1. Introduction
Metal–organic frameworks (MOFs) are mainly composed of metals/metal clusters and organiclinkers [
1,
2,
3]. Due to their high specific surface area, large pore size, high thermal and chemical stability, and novel and diverse structures, they are widely used in gas separation, catalysis, fluorescence detection, molecular magnetism, ion recognition, and other fields [
4,
5,
6,
7,
8,
9,
10]. Compared with conventional MOFs, rare earth metal–organic frameworks (RE-MOFs) not only have many of the same properties but also have their own features [
11,
12,
13,
14,
15]. (1) Rare earth metal nodes are divided into different structures, such as rare earth metal ions, rare earth metal chains, or rare earth metal clusters. The f-f jumps produce unique narrow-band emission with high color purity. By adjusting the types and ratios of rare earth ions (such as Eu
3+/Tb
3+), a multi-color fluorescence gradient from green to red can be achieved for multifunctional display and encryption technology. (2) The high strength of coordination bonds formed between rare earth metal ions and polydentate ligands (e.g., carboxylic acids) makes RE-MOFs chemically more stable than ordinary MOFs, and able to remain thermally stable above 450 °C, while ordinary MOFs (e.g., Zn-MOFs, Cu-MOFs) usually decompose at 200–300 °C. (3) Certain rare earth ions exhibit excellent emission properties in the near-infrared (NIR) region, which is particularly beneficial for bio-imaging and sensing applications due to the reduction in tissue autofluorescence and the increase in penetration depth. In summary, rare earth ions with MOFs show a range of advantages, including enhanced luminescence, tailored photonic properties, and unique electronic properties. These unique features make RE-MOFs ideally suited for advanced photonic applications, including optical sensors, fluorescence detection probes, photocatalytic materials, quantum memory devices, and bioimaging. Notably, coupling MOFs with sensing devices can yield exceptional quantum memory devices. Their highly ordered porous structures and tunable electronic states enable high-fidelity storage and efficient manipulation of quantum information, providing a high-density, low-power-consumption hardware foundation for future quantum computing and communication systems [
16,
17,
18,
19,
20,
21].
Although the exploration of RE-MOFs is still at an early stage, there are several key issues that deserve further investigation. Firstly, the structural design principles and strategies based on luminescence enhancement are crucial in the study of RE-MOFs. The systematic analysis of the selection of organic ligands and the design of the RE-MOF backbone structure are the key elements for the high-quality luminescence of RE-MOF materials, which can provide theoretical guidance and practical references for the research and development of new rare earth materials. Secondly, there is an urgent need for environmentally friendly and efficient synthesis methods. Organic pollutants contribute to approximately 3 million premature deaths annually, with economic losses reaching
$1.5 trillion, primarily due to water pollution, soil degradation, and biodiversity loss [
22,
23,
24]. Establishing sustainable and robust methods to generate high-quality renewable MOFs is essential to ensure their scalability and practical applications. Finally, fluorescence detection mechanisms remain a key research area in the field. The chemical reaction between the recognition group and the analyte is cleverly used to change the fluorescence intensity, and the chemical signal of the target is converted into an optical signal to achieve the purpose of transmitting information. These challenges highlight the exciting potential of remote controls, while also emphasizing the need for continued research to unleash their full functionality and applications.
In this paper, the design principles and strategies, synthesis methods, fluorescence monitoring mechanisms, and applications of rare earth metal–organic frameworks are comprehensively reviewed. The review reports recent advances in rare earth metal–organic frameworks for pollutant monitoring, covering design strategies and synthesis methods for monitoring gaseous, ionic, and organic pollutants. A new aspect of this review related to the design strategies of rare earth metal–organic frameworks, including the effect of organic ligands on RE-MOF formation and contaminant monitoring, which fills the systematic gap in the research on mechanism-performance synergistic optimization within conventional reviews. The paper also discusses the intricate details of the spectroscopy and energy transfer processes of RE-MOFs, providing a comprehensive understanding of their underlying mechanisms. Finally, the current challenges and potential future directions in the field of MOFs are provided. This review provides valuable insights into the evolving RE-MOFs and their applications in the fluorescence detection of pollutants, making them an invaluable resource for researchers in the field.
2. Design Principles and Strategies for High-Quality Luminescent RE-MOFs
Rare earth elements are often found in the form of trivalent ions, which have an electronic arrangement of 4f
0–14 with fully filled 5s
25p
6 electron orbitals and unfilled 4f electron orbitals [
25]. According to Laporte’s rule, the f-f leap of rare earth ions is a forbidden barrier leap. Therefore, the molar absorption coefficient of rare earth ions for UV-visible light is small, and the direct absorption of light is weak, which leads to the weak fluorescence of rare earth ions, limiting the popularization of their applications. RE-MOFs suffer from drawbacks and limitations such as low quantum yield, poor stability, short fluorescence lifetime, and small Stokes shift [
26,
27]. By strategically combining rare earth ions with organic ligands, the antenna effect can be exploited to overcome their inherent low luminescence efficiency, resulting in the creation of high-quality luminescent materials with high quantum yields, long fluorescence lifetimes, and large Stokes shifts [
28,
29]. The selectivity of RE-MOFs is governed by the synergistic interplay of ligand chemistry, framework topology, and dynamic responsiveness. Through molecular-level precision engineering, these materials can be endowed with ‘intelligent recognition’ capabilities, such as incorporating stimuli-responsive moieties to design switchable sensing platforms that adaptively target specific pollutants. So, the systematic analysis of the selection of organic ligands, the design of RE-MOFs backbone structure, and the post-modification design of RE-MOFs are the key elements for the high-quality luminescence of RE-MOFs materials, which can provide theoretical guidance and practical references for the research and development of new rare earth materials.
2.1. Selection of Organic Ligands
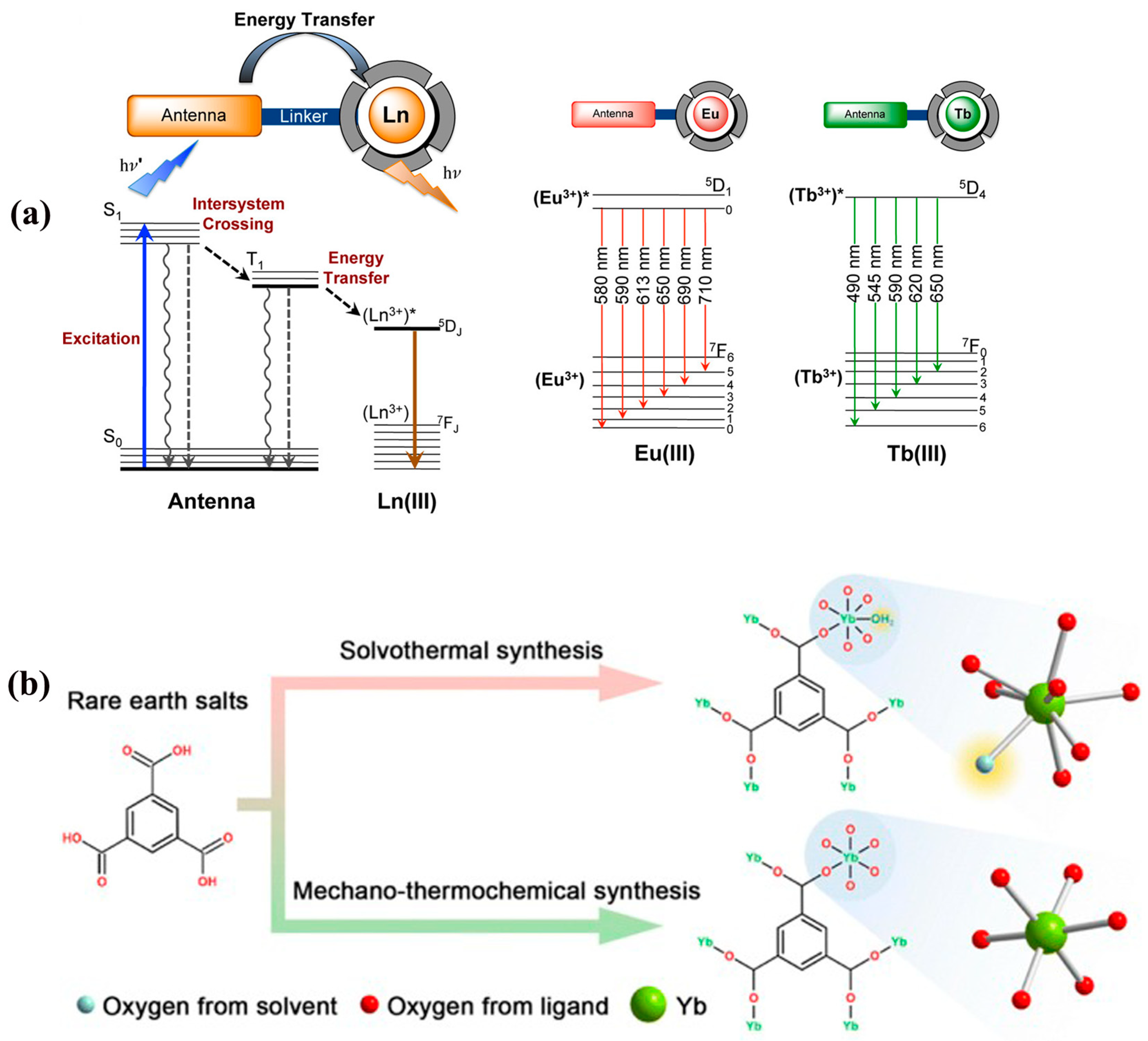
Organic ligands are important components in the construction of MOFs and are responsible for linking the metal nodes into a multidimensional mesh structure through ligand bonds. Through the precise design of the functional groups, rigidity, and topological structures of ligands, RE-MOFs can be endowed with ‘molecular recognition’ capabilities for specific pollutants, even enabling simultaneous detection of multiple contaminants with a single versatile material. This property increases the fluorescence yield and fluorescence lifetime, thus optimizing the luminescent properties of the material. The most widely used organic ligands in combination with rare earth ions are polydentate or rigid organic complexes with various functional groups. These functional groups include o-phenanthroline, sulphate (SO
42−), nicotinic acid, succinic acid, and tartaric acid, as well as chemical derivatives of benzene rings, pyridine, pyrazine, and porphyrin. These ligands are chosen based on their specific properties and ability to sensitize rare earth ions and enhance their luminescent properties. The whole process of their sensitization consists of the following steps (
Figure 1): (i) the organic ligand is excited to the singlet state; (ii) a portion of the energy is transferred from the singlet state to the triplet state by intersystem crossing (ISC); and (iii) the ligand in the excited state transfers energy to the rare earth metals and sensitizes the luminescence. This process can effectively increase the luminescence quantum yield of rare earth ions at room temperature [
30]. Therefore, RE-MOFs have strong luminescent properties and can be used as chemical sensors.
