Abstract
Uric acid (UA), the final metabolic product of purines, plays a crucial role in human health monitoring. The UA concentration in biological fluids serves as a diagnostic marker for various disorders, particularly kidney diseases, and represents a potential therapeutic target. Given the growing emphasis on preventive healthcare, developing methods for real-time UA detection has become increasingly significant. Here, we demonstrate the synthesis of novel tumbleweed-like molybdenum diselenide (MoSe2) nanostructures through a single-step hydrothermal process. The synthesized MoSe2 was subsequently hybridized with reduced graphene oxide (rGO) to construct electrodes for UA sensing. Differential pulse voltammetry (DPV) measurements revealed that the MoSe2/rGO-modified glassy carbon electrode (GCE) exhibited excellent UA detection capabilities under optimized conditions. The sensor demonstrated a remarkably low limit of detection (LOD) of 28.4 nM and maintained linearity across a wide concentration range (40 nM to 200 μM). Notably, the sensor showed high selectivity for UA detection even in the presence of common interfering species, including citric acid (CA), dopamine (DA), ascorbic acid (AA), cysteine (Cys), glucose (Glu), oxalic acid (OA), sodium ions (Na+), and potassium ions (K+). The developed sensor displayed outstanding selectivity, stability, and reproducibility characteristics. This synthetic approach offers promising opportunities for developing MoSe2-based electrochemical sensing platforms suitable for diverse bioanalytical applications.
1. Introduction
UA, a crucial end product of purine metabolism in humans, requires careful monitoring of its concentrations in serum and urine for effective health management [1]. Contemporary improvements in dietary standards have significantly influenced UA metabolism, leading to an increased prevalence of UA-related disorders [2,3]. In healthy individuals, the normal range of UA concentration in serum is typically 200–420 μM for males and 140–360 μM for females. In human urine, the normal range of UA excretion is generally 1.5–4.5 mmol/24 h (≈0.7–4.4 mM) [4,5]. Insufficient UA levels correlate with elevated risks of various conditions, including diabetes mellitus and multiple sclerosis [6,7]. Conversely, elevated UA concentrations contribute to the development of multiple pathological conditions, including nephrolithiasis, gouty arthritis, obesity, and various cardiovascular and neurological disorders [8,9]. Although numerous analytical techniques have been established for UA detection—including ion chromatography [10], fluorescence spectroscopy [11], spectrophotometry [12], capillary electrophoresis [13], colorimetry [14], and high-performance liquid chromatography [15]—these conventional methods present significant limitations. Their time-intensive nature, requirement for extensive sample preparation, and high operational costs render them impractical for rapid, point-of-care UA monitoring [16,17]. In contrast, electrochemical detection methods have emerged as superior alternatives [18], offering the advantages of cost-effectiveness, operational simplicity [19], enhanced sensitivity [20], and excellent selectivity in UA quantification [21,22].
Graphene and its derivatives have emerged as prominent materials for electrochemical UA detection sensors, owing to their rapid electron transport, high surface area, excellent biocompatibility, and superior mechanical properties [23,24,25,26]. Despite these advantages, electrochemical electrodes fabricated from unmodified carbon-based materials exhibit insufficient sensitivity for practical UA detection applications [27,28]. To enhance performance, researchers have focused on modifying graphene and its derivatives to optimize charge transfer during UA electrocatalytic oxidation, resulting in composite materials with improved catalytic capabilities [29,30]. Notable examples include the work of Aparna et al. [31], who developed Au-Cu2O/rGO nanocomposites through a one-pot synthesis method, achieving UA detection with a linear range of 100–900 μM and a LOD of 6.5 μM. Similarly, Darabi et al. [32] employed microwave-assisted synthesis to create rGO/polypyrrole–Pt nanoparticle composites, yielding an electrochemical UA sensor with a linear range of 100–350 μM and an impressive LOD of 0.16 μM. While these composite materials demonstrate enhanced catalytic activity for UA oxidation, their reliance on expensive noble metal nanomaterials (Au and Pt) and complex synthesis protocols increases production costs and hinders widespread adoption. Consequently, there remains a critical need for simple, stable, and cost-effective composite materials for highly sensitive UA electrochemical biosensors [33]. Transition metal dichalcogenides (TMDs) have recently emerged as promising alternatives to graphene-based materials for electrochemical sensors, offering remarkable physical, chemical, catalytic, optical, and electronic properties while maintaining cost-effectiveness and straightforward synthesis procedures [34]. TMDs facilitate efficient electron transfer through their large surface area and abundant active sites, while surface defects serve as additional binding sites for small biomolecules, thereby enhancing sensor conductivity and sensitivity [35]. Recent investigations into TMD-based composite materials for UA electrochemical sensing have yielded promising results [36]. For instance, Cogal et al. [37] synthesized two-dimensional WSe2 nanosheet–carbon black composites via hydrothermal methods, developing a WSe2@C electrode that demonstrated high sensitivity, selectivity, reproducibility, and stability, with a linear range of 1–185.2 μM and an LOD of 0.42 μM. Similarly, Sha et al. [38] combined electrospinning and hydrothermal synthesis to fabricate MoS2 nanosheet/nanocarbon fiber composites, producing a UA electrochemical sensor with a linear range of 1–60 μM and an LOD of 0.91 μM, exhibiting excellent reproducibility and stability. These results underscore the exceptional potential of TMD-based composite materials for electrochemical UA detection.
