Electrochemical Detection of Dopamine with a Non-Enzymatic Sensor Based on Au@SiO2-APTES Composite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Au@SiO2-APTES
2.3. Assembly of Au@SiO2-APTES/GCEs
2.4. Characterisation and Electrochemical Measurements
3. Results and Discussion
3.1. Material Characterisation
3.1.1. Morphology and Structure of Au@SiO2-APTES
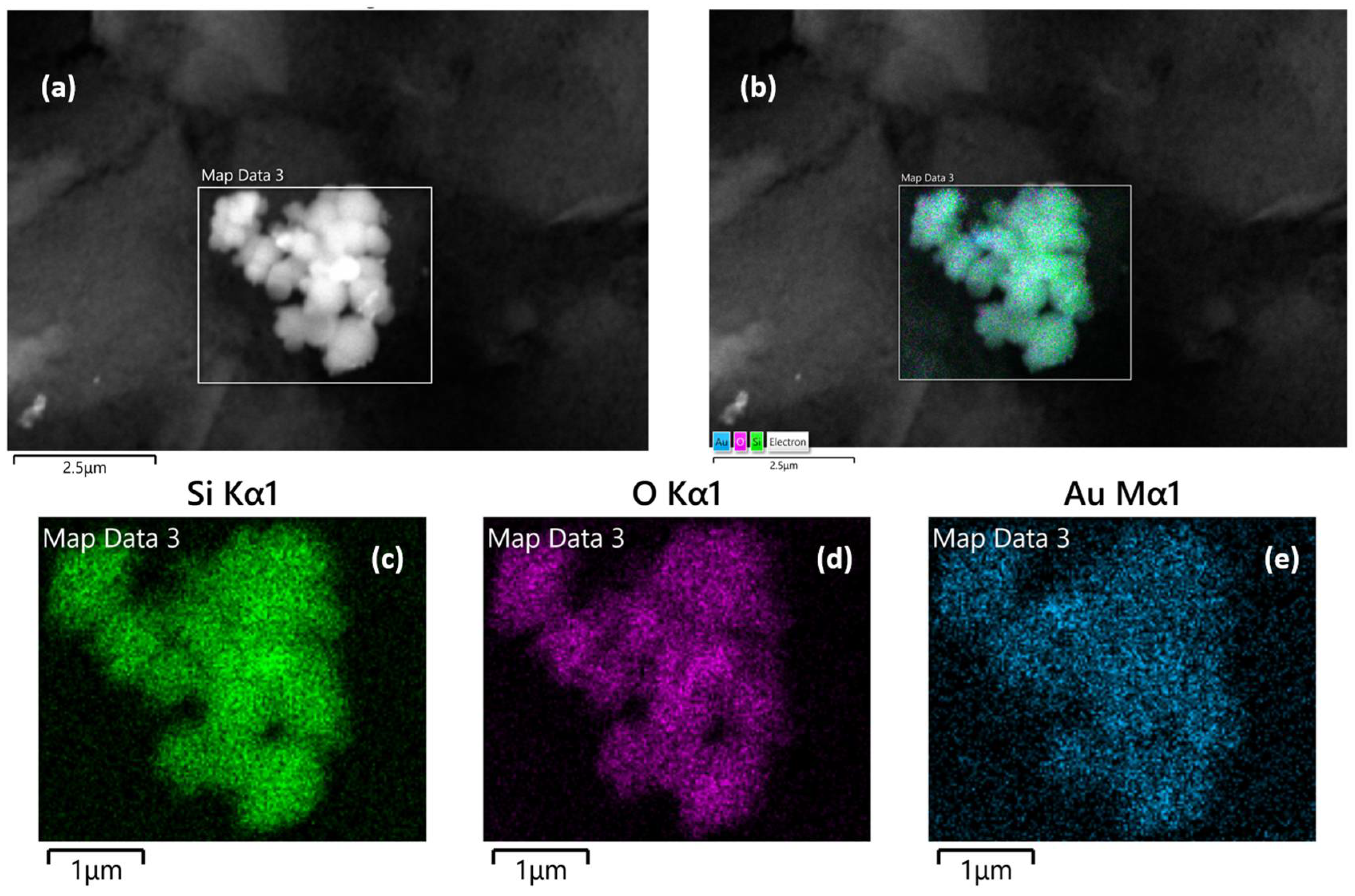
3.1.2. Electron Microscopy Analysis
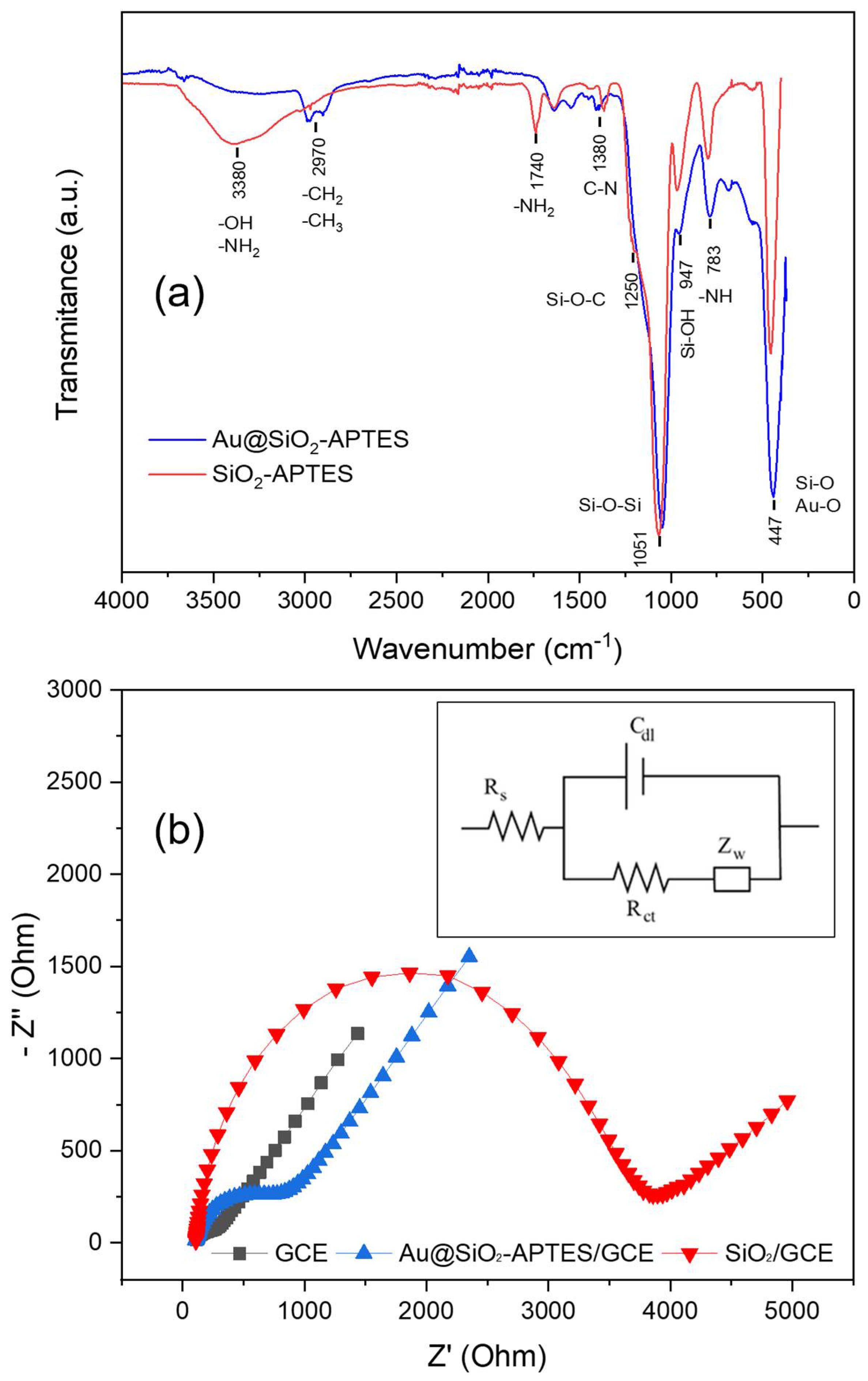
3.1.3. Infrared Spectrum Characterisation
3.1.4. Electrochemical Characterisation of SiO2-APTES and Au@SiO2-APTES Nanocomposites
3.2. Analytical and Electrochemical Optimisations
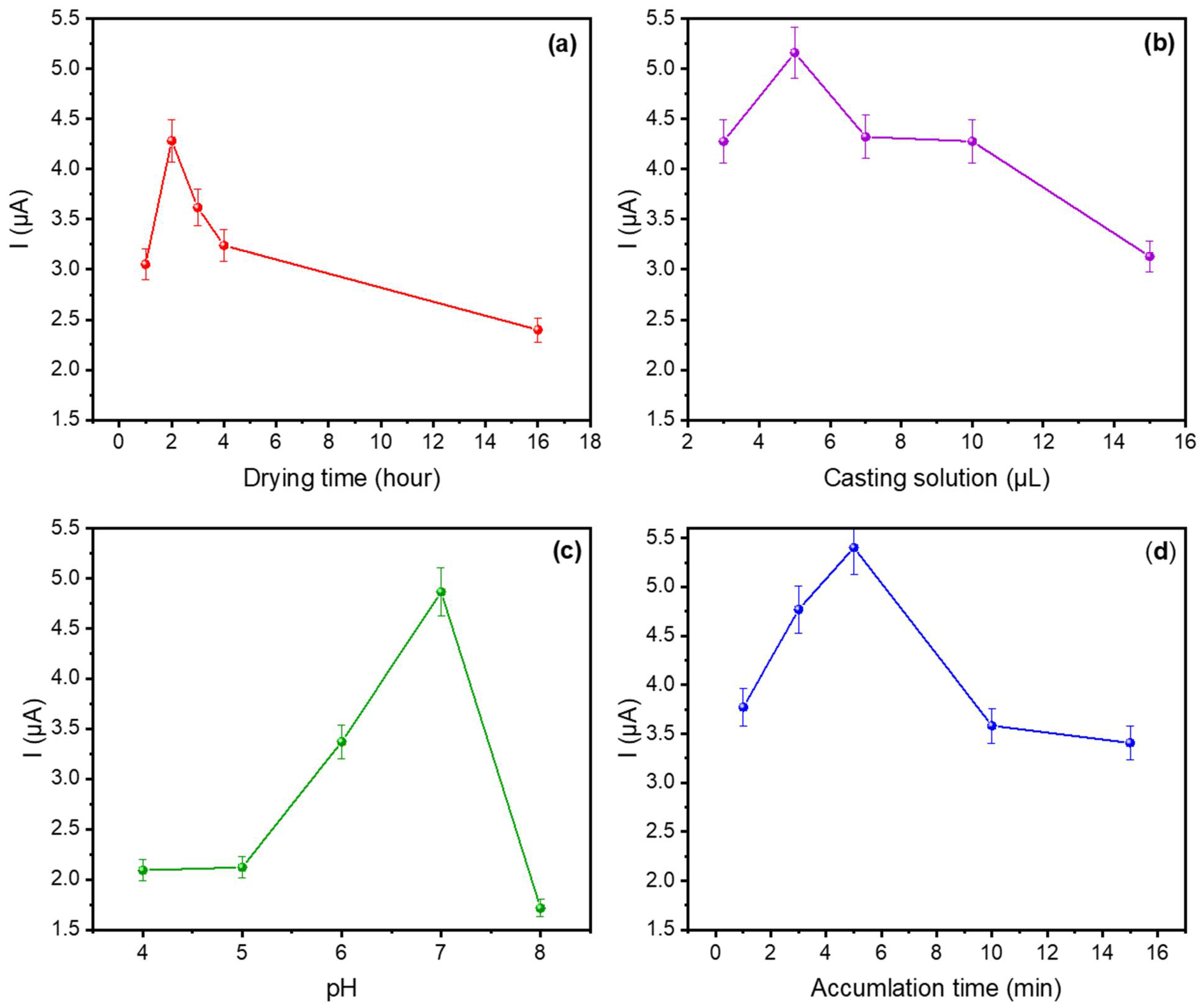
3.2.1. Optimisation of Experimental Conditions
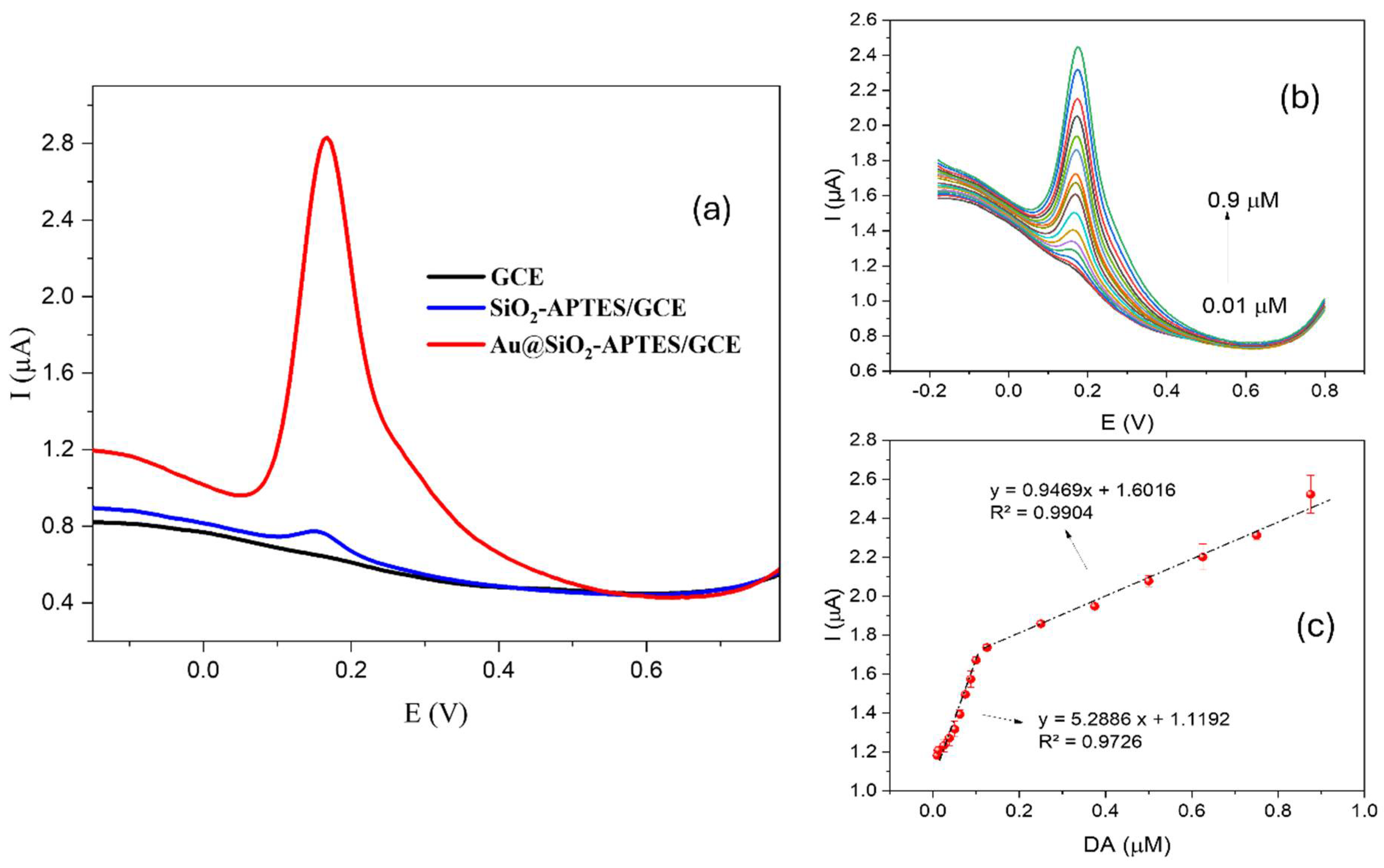
3.2.2. DA Detection
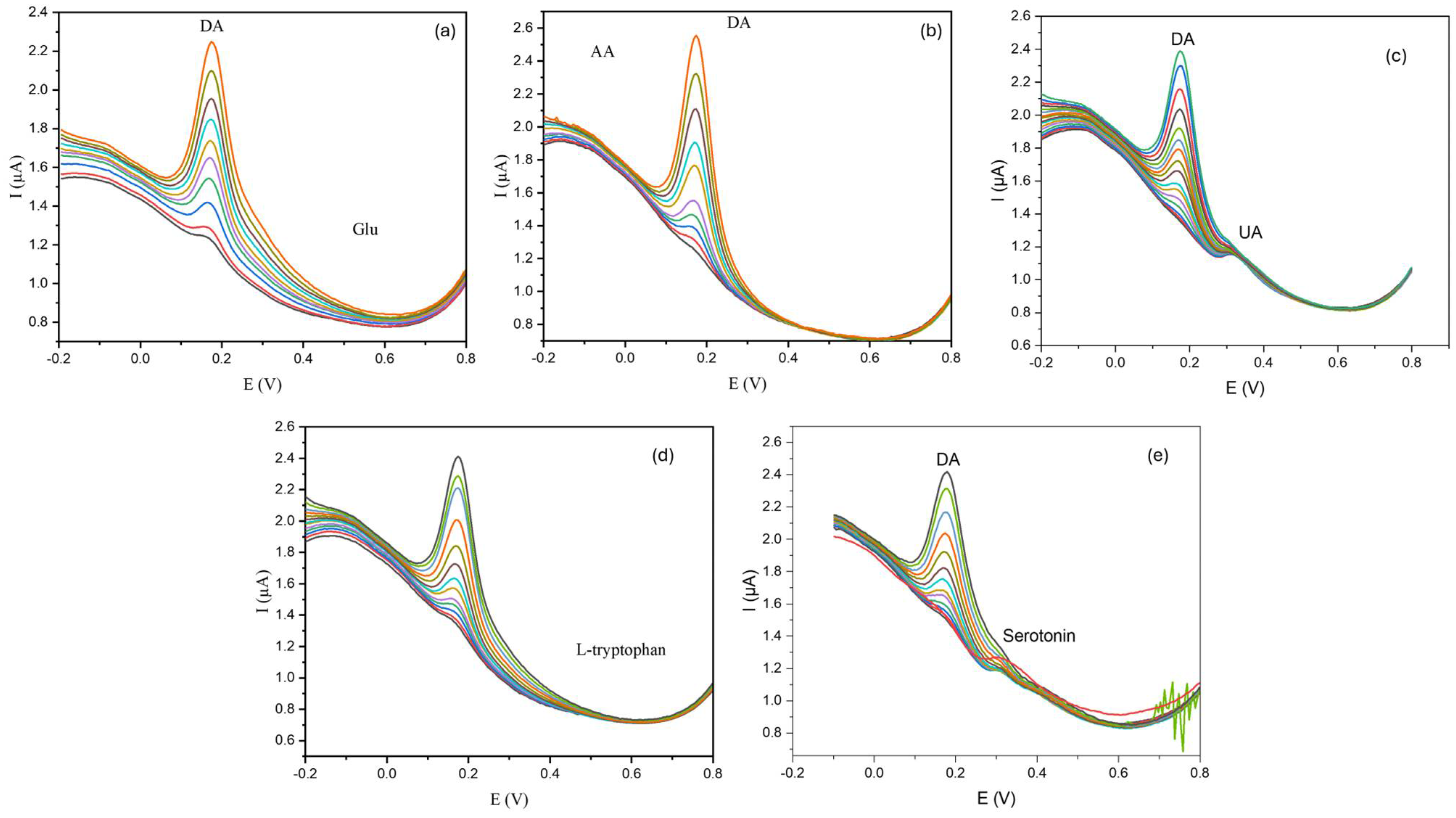
3.2.3. Interference Study
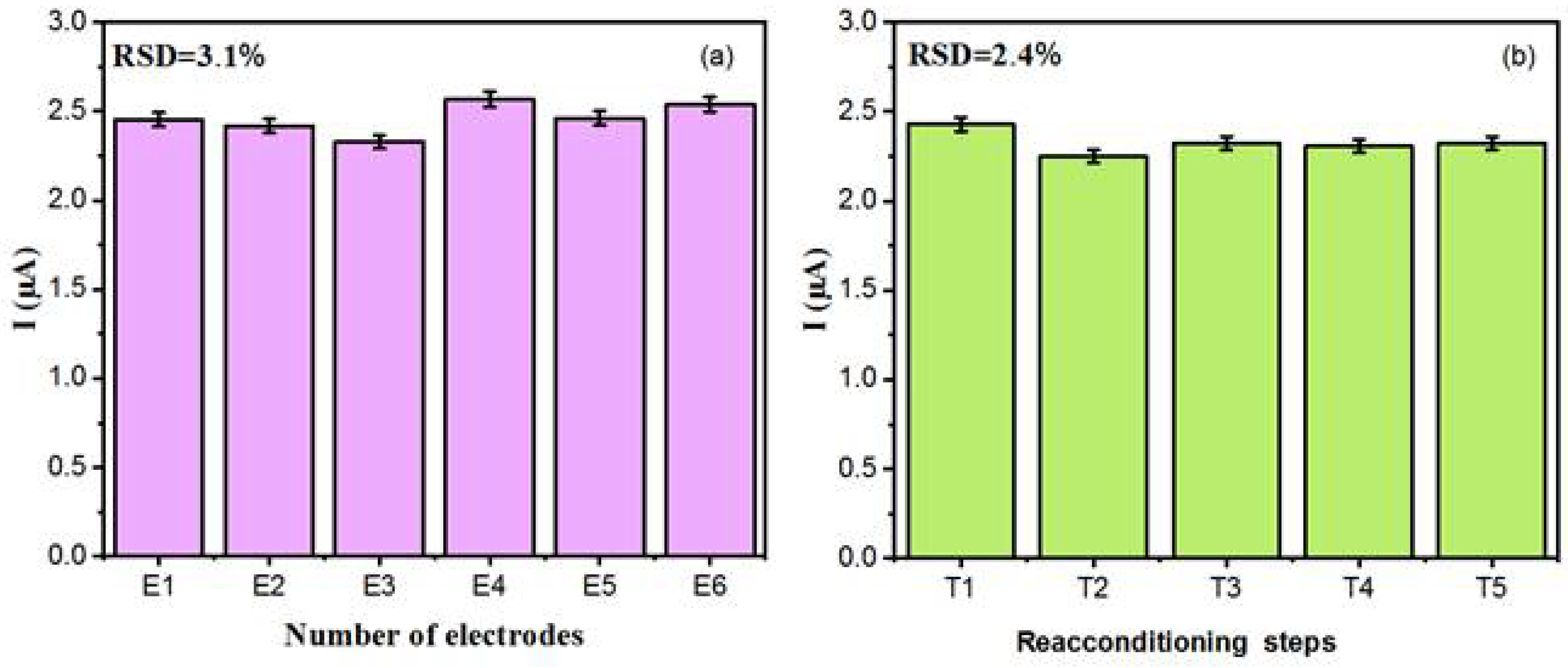
3.2.4. Reproducibility and Repeatability Test
3.2.5. Applications in Biological Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Mekuye, B.; Abera, B. Nanomaterials: An overview of synthesis, classification, characterization, and applications. Nano Sel. 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Wang, P.; Lombi, E.; Zhao, F.-J.; Kopittke, P.M. Nanotechnology: A New Opportunity in Plant Sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Dimkić, I.; Stanković, S.; Nišavić, M.; Petković, M.; Ristivojević, P.; Fira, D.; Berić, T. Corrigendum: The Profile and Antimicrobial Activity of Bacillus Lipopeptide Extracts of Five Potential Biocontrol Strains. Front. Microbiol. 2017, 8, 1500. [Google Scholar] [CrossRef]
- Yang, D.; Hartman, M.R.; Derrien, T.L.; Hamada, S.; An, D.; Yancey, K.G.; Cheng, R.; Ma, M.; Luo, D. DNA Materials: Bridging Nanotechnology and Biotechnology. Acc. Chem. Res. 2014, 47, 1902–1911. [Google Scholar] [CrossRef]
- Cao, G.; Wang, Y. Nanostructures and Nanomaterials: Synthesis, Properties, and Applications; World Scientific: Singapore, 2011; Volume 2, p. 596. [Google Scholar]
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef]
- Selvarajan, S.; Suganthi, A.; Rajarajan, M. A facile approach to synthesis of mesoporous SnO2/chitosan nanocomposite modified electrode for simultaneous determination of ascorbic acid, dopamine and uric acid. Surf. Interfaces 2017, 7, 146–156. [Google Scholar] [CrossRef]
