Abstract
Cannabis remains the most widely used illicit drug worldwide, identifying it is a routine procedure in forensic toxicology. Due to its widespread use, there is a need for analytical methods that can detect it in biological samples. Hair is of particular interest in forensic toxicology as it is the only biological sample that enables retrospective analysis of consumption. In addition, collecting hair is non-invasive, and the specimens can be stored at room temperature. However, the sample preparation process for hair is tedious and multi-step. To address this issue, this study introduces a novel approach to preparing hair samples for analysis, based on air-assisted liquid–liquid microextraction (AALLME). This technique is a modification of dispersive liquid–liquid microextraction (DLLME), which eliminates the need for dispersants and chlorinated organic solvents as extractants. Both techniques offer sustainable alternatives to conventional liquid–liquid extraction (LLE) and solid-phase extraction (SPE), making them of interest in forensic toxicology. This study is the first to report the application of AALLME to the hair matrix. A mixture of cyclohexane and ethyl acetate (9:1) was used as the extractant solvent. Gas chromatography–mass spectrometry (GC–MS) was then used to determine and quantify THC. The method was validated according to FDA guidelines and demonstrated good linearity within the 0.01–4 ng/mg range. The limits of detection (LOD) and quantification (LOQ) were 0.008 and 0.01 ng/mg, respectively. Finally, the applicability of the method was evaluated by analyzing hair samples received by the Forensic Toxicology Service.
1. Introduction
It is estimated that around 147 million people worldwide use cannabis, representing 2.5% of the population. This is in contrast to the prevalence of cocaine and opiate abuse, which is estimated at 0.2%. Cannabis thus remains the most widely used illicit drug [1]. In recent years, cannabis has increasingly been implicated in deaths, particularly when used alongside other substances such as hypnotics, opioids, cocaine, and alcohol [2]. Furthermore, the long-term effects of regular cannabis use are a significant public health concern [3,4,5].
Hair can be used as an alternative to blood and urine for toxicological analysis. Its main advantage is its wide detection window, which makes it useful for retrospective analyses. This makes it possible to study long-term use, conduct epidemiological research, and assess the presence of environmental contaminants. In addition, hair sampling is non-invasive and can be stored at room temperature; it is also difficult to adulterate. However, the main limitation of hair analysis is the complex pharmacokinetics of drug uptake. Furthermore, the complexity of the matrix necessitates sensitive, multi-step sample preparation methods to ensure accurate and reliable results [3,6,7,8].
Hair sample preparation typically consists of three main steps: a decontamination procedure to remove external contaminants with rigorous washing, the drying of the sample, the cutting and weighing of the sample, a pre-treatment step to release and isolate target substances from the matrix, and finally, a further extract clean-up and/or target pre-concentration stage before analysis. Due to the low concentrations detected in this biological matrix, the use of sensitive analytical methodologies is required. Clean-up procedures commonly involve liquid–liquid extraction (LLE) or solid-phase extraction (SPE) techniques [3,6,9,10]. However, both methods require large volumes of organic solvents and often involve multiple steps, resulting in lengthy procedures. According to the principles of green analytical chemistry, an ideal sample preparation method should minimise the number of steps while maintaining high performance, reproducibility, and sensitivity [11]. Furthermore, the process should be environmentally friendly and reduce the use of hazardous substances. To address these challenges, microextraction techniques have been developed as sustainable alternatives. Notably, dispersive liquid–liquid microextraction (DLLME), solid-phase microextraction (SPME), and microextraction by packed sorbents (MEPS) techniques are prominent in hair analysis [3,6,9,10,11,12].
DLLME was developed as an environmentally friendly alternative to LLE and has been widely used in toxicological studies [13,14,15,16]. This technique uses a ternary solvent system consisting of an organic extractant that is immiscible with the aqueous phase and a dispersant that is miscible with both the extractant and the aqueous phase. This allows an emulsion to form. This mixture promotes phase contact, accelerates equilibration, and improves extraction efficiency. However, the technique’s main limitations are the need to use halogenated organic solvents as extractants and the requirement for relatively large volumes of dispersant [17,18]. To overcome these limitations, several DLLME variants have been developed. In a first approach, the main objective was to replace the conventional organic solvents with more sustainable alternatives such as ionic liquids, eutectic solvents, supramolecular solvents, and low-density solvents. Other approaches aim to eliminate the dispersant solvent by enhancing phase-to-phase contact through mechanical or chemical methods, whether or not they are combined with green solvents. These innovations mainly include vortex-assisted (VA-DLLME), ultrasonic (US-DLLME), microwave (MA-DLLME), and air-assisted (AALLME) agitation. In addition, alternative dispersion techniques have been explored, including bubbling techniques, effervescent chemical reactions (EA-DLLME), surfactants (SA-DLLME), or dispersion in a solid base (BS-DLLME). AALLME uses a single extraction solvent in smaller volumes than LLE and optimizes phase contact by aspiration–dispersion cycles of the sample-extractant mixture. These cycles generate fine droplets of the extraction solvent, significantly increasing the contact area and improving extraction efficiency, similar to an emulsion process [13,19]. Although AALLME has been successfully used to analyze drugs and pharmaceuticals in biological matrices, its potential for analyzing hair samples remains to be explored [20,21,22,23,24,25].
The purpose of this research was to develop a gas chromatography–mass spectrometry (GC–MS) method for identifying and determining THC in hair by studying two variants of DLLME. Additionally, the method was validated according to FDA regulations [26] for AALLME. To the authors’ knowledge, this has not previously been used for analyzing illicit substances in hair.
2. Material and Methods
2.1. Chemical Reagents and Standards
Cyclohexane, ethyl acetate, isopropanol, chloroform, dichloromethane, sodium chloride, octanol, and methanol were purchased from Merck® (Darmstadt, Germany). THC and THC 13C-D3 were purchased from Cerilliant (Round Rock, TX, USA). Ultrapure water was processed through a Milli-Q water system (Millipore, Bedford, MA, USA).
