Polydopamine-Coated Magnetite as a Sensing Material for the Optical Detection of Cationic Surfactants
Abstract
1. Introduction
2. Material and Methods
2.1. Reagents
2.2. Instrumentation
2.3. Synthesis of the Materials
2.4. Dye Adsorption Studies
2.5. Dye Desorption Studies
2.6. Sensing Studies
3. Results and Discussion
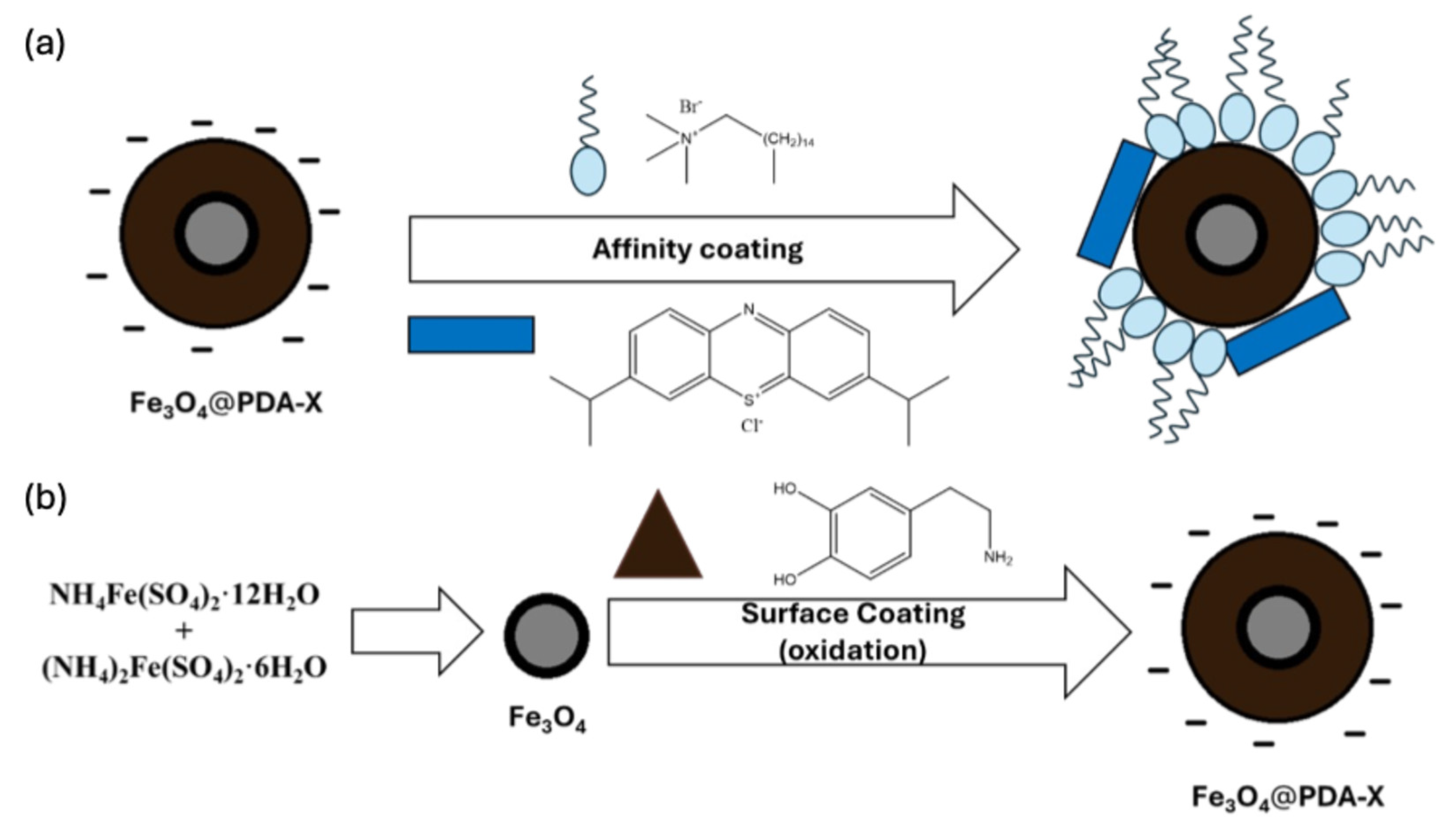
3.1. Sensing Design
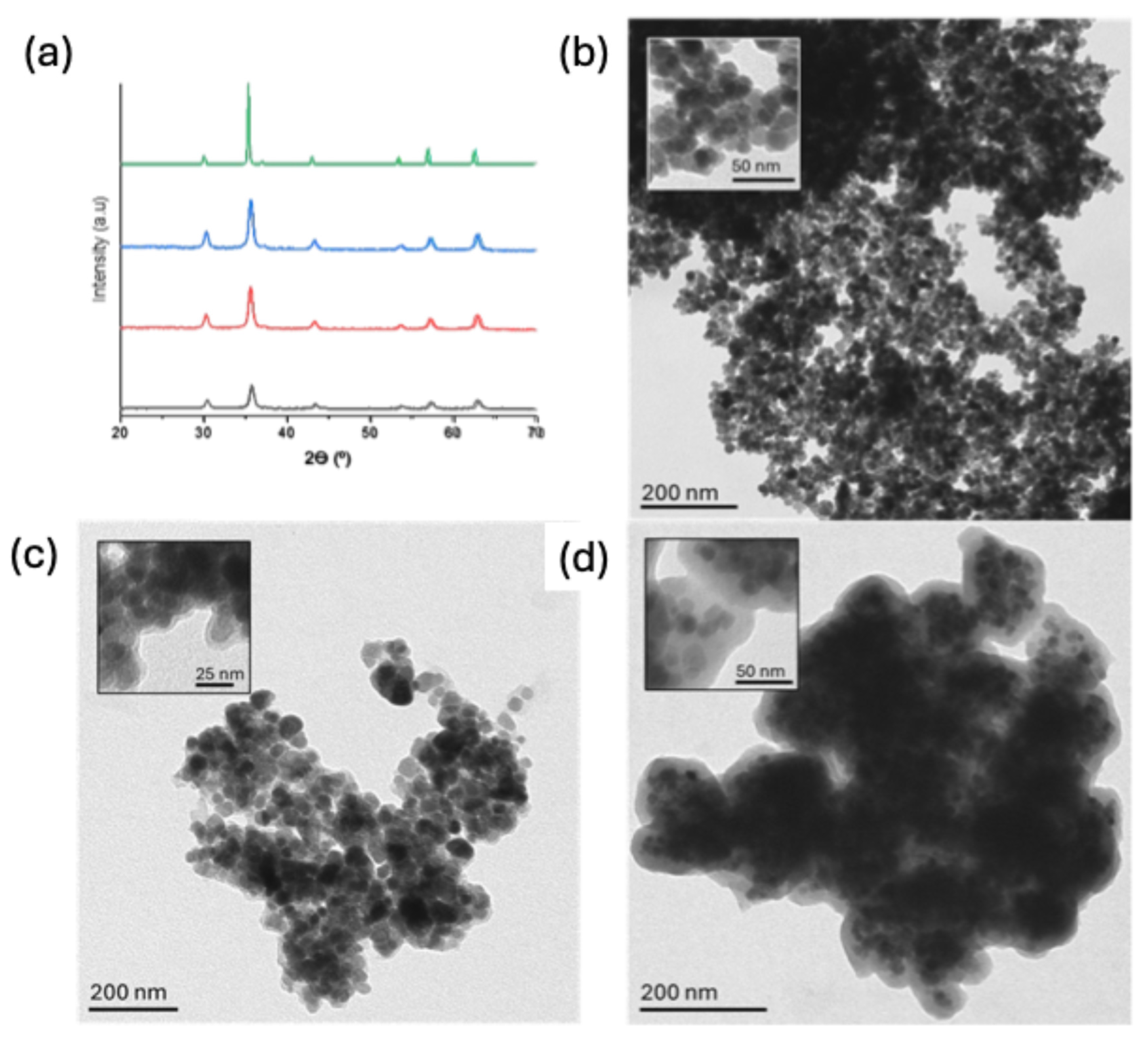
3.2. Synthesis and Characterization of the Materials
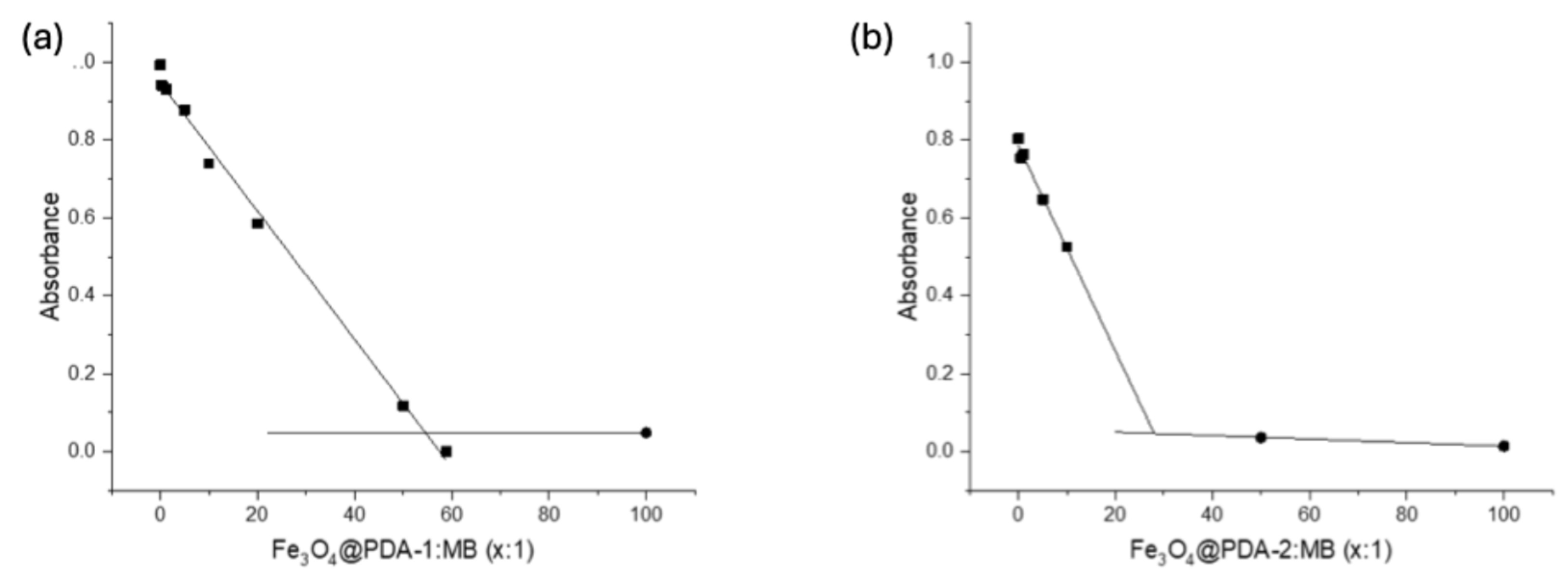
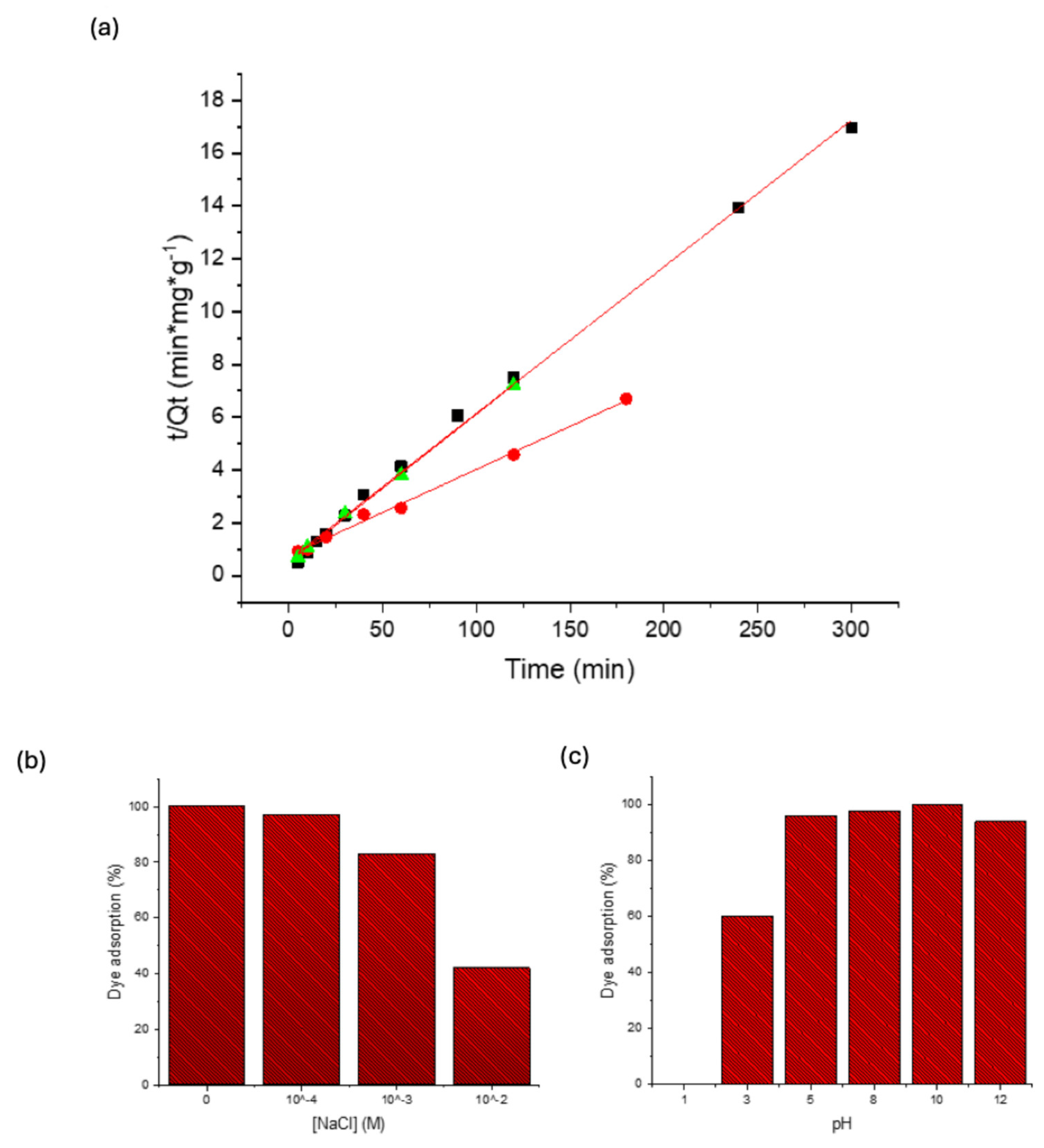
3.3. Dye Adsorption Studies
3.4. Dye Desorption Studies
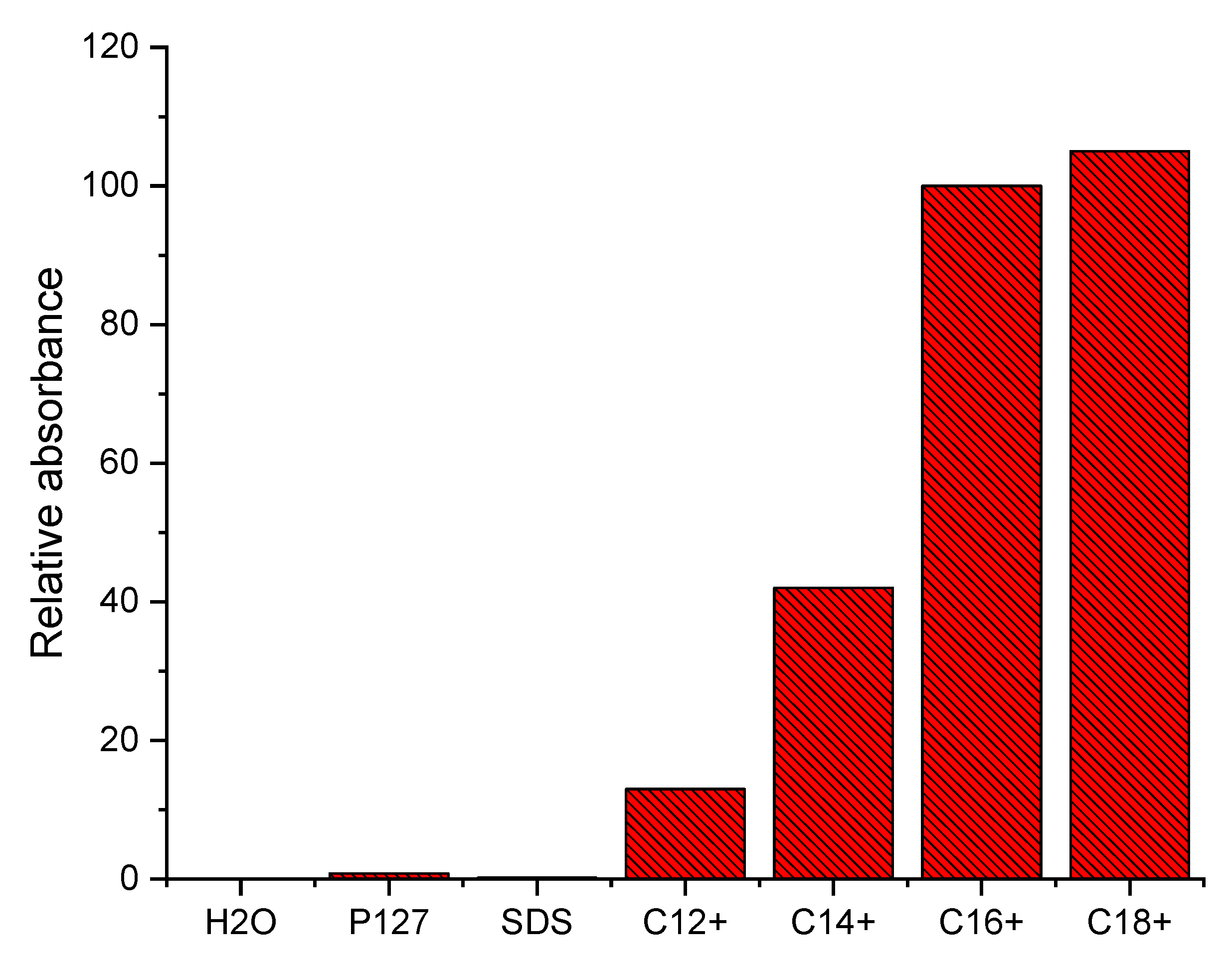
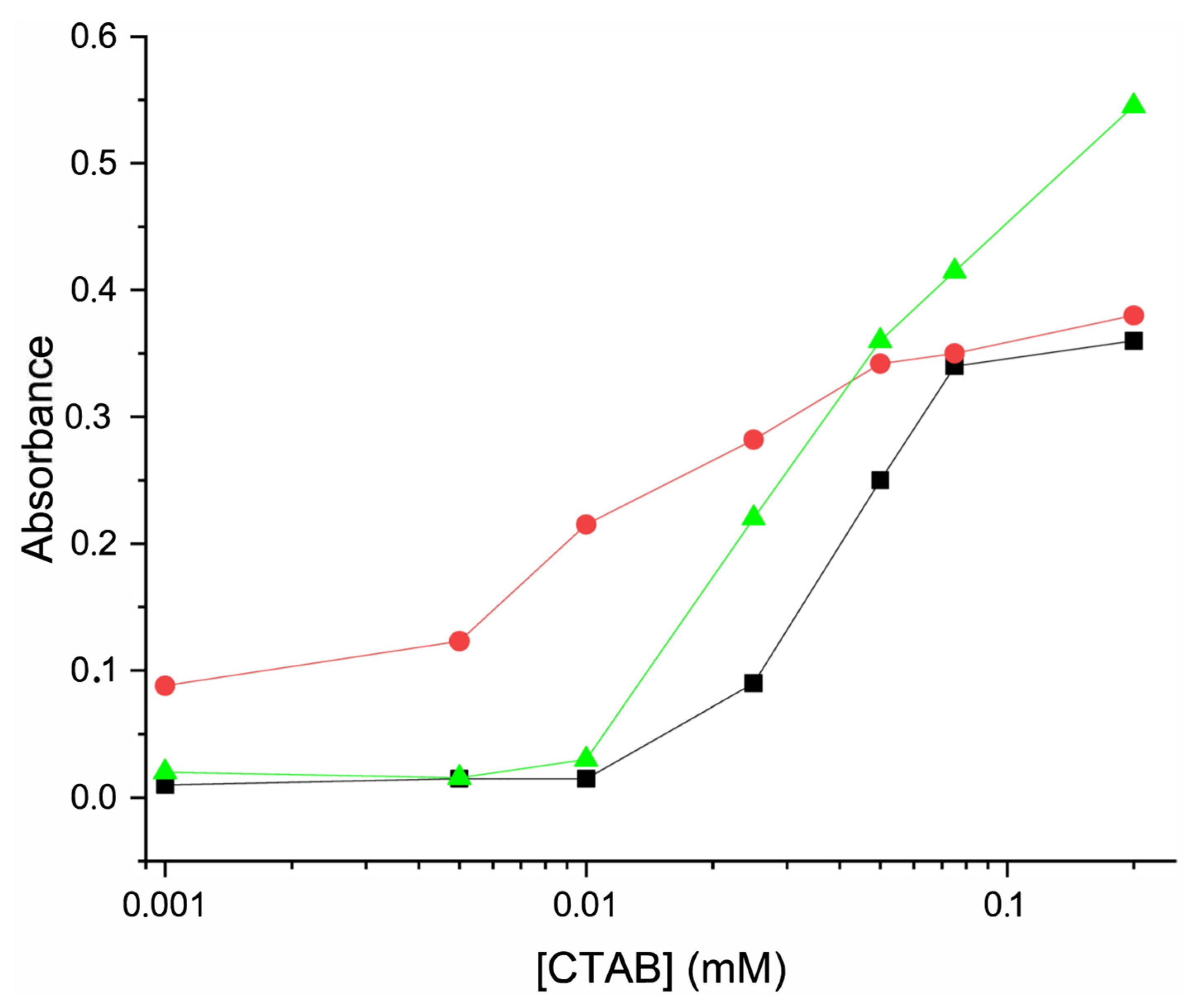
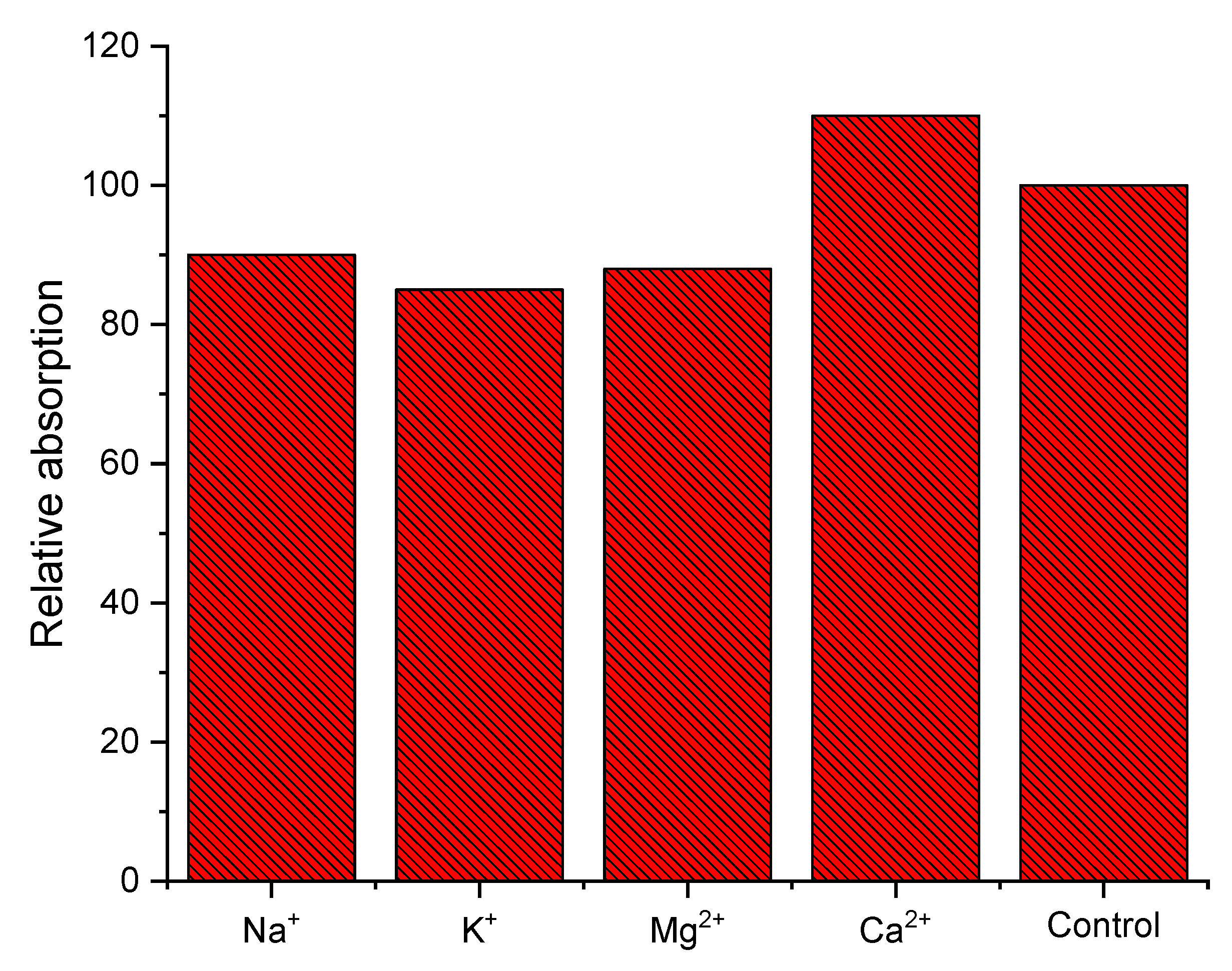
3.5. Sensing Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sivaramakrishnan, C.N. The Use of Surfactants in the Finishing of Technical Textiles. In Advances in the Dyeing and Finishing of Technical Textiles; Elsevier: Amsterdam, The Netherlands, 2013; pp. 199–235. [Google Scholar] [CrossRef]
- Le Marechal, A.M.; Krianec, B.; Vajnhandl, S.; Volmajer, J. Textile Finishing Industry as an Important Source of Organic Pollutants. In Organic Pollutants Ten Years After the Stockholm Convention—Environmental and Analytical Update; Puzyn, T., Ed.; InTech: Houston, TX, USA, 2012. [Google Scholar] [CrossRef]
- Brycki, B.E.; Kowalczyk, I.H.; Szulc, A.; Kaczerewska, O.; Pakiet, M. Multifunctional Gemini Surfactants: Structure, Synthesis, Properties and Applications. In Application and Characterization of Surfactants; Najjar, R., Ed.; InTech: Houston, TX, USA, 2017. [Google Scholar] [CrossRef]