- Sangamithirai, D.; Munusamy, S.; Narayanan, V.; Stephen, A. Fabrication of neurotransmitter dopamine electrochemical sensor based on poly(o-anisidine)/CNTs nanocomposite. Surf. Interfaces 2016, 4, 27–34. [Google Scholar] [CrossRef]
- Immanuel, S.; Aparna, T.K.; Sivasubramanian, R. A facile preparation of Au—SiO2 nanocomposite for simultaneous electrochemical detection of dopamine and uric acid. Surf. Interfaces 2019, 14, 82–91. [Google Scholar] [CrossRef]
- Ramya, M.; Senthil Kumar, P.; Rangasamy, G.; Uma Shankar, V.; Rajesh, G.; Nirmala, K.; Saravanan, A.; Krishnapandi, A. A recent advancement on the applications of nanomaterials in electrochemical sensors and biosensors. Chemosphere 2022, 308, 136416. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Wang, Z.; Zhang, W.; Liu, X.; Li, M.; Li, G.; Zhang, B.; Singh, R. Optically Active Nanomaterials and Its Biosensing Applications & mdash;A Review. Biosensors 2023, 13, 85. [Google Scholar]
- Pirzada, M.; Altintas, Z. Nanomaterials for Healthcare Biosensing Applications. Sensors 2019, 19, 5311. [Google Scholar] [CrossRef]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2, 63. [Google Scholar] [CrossRef]
- Su, S.; Wu, W.; Gao, J.; Lu, J.; Fan, C. Nanomaterials-based sensors for applications in environmental monitoring. J. Mater. Chem. 2012, 22, 18101–18110. [Google Scholar] [CrossRef]
- Kujawska, M.; Bhardwaj, S.K.; Mishra, Y.K.; Kaushik, A. Using Graphene-Based Biosensors to Detect Dopamine for Efficient Parkinson’s Disease Diagnostics. Biosensors 2021, 11, 433. [Google Scholar] [CrossRef]
- Nichkova, M.; Wynveen, P.M.; Marc, D.T.; Huisman, H.; Kellermann, G.H. Validation of an ELISA for urinary dopamine: Applications in monitoring treatment of dopamine-related disorders. J. Neurochem. 2013, 125, 724–735. [Google Scholar] [CrossRef]
- Ryding, E.; Lindström, M.; Träskman-Bendz, L. The role of dopamine and serotonin in suicidal behaviour and aggression. In Progress in Brain Research; Di Giovann, G., Di Matteo, V., Esposito, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 172, pp. 307–315. [Google Scholar]
- Dong, M.-X.; Chen, G.-H.; Hu, L. Dopaminergic System Alteration in Anxiety and Compulsive Disorders: A Systematic Review of Neuroimaging Studies. Front. Neurosci. 2020, 14, 608520. [Google Scholar] [CrossRef]