2.2. Instrumentation
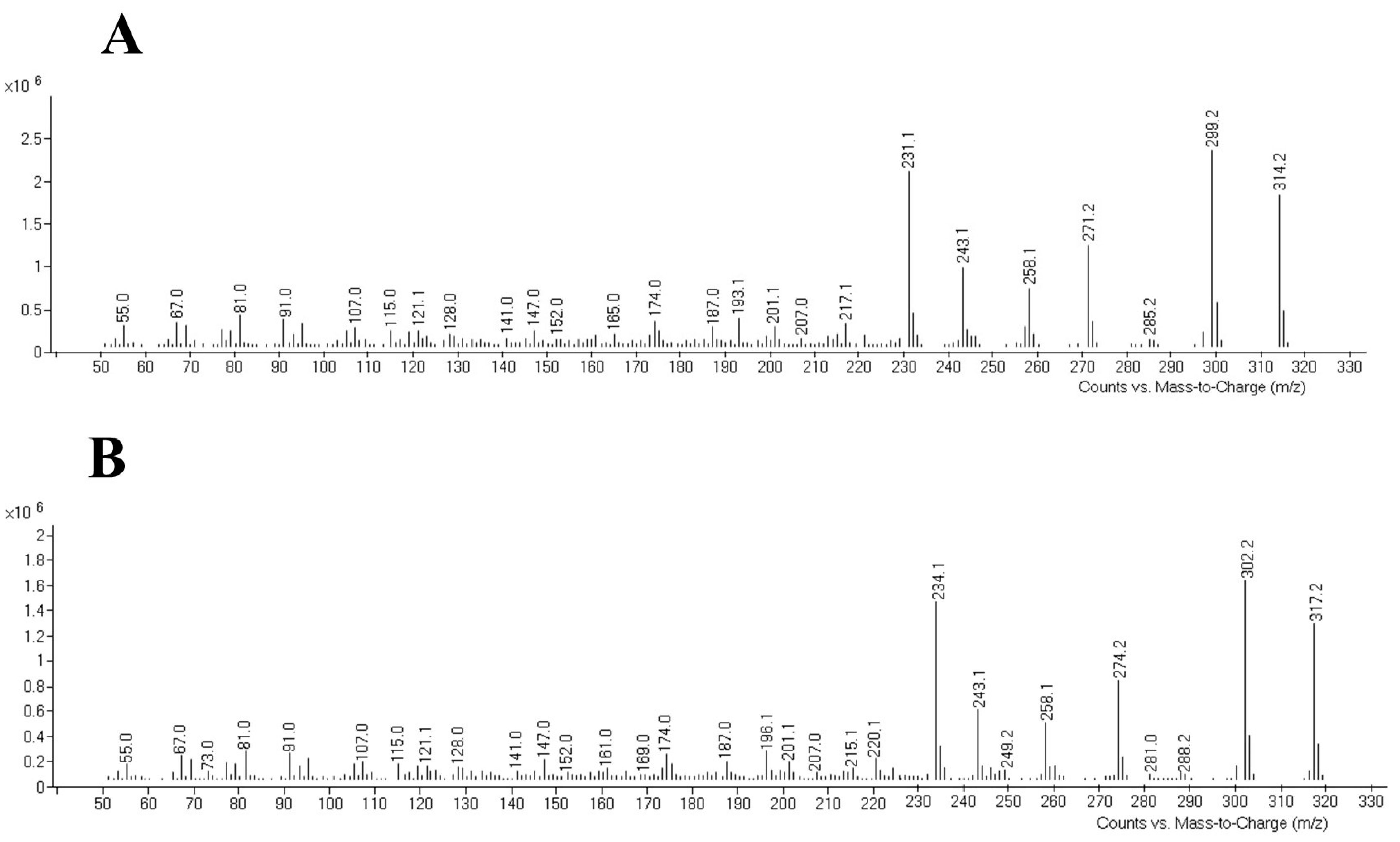
Chromatographic analyses were performed using a 7890 B gas chromatograph from Agilent Technologies (Santa Clara, CA, USA) with an electron impact ionization at 70 eV interfaced to a 5977 B mass selector detector (MSD), also from Agilent Technologies. The selected column was a HP-5MS capillary column (30 m × 250 µm i.d., 0.5 µm film thickness; Agilent Technologies) with helium as carrier gas (1.0 mL/min). The injector temperature was set at 240 °C and a purge time of 2.0 min was used. Samples were injected in the splitless mode. The following temperature program was applied: the initial temperature of the column was kept constant at 90 °C for 1.0 min and then ramped up at 40 °C/min up to 210 °C and 5.0 °C/min up to 250 °C. This temperature was held for 11 min. After, the temperature was increased to 300 °C for 30 s to clean the column. The observed retention time was 18.99 min for THC and 18.92 min for THC 13C-D3, and the total run time was 24.43 min. The MSD was kept at 300 °C, the ion source at 230 °C, and the quadrupole at 150 °C. The SCAN mode, scanning from 50 to 550 amu, was used for evaluating THC standard and internal standard THC 13C-D3 at first, obtaining the retention time and mass spectra of each compound (Figure 1). Quantifier and qualifier ions were chosen based on their abundances and mass-to-charge ratios (m/z). Once the compounds were identified, the SIM (selected ion monitoring) mode was selected to increase the sensitivity of the method. The chosen ions are listed in Table 1 together with the retention times.
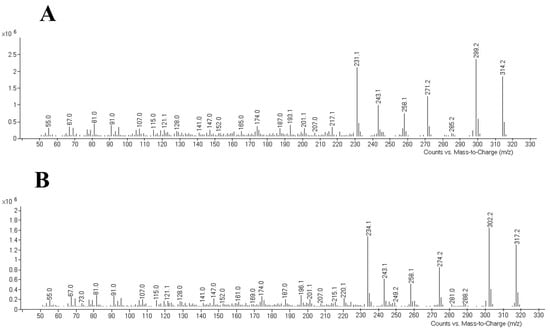
Figure 1.
Mass spectra for THC (A) and its internal standard (B), respectively. THC: tetrahydrocannabinol.

Table 1.
Retention times and characteristic ions for THC and the internal standard THC 13C-D3. THC: tetrahydrocannabinol; THC 13C-D3: tetrahydrocannabinol-D3.
2.3. Hair Samples Collection
For the validation process, hair samples were taken from abstaining volunteers, including children and adults who were not using any substances. The samples were cut from the posterior vertex region of the head as close to the scalp as possible, with the proximal section being identified. To prevent external contamination and sebum interfering with the analysis, all samples were thoroughly washed with pH-neutral liquid soap (Tween 20, Panreac®, Barcelona, Spain) and deionized water prior to analysis. The samples were then dried in an oven at 40 °C. Finally, they were cut into pieces approximately 5 mm long and weighed to a mass of 50 mg.
2.4. Sample Preparation Procedure (AALLME)
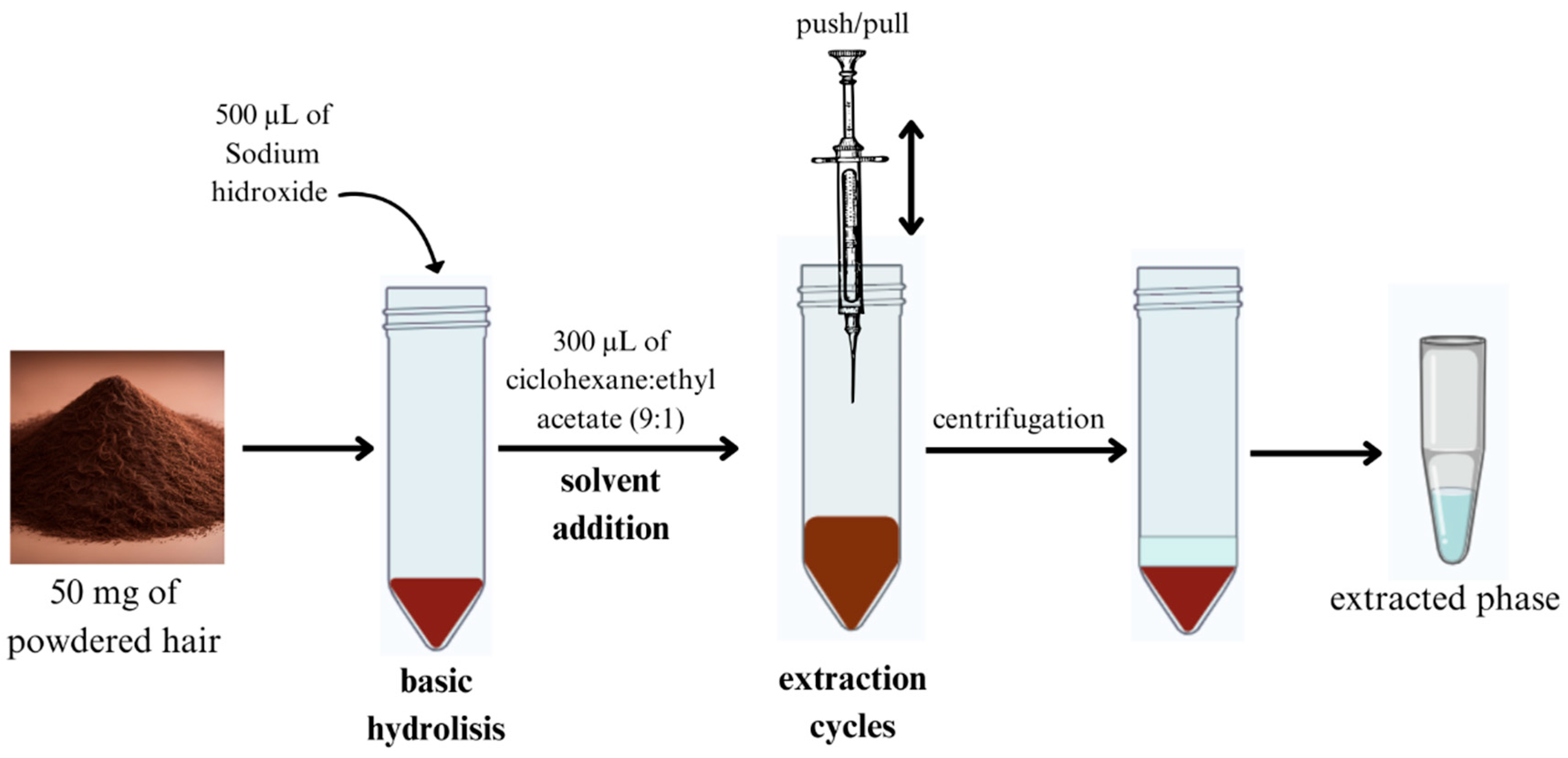
The hair sample (50 mg) was hydrolyzed in a basic medium containing 500 µL of 2.0 M NaOH at 100 °C for 10 min. Then, 40 µL of 2 ppm THC-D3 was added. AALLME extraction was then performed using the following procedure (Figure 2): 300 µL of a cyclohexane/ethyl acetate (9:1) mixture was injected into the hydrolyzed sample as the extraction solvent. The mixture was then aspirated six times with a Pasteur pipette and centrifuged at 3000 rpm for 4.0 min. This process led to the formation of an organic phase, which was collected at the top and transferred to a glass tube. The extracted organic solvent was then evaporated using a stream of nitrogen gas on an aluminum plate heated to 40 °C (VLM GmbH, Bielefeld, Germany). The dried residue was reconstituted with 40 µL of methanol, after which a 2.0 µL aliquot was injected into the GC–MS system.