- Jackson, M.; Eadsforth, C.; Schowanek, D.; Delfosse, T.; Riddle, A.; Budgen, N. Comprehensive Review of Several Surfactants in Marine Environments: Fate and Ecotoxicity. Environ. Toxic. Chem. 2016, 35, 1077–1086. [Google Scholar] [CrossRef]
- Kaczerewska, O.; Martins, R.; Figueiredo, J.; Loureiro, S.; Tedim, J. Environmental Behaviour and Ecotoxicity of Cationic Surfactants towards Marine Organisms. J. Hazard. Mater. 2020, 392, 122299. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.T.; Ribosa, I.; Kowalczyk, I.; Pakiet, M.; Brycki, B. Biodegradability and Aquatic Toxicity of New Cleavable Betainate Cationic Oligomeric Surfactants. J. Hazard. Mater. 2019, 371, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Badmus, S.O.; Amusa, H.K.; Oyehan, T.A.; Saleh, T.A. Environmental Risks and Toxicity of Surfactants: Overview of Analysis, Assessment, and Remediation Techniques. Environ. Sci. Pollut. Res. 2021, 28, 62085–62104. [Google Scholar] [CrossRef]
- Fernández-Núñez, A.; El Haskouri, J.; Amorós, P.; Ros-Lis, J.V. Graphene Oxide as Inhibitor on the Hydrolysis of Fats under Simulated in Vitro Duodenal Conditions. Heliyon 2024, 10, e28624. [Google Scholar] [CrossRef]
- Budetić, M.; Jozanović, M.; Pukleš, I.; Samardžić, M. Review of Potentiometric Determination of Cationic Surfactants. Rev. Anal. Chem. 2024, 43, 20230078. [Google Scholar] [CrossRef]
- Coll, C.; Ros-Lis, J.V.; Martínez-Máñez, R.; Marcos, M.D.; Sancenón, F.; Soto, J. A New Approach for the Selective and Sensitive Colorimetric Detection of Ionic Surfactants in Water. J. Mater. Chem. 2010, 20, 1442–1451. [Google Scholar] [CrossRef]