- Post, M.R.; Sulzer, D. The chemical tools for imaging dopamine release. Cell Chem. Biol. 2021, 28, 748–764. [Google Scholar] [CrossRef]
- Juárez Olguín, H.; Calderón Guzmán, D.; Hernández García, E.; Barragán Mejía, G. The Role of Dopamine and Its Dysfunction as a Consequence of Oxidative Stress. Oxidative Med. Cell. Longev. 2016, 2016, 9730467. [Google Scholar] [CrossRef]
- Wise, R.A.; Jordan, C.J. Dopamine, behavior, and addiction. J. Biomed. Sci. 2021, 28, 83. [Google Scholar] [CrossRef] [PubMed]
- Davidson, D.F. Elevated urinary dopamine in adults and children. Ann. Clin. Biochem. 2005, 42, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, C.; Wang, C.; Pu, F.; Ren, J.; Qu, X. Silver nanoprobe for sensitive and selective colorimetric detection of dopaminevia robust Ag–catechol interaction. Chem. Commun. 2011, 47, 1181–1183. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, A.; Khajehzadeh, A.; Ghaffarinejad, A. A simple and cost-effective method, as an appropriate alternative for visible spectrophotometry: Development of a dopamine biosensor. Analyst 2009, 134, 1692–1698. [Google Scholar] [CrossRef]
- Abrantes Dias, A.S.; Amaral Pinto, J.C.; Magalhães, M.; Mendes, V.M.; Manadas, B. Analytical methods to monitor dopamine metabolism in plasma: Moving forward with improved diagnosis and treatment of neurological disorders. J. Pharm. Biomed. Anal. 2020, 187, 113323. [Google Scholar] [CrossRef]
- He, C.; Tao, M.; Zhang, C.; He, Y.; Xu, W.; Liu, Y.; Zhu, W. Microelectrode-Based Electrochemical Sensing Technology for in Vivo Detection of Dopamine: Recent Developments and Future Prospects. Crit. Rev. Anal. Chem. 2022, 52, 544–554. [Google Scholar] [CrossRef]
- Anuar, N.S.; Basirun, W.J.; Shalauddin, M.; Akhter, S. A dopamine electrochemical sensor based on a platinum–silver graphene nanocomposite modified electrode. RSC Adv. 2020, 10, 17336–17344. [Google Scholar] [CrossRef]
- Lakard, S.; Pavel, I.A.; Lakard, B. Electrochemical Biosensing of Dopamine Neurotransmitter: A Review. Biosensors 2021, 11, 179. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Holmes, C.; Sharabi, Y. Cerebrospinal fluid biomarkers of central catecholamine deficiency in Parkinson’s disease and other synucleinopathies. Brain 2012, 135, 1900–1913. [Google Scholar] [CrossRef]
