Enzyme Inhibition-Mediated Distance-Based Paper Biosensor for Organophosphate Pesticide Detection in Food Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Construction of EIDP Biosensor
2.3. Optimization Reagent Concentration and Incubation Time
2.4. Sensitivity and Selectivity of EIDP Biosensor
2.5. Analysis of Food Samples
3. Results
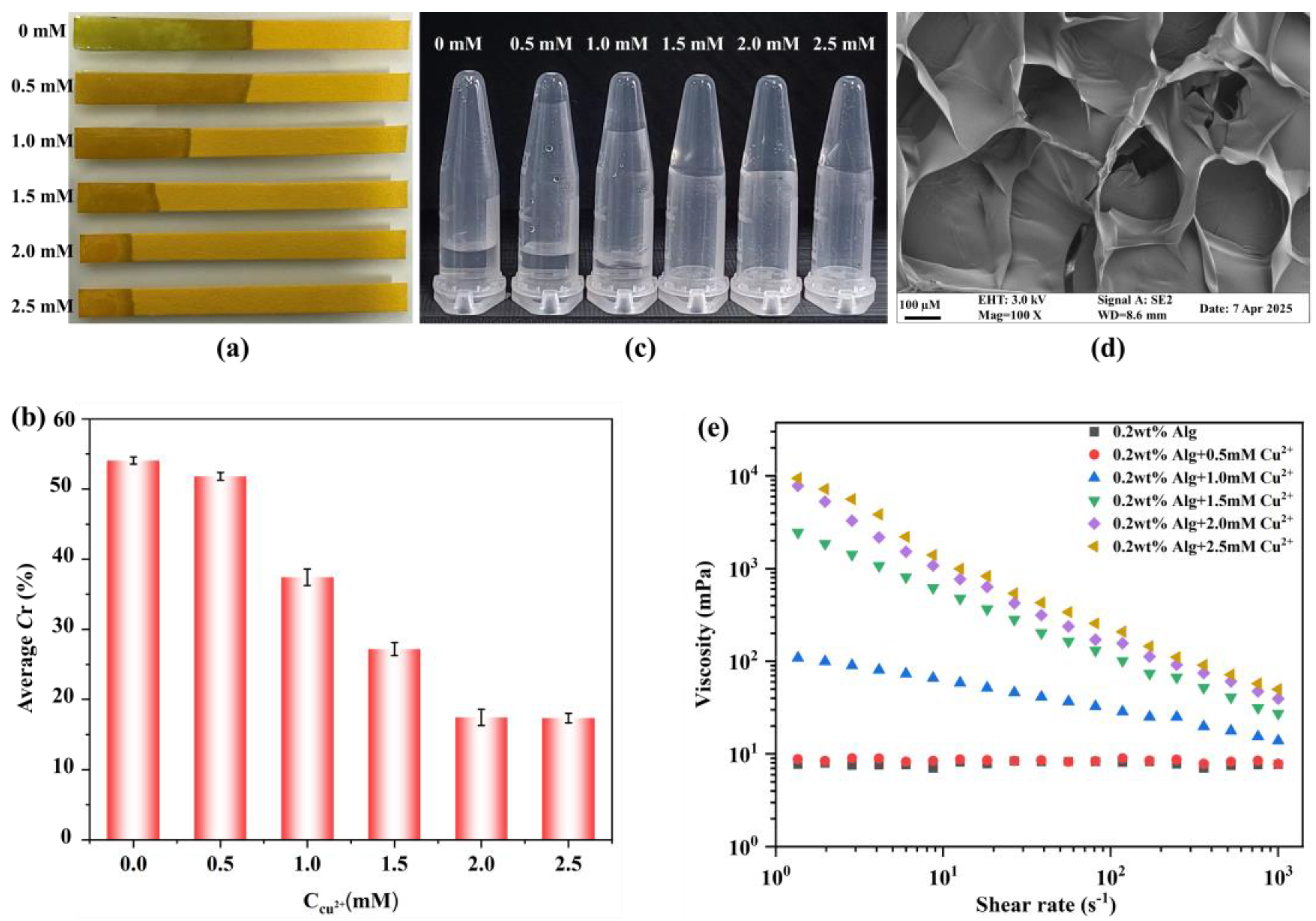
3.1. The Water Flow Distance as a Function of Cu-Alg Hydrogel Formation
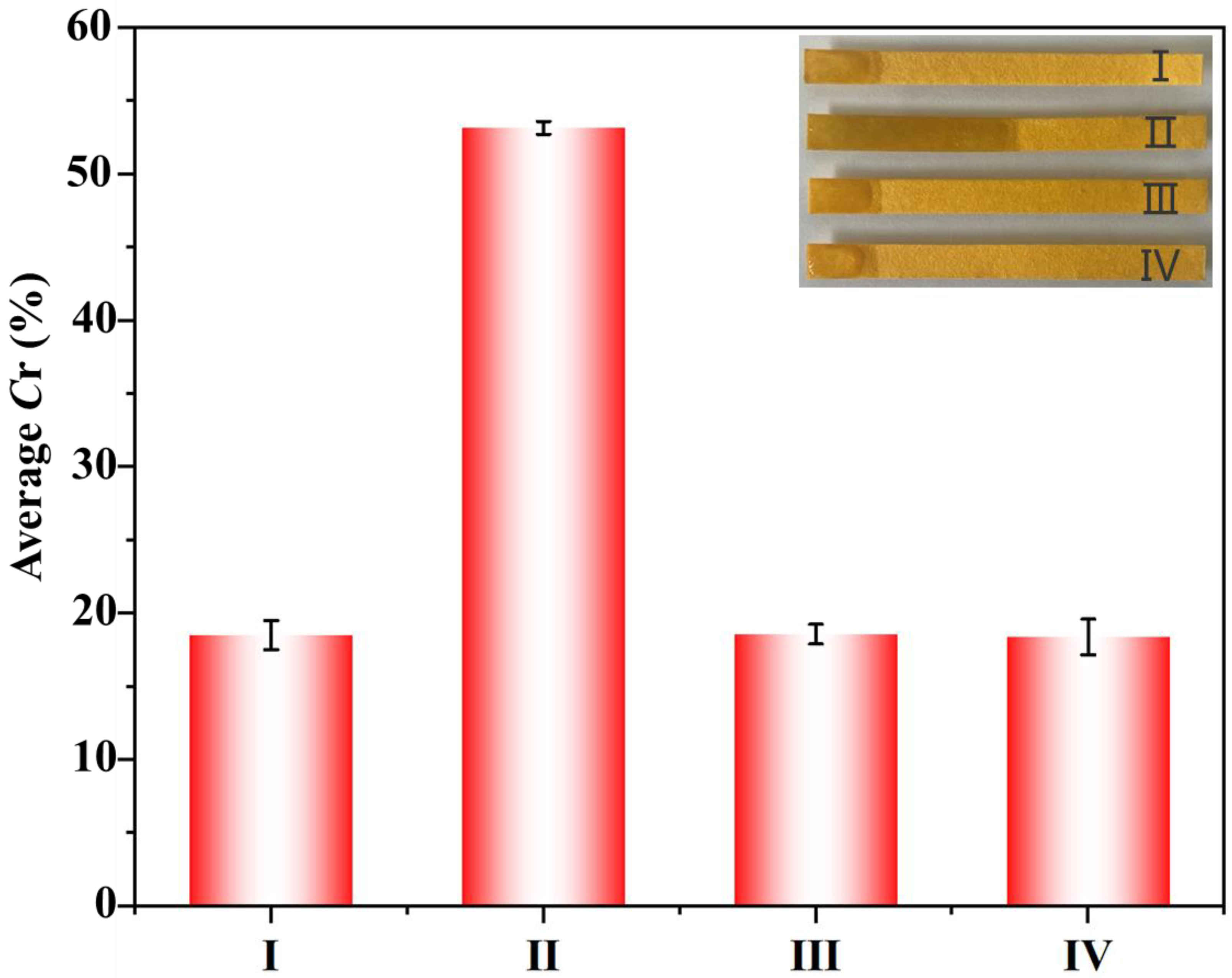
3.2. Feasibility of EIDP Biosensor for OP Detection
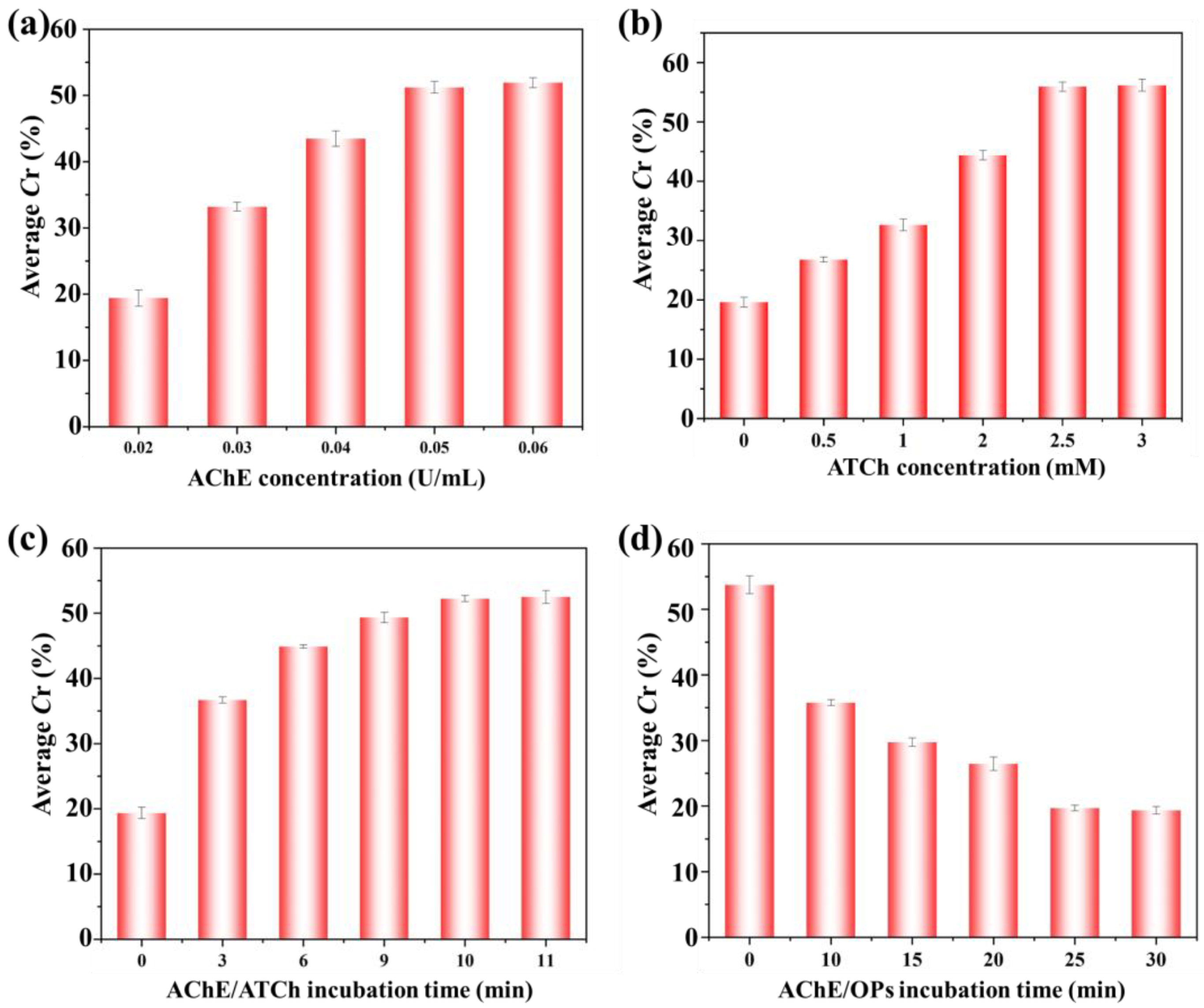
3.3. The Optimization of the Experimental Parameters
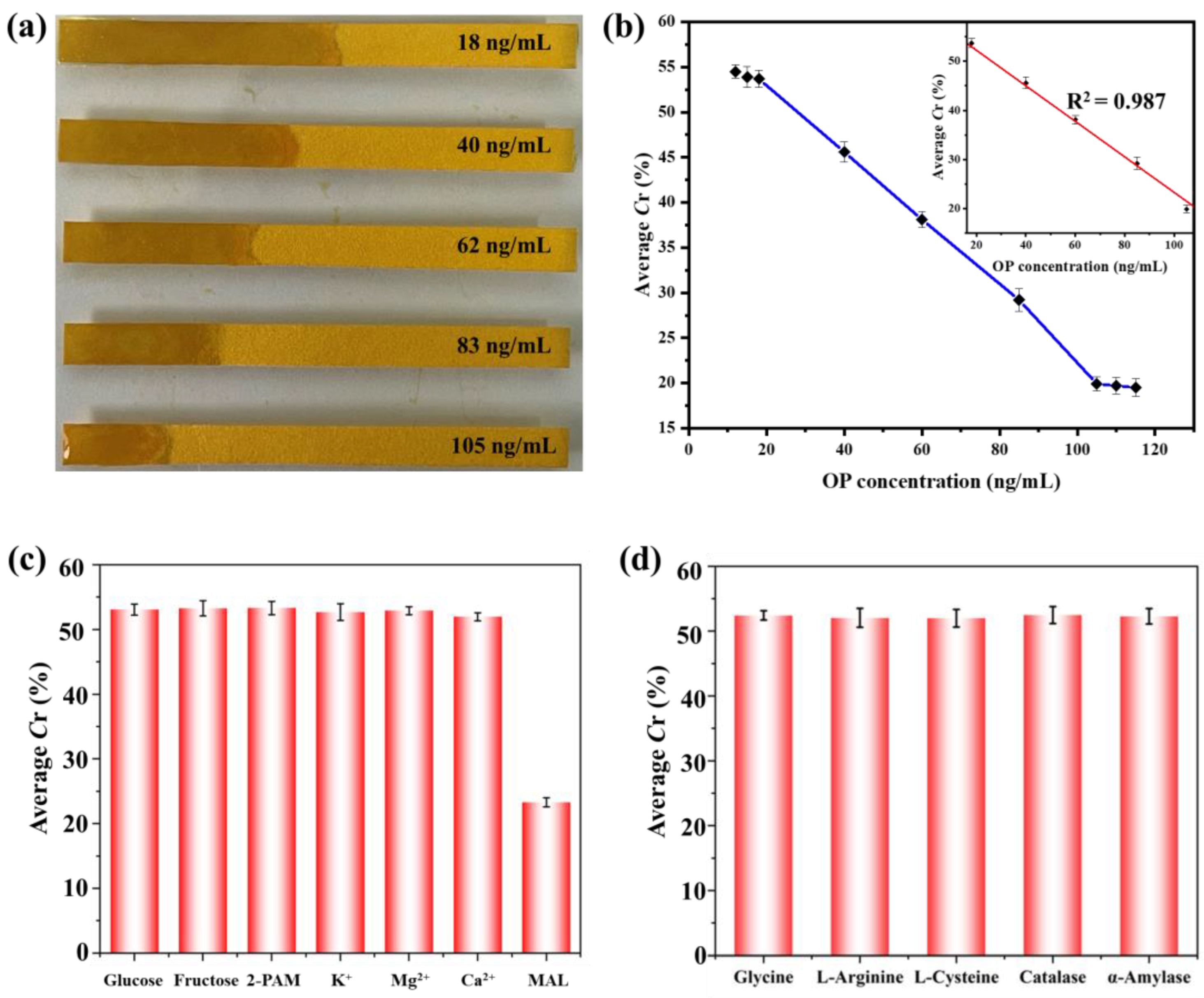
3.4. Sensitivity and Selectivity of OP Detection
3.5. OPs Detection in Pumpkin and Rice Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EIDP | Enzyme inhibition-mediated distance-based paper |
| OPs | Organophosphate pesticides |
| ATCh | Acetylthiocholine iodide |
| AChE | Acetylcholinesterase |
| Cu-Alg | Copper alginate |
| 2-PAM | 2-Pyridinealdoxime methochlorid |
References
- Songa, E.A.; Okonkwo, J.O. Recent approaches to improving selectivity and sensitivity of enzyme-based biosensors for organophosphorus pesticides: A review. Talanta 2016, 155, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.-T.; Zhang, Y.-H.; Zhu, Y.; Du, S.-L.; Zhang, J.; Cao, Y.; Wang, Y.-Z.; Wang, G.-T.; He, L.-S. Deriving water quality criteria for China for the organophosphorus pesticides dichlorvos and malathion. Environ. Sci. Pollut. Res. 2019, 26, 34622–34632. [Google Scholar] [CrossRef] [PubMed]