- Hnaien, M.; Dzyadevych, S.; Lagarde, F.; Jaffrezic-Renault, N. A Conductometric Sensor Specific for Cationic Surfactants. Electroanalysis 2012, 24, 1441–1445. [Google Scholar] [CrossRef]
- Descalzo, A.B.; Martínez-Máñez, R.; Sancenón, F.; Hoffmann, K.; Rurack, K. The Supramolecular Chemistry of Organic–Inorganic Hybrid Materials. Angew. Chem. Int. Ed. 2006, 45, 5924–5948. [Google Scholar] [CrossRef]
- Calero, P.; Aznar, E.; Lloris, J.M.; Marcos, M.D.; Martínez-Máñez, R.; Ros-Lis, J.V.; Soto, J.; Sancenón, F. Chromogenic Silica Nanoparticles for the Colorimetric Sensing of Long-Chain Carboxylates. Chem. Commun. 2008, 14, 1668. [Google Scholar] [CrossRef]
- Novoselova, L.Y. Nanoscale Magnetite: New Synthesis Approach, Structure and Properties. Appl. Surf. Sci. 2021, 539, 148275. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Chircov, C.; Grumezescu, A.M. Magnetite Nanoparticles: Synthesis Methods—A Comparative Review. Methods 2022, 199, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Cotar, A.I.; Grumezescu, A.M.; Huang, K.-S.; Voicu, G.; Chifiriuc, C.M.; Radulescu, R. Magnetite Nanoparticles Influence the Efficacy of Antibiotics against Biofilm Embedded Staphylococcus Aureus Cells. Biointerface Res. Appl. Chem. 2013, 3, 2. [Google Scholar]
- Cabeza, V.S. High and Efficient Production of Nanomaterials by Microfluidic Reactor Approaches. In Advances in Microfluidics—New Applications in Biology, Energy, and Materials Sciences; Yu, X.-Y., Ed.; InTech: Houston, TX, USA, 2016. [Google Scholar] [CrossRef]
- Carriazo, J.G.; Noval, V.E.; Ochoa Puentes, C. Magnetita (Fe3O4): Una estructura inorgánica con múltiples aplicaciones en catálisis heterogénea. Rev. Colomb. Quim. 2017, 46, 42. [Google Scholar] [CrossRef]