- Andersen, A.D.; Blaabjerg, M.; Binzer, M.; Kamal, A.; Thagesen, H.; Kjaer, T.W.; Stenager, E.; Gramsbergen, J.B.P. Cerebrospinal fluid levels of catecholamines and its metabolites in Parkinson’s disease: Effect of l-DOPA treatment and changes in levodopa-induced dyskinesia. J. Neurochem. 2017, 141, 614–625. [Google Scholar] [CrossRef]
- Beatto, T.G.; Gomes, W.E.; Etchegaray, A.; Gupta, R.; Mendes, R.K. Dopamine levels determined in synthetic urine using an electrochemical tyrosinase biosensor based on ZnO@Au core–shell. RSC Adv. 2023, 13, 33424–33429. [Google Scholar] [CrossRef] [PubMed]
- Ping, J.; Wu, J.; Wang, Y.; Ying, Y. Simultaneous determination of ascorbic acid, dopamine and uric acid using high-performance screen-printed graphene electrode. Biosens. Bioelectron. 2012, 34, 70–76. [Google Scholar] [CrossRef]
- Huang, K.-J.; Jing, Q.-S.; Wu, Z.-W.; Wang, L.; Wei, C.-Y. Enhanced sensing of dopamine in the present of ascorbic acid based on graphene/poly(p-aminobenzoic acid) composite film. Colloids Surf. B Biointerfaces 2011, 88, 310–314. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Meng, W.; Han, C.; Leng, C. Electrochemical Dopamine Detection using a Fe/Fe3O4@C Composite derived from a Metal-Organic Framework. ChemistrySelect 2022, 7, e202201534. [Google Scholar] [CrossRef]
- Kim, D.-S.; Kang, E.-S.; Baek, S.; Choo, S.-S.; Chung, Y.-H.; Lee, D.; Min, J.; Kim, T.-H. Electrochemical detection of dopamine using periodic cylindrical gold nanoelectrode arrays. Sci. Rep. 2018, 8, 14049. [Google Scholar] [CrossRef]
- Castagnola, E.; Robbins, E.M.; Wu, B.; Pwint, M.Y.; Garg, R.; Cohen-Karni, T.; Cui, X.T. Flexible Glassy Carbon Multielectrode Array for In Vivo Multisite Detection of Tonic and Phasic Dopamine Concentrations. Biosensors 2022, 12, 540. [Google Scholar] [CrossRef]
- Demuru, S.; Nela, L.; Marchack, N.; Holmes, S.J.; Farmer, D.B.; Tulevski, G.S.; Lin, Q.; Deligianni, H. Scalable Nanostructured Carbon Electrode Arrays for Enhanced Dopamine Detection. ACS Sens. 2018, 3, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.-H.; Zheng, X.-Q.; Xu, J.-Y.; Bao, W.-J.; Wang, F.-B.; Xia, X.-H. Electrochemical sensor based on nitrogen doped graphene: Simultaneous determination of ascorbic acid, dopamine and uric acid. Biosens. Bioelectron. 2012, 34, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Kan, X.; Zhao, Y.; Geng, Z.; Wang, Z.; Zhu, J.-J. Composites of Multiwalled Carbon Nanotubes and Molecularly Imprinted Polymers for Dopamine Recognition. J. Phys. Chem. C 2008, 112, 4849–4854. [Google Scholar] [CrossRef]