- Čadež, T.; Kolić, D.; Šinko, G.; Kovarik, Z. Assessment of four organophosphorus pesticides as inhibitors of human acetylcholinesterase and butyrylcholinesterase. Sci. Rep. 2021, 11, 21486. [Google Scholar] [CrossRef] [PubMed]
- Zoofaghari, S.; Maghami-Mehr, A.; Abdolrazaghnejad, A. Organophosphate Poisoning: Review of Prognosis and Management. Adv. Biomed. Res. 2024, 13, 82. [Google Scholar] [CrossRef]
- Shekhar, C.; Khosya, R.; Thakur, K.; Mahajan, D.; Kumar, R.; Kumar, S.; Sharma, A.K. A systematic review of pesticide exposure, associated risks, and long-term human health impacts. Toxicol. Rep. 2024, 13, 101840. [Google Scholar] [CrossRef]
- Kumaran, A.; Vashishth, R.; Singh, S.; U, S.; James, A.; Velayudhaperumal Chellam, P. Biosensors for detection of organophosphate pesticides: Current technologies and future directives. Microchem. J. 2022, 178, 107420. [Google Scholar] [CrossRef]
- Zhu, B.; Xu, X.; Luo, J.; Jin, S.; Chen, W.; Liu, Z.; Tian, C. Simultaneous determination of 131 pesticides in tea by on-line GPC-GC–MS/MS using graphitized multi-walled carbon nanotubes as dispersive solid phase extraction sorbent. Food Chem. 2019, 276, 202–208. [Google Scholar] [CrossRef]