- Beveridge, J.S.; Stephens, J.R.; Williams, M.E. The Use of Magnetic Nanoparticles in Analytical Chemistry. Annu. Rev. Anal. Chem. 2011, 4, 251–273. [Google Scholar] [CrossRef]
- Öztürk Er, E.; Dalgıç Bozyiğit, G.; Büyükpınar, Ç.; Bakırdere, S. Magnetic Nanoparticles Based Solid Phase Extraction Methods for the Determination of Trace Elements. Crit. Rev. Anal. Chem. 2022, 52, 231–249. [Google Scholar] [CrossRef]
- Marcelo, L.R.; De Gois, J.S.; Da Silva, A.A.; Cesar, D.V. Synthesis of Iron-Based Magnetic Nanocomposites and Applications in Adsorption Processes for Water Treatment: A Review. Environ. Chem. Lett. 2021, 19, 1229–1274. [Google Scholar] [CrossRef]
- El-Deen, A.K.; Hussain, C.M. Advances in Magnetic Analytical Extraction Techniques for Detecting Antibiotic Residues in Edible Samples. Food Chem. 2024, 450, 139381. [Google Scholar] [CrossRef]
- Wan Ibrahim, W.A.; Nodeh, H.R.; Aboul-Enein, H.Y.; Sanagi, M.M. Magnetic Solid-Phase Extraction Based on Modified Ferum Oxides for Enrichment, Preconcentration, and Isolation of Pesticides and Selected Pollutants. Crit. Rev. Anal. Chem. 2015, 45, 270–287. [Google Scholar] [CrossRef]
- de Queiroz, D.F.; de Camargo, E.R.; Martines, M.A.U. Synthesis and Characterization of Magnetic Nanoparticles of Cobalt Ferrite Coated with Silica. Biointerface Res. Appl. Chem. 2019, 10, 4908–4913. [Google Scholar] [CrossRef]
- Jaime, J.; Rangel, G.; Muñoz-Bonilla, A.; Mayoral, A.; Herrasti, P. Magnetite as a Platform Material in the Detection of Glucose, Ethanol and Cholesterol. Sens. Actuators B Chem. 2017, 238, 693–701. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, X.; Zeng, T.; Cao, D.; Zhou, Z.; Li, W.; Niu, H.; Cai, Y. Polydopamine-Coated Magnetic Nanoparticles for Enrichment and Direct Detection of Small Molecule Pollutants Coupled with MALDI-TOF-MS. ACS Appl. Mater. Interfaces 2013, 5, 1024–1030. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Zhou, Q.; Lei, P.; Cheng, S.; Wang, H.; Dong, W.; Pan, X. Recent Progress in Magnetic Polydopamine Composites for Pollutant Removal in Wastewater Treatment. Int. J. Biol. Macromol. 2024, 262, 130023. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Elucidating the Structure of Poly(Dopamine). Langmuir 2012, 28, 6428–6435. [Google Scholar] [CrossRef]
- Deng, Z.; Shang, B.; Peng, B. Polydopamine Based Colloidal Materials: Synthesis and Applications. Chem. Rec. 2018, 18, 410–432. [Google Scholar] [CrossRef]
- Kim, S.; Kim, E.M.; Yamamoto, M.; Park, H.; Shin, H. Engineering Multi-Cellular Spheroids for Tissue Engineering and Regenerative Medicine. Adv. Healthc. Mater. 2020, 9, 2000608. [Google Scholar] [CrossRef]
- Cheng, W.; Nie, J.; Xu, L.; Liang, C.; Peng, Y.; Liu, G.; Wang, T.; Mei, L.; Huang, L.; Zeng, X. pH-Sensitive Delivery Vehicle Based on Folic Acid-Conjugated Polydopamine-Modified Mesoporous Silica Nanoparticles for Targeted Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 18462–18473. [Google Scholar]
- Bardhan, R.; Lal, S.; Joshi, A.; Halas, N.J. Theranostic Nanoshells: From Probe Design to Imaging and Treatment of Cancer. Acc. Chem. Res. 2011, 44, 936–946. [Google Scholar] [CrossRef]
- Lin, J.-H.; Yu, C.-J.; Yang, Y.-C.; Tseng, W.-L. Formation of Fluorescent Polydopamine Dots from Hydroxyl Radical-Induced Degradation of Polydopamine Nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 15124–15130. [Google Scholar] [CrossRef]