- Muddapur, U.M.; Alshehri, S.; Ghoneim, M.M.; Mahnashi, M.H.; Alshahrani, M.A.; Khan, A.A.; Iqubal, S.M.S.; Bahafi, A.; More, S.S.; Shaikh, I.A.; et al. Plant-Based Synthesis of Gold Nanoparticles and Theranostic Applications: A Review. Molecules 2022, 27, 1391. [Google Scholar] [CrossRef]
- Patel, K.N.; Trivedi, P.G.; Thakar, M.S.; Prajapati, K.V.; Prajapati, D.K.; Sindhav, G.M. Gold nanoparticles synthesis using Gymnosporia montana L. and its biological profile: A pioneer report. J. Genet. Eng. Biotechnol. 2023, 21, 71. [Google Scholar] [CrossRef] [PubMed]
- Dhaffouli, A.; Salazar-Carballo, P.A.; Carinelli, S.; Holzinger, M.; Barhoumi, H. Improved electrochemical sensor using functionalized silica nanoparticles (SiO2-APTES) for high selectivity detection of lead ions. Mater. Chem. Phys. 2024, 318, 129253. [Google Scholar] [CrossRef]
- Rao, X.; Abou Hassan, A.; Guyon, C.; Zhang, M.; Ognier, S.; Tatoulian, M. Plasma Polymer Layers with Primary Amino Groups for Immobilization of Nano- and Microparticles. Plasma Chem. Plasma Process. 2020, 40, 589–606. [Google Scholar] [CrossRef]
- Dhaffouli, A.; Salazar-Carballo, P.A.; Mabrouk, C.; Carinelli, S.; Holzinger, M.; Barhoumi, H. Synthesis, characterization, and application of ZnO@SiO2-APTES core-shell composite for selective electrochemical detection of Pb2+ ions. Sens. Actuators A Phys. 2024, 373, 115416. [Google Scholar] [CrossRef]
- Yamada, K.; Yoshii, S.; Kumagai, S.; Fujiwara, I.; Nishio, K.; Okuda, M.; Matsukawa, N.; Yamashita, I. High-Density and Highly Surface Selective Adsorption of Protein–Nanoparticle Complexes by Controlling Electrostatic Interaction. Jpn. J. Appl. Phys. 2006, 45, 4259. [Google Scholar] [CrossRef]
- Carinelli, S.; Fernández, I.; Luis González-Mora, J.; Salazar-Carballo, P.A. Hemoglobin-modified nanoparticles for electrochemical determination of haptoglobin: Application in bovine mastitis diagnosis. Microchem. J. 2022, 179, 107528. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, R.; Chai, Y.; Li, W.; Zhong, X.; Zhong, H. Simultaneous voltammetric determination for DA, AA and NO2− based on graphene/poly-cyclodextrin/MWCNTs nanocomposite platform. Biosens. Bioelectron. 2011, 26, 3977–3980. [Google Scholar] [CrossRef]
