEA molecules and the adsorbed oxygen species present on the LaFeO3 surface promoted the release of electrons back into the materials, increasing the resistance.
As shown in Figure 13, LaFeO3 is a p-type semiconductor [52], and SnS2 is an n-type semiconductor [53]. Affected by their different work functions, the electrons of LaFeO3 can flow to SnS2 and the holes of SnS2 flow to LaFeO3 until the Fermi level achieves equilibrium [54,55]. This charge transfer can be verified by XPS characterization (Figure 5).
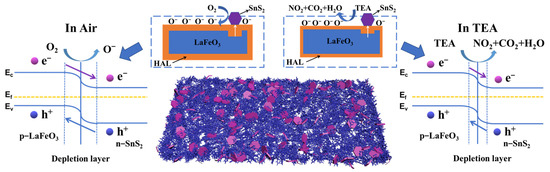
Figure 13.
Schematic diagram of the sensing mechanism of the SnS2/LaFeO3 sensor.
This further increases the hole concentration in LaFeO3 and thickens the HAL in an air atmosphere, thereby reducing the electronic resistance Ra and providing the potential for a lager change between Ra and Rg. As shown in Figure 7b, a small amount (5%) of SnS2-composition significantly reduces the resistance value of Ra. Moreover, the p-n heterojunction improves the charge transport during the reaction process on the surface, enhancing the sensing response. The 5% SnS2/LaFeO3 composite exhibits the highest content of OV and OC. This promotes the participation of more target gas molecules in surface redox reactions. Furthermore, BET surface area measurements indicate that the introduction of SnS2 effectively increases the specific surface area of the material. This provides more active sites and accelerates the redox reaction process, thus enhancing the gas-sensing performance.
4. Conclusions
In conclusion, the SnS2/LaFeO3 composites were successfully synthesized by a high-temperature annealing treatment and hydrothermal method. The 5% SnS2/LaFeO3 sensor demonstrates a response of 124.5 to 100 ppm TEA at 140 °C, 3.2 times higher than the pure LaFeO3 sensor. Furthermore, for the SnS2/LaFeO3 sensor, the sensor response exhibits a linear relevance to the gas concentration, anti-interference capability, and long-term stability. Furthermore, the formed heterojunction elevates the OV content and expands the HAL on the surface, thereby improving the TEA-sensing property. The findings in the study will assist researchers in designing and synthesizing MOS gas sensors for TEA detection.
Author Contributions
Conceptualization, X.W. (Xiaofeng Wang); methodology, H.W. and X.W. (Xiaofeng Wang); data curation, H.W.; validation, X.W. (Xiaobing Wang) and Y.C.; investigation, X.W. (Xiaobing Wang) and Y.C.; writing—original draft preparation, H.W. and X.W. (Xiaofeng Wang); writing—review and editing, X.W. (Xiaofeng Wang); supervision, X.W. (Xiaofeng Wang); funding acquisition, X.W. (Xiaofeng Wang). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Liaoning Province of China (No. 2023-MSBA-012) and the Fundamental Research Funds for the Central Universities of China (DUT24MS012).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Banga, I.; Paul, A.; Poudyal, D.C.; Muthukumar, S.; Prasad, S. Recent Advances in Gas Detection Methodologies with a Special Focus on Environmental Sensing and Health Monitoring Applications—A Critical Review. ACS Sens. 2023, 8, 3307–3319. [Google Scholar] [CrossRef] [PubMed]
