Abstract
Bio-sniffers represent a novel detection technology that demonstrates significant potential in medical diagnostics. Specifically, they assess disease conditions and metabolic status through the detection of volatile organic compounds (VOCs) in exhaled breath. Unlike conventional methods such as gas chromatography-mass spectrometry (GC-MS) and gas chromatography time-of-flight mass spectrometry (GC-TOF-MS), bio-sniffers provide rapid, sensitive, and portable detection capabilities. In this review, we examine the metabolic pathways and detection methods of specific VOCs in the human body, and their roles as disease biomarkers, and focus on the detection principles, performance characteristics, and medical applications of two bio-sniffer types: electrical and optical sensors. Finally, we systematically discuss the current challenges facing bio-sniffers in VOC monitoring, outline future development directions, and provide suggestions for improving sensitivity and reducing environmental interference.
1. Introduction
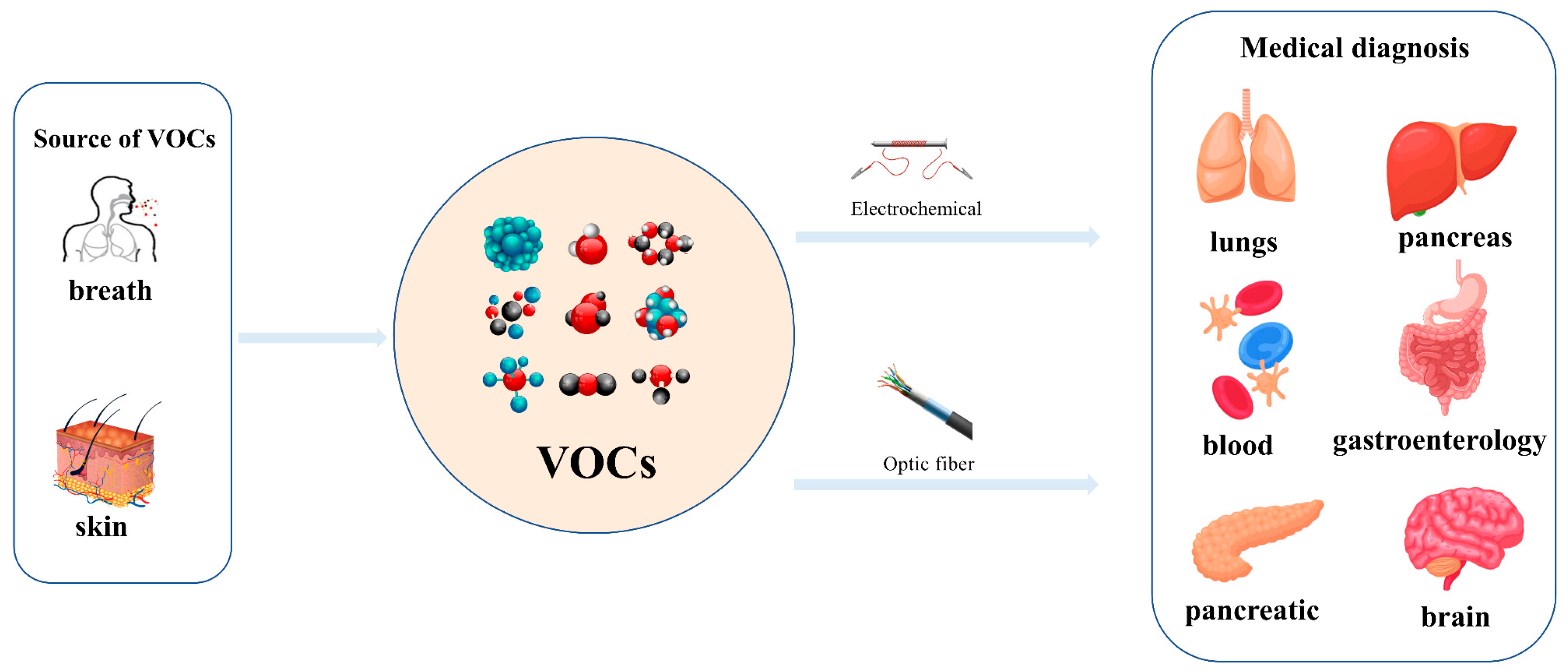
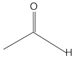
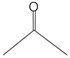
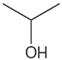
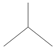
Exhaled breaths serve as a rich source of biological information, encompassing indicators that reflect metabolic status [1], biological rhythms, and early signals of certain diseases [2,3,4,5,6]. Human breath harbors in excess of 3500 volatile organic compounds (VOCs) [7], some of which are considered to possess the potential to function as biomarkers for various diseases [8,9,10,11,12]. Acetone is strongly associated with diabetic ketoacidosis (DKA), and its concentration in exhaled breath is significantly elevated (75 ppm–1200 ppm), making it a potential biomarker for non-invasive diagnosis. Ethanol is associated with liver disease, and changes in the concentration of its metabolites (e.g., acetaldehyde) in exhaled breath can be used to assess liver function. Isopropanol is found at elevated concentrations in the exhaled breath of patients with certain cancers (e.g., lung cancer) and can be used for early screening. Methanol correlates with the activity of the gut microbiota and can be used to assess gut health. In the realm of metabolomics, biological samples, including exhaled breath, urine, blood, and cerebrospinal fluid obtained from humans and animals, are rich in chemical, physical, and biological information, particularly VOCs [13,14,15]. Metabolites, intimately linked to the metabolic activities of organisms, are pivotal in the genesis and progression of diseases. Within the sphere of human metabolomics, exhaled breath and skin emerge as the predominant sources of VOCs, each contributing 54% to the total VOC profile. Feces, saliva, urine, and blood account for 15%, 14%, 11%, and 6% of the total VOCs, respectively [16]. Additionally, 84, 360, and 532 different exogenous and endogenous VOCs were detected in respiratory samples, saliva, and the skin, respectively [17,18]. As a result, VOCs have risen to prominence as potential biomarkers in the realm of disease diagnostics. The examination of VOCs in breath composition enables real-time monitoring of the human body’s metabolic status, leveraging its non-invasive and continuous monitoring characteristics, which eliminate the need for sample collection or processing. Breath analysis has demonstrated significant application potential in the field of clinical medicine [19,20]. In contrast to blood tests, the non-invasive characteristic of breath analysis not only alleviates the discomfort experienced by subjects but also offers several additional advantages [21], while also providing an effective means of continuously monitoring physiological changes in the human body [22]. As time progresses, gas sensor technology has advanced rapidly and has been widely applied in environmental quality testing [23,24], food safety testing [25,26], and health care [27,28,29]. This article focuses on the challenges and applications of this technology in the field of medical diagnostics (Figure 1).
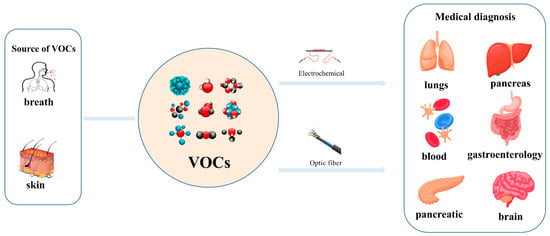
Figure 1.
Detection of VOCs in human exhaled and skin gases using electrochemical bio-sniffers and optical bio-sniffers to identify biomarkers associated with specific diseases (e.g., lung diseases, liver diseases, gastrointestinal diseases, etc.).
To ensure the accurate capture and analysis of these trace yet clinically significant gas components, the detection of trace VOCs necessitates highly sensitive and accurate methods. Over the past two decades, researchers across the globe have utilized techniques including gas chromatography and gas chromatography-mass spectrometry, in conjunction with a variety of detectors, for the detection of VOCs in the breath. This approach has been employed for disease detection and health monitoring purposes [30,31,32]. Consequently, these techniques dominate laboratory analysis [33]. Sanchez et al. identified approximately 25 components in human breath by employing a sophisticated analytical setup. This setup included a series of dual-column collections featuring polar columns with stationary phases of trifluoropropyl methylpolysiloxane and poly(ethylene glycol), as well as non-polar columns with dimethylpolysiloxane stationary phases. The system was further enhanced with a four-layer adsorption trap, utilizing a flame ionization detector for sensitive and accurate detection [34]. Schnabel and colleagues employed gas chromatography time-of-flight mass spectrometry (GC-TOF-MS) to conduct the noninvasive detection of ventilator-associated pneumonia (VAP) in intensive care unit (ICU) patients via the analysis of respiratory VOCs, thereby pinpointing 12 distinct VOCs [35]. These traditional detection methods are expensive, complex, time-consuming, and require specialized personnel for analysis, leading to the increasing importance of sensor technology in gas detection (Table 1). Currently, bio-sniffers are emerging as promising gas sensors, representing a highly intelligent device that is capable of the real-time detection and accurate analysis of gases through the affinity between biological receptors and specific gases or compounds [36]. The working mechanism is based on the interaction of biological receptors with target molecules, generating measurable signals to ascertain the presence or absence of target substances and their concentrations [37]. The development of bio-sniffers has benefited from the continuous advancement of biotechnology and sensor technology [38,39,40]. Compared to traditional methods, sensors provide the benefits of rapid response, heightened sensitivity, compact design, and the capability for real-time monitoring. The advantages of these sensors hinge on the operational principles of the sensing technologies employed; sensors can be classified according to their detection principles as electrical sensors (e.g., resistive and electrochemical), optical sensors (e.g., fluorescence and colorimetric), surface acoustic wave (SAW) sensors, and microbalance sensors. Each type of sensor has unique advantages and limitations when detecting VOCs. Among these, electrochemical sensors are used to detect electrically active gases and typically consist of sulfuric acid electrolytes, porous carbon working electrodes, reference electrodes, and counter electrodes, which are simple, portable, and capable of a rapid response [41]. A novel yttrium-stabilized zirconia (YSZ)-based electrochemical gas sensor has been devised for the detection of acetone concentrations in the exhaled breath of individuals with diabetes. Sm2-xSrxNiO4, synthesized by sol-gel synthesis as a sensing material, has demonstrated excellent performance in detecting acetone, with a detection limit as low as 300 ppb. This sensor can accurately detect acetone in exhaled breath, and it features a rapid response and recovery time, rendering it suitable for real-time monitoring applications [42]. Another study proposed a method to detect butylated hydroxytoluene (BHT) in the exhaled breath of individuals with Alzheimer’s disease (AD). This method involves depositing a thin layer of graphene on a glass carbon substrate to create a three-layer sensor and using electrochemical polymerization technology to form a polypyrrole layer that is selective for BHT on the sensor surface. These sensors exhibit remarkable sensitivity and are capable of detecting BHT at exceedingly low concentrations, spanning from 0.02 ppb to 1 ppb, demonstrating good sensitivity and selectivity [43]. It is important to note that these values are not absolute and can be affected by a number of factors. Optical sensors have become a new trend due to their fast response, wide dynamic detection range, and high cost-effectiveness in VOC detection [44]. Researchers have devised a diverse array of single-use chromatography sensors based on a variety of chemically responsive pigments that can rapidly identify 20 VOCs associated with lung cancer [45]. The construction of a fluorescent sensor comprising CdSe quantum dots and carbon dots has been reported to detect exhaled methyl nicotinate in patients with tuberculosis (TB) [46]. In the field of medical diagnosis, bio-sniffers can be used for respiratory analysis, body odor analysis, fecal gas analysis, and other applications, detecting VOCs associated with specific diseases and providing strong support for early disease diagnosis [47,48,49]. In this paper, we focus on the application of electrical and optical bio-sniffers for the detection of VOCs. These two types of bio-sniffers were selected based on their unique advantages in terms of detection principle, performance, and future trends. Electrical sensors are widely used in fields such as food safety and medical diagnostics due to their fast response, high sensitivity, and portability, while optical sensors have garnered significant research attention in recent years due to their non-contact detection, wide dynamic range, and high selectivity. ‘Non-contact detection’ in this context refers to the ability of the sensor to analyze the gas composition without requiring physical contact with the measured gas, utilizing optical methods such as spectroscopy. However, the active medium of some optical sensors (e.g., fluorescent sensors) may still come into contact with the gas to perform a specific detection function. These characteristics make them particularly effective for detecting VOCs in human exhalations, especially in complex biological samples. However, bio-sniffers face numerous challenges in terms of calibration, selectivity, and long-term stability. Regular calibration is crucial to ensure their accuracy under varying environmental conditions, while enhancing the selectivity of the sensor is essential for differentiating complex VOC mixtures. Moreover, long-term operation may result in performance degradation; thus, the development of anti-degradation strategies, such as the use of more stable materials or the design of self-cleaning mechanisms, is important to extend the sensor’s service life and maintain its performance. In addition, electrical and optical sensor technologies are rapidly evolving, and new materials and detection principles are emerging, such as nanocomposites, which can significantly improve the sensitivity and selectivity of sensors [50]. This offers the possibility of developing portable and wearable devices. This interdisciplinary research paradigm is not only accelerating the innovation of sensor technologies but also driving their application across diverse fields. In the field of medical diagnostics, the non-invasive and real-time monitoring capabilities of these sensors render them valuable for critical clinical applications, providing results within minutes and facilitating ease of use without complex sample pre-treatment. Thus, electrical and optical bio-sniffers not only excel in the current detection of VOCs but also offer new directions for future medical diagnostics and health monitoring.
This article reviews the application of VOC bio-sniffers in medical diagnostics. The article first examines the nature and metabolic pathways of VOCs and their roles as disease biomarkers. It then discusses the detection principles and performance characteristics of two types of bio-sniffers—electrical and optical sensors—as well as their sensing mechanisms and applications in medical diagnostics. In addition to discussing current challenges, the article also envisions future directions for the medical detection of VOCs.

Table 1.
The application and advantages and disadvantages of traditional detection methods for the detection of VOCs.
Table 1.