- Erhard, M.H.; Schmidt, P.; Kühlmann, R.; Lösch, U. Development of an ELISA for detection of an organophosphorus compound using monoclonal antibodies. Arch. Toxicol. 1989, 63, 462–468. [Google Scholar] [CrossRef]
- Ioerger, B.P.; Smith, J.S. Multiresidue method for the extraction and detection of organophosphate pesticides and their primary and secondary metabolites from beef tissue using HPLC. J. Agric. Food Chem. 1993, 41, 303–307. [Google Scholar] [CrossRef]
- Qiu, L.; Lv, P.; Zhao, C.; Feng, X.; Fang, G.; Liu, J.; Wang, S. Electrochemical detection of organophosphorus pesticides based on amino acids conjugated nanoenzyme modified electrodes. Sens. Actuators B Chem. 2019, 286, 386–393. [Google Scholar] [CrossRef]
- Loguercio, L.F.; Griep, J.; Demingos, P.G.; Morawski, R.; Brolo, A.G.; Andrade, G.F.S.; Santos, J.F.L. Enhanced enzymatic electrochemical detection of an organophosphate Pesticide: Achieving Wide linearity and femtomolar detection via gold nanoparticles growth within polypyrrole films. Talanta 2025, 281, 126714. [Google Scholar] [CrossRef] [PubMed]
- Hassani, S.; Momtaz, S.; Vakhshiteh, F.; Maghsoudi, A.S.; Ganjali, M.R.; Norouzi, P.; Abdollahi, M. Biosensors and their applications in detection of organophosphorus pesticides in the environment. Arch. Toxicol. 2017, 91, 109–130. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Singh, P.K. Enzyme-based optical biosensors for organophosphate class of pesticide detection. Phys. Chem. Chem. Phys. 2020, 22, 15105–15119. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-L.; Yu, L.-H.; Pang, Y.-H.; Shen, X.-F. A ratiometric fluorescence sensor for detection of organophosphorus pesticides based on enzyme-regulated multifunctional Fe-based metal-organic framework. Talanta 2024, 278, 126516. [Google Scholar] [CrossRef]
- Yousefi, R.; Asgari, S.; Banitalebi Dehkordi, A.; Mohammadi Ziarani, G.; Badiei, A.; Mohajer, F.; Varma, R.S.; Iravani, S. MOF-based composites as photoluminescence sensing platforms for pesticides: Applications and mechanisms. Environ. Res. 2023, 226, 115664. [Google Scholar] [CrossRef]
- Jing, W.; Shi, Q.; Qiang, S.; Wang, Y.; Li, Y.; Zhao, T.; Li, Y.; Liu, D.; Liu, Y.; Liu, F. Dual-mode colorimetric and chemiluminescence aptasensor for organophosphorus pesticides detection using aptamer-regulated peroxidase-like activity of TA-Cu. Talanta 2025, 285, 127410. [Google Scholar] [CrossRef]
- Yu, H.; Wang, M.; Cao, J.; She, Y.; Zhu, Y.; Ye, J.; Abd El-Aty, A.M.; Hacımüftüoğlu, A.; Wang, J.; Lao, S. Dual-mode detection of organophosphate pesticides in pear and Chinese cabbage based on fluorescence and AuNPs colorimetric assays. Food Chem. 2021, 364, 130326. [Google Scholar] [CrossRef]
- Meng, X.; Wei, J.; Ren, X.; Ren, J.; Tang, F. A simple and sensitive fluorescence biosensor for detection of organophosphorus pesticides using H2O2-sensitive quantum dots/bi-enzyme. Biosens. Bioelectron. 2013, 47, 402–407. [Google Scholar] [CrossRef]