- Zhou, J.; Duan, B.; Fang, Z.; Song, J.; Wang, C.; Messersmith, P.B.; Duan, H. Interfacial Assembly of Mussel-Inspired Au@Ag@ Polydopamine Core–Shell Nanoparticles for Recyclable Nanocatalysts. Adv. Mater. 2014, 26, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Zhao, Q.; Li, X.; Wang, L. Multifunctional Plasmonic Co-Doped Fe2O3 @polydopamine-Au for Adsorption, Photocatalysis, and SERS-Based Sensing. Part. Part. Syst. Charact. 2016, 33, 602–609. [Google Scholar] [CrossRef]
- Fang, Q.; Duan, S.; Zhang, J.; Li, J.; Leung, K.C.-F. Dual Shelled Fe3O4/Polydopamine Hollow Microspheres as an Effective Eu(III) Adsorbent. J. Mater. Chem. A 2017, 5, 2947–2958. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Y.; Zhang, Y.; Guo, Z.; Zhang, H.; Xin, J.H.; Zhang, L. Bioinspired Superhydrophobic Fe3O4 @Polydopamine@Ag Hybrid Nanoparticles for Liquid Marble and Oil Spill. Adv. Mater. Inter. 2015, 2, 1500234. [Google Scholar] [CrossRef]
- Kuznowicz, M.; Jędrzak, A.; Leda, A.; Rębiś, T.; Jesionowski, T. Measurements of Working Parameters of External Mediators for Biodetectors Based on the Polydopamine@magnetite Nanoparticles. Measurement 2021, 184, 109950. [Google Scholar] [CrossRef]
- Bui, T.Q.; Ton, S.N.-C.; Duong, A.T.; Tran, H.T. Size-Dependent Magnetic Responsiveness of Magnetite Nanoparticles Synthesised by Co-Precipitation and Solvothermal Methods. J. Sci. Adv. Mater. Devices 2018, 3, 107–112. [Google Scholar] [CrossRef]