- Tan, L.; Zhou, K.-G.; Zhang, Y.-H.; Wang, H.-X.; Wang, X.-D.; Guo, Y.-F.; Zhang, H.-L. Nanomolar detection of dopamine in the presence of ascorbic acid at β-cyclodextrin/graphene nanocomposite platform. Electrochem. Commun. 2010, 12, 557–560. [Google Scholar] [CrossRef]
- Liu, B.; Lian, H.T.; Yin, J.F.; Sun, X.Y. Dopamine molecularly imprinted electrochemical sensor based on graphene–chitosan composite. Electrochim. Acta 2012, 75, 108–114. [Google Scholar] [CrossRef]
- Fan, Y.; Lu, H.-T.; Liu, J.-H.; Yang, C.-P.; Jing, Q.-S.; Zhang, Y.-X.; Yang, X.-K.; Huang, K.-J. Hydrothermal preparation and electrochemical sensing properties of TiO2–graphene nanocomposite. Colloids Surf. B Biointerfaces 2011, 83, 78–82. [Google Scholar] [CrossRef]
- Ma, X.; Chao, M.; Wang, Z. Electrochemical detection of dopamine in the presence of epinephrine, uric acid and ascorbic acid using a graphene-modified electrode. Anal. Methods 2012, 4, 1687–1692. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Hou, H.; You, T. Simultaneous electrochemical determination of dopamine, uric acid and ascorbic acid using palladium nanoparticle-loaded carbon nanofibers modified electrode. Biosens. Bioelectron. 2008, 24, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-F.; Kumar, S.A.; Chen, S.-M. Zinc oxide/redox mediator composite films-based sensor for electrochemical detection of important biomolecules. Anal. Biochem. 2008, 380, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.-S.; Chen, Y.-L.; Lee, C.-Y.; Chiu, H.-T. Gold Nanostructures on Flexible Substrates as Electrochemical Dopamine Sensors. ACS Appl. Mater. Interfaces 2012, 4, 5570–5575. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhao, Q.; Tan, F.; Wang, X.; Gao, J. Simultaneous detection of dopamine, uric acid, and ascorbic acid using SnO2 nanoparticles/multi-walled carbon nanotubes/carbon paste electrode. Anal. Methods 2012, 4, 3283–3289. [Google Scholar] [CrossRef]
- Peik-See, T.; Pandikumar, A.; Nay-Ming, H.; Hong-Ngee, L.; Sulaiman, Y. Simultaneous Electrochemical Detection of Dopamine and Ascorbic Acid Using an Iron Oxide/Reduced Graphene Oxide Modified Glassy Carbon Electrode. Sensors 2014, 14, 15227–15243. [Google Scholar] [CrossRef]