2.1.1. Multi-Dentate Ligands
The polydentate ligand combines with rare earth ions to form a strong coordination bond through multiple coordination sites, which reduces the coordination of water molecules while improving the thermal and chemical stability of the framework structure. The fluorescence quenching caused by the vibration of water molecules is avoided to a certain extent, and the fluorescence emission of rare earth ions is effectively excited. Cui et al. [
31] constructed a metal–organic framework by using nitrogen-containing tricarboxylic acid (H
3ttc) as an antenna sensitization ligand and lanthanide metals (Dy
3+, Eu
3+, Nd
3+, Gd
3+) to solve the problem of low molar absorbance coefficient of Ln
3+ and weak ability to directly absorb light. Wang et al. [
32] selected a combination of flexible and rigid tricarboxylic acid (H
3L) as an organic ligand, which provides both a multicoordination mode to enhance the stability of the framework and a π-conjugated system to effectively enhance the fluorescence emission intensity of terbium (Tb
3+) ions with an emission characteristic wavelength of 544 nm (green light). The carboxyl group in the ligand coordinates with the Tb
3+ metal center to form a stable framework with nine-coordination and ultimately RE-MOFs with three-dimensional porous structures are formed, which improves the interaction efficiency with target molecules. Unlike the study of Wang et al. [
32], Karmaker et al. [
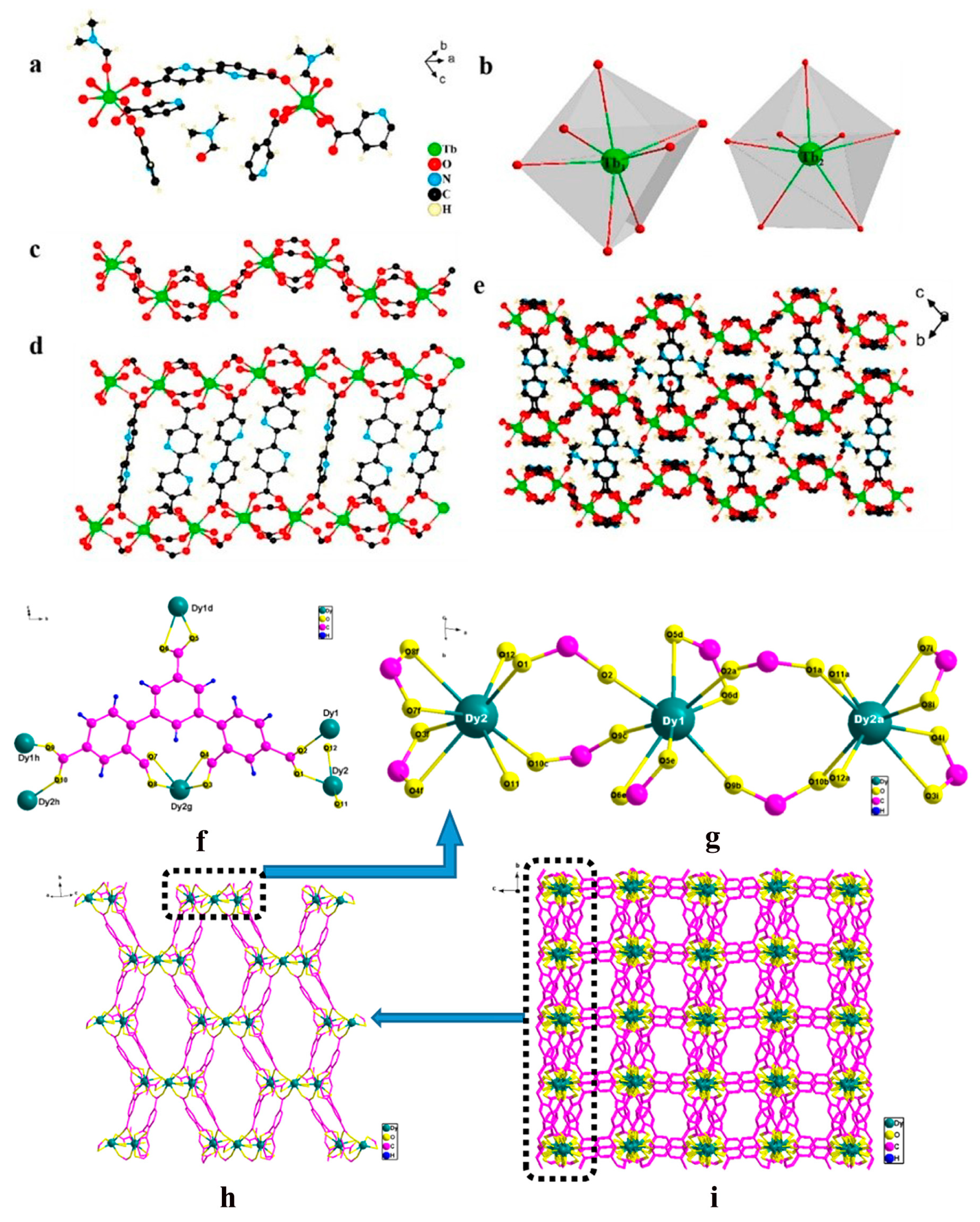
33] chose 2,2′-bipyridine-5,5′-dicarboxylic acid (H
2BPDC) with strong UV absorption capacity and good coordination capacity as an organic ligand, which can efficiently stimulate the green fluorescence emission of Tb
3+ upon binding with Tb
3+. The resulting Tb-MOFs frameworks were bridged with organic ligands to form one-dimensional chains, two-dimensional layers, and three-dimensional network structures through seven-coordinated Tb
3+ ions, which enhanced the fluorescence properties and water stability of the materials (
Figure 2). Ren et al. [
34] employed 3,5-bis(2′,4′-dicarboxyphenyl) benzoic acid (H5L) with pentacarboxylic acid structure as an organic ligand due to its abundant coordination capacity, which can form a stable three-dimensional framework with multiple rare earth metal centers. The resulting La-MOFs and Ce-MOFs were constructed to form a triangular channel structure, and the Dy-MOFs were constructed to form a three-dimensional network with rectangular channels, which improved the pore distribution and molecular recognition ability of the materials (
Figure 2). Wen et al. [
35] employed 2′,5′-dihydroxy-1,1′:4′,1′′-triphenylene-3,3′′,5,5′′- with abundant carboxyl and phenolic hydroxyl groups tetracarboxylic acid (H4DTTP-2OH) as an organic ligand, and used its multifunctional active site to optimize the proton transport path through hydrogen bonding and pore design in a three-dimensional network at the same time, and at the same time, the high luminescence efficiency of europium ions was used to achieve multifunctional performance.
2.1.2. Rigid Ligands
Absorption of UV light by organic ligands is a prerequisite for the luminescence of rare earth ion complexes; therefore, ligands with high molar absorption coefficients are the key to improving the fluorescence intensity of rare earth ions [
36,
37]. The presence of rigid structures and electron-rich conjugated systems in organic ligands contributes to the enhancement of UV absorption. The larger the conjugated system of the ligand, the stronger the absorption of UV light and the easier the π-π jumps can occur [
25]. In addition, when the degree of conjugation of rare earth ionic complexes is larger and more rigid, their structures are more stable, the mobility of electrons is better, the energy loss in the luminescence process is less, and the luminescence efficiency is higher [
38]. Wu et al. [
39] used bis-phenyl-2,2′-dicarboxylic acid (BPDA), which has stronger π–π interaction and energy transfer capacity, as a ligand to construct a three-dimensional Eu-MOF network with Eu
3+ to promote enhanced luminescence performance of Eu
3+. Chen et al. [
40] employed rigid 2,5-benzothiophene dicarboxylic acid (H
2btpdc) to bind with Eu
3+ to form a stable three-dimensional structure that provides high thermal and chemical stability while enhancing the fluorescence emission efficiency of Eu
3+.
With the deepening of research, People’s demand for organic ligands is not only limited to the enhancement of fluorescence intensity of rare earth metals but also desires to have concomitant properties such as luminescence properties and functional sites. Xing et al. [
41] exploited the strong conjugation utility and unique optical properties of 1,3,6,8-tetrakis(p-carboxybenzoic acid)pyrene (H4TBAPy) ligand by coordinating with lanthanide metal ions, such as Sm
3+, La
3+, and Nd
3+ lanthanide metal ions, to form RE-MOFs with porous three-dimensional structure, which facilitates the entry of the molecules to be measured into the channel for binding to the sensing site. This ligand not only facilitates photosensitization but also provides more active sites through the coordination of its carboxyl group with the metal (
Figure 3). Zheng et al. [
42] chose 4,4′-(quinoline-5,8-diyl)benzoic acid (QBA) with strong light-absorbing capacity as an organic ligand to coordinate with Yb
3+. The “antenna effect” was used to significantly enhance the near-infrared fluorescence emission of Yb
3+, and the abundant nitrogen-oxygen functional sites were used to promote strong interactions between the target molecules and the frameworks. Yang et al. [
43] chose 2-amino-terephthalic acid (H
2ATPA) as a ligand for coordinating with Sm
3+ to form a three-dimensional network structure with high rigidity and open coordination sites. Among them, the free amino group was not involved in the coordination of Sm
3+, which provided a reaction site for the detection of Hg
2+ and enhanced the fluorescence emission of Sm
3+ to achieve the “open” fluorescence response. Zhang et al. [
44] used 4,4′-(4,4′-bipyridyl-2,6-diyl) dibenzoic acid (bpydbH
2) as a ligand to form a three-dimensional network structure with europium ions, resulting in a highly rigid framework with open coordination sites. This structure provides open Lewis basic sites for enhanced selective binding to the metal ions being detected.
2.2. Design of RE-MOFs Framework Structure
The framework structure of RE-MOFs has a significant effect on their luminescence performance, which is determined by the synergistic effects of rare earth metal nodes, the nature of the ligands, and the porous structure. For the characteristics of hybrid rare earth metal–organic frameworks, host-guest rare earth metal–organic frameworks, and post-modified rare earth metal–organic frameworks, the effects of their design on luminescence performance are summarized as follows:
2.2.1. Framework for Hybrid RE-MOFs
Compared with single rare earth metal–organic frameworks, hybrid rare earth metal–organic frameworks have the following advantages: (1) in the system of multiple luminescent centers, a self-calibration mechanism can be provided by comparing the intensity of luminescence of different rare earth ions, which reduces the systematic error without the need to introduce other references; (2) through the modulation of energy transfer between different luminescent centers, luminescence enhancement, and extinction can be produced, respectively, and the luminescence color conversion of sensing materials can be achieved. These advantages are not only conducive to enhancing the stability of the performance of the sensing material but also improve the sensitivity and selectivity of the identification of specific chemical species.
Ma et al. [
45] achieved self-calibrated ratiometric fluorescence sensing through an energy transfer mechanism using bimetallic Tb
3+ /Eu
3+ as the luminescence center. The bimetallic (Tb/Eu) system is capable of simultaneously realizing the green emission of Tb
3+ and the red emission of Eu
3+ and enhances the sensing sensitivity by regulating the energy transfer between the two. Xu et al. [
46], on the other hand, modulated the triplet state energy levels of three different ligands, namely, 2,6-naphthalenedicarboxylic acid (H
2NDC), 4,4′-biphenyldicarboxylic acid (H
2BPDC), and 1,4-benzenyldicarboxylic acid (H
2BDC), via the triplet energy level (T
1) of Ln
3+ center. modulate the luminescence properties of the Ln
3+ center to achieve control of the energy transfer between Eu
3+ and Tb
3+ and enhance the self-calibration sensitivity based on ratiometric fluorescence (
Figure 4). Unlike the bimetallic independent luminescent center, Huo et al. [
47] designed a fluorescent sensor array based on a bis-lanthanide metal–organic framework (Tb@La-MOF), which exhibits four characteristic emission peaks (491 nm, 546 nm, 586 nm, and 621 nm) under the excitation light of 327 nm through the combined effect of Tb
3+ and La
3+, which are used as the four array points of the sensor array. Based on the multidimensional signal output of the array points, the quantitative detection of acetaldehyde concentration and the classification of different liquor samples were achieved by combining with pattern recognition methods such as principal component analysis and linear discriminant analysis (
Figure 4).
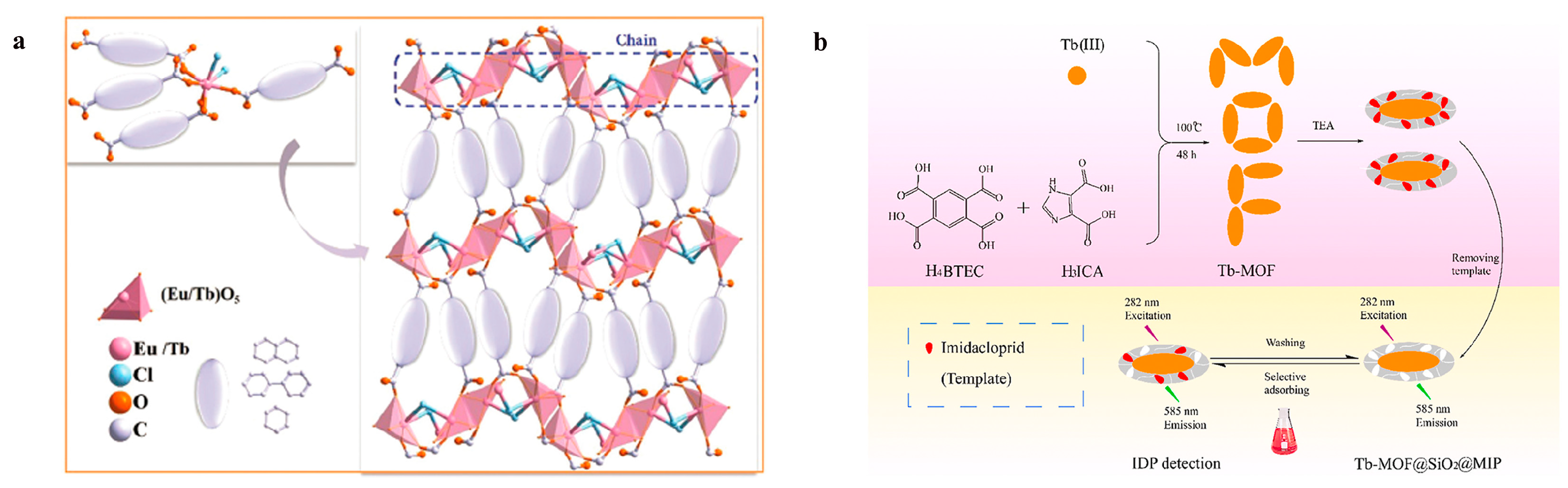
Xu et al. [
48] proposed a new luminescent rare earth metal–organic framework counting strategy, which employs a bi-ligand structure of benzene tetracarboxylic acid and imidazolium dicarboxylic acid to construct the Tb-MOF backbone, enhance its stability in an aqueous environment, and provide a multisite coordination structure to optimize fluorescence performance. SiO
2 layers and molecularly imprinted polymers were introduced on the Tb-MOF surface to improve the selective recognition of the insecticide imidacloprid (IDP).