In this study, we developed an efficient hydrothermal method for synthesizing tumbleweed-like MoSe2 nanostructures, offering the advantages of simplicity, cost-effectiveness, and high yield. The composite electrode, fabricated using MoSe2/rGO, with its distinctive roll-like morphology, functioned as an electrochemical UA sensor, exhibiting a broad linear detection range from 40 nM to 200 μM and achieving a remarkably low LOD of 28.4 nM under optimized conditions. The electrode demonstrated superior analytical performance in terms of repeatability, anti-interference capability, and long-term stability. Notably, this work represents the first reported application of MoSe2 in high-sensitivity bioelectronic sensing for nanomolar-level UA detection.
2. Materials and Methods
2.1. Reagents
Phosphate buffer solution (PBS, containing 2.67 mM KCl, 136.89 mM NaCl, 1.76 mM KH2PO4, and 8.10 mM Na2HPO4) was purchased from Sangon Biotech (Shanghai, China) Co., Ltd. (Shanghai, China). Ammonium molybdate tetrahydrate ((NH4)6Mo7O24·4H2O), selenium powder (Se), sulfuric acid (H2SO4), potassium hexacyanoferrate(Ⅱ) (K4[Fe(CN)6]), potassium ferrocyanide (K3[Fe(CN)6]), dopamine (C8H11NO2), citric acid (C6H8O7), cystine (C6H12N2O4S2), sodium chloride (NaCl), potassium chloride (KCl), D(+)-glucose anhydrous (C6H12O6), ascorbic acid (C6H8O6), dopamine hydrochloride (C8H11NO2·HCl), anhydrous oxalic acid (C2H2O4), and UA were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). rGO was obtained from Nanjing XFNANO Materials Tech Co., Ltd. (Nanjing, China). All chemicals used in the experiments were of analytical grade and were used without further purification. Deionized water (Milli-Q) was used throughout all experiments.
2.2. Synthesis of Tumbleweed-like MoSe2/rGO and Electrode Fabrication
First, 0.5 g of (NH4)6Mo7O24·4H2O and 0.32 g of Se were added to 40 mL of deionized water, and the solution was stirred with a magnetic stirrer for 20 min to achieve homogeneous dispersion. Subsequently, 1.12 g of OA was introduced into the solution and stirred for an additional 15 min. The homogeneous mixture was placed into a 50 mL Teflon-lined stainless-steel autoclave and underwent hydrothermal treatment at 200 °C for 24 h in a muffle furnace. Upon natural cooling to ambient temperature, the reaction product underwent three successive washing cycles with deionized water and ethanol. The resultant black precipitate was isolated via centrifugation and vacuum-dried at 60 °C for 4 h to obtain MoSe2 powder.