- Sun, H.; Chao, J.; Zuo, X.; Su, S.; Liu, X.; Yuwen, L.; Fan, C.; Wang, L. Gold nanoparticle-decorated MoS2 nanosheets for simultaneous detection of ascorbic acid, dopamine and uric acid. RSC Adv. 2014, 4, 27625–27629. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Y.; Xu, Z.; Wang, S.; Chen, B.; Zhang, D.; Fang, Y. Simultaneous Detection of Ascorbic Acid, Dopamine, and Uric Acid Using a Novel Electrochemical Sensor Based on Palladium Nanoparticles/Reduced Graphene Oxide Nanocomposite. Int. J. Anal. Chem. 2020, 2020, 8812443. [Google Scholar] [CrossRef]
- Sun, C.-L.; Lee, H.-H.; Yang, J.-M.; Wu, C.-C. The simultaneous electrochemical detection of ascorbic acid, dopamine, and uric acid using graphene/size-selected Pt nanocomposites. Biosens. Bioelectron. 2011, 26, 3450–3455. [Google Scholar] [CrossRef]
- Murali, A.; Lan, Y.P.; Sarswat, P.K.; Free, M.L. Synthesis of CeO2/reduced graphene oxide nanocomposite for electrochemical determination of ascorbic acid and dopamine and for photocatalytic applications. Mater. Today Chem. 2019, 12, 222–232. [Google Scholar] [CrossRef]
- Vinay, M.M.; Arthoba Nayaka, Y. Iron oxide (Fe2O3) nanoparticles modified carbon paste electrode as an advanced material for electrochemical investigation of paracetamol and dopamine. J. Sci. Adv. Mater. Devices 2019, 4, 442–450. [Google Scholar] [CrossRef]
- Aparna, T.K.; Sivasubramanian, R.; Dar, M.A. One-pot synthesis of Au-Cu2O/rGO nanocomposite based electrochemical sensor for selective and simultaneous detection of dopamine and uric acid. J. Alloys Compd. 2018, 741, 1130–1141. [Google Scholar] [CrossRef]
- Maseed, H.; Reddy Yenugu, V.M.; Devarakonda, S.S.; Petnikota, S.; Gajulapalli, M.; Srikanth, V.V.S.S. Peroxidase-like Fe3O4 Nanoparticle/Few-Layered Graphene Composite for Electrochemical Detection of Dopamine, Ascorbic Acid, and Uric Acid. ACS Appl. Nano Mater. 2023, 6, 18531–18538. [Google Scholar] [CrossRef]
- Tan, C.; Zhao, J.; Sun, P.; Zheng, W.; Cui, G. Gold nanoparticle decorated polypyrrole/graphene oxide nanosheets as a modified electrode for simultaneous determination of ascorbic acid, dopamine and uric acid. New J. Chem. 2020, 44, 4916–4926. [Google Scholar] [CrossRef]


| Modified Electrode | Electrochemical Technique | Working Range (µmolL−1) | LOD (µmolL−1) | References |
|---|---|---|---|---|
| Au-SiO2/GCE | DPV | 10–100, 200–500 | 1.98 | [11] |
| Graphene/poly cyclodextrin/MWCNTs | DPV | 0.15–21.65 | 0.05 | [49] |
| Au nanoparticles-graphene | DPV | 0.1–10 | 0.04 | [50] |
| Graphene-CTAB | DPV | 4–52 | 0.6 | [51] |