2.2.2. Framework for Subject-Object RE-MOFs
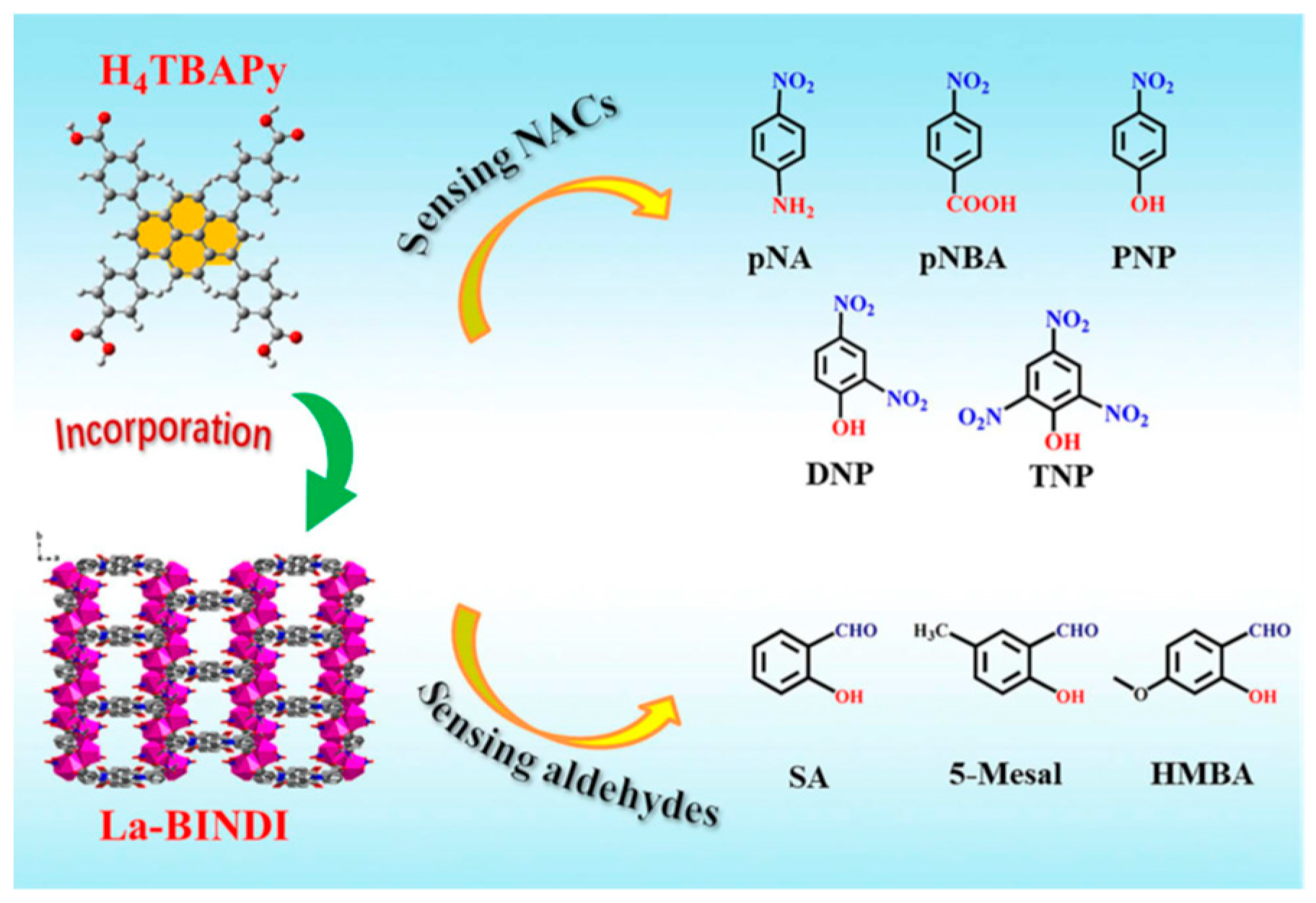
Although the above approaches are very attractive for the development of ratiometric luminescence, the available emission is limited to the backbone of rare earth metal–organic frameworks, i.e., rare earth metal ions and ligands. Encapsulation of organic fluorescent dyes into the pores/channels of rare earth metal–organic frameworks to form MOF@dye composites not only significantly enhances the fluorescence properties but also endows the materials with the ability to respond to specific molecules or environmental conditions, making them important for applications in sensing, monitoring, and optics. Xing et al. [
49] constructed naphthalene dimide (H4BINDI)-based Ln-MOF as an electron acceptor and formed an acceptor-donor (D-A) type composite by surface modification of an electron-rich pyrene derivative (H4TBAPy). This design allows the transfer of electrons from H4TBAPy to H4BINDI, where they interact with the target molecule via hydrogen bonding or π-π stacking to enhance the fluorescence response (
Figure 5). Sun et al. [
50] employed 1,4-naphthalenedicarboxylic acid (NDC) as a ligand, which was bonded to the lanthanide metal Eu
3+, and utilized the characteristic emission peaks of Eu
3+ (617 nm) and the ligand emission peaks (485 nm) to form a dual-emission fluorescence signal, which was detected by the ratio of fluorescence intensity for self-calibration.
2.2.3. Post-Modified RE-MOFs
Post-synthesis modification of RE-MOFs is an effective strategy for the preparation of rare earth organic backbone materials and derivation of high-performance functional materials, which usually have better properties than the original parent frameworks [
51]. This strategy involves changing the backbone composition of MOFs by ligand functional group modification, surface chemical modification, or metal center modification based on the reported MOFs to obtain luminescent properties that cannot be obtained by direct synthesis [
52]. As a result of the change in chemical composition, new chemical and physical properties are installed in MOFs, providing new opportunities to develop multiple application areas [
53].
Liu et al. [
54] took advantage of the good stability and macroporous properties of bismuth metal–organic frameworks (Bi-MOFs) to enhance their fluorescence properties by doping Eu
3+. That is, in water, the Eu
3+ fluorescence of Eu/Bi-MOF is quenched due to the coupling of the O-H vibration of water molecules to the excited state of Eu
3+, which weakens the energy transfer from the ligand to Eu
3+. After the addition of histidine, histidine interacts with the ligand through π–π stacking and through the π-bond resonance between the lone pair of electrons of the imidazole ring and the ligand carboxyl group, which promotes the energy transfer efficiency from the ligand to Eu
3+, thus restoring the fluorescence of Eu
3+ and forming a “fluorescence-on” proportional sensing system. Yan et al. [
55] used Zn-based MOF-SO
3− with uncoordinated sulfonic acid groups as the carrier framework and introduced Ln(III) to form a luminescent hybrid material. Guo et al. [
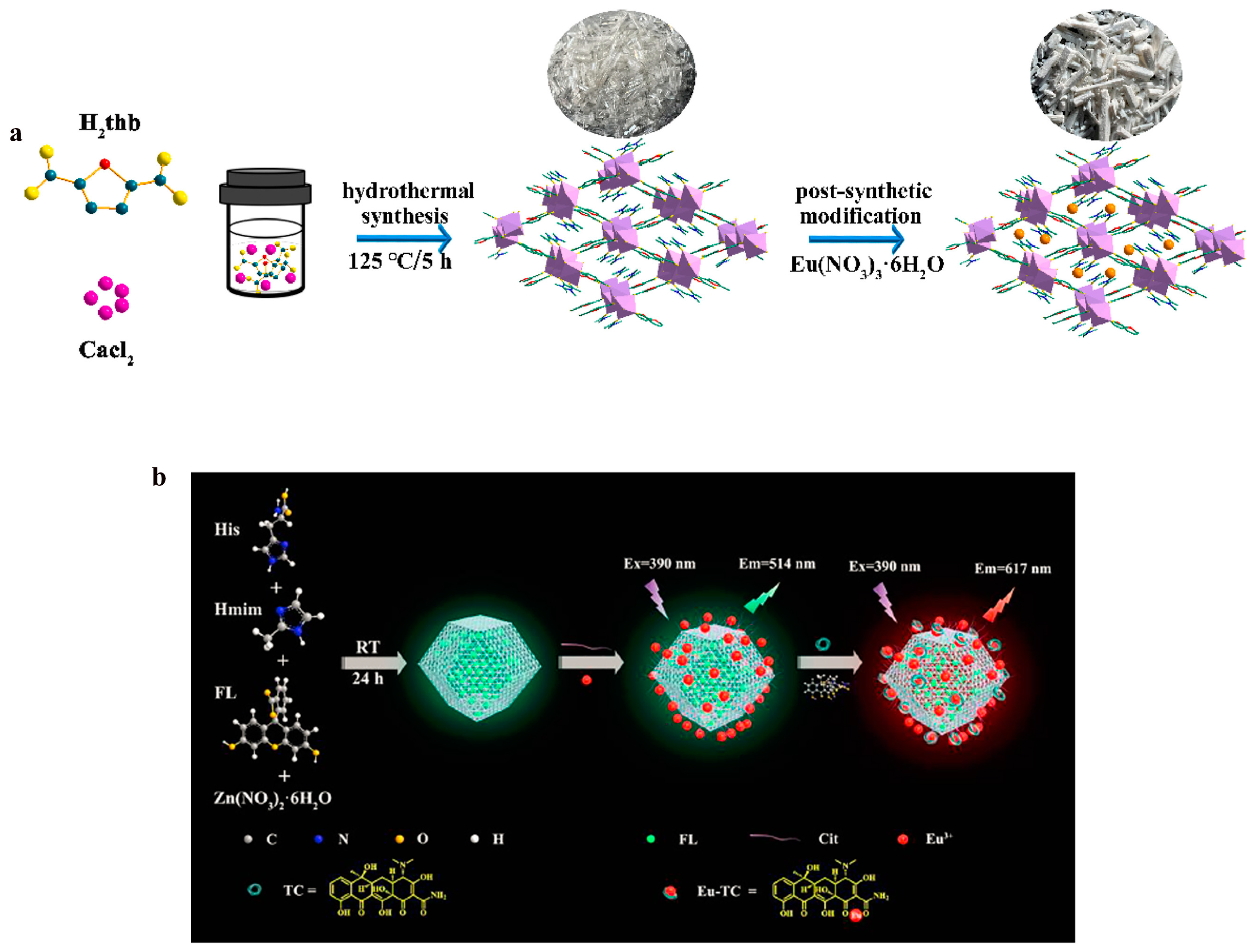
56] used Ca-MOF as the parent framework and utilized the carboxyl groups in its pores to provide the active sites to introduce Eu
3+ into the framework via a post-synthesis method to achieve the functionalization of Eu-Ca-MOFs (
Figure 6).