The MoSe2/rGO composite was synthesized by dispersing MoSe2 and rGO powders in deionized water at a mass ratio of 3:1, followed by 20 min of sonication to achieve a homogeneous dispersion. The GCE (3 mm diameter) was prepared through sequential surface treatments, beginning with mechanical polishing using 50 nm alumina slurry. Successively, the electrode underwent ultrasonic cleaning in deionized water and ethanol to remove residual particles. Electrochemical activation of the GCE was accomplished through cyclic voltammetry (CV) in 0.5 M H2SO4 solution, applying potential sweeps between −1.0 and 1.0 V at 100 mV/s. The modified electrode was fabricated by drop-casting 7 μL of the MoSe2/rGO aqueous dispersion (1 mg/mL) onto the activated GCE surface, followed by thermal drying at 50 °C for 10 min.
2.3. Electrochemical Sensing of UA
For electrochemical measurements, the electrolyte was prepared using PBS. A three-electrode system was employed, comprising MoSe2/rGO as the working electrode, platinum (Pt) as the counter electrode, and a saturated calomel electrode (SCE) as the reference electrode. The electrochemical characterization included DPV, CV, and electrochemical impedance spectroscopy (EIS) to evaluate the voltametric responses of the modified electrodes. DPV analysis was conducted from −0.1 to 0.5 V using optimized parameters (pulse period: 0.5 s; step potential: 0.004 V; amplitude: 0.005 V). CV measurements were performed over five cycles within a potential window of −0.2 to 0.6 V at 0.1 V/s. EIS measurements were conducted at the open circuit potential (OCP), with the equilibrated OCP being 0.193 V. The AC amplitude was set to 10 mV, and the frequency range was 0.1 Hz to 100 kHz. All electrochemical analyses were performed using a CHI660e electrochemical workstation (CH Instruments, Shanghai, China).
2.4. Characterization
The morphology of the modified materials was observed using a transmission electron microscope (TEM, JEM–2100F, JEOL, Tokyo, Japan), energy-dispersive spectroscopy (EDS), and a field-emission scanning electron microscope (FE–SEM, QUANTA 250 FEG, FEI, Hillsboro, OR, USA). Raman spectroscopy (Renishaw inVia Reflex, Renishaw plc, Wotton–under–Edge, London, UK) with a 532 nm laser wavelength was used to analyze the synthesized materials. X-ray diffraction (XRD, D8 Advance, Bruker, Karlsruhe, Germany) with Cu Kα radiation (λ = 1.54 Å) was employed for the elemental and crystallographic analysis of the materials. X-ray photoelectron spectroscopy (XPS, Axis SUPRA+, Shimadzu, Japan) was used to characterize the surface composition and chemical states of the materials.
3. Results and Discussion
3.1. Morphological Characterization of Tumbleweed-like MoSe2
As shown in Figure 1a, the MoSe2 exhibited a clustered, tumbleweed-like structure with an average spherical diameter of approximately 300 nm and a nanoflake-rich composition. EDS analysis (Figure 1b,c) demonstrated a uniform distribution of Mo and Se elements throughout the sample. Additionally, Figure 1b and c show that both Mo and Se are evenly distributed across the material. The TEM and HRTEM images (Figure 1d,e) reveal the layered structure of MoSe2, with an interlayer spacing of approximately 0.649 nm corresponding to the (002) plane. Selected area electron diffraction (SAED) (Figure 1f) indicated that the MoSe2 material is polycrystalline. These comprehensive morphological and structural characterizations validate the successful synthesis of tumbleweed-like MoSe2, featuring a well-defined, nanoflake-rich structure suitable for electrochemical applications.
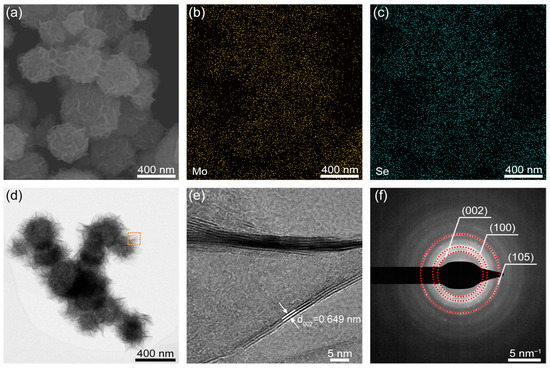
Figure 1.
(a) SEM image of MoSe2 nanostructures. EDS mapping of (b) Mo and (c) Se elements. (d) TEM image. (e) High-resolution TEM image of the red square region in (d) and (f) corresponding selected area electron diffraction.