- Sánchez, E.G.; Melero, M.M.; Úbeda Picot, M.Á.; Pérez-Pla, F.; Marcos, M.D.; El Haskouri, J.; Amorós, P. Nitroarene Hydrogenation Catalysts Based on Pd Nanoparticles Glued with PDA on Inorganic Supports: Multivariate Curve Resolution as an Useful Tool to Compare the Catalytic Activity in Multi-Step Reactions. Appl. Catal. A Gen. 2021, 619, 118125. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Glazyrin, K.; McCammon, C.; Dubrovinsky, L.; Merlini, M.; Schollenbruch, K.; Woodland, A.; Hanfland, M. Effect of High Pressure on the Crystal Structure and Electronic Properties of Magnetite below 25 GPa. Am. Mineral. 2012, 97, 128–133. [Google Scholar] [CrossRef]
- Mazario, E.; Sánchez-Marcos, J.; Menéndez, N.; Herrasti, P.; García-Hernández, M.; Muñoz-Bonilla, A. One-Pot Electrochemical Synthesis of Polydopamine Coated Magnetite Nanoparticles. RSC Adv. 2014, 4, 48353–48361. [Google Scholar] [CrossRef]
- Brown, H.K.; El Haskouri, J.; Marcos, M.D.; Ros-Lis, J.V.; Amorós, P.; Úbeda Picot, M.Á.; Pérez-Pla, F. Synthesis and Catalytic Activity for 2, 3, and 4-Nitrophenol Reduction of Green Catalysts Based on Cu, Ag and Au Nanoparticles Deposited on Polydopamine-Magnetite Porous Supports. Nanomaterials 2023, 13, 2162. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Xin, Q.; Wu, X.; Chen, Z.; Yan, Y.; Liu, S.; Wang, M.; Xu, Q. Selective Adsorption and Separation of Organic Dyes from Aqueous Solution on Polydopamine Microspheres. J. Colloid Interface Sci. 2016, 461, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Fang, W.; Chen, F.; Zhong, M. Preparation and Properties of a pH-Responsive PDMS Platform. In AIP Conference Proceedings; AIP: Kelantan, Malaysia, 2019; p. 040004. [Google Scholar] [CrossRef]
- International Standard ISO 2871-2.; Surface active agents – Detergents – Determination of cationic-active matter content. ISO: Geneva, Switzerland, 2010.