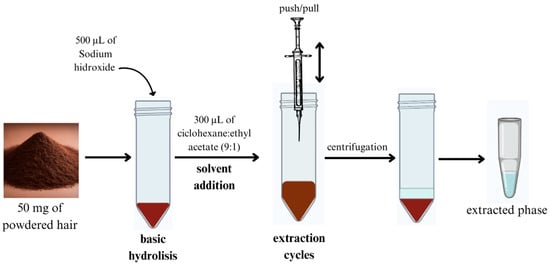
Figure 2.
Optimized hair hydrolysis and AALLME pretreatment for THC isolation from hair. AALLME: air-assisted liquid–liquid microextracion; THC: tetrahydrocannabinol.
2.5. Sample Preparation Procedure (DLLME)
A 50 mg hair sample was hydrolyzed in a basic medium containing 500 µL of 2 M NaOH at 100 °C for 10 min. Then, 40 µL of 2 ppm THC-13C-D3 was added. DLLME extraction was then performed by injecting a volume of 300 µL of a mixture of extractant and dispersant (500:150 µL) into the hydrolyzed sample. The mixture was then centrifuged at 3000 rpm for 4.0 min. This led to the formation of an organic phase, which was collected at the top and transferred to a glass tube. The extracted organic solvent was then evaporated using a stream of nitrogen gas on an aluminum plate heated to 40 °C (VLM GmbH, Bielefeld, Germany). The residue was then reconstituted with 40 µL of methanol before a 2.0 µL aliquot was injected into the GC–MS system.
2.6. Validation Parameters Studied
The method was fully validated in accordance with FDA guidelines on the validation of bioanalytical methods, with the selectivity, sensitivity, linearity, precision, bias, and recovery parameters evaluated [26].
Drug-free control hair samples spiked with standard THC solutions were used to achieve the desired concentration range. Standard addition curves were prepared after the AALLME method was performed as described. These curves were obtained by plotting the ratio of the peak areas of the analyte to the internal standard against THC concentration.
Sensitivity was determined by calculating the limit of detection (LOD) and the limit of quantification (LOQ). The limit of detection was set as the lowest concentration that the instrument can detect, calculated from the calibration curve (Equation (1)).
where s is standard deviation of the ordinate at the origin and µ is the slope of the calibration curve.
The LOQ represents the lowest concentration on the calibration curve that could be quantified with the required accuracy and precision, following Equation (2).
where s is the standard deviation of the ordinate at the origin and µ is the slope of the calibration curve.
The upper limit of quantification (ULOQ) was determined to be the concentration at which the calibration curve ceased to be linear.
Selectivity is the ability of an analytical method to accurately measure a specific analyte within a complex mixture without interference from other substances. To assess this parameter, six blank hair samples from different sources were analyzed to confirm the absence of significant interfering signals.
Bias (or error) is defined as the difference between the average results obtained using a specific analytical method and the established reference value. Precision is defined as the degree of agreement between results obtained from repeated analyses of a homogeneous sample under predefined conditions. Both parameters were assessed using inter-day and intra-day evaluations.
To assess inter-day precision and accuracy, blank hair samples spiked with three different concentrations of THC were analyzed. This involved analyzing five replicates daily at each concentration level, including the LOQ, ULOQ, and an intermediate level. Intra-day precision and accuracy were measured by analyzing five replicates at the same three concentration levels on the same day.
Recovery was determined as the ratio of the measured experimental analyte concentration to the concentration added to the sample. This parameter reflects the efficiency of the extraction method, which was evaluated by analyzing quintuplicates of blank hair samples spiked with high, medium, and low THC concentrations over several days.
3. Results and Discussion
3.1. Preliminary Study
3.1.1. Standard Hydrolysis Procedure
The alkaline hydrolysis process and the amount of sample used were not evaluated in this study as they follow a standardized, previously validated procedure [27]. Adding 500 µL of NaOH made the method suitable for the sample volume required for the AALLME application, even though a larger sample volume is normally used for biological samples, between 5 and 10 mL [20,21,22,23,24,25,28,29].
3.1.2. Comparison Between DLLME and AALLME
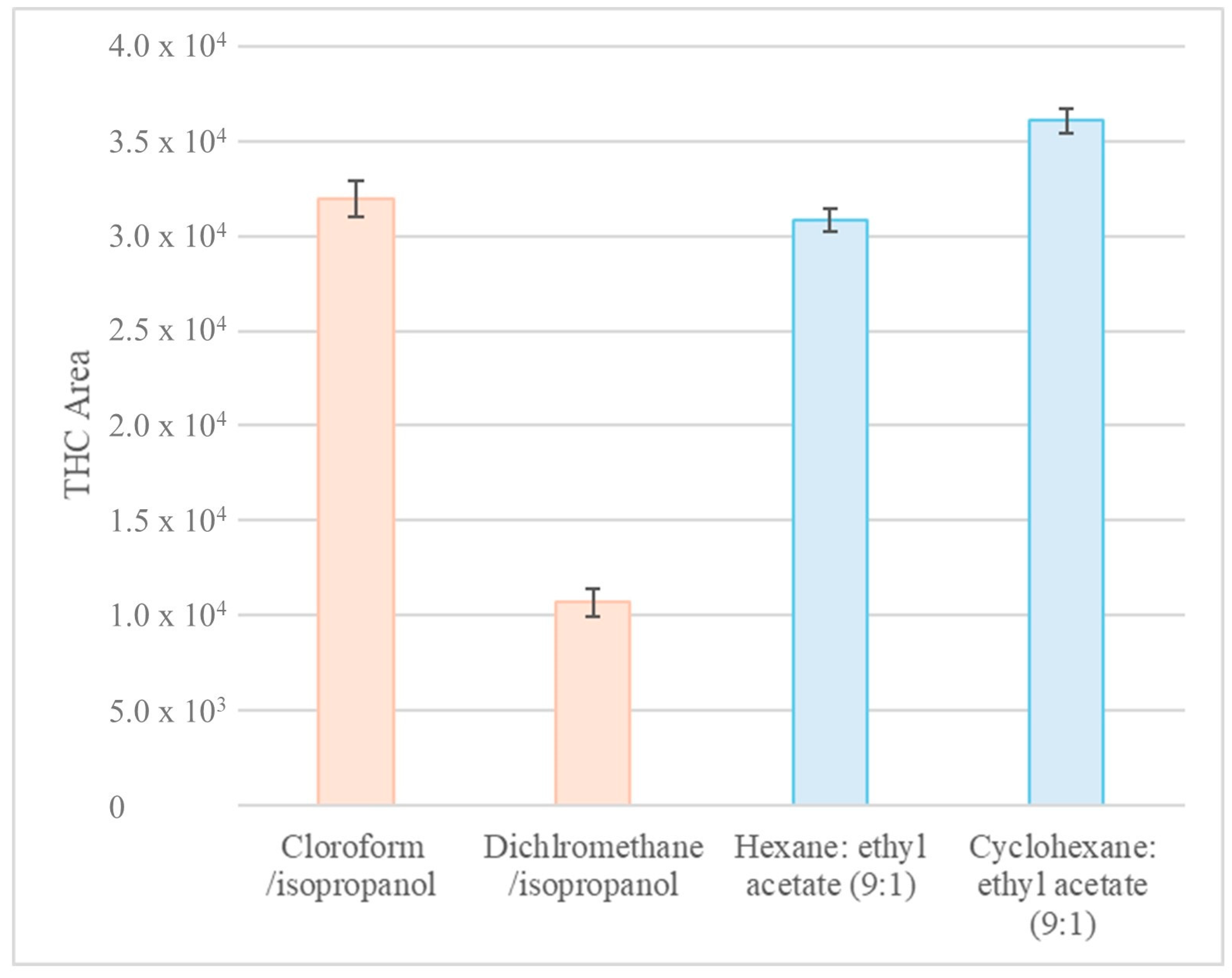
A series of experiments were conducted to compare the effectiveness of DLLME and AALLME in extracting THC from hair (Figure 3). Firstly, DLLME was investigated using isopropanol as the dispersant and chloroform, dichloromethane, and octanol as the extractants. This approach was then compared with the AALLME technique, which uses hexane and cyclohexane (solvent that is more environmentally and operator health friendly) as extraction solvents in combination with ethyl acetate in a 9:1 ratio [13,19]. Figure 3 shows the results obtained for DLLME and AALLME, with the exception of the octanol experiment, for which the required extraction droplet did not form.