- Bhattu, M.; Verma, M.; Kathuria, D. Recent advancements in the detection of organophosphate pesticides: A review. Anal. Methods 2021, 13, 4390–4428. [Google Scholar] [CrossRef]
- Chaudhari, P.; Chau, L.-K.; Ngo, L.T.; Chang, T.-C.; Chen, Y.-L.; Huang, K.-T. Competitive Assay for the Ultrasensitive Detection of Organophosphate Pesticides Based on a Fiber-Optic Particle Plasmon Resonance Biosensor and an Acetylcholinesterase Binding Peptide. Anal. Chem. 2023, 95, 14600–14607. [Google Scholar] [CrossRef]
- Khan, M.; Zhao, B.; Wu, W.; Zhao, M.; Bi, Y.; Hu, Q. Distance-based microfluidic assays for instrument-free visual point-of-care testing. TrAC Trends Anal. Chem. 2023, 162, 117029. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, F.; Zhang, K.; Min, T.; Chen, D.; Wen, Y. Distance-Based Biosensor for Ultrasensitive Detection of Uracil-DNA Glycosylase Using Membrane Filtration of DNA Hydrogel. ACS Sens. 2021, 6, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, X.; Yin, Y.; Yin, F.; Li, J.; Hou, Z.; Khan, M.; Zhao, R.; Wu, W.; Hu, Q. Distance-based lateral flow biosensor for the quantitative detection of bacterial endotoxin. Chin. Chem. Lett. 2024, 35, 109718. [Google Scholar] [CrossRef]