- Min, K.; Yang, Q.; Zhong, X.; Yan, D.; Luo, W.; Fang, Z.; Xiao, J.; Maa, M.; Chen, B. Rapid analysis of anionic and cationic surfactants in water by paper spray mass spectrometry. Anal. Methods 2021, 13, 986–995. [Google Scholar] [PubMed]
- Patel, R.; Patel, R.S. Simple and specific method for flow injection analysis determination of cationic surfactants in environmental and commodity samples. Talanta 1999, 48, 923–931. [Google Scholar] [CrossRef]
- Agrawal, K.; Agnihotri, G.; Shrivas, K.; Mundhara, G.L.; Patel, K.S.; Hoffmann, P. Determination of Cationic Surfactants in Environmental Samples by Flow Injection Analysis. Microchim. Acta 2004, 147, 273–278. [Google Scholar] [CrossRef]
- El Hamd, M.A.; El-Maghrabey, M.; Abdel-Lateef, M.A.; Ali, S.M.; Ibrahim, M.M.; El-Shahat, M.F.; Azeem, S.M.A. Flow Injection Sensing Strategy for Determining Cationic Surfactants in Commodity and Water Samples. Chemosensors 2022, 10, 434. [Google Scholar] [CrossRef]

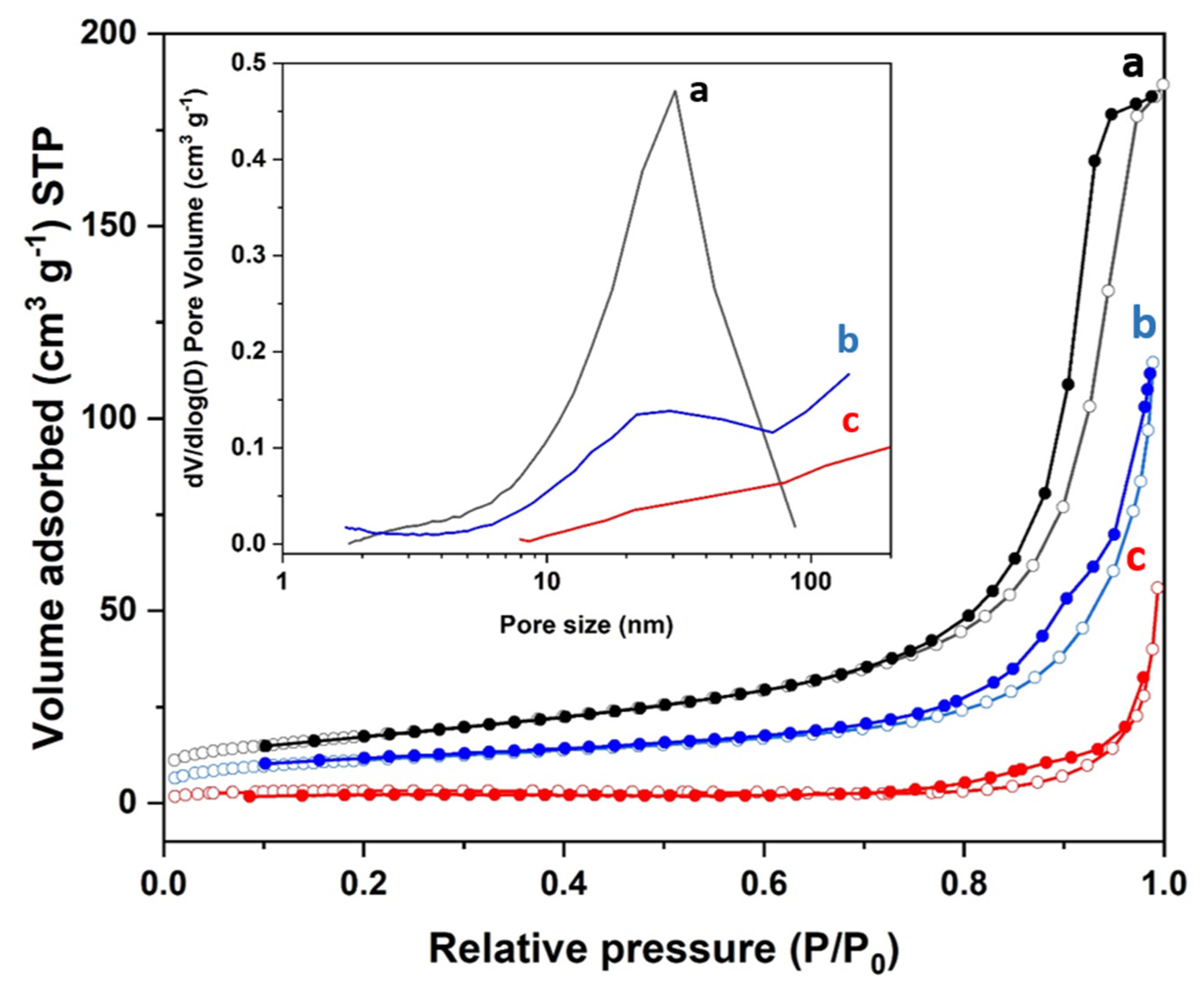

| Sample | Z Potential (mV) | Particle Size (nm) a | Organic Content (mg/g of Material) b | Area (m2/g of Material) c | Pore Size (nm) d | Pore Volume (cm3/g of Material) d |
|---|---|---|---|---|---|---|
| Fe3O4 | −1.5 | 2700 ± 200 | 0 | 61.9 | 18.9 | 0.28 |
| Fe3O4@PDA-1 | −31.7 | 369 ± 9 | 170 | 39.5 | 22.1 | 0.18 |
| Fe3O4@PDA-2 | −30 | 781 ± 17 | 410 | 9.8 | 53.2 | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Núñez, A.; Pérez-Pla, F.; Úbeda, M.Á.; Amorós, P.; Ros-Lis, J.V. Polydopamine-Coated Magnetite as a Sensing Material for the Optical Detection of Cationic Surfactants. Chemosensors 2025, 13, 125. https://doi.org/10.3390/chemosensors13040125
Fernández-Núñez A, Pérez-Pla F, Úbeda MÁ, Amorós P, Ros-Lis JV. Polydopamine-Coated Magnetite as a Sensing Material for the Optical Detection of Cationic Surfactants. Chemosensors. 2025; 13(4):125. https://doi.org/10.3390/chemosensors13040125
Chicago/Turabian StyleFernández-Núñez, Alberto, Francisco Pérez-Pla, Maria Ángeles Úbeda, Pedro Amorós, and José V. Ros-Lis. 2025. "Polydopamine-Coated Magnetite as a Sensing Material for the Optical Detection of Cationic Surfactants" Chemosensors 13, no. 4: 125. https://doi.org/10.3390/chemosensors13040125
APA StyleFernández-Núñez, A., Pérez-Pla, F., Úbeda, M. Á., Amorós, P., & Ros-Lis, J. V. (2025). Polydopamine-Coated Magnetite as a Sensing Material for the Optical Detection of Cationic Surfactants. Chemosensors, 13(4), 125. https://doi.org/10.3390/chemosensors13040125