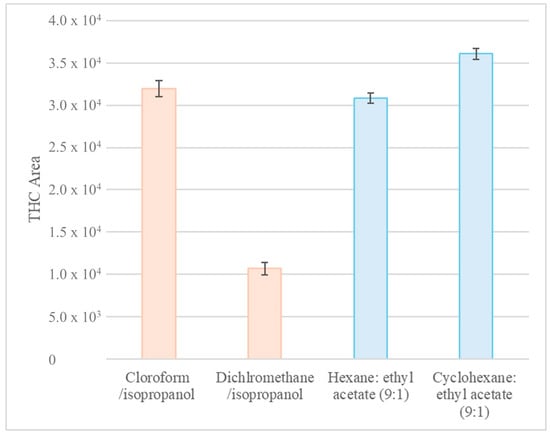
Figure 3.
Comparison of the chromatographic area of the THC peak obtained using DLLME (orange) and AALLME (blue) extraction techniques with different extraction solvents. The error bars represent the variability associated with a point after several measurements of the same experiment. THC: tetrahydrocannabinol.
AALLME demonstrated comparable or marginally superior extraction efficiency to DLLME for THC (Figure 3). In addition, it used less solvent and did not require the use of chlorinated organic solvents. Therefore, AALLME was chosen as the extraction technique. To demonstrate the accuracy of the measurements, each experiment was carried out several times. This is represented by the error bars in Figure 3.
3.2. AALLME Optimization
3.2.1. Selection of the Extraction Solvent
The selection of the solvent was based on a previously validated LLE method using an hexane–ethyl acetate mixture (9:1), with the aim of miniaturizing the conventional extraction process [27]. In addition, the possibility of replacing hexane with the less harmful alternative cyclohexane was also considered.
N-hexane is recognized as a chronic neurotoxicant. It exhibits reactive oxygen species (ROS)-mediated toxicity and alters gene expression related to DNA methylation and ovarian hormone production [30,31]. Cyclohexane, on the other hand, is slightly less volatile and more chemically stable. Consequently, it poses a lower risk and has higher permissible exposure limits than n-hexane [32]. According to the Pfizer solvent selection guide, cyclohexane is classified as a usable solvent, whereas the use of hexane is not recommended [30,33,34].
Figure 3 shows that cyclohexane provided an extraction efficiency comparable to that of hexane for the target analyte. Therefore, it was selected as a sustainable alternative extraction solvent.
3.2.2. Multivariate Optimization of Experimental Factors Affecting AALLME Procedure
To improve extraction efficiency, it is essential to optimise critical parameters. To this end, a multivariate optimization was performed using Statgraphics Centurion 19 software to account for any interactions between the factors. First, a two-level, four-component, 24−1 fractional factorial screening design with a p value of 0.05 was performed to determine which factors significantly influenced THC extraction. To minimise the effect of noise factors and systematic error, randomization and two centre points were included, resulting in a total of ten experiments. The parameters evaluated at low and high levels are listed in Table 2.

Table 2.
Experimental factors and dependent variables defined in Statgraphics Centurion 19 software.
Following statistical analysis and evaluation, the results of the ANOVA test are listed in Table 3. Initially, no significant effects were found, possibly due to low statistical values. To address this issue, the factor with the lowest calculated effect was eliminated. Ionic strength was not significant at the established confidence level. The presence of salts increases the ionic strength of the aqueous phase, which can improve the extraction process. However, in DLLME, this effect may not always improve extraction efficiency due to potential physicochemical changes in the aqueous phase [18]. The same argument seems to apply to AALLME: it has been reported that adding salt rarely improves extraction [20,21,29] and that added viscosity can sometimes reduce extraction efficiency by lowering diffusion coefficients and thus mass transfer efficiency at higher viscosities [35,36]. It was therefore decided not to include salt in the extraction procedure.

Table 3.
ANOVA results of the fractional factorial screening design, including and excluding salt addition as a factor, and the surface response design without the factor salt addition. Resultant significant effects, with p values < 0.05, are highlighted in red.