The application and advantages and disadvantages of traditional detection methods for the detection of VOCs.
| Methods | VOCs | Applications | Results | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|
| GC-MS | Styrene | Cancer | Detection of five VOCs associated with lung cancer in the nanomolar concentration range | High resolution and good detection sensitivity | High equipment cost, difficult to operate, and long analysis time | [51] |
| GC-TOF-MS | Ethanol, isopropanol, acetone, ethylbenzene, tetrahydrofuran | VAP | Distinguishing ICU patients with and without VAP, based on 12 VOCs | Better response to high-molecular-weight VOCs | Low automation, High equipment cost | [35] |
| PTR-MS | Isoprene | Head and neck tumors | VOCs can screen for HNSCC | High precision | High response to alcohols and low response to aldehydes | [52] |
| SIFT-MS | 2-propanol, acetaldehyde, acetone, ethanol, pentane, and trimethylamine (TMA) | Alcoholic hepatitis | A low correlation between exhaled TMA levels and AH severity was determined (r = 0.38) | Highly sensitive analysis | Complicated operation | [53] |
| SIFT-MS | Acetone | Diabetes mellitus | A linear correlation between acetone levels, as quantified by SIFT-MS and blood glucose concentrations. | Highly sensitive analysis | Complicated operation | [54] |
2. Characteristics of VOCs
2.1. Physical Properties and Chemical Structure of VOCs
VOCs represent a large class of organic chemicals that are volatile at room temperature, and their chemical structures and physical properties exhibit a wide diversity. Ranging from simple alkanes such as methane to complex polycyclic aromatic hydrocarbons, these diverse chemical structures confer upon VOCs distinct physical characteristics, including color, state at room temperature and at the pressure boiling point, and water solubility and fat solubility, which determine their behavior and migration pathways in the environment. For instance, VOCs with lower boiling points are more likely to volatilize at room temperature, whereas compounds with higher vapor pressures are more likely to escape from liquid or solid surfaces into the atmosphere [55]. VOCs with higher water solubility may be more easily transported through the aqueous phase, whereas VOCs with better fat solubility may accumulate in organisms. Understanding the chemical structure and physical properties of VOCs is essential for predicting their fate in the environment and assessing their potential ramifications for human health and the living environment. The physical properties of some common VOCs are listed in Table 2.

Table 2.
Properties and chemical structures of common VOCs.
2.2. Metabolic Processes in the Human Body
When VOCs are ingested into the body via breathing, skin contact, or diet, these compounds are rapidly distributed into the blood and tissues. Metabolic processes not only affect the toxicity and biological activity of VOCs but also determine their half-life and potential health risks within the body. For instance, the metabolic intermediates of certain VOCs, such as benzene, are highly reactive and can directly interact with cellular components, leading to DNA damage and the formation of protein adducts, which can result in cellular dysfunction and even carcinogenesis [56]. Consequently, understanding the metabolic pathways of VOCs in the human body is crucial for assessing their health risks.
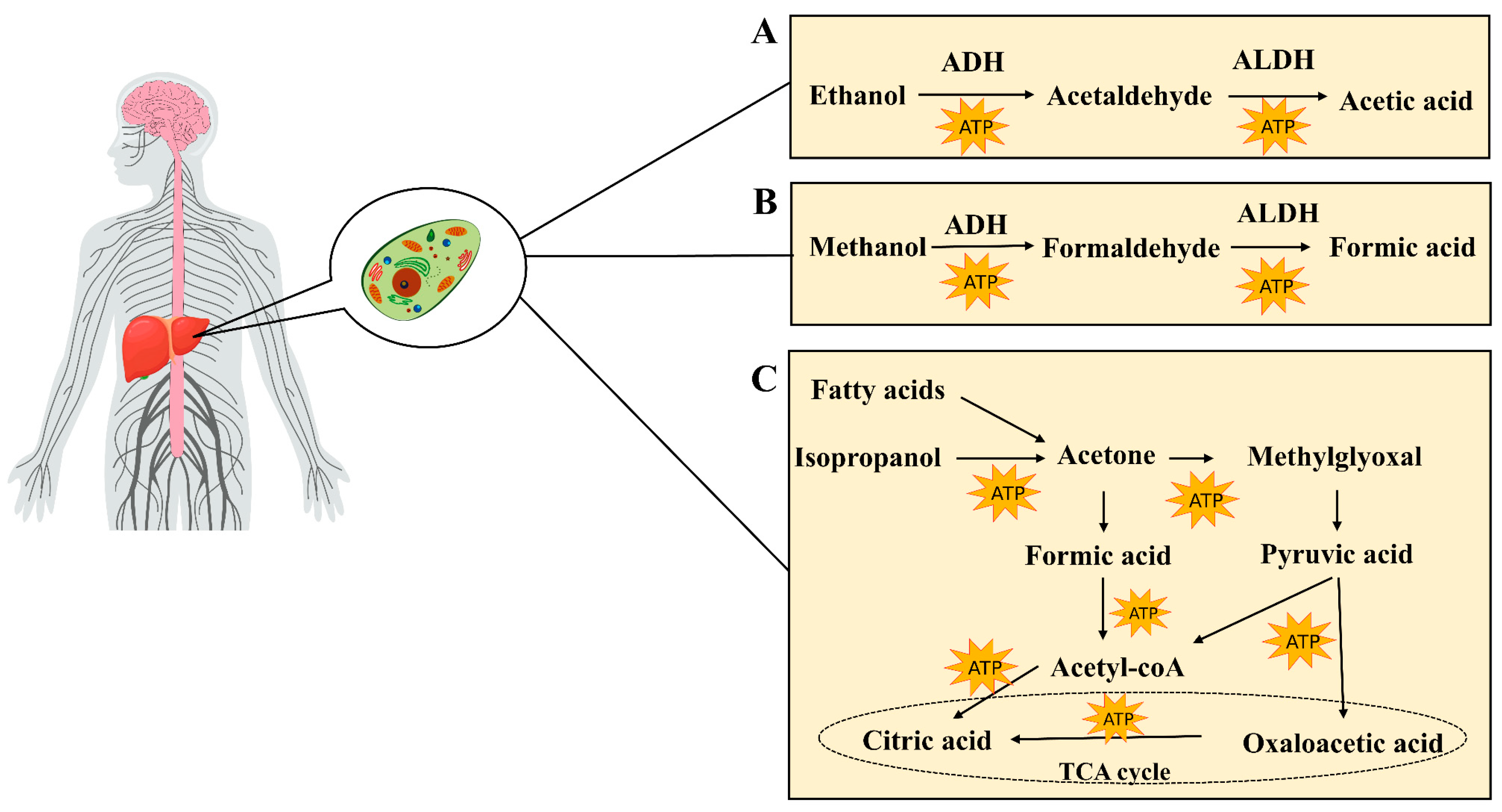
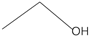
Most of the ethanol (80–90%) is absorbed in the gastric mucosa and intestine, and, upon entering the bloodstream, it is metabolized into acetaldehyde within the cytoplasm of liver cells via the action of alcohol dehydrogenase (ADH). Acetaldehyde is subsequently oxidized to acetic acid via the action of aldehyde dehydrogenase (ALDH). Ultimately, acetic acid is further metabolized to carbon dioxide and water, releasing energy (Figure 2A). This biochemical reaction generates substantial quantities of reactive oxygen species (ROS), thereby perturbing the hepatic homeostatic equilibrium. Such alterations may precipitate the onset of alcoholic liver disease (ALD) [57]. A minor proportion of ethanol, ranging from 10 to 20 percent, undergoes metabolism within hepatic microsomes via the cytochrome P450 enzyme system. The principal enzyme involved in this process is cytochrome P450 family 2 member E1 (CYP2E1) [58]. In addition, ethanol may also undergo metabolism through non-oxidative pathways. These include sulfation, where ethanol is converted to ethyl sulfate (EtS) by sulfate transferases (SULTs) [59]; glucuronidation, which uses uridine diphosphate (UDP)-glucuronosyltransferase (UGT) to combine ethanol with uridine diphosphate glucuronic acid, yielding ethyl glucuronic acid (EtG) [60]; phosphorylation reactions, which produce phospholipid ethanol (PEth) [61]; and esterification reactions, which give rise to the production of fatty acid ethyl esters (FAEEs) [62]. The enzymes involved in these non-oxidative metabolic pathways are primarily expressed in the liver, and their metabolites are generally water-soluble and can be excreted in the urine. These non-oxidative pathways are relatively less involved in the overall metabolism of ethanol, but their effects may become more pronounced in cases of excessive alcohol intake, helping to reduce the toxic accumulation of ethanol and its metabolites in the body. Clinically, the metabolites of non-oxidative metabolic pathways may also serve as biomarkers to help assess chronic or excessive alcohol intake, while the products of these pathways may contribute to organ-specific damage, such as the accumulation of FAEEs in the pancreas, which is associated with pancreatitis.
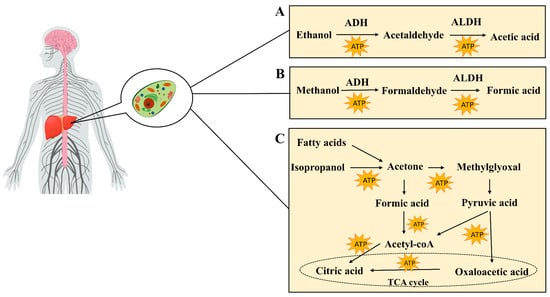
Figure 2.
(A) Metabolism of ethanol in the liver, including oxidative metabolism into acetaldehyde and acetic acid. (B) Metabolism of methanol in the liver, including oxidative metabolism into formaldehyde and formic acid. (C) Sources of acetone (e.g., fatty acid beta-oxidation and isopropanol metabolism), along with its metabolic pathways.
The concentration of acetone in exhaled breaths correlates directly with the levels of ketone bodies in the bloodstream. Within the human body, fatty acids are subjected to β-oxidation in the liver, leading to the production of ketone bodies. These ketone bodies primarily comprise acetone, acetoacetic acid, and β-hydroxybutyric acid. Additionally, the oxidation of isopropanol, which is primarily catalyzed by ADH, contributes to acetone levels in the body, as do external sources such as occupational exposure [63]. The metabolic pathways of acetone in the body (Figure 2B) include its conversion to compounds such as acetyl phosphoryl, formic acid, and methylglyoxal. Acetone can first be converted into acetyl phosphoric acid, which is further converted to formic acid and then to acetyl-CoA, entering the tricarboxylic acid cycle to produce energy. Concurrently, acetone can also be converted to methylglyoxal, which, in turn, forms D-lactic acid, eventually being converted to pyruvate, which can enter the TCA cycle or be converted to acetyl-CoA [64]. Due to insulin deficiency, fatty acids in diabetic patients are preferentially used as an energy source [65]. This makes respiratory acetone an ideal non-invasive biomarker for detecting DKA [66,67]. In non-diabetic individuals, respiratory acetone concentrations are typically below 1 ppm. In contrast, in well-managed patients with type 1 and type 2 diabetes, these levels are moderately elevated, generally remaining below 2 ppm. However, in the setting of DKA, respiratory acetone concentrations can increase significantly, ranging from 75 ppm to 1200 ppm, providing an important clinical basis for diagnosis [68].
In mammals, the gut microbiota is instrumental in methanol production [69,70]. These microorganisms produce methanol through their metabolic activity, which subsequently undergoes a series of enzymatic reactions in the liver (Figure 2C). First, methanol is oxidized into formaldehyde by alcohol dehydrogenase. Subsequently, the formaldehyde is further oxidized into formic acid by aldehyde dehydrogenase [71]. These metabolites, namely, formaldehyde and formic acid, can either be excreted in the urine or further metabolized into carbon dioxide, thereby completing the detoxification process. This continuous process of enzymatic oxidation is the primary mechanism by which methanol is removed in vivo. Thus, the presence of methanol in exhaled breaths serves as a non-invasive indicator for assessing the gut microbiota [72].
3. Application of Bio-Sniffers in VOC Detection
3.1. Electrical Bio-Sniffers
Electrical bio-sniffers produce a variety of electrical signals upon contact with VOCs. These bio-sniffers can be categorized into various types according to variations in their electrical signals, such as chemical resistors, conductivity bio-sniffers, and electrochemical bio-sniffers (Table 3) [73]. Electrical sensors usually require periodic calibration to maintain high sensitivity and may face reproducibility problems in high-temperature and high-humidity environments. Their specificity is mainly dependent on selective coatings or surface modifications to nanomaterials, but their ability to discriminate between complex VOC mixtures is limited.