The introduction of rare earth metals after the modification of functional groups of MOFs is also an important means of post-modification of RE-MOFs. Saikia et al. [
57] introduced Eu
3+ after the modification of UiO-66 amine functionalization, which, on the one hand, enhances the luminescence efficiency of the materials by ligand–metal charge transfer, and, on the other hand, provides an active site for the specific binding of the hazardous substance. Zhu et al. [
58] chose zeolite imidazolium skeleton material (ZIF-8) with a large specific surface area, high porosity, and water stability as the base material, and introduced L-histidine to enhance the ligand ability on the surface of the material and enhance the binding stability to lanthanide metal ions while embedding fluorescein in the framework as a reference signal to provide stable green fluorescence for ratiometric fluorescence detection. The citrateeuropium complex was utilized as a response signal to generate red fluorescence by energy transfer in the presence of tetracycline TC, achieving precise detection of fluorescent ratiometric signals (
Figure 6).
Figure 6.
(
a) Schematic illustration of the synthesis of Eu-Ca-MOF [
56]. Copyright 2022, Elsevier. (
b) Fabrication process of FL:LZIF-8-Cit-Eu nanosensor and its fluorescence sensing diagram for TC [
58]. Copyright 2022, Elsevier.
Figure 6.
(
a) Schematic illustration of the synthesis of Eu-Ca-MOF [
56]. Copyright 2022, Elsevier. (
b) Fabrication process of FL:LZIF-8-Cit-Eu nanosensor and its fluorescence sensing diagram for TC [
58]. Copyright 2022, Elsevier.
3. Synthesis of RE-MOFs
The synthesis of RE-MOFs is often influenced by a variety of factors, and in general, even if the reaction mixture is the same, different synthetic methods may result in RE-MOFs materials with completely different morphologies, structures, particle sizes, and yields (
Table 1). Given the huge number of candidate nodes (inorganic metal ions or clusters) and linkers (organic ligands), it is possible to synthesize RE-MOFs in huge quantities. However, achieving rational and purposeful design and functional RE-MOFs with desired functions is still a great challenge and still requires continuous practice and exploration. Through the continuous efforts and practical experience of researchers, many synthetic methods with different characteristics have been obtained.
Initially, MOFs were synthesized by the diffusion method, in which the reactants were dissolved separately in a suitable solvent, and the solvents containing the reactants reacted by mutual diffusion at the interface or in the medium to form the products. Although this method can yield larger-sized crystals, it has largely been replaced by hydrothermal/solvothermal methods due to its low product yield and time-consuming nature, with yields typically ranging between 20% and 60%. The mixture of rare earth metal salts and ligands with suitable linking groups is placed in a suitable solvent (dimethyl formamide DMF, dimethylsulfoxide DMSO, H
2O, etc.) and stirred with a magnetic stirrer until the solids are partially or completely dissolved and then transferred to the inner liner of a reactor vessel to seal it, and heated in an oven at the target temperature for three to five days to allow the functional ligands, the inorganic metal salts, and the solvent to react at a certain temperature and pressure. The solvent is one of the most important parameters in the synthesis of RE-MOFs, and although no direct interaction with the framework is usually observed, the solvent is almost always incorporated into the structure of the synthesized RE-MOFs, thus acting as a space-filling molecule. This synthesis method is relatively simple and offers high yields, typically ranging between 60% and 90%, making it the most common approach for synthesizing RE-MOFs (
Figure 7) [
59,
60,
61,
62].
RE-MOFs can also be obtained by mechanical methods, acoustochemical methods, and microwave-assisted solvothermal synthesis methods, and their yield is relatively high, between 70% and 95%. Mechanochemical methods have the advantage of not using organic solvents compared to hydrothermal/solvothermal methods. However, there have been few studies on the luminescence of mechanochemically synthesized materials. The first person to prepare MOFs using mechanochemical synthesis was James [
63]. He and his research team prepared Cu(ina)
2 according to the acid–base reaction principle using ethyl acetate and maleic enzyme reactants. Both acetic acid and water, as by-products, were partially involved in the composition of Cu(ina)
2, i.e., embedded in the framework pores. Alammar et al. [
64] ball-milled organic ligands with lanthanide carbonate hydrates at a 2:1 molar ratio for 2 h. Mechanical force-induced chemical reactions took place during ball-milling, resulting in the formation of 19 lanthanide-based MIL-78 MOFs. The products exhibited luminescence properties of remarkable red, green, and yellow emission properties, providing a new material platform for solid-state lighting, sensing, and other applications. Ji et al. [
65] described a method for the preparation of two-dimensional La-based metal–organic framework nanosheets based on microwave- and ultrasonic-assisted synthesis techniques. The dissolved ligand solution was mixed with lanthanide ion solution, and the mixed solution was transferred to a four-neck flask and heated in a multifunctional microwave reaction device. The temperature was increased from room temperature to 90 °C at a rate of 9 °C/min. While heating, ultrasonic oscillation at 200 kHz was applied to further enhance the reaction rate and homogeneity. The total reaction time was 10 min, followed by cooling to room temperature. The resulting material can be used to enhance the adsorption properties of phosphates.
4. Mechanism of Contaminant Detection in RE-MOFs
Fluorescent probes of rare earth complexes are composed of fluorescent groups and recognition groups, and the chemical reaction between the recognition group and the analyte is used to change the fluorescence intensity, and the chemical signal of the target is converted into an optical signal to achieve the purpose of transmitting information. According to the different ways fluorescence intensity changes after the fluorescent probe interacts with the substance to be measured, it can be divided into quenching (Turn-off type) fluorescent probe, enhancement (Turn-on type) fluorescent probe, and ratiometric (Ratiometric type) fluorescent probe.
4.1. Turn-Off Fluorescent Probes
Turn-off fluorescent probes possess strong fluorescence on their own, which decreases or even disappears when interacting with the DUT. Xing et al. [
66] efficiently detected Th
4+, UO
22+, Cr
2O
72−, and aldehydes in the liquid phase using MOF and its post-modification products by the fluorescence quenching detection mechanism, and aldehydes in the gas phase. This method showed high sensitivity and selectivity as well as good stability. Wen et al. [
35] prepared Eu-MOF, whose excitation spectra overlapped with the UV absorption spectra of tetracycline antibiotics, which induced excitation photoinhibition of Eu(III), leading to a decrease in the fluorescence intensity. Pedrosa et al. [
67] quenched the fluorescence of MOF by using them as π electron acceptors based on the strong electron absorption capacity of nitroaromatic explosives, seizing electrons from excited ligands.
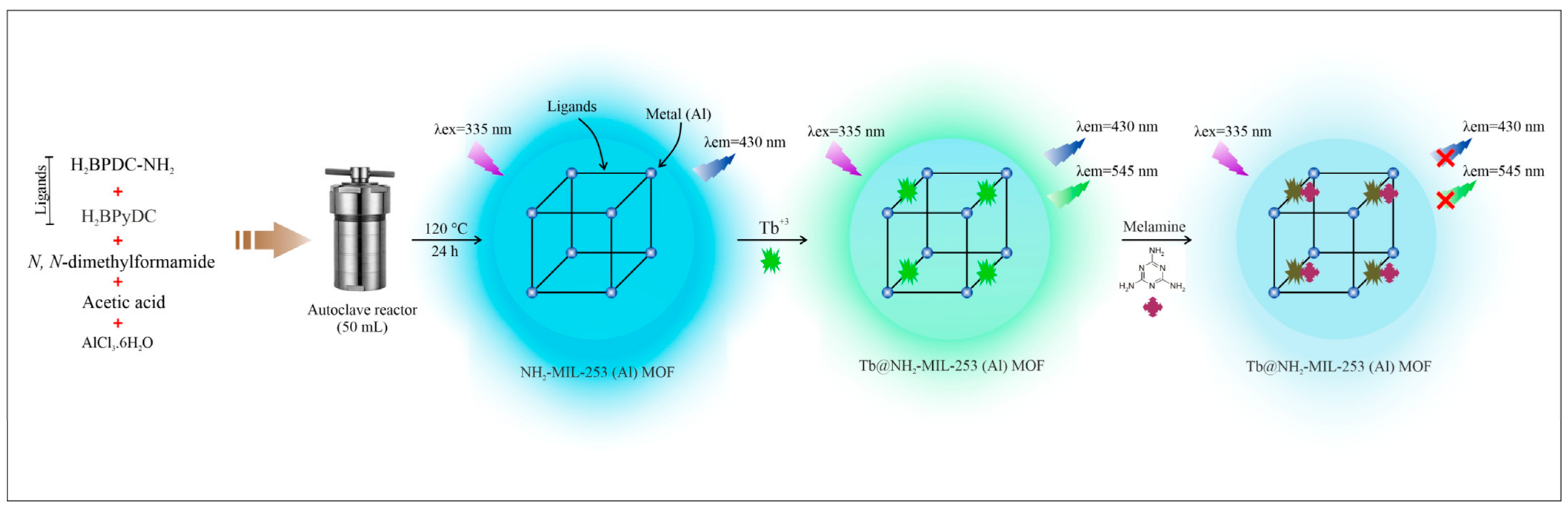
Studies have shown that the degree of quenching of different nitro compounds is directly related to their electron uptake capacity. The higher the redox potential, the more obvious the quenching effect. The lowest unoccupied molecular orbital (LUMO) energy level of the vomitoxin (VT) molecule detected by Qin et al. [
68] was lower than that of the HOMO energy level of the ligand, which resulted in the transfer of energy from the ligand to the VT molecule, weakening the photosensitization effect of the ligand on the Tb
3+ and leading to fluorescence quenching. The detection limit is better than most of the reported methods, demonstrating the potential for a wide range of applications in food safety and environmental monitoring. The melamine detected by Jahed-Khaniki et al. [
69] forms a hydrogen bond with the ligand in the sensor via a nitrogen heterocycle. Melamine acts as an electron donor and interferes with the energy transfer between the ligand and the Tb
3+ ion. The fluorescence intensity decreases at 430 nm and 545 nm, respectively (
Figure 8).
4.2. Turn-On Fluorescent Probes
The autofluorescence intensity of the enhanced (Turn-on type) fluorescent probe is weak, and the fluorescence intensity increases after interaction with the DUT so that the target can be monitored. Xia et al. [
70] used glutamic acid as a target pollutant for detection, in which the carboxyl group interacted with the phenolic hydroxyl group in the MOF ligand through hydrogen bonding to inhibit the light-induced electron transfer of the ligand, thus significantly enhancing the fluorescence intensity of the ligand (
Figure 9). Wu et al. [
71] replaced the water molecules around the metal center by the interaction of the DEA molecules with the surface of the MOFs, reducing the non-radiative energy loss due to O-H vibrations, thus enhancing the characteristic emission of Eu
3+, with a significant enhancement of the intensity of the red emission peak at 614 nm. Peng et al. [
72] prepared the ligands of RE-MOFs containing anthracene moieties with their π–π conjugated structure endowed the material with fluorescence properties. The qubits Al
3+ and Ga
3+ interacted with the framework and enhanced the specific absorption bands of the MOF, leading to fluorescence blue-shifting and enhancement.