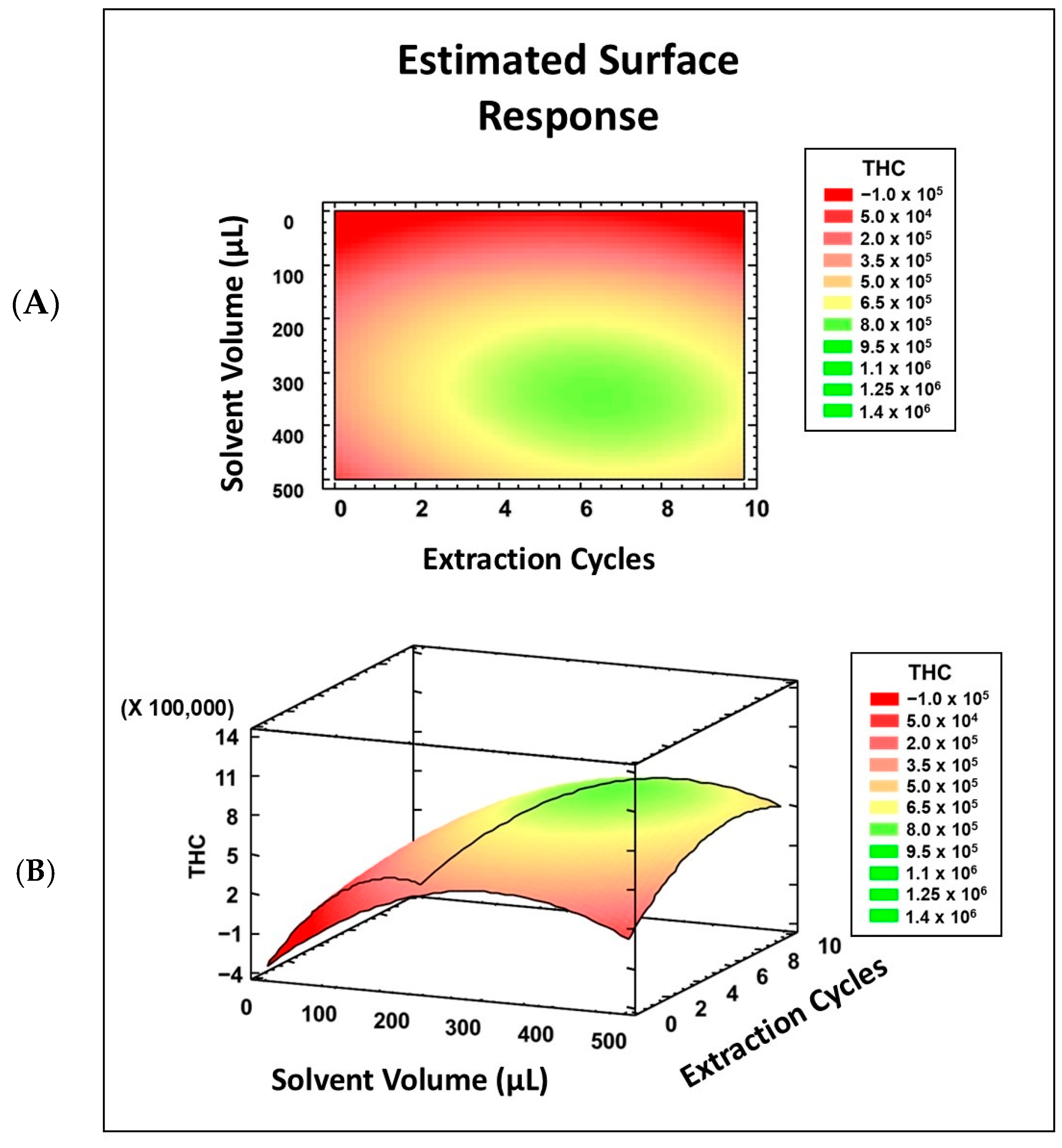
Excluding the factor of salt addition resulted in more effects becoming significant, giving an R2 of 90.65% with the screening model, which is acceptable. The factors found to be significant were the solvent volume, extraction cycles, and their interaction. Previous AALLME methods have reported that the number of extraction cycles increases extraction up to a certain level. After this point, the number of cycles either remains constant as equilibrium is reached, or starts to decrease [20,29,35,36,37,38]. Therefore, a surface response design was used to examine the curvature of these factors through ten additional experiments at three levels, in order to properly fit the model to the THC response (Figure 4).
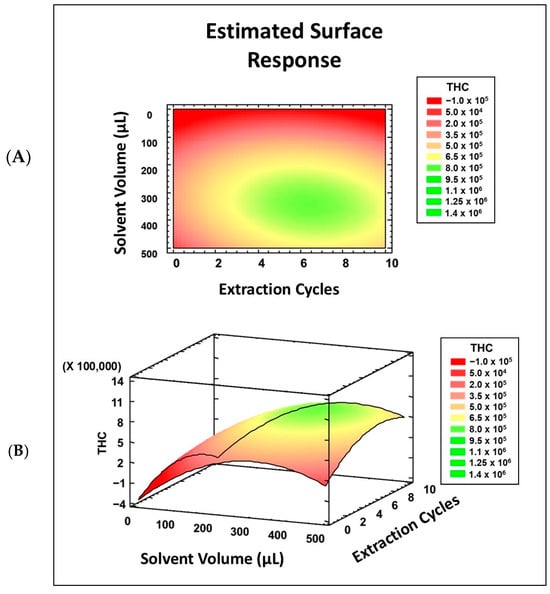
Figure 4.
Surface response design for number of cycles (A) and solvent volume (B).
The ANOVA results are shown again in Table 3, with an R2 of 93.75%, which indicates that the model fits the data better. However, the ultrasound-assisted process was not found to significantly influence extraction efficiency in either experiment, probably because equilibrium had already been reached by the end of the extraction cycles.
As the fractional factorial design did not provide sufficient information to define the values of the significant variables, a surface response design was performed (see Figure 4). Figure 4 shows the study range for each factor, the number of cycles (Figure 4A), and the solvent volume (Figure 4B), as well as the area of maximum signal, which is marked in green. Based on this, an optimal solvent volume of 300 µL and a number of cycles of n = 6 were selected.
3.3. Method Validation
To perform the validation, various parameters such as selectivity, linearity, and sensitivity; precision and accuracy; and recovery were monitored according to the FDA Bioanalytical Methods Validation Guideline [26].
The method was fully validated demonstrating high specificity and selectivity. The selectivity study involved analyzing six blank hair samples from different sources to confirm the absence of interferences at the THC retention time. Figure 5 shows the chromatogram obtained from one of the blank hair samples, confirming the absence of interferences at the THC retention time.
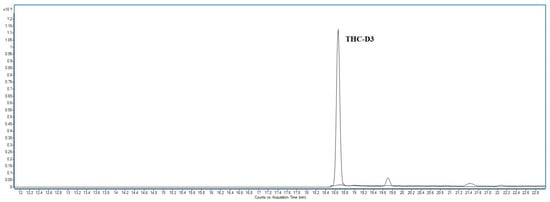
Figure 5.
Chromatogram of a blank hair sample.
The sensitivity of the method was determined by substituting the standard deviation and the slope of the calibration curve into Equation (2) after the LOQ study. A similar procedure was performed to determine the lowest concentration that the instrument can detect (LOD), substituting the aforementioned parameters into equation 1. The LOD and LOQ values were found to be 0.008 ng/mg and 0.01 ng/mg, respectively.
Addition curves were prepared using drug-free hair samples. The y-axis represented the ratio between the peak areas of the analyte and the internal standard, while the x-axis represented the THC concentration. The method exhibited a linear response within the 0.01–4.0 ng/mg range with correlation coefficients greater than 0.99. The resulting calibration curve was defined by the equation Area = 0.001169 (0.000147922) [THC] + 0.01256867 (0.002866044).
The intra- and inter-day precision, bias, and recovery were determined for three concentrations within the calibration range, with five replicates of each concentration. The proposed method met the requirements for intra- and inter-day precision, bias, and recovery. Precision and bias were less than 15% and 20%, respectively, at LOQ. The average recovery also met the requirements, ranging from 83% to 112%. The intra- and inter-day results for low, medium, and high concentration levels are shown in Table 4.

Table 4.
Intra- and inter-day precision (RSD), bias (ME), and analytical recovery.
The application of AALLME to hair samples has not previously been reported. The most common sample preparation methods for cannabinoid analysis are liquid–liquid extraction (LLE) and solid-phase extraction (SPE), combined with gas chromatography (GC) or high-performance liquid chromatography (HPLC), coupled with mass spectrometry (MS) or tandem mass spectrometry (MS/MS) for detection [39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55]. Microextraction techniques mainly rely on headspace (HS) or hollow fibre (HF) solid-phase microextraction (SPME) combined with gas chromatography–mass spectrometry (GC–MS) or GC–MS/MS analysis [56,57,58,59,60,61]. Additionally, dispersive solid-phase extraction (d-SPE) has been used alongside GC–MS for cannabinoid detection [3]. Most of the described methods involve basic hydrolysis, similar to that proposed in this study. The most common form of hydrolysis uses NaOH, particularly at a concentration of 1 M [40,42,43,44,46,48,51], either alone or in combination with carbonates [3], hydrochloric acid, or methanol [39,45]. Alternative approaches have included acid hydrolysis [58] or enzymatic hydrolysis [47], as well as the use of other bases, such as KOH combined with methanol [52], acetonitrile [55], or pressurised liquid extraction (PLE) [54]. Another common strategy involves a single hydrolysis and clean-up step using methanol [4,62,63,64,65].