- Han, C.; Yuan, X.; Shen, Z.; Xiao, Y.; Wang, X.; Khan, M.; Liu, S.; Li, W.; Hu, Q.; Wu, W. A paper-based lateral flow sensor for the detection of thrombin and its inhibitors. Anal. Chim. Acta 2022, 1205, 339756. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chu, W.; Liu, W.; Guo, X. Distance-based carcinoembryonic antigen assay on microfluidic paper immunodevice. Sens. Actuators B Chem. 2018, 260, 452–459. [Google Scholar] [CrossRef]
- Tai, W.; Yin, F.; Bi, Y.; Lin, J.-M.; Zhang, Q.; Wei, Y.; Hu, Q.; Yu, L. Paper-based sensors for visual detection of alkaline phosphatase and alpha-fetoprotein via the distance readout. Sens. Actuators B Chem. 2023, 384, 133666. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Xiao, J.; Li, C.; Wang, J.; Xiao, X.; Huang, H.; Shrestha, B.; Tang, L.; Deng, K.; et al. Visual Quantitative Detection of Glutathione and Cholesterol in Human Blood Based on the Thiol–Ene Click Reaction-Triggered Wettability Change of the Interface. Anal. Chem. 2021, 93, 7292–7299. [Google Scholar] [CrossRef]
- Ho, M.; Sathishkumar, N.; Sklavounos, A.A.; Sun, J.; Yang, I.; Nichols, K.P.; Wheeler, A.R. Digital microfluidics with distance-based detection—A new approach for nucleic acid diagnostics. Lab Chip 2023, 24, 63–73. [Google Scholar] [CrossRef]
- Allameh, S.; Rabbani, M. A Distance-Based Microfluidic Paper-Based Biosensor for Glucose Measurements in Tear Range. Appl. Biochem. Biotechnol. 2022, 194, 2077–2092. [Google Scholar] [CrossRef]
- Jiang, C.; Li, Y.; Wang, H.; Chen, D.; Wen, Y. A portable visual capillary sensor based on functional DNA crosslinked hydrogel for point-of-care detection of lead ion. Sens. Actuators B Chem. 2020, 307, 127625. [Google Scholar] [CrossRef]