4.3. Ratiometric Fluorescent Probes
Ratiometric fluorescent probe is a detection technique based on the different intensities of the target on different luminescent substances in MOFs to achieve the change of fluorescence intensity ratios at different wavelengths and finally determine the content of the target. This kind of probe is independent of the intensity of the light source and the sensitivity of the instrument, so it has a higher sensitivity, selectivity, and detection range. Sun et al. [
50] based on ligand luminescence of RE-MOFs and Eu
3+ luminescence to constitute ratiometric fluorescent probes. At elevated temperatures, the fluorescence intensity of the ligand was almost unchanged, and that of Eu
3+ was significantly reduced, leading to a temperature-dependent change in the fluorescence intensity ratio (ligand/Eu). Ma et al. [
45] replaced the water molecule with the coordination of Tb
3+ by 2,6-dipyridylcarboxylic acid (DPA) and formed a complex with it, which significantly increased the green emission of Tb
3+ and interrupted the energy transfer from Tb
3+ to Eu
3+, resulting in a decrease in the red emission of Eu
3+ and an enhancement of the fluorescence intensity ratio. Guo et al. [
56] bound Hg
2+ to the sulfur atom in the ligand of RE-MOFs, and the electron density of the ligand changed, leading to an enhancement of the fluorescence at 381 nm. On the other hand, Hg
2+ inhibited the characteristic jump of Eu
3+ and weakened the fluorescence intensity. The fluorescence intensity ratio of the probe was eventually enhanced. Zhu et al. [
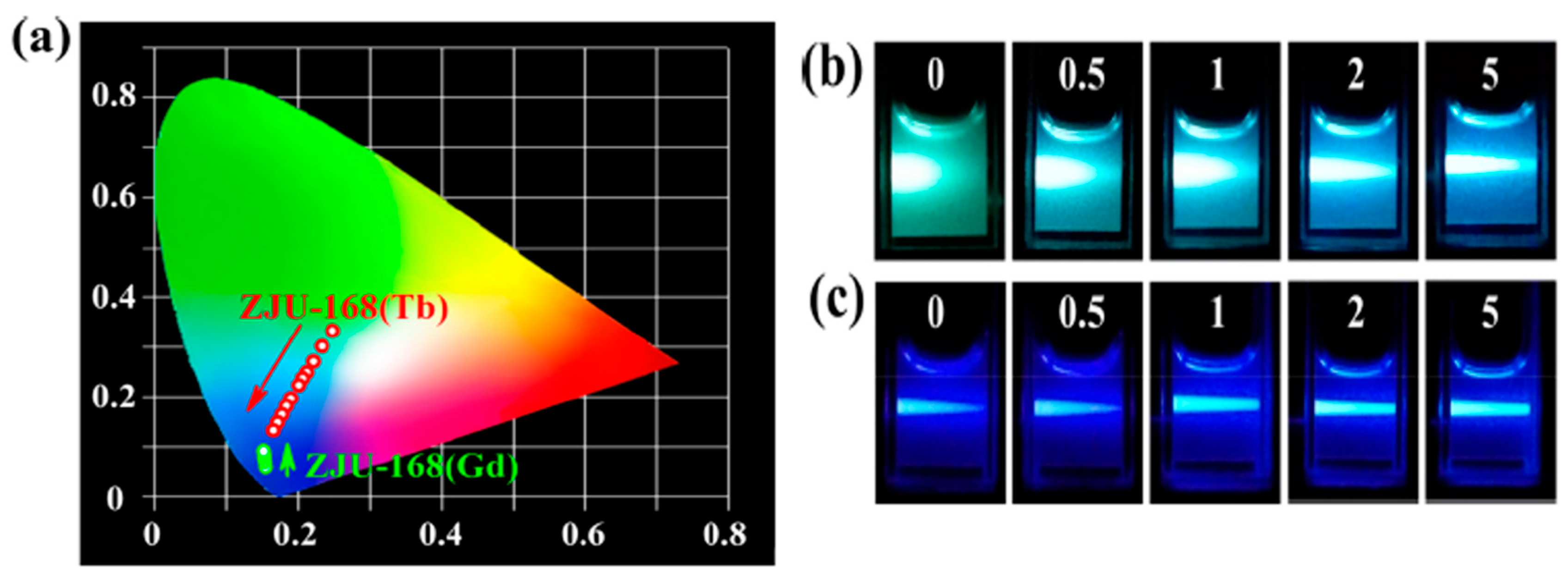
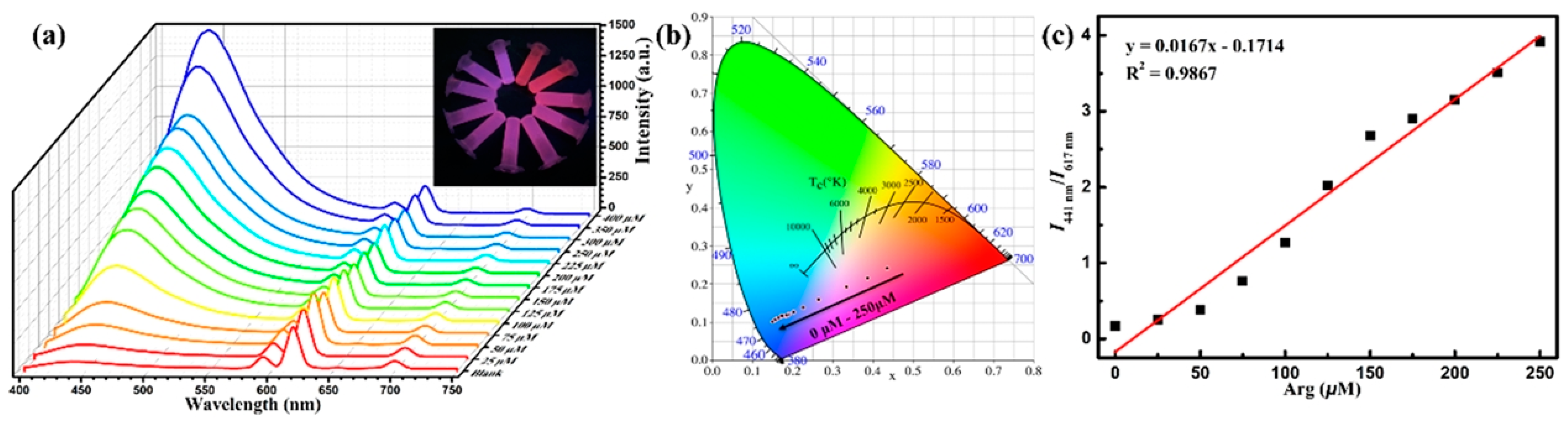
73] utilized the strong hydrogen bonding interactions between the carboxyl group (COOH) in p-mercapto diethanolic acid and the amide group (CONH) in the MOF ligand to promote changes in the ratio of the two luminescent signals of the MOFs and to achieve double-ratio fluorescence detection. In addition, it was found that p-mercapto diethanolic acid triggered the appearance of a new ligand emission peak (452 nm), which made the sensing system more sensitive. Wen et al. [
74] utilized the emission peak of the ligand (441 nm) and the characteristic emission peak of Eu
3+ (617 nm) to form a dual fluorescence signal. The cations in lysine and arginine bind to the methoxy group of the ligand of RE-MOFs through electrostatic interaction and hydrogen bonding, which inhibits the light-induced electron transfer process and enhances the fluorescence intensity of the ligand, whereas the characteristic emission signals of Eu
3+ remain stable to achieve ratiometric fluorescence detection (
Figure 10).
In summary, when RE-MOFs are employed as fluorescent sensors, their core principle relies on the interaction between pollutants and the RE-MOFs, which alters their fluorescence signals (e.g., quenching, enhancement, or wavelength shifts). However, the photobleaching effect significantly compromises their performance, leading to issues such as signal stability degradation, limited service life, and background noise interference. Through material design, composite system construction, and excitation condition optimization, the photostability of RE-MOFs can be markedly enhanced. These advancements will accelerate their practical application in real-time monitoring of environmental pollutants, enabling robust and long-term sensing platforms for industrial and ecological systems.
5. Application of RE-MOFs for Fluorescent Sensing of Pollutants
Fluorescence sensing refers to the change of luminescence properties (intensity, peak position, lifetime, half-height width, etc.) of a fluorescent probe when the object to be measured interacts with it, thus achieving qualitative and quantitative identification of the object to be measured [
75]. Fluorescence sensing technology has received extensive attention from researchers due to its advantages of fast response speed, high sensitivity, good selectivity, high spatial resolution, non-contact, non-invasive, and simple equipment operation, and has demonstrated broad application prospects in the fields of biology, medicine, etc. [
76,
77,
78]. Among many fluorescence sensing materials, MOFs have abundant active sites and diverse structures, which makes them widely used in fluorescence sensing [
79,
80].
5.1. Fluorescence Sensing of Gaseous Pollutants
The importance of gas sensing is rapidly being recognized in a number of areas including environmental protection, safety measures, food safety protocols, and medical diagnostic processes. As the demand for these applications continues to escalate, so does the need for gas sensors that can provide better sensing performance, especially for the detection of sulfur-containing toxic gases, nitrogen compounds, and volatile organic compounds.
5.1.1. Fluorescence Sensing of Sulfur-Containing Toxic Gases
Currently, the detection of sulfur-containing toxic gases such as H
2S and SO
2 can be achieved by using lanthanide metal–organic frameworks (Ln-MOFs), copper-based metal–organic skeletons (Cu-MOFs), and MOF composites. Zhu et al. [
81] developed a novel sensing platform by growing MOF-5-NH
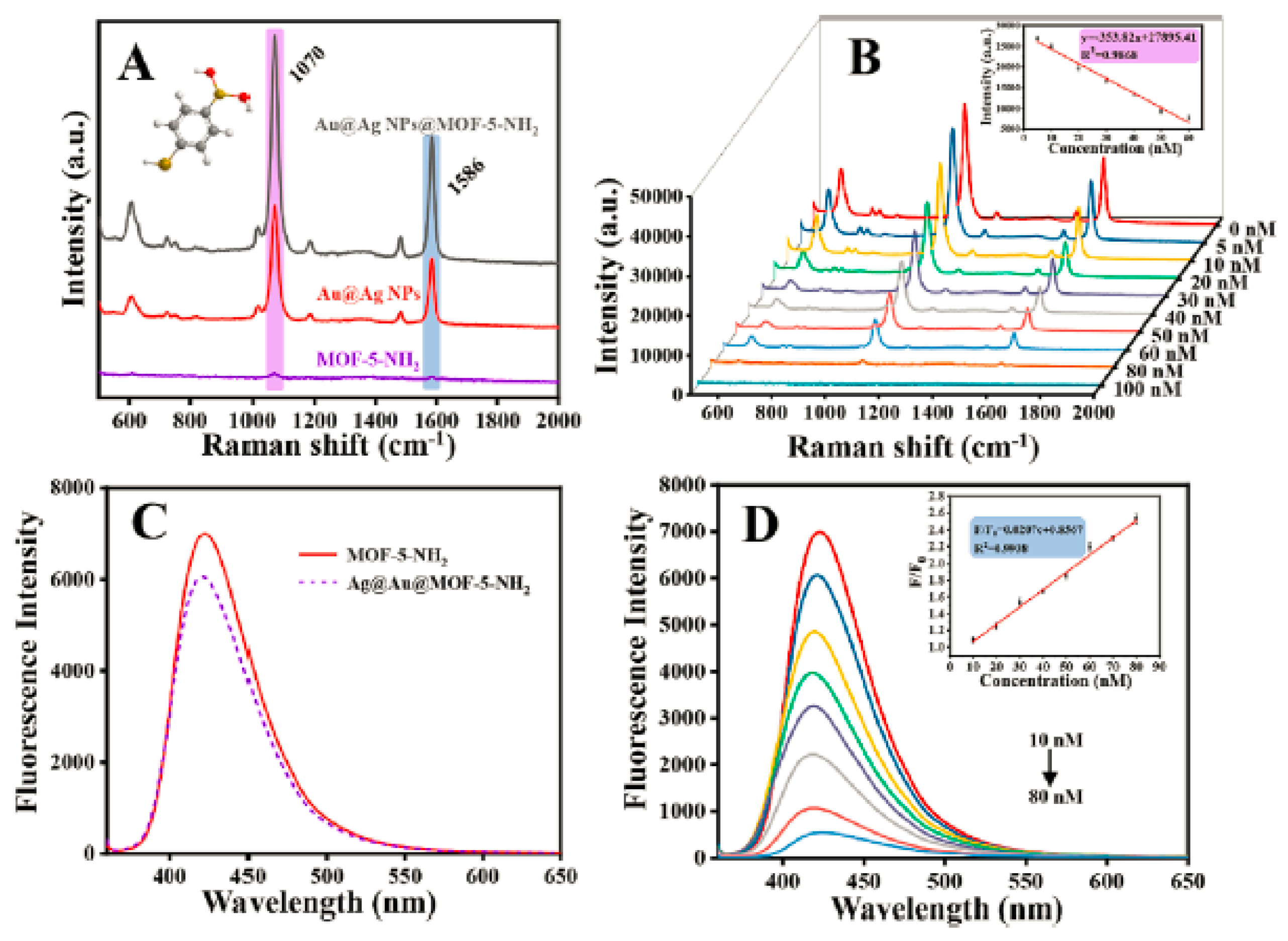
2 on the surface. This Au@Ag NPs@MOF-5-NH2 hybrid system is capable of detecting two sulfur-containing gases, H
2S and SO
2, simultaneously, achieving an ultra-low detection limit of 2.26 nM by utilizing a surface-enhanced Raman scattering and fluorescence “turn-on” mechanism (
Figure 11). Wang et al. [
82] described a reaction-triggered Ce
4+/Tb
3+ MOF fluorescent probe that utilizes Ce
4+ as the reactive ion and Tb
3+ as the fluorescent ion. The energy generated by the reduction reaction between Ce
4+ and SO
2 or sulphite is used to excite the Tb
3+ ion, leading to its fluorescence emission. SO
2 sensors based on this principle offer excellent selectivity and sensitivity. The detection limits of the technique were 50 nM for sulphite and 0.093 mM for SO
2, demonstrating the effectiveness of the method in detecting very low concentrations of SO
2.