3.1.1. Resistance Bio-Sniffers
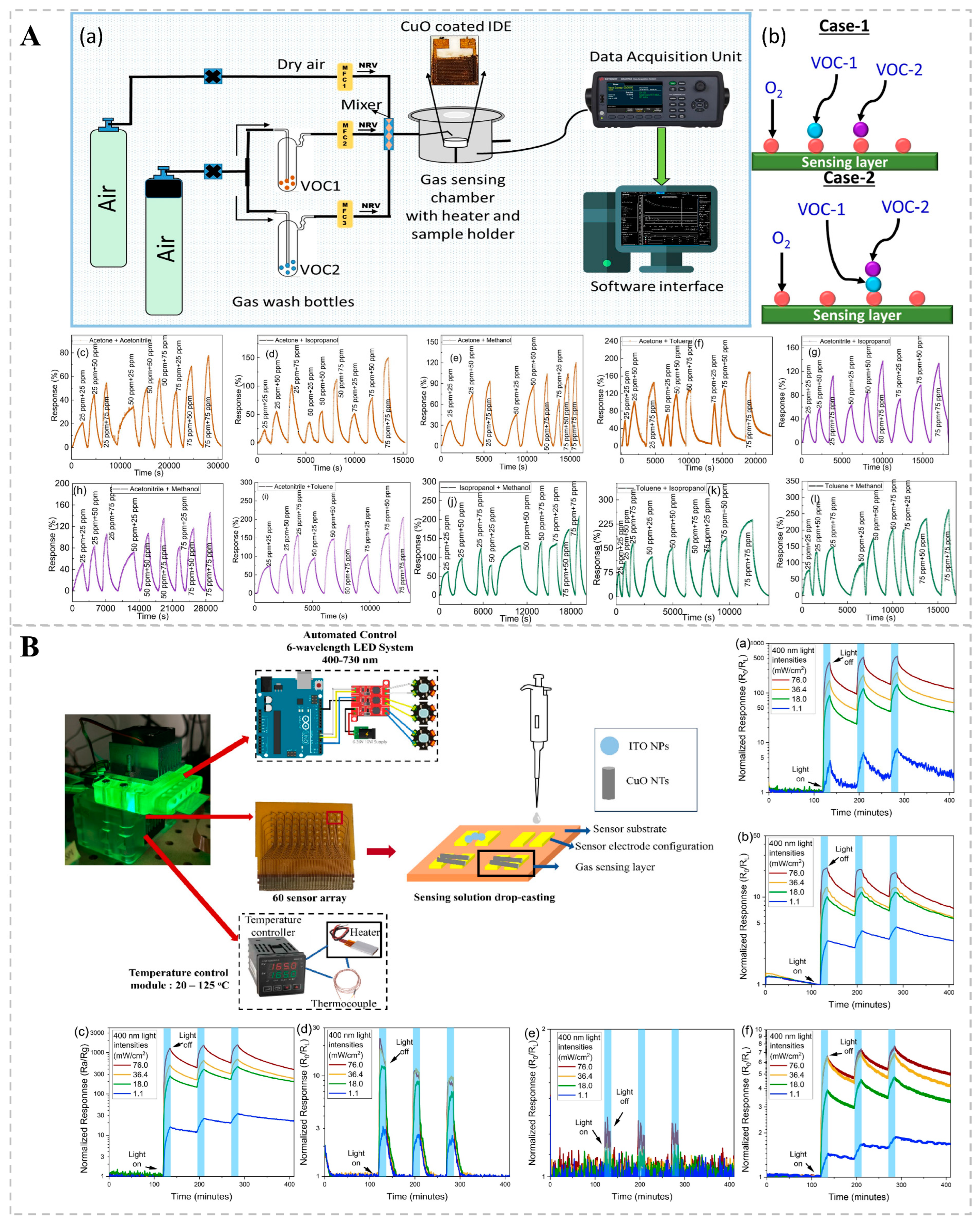
Resistance-based bio-sniffers are extensively utilized in gas detection owing to their simplicity, affordability, and user-friendliness. A resistance bio-sniffer contains a sensitive material, typically a semiconductor, the resistance of which changes in certain environmental conditions, such as gas concentration. When the target gas comes into contact with a sensitive material, physical or chemical adsorption occurs, altering the charge carrier (electron or hole) concentration on the surface of the material, thereby changing the resistance of the material. A chemo-resistive bio-sniffer synthesized by Saraswati Kulkarni’s team using 2D CuO (copper dioxide) nanosheets via hydrothermal synthesis exhibited good catalytic activity (Figure 3A). During operation, oxygen adsorbed onto the surface of CuO, forming reactive oxygen species that interacted with each other to cause electron transfer, changing the bio-sniffer’s resistance and enabling VOC detection. The RF classification algorithm was then used to classify the dynamic response of CuO nanosheets to VOC mixtures, achieving an accuracy of 93.8%. This high accuracy demonstrates that the method is effective in distinguishing and quantifying the individual components in the VOC mixture [74]. A major limitation of chemical resistance gas bio-sniffers based on metal oxides (MOS) is the necessity for high operating temperatures, which range from 250 to 450 °C. This requirement results in substantial power consumption and reduced lifespans. Suporna Paul and her colleagues, however, managed to detect VOCs at ambient temperatures by employing UV light excitation (Figure 3B). The sensing capabilities of various MOS nanostructures (e.g., SnO2, In2O3, ZnO, WO3, CuO, and ITO) for VOCs were compared with and without 400 nm UV illumination. It was found that WO3 nanoparticles could increase the response value of p-ethylbenzene and xylene under UV light excitation, and the light response of SnO2 increased by approximately 83 times with increasing UV light intensity. This method enables highly sensitive detection without the need for heating, thereby reducing energy consumption and potentially extending the bio-sniffer’s functional lifetime [75].
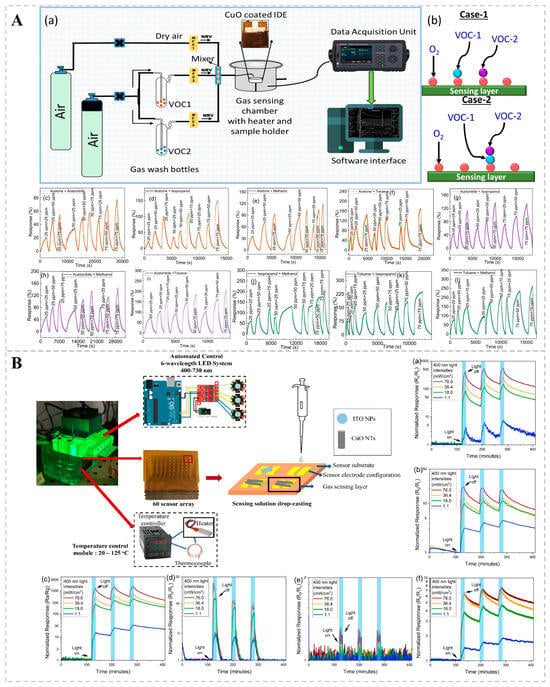
Figure 3.
(A) Detection of VOC mixtures by a chemo-resistive bio-sniffer based on 2D CuO nanosheets. (a) Schematic diagram of the sensing setup utilized for analyzing binary mixtures of VOCs. (b) Illustrations of Case-1 and Case-2 within the Eley–Rideal mechanism. (c–l) Dynamic response maps for 10 binary mixtures of 5 VOCs at 300 °C. Reprinted from Ref. [74]. (B) Enhanced gas sensing performance of metal-oxide semiconductor chemo-resistors at room temperature using ultraviolet excitation. Transient optical responses of (a) SnO2, (b) In2O3, (c) ZnO, (d) WO3, (e) CuO, and (f) ITO under varying light intensities (1.1, 18.0, 36.4, 76.0 mW/cm2) during UV irradiation at 400 nm. Reprinted from Ref. [75].
3.1.2. Conductivity Bio-Sniffers
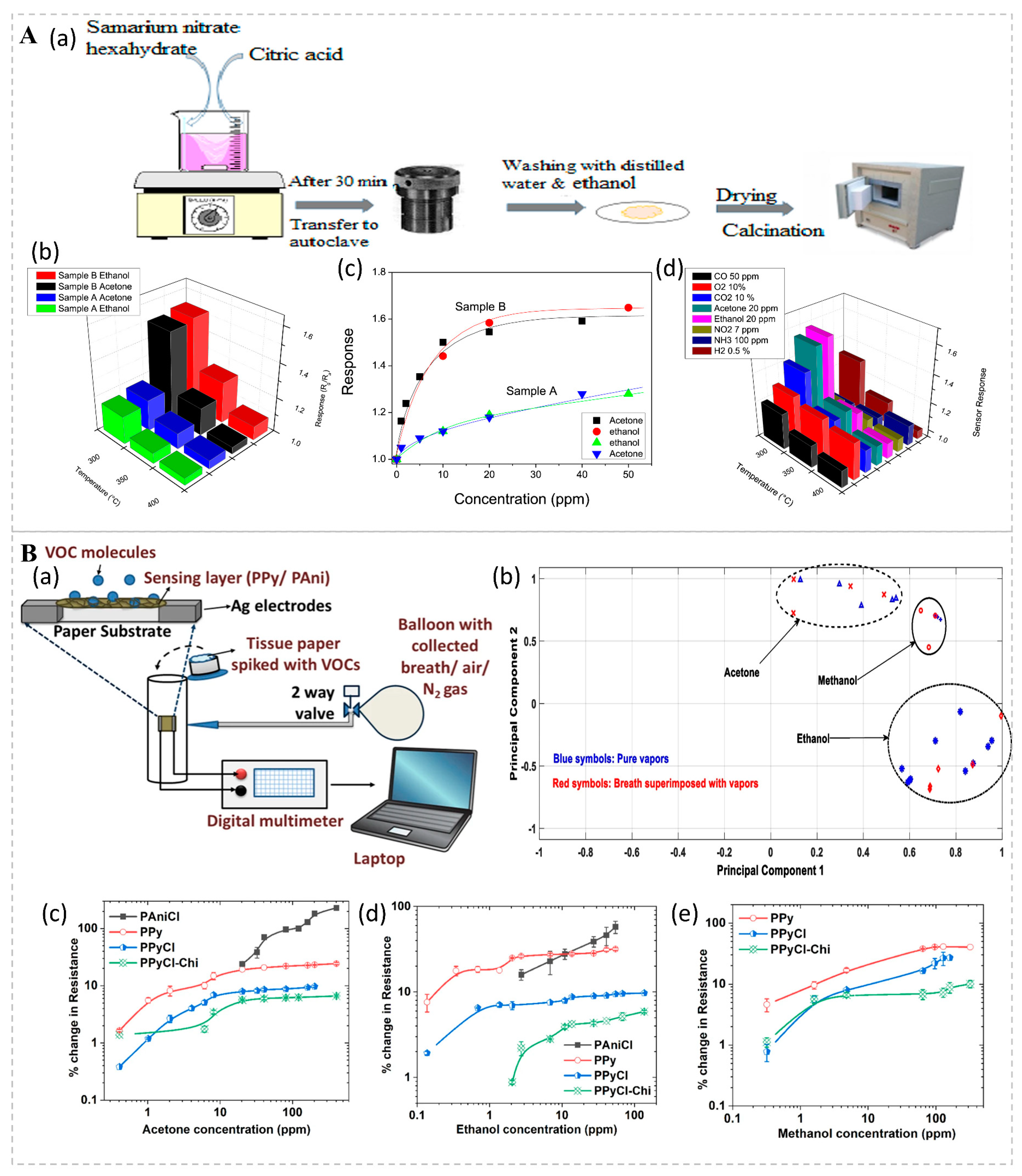
Conductivity bio-sniffers rely on alterations in the material’s conductivity, which can be caused by the migration of ions, the movement of electrons, or changes in the material’s structure. Jamnani et al. developed conductivity bio-sniffers for VOC detection based on samarium oxide (Sm2O3) nanoparticles (Figure 4A). Upon introduction, target gases undergo chemical adsorption onto the bio-sniffer’s surface, initiating reactions with surface reactive oxygen species ions. This process liberates electrons into the bulk Sm2O3 material, diminishing the concentration of majority carriers (holes) and thereby augmenting the bio-sniffer’s resistance. Research findings demonstrate that the hierarchical self-assembly structure of Sm2O3 affords an enlarged specific surface area and a heightened number of active sites, both of which contribute to enhanced gas adsorption and reaction efficiency. Owing to its high sensitivity, rapid response, exceptional selectivity, robust stability, and economic viability, it has demonstrated significant potential in the realm of VOC monitoring [76]. VOCs are usually physically adsorbed onto conductive polymers (p-type semiconductors) in ambient conditions, leading to the expansion of the polymer matrix and hindering the electronic transition process, thereby reducing the conductivity of the conductive polymers and increasing their resistance. Mondal studied a chemo-resistive filter paper bio-sniffer based on a conductive polymer coating, with detection thresholds of 400 ppb for acetone, 150 ppb for ethanol, and 300 ppb for methanol, all of which were detected within 2 min (Figure 4B). When exposed to the same concentrations of VOCs multiple times, the deviation of the resistance change is less than 10%, indicating good stability and repeatability. The bio-sniffer reduces the risk of cross-contamination by replacing the filter paper, making it easy to carry and deploy, and offers significant potential and advantages for future development [77].
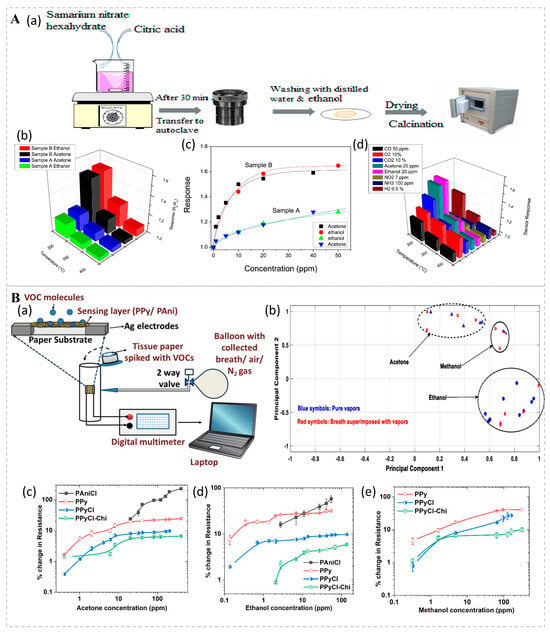
Figure 4.
(A) (a) Schematic illustration of the synthesis process for Sm2O3 nanoparticles. (b) Sensor responses to acetone and ethanol across a range of operating temperatures from 300 to 400 °C. (c) Calibration curves for sensors A and B in response to low concentrations of ethanol and acetone at 300 °C. (d) Selective response of sensor B to various gases at 300 °C. Reprinted from Ref. [76]. (B) (a) Schematic diagram of the experimental setup for vapor sensing. (b) Principal component analysis (PCA) plots for various VOCs in their pure forms (blue symbols) and when superimposed with breath samples (red symbols), demonstrating distinct clusters for each VOC. Sensor responses to (c) acetone, (d) ethanol, and (e) methanol vapors. Lines are included for visual guidance. Reprinted from Ref. [77].
3.1.3. Cyclic Voltammetry Bio-Sniffers
Electrochemical bio-sniffers oxidize or reduce VOC molecules through working electrodes in electrochemical cells, which pass through the electrolyte layer (which may be solid, liquid, or gaseous ionic conductors) to the electrode surface [56]. The operational mechanism of the bio-sniffer hinges on the electrochemical reactions occurring at the electrode surface, which entail electron transfer. In electrochemical sensors, the target gas undergoes an oxidation or reduction reaction at the working electrode (or sensing electrode) that produces or consumes electrons, resulting in an electric current. This current is supplied to the sensor through the counter electrode (or counter electrode) to complete the circuit. The resultant current is directly proportional to the concentration of VOCs [78,79]. Electrochemical bio-sniffers can be amperometric, to monitor redox currents, or potentiometric, to monitor potential changes. In addition, cyclic voltammetry (CV) is a commonly used technique in electrochemical bio-sniffers to study the kinetics and mechanisms of electrode reactions [80,81].