Alkanes such as pentane and hexane, which are often mixed with ethyl acetate in a 9:1 ratio, are the most commonly used extractants in LLE approaches. However, some exceptions employ ethyl acetate [46] or chloroform–propanol combined with solid-phase extraction (SPE) [40]. The required solvent volumes typically range from 1.2 to 6 mL, whereas the optimized method significantly reduces this to 300 µL [39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55]. In addition, cyclohexane has been used as a sustainable alternative to the most commonly used extraction solvent.
The analytical performances of GC–MS-based methods vary. LLE approaches report LODs ranging from 0.006 to 0.05 ng/mg and LOQs ranging from 0.01 to 0.85 ng/mg [39,41,42,43,48]. By contrast, SPME-based methods report LODs of between 0.01 and 0.05 ng/mg and LOQs of between 0.02 and 0.27 ng/mg [56,57,58,59,60,61]. Table 5 provides a comparison of the above methods, which also employ similar hydrolysis techniques coupled with GC–MS analysis. The proposed method achieves lower detection limits than most published techniques and is comparable with conventional approaches. Furthermore, the linear range of the method is consistent with the Society of Hair Testing (SoHT) consensus cut-off value of 0.05 ng/mg for cannabinoid analysis in hair [66].

Table 5.
Comparison of the proposed method with other methods in the literature using NaOH hydrolysis and GC–MS analysis for THC, cannabinol (CBN), and cannabidiol (CBD) assessment.
3.4. Application to Forensic Cases
The developed method was applied to thirteen hair samples received by the Forensic Toxicology Service at the Institute of Forensic Sciences in Santiago de Compostela. These were judicial samples sent to the service for toxicological analysis. Once the analyses had been completed, the samples were stored for one year. After this period, the samples can be destroyed or anonymised for research purposes. The results are summarized in Table 6, which shows that fifteen cases were positive, three were negative, one was lower than the lower limit of quantification (LLOQ) of the method, and another was lower than the upper limit of quantification (ULOQ). These findings confirm that the limit of detection of the method is well defined for its intended application. Figure 6 shows the chromatogram of a positive case (number 9).

Table 6.
THC concentrations in the studied forensic cases.
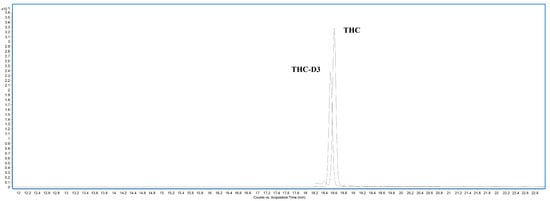
Figure 6.
Chromatogram of real case number 9.
4. Conclusions
This study aimed to validate a method for quantifying THC in hair using AALLME as a pre-treatment sample for the first time. AALLME is a more environmentally sustainable alternative to the traditional LLE method, as it modifies DLLME by omitting halogenated organic solvents and large quantities of dispersant. The optimal extraction solvent mixture found was cyclohexane and ethyl acetate in a ratio of 9:1, which effectively miniaturizes conventional LLE for THC analysis and replaces hexane with a more sustainable option. Successful separation and identification of THC was achieved using GC–MS. According to FDA guidelines, the proposed method was validated and showed a detection limit of 0.008 and a linear range of 0.01–4 ng/mg. The method’s precision and accuracy were confirmed with errors below 20% and recovery rates ranging from 82% to 112%. These results indicate that the method is highly selective, sensitive, and accurate, making it suitable for forensic applications. The developed method was applied to analyze twenty hair samples, with THC detected in fifteen cases and three yielding negative results. Future research will focus on extending the application of this technique to other target drugs.
Author Contributions
Conceptualization: A.M.B.-B. and A.M.-P.; Methodology: A.M.B.-B., P.C.-F., A.M.-P. and M.J.T.-D.; Validation: L.B.-G. and P.C.-F.; Investigation: L.B.-G. and P.C.-F.; Writing—original draft preparation: L.B.-G.; Writing—review and editing: A.M.-P., A.M.B.-B., A.M.-P., I.Á.-F. and M.J.T.-D.; Supervision: A.M.B.-B., A.M.-P. and P.C.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Approval from Galicia’s Ethics Committee was not required because the toxicological data used in this work did not allow for the identification of the subjects.
Informed Consent Statement
The approval of the Ethics Committee of Galicia was not required because the toxicological data used in this work do not allow the identification of the subjects.
Data Availability Statement
The data that has been used is confidential.
Conflicts of Interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
References
- World Health Organization. Cannabis. Organización Mundial de la Salud. Available online: https://www.who.int/teams/mental-health-and-substance-use/alcohol-drugs-and-addictive-behaviours/drugs-psychoactive/cannabis (accessed on 3 March 2025).
- Observatorio Español de las Drogas y las Adicciones. Informe 2024: Alcohol, Tabaco y Drogas Ilegales en España; Ministerio de Sanidad, Delegación del Gobierno para el Plan Nacional sobre Drogas: Madrid, Spain, 2024; Available online: https://pnsd.sanidad.gob.es/ (accessed on 15 January 2025).
- Kale, R.; Chaturvedi, D.; Dandekar, P.; Jain, R. Analytical techniques for screening of cannabis and derivatives from human hair specimens. Anal. Methods 2024, 16, 1133–1149. [Google Scholar] [CrossRef] [PubMed]
- Nestoros, J.N.; Vakonaki, E.; Tzatzarakis, M.N.; Alegakis, A.; Skondras, M.D.; Tsatsakis, A.M. Long lasting effects of chronic heavy cannabis abuse. Am. J. Addict. 2017, 26, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.; Weizman, A.; Weinstein, A. Positive and negative effects of cannabis and cannabinoids on health. Clin. Pharmacol. Ther. 2019, 105, 1139–1147. [Google Scholar] [CrossRef]
- Ferreira, C.; Paulino, C.; Quintas, A. Extraction procedures for hair forensic toxicological analysis: A mini-review. Chem. Res. Toxicol. 2019, 32, 2367–2381. [Google Scholar] [CrossRef]
- Baciu, T.; Borrull, F.; Aguilar, C.; Calull, M. Recent trends in analytical methods and separation techniques for drugs of abuse in hair. Anal. Chim. Acta 2015, 856, 1–26. [Google Scholar] [CrossRef]
- Kintz, P. Hair analysis in forensic toxicology. Wiley Interdiscip. Rev. Forensic Sci. 2019, 1, e1196. [Google Scholar] [CrossRef]
- Chen, X.; Wu, X.; Luan, T.; Jiang, R.; Ouyang, G. Sample preparation and instrumental methods for illicit drugs in environmental and biological samples: A review. J. Chromatogr. A 2021, 1640, 461961. [Google Scholar] [CrossRef]
- Khajuria, H.; Nayak, B.P.; Badiye, A. Toxicological hair analysis: Pre-analytical, analytical and interpretive aspects. Med. Sci. Law 2018, 58, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Garrigues, S.; de la Guardia, M. (Eds.) Challenges in Green Analytical Chemistry, 2nd ed.; Royal Society of Chemistry: Tokyo, Japan, 2020. [Google Scholar] [CrossRef]
- Rosado, T.; Barroso, M.; Vieira, D.N.; Gallardo, E. Trends in microextraction approaches for handling human hair extracts—A review. Anal. Chim. Acta 2021, 1185, 338792. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Zhang, Y.; Bian, Y.; Liu, Y.J.; Ren, A.; Zhou, Y.; Shi, D.; Feng, X.S. Benzodiazepines in complex biological matrices: Recent updates on pretreatment and detection methods. J. Pharm. Anal. 2023, 13, 442–462. [Google Scholar] [CrossRef]