5.1.2. Fluorescence Sensing of Nitrides
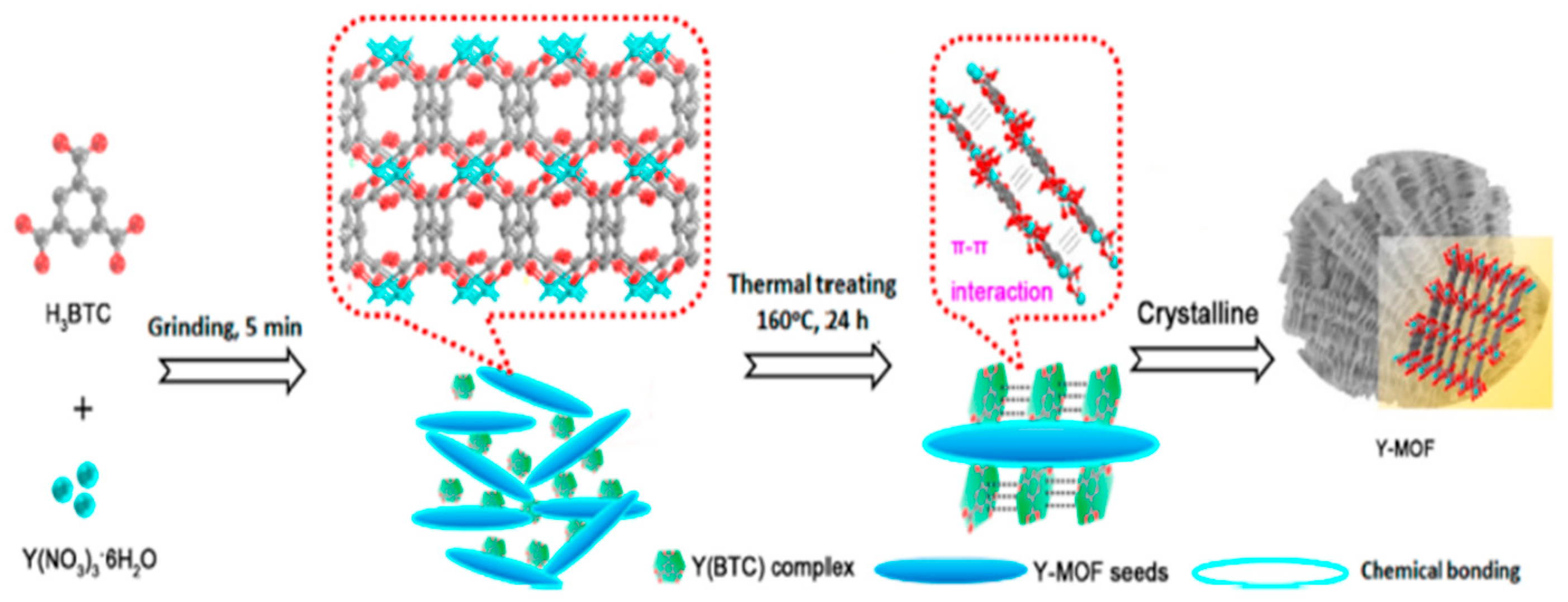
Nitrogen compounds can react with atmospheric hydroxides to produce acid rain, which causes damage to soil, water bodies, and plants, with significant impacts on the environment and human health. Moscoso et al. [
83] reported a typical work using a mechanism to achieve effective NO
2 vapor detection. They synthesized a terbium-based MOF (Tb(BTC)) using benzene-1,3,5-tricarboxylate (BTC) as an organic ligand. NO
2 acts as an electron acceptor that can open new energy transfer channels from the BTC ligand to the analyte molecule, resulting in quenching of the intrinsic fluorescence of Tb(BTC). The sensing sensitivity was further improved by doping the MOF particles into the polymer matrix, which facilitated the full access of the analyte into the cage of the sensing material. This approach ultimately achieved a sub-ppb detection range for nitroaromatic vapors. Goel et al. [
84] utilized a suite of transition metal- and terbium-based MOFs as inks to integrate inkjet printing onto flexible substrates, such as paper-based polyethylene terephthalate, in order to fabricate patterned films. Notably, two MOF films have demonstrated the ability to perform fluorescence and colorimetric sensing of ammonia, with the sensor achieving a detection limit of 0.3 ppm for ammonia (
Figure 12).
5.1.3. Fluorescence Sensing of Volatile Organic Compounds
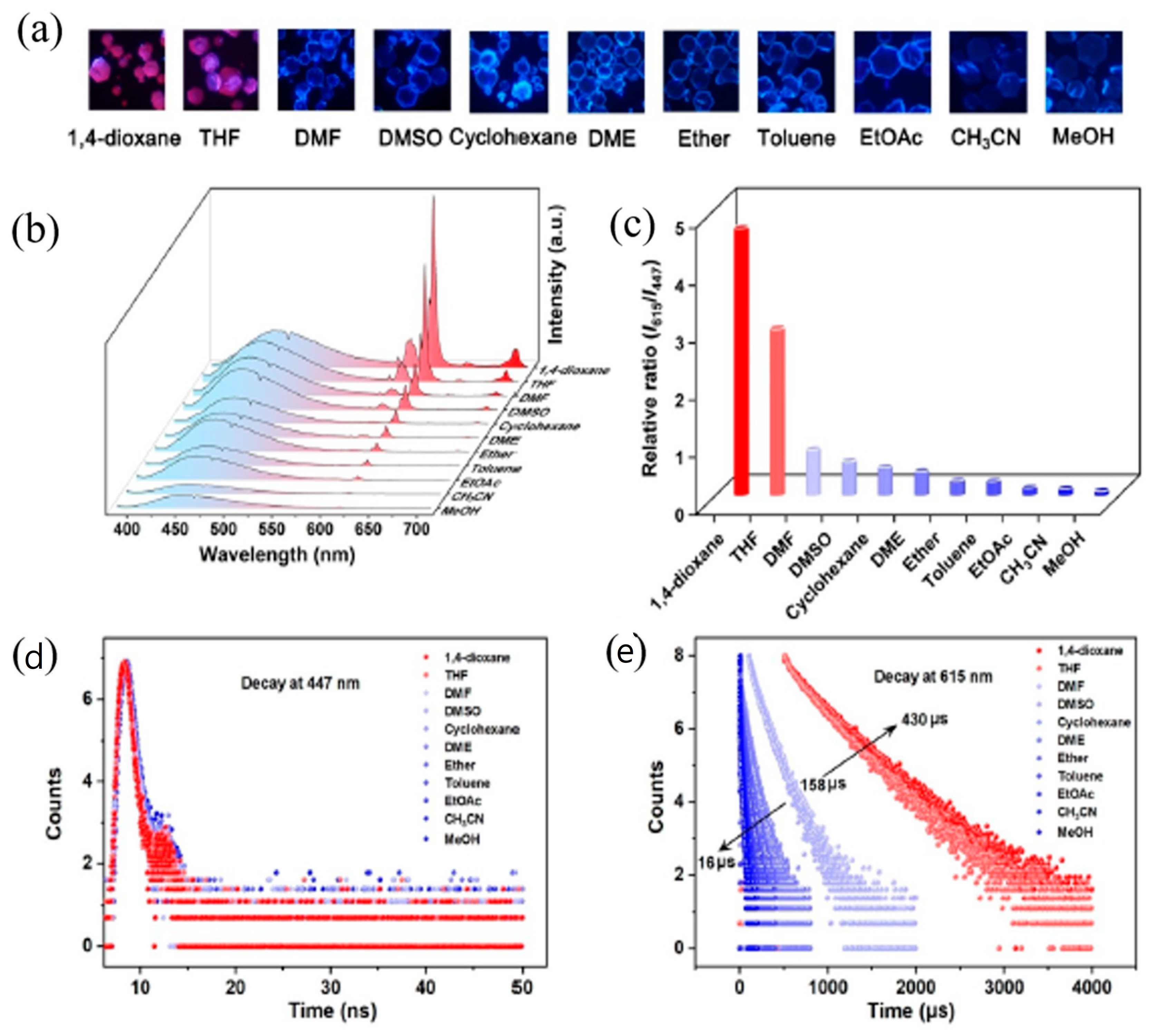
Volatile organic compounds (VOCs) come from a variety of sources, including naturally occurring and anthropogenic activities. Most of these compounds have negative impacts on the environment. Xu et al. [
85] synthesized an Eu-MOF ratiometric fluorescent sensor that can be used for fluorescence detection of a wide range of VOC molecules. The structure of the Eu-MOF was cleverly designed by choosing a suitable organic ligand to endow the Eu-MOF with multiple unsaturated metal sites and multi-restricted space, and the Eu-MOF consists of Eu9 clusters with unsaturated ligand sites, which gives the Eu-MOF a “binding pocket” consisting of potential metal open sites, which is favorable for the pre-enrichment of VOC molecules. Moreover, the luminescence property of the Eu-MOF is very bright, with both Eu
3+ and organic ligand luminescent centers, which can be used as a ratiometric fluorescent sensor. When the VOC molecules enter the “binding pocket” of the Eu-MOF, the VOC molecules not only displace the hydroxyl groups in the first coordination layer of Eu
3+ but also “bind” the adjacent Eu9 clusters in the second coordination layer through hydrogen bonding. In this process, the VOC molecules attenuate the molecular thermal vibrations in the first and second coordination layers of Eu
3+, which reduces the energy loss of the non-radiative jump of Eu-MOF, leading to the enhancement of Eu-MOF fluorescence. In addition, the fluorescence enhancement of Eu-MOF after binding to different VOC molecules varies due to the different strengths of the binding pockets of different VOC molecules, which makes it possible to selectively identify VOC molecules by the value of the I615/I447 ratio. Interestingly, this Eu-MOF enables the visual detection of tetrahydrofuran vapor, with the fluorescence color changing from blue to red as the concentration increases, and has the advantages of fast response, strong fatigue resistance, easy recovery, and quantitative detection (
Figure 13).