- Bilge, S.; Dogan-Topal, B.; Yücel, A.; Sınağ, A.; Ozkan, S.A. Recent advances in flower-like nanomaterials: Synthesis, characterization, and advantages in gas sensing applications. TrAC Trends Anal. Chem. 2022, 153, 116638. [Google Scholar] [CrossRef]
- Bulemo, P.M.; Kim, D.-H.; Shin, H.; Cho, H.-J.; Koo, W.-T.; Choi, S.-J.; Park, C.; Ahn, J.; Güntner, A.T.; Penner, R.M.; et al. Selectivity in Chemiresistive Gas Sensors: Strategies and Challenges. Chem. Rev. 2025, 125, 4111–4183. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zeng, W.; Li, Y. Metal oxide gas sensors for detecting NO2 in industrial exhaust gas: Recent developments. Sens. Actuators B Chem. 2022, 359, 131579. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, T.; Zhai, C.; Du, L.; Zhang, J.; Zhang, M. Fast triethylamine gas sensing response properties of Ho-doped SnO2 nanoparticles. J. Alloys Compd. 2020, 817, 152724. [Google Scholar] [CrossRef]
- Wang, H.; Luo, Y.; Li, K.; Liu, B.; Gao, L.; Duan, G. Porous α-Fe2O3 gas sensor with instantaneous attenuated response toward triethylamine and its reaction kinetics. Chem. Eng. J. 2022, 427, 131631. [Google Scholar] [CrossRef]
- Gui, Y.; Tian, K.; Liu, J.; Yang, L.; Zhang, H.; Wang, Y. Superior triethylamine detection at room temperature by {-112} faceted WO3 gas sensor. J. Hazard. Mater. 2019, 380, 120876. [Google Scholar] [CrossRef]
- Pereira, P.F.M.; de Sousa Picciani, P.H.; Calado, V.; Tonon, R.V. Electrical gas sensors for meat freshness assessment and quality monitoring: A review. Trends Food Sci. Technol. 2021, 118, 36–44. [Google Scholar] [CrossRef]
- Wu, K.; Debliquy, M.; Zhang, C. Metal-oxide-semiconductor resistive gas sensors for fish freshness detection. Compr. Rev. Food Sci. Food Saf. 2023, 22, 913–945. [Google Scholar] [CrossRef]
- Zeng, J.; Rong, Q.; Xiao, B.; Yu, R.; Zi, B.; Kuang, X.; Deng, X.; Ma, Y.; Zhang, J.; Wu, J.; et al. Single-atom silver loaded on tungsten oxide with oxygen vacancies for high performance triethylamine gas sensors. J. Mater. Chem. A 2021, 9, 8704–8710. [Google Scholar] [CrossRef]
- Yang, J.; Han, W.; Jiang, B.; Wang, X.; Sun, Y.; Wang, W.; Lou, R.; Ci, H.; Zhang, H.; Lu, G. Electrospinning Derived NiO/NiFe2O4 Fiber-in-Tube Composite for Fast Triethylamine Detection under Different Humidity. ACS Sens. 2022, 7, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Sun, N.; Wang, Y.; Li, Z.; Qu, Y.; Jing, L. Synthesis of SnO2/yolk-shell LaFeO3 nanocomposites as efficient visible-light photocatalysts for 2,4-dichlorophenol degradation. Mater. Res. Bull. 2020, 127, 110857. [Google Scholar] [CrossRef]
- Kou, X.; Meng, F.; Chen, K.; Wang, T.; Sun, P.; Liu, F.; Yan, X.; Sun, Y.; Liu, F.; Shimanoe, K.; et al. High-performance acetone gas sensor based on Ru-doped SnO2 nanofibers. Sens. Actuators B Chem. 2020, 320, 128292. [Google Scholar] [CrossRef]
- Lee, J.-H. Gas sensors using hierarchical and hollow oxide nanostructures: Overview. Sens. Actuators B Chem. 2009, 140, 319–336. [Google Scholar] [CrossRef]
- Lee, J.; Jung, Y.; Sung, S.-H.; Lee, G.; Kim, J.; Seong, J.; Shim, Y.-S.; Jun, S.C.; Jeon, S. High-performance gas sensor array for indoor air quality monitoring: The role of Au nanoparticles on WO3, SnO2, and NiO-based gas sensors. J. Mater. Chem. A 2021, 9, 1159–1167. [Google Scholar] [CrossRef]