| Sample | Spiked OPs (ng/mL) | Measured (ng/mL) | Recovery (%) | RSD (%), n = 5 |
|---|---|---|---|---|
| Pumpkin | 20 | 18.71 | 93.6 | 7.6 |
| 50 | 47.53 | 95.1 | 8.8 | |
| 100 | 102.55 | 102.6 | 5.6 | |
| Rice | 20 | 18.12 | 90.6 | 6.5 |
| 50 | 46.53 | 93.1 | 9.4 | |
| 100 | 101.34 | 101.3 | 6.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Dong, L.; Hu, Q.; Chen, J.; Khan, M. Enzyme Inhibition-Mediated Distance-Based Paper Biosensor for Organophosphate Pesticide Detection in Food Samples. Chemosensors 2025, 13, 147. https://doi.org/10.3390/chemosensors13040147
Liu Y, Dong L, Hu Q, Chen J, Khan M. Enzyme Inhibition-Mediated Distance-Based Paper Biosensor for Organophosphate Pesticide Detection in Food Samples. Chemosensors. 2025; 13(4):147. https://doi.org/10.3390/chemosensors13040147
Chicago/Turabian StyleLiu, Yulin, Longzhan Dong, Qiognzheng Hu, Jingbo Chen, and Mashooq Khan. 2025. "Enzyme Inhibition-Mediated Distance-Based Paper Biosensor for Organophosphate Pesticide Detection in Food Samples" Chemosensors 13, no. 4: 147. https://doi.org/10.3390/chemosensors13040147
APA StyleLiu, Y., Dong, L., Hu, Q., Chen, J., & Khan, M. (2025). Enzyme Inhibition-Mediated Distance-Based Paper Biosensor for Organophosphate Pesticide Detection in Food Samples. Chemosensors, 13(4), 147. https://doi.org/10.3390/chemosensors13040147