In addition to environmental concerns, volatile organic compounds (VOCs) are also important biomarkers for human and plant health. For example, analysis of VOCs present in human exhaled gas can help in the early detection of various diseases including cancer. Zhang et al. [
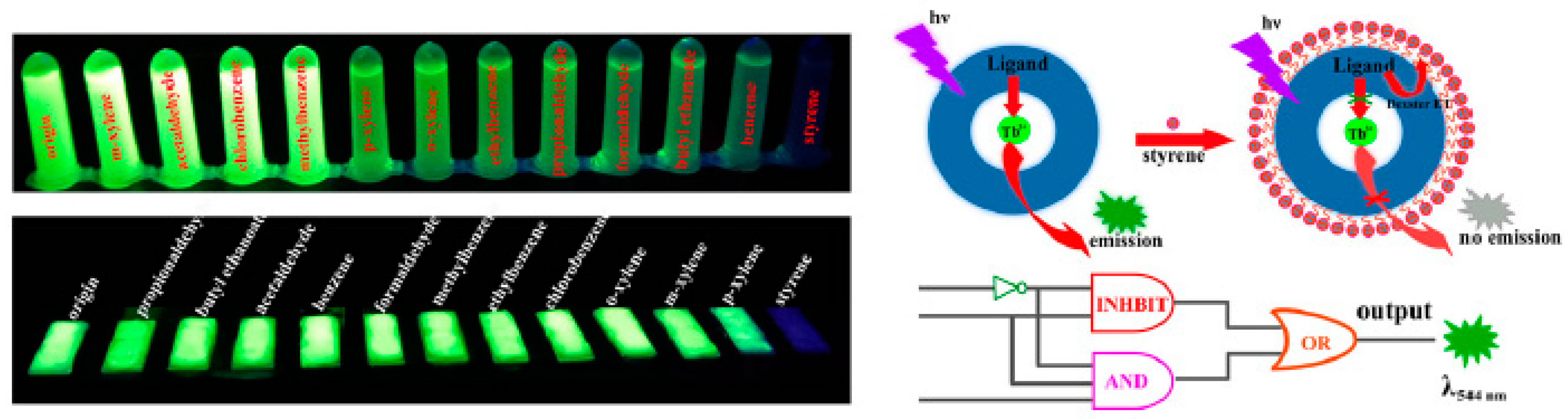
86] used the bimetallic material Tb-UiO-66 for the detection of respiratory volatile biomarkers associated with lung cancer, including Styrene (Styrene) and Ethylbenzene (EB). The limit of detection (LOD) for p-Styrene was 18.3 ppb and for Ethylbenzene (EB) was 0.45 ppm. In addition, the MOF material retained its crystal structure intact at 472 °C and was stable for more than 7 days in extremely acidic (pH = 2) and alkaline (pH = 12) environments. Li et al. [
87] proposed and synthesized a Tb-MOF material that utilizes a trimellitic acid (TMA) ligand as the detection agent for styrene and ethylbenzene (EB). The (TMA) ligand is a sensitive site for the detection of styrene vapor. Taking advantage of the fact that resonance energy transfer (FRET) and Dexter electron transfer occur only between styrene and TMA ligand, the Tb-MOF was endowed with the ability to detect styrene with high selectivity. In addition to selectivity, the Tb-MOF exhibits excellent response characteristics. The detection limit for styrene was as low as 0.0017% (
Figure 14). In order to improve the sensitivity, Che et al. [
88] obtained a thin film-based fluorescent sensor by crosslinking the prepared Eu@UiO-66 (COOH), which exhibited an ultrasensitive detection of aniline vapor with a limit of detection of 0.086 ppb. Zhang et al. [
89] employed a complex multistep tandem functionalization strategy to modify europium-doped (Eu
3+) ZnMOF-5-NH
2. This modification involved the introduction of methacrylic anhydride to introduce unsaturated bonds in the molecular structure. Subsequently, the modified molecule was copolymerized with butyl methacrylate, resulting in the development of europium-based luminescent MOF-derived hybrid polymer films. It showed an excellent ability to identify ethylenediamine vapor from volatile organic compounds.
5.2. Fluorescence Sensing of Ionic Contaminants
The detection of ions has always been an important field of chemical detection, especially the detection of heavy metal ions (Hg2+, Pb2+, etc.) and toxic and harmful anions (Cr2O72−, F−, CN−, etc.). In Ln-MOFs ion fluorescence sensors, Ln3+ as the luminescence center is susceptible to fluorescence quenching by some metal ions (Fe3+, etc.) in the open-shell layer, whereas some hydrogen-bonding sites and reactive sites in the pores and surfaces of the Ln-MOFs enhance the ionic perturbation of the luminescence behavior of Ln3+. Therefore, the Ln-MOFs ion fluorescence sensor has the advantages of low detection limit and high sensitivity.
5.2.1. Fluorescence Sensing of Metal Ions
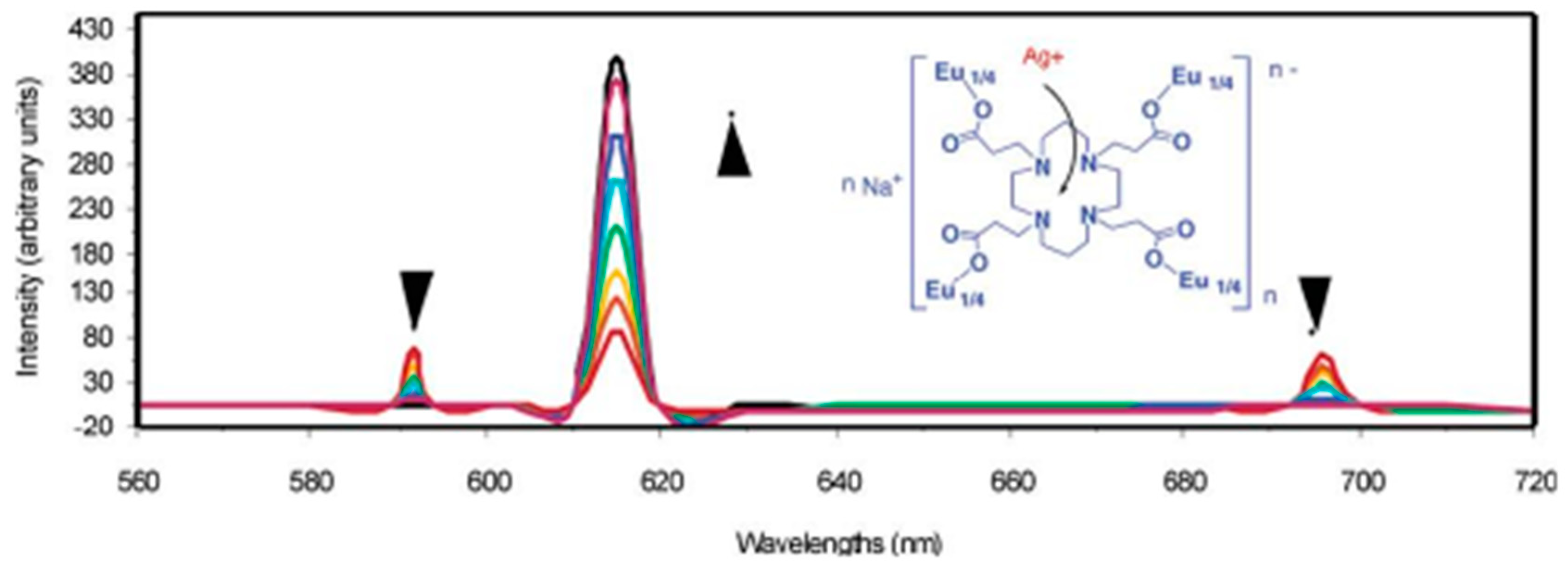
Since the birth of the first Ln-MOFs fluorescence sensor, more and more structurally novel Ln-MOFs have been used for the fluorescence detection of metal ions. Liu et al. [
90] reported the first Ln-MOFs fluorescence sensor for the detection of metal ions, and the synthesized NaEuL
5(H
2O)(4)-2H
2O can be used for the fluorescence detection of Ag
+, Cu
2+, Zn
2+, Cd
2+, and Hg
2+, in which the coordination of Ag
+ with the coordination site of the nitrogen ring vacancies leads to the enhancement of europium ions’ characteristic emission by a factor of 4.9, and the coordination of Cu
2+, Zn
2+, Cd
2+, and Hg
2+ with the coordination site of the nitrogen ring vacancies leads to the weakening of the characteristic emission of europium ions, which mainly stems from the fact that the coordination of Ag
+ alters this Ln-MOF rigidity with the paramagnetic spin state of Eu
3+ (
Figure 15). Chen Di-Ming and his colleagues [
91] designed a Tb(III)-based luminescent MOF using 4,4′-oxybis(benzoic acid) (H2oba) as ligand and 3-amino-1,2,4-benzotriazole (Hatz) as template reagent, and single-crystal shows that the structure has two types of channels: a large one-dimensional hexagonal mesoporous channel with a diameter of 2.6 nm and six surrounding one-dimensional triangular channels with a diameter of 0.48 nm. Tb-MOF can selectively detect Fe
3+ and Al
3+ in aqueous solution and can be recycled. The detection limits and Ksv values for Fe
3+ are 1.0 × 10
−6 M and 3.4 × 10
−4 M
−1, respectively. In Ref. [
92], the MOF-808-based metal–organic framework material was used for the detection of aluminum ions (Al
3+) and uranyl ions (UO
22+), and the limit of detection of Al
3+ was 0.111 ppm in a real serum sample. The limit of detection of UO
22+ in a real river water sample was 0.128 ppm. It showed high selectivity and anti-interference ability against common interfering ions such as Ca
2+, Mg
2+. Bu et al. [
93] reported a case of an ultra-stable Tb-MOF based on an AIE-type organic ligand for the fluorescence detection of trivalent iron and hexavalent chromium in aqueous solutions. Although the energy level of the introduced organic ligand is relatively low, Tb
3+ cannot be sensitized for luminescence. However, the Tb-MOF has excellent water stability and good ligand luminescence. When the pH value of the aqueous solution increases from 1 to 14, the Tb-MOF can maintain its structural stability in sodium hydroxide solution or hydrogen chloride solutions for 4 days. At the same time, the Tb-MOF emits indigo fluorescence of organic ligands and selectively detects Fe(III) and Cr(VI) by a light quenching process. The detection of Hg
2+, which is more toxic than Cr(VI), is also necessary. Qu et al. [
94] reported an example of a boronic acid-functionalized Ln-MOFs fluorescence sensor (BA-Eu-MOF), which selectively detects Hg
2+ by fluorescence enhancement and detects Hg
2+ at the concentration ranging from 1 to 60 μM, which corresponds to a detection limit of 220 nM.
5.2.2. Fluorescence Sensing of Anions
Yi et al. [
95] developed a dual-mode visual phosphate detection method using smartphone and test paper coupling by taking advantage of the superior fluorescence properties of UiO-66-NH
2 and Eu
3+@MOF-808 metal–organic frameworks. The experimental results showed that the blue fluorescence emission peak of UiO-66-NH
2 and the red fluorescence emission peak of Eu
3+@MOF-808 were regularly enhanced and quenched, respectively, when phosphate was added to the system, and the fluorescence response of this detection strategy to phosphate showed a color change process of (Red→Pink→Blue). Su et al. [
96] synthesized water-stable Eu-MOF and Tb-MOF fluorescence sensors using two organic ligands, pyromellitic acid, and imidazole-4,5-dicarboxylic acid, which can rapidly and accurately detect Fe
3+, CrO
42− and Cr
2O
72− in aqueous solutions. The synthesized Eu-MOFs and Tb-MOFs maintain stable crystal structures and luminescent properties in aqueous environments for up to 30 days, and they are stable not only in aqueous solutions with pH values ranging from 3 to 12 but also in ice and hot water. The efficient “antenna effect” of the two organic ligands gives Ln
3+ excellent luminescence and good fluorescence sensing properties, and the synthesized Eu-MOFs can detect Fe
3+ with a sensitivity up to 20,280 M
−1, Cr
2O
72− with a sensitivity up to 19,150 M
−1, CrO
42− with a sensitivity up to 11,400 M
−1, and CrO
42− with a sensitivity up to 11,400 M
−1, respectively. The sensitivity of the synthesized Tb-MOF can reach 12040 M
−1 for Fe
3+, 14100 M
−1 for Cr
2O
72−, and 8230 M
−1 for CrO
42− (
Figure 16).
5.3. Fluorescence Sensing of Organic Pollutants
The demand for the detection of organic molecules (organic solvents, biomarkers, nitro explosives, etc.) exists in every aspect of production and life, and the traditional detection methods (nuclear magnetic resonance hydrogen spectroscopy, ultraviolet-visible absorption spectroscopy, etc.) have deficiencies of cumbersome operation, non-intuitive results, etc. Ln-MOFs can realize the enrichment and detection of small organic molecules by the introduction of the functional sites and the regulation of spectroscopic properties of organic ligands, with the advantages of low detection limit, intuitive results, and easy operation. Equally critical is the rational selection of regeneration methods—such as solvent washing, thermal treatment, or photocatalytic degradation—based on pollutant-binding mechanisms, which enables multiple reuse cycles of RE-MOFs without compromising their sensing performance.
5.3.1. Fluorescence Sensing of Toxic Substances
Nitrofurans are a class of synthetic antibiotics. When nitrofurans and their metabolites are absorbed by the human body through animal food, they can accumulate in the body, leading to chronic poisoning and even carcinogenicity, teratogenicity, and mutagenicity. Li Guangming et al. [
97] prepared RhB@Tb-dcpcpt, which can achieve the detection of nitrofuran antibiotics by fluorescence quenching and the detection of quinolone antibiotics (ciprofloxacin and norfloxacin) by luminescence discoloration process. The detection limits were as low as 0.502 M (99 ppb) and 0.448 M (107 ppb), respectively.
Compared to nitrofurans, nitrobenzene, as a chemical agent, is toxic and carcinogenic. Therefore, it may cause adverse effects on human health and ecology when exposed to the environment. Yang et al. [
98] used the prepared lanthanide metal–organic frameworks for the detection of nitrobenzene, which showed a highly selective fluorescence response to a wide range of organic solvents, with significant quenching of fluorescence by nitrobenzene only and a limited detection of 3.96 × 10
−5 M. The detection sensitivity (Ksv value) was 1.5143 × 10
4 M
−1 with a detection efficiency of 99%. In addition, the material maintained the framework integrity below 430 °C, and the framework structure was not damaged under the action of acids, bases, and solvents (
Figure 17).