- Zhang, S.; Song, P.; Zhang, J.; Yan, H.; Li, J.; Yang, Z.; Wang, Q. Highly sensitive detection of acetone using mesoporous In2O3 nanospheres decorated with Au nanoparticles. Sens. Actuators B Chem. 2017, 242, 983–993. [Google Scholar] [CrossRef]
- Hao, P.; Qu, G.-M.; Song, P.; Yang, Z.-X.; Wang, Q. Synthesis of Ba-doped porous LaFeO3 microspheres with perovskite structure for rapid detection of ethanol gas. Rare Met. 2021, 40, 1651–1661. [Google Scholar] [CrossRef]
- Nakhostin Panahi, P.; Rasoulifard, M.H.; Babaei, S. Photocatalytic activity of cation (Mn) and anion (N) substitution in LaCoO3 nanoperovskite under visible light. Rare Met. 2020, 39, 139–146. [Google Scholar] [CrossRef]
- Voznyy, O. Realizing ultra-pure red emission with Sn-based lead-free perovskites. Rare Met. 2020, 39, 330–331. [Google Scholar] [CrossRef]
- Ochoa-Muñoz, Y.H.; Mejía de Gutiérrez, R.; Rodríguez-Páez, J.E.; Gràcia, I.; Vallejos, S. Gas Sensors Based on Porous Ceramic Bodies of MSnO3 Perovskites (M = Ba, Ca, Zn): Formation and Sensing Properties towards Ethanol, Acetone, and Toluene Vapours. Molecules 2022, 27, 2889. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, X.; Ma, Z.; Yang, K.; Gao, X.; Wang, H.; Jia, L. Formaldehyde gas sensor with 1 ppb detection limit based on In-doped LaFeO3 porous structure. Sens. Actuators B Chem. 2022, 371, 132558. [Google Scholar] [CrossRef]
- Wang, B.; Yu, Q.; Zhang, S.; Wang, T.; Sun, P.; Chuai, X.; Lu, G. Gas sensing with yolk-shell LaFeO3 microspheres prepared by facile hydrothermal synthesis. Sens. Actuators B Chem. 2018, 258, 1215–1222. [Google Scholar] [CrossRef]
- Li, F.; Wang, S.; Wu, Z.; Xiong, X.; Li, J.; Zhou, J.; Gao, X. Excellent ethanol sensor based on LaFeO3 modified with gold nanoparticles. J. Mater. Sci. Mater. Electron. 2021, 32, 27587–27595. [Google Scholar] [CrossRef]
- Xiao, H.; Xue, C.; Song, P.; Li, J.; Wang, Q. Preparation of porous LaFeO3 microspheres and their gas-sensing property. Appl. Surf. Sci. 2015, 337, 65–71. [Google Scholar] [CrossRef]
- Liu, W.-D.; Xiong, Y.; Shen, A.; Wang, X.-Z.; Chang, X.; Lu, W.-B.; Tian, J. SnS2 nanosheets decorated SnO2 hollow multishelled nanostructures for enhanced sensing of triethylamine gas. Rare Met. 2024, 43, 2339–2348. [Google Scholar] [CrossRef]
- Yan, W.; Lv, C.; Zhang, D.; Chen, Y.; Zhang, L.; Ó Coileáin, C.; Wang, Z.; Jiang, Z.; Hung, K.-M.; Chang, C.-R.; et al. Enhanced NO2 Sensitivity in Schottky-Contacted n-Type SnS2 Gas Sensors. ACS Appl. Mater. Interfaces 2020, 12, 26746–26754. [Google Scholar] [CrossRef]
- Dong, X.; Han, Q.; Kang, Y.; Li, H.; Huang, X.; Fang, Z.; Yuan, H.; Elzatahry, A.A.; Chi, Z.; Wu, G.; et al. Rational construction and triethylamine sensing performance of foam shaped α-MoO3@SnS2 nanosheets. Chin. Chem. Lett. 2022, 33, 567–572. [Google Scholar] [CrossRef]
- Xu, X.; Ma, S.; Xu, X.; Pei, S.; Han, T.; Liu, W. Transformation synthesis of heterostructured SnS2/ZnS microspheres for ultrafast triethylamine detection. J. Alloys Compd. 2021, 868, 159286. [Google Scholar] [CrossRef]
- Gao, X.; Li, F.; Wang, R.; Zhang, T. A formaldehyde sensor: Significant role of p-n heterojunction in gas-sensitive core-shell nanofibers. Sens. Actuators B Chem. 2018, 258, 1230–1241. [Google Scholar] [CrossRef]