Researchers have developed an electrochemical nasal system (ZENose), based on bipyridine encapsulated in ZIF-8, for detecting ammonia levels in the breath with high sensitivity and specificity, aiding in the monitoring of health conditions related to kidney function and protein metabolism pathways (Figure 5A). The electrochemical characterization of Fc@ZIF-8 nanocomposites by CV, dual-potentiometric chronoamperometry, and chronocoulometric methods confirmed their effectiveness in detecting ammonia gas down to 400 ppb. The experimental results showed that Fc@ZIF-8 had a clear response to ammonia, which was about three times more specific than the non-specific signal of cross-reactive gases. This electrochemical bio-sniffer provides a potentially non-invasive approach for the early diagnosis of chronic kidney disease [82]. Alcohols undergo electrocatalytic oxidation on the surface of Ptm@Auto nanoparticles, forming intermediates that are further oxidized to carbon dioxide (CO2). The platinum-coated octahedral gold (Au) nanoparticles (Ptm@Auto) electrochemical bio-sniffer, specially designed by Ma et al., can detect and differentiate between alcohol congeners such as methanol, ethanol, and isopropanol. It differentiates between alcohol molecules by measuring the oxidation peaks in the CV, which show different oxidative behaviors at specific potentials (Figure 5B). Pt0.8@Auto nanoparticles exhibited excellent electrochemical activity, and Pt0.8@Auto NPs catalyzed the electrocatalytic oxidation of methanol, ethanol, and isopropanol in a 1.0 M KOH solution. After 100 cycles, the normalized currents of Pt0.8@Auto NPs in methanol, ethanol, and isopropanol were maintained at 69.6%, 68.7%, and 112.9%, respectively, demonstrating good stability and repeatability. Furthermore, the bio-sniffer has been successfully integrated into wearable gloves with good mechanical properties and repeatability; these are capable of withstanding 1500 bending or stretching cycles, which is a significant advantage for convenient real-time detection in the future [83].
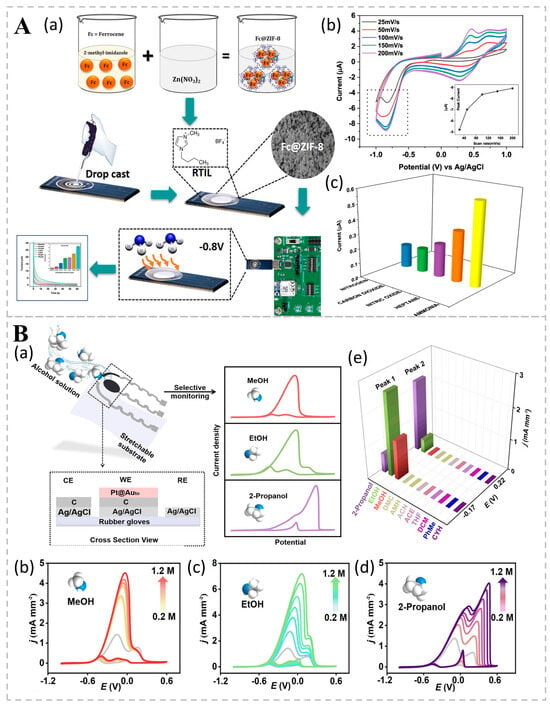
Figure 5.
(A) A ZIF-8 packaged bipyridine-based electrochemical nasal system for highly sensitive and specific detection of ammonia in human breath. (a) Schematic illustration of the optimized preparation method. (b) CV results for the SENCE device from −1 to +1 V, with increasing scan rates from 25 to 250 mV/s for ammonia sensing at 400 ppb. The inset shows the peak current plotted against the scan rate, validating the Randles–Sevcik equation and demonstrating linearity in the anodic peak current. (c) Selective sensing response of the Fc@ZIF-8-modified electrode for the detection of various gases and volatile chemical compounds, including nitrogen, carbon dioxide, nitric oxide, heptane, and ammonia. Reprinted from Ref. [82]. (B) Electrochemical bio-sniffer based on Pt-coated octahedral Au nanoparticles for the differentiation of different alcohol congeners. (a) Schematic representation of flexible Ptm@Auto-based alcohol bio-sniffers. The selective monitoring of alcohols is achieved through distinct CV curve shapes. CV curves for varying concentrations of (b) methanol (MeOH); (c) ethanol (EtOH); and (d) 2-propanol within the concentration range of 0.2–1.2 M in mixtures, in the presence of 1 M MeOH, EtOH, and 2-propanol, respectively. The gray lines in (b–d) represent the original curves in 1.0 M MeOH, EtOH, and 2-propanol. (e) Selectivity performance of alcohol sensors among methanol, ethanol, 2-propanol, dimethyl carbonate (DMC), ammonia monohydrate (AMH), acetonitrile (ACN), acetone (ACE), tetrahydrofuran (THF), dichloromethane (DCM), toluene (PhMe), and cyclohexane (CYH). The concentration of VOCs was 1.0 M, with applied potentials of −0.22 V and 0.17 V for peak 1 and peak 2, respectively. Reprinted from Ref. [83].
Figure 5.
(A) A ZIF-8 packaged bipyridine-based electrochemical nasal system for highly sensitive and specific detection of ammonia in human breath. (a) Schematic illustration of the optimized preparation method. (b) CV results for the SENCE device from −1 to +1 V, with increasing scan rates from 25 to 250 mV/s for ammonia sensing at 400 ppb. The inset shows the peak current plotted against the scan rate, validating the Randles–Sevcik equation and demonstrating linearity in the anodic peak current. (c) Selective sensing response of the Fc@ZIF-8-modified electrode for the detection of various gases and volatile chemical compounds, including nitrogen, carbon dioxide, nitric oxide, heptane, and ammonia. Reprinted from Ref. [82]. (B) Electrochemical bio-sniffer based on Pt-coated octahedral Au nanoparticles for the differentiation of different alcohol congeners. (a) Schematic representation of flexible Ptm@Auto-based alcohol bio-sniffers. The selective monitoring of alcohols is achieved through distinct CV curve shapes. CV curves for varying concentrations of (b) methanol (MeOH); (c) ethanol (EtOH); and (d) 2-propanol within the concentration range of 0.2–1.2 M in mixtures, in the presence of 1 M MeOH, EtOH, and 2-propanol, respectively. The gray lines in (b–d) represent the original curves in 1.0 M MeOH, EtOH, and 2-propanol. (e) Selectivity performance of alcohol sensors among methanol, ethanol, 2-propanol, dimethyl carbonate (DMC), ammonia monohydrate (AMH), acetonitrile (ACN), acetone (ACE), tetrahydrofuran (THF), dichloromethane (DCM), toluene (PhMe), and cyclohexane (CYH). The concentration of VOCs was 1.0 M, with applied potentials of −0.22 V and 0.17 V for peak 1 and peak 2, respectively. Reprinted from Ref. [83].

Table 3.
Electro-bio-sniffer for the detection of VOCs.
Table 3.
Electro-bio-sniffer for the detection of VOCs.
| Sensing Methods | Materials | VOCs | Performance | Results | Ref. |
|---|---|---|---|---|---|
| Resistance | CuO | Toluene, methanol, isopropanol, acetonitrile, and acetone | Accurately and selectively detect volatile organic compound mixtures | 93.8% accuracy rate | [74] |
| Resistance | MOS | Ethylbenzene and xylene | High sensitivity and long service life | Different nanoparticles are able to increase the response value under UV light excitation | [75] |
| Conductance | Sm2O3 | Ethanol and acetone | High sensitivity, fast response, high selectivity, and stability | The tested bio-sniffer exhibits high sensitivity to low concentrations of ethanol and acetone, and shows a fast response to 20 ppm of ethanol | [76] |
| Conductance | Conductive polymers | Acetone, ethanol, and methanol | Good stability and repeatability | 400 ppb for acetone, 150 ppb for ethanol, 300 ppb for methanol, and less than 10% of deviation in resistance change | [77] |
| CV | Fc@ZIF-8 | Ammonia | High sensitivity and specificity | Fc@ZIF-8 has a clear response to ammonia, which is about 3 times more specific than the non-specific signal of cross-reactive gases | [82] |
| CV | Ptm@Auto | Alcohol homologs | Good stability and repeatability | The normalized currents of Pt0.8@Auto NPs in methanol, ethanol, and isopropanol remained at the initial values of 69.6%, 68.7%, and 112.9%, respectively | [83] |
3.2. Optical Bio-Sniffers
Optical bio-sniffers constitute a diverse category of instruments designed to discern chemical interactions through the measurement of radiation intensity across infrared, visible, and ultraviolet spectra [84]. Table 4 summarizes the most common optical sensing methods for VOCs: colorimetric, fluorescent, and optical fiber bio-sniffers. Fluorescent sensors have high specificity and can achieve the detection of specific VOCs with selective fluorescent probes. However, their long-term stability may be affected by fluorescence bursts and the periodic replacement of fluorescent probes is required to maintain performance.
3.2.1. Colorimetric Bio-Sniffers
The dyes or indicators in colorimetric bio-sniffers react with specific chemicals, such as VOCs in gases or liquids, causing changes to their molecular structure. This structural change causes a shift in the dye’s absorption spectrum, altering its color [85]. Hee Nah’s team dispersed silica nanoparticles (NPs) in photo-crosslinked monomers and fabricated photonic crystals on silicon wafers using spin-coating techniques, providing a simple and scalable method for preparing structural colors. Through patterning and PCA analysis, VOCs could be identified and differentiated, reducing the data dimension and making the results easier to interpret. The results were rapid and showed high sensitivity, with color changes occurring rapidly within 1 min of VOC exposure, with detection limits of 1 ppm for acetone, 0.1 ppm for ethanol, and 0.02 ppm for acetic acid [86]. Some studies have used PVDF and copper metal–organic frameworks (Cu MOFs), graphene aerogels (GAs), and dyes as response materials to construct bio-sniffer arrays. In one study, 28 VOCs were placed in contact with a bio-sniffer array in a sealed reaction chamber to generate specific recognition patterns or finger-maps through intermolecular interactions. Using a smartphone to take images before and after the response of the bio-sniffer array, the changes in ΔR, ΔG, and ΔB were obtained by extracting the RGB values (the values of the red, green, and blue color channels). This convenient bio-sniffer, which could be monitored in real time with a smartphone, had high sensitivity, high selectivity, and good stability. Using methods such as PCA, LDA, and HCA, the bio-sniffer array was able to successfully distinguish between 28 VOCs and achieved 100% accuracy for LDA analysis at concentrations of 100 μM and 300 μM, all of which were detected in less than 15 min [87].
3.2.2. Fluorescence Bio-Sniffers
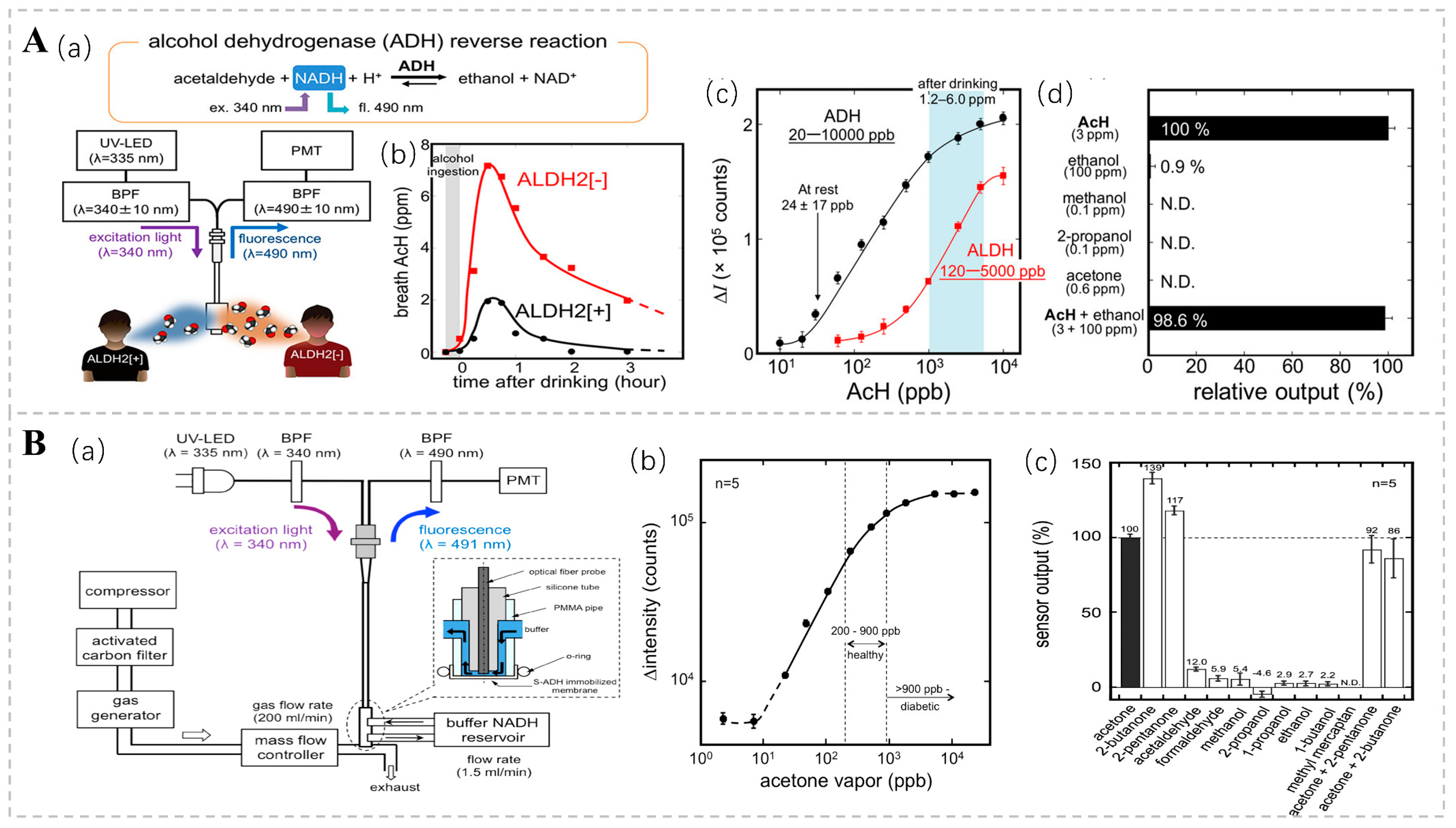
Fluorescence bio-sniffers possess the ability to detect signals based on a diverse array of fluorescence properties, encompassing fluorescence intensity, anisotropy, lifetime, emission and excitation spectra, fluorescence quenching, and quantum yield [88]. Acetaldehyde (AcH) is an intermediate product of ethanol metabolism, and Koji T. and his team used an ethanol bio-sniffer to detect acetaldehyde in exhaled gas using the reverse reaction of ADH. In this reaction, ADH reduces acetaldehyde to ethanol and consumes the coenzyme nicotinamide adenine dinucleotide (NADH). Given that NADH exhibits distinctive fluorescence at 490 nm upon excitation with UV light at 340 nm, the quantification of acetaldehyde concentration can be achieved by monitoring alterations in NADH fluorescence intensity (Figure 6A). This approach boasts high sensitivity and specificity, with a dynamic detection range spanning from 20 ppb to 10 ppm, thereby encompassing the acetaldehyde levels observed in both healthy individuals and post-alcohol consumption [89]. There is a correlation between the concentration of acetone in exhalation and the concentration of blood glucose [90]. In diabetic patients, acetone concentrations are markedly elevated compared to those in healthy individuals, with levels sometimes surpassing 900 ppb [91]. Consequently, the acetone present in exhaled breath is regarded as a prospective non-invasive biomarker for diabetes diagnosis. Ming Ye and colleagues engineered a graphene-based bio-sniffer utilizing NADH-dependent secondary alcohol dehydrogenase (S-ADH) [92]. Among these, the UV-LED excitation system stands out for its simplicity and compactness, low power consumption, and minimal heat generation. These features provide enhanced safety and controllability compared to conventional ultraviolet lamps. The fluorescence intensity was shown to be linearly correlated with acetone concentrations in the range of 20 to 5300 ppb (Figure 6B).