5.3.2. Fluorescence Sensing of Biomarkers
Fluorescence detection of some markers related to life and health is also necessary, Wang et al. [
99] used a fluorescent probe based on UiO-66-NH
2 for the highly sensitive detection of dopamine (DA) and reduced glutathione (GSH). The probe itself has fluorescence emission at 450 nm, and when DA is added to the probe system, DA is oxidized in polyethyleneimine (PEI) solution to form a copolymer (PDA-PEI), which can emit yellow-green fluorescence at 530 nm. Due to the fluorescence resonance energy transfer effect, the fluorescence of the probe was quenched at 450 nm and the fluorescence intensity increased at 530 nm. Subsequently, the fluorescence of UiO-66-NH
2 was restored by adding GSH to the above system, and the strong reducing property of GSH prevented the formation of PDA-PEI. The detection limits of the probes for DA and GSH were low at 0.68 μM and 0.57 μM, respectively. Yang et al. [
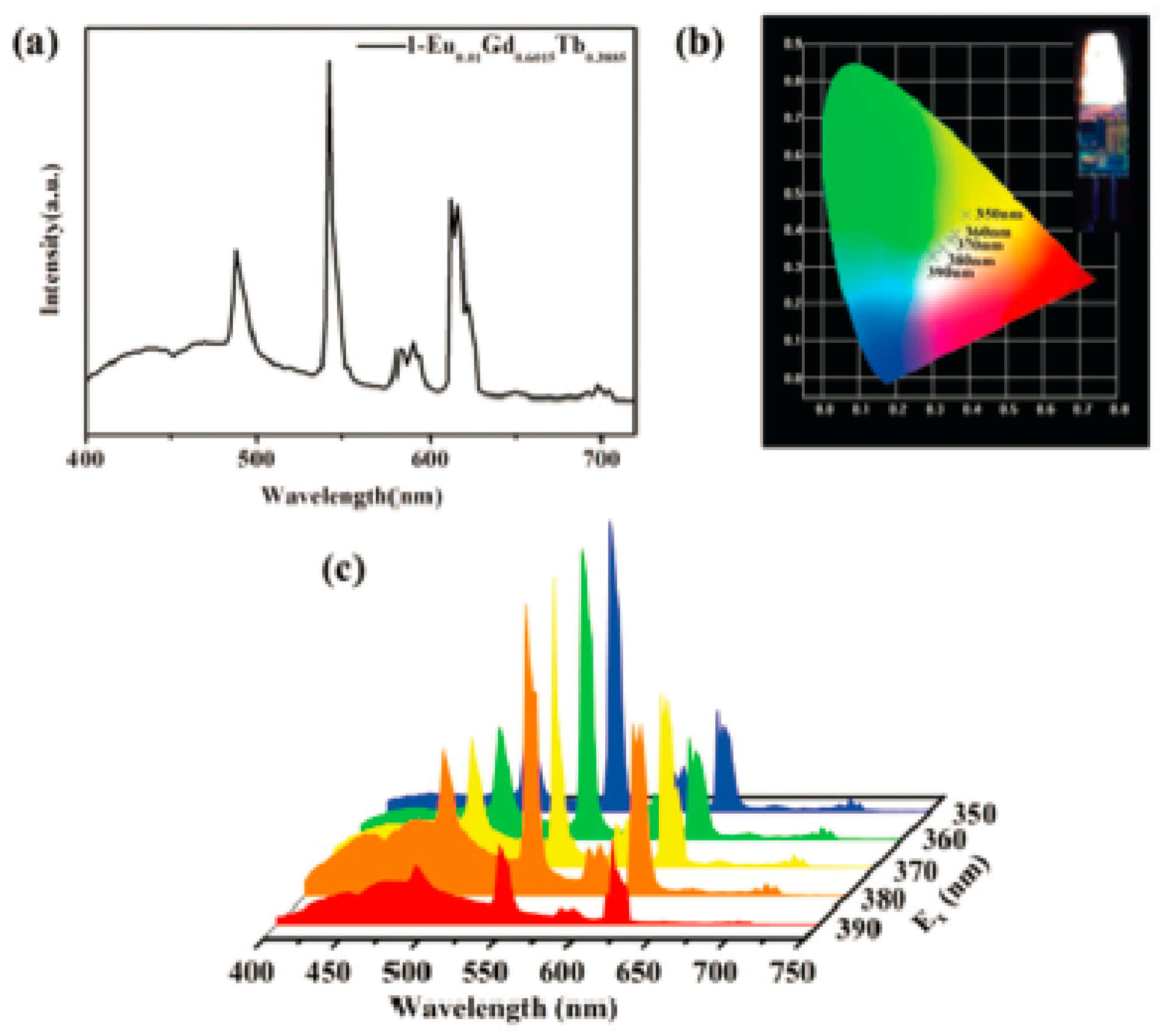
100] constructed lanthanide metal–organic framework materials for the detection of methylmalonic acid (MMA), a potential marker of vitamin B12 deficiency, in simulated urine samples, with the detection limits much lower than the range of MMA concentrations in urine from healthy people (3–11 mM). The material showed good acid-base stability in the pH range of 4.0–9.0. It maintains a stable crystal structure when immersed in water for a long period of time. And it can overcome the interference of other similar biosignaling molecules (e.g., glucose, urea, etc.). In addition, the material can still be reused without any significant decrease in stability and performance by washing and centrifugation recovery after the assay (
Figure 18).
Cheng et al. [
101] reported an example of water-stabilized Ln-MOFs fluorescent sensors that can be used for the fluorescence detection of 5-hydroxytryptamine (HT) and hydroxytryptamine indoleacetic acid (HIAA), which are markers of carcinoid tumors. The synthesized Eu-MOF fluorescent sensor selectively recognized HT and HIAA through a strong quenching effect, enables rapid detection of HT and HIAA in serum and urine, and overcomes interference from other similar biosignaling molecules. In addition, the Eu-MOF fluorescence sensor has good reproducibility and low detection limits of 0.66 × 10
−6 M
−1 for HT and 0.54 × 10
−6 M
−1 for HIAA, which can meet the concentration requirements for physiological environment detection. Meanwhile, the relationship between the relative luminescence intensity and the substrate concentration of this Eu-MOF fluorescence sensor conforms to a linear equation in the lower concentration range, which means that the quantitative fluorescence detection of HT and HIAA can be achieved.
Amino acids are the basic structural units of life-active substances such as peptides and proteins, which are closely related to cell metabolism and life and health, and the monitoring of amino acid concentration changes in the microenvironment of human cells plays a very important role in the study of cell metabolic activities and human life and health. Liu et al. [
102] reported a case of a pH-responsive Eu-MOF fluorescence sensor (JXUST-29) for fluorescence monitoring of arginine and lysine in living cells. The Eu-MOF was synthesized based on two organic ligands, lactic acid and azole carboxylic acid (H4BTDBA = 4′,4′′′-(benzo[1] [1,2,5]thiadiazole-4,7-diyl)bis([1,1′-biphenyl-3,5-dicarboxylic acid )), and has good water stability, pH stability and solvent stability. However, due to the insufficient energy level of the organic ligands, the fluorescence emission spectra of JXUST-29 showed only the broad emission peaks of the ligands, but not the sharp characteristic emission peaks of Eu
3+. As the pH of the aqueous solution increased from 2 to 5, the intensity of the fluorescence emission peaks of JXUST-29 increased to 54 times the initial intensity, accompanied by a certain degree of emission peak shift, which was attributed to the fact that the organic ligand, H4BTDBA, contained uncoordinated nitrogen atoms, which would lead to a change in the fluorescence emission behavior of JXUST-29 when the nitrogen atoms interacted with the hydrogen ions in the aqueous solution. In addition, JXUST-29 was also able to detect arginine and lysine by fluorescence enhancement effect and blue-shifted fluorescence of the emission peaks, with detection limits of 0.023 M and 0.077 M, respectively.
6. Conclusions and Future Prospective
In conclusion, this paper describes recent advances in the selection of organic ligands, design of framework structures, synthesis techniques, and applications related to the fluorescence monitoring of pollutants for RE-MOF materials with the aim of high-quality luminescent properties. Based on the unique luminescence, structural and dimensional tunability, as well as structural rigidity and porosity (e.g., MOF) RE-MOFs are attractive for monitoring gaseous pollutants, ionic pollutants, and organic pollutants. By appropriately designing the ligands and MOF framework structures, and following different synthesis methods, RE-MOF nanomaterials can be synthesized to form various nanostructures. From the design of high-quality luminescent RE-MOFs, the selection of organic ligands is no longer limited to the enhancement of the fluorescence intensity and stability of rare earth metals but gradually focuses on the concomitant luminescence, functional sites, and other concomitant properties. The metal linkers in the MOF framework structure range from single rare earth metals to bimetallic, from simple MOF structures to post-synthesis modifications. The synthesis methods are also not limited to hydrothermal/solvothermal methods, but also the birth of mechanochemical synthesis means without the use of organic solvents. From the current discussion on contaminant monitoring of RE-MOFs, we can conclude that it has been applied in several research areas. These materials have potential applications in ion, small molecule, and biomolecule sensing, as well as tunable photoluminescent materials. The paucity of reports on 2D metal–organic framework (MOF) nanosheets suggests that this area needs to be further explored as it has the potential to be a multifunctional material and may have comparable applications with pure organic and inorganic nanosheets. Furthermore, the stability of MOFs in aqueous media is limited by the degradable nature of the metal–ligand bond. In this regard, the development of composites may be a good option to improve stability. Meanwhile, the synthesis and application of RE-MOF composites have also gained great attention from researchers, mainly including the combination with aggregation-induced luminescent materials, quantum dots, dyes, and other materials, which can be used to prepare composites with excellent performance. The use of luminescent MOFs as a platform for the development of various complex optical barcodes and anti-counterfeiting labels for information encryption has also been a research direction that researchers have paid great attention to in recent years. In recent years, RE-MOFs have been found to have new optical properties such as two-photon fluorescence, room temperature phosphorescence, thermally activated delayed fluorescence, near-infrared two-region fluorescence, and circularly polarized luminescence, which have endowed the rare earth metal–organic frameworks with new combined luminescence applications. It is clear that this field of research is growing at a phenomenal rate, leading to exciting new developments. The future of RE-MOFs as novel multifunctional materials is clearly very bright.
To advance the in-depth development of RE-MOFs in pollutant monitoring and photofunctional materials, future research should focus on the following directions:
(1) Multidimensional Structural Design: Explore the controlled synthesis of 2D/3D RE-MOF nanosheets and their interfacial engineering. Combine theoretical calculations to elucidate the structure–property relationships between framework architecture and luminescence performance, expanding their applications in flexible sensing and micro/nano optoelectronic devices.
(2) Stability Enhancement: Develop RE-MOF composites (e.g., with polymers or carbon-based materials) with high hydrothermal stability, or enhance material stability in complex environments through ligand functionalization (e.g., introducing hydrophobic groups or dynamic covalent bonds).
(3) Smart Functional Integration: Leverage the multimodal luminescent properties of RE-MOFs (e.g., circularly polarized luminescence, NIR-II fluorescence) to design self-responsive sensors or adaptive optical labels for in situ pollutant identification and dynamic tracking.
(4) Green Synthesis Techniques: Advance low-energy, solvent-free mechanochemical synthesis routes. Integrate artificial intelligence to screen efficient ligands and synthesis conditions, promoting large-scale production and environmental compatibility of RE-MOFs.
(5) Interdisciplinary Application Expansion: Collaborate with optoelectronics, biomedicine, and data science fields to explore cross-disciplinary applications of RE-MOFs in in vivo bioimaging, photodynamic therapy, and optical information encryption.
(6) Technical optimization: Relieve the dual challenges of high rare earth element costs and supply limitations in the application potential of RE MOFs in pollutant monitoring through technical optimization and strategic design.
Through these strategies, RE-MOFs are expected to transition from laboratory research to practical applications, offering next-generation high-performance solutions for environmental monitoring, medical diagnostics, and information security.