- Shuai, Y.; Peng, R.; He, Y.; Liu, X.; Wang, X.; Guo, W. NiO/BiVO4 p-n heterojunction microspheres for conductometric triethylamine gas sensors. Sens. Actuators B Chem. 2023, 384, 133625. [Google Scholar] [CrossRef]
- Wu, R.; Liu, T.; Chen, X.; Yin, X.-T. Shape-controlled multi-dimensional In2O3/Mn2O3 p-n heterojunction for triethylamine detection. J. Alloys Compd. 2023, 960, 170527. [Google Scholar] [CrossRef]
- Hao, P.; Song, P.; Yang, Z.; Wang, Q. Synthesis of novel RuO2/LaFeO3 porous microspheres its gas sensing performances towards triethylamine. J. Alloys Compd. 2019, 806, 960–967. [Google Scholar] [CrossRef]
- Ma, N.; Ma, S.; Guo, J.; Fan, G.; Ni, P.; Wang, Y.; Zhu, J.; Wang, H. Enhanced triethylamine sensing with novel WO3 composite SnWO4 nanorod-based gas sensor. Surf. Interfaces 2025, 58, 105870. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, S.; Lv, M.; Liu, J.; Chen, Y.; Wang, Y.; Zhou, H.; Che, X.; Kang, B.; Zhang, Q. An ultra-sensitive triethylamine sensor based on WO3 modified ErVO4 nanoparticles. Vacuum 2025, 239, 114440. [Google Scholar] [CrossRef]
- Ni, P.; Ma, S.; Cai, Y.; Guo, J.; Fan, G.; Ma, N.; Wang, H.; Wang, Y.; Zhu, J. Triethylamine sensing behavior of p-LaFeO3/n-ZnO composite synthesized by one-step hydrothermal method. Sens. Actuators B Chem. 2025, 442, 138051. [Google Scholar] [CrossRef]
- Dhahri, R.; Benamara, M.; Nassar, K.I.; Elkenany, E.B.; Al-Syadi, A.M. Zinc oxide-based sensor prepared by modified sol–gel route for detection of low concentrations of ethanol, methanol, acetone, and formaldehyde. Semicond. Sci. Technol. 2024, 39, 115021. [Google Scholar] [CrossRef]
- Bharathi, P.; Harish, S.; Shimomura, M.; Krishna Mohan, M.; Archana, J.; Navaneethan, M. Controlled growth and fabrication of edge enriched SnS2 nanostructures for room temperature NO2 gas sensor applications. Mater. Lett. 2023, 335, 133691. [Google Scholar] [CrossRef]
- Liang, H.; Liu, N.; Lin, J.; Meng, Y.; Ni, J.; Gao, B.; Tan, Z.; Song, X.-Z.; Wang, X. Narrow-Band HoFeO3 Nanostructure-Based Gas Sensor for n-Propanol Detection. ACS Appl. Nano Mater. 2024, 7, 7573–7581. [Google Scholar] [CrossRef]
- Shingange, K.; Swart, H.; Mhlongo, G.H. Ultrafast Detection of Low Acetone Concentration Displayed by Au-Loaded LaFeO3 Nanobelts owing to Synergetic Effects of Porous 1D Morphology and Catalytic Activity of Au Nanoparticles. ACS Omega 2019, 4, 19018–19029. [Google Scholar] [CrossRef]
- Wei, H.; Hou, C.; Zhang, Y.; Nan, Z. Scalable low temperature in air solid phase synthesis of porous flower-like hierarchical nanostructure SnS2 with superior performance in the adsorption and photocatalytic reduction of aqueous Cr(VI). Sep. Purif. Technol. 2017, 189, 153–161. [Google Scholar] [CrossRef]
- Liu, Y.; Geng, P.; Wang, J.; Yang, Z.; Lu, H.; Hai, J.; Lu, Z.; Fan, D.; Li, M. In-situ ion-exchange synthesis Ag2S modified SnS2 nanosheets toward highly photocurrent response and photocatalytic activity. J. Colloid Interface Sci. 2018, 512, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Sun, C.; Liu, H.; He, P.; Liu, Q.; Sun, J.; Li, J.; Pan, G.; Yang, X. Insight into Au functionalization on core-shell LaFeO3 spheres for high-response and selectivity n-butanol gas sensors with DFT study. Sens. Actuators B Chem. 2023, 382, 133506. [Google Scholar] [CrossRef]