- Jain, R.; Singh, R. Applications of dispersive liquid–liquid micro-extraction in forensic toxicology. TrAC Trends Anal. Chem. 2016, 75, 227–237. [Google Scholar] [CrossRef]
- Manousi, N.; Samanidou, V. Green sample preparation of alternative biosamples in forensic toxicology. Sustain. Chem. Pharm. 2021, 20, 100388. [Google Scholar] [CrossRef]
- Oliveira, J.R.I.L.; Rodrigues, L.C.; Kahl, J.M.M.; Berlinck, D.Z.; Costa, J.L. Green Analytical Toxicology procedure for determination of ketamine, its metabolites and analogues in oral fluid samples using dispersive liquid–liquid microextraction (DLLME). J. Anal. Toxicol. 2024, 48, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Zuloaga, O.; Olivares, M.; Navarro, P.; Vallejo, A.; Prieto, A. Dispersive liquid–liquid microextraction: Trends in the analysis of biological samples. Bioanalysis 2015, 7, 2211–2225. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Ouyang, G. Fast analytical techniques based on microextraction. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; Volume 67, pp. 85–134. [Google Scholar] [CrossRef]
- Azooz, E.A.; Al-Wani, H.S.A.; Gburi, M.S.; Al-Muhanna, E.H.B. Recent modified air-assisted liquid–liquid microextraction applications for medicines and organic compounds in various samples: A review. Open Chem. 2022, 20, 525–540. [Google Scholar] [CrossRef]
- Lamei, N.; Ezoddin, M.; Abdi, K. Air assisted emulsification liquid-liquid microextraction based on deep eutectic solvent for preconcentration of methadone in water and biological samples. Talanta 2017, 165, 176–181. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Reza, M.; Mogaddam, A.; Bamorowat, M. Determination of unconjugated non-steroidal anti-inflammatory drugs in biological fluids using air-assisted liquid–liquid microextraction combined with back extraction followed by high performance liquid chromatography. Anal. Methods 2015, 7, 1372–1379. [Google Scholar] [CrossRef]
- Ghadi, M.; Hadjmohammadi, M.R. Extraction and determination of three benzodiazepines in aqueous and biological samples by air-assisted liquid–liquid microextraction and high-performance liquid chromatography. J. Iran. Chem. Soc. 2019, 16, 1147–1155. [Google Scholar] [CrossRef]
- Barfi, B.; Asghari, A.; Rajabi, M.; Moghadam, A.G.; Mirkhani, N.; Ahmadi, F. Comparison of ultrasound-enhanced air-assisted liquid–liquid microextraction and low-density solvent-based dispersive liquid–liquid microextraction methods for determination of nonsteroidal anti-inflammatory drugs in human urine samples. J. Pharm. Biomed. Anal. 2015, 111, 297–305. [Google Scholar] [CrossRef]
- Majidi, S.M.; Hadjmohammadi, M.R. Air-assisted surfactant-enhanced emulsification liquid–liquid microextraction based on the solidification of floating organic droplets followed by high-performance liquid chromatography with ultraviolet detection for the determination of clozapine in biological samples. J. Iran. Chem. Soc. 2019, 16, 2307–2314. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Mohebbi, A.; Pazhohan, A.; Nemati, M.; Mogaddam, M.R.A. Air–assisted liquid–liquid microextraction; principles and applications with analytical instruments. TrAC Trends Anal. Chem. 2020, 122, 115734. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services, Food and Drug Administration. Bioanalytical Method Validation: Guidance for Industry; U.S. Department of Health and Human Services, Food and Drug Administration: Silver Spring, MD, USA, 2018. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 10 January 2025).
- Villamor, J.L.; Bermejo, A.M.; Tabernero, M.J.; Fernandez, P. Determination of cannabinoids in human hair by GC/MS. Anal. Lett. 2004, 37, 517–528. [Google Scholar] [CrossRef]
- Ferrone, V.; Cotellese, R.; Carlucci, M.; Di Marco, L.; Carlucci, G. Air assisted dispersive liquid-liquid microextraction with solidification of the floating organic droplets (AA-DLLME-SFO) and UHPLC-PDA method: Application to antibiotics analysis in human plasma of hospital acquired pneumonia patients. J. Pharm. Biomed. Anal. 2018, 151, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Bazregar, M.; Rajabi, M.; Yamini, Y.; Asghari, A.; Hemmati, M. Tandem air-agitated liquid–liquid microextraction as an efficient method for determination of acidic drugs in complicated matrices. Anal. Chim. Acta 2016, 917, 44–52. [Google Scholar] [CrossRef]
- Joshi, D.R.; Adhikari, N. An overview on common organic solvents and their toxicity. J. Pharm. Res. Int. 2019, 28, 1–18. [Google Scholar] [CrossRef]
- Cravotto, C.; Fabiano-Tixier, A.S.; Claux, O.; Abert-Vian, M.; Tabasso, S.; Cravotto, G.; Chemat, F. Towards substitution of hexane as extraction solvent of food products and ingredients with no regrets. Foods 2022, 11, 3412. [Google Scholar] [CrossRef] [PubMed]
- Kreckmann, K.H.; Baldwin, J.K.; Roberts, L.G.; Staab, R.J.; Kelly, D.P.; Saik, J.E. Inhalation developmental toxicity and reproduction studies with cyclohexane. Drug Chem. Toxicol. 2000, 23, 555–573. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. Safety Data Sheet: Hexane (Revision Number 12). 2023. Available online: https://origin-beta.fishersci.it/chemicalProductData_uk/wercs?itemCode=12347083&lang=EN (accessed on 29 November 2024).
- Thermo Fisher Scientific. Safety Data Sheet: Cyclohexane (Revision Number 12). 2023. Available online: https://www.thermofishersci.in/msds/cyclohexane.pdf (accessed on 29 November 2024).
- Farajzadeh, M.A.; Sattari Dabbagh, M.; Yadegari, A.; Alizadeh Nabil, A.A. Air-assisted liquid-liquid microextraction vs. dispersive liquid-liquid microextraction; a comparative study for the analysis of multiclass pesticides. Anal. Bioanal. Chem. Res. 2019, 6, 29–46. [Google Scholar] [CrossRef]
- Wang, L.; Huang, T.; Cao, H.X.; Yuan, Q.X.; Liang, Z.P.; Liang, G.X. Application of air-assisted liquid-liquid microextraction for determination of some fluoroquinolones in milk powder and egg samples: Comparison with conventional dispersive liquid-liquid microextraction. Food Anal. Methods 2016, 9, 2223–2230. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Mogaddam, M.R.A.; Aghdam, A.A. Comparison of air-agitated liquid–liquid microextraction technique and conventional dispersive liquid–liquid micro-extraction for determination of triazole pesticides in aqueous samples by gas chromatography with flame ionization detection. J. Chromatogr. A 2013, 1300, 70–78. [Google Scholar] [CrossRef]
- Zhou, Q.; Jin, Z.; Li, J.; Wang, B.; Wei, X.; Chen, J. A novel air-assisted liquid-liquid microextraction based on in-situ phase separation for the HPLC determination of bisphenols migration from disposable lunch boxes to contacting water. Talanta 2018, 189, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Tassoni, G.; Cippitelli, M.; Ottaviani, G.; Froldi, R.; Cingolani, M. Detection of cannabinoids by ELISA and GC–MS methods in a hair sample previously used to detect other drugs of abuse. J. Anal. Toxicol. 2016, 40, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Angeli, I.; Casati, S.; Ravelli, A.; Minoli, M.; Orioli, M. A novel single-step GC–MS/MS method for cannabinoids and 11-OH-THC metabolite analysis in hair. J. Pharm. Biomed. Anal. 2018, 155, 1–6. [Google Scholar] [CrossRef]