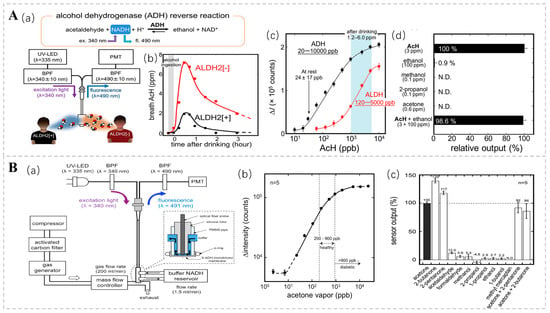
Figure 6.
(A) (a) Experimental setup of the AcH bio-sniffer, featuring the UV-LED, PMT, and optical fiber probe. The inset illustrates the structure of the flow cell. (b) Temporal profile of breath AcH concentration following alcohol ingestion, showing a nearly threefold difference between ALDH2 [+] subjects (●) and ALDH2 [−] subjects (■). (c) Comparison of the dynamic range of the AcH bio-sniffer with ADH (●) and ALDH (■). The typical post-drinking AcH concentration ranges from 1.2 ± 0.8 to 6.0 ± 3.0 ppm. (d) Selectivity of the AcH bio-sniffer towards common breath components at typical post-drinking concentrations. Reprinted from Ref. [89]. (B) (a) Experimental setup for evaluating the fiber-optic acetone bio-sniffer’s performance. (b) Calibration curve of the acetone bio-sniffer in reaction to acetone vapor, where fluorescence intensity is proportional to acetone vapor concentrations from 20 to 5300 ppb. (c) Gas selectivity of the S-ADH bio-sniffer, showing responses of 139% and 117% to 2-butanone and 2-pentanone, respectively, with lower responses to other chemicals. Reprinted from Ref. [92].
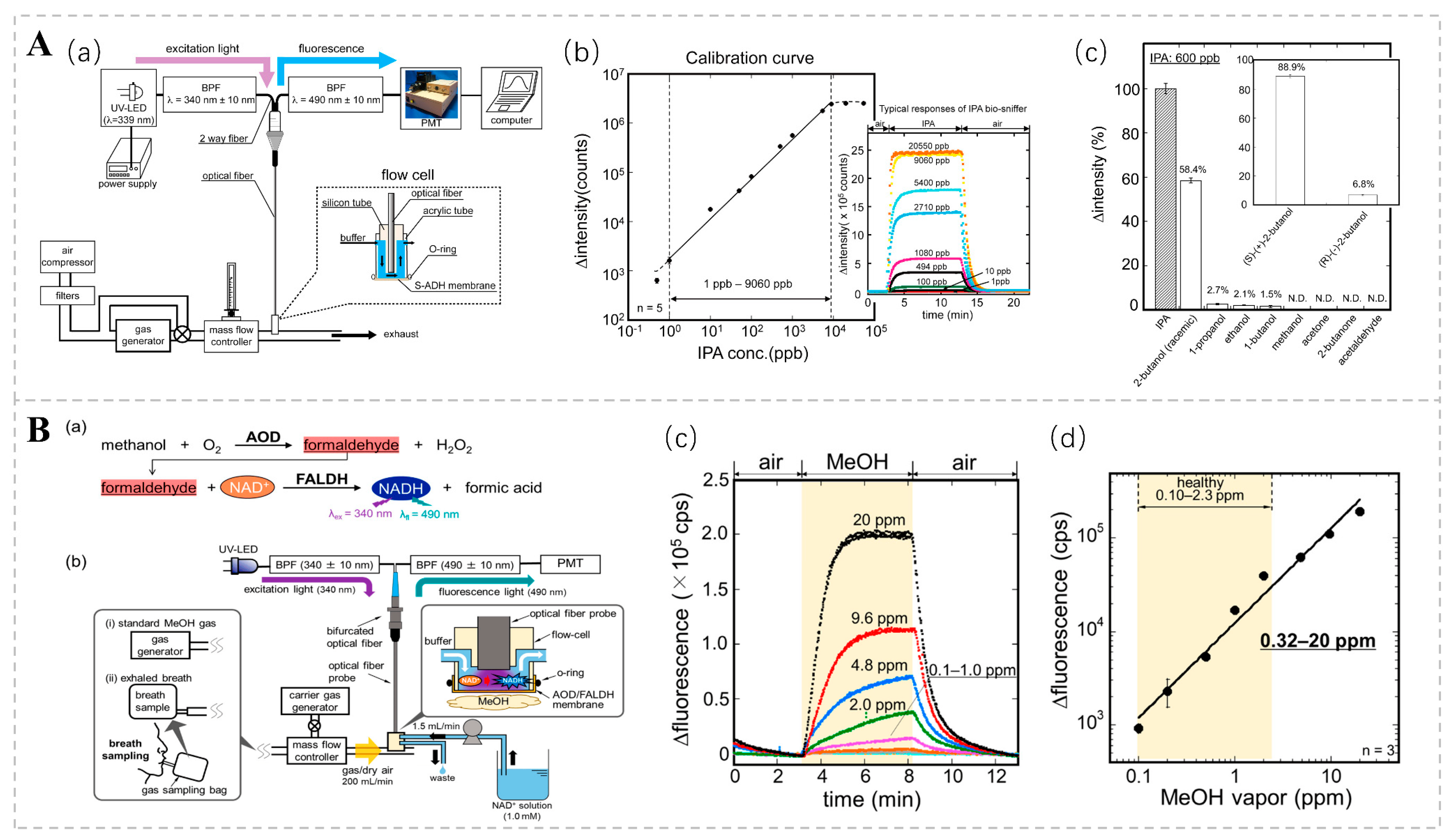
Concentrations of isopropyl alcohol (IPA) in the breath may be correlated with an array of pathologies. According to Hanouneh et al., patients with liver disease had higher levels of exhaled IPA than healthy individuals [53]. IPA levels have been shown to predict the development of breast cancer [93]. Furthermore, there is an observed trend of elevated IPA concentrations in the exhaled breath of individuals suffering from pulmonary hypertension [94]. Several studies have also shown that expiratory concentrations of IPA may be higher in patients with lung cancer [95,96,97]. In addition to respiratory IPA, some studies have found an increase in the blood concentrations of IPA in patients with ketoacidosis [98]. Koji T., in conjunction with his research group, engineered an isopropanol bio-sniffer characterized by its high sensitivity and selectivity, expansive dynamic range, and capacity for real-time monitoring [99]. The sniffer utilizes an immobilized S-ADH to catalyze an enzymatic reaction that oxidizes IPA to acetone and simultaneously generates NADH. The concentration of IPA can be indirectly ascertained by examining the fluorescence characteristics of NADH. The detection range spanned from 1 ppb to 9060 ppb, covering the range of respiratory IPA concentrations in healthy individuals and patients with certain diseases (Figure 7A). The research team further refined the bio-sniffer by incorporating a C8855 photomultiplier tube (PMT) to augment fluorescence collection. Additionally, they expanded the area of the enzyme-fixation film in contact with the air sample. These modifications led to a notable reduction in the detection limit to 0.5 ppb, a substantial improvement over the previous limit of 1.0 ppb [100].
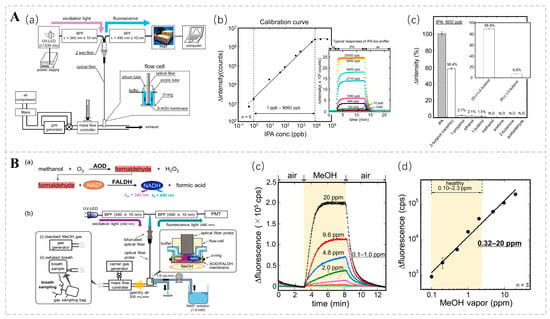
Figure 7.
(A) (a) Diagrammatic representation of the isopropanol (IPA) bio-sniffer. (b) Calibration curve for the isopropanol (IPA) bio-sniffer. (c) Selectivity of the S-ADH evaluation by bio-sniffer. Reprinted from Ref. [99]. (B) (a) Measurement principle and (b) schematic diagram of the MeOH bio-sniffer. (c) Sensor responses to varying concentrations of MeOH vapor. (d) Calibration curve of the bio-sniffer for MeOH vapor. Reprinted from Ref. [101].
Moreover, the presence of MeOH in exhaled breaths serves as a non-invasive biomarker for evaluating the composition and activity of the gut microbiota. Although significant progress has been made in the bio-sniffer detection of methanol, achieving highly selective measurements remains challenging. To address this challenge, Koji Toma and colleagues devised a method for detecting methanol in the gas phase by employing a tandem enzymatic reaction involving alcohol oxidase (AOD) and formaldehyde dehydrogenase (FALDH). This coupled reaction results in the generation of the reduced form of NADH; the concentration of methanol vapor is determined by measuring the autofluorescence of NADH. The dynamic measurement range of this system spans from 0.32 to 20 ppm, encompassing the range of exhaled methanol concentrations observed in healthy individuals (Figure 7B). This methanol bio-sniffer, which leverages a cascade reaction, represents a robust non-invasive approach for detecting intestinal microbiota [101].