| TiO2-graphene | DPV | 5–200 | 2 | [52] |
| MIPs-GR | DPV | 0.001–0.1, 0.1–100 | 0.00001 | [51] |
| Graphene | CV | 2.5–100 | 0.5 | [53] |
| Pd-CNFs | DPV | 0.5–160 | 0.2 | [54] |
| ZnO/RM | CV | 6–960 | 0.7 | [55] |
| Au NS/PET | Amperometry | 0.2− 600 | 0.026 | [56] |
| SWCNTs | DPV | 0.3–50 | 0.03 | [57] |
| Fe3O4/rGO | DPV | 0.5–10 | 0.12 | [58] |
| AuNPs@MoS2 | DPV | 0.05–30 | 0.05 | [59] |
| nanoSnO2/MWCNTs | DPV | 0.3–50 | 0.03 | [59] |
| Au@Pd-RGO | DPV | 0.01–100 | 0.002 | [60] |
| Graphene@Pt/GCE | Amperometry | 0.03–8.13 | 0.03 | [61] |
| Fe3O4@PPy/rGO | DPV | 0–100 | 0.063 | [61] |
| CeO2/rGO | DPV | 10–150 | 2 | [62] |
| Fe2O3/CPE | DPV | 2–170 | 0.79 | [63] |
| Au-Cu2O/rGO | DPV | 10–90 | 3.9 | [64] |
| Fe3O4/FLG | Amperometry | 0.003−0.019 0.023−0.062 | 0.0004 | [65] |
| AuNPs@GO/PPy/CFP | DPV | 0.2–60 | 0.12 | [66] |
| Au@SiO2-APTES | DPV | 0.047–0.125 0.125–0.875 | 0.014 | Current work |
| Sample | Added (×10−7 molL−1) | Found (×10−7 molL−1) | Recovery (%) |
|---|---|---|---|
| Blood | - | 0.7 | - |
| 3.7 | 4.4 | 100.0 | |
| 6.2 | 6.4 | 92.8 | |
| Urine | - | 0.9 | - |
| 1 | 1.7 | 89.5 | |
| 7.5 | 8.6 | 102.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhaffouli, A.; Salazar-Carballo, P.A.; Carinelli, S.; Holzinger, M.; Rodrigues, B.V.M.; Barhoumi, H. Electrochemical Detection of Dopamine with a Non-Enzymatic Sensor Based on Au@SiO2-APTES Composite. Chemosensors 2025, 13, 87. https://doi.org/10.3390/chemosensors13030087
Dhaffouli A, Salazar-Carballo PA, Carinelli S, Holzinger M, Rodrigues BVM, Barhoumi H. Electrochemical Detection of Dopamine with a Non-Enzymatic Sensor Based on Au@SiO2-APTES Composite. Chemosensors. 2025; 13(3):87. https://doi.org/10.3390/chemosensors13030087
Chicago/Turabian StyleDhaffouli, Afef, Pedro A. Salazar-Carballo, Soledad Carinelli, Michael Holzinger, Bruno V. M. Rodrigues, and Houcine Barhoumi. 2025. "Electrochemical Detection of Dopamine with a Non-Enzymatic Sensor Based on Au@SiO2-APTES Composite" Chemosensors 13, no. 3: 87. https://doi.org/10.3390/chemosensors13030087
APA StyleDhaffouli, A., Salazar-Carballo, P. A., Carinelli, S., Holzinger, M., Rodrigues, B. V. M., & Barhoumi, H. (2025). Electrochemical Detection of Dopamine with a Non-Enzymatic Sensor Based on Au@SiO2-APTES Composite. Chemosensors, 13(3), 87. https://doi.org/10.3390/chemosensors13030087