- Haron, W.; Wisitsoraat, A.; Wongnawa, S. Nanostructured perovskite oxides—LaMO3 (M=Al, Co, Fe) prepared by co-precipitation method and their ethanol-sensing characteristics. Ceram. Int. 2017, 43, 5032–5040. [Google Scholar] [CrossRef]
- Liu, X.; Hu, J.; Cheng, B.; Qin, H.; Zhao, M.; Yang, C. First-principles study of O2 adsorption on the LaFeO3 surface. Sens. Actuators B Chem. 2009, 139, 520–526. [Google Scholar] [CrossRef]
- Wang, S.; Xiong, H.; Tang, Y.; Zhang, W.; Zhang, Y.; Liu, Q.; Zhang, J. High sensitivity and selectivity triethylamine gas sensor based on ZnO–SmFeO3 molecular imprinted polymers. Mater. Res. Bull. 2023, 161, 112147. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, L.; Huang, C.; Liang, S.; Jiang, G. Enhanced acetone gas sensing performance of ZnO polyhedrons decorated with LaFeO3 nanoparticles. Mater. Res. Express 2023, 10, 095902. [Google Scholar] [CrossRef]
- Cao, E.; Zhang, Y.; Zhang, Y.; Hao, W.; Sun, B.; Sun, L. Acetone sensing characteristics of TiO2-LaFeO3 nanocomposites. Mater. Lett. 2023, 351, 135056. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Z.; Ding, H.; Wei, Y.; Huang, W.; Yang, X.; Li, Z.; Qiu, L.; Wang, X. Three-Dimensional Graphene Hydrogel Decorated with SnO2 for High-Performance NO2 Sensing with Enhanced Immunity to Humidity. ACS Appl. Mater. Interfaces 2020, 12, 2634–2643. [Google Scholar] [CrossRef]
- Wærnhus, I.; Vullum, P.E.; Holmestad, R.; Grande, T.; Wiik, K. Electronic properties of polycrystalline LaFeO3. Part I: Experimental results and the qualitative role of Schottky defects. Solid State Ion. 2005, 176, 2783–2790. [Google Scholar] [CrossRef]
- Wærnhus, I.; Grande, T.; Wiik, K. Electronic properties of polycrystalline LaFeO3. Part II: Defect modelling including Schottky defects. Solid State Ion. 2005, 176, 2609–2616. [Google Scholar] [CrossRef]
- Baek, J.W.; Kim, Y.H.; Ahn, J.; Kim, D.-H.; Shin, H.; Ko, J.; Park, S.; Park, C.; Shin, E.; Jang, J.-S.; et al. Galvanic replacement reaction in perovskite oxide for superior chemiresistors. J. Mater. Chem. A 2022, 10, 23282–23293. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, K.; Xiao, C.; Jia, L. C-doped LaFeO3 Porous Nanostructures for Highly Selective Detection of Formaldehyde. Sens. Actuators B Chem. 2021, 347, 130550. [Google Scholar] [CrossRef]
- Yang, Z.; Su, C.; Wang, S.; Han, Y.; Chen, X.; Xu, S.; Zhou, Z.; Hu, N.; Su, Y.; Zeng, M. Highly sensitive NO2 gas sensors based on hexagonal SnS2 nanoplates operating at room temperature. Nanotechnology 2020, 31, 075501. [Google Scholar] [CrossRef] [PubMed]
- Kumarage, G.W.C.; Jayawardena, S.; Zappa, D.; Shimomura, M.; Comini, E. Unlocking superior acetone sensitivity with Co3O4/ZnO nanowire innovations. Sens. Actuators B Chem. 2025, 431, 137450. [Google Scholar] [CrossRef]
- Vikraman, H.K.; George, J.; Ghuge, R.S.; Painappallil Reji, R.; Jayaraman, S.V.; Kawazoe, Y.; Sivalingam, Y.; Mangalampalli, S.R.N.K. Highly Selective, Room-Temperature Triethylamine Sensor Using Humidity-Resistant Novel TiZn Alloy Nanoparticles-Decorated MoS2 Nanosheets. Small 2025, 21, 2408500. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).