3.2.3. Fiberoptic Bio-Sniffers
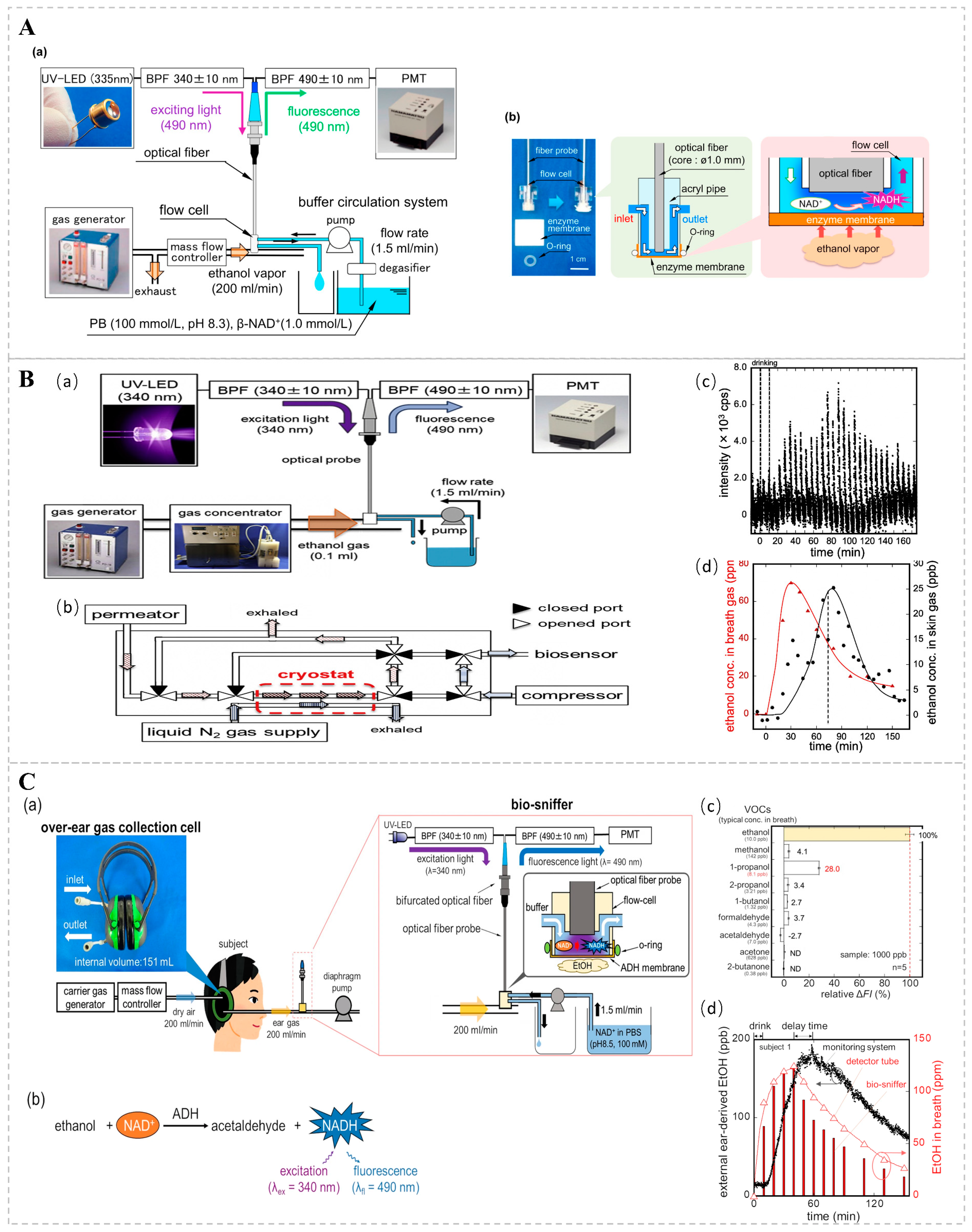
Optical bio-sniffers examine the effects of analytes on intact cells, primarily, microorganisms. Optical detection is performed using an indicator (pH or oxygen) or the optical properties of the cell itself, such as fluorescence or bioluminescence, which occur or disappear [85,102]. One study suggests that respiratory ethanol may be associated with hepatic steatosis [103]. Existing studies typically use an ethanol dehydrogenase (ADH)-based bio-sniffer, which uses ADH-catalyzed ethanol oxidation to produce acetaldehyde and reduced nicotinamide adenine dinucleotides (NADH), quantifying ethanol by detecting fluorescence changes in NADH. Building on these findings, Koji T. and colleagues engineered a fiber-optic bio-sniffer and assembled an ethanol gas bio-sniffer utilizing a flow cell to facilitate the continuous quantification of ethanol vapor (Figure 8A). During the experimental procedure, a buffer solution enriched with 1.0 mmol/L NAD+ was continuously infused into the flow cell at a rate of 0.5 mL/min. As ethanol molecules traversed the cell and underwent oxidation on the porous membrane, the coenzyme NADH was generated. The resultant fluctuations in NADH fluorescence intensity were captured by an optical fiber, thereby permitting the assessment of ethanol vapor concentrations. Moreover, the team conducted continuous monitoring of ethanol vapor concentrations on human skin post-alcohol ingestion, spanning a range from 25 ppb to 128 ppm, thereby underscoring the system’s high sensitivity [104]. Takahiro A. and his research group further refined the system by integrating a gas concentrator, meticulously optimizing the length, inner diameter, and flow rate of the buffer solution within the gas conveyance conduit. This enhancement led to a marked amplification in the system’s sensitivity and responsiveness. Compared to Koji T.’s method, this system adds a gas concentrator to concentrate its VOC components to a volume of 0.1 mL by cooling the sample gas with liquid nitrogen (Figure 8B). Through the optimization of the measurement system, the diffusion of low-concentration gases was effectively suppressed, and the results showed that ethanol gases could be measured within a range of 1–3100 ppb with low detection limits [105]. The team also found that the sweat glands of the outer ear interfered with the bio-sniffer signal very little; therefore, they developed an over-the-ear gas collection unit for collecting gas from the outer ear, thereby improving the stability and reliability of the signal by optimizing the bio-sniffer parameters (Figure 8C). During the monitoring of gases originating from the external ear, the subject’s sample gas is captured by an over-ear gas collection cell and promptly conveyed to a detector for immediate analysis. The monitoring system has the capability for continuous measurements, with a dynamic range of 26 ppb to 554 ppm, and exhibits high selectivity for ethanol, suggesting that the outer ear is suitable for the non-invasive monitoring of ethanol in VOCs [106].
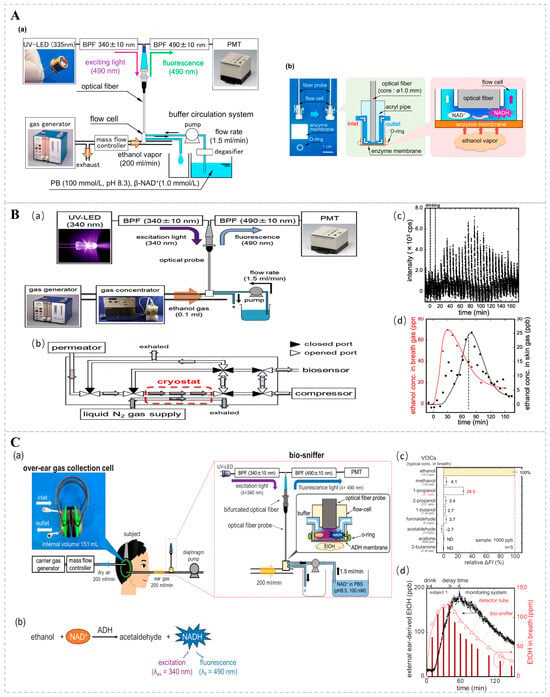
Figure 8.
(A) Diagrammatic representation of an ADH-immobilized biosensor for ethanol vapor detection. (a) The experimental configuration of the ethanol biosensor, employing a UV light-emitting diode (UV-LED) for excitation. (b) An image of the biosensor and a schematic of the flow cell, which comprises an enzyme-coated membrane and an optical fiber probe. Reprinted from [104]. (B) Schematic representation of a skin ethanol vapor measurement system utilizing a bio-sniffer and a gas concentrator. (a) System for measuring skin gas with a gas concentrator. (b) Internal structure of the gas concentrator and the flow path during concentration. (c) Signal corresponding to ethanol in skin gas detected at the wrist using the skin gas measurement system. (d) Ethanol concentrations in skin gas derived from peak values and ethanol concentrations in exhaled air, determined using a gas detector tube. Reprinted from Ref. [105]. (C) (a) Schematic diagram of the monitoring system for ethanol derived from the external ear. The system consists of an over-ear gas collection cell and a bio-sniffer. (b) Principle of ethanol measurement utilizing ADH and NADH. (c) Relative sensor output in response to representative VOCs found in the breath. (d) Monitoring of ethanol concentration in ear gas following alcohol consumption with the developed system for subject 1. Symbols represent ethanol concentration in ear gas (●) in the breath, measured by detector tubes (Δ) and by the ethanol bio-sniffer (bars). Reprinted from Ref. [106].

Table 4.
Reports common optical bio-sniffers for the detection of VOCs.
Table 4.
Reports common optical bio-sniffers for the detection of VOCs.
| Sensing Methods | VOCs | Performance | Related Applications | Detection Range | Ref. |
|---|---|---|---|---|---|
| Colorimetric | Acetone, ethanol, and acetic acid | Results are provided fast and show high sensitivity | - | 1 ppm, 0.1 ppm, 0.02 ppm | [86] |
| Colorimetric | 28 VOCs | High sensitivity, high selectivity, and good stability | Efficiently differentiated between 5 human cancer cells and 2 normal human cells. | – | [87] |
| Luminous | Acetaldehyde | Has high sensitivity and specificity | Assess alcohol metabolism | 20 ppb–10 ppm | [89] |
| Luminous | Acetone | Low power consumption and heat generation | Screening for diabetes | 20 ppb–5300 ppb | [92] |
| Luminous | Isopropanol | High sensitivity and selectivity, wide dynamic range and real-time monitoring capability | Screening for type 2 diabetes | 1 ppb–9060 ppb | [99] |
| Luminous | Methanol | High sensitivity | Assess the intestinal microbiota | 0.32–20 ppm | [101] |
| Optic fiber | Ethanol | High sensitivity | Assess alcohol metabolism | 25 ppb–128 ppm | [104] |
| Optic fiber | Ethanol | High sensitivity | Assess alcohol metabolism | 1 ppb–3100 ppb | [105] |
| Optic fiber | Ethanol | High sensitivity | Assess alcohol metabolism | 26 ppb–554 ppm | [106] |
4. Challenges and Prospects
When exploring the application of bio-sniffers for monitoring VOCs in medical diagnostics, we face a number of challenges. Firstly, technical sensitivity and selectivity are among the main challenges facing current monitoring technologies. Although electrical and optical bio-sniffers have made some progress in detecting VOCs, their selectivity still needs to be improved. To enhance the detection of specific VOCs, researchers are exploring more efficient pre-concentration techniques and more sensitive bio-sniffers. In addition, the selectivity and sensitivity of VOC detection can be enhanced by establishing a more precise correlation between VOC detection outcomes and the presence of diseases. This enhancement can be achieved by identifying more characteristic VOC targets, thereby expanding the range of diseases that can be detected [84]. Furthermore, environmental factors, including fluctuations in humidity and temperature, have the potential to impact the performance of bio-sniffers, thereby causing inaccuracies in the measurement results. Temperature fluctuations significantly influence the performance of bio-sniffers, as both the electrode reaction rate in electrochemical sensors and the fluorescence intensity in optical sensors are affected by temperature changes, resulting in biased detection outcomes. The development of sensors capable of stable operation across a broad temperature range is essential. For instance, utilizing high-temperature stable materials (e.g., ceramic substrates) and sensors with low thermal coefficients can mitigate the effects of temperature fluctuations on performance. Therefore, improving the immunity of the bio-sniffer to environmental changes is an important research direction. Stability against humidity changes can be realized through the use of hydrophobic substrates with porous micro-nano structures [45]. At the same time, due to the complexity of VOCs in respiratory samples, accurately interpreting the data and identifying the patterns associated with specific diseases is a challenge requiring more advanced data processing techniques and algorithms to improve the accuracy of diagnosis. AI technology has the capability to extract a greater amount of information from the detection results, enabling more accurate and extensive judgments to be made [107]. For example, neural networks are used to handle complex VOC pattern recognition, while support vector machines (SVMs) excel in classification and regression analysis. PCA is used for dimensionality reduction and feature extraction [108,109,110]. Device integration is also one of the technical challenges, but integrating multiple bio-sniffers into a single portable, user-friendly device while maintaining their detection performance is key to achieving a wide range of applications, for example, as VOC bio-sniffers [111]. In addition, the aforementioned PVDF-based bio-sniffer arrays have been built using Cu MOFs, GAs, and dyes as responsive materials, and the convenience of connecting to smartphones has made these arrays widely available. The electrical and optical bio-sniffers mentioned in this article are both future development trends, each showing unique advantages and potential in different application fields and technology development directions. The electrical bio-sniffer has great development potential in terms of integration and intelligence, while the optical bio-sniffer shows outstanding performance for intelligence and miniaturization. Both are innovative for use in a wider range of applications and for more efficient monitoring.
Looking to the future, the application of new materials and nanotechnology offers new possibilities for improving bio-sniffer performance. For example, bio-sniffers based on 2D materials have shown potential in the field of medical monitoring, due to their unique physicochemical properties [112,113,114]. The development of biofluorescence technology, especially for the detection of volatiles in humans, has shown great potential, particularly for early disease screening and the real-time monitoring of metabolic status [115,116]. The development of this technology may lead to a new direction for bio-sniffers. The application of deep learning algorithms can enhance the ability to identify disease-related VOC patterns, and the bio-sniffer array mentioned in this paper can successfully distinguish 28 types of VOCs through the calculation of PCA, LDA, and HCA, which greatly improves the accuracy and reliability of diagnosis. Interdisciplinary collaboration is key to advancing bio-sniffers and requires chemistry, biology, materials science, engineering, and data science to work together to solve technical challenges and drive innovation. For example, as mentioned in the introduction, an electronic nose based on nanomaterials for the detection of VOCs enables interdisciplinary co-operation. Bio-sniffers combining electrochemistry and optics represent a future trend in biosensing technology, providing highly sensitive and selective monitoring capabilities by leveraging the advantages of both sensing technologies. This convergence of technologies enables bio-sniffers to achieve the precise identification of biomarkers and VOCs, which are essential for early disease diagnosis and health monitoring. In addition, their multimodal detection capability enables these devices to integrate multiple detection methods on the same platform, providing more comprehensive and accurate analytical results. Finally, for these technologies to be widely used in the clinic, large-scale clinical validation is required, and they must meet the regulatory requirements of medical devices. In summary, the application of bio-sniffers in medical diagnosis is promising, but it also faces many challenges in technology, data analysis, and clinical application. Future research requires breakthroughs in improving bio-sniffer performance, data processing capabilities, and device integration for more accurate disease diagnosis and health surveillance.
5. Conclusions
This paper systematically examines the applications and challenges of using bio-sniffers for monitoring VOCs in medical diagnostics, with a focus on the detection principles, performance characteristics, and clinical application potential of electrical and optical bio-sniffers. Electrical bio-sniffers excel in VOC detection and are characterized by their fast response, high sensitivity, and portability. Optical bio-sniffers, on the other hand, are of interest due to their non-contact detection, wide dynamic range, and high selectivity. Although electrical and optical bio-sniffers perform well in VOC detection, they also face several challenges. For instance, environmental factors such as temperature and humidity variations may affect sensor performance. To further advance the field, future research should prioritize multidisciplinary collaborations to overcome current challenges and accelerate the application of biosensor technology in medical diagnostics. As these challenges are progressively resolved, bio-sniffers are poised to play a critical role in future medical diagnosis and health monitoring.