- Míguez-Framil, M.; Cocho, J.Á.; Tabernero, M.J.; Bermejo, A.M.; Moreda-Piñeiro, A.; Bermejo-Barrera, P. An improved method for the determination of∆ 9-tetrahydrocannabinol, cannabinol and cannabidiol in hair by liquid chromatography–tandem mass spectrometry. Microchem. J. 2014, 117, 7–17. [Google Scholar] [CrossRef]
- Kim, J.Y.; Suh, S.; In, M.K.; Paeng, K.J.; Chung, B.C. Simultaneous determination of cannabidiol, cannabinol, and gD9 9-tetrahydrocannabinol in human hair by gas chromatography-mass spectrometryin human hair by gas chromatography-mass spectrometry. Arch. Pharmacal Res. 2005, 28, 1086–1091. [Google Scholar] [CrossRef]
- Heinl, S.; Lerch, O.; Erdmann, F. Automated GC–MS Determination of Δ9-Tetrahydrocannabinol, Cannabinol and Cannabidiol in Hair. J. Anal. Toxicol. 2016, 40, 498–503. [Google Scholar] [CrossRef]
- Han, E.; Park, Y.; Kim, E.; In, S.; Yang, W.; Lee, S.; Choi, H.; Lee, S.; Chung, H.; myong Song, J. Simultaneous analysis of Δ9-tetrahydrocannabinol and 11-nor-9-carboxy-tetrahydrocannabinol in hair without different sample preparation and derivatization by gas chromatography–tandem mass spectrometry. J. Pharm. Biomed. Anal. 2011, 55, 1096–1103. [Google Scholar] [CrossRef]
- Paul, R.; Williams, R.; Hodson, V.; Peake, C. Detection of cannabinoids in hair after cosmetic application of hemp oil. Sci. Rep. 2019, 9, 2582. [Google Scholar] [CrossRef]
- Mercolini, L.; Mandrioli, R.; Protti, M.; Conti, M.; Serpelloni, G.; Raggi, M.A. Monitoring of chronic Cannabis abuse: An LC–MS/MS method for hair analysis. J. Pharm. Biomed. Anal. 2013, 76, 119–125. [Google Scholar] [CrossRef]
- Breidi, S.E.; Barker, J.; Petroczi, A.; Naughton, D.P. Enzymatic digestion and selective quantification of underivatised delta-9-tetrahydrocannabinol and cocaine in human hair using gas chromatography-mass spectrometry. J. Anal. Methods Chem. 2012, 2012, 907893. [Google Scholar] [CrossRef]
- Kronstrand, R.; Nyström, I.; Forsman, M.; Käll, K. Hair analysis for drugs in driver’s license regranting. A Swedish pilot study. Forensic Sci. Int. 2010, 196, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Auwärter, V.; Wohlfarth, A.; Traber, J.; Thieme, D.; Weinmann, W. Hair analysis for Δ9-tetrahydrocannabinolic acid A—New insights into the mechanism of drug incorporation of cannabinoids into hair. Forensic Sci. Int. 2010, 196, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Cobo-Golpe, M.; de-Castro-Ríos, A.; Cruz, A.; López-Rivadulla, M.; Lendoiro, E. Determination and distribution of cannabinoids in nail and hair samples. J. Anal. Toxicol. 2021, 45, 969–975. [Google Scholar] [CrossRef]
- Kieliba, T.; Lerch, O.; Andresen-Streichert, H.; Rothschild, M.A.; Beike, J. Simultaneous quantification of THC-COOH, OH-THC, and further cannabinoids in human hair by gas chromatography–tandem mass spectrometry with electron ionization applying automated sample preparation. Drug Test. Anal. 2019, 11, 267–278. [Google Scholar] [CrossRef]
- Hill, V.A.; Schaffer, M.I.; Paulsen, R.B.; Stowe, G.N. Cannabinoids Tetrahydrocannabinol, Cannabinol, Cannabidiol, Tetrahydrocannabivarin and 11-nor-9-carboxy-∆ 9-THC in Hair. J. Anal. Toxicol. 2022, 46, 487–493. [Google Scholar] [CrossRef]
- Rodrigues, A.; Yegles, M.; Van Elsué, N.; Schneider, S. Determination of cannabinoids in hair of CBD rich extracts consumers using gas chromatography with tandem mass spectrometry (GC/MS–MS). Forensic Sci. Int. 2018, 292, 163–166. [Google Scholar] [CrossRef]
- Montesano, C.; Simeoni, M.C.; Vannutelli, G.; Gregori, A.; Ripani, L.; Sergi, M.; Compagnone, D.; Curini, R. Pressurized liquid extraction for the determination of cannabinoids and metabolites in hair: Detection of cut-off values by high performance liquid chromatography–high resolution tandem mass spectrometry. J. Chromatogr. A 2015, 1406, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Lendoiro, E.; Quintela, O.; de Castro, A.; Cruz, A.; Lopez-Rivadulla, M.; Concheiro, M. Target screening and confirmation of 35 licit and illicit drugs and metabolites in hair by LC–MSMS. Forensic Sci. Int. 2012, 217, 207–215. [Google Scholar] [CrossRef]
- Emídio, E.S.; de Menezes Prata, V.; Dórea, H.S. Validation of an analytical method for analysis of cannabinoids in hair by headspace solid-phase microextraction and gas chromatography–ion trap tandem mass spectrometry. Anal. Chim. Acta 2010, 670, 63–71. [Google Scholar] [CrossRef]
- Emídio, E.S.; de Menezes Prata, V.; De Santana, F.J.M.; Dórea, H.S. Hollow fiber-based liquid phase microextraction with factorial design optimization and gas chromatography–tandem mass spectrometry for determination of cannabinoids in human hair. J. Chromatogr. B 2010, 878, 2175–2183. [Google Scholar] [CrossRef]
- Merola, G.; Gentili, S.; Tagliaro, F.; Macchia, T. Determination of different recreational drugs in hair by HS-SPME and GC/MS. Anal. Bioanal. Chem. 2010, 397, 2987–2995. [Google Scholar] [CrossRef] [PubMed]
- Musshoff, F.; Junker, H.P.; Lachenmeier, D.W.; Kroener, L.; Madea, B. Fully automated determination of cannabinoids in hair samples using headspace solid-phase microextraction and gas chromatography-mass spectrometry. J. Anal. Toxicol. 2002, 26, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Nadulski, T.; Pragst, F. Simple and sensitive determination of Δ9-tetrahydrocannabinol, cannabidiol and cannabinol in hair by combined silylation, headspace solid phase microextraction and gas chromatography–mass spectrometry. J. Chromatogr. B 2007, 846, 78–85. [Google Scholar] [CrossRef]
- Dizioli Rodrigues de Oliveira, C.; Yonamine, M.; de Moraes Moreau, R.L. Headspace solid-phase microextraction of cannabinoids in human head hair samples. J. Sep. Sci. 2007, 30, 128–134. [Google Scholar] [CrossRef]
- Odoardi, S.; Valentini, V.; De Giovanni, N.; Pascali, V.L.; Strano-Rossi, S. High-throughput screening for drugs of abuse and pharmaceutical drugs in hair by liquid-chromatography-high resolution mass spectrometry (LC-HRMS). Microchem. J. 2017, 133, 302–310. [Google Scholar] [CrossRef]
- Di Corcia, D.; D’urso, F.; Gerace, E.; Salomone, A.; Vincenti, M. Simultaneous determination in hair of multiclass drugs of abuse (including THC) by ultra-high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2012, 899, 154–159. [Google Scholar] [CrossRef]
- Roth, N.; Moosmann, B.; Auwärter, V. Development and validation of an LC-MS/MS method for quantification of Δ9-tetrahydrocannabinolic acid A (THCA-A), THC, CBN and CBD in hair. J. Mass Spectrom. 2013, 48, 227–233. [Google Scholar] [CrossRef]
- Domínguez-Romero, J.C.; García-Reyes, J.F.; Molina-Díaz, A. Screening and quantitation of multiclass drugs of abuse and pharmaceuticals in hair by fast liquid chromatography electrospray time-of-flight mass spectrometry. J. Chromatogr. B 2011, 879, 2034–2042. [Google Scholar] [CrossRef]
- SoHT. SoHT Consensus on Drugs of Abuse (DoA) Testing in Hair. Sociedad de Toxicología del Cabello (SoHT). 2021. Available online: https://www.soht.org/consensus (accessed on 20 January 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).