Author Contributions
Y.W.: writing—original draft and investigation. X.Z.: writing—original draft and investigation. S.M.: methodology and conceptualization. S.C.: Methodology and conceptualization. Z.G.: Supervision, review, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This project is part of the Hubei Provincial Advantage Characteristic Disciplines Project of Wuhan University of Science and Technology under “The 14th Five-Year Plan”, China (grant number 2023C0308); Joint Funding of Hubei Province Key Laboratory of Occupational Hazards Identification and Control, Wuhan University of Science and Technology (2023).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bruderer, T.; Gaisl, T.; Gaugg, M.T.; Nowak, N.; Streckenbach, B.; Müller, S.; Moeller, S.; Kohler, S.; Zenobi, R. On-Line Analysis of Exhaled Breath. Chem. Rev. 2019, 119, 10803–10828. [Google Scholar]
- Moor, C.C.; Oppenheimer, J.C.; Nakshbandi, G.; Aerts, J.G.J.V.; Brinkman, P. Exhaled breath analysis by use of eNose technology: A novel diagnostic tool for interstitial lung disease. Eur. Respir. J. 2021, 57, 2002042. [Google Scholar] [PubMed]
- Wlodzimirow, K.A.; Abu-Hanna, A.; Schultz, M.J. Exhaled breath analysis with electronic nose technology for detection of acute liver failure in rats. Biosens. Bioelectron. 2014, 53, 129–134. [Google Scholar] [PubMed]
- Chen, T.; Liu, T.; Li, T. Exhaled breath analysis in disease detection. Clin. Chim. Acta 2021, 515, 61–72. [Google Scholar] [PubMed]
- Wallace, M.A.G.; Pleil, J.D. Evolution of clinical and environmental health applications of exhaled breath research: Review of methods and instrumentation for gas-phase, condensate, and aerosols. Anal. Chim. Acta 2018, 1024, 18–38. [Google Scholar] [PubMed]
- Bakali, U.; Killawala, C.; Monteagudo, E. Exhaled breath analysis applications for evaluating occupational and environmental exposures. Trends Anal. Chem. 2024, 177, 117787. [Google Scholar]
- Popov, T.A. Human exhaled breath analysis. Ann. Allergy Asthma Immunol. 2011, 106, 451–456. [Google Scholar] [PubMed]
- Zhou, J.; Huang, Z.-A.; Kumar, U.; Chen, D.D.Y. Review of recent developments in determining volatile organic compounds in exhaled breath as biomarkers for lung cancer diagnosis. Anal. Chim. Acta 2017, 996, 1–9. [Google Scholar] [CrossRef]
- Bax, C.; Lotesoriere, B.J.; Sironi, S.; Capelli, L. Review and Comparison of Cancer Biomarker Trends in Urine as a Basis for New Diagnostic Pathways. Cancers 2019, 11, 1244. [Google Scholar] [CrossRef]
- Xu, W.; Zou, X.; Ding, Y. Rapid screen for ventilator associated pneumonia using exhaled volatile organic compounds. Talanta 2023, 253, 124069. [Google Scholar]
- Wojciech, F.; Pawel, M.; Anna, F. A Compendium of Volatile Organic Compounds (VOCs) Released By Human Cell Lines. Curr. Med. Chem. 2016, 23, 2112–2131. [Google Scholar]
- Moura, P.C.; Raposo, M.; Vassilenko, V. Breath volatile organic compounds (VOCs) as biomarkers for the diagnosis of pathological conditions: A review. Biomed. J. 2023, 46, 100623. [Google Scholar] [CrossRef]
- Bajo-Fernández, M.; Souza-Silva, É.A.; Barbas, C. GC-MS-based metabolomics of volatile organic compounds in exhaled breath: Applications in health and disease. A review. Front. Mol. Biosci. 2024, 10, 1295955. [Google Scholar] [CrossRef]
- Baranska, A.; Mujagic, Z.; Smolinska, A. Volatile organic compounds in breath as markers for irritable bowel syndrome: A metabolomic approach. Aliment. Pharmacol. Ther. 2016, 44, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Yang, J.; Zhu, H. Volatile organic compound exposure in relation to lung cancer: Insights into mechanisms of action through metabolomics. J. Hazard. Mater. 2024, 480, 135856. [Google Scholar] [CrossRef] [PubMed]
- Broza, Y.Y.; Mochalski, P.; Ruzsanyi, V. Hybrid Volatolomics and Disease Detection. Angew. Chem. Int. Ed. 2015, 54, 11036–11048. [Google Scholar] [CrossRef] [PubMed]
- Arulvasan, W.; Chou, H.; Greenwood, J. High-quality identification of volatile organic compounds (VOCs) originating from breath. Metabolomics 2024, 20, 102. [Google Scholar] [CrossRef] [PubMed]
- Amann, A.; de Lacy Costello, B.; Miekisch, W. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014, 8, 034001. [Google Scholar] [CrossRef]
- Das, S.; Pal, M. Review—Non-Invasive Monitoring of Human Health by Exhaled Breath Analysis: A Comprehensive Review. J. Electrochem. Soc. 2020, 167, 037562. [Google Scholar] [CrossRef]
- Jalal, A.H.; Alam, F.; Roychoudhury, S. Prospects and Challenges of Volatile Organic Compound Sensors in Human Healthcare. ACS Sens. 2018, 3, 1246–1263. [Google Scholar] [CrossRef]
- Velusamy, P.; Su, C.-H.; Ramasamy, P. Volatile Organic Compounds as Potential Biomarkers for Noninvasive Disease Detection by Nanosensors: A Comprehensive Review. Crit. Rev. Anal. Chem. 2023, 53, 1828–1839. [Google Scholar] [CrossRef] [PubMed]
- Mohaghegh Montazeri, M.; O’brien, A.; Hoorfar, M. Understanding microfluidic-based gas detectors: A numerical model to investigate fundamental sensor operation, influencing phenomena and optimum geometries. Sens. Actuators B Chem. 2019, 300, 126904. [Google Scholar]
- Novikov, S.; Lebedeva, N.; Satrapinski, A. Graphene based sensor for environmental monitoring of NO2. Sens. Actuators B Chem. 2016, 236, 1054–1060. [Google Scholar]
- Kaushik, A.; Kumar, R.; Arya, S.K. Organic–Inorganic Hybrid Nanocomposite-Based Gas Sensors for Environmental Monitoring. Chem. Rev. 2015, 115, 4571–4606. [Google Scholar] [PubMed]
- Loutfi, A.; Coradeschi, S.; Mani, G.K. Electronic noses for food quality: A review. J. Food Eng. 2015, 144, 103–111. [Google Scholar]
- Ghasemi-Varnamkhasti, M.; Mohtasebi, S.S.; Siadat, M. Biomimetic-based odor and taste sensing systems to food quality and safety characterization: An overview on basic principles and recent achievements. J. Food Eng. 2010, 100, 377–387. [Google Scholar]
- Kim, I.-D.; Choi, S.-J.; Kim, S.-J.; Jang, J.-S. Exhaled Breath Sensors. In Smart Sensors for Health and Environment Monitoring; Kyung, C.-M., Ed.; Springer: Dordrecht, The Netherlands, 2015; pp. 19–49. [Google Scholar]
- Kim, K.-H.; Jahan, S.A.; Kabir, E. A review of breath analysis for diagnosis of human health. Trends Anal. Chem. 2012, 33, 1–8. [Google Scholar] [CrossRef]
- Gregis, G.; Sanchez, J.-B.; Bezverkhyy, I. Detection and quantification of lung cancer biomarkers by a micro-analytical device using a single metal oxide-based gas sensor. Sens. Actuators B Chem. 2018, 255, 391–400. [Google Scholar]
- Pang, X.; Nan, H.; Zhong, J. Low-cost photoionization sensors as detectors in GC × GC systems designed for ambient VOC measurements. Sci. Total Environ. 2019, 664, 771–779. [Google Scholar] [CrossRef]
- Edwards, S.J.; Lewis, A.C.; Andrews, S.J. A compact comprehensive two-dimensional gas chromatography (GC×GC) approach for the analysis of biogenic VOCs. Anal. Methods 2013, 5, 141–150. [Google Scholar] [CrossRef]
- Wyszynski, B.; Yatabe, R.; Nakao, A. Array of Chemosensitive Resistors with Composites of Gas Chromatography (GC) Materials and Carbon Black for Detection and Recognition of VOCs: A Basic Study. Sensors 2017, 17, 1606. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, F.; Talebpour, Z.; Sanati-Nezhad, A. Through the years with on-a-chip gas chromatography: A review. Lab A Chip 2015, 15, 2559–2575. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.M.; Sacks, R.D. GC Analysis of Human Breath with A Series-Coupled Column Ensemble and A Multibed Sorption Trap. Anal. Chem. 2003, 75, 2231–2236. [Google Scholar] [CrossRef]
- Schnabel, R.; Fijten, R.; Smolinska, A. Analysis of volatile organic compounds in exhaled breath to diagnose ventilator-associated pneumonia. Sci. Rep. 2015, 5, 17179. [Google Scholar]
- Branković, Z.; Rostovtsev, Y. A resonant single frequency molecular detector with high sensitivity and selectivity for gas mixtures. Sci. Rep. 2020, 10, 1537. [Google Scholar]
- Cheng, F.; Zhang, A.; Wang, T. Research based on double coverage rate and reliability of gas detector layout optimization. J. Loss Prev. Process Ind. 2020, 68, 104285. [Google Scholar] [CrossRef]
- Davies, S.; Hu, Y.; Jiang, N. Holographic Sensors in Biotechnology. Adv. Funct. Mater. 2021, 31, 2105645. [Google Scholar] [CrossRef]
- Giraldo, J.P.; Wu, H.; Newkirk, G.M.; Kruss, S. Nanobiotechnology approaches for engineering smart plant sensors. Nat. Nanotechnol. 2019, 14, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Patwari, P.; Pruckner, F.; Fabris, M. Biosensors in microalgae: A roadmap for new opportunities in synthetic biology and biotechnology. Biotechnol. Adv. 2023, 68, 108221. [Google Scholar]
- Farquhar, A.K.; Henshaw, G.S.; Williams, D.E. Errors in ambient gas concentration measurement caused by acoustic response of electrochemical gas sensors. Sens. Actuators A Phys. 2023, 354, 114254. [Google Scholar]
- Hao, X.; Wu, D.; Wang, Y. Gas sniffer (YSZ-based electrochemical gas phase sensor) toward acetone detection. Sens. Actuators B Chem. 2019, 278, 1–7. [Google Scholar]
- Emam, S.; Adedoyin, A.; Geng, X. A Molecularly Imprinted Electrochemical Gas Sensor to Sense Butylated Hydroxytoluene in Air. J. Sens. 2018, 2018, 3437149. [Google Scholar]
- Liao, H.-C.; Hsu, C.-P.; Wu, M.-C. Conjugated Polymer/Nanoparticles Nanocomposites for High Efficient and Real-Time Volatile Organic Compounds Sensors. Anal. Chem. 2013, 85, 9305–9311. [Google Scholar] [CrossRef]
- Zhong, X.; Li, D.; Du, W. Rapid recognition of volatile organic compounds with colorimetric sensor arrays for lung cancer screening. Anal. Bioanal. Chem. 2018, 410, 3671–3681. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, D.; Sarswat, P.K.; Free, M.L. Quantum dots and carbon dots based fluorescent sensors for TB biomarkers detection. Vacuum 2017, 146, 606–613. [Google Scholar]
- Kaloumenou, M.; Skotadis, E.; Lagopati, N. Breath Analysis: A Promising Tool for Disease Diagnosis—The Role of Sensors. Sensors 2022, 22, 1238. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Gopal, R.A.; Lkhagvaa, T.; Choi, D. Metal-oxide gas sensors for exhaled-breath analysis: A review. Meas. Sci. Technol. 2021, 32, 102004. [Google Scholar]
- Zhang, L.; Khan, K.; Zou, J. Recent Advances in Emerging 2D Material-Based Gas Sensors: Potential in Disease Diagnosis. Adv. Mater. Interfaces 2019, 6, 1901329. [Google Scholar]
- Shooshtari, M.; Salehi, A. An electronic nose based on carbon nanotube -titanium dioxide hybrid nanostructures for detection and discrimination of volatile organic compounds. Sens. Actuators B Chem. 2022, 357, 131418. [Google Scholar] [CrossRef]
- Giardina, M.; Olesik, S.V. Application of Low-Temperature Glassy Carbon-Coated Macrofibers for Solid-Phase Microextraction Analysis of Simulated Breath Volatiles. Anal. Chem. 2003, 75, 1604–1614. [Google Scholar] [CrossRef]
- Schmutzhard, J.; Rieder, J.; Deibl, M. Pilot study: Volatile organic compounds as a diagnostic marker for head and neck tumors. Head Neck 2008, 30, 743–749. [Google Scholar] [PubMed]
- Hanouneh, I.A.; Zein, N.N.; Cikach, F. The breathprints in patients with liver disease identify novel breath biomarkers in alcoholic hepatitis. Clin. Gastroenterol. Hepatol. 2014, 12, 516–523. [Google Scholar] [PubMed]
- Walton, C.; Patel, M.; Pitts, D. The use of a portable breath analysis device in monitoring type 1 diabetes patients in a hypoglycaemic clamp: Validation with SIFT-MS data. J. Breath Res. 2014, 8, 037108. [Google Scholar] [PubMed]
- Jakubowski, M.; Czerczak, S. Calculating the retention of volatile organic compounds in the lung on the basis of their physicochemical properties. Environ. Toxicol. Pharmacol. 2009, 28, 311–315. [Google Scholar] [CrossRef]
- Spinelle, L.; Gerboles, M.; Kok, G. Review of Portable and Low-Cost Sensors for the Ambient Air Monitoring of Benzene and Other Volatile Organic Compounds. Sensors 2017, 17, 1520. [Google Scholar] [CrossRef]
- Yan, C.; Hu, W.; Tu, J. Pathogenic mechanisms and regulatory factors involved in alcoholic liver disease. J. Transl. Med. 2023, 21, 300. [Google Scholar] [CrossRef] [PubMed]
- Quintans, L.N.; Castro, G.D.; Castro, J.A. Oxidation of ethanol to acetaldehyde and free radicals by rat testicular microsomes. Arch. Toxicol. 2005, 79, 25–30. [Google Scholar] [CrossRef]
- Helander, A.; Beck, O. Ethyl Sulfate: A Metabolite of Ethanol in Humans and a Potential Biomarker of Acute Alcohol Intake. J. Anal. Toxicol. 2005, 29, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Chen, G. Ethanol up-regulates phenol sulfotransferase (SULT1A1) and hydroxysteroid sulfotransferase (SULT2A1) in rat liver and intestine. Arch. Physiol. Biochem. 2015, 121, 68–74. [Google Scholar] [CrossRef]
- Kechagias, S.; Dernroth, D.N.; Blomgren, A. Phosphatidylethanol Compared with Other Blood Tests as a Biomarker of Moderate Alcohol Consumption in Healthy Volunteers: A Prospective Randomized Study. Alcohol Alcohol. 2015, 50, 399–406. [Google Scholar] [CrossRef]
- Vela, S.; Guerra, A.; Farrell, G. Pathophysiology and Biomarker Potential of Fatty Acid Ethyl Ester Elevation During Alcoholic Pancreatitis. Gastroenterology 2021, 161, 1513–1525. [Google Scholar] [CrossRef]
- Saasa, V.; Beukes, M.; Lemmer, Y.; Mwakikunga, B. Blood Ketone Bodies and Breath Acetone Analysis and Their Correlations in Type 2 Diabetes Mellitus. Diagnostics 2019, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Ruzsányi, V.; Péter Kalapos, M. Breath acetone as a potential marker in clinical practice. J. Breath Res. 2017, 11, 024002. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Mizukoshi, N.; Iitani, K. A Bio-Fluorometric Acetone Gas Imaging System for the Dynamic Analysis of Lipid Metabolism in Human Breath. Chemosensors 2021, 9, 258. [Google Scholar] [CrossRef]
- Alkedeh, O.; Priefer, R. The Ketogenic Diet: Breath Acetone Sensing Technology. Biosensors 2021, 11, 26. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, C.; Fan, T. Mixed Potential Type Acetone Sensor with Ultralow Detection Limit for Diabetic Ketosis Breath Analysis. ACS Sens. 2024, 9, 464–473. [Google Scholar] [CrossRef]
- Wei, S.; Li, Z.; Murugappan, K. Nanowire Array Breath Acetone Sensor for Diabetes Monitoring. Adv. Sci. 2024, 11, 2309481. [Google Scholar]
- Dorokhov, Y.L.; Shindyapina, A.V.; Sheshukova, E.V.; Komarova, T.V. Metabolic Methanol: Molecular Pathways and Physiological Roles. Physiol. Rev. 2015, 95, 603–644. [Google Scholar] [PubMed]
- Toma, K.; Iwasaki, K.; Arakawa, T. Sensitive and selective methanol biosensor using two-enzyme cascade reaction and fluorometry for non-invasive assessment of intestinal bacteria activity. Biosens. Bioelectron. 2021, 181, 113136. [Google Scholar]
- Razzaghy-Azar, M.; Nourbakhsh, M.; Vafadar, M. A novel metabolic disorder in the degradation pathway of endogenous methanol due to a mutation in the gene of alcohol dehydrogenase. Clin. Biochem. 2021, 90, 66–72. [Google Scholar]
- Laakso, O.; Haapala, M.; Jaakkola, P. FT-IR Breath Test in the Diagnosis and Control of Treatment of Methanol Intoxications. J. Anal. Toxicol. 2001, 25, 26–30. [Google Scholar] [PubMed]
- Chen, H.; Gao, P.; Zhu, X. Monitoring, fate and transport, and risk assessment of organic pollutants in the environment: CREST publications during 2019–2023. Crit. Rev. Environ. Sci. Technol. 2024, 54, 1–12. [Google Scholar]
- Kulkarni, S.; Ghosh, R. Development of 2D CuO based chemiresistive sensors for detecting binary mixture of volatile organic compounds and investigation of the adsorption kinetics via Eley-Rideal mechanism. Appl. Surf. Sci. 2024, 665, 160328. [Google Scholar] [CrossRef]
- Paul, S.; Mendoza, E.R.; To, D.T.H. Enhancing room-temperature gas sensing performance of metal oxide semiconductor chemiresistors through 400 nm UV photoexcitation. Sens. Actuators Rep. 2024, 7, 100194. [Google Scholar]
- Jamnani, S.R.; Moghaddam, H.M.; Leonardi, S.G.; Neri, G. A novel conductometric sensor based on hierarchical self-assembly nanoparticles Sm2O3 for VOCs monitoring. Ceram. Int. 2018, 44, 16953–16959. [Google Scholar]
- Mondal, D.; Nair, A.M.; Mukherji, S. Volatile organic compound sensing in breath using conducting polymer coated chemi-resistive filter paper sensors. Med. Biol. Eng. Comput. 2023, 61, 2001–2011. [Google Scholar]
- Xiong, L.; Compton, R.G. Amperometric Gas detection: A Review. Int. J. Electrochem. Sci. 2014, 9, 7152–7181. [Google Scholar]
- Ueda, T.; Abe, H.; Kamada, K. Enhanced sensing response of solid-electrolyte gas sensors to toluene: Role of composite Au/metal oxide sensing electrode. Sens. Actuators B Chem. 2017, 252, 268–276. [Google Scholar]
- Gross, P.-A.; Jaramillo, T.; Pruitt, B. Cyclic-Voltammetry-Based Solid-State Gas Sensor for Methane and Other VOC Detection. Anal. Chem. 2018, 90, 6102–6108. [Google Scholar]
- Tan, W.C.; Ang, K.-W. Volatile Organic Compound Sensors Based on 2D Materials. Adv. Electron. Mater. 2021, 7, 2001071. [Google Scholar]
- Banga, I.; Paul, A.; Muthukumar, S.; Prasad, S. ZENose (ZIF-Based Electrochemical Nose) Platform for Noninvasive Ammonia Detection. ACS Appl. Mater. Interfaces 2021, 13, 16155–16165. [Google Scholar] [PubMed]
- Ma, H.; Cheng, P.; Chen, C. Highly Selective Wearable Alcohol Homologue Sensors Derived from Pt-Coated Truncated Octahedron Au. ACS Sens. 2022, 7, 3067–3076. [Google Scholar] [PubMed]
- Qu, X.; Hu, Y.; Xu, C. Optical sensors of volatile organic compounds for non-invasive diagnosis of diseases. Chem. Eng. J. 2024, 485, 149804. [Google Scholar]
- Khatib, M.; Haick, H. Sensors for Volatile Organic Compounds. ACS Nano 2022, 16, 7080–7115. [Google Scholar]
- Nah, S.H.; Kim, J.B.; Chui, H.N.T. Enhanced Colorimetric Detection of Volatile Organic Compounds Using a Dye-Incorporated Photonic Crystal-Based Sensor Array. Adv. Mater. 2024, 36, 2409297. [Google Scholar]
- Hou, J.; Liu, X.; Hou, C. A PVDF-based colorimetric sensor array for noninvasive detection of multiple disease-related volatile organic compounds. Anal. Bioanal. Chem. 2023, 415, 6647–6661. [Google Scholar] [PubMed]
- Shin, Y.-H.; Teresa Gutierrez-Wing, M.; Choi, J.-W. Review—Recent Progress in Portable Fluorescence Sensors. J. Electrochem. Soc. 2021, 168, 017502. [Google Scholar] [CrossRef]
- Iitani, K.; Chien, P.-J.; Suzuki, T. Fiber-Optic Bio-sniffer (Biochemical Gas Sensor) Using Reverse Reaction of Alcohol Dehydrogenase for Exhaled Acetaldehyde. ACS Sens. 2018, 3, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Blaikie, T.P.J.; Edge, J.A.; Hancock, G. Comparison of breath gases, including acetone, with blood glucose and blood ketones in children and adolescents with type 1 diabetes. J. Breath Res. 2014, 8, 046010. [Google Scholar] [CrossRef] [PubMed]
- Toyooka, T.; Hiyama, S.; Yamada, Y. A prototype portable breath acetone analyzer for monitoring fat loss. J. Breath Res. 2013, 7, 036005. [Google Scholar] [PubMed]
- Ye, M.; Chien, P.-J.; Toma, K. An acetone bio-sniffer (gas phase biosensor) enabling assessment of lipid metabolism from exhaled breath. Biosens. Bioelectron. 2015, 73, 208–213. [Google Scholar]
- Phillips, M.; Cataneo, R.N.; Ditkoff, B.A. Prediction of breast cancer using volatile biomarkers in the breath. Breast Cancer Res. Treat. 2006, 99, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Cikach, F.S.; Tonelli, A.R.; Barnes, J. Breath Analysis in Pulmonary Arterial Hypertension. Chest 2014, 145, 551–558. [Google Scholar] [CrossRef]
- Buszewski, B.; Ligor, T.; Jezierski, T. Identification of volatile lung cancer markers by gas chromatography-mass spectrometry: Comparison with discrimination by canines. Anal. Bioanal. Chem. 2012, 404, 141–146. [Google Scholar] [PubMed]
- Rudnicka, J.; Walczak, M.; Kowalkowski, T. Determination of volatile organic compounds as potential markers of lung cancer by gas chromatography–mass spectrometry versus trained dogs. Sens. Actuators B Chem. 2014, 202, 615–621. [Google Scholar] [CrossRef]
- Wehinger, A.; Schmid, A.; Mechtcheriakov, S. Lung cancer detection by proton transfer reaction mass-spectrometric analysis of human breath gas. Int. J. Mass Spectrom. 2007, 265, 49–59. [Google Scholar]
- Palmiere, C.; Sporkert, F.; Werner, D. Blood, urine and vitreous isopropyl alcohol as biochemical markers in forensic investigations. Leg. Med. 2012, 14, 17–20. [Google Scholar] [CrossRef]
- Chien, P.-J.; Suzuki, T.; Tsujii, M. Bio-sniffer (gas-phase biosensor) with secondary alcohol dehydrogenase (S-ADH) for determination of isopropanol in exhaled air as a potential volatile biomarker. Biosens. Bioelectron. 2017, 91, 341–346. [Google Scholar] [CrossRef]
- Chien, P.-J.; Suzuki, T.; Ye, M. Ultra-Sensitive Isopropanol Biochemical Gas Sensor (Bio-Sniffer) for Monitoring of Human Volatiles. Sensors 2020, 20, 6827. [Google Scholar] [CrossRef]
- Toma, K.; Iwasaki, K.; Zhang, G. Biochemical Methanol Gas Sensor (MeOH Bio-Sniffer) for Non-Invasive Assessment of Intestinal Flora from Breath Methanol. Sensors 2021, 21, 4897. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, M.; Zamarreño, C.R.; Melendi-Espina, S. Optical Fibre Sensors Using Graphene-Based Materials: A Review. Sensors 2017, 17, 155. [Google Scholar] [CrossRef] [PubMed]
- Mathur, M.; Yeh, Y.-T.; Arya, R.K. Adipose lipolysis is important for ethanol to induce fatty liver in the National Institute on Alcohol Abuse and Alcoholism murine model of chronic and binge ethanol feeding. Hepatology 2023, 77, 1688–1701. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Suzuki, T.; Tsujii, M. Real-time monitoring of skin ethanol gas by a high-sensitivity gas phase biosensor (bio-sniffer) for the non-invasive evaluation of volatile blood compounds. Biosens. Bioelectron. 2019, 129, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Aota, T.; Iitani, K. Skin ethanol gas measurement system with a biochemical gas sensor and gas concentrator toward monitoring of blood volatile compounds. Talanta 2020, 219, 121187. [Google Scholar] [CrossRef] [PubMed]
- Toma, K.; Suzuki, S.; Arakawa, T. External ears for non-invasive and stable monitoring of volatile organic compounds in human blood. Sci. Rep. 2021, 11, 10415. [Google Scholar] [CrossRef]
- Yammouri, G.; Ait Lahcen, A. AI-Reinforced Wearable Sensors and Intelligent Point-of-Care Tests. J. Pers. Med. 2024, 14, 1088. [Google Scholar] [CrossRef]
- Cao, H.; Shi, H.; Tang, J. Ultrasensitive discrimination of volatile organic compounds using a microfluidic silicon SERS artificial intelligence chip. iScience 2023, 26, 107821. [Google Scholar] [CrossRef] [PubMed]
- Mansour, E.; Palzur, E.; Broza, Y.Y. Noninvasive Detection of Stress by Biochemical Profiles from the Skin. ACS Sens. 2023, 8, 1339–1347. [Google Scholar] [CrossRef]
- Syed, I.B.; Sundaram, B.; Ayothiraman, S.; Yuvaraj, S. Planar Microwave Sensor suitable for Artificial-Intelligence (AI) based detection of Volatile Organic Compounds. AEU-Int. J. Electron. Commun. 2024, 185, 155444. [Google Scholar] [CrossRef]
- Li, L.; Fu, C.; Lou, Z. Flexible planar concentric circular micro-supercapacitor arrays for wearable gas sensing application. Nano Energy 2017, 41, 261–268. [Google Scholar] [CrossRef]
- Hong, G.; Kim, M.E.; Lee, J.S. Respiration Monitoring Using Humidity Sensor Based on Hydrothermally Synthesized Two-Dimensional MoS2. Nanomaterials 2024, 14, 1826. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Pan, Z.; Ye, S. Interface engineering of NiCo LDH and 2D materials for advanced non-invasive glucose sensors. J. Alloys Compd. 2025, 1010, 177231. [Google Scholar] [CrossRef]
- Ismail, S.N.A.; Nayan, N.A.; Mohammad Haniff, M.A.S. Wearable Two-Dimensional Nanomaterial-Based Flexible Sensors for Blood Pressure Monitoring: A Review. Nanomaterials 2023, 13, 852. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.T.T.; Tohl, D.; Wallace, A. Developing a fluorescent sensing based portable medical open-platform—A case study for albuminuria measurement in chronic kidney disease screening and monitoring. Sens. Bio-Sens. Res. 2022, 37, 100504. [Google Scholar]
- Senthilnathan, N.; Oral, C.M.; Novobilsky, A.; Pumera, M. Intelligent Magnetic Microrobots with Fluorescent Internal Memory for Monitoring Intragastric Acidity. Adv. Funct. Mater. 2024, 34, 2401463. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).