3.2. Chemical Composition and Crystal Structure Analysis of Tumbleweed-like MoSe2
Raman spectroscopy analysis revealed the characteristic chemical structure of the synthesized MoSe2, as illustrated in Figure 2a. The peaks at 263.0 cm−1 and 292.2 cm−1 correspond to the out-of-plane (A1g) and in-plane (E1 2g) vibrational modes of the Se–Mo–Se bond, respectively [39]. The XRD pattern in Figure 2b shows that all the observed diffraction peaks are attributable to MoSe2 (JCPDS# 29–0914) [40]. These comprehensive characterization results provide compelling evidence for the successful formation of hexagonal MoSe2 crystals.
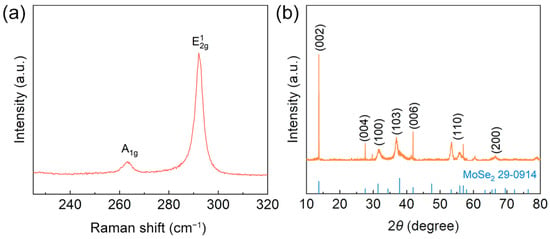
Figure 2.
(a) Raman spectra of MoSe2. (b) XRD patterns.
XPS analysis was employed to analyze the chemical binding energies and elemental composition of the sample. The XPS spectra of MoSe2 are presented in Figure 3a–c. The survey spectrum (Figure 3a) confirmed the presence of Mo and Se as the primary constituent elements in MoSe2. Figure 3b displays the Mo 3d core-level spectrum, with two characteristic peaks at 228.66 eV and 231.77 eV, which are attributed to the Mo 3d5/2 and Mo 3d3/2 peaks of Mo4+ in MoSe2, respectively. The Se 3d spectrum in Figure 3c shows two characteristic peaks at approximately 54.22 eV and 55.06 eV, corresponding to Se 3d5/2 and Se 3d3/2, respectively [41].
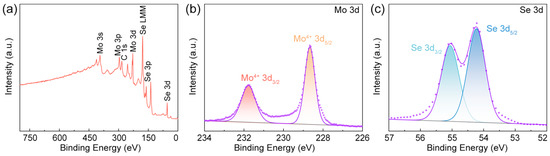
Figure 3.
(a) XPS survey spectrum of tumbleweed-like MoSe2 and high-resolution XPS spectra at (b) Mo 3d and (c) Se 3d peaks.
3.3. Sensitivity Optimization of MoSe2/rGO Electrodes for UA Detection
To evaluate the electrochemical performance of various electrode modification materials for UA detection, DPV measurements were performed using bare GCE, rGO, MoSe2, and MoSe2/rGO electrodes in PBS electrolyte containing 10 µM UA across a potential window of −0.1 to 0.5 V (Figure 4a). The DPV curves exhibited a characteristic UA oxidation peak at 0.271 V, which served as the analytical signal for UA detection. In comparison to the bare GCE, the MoSe2/rGO, rGO, and MoSe2 electrodes demonstrated enhanced current densities of 300.0%, 158.3%, and 32.4%, respectively. The superior electrochemical performance of the MoSe2/rGO electrode suggested a synergistic interaction between the MoSe2 and rGO components. This enhancement is attributed to the incorporation of rGO, which provided additional electrochemically active sites and established efficient electron transfer pathways throughout the MoSe2 nanostructures. The results confirm that the MoSe2/rGO electrode exhibited remarkable electrochemical sensing capabilities for UA detection.
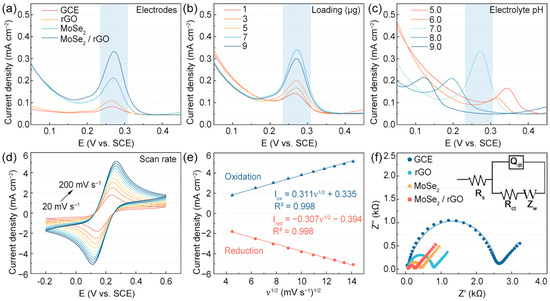
Figure 4.
(a) DPV curves of tumbleweed-like MoSe2/rGO and other modified electrodes in PBS containing 10 µM of UA. DPV tests under different sensing conditions in PBS solution containing 10 µM of UA: (b) loading mass; (c) pH. (d) CV tests of MoSe2/rGO electrodes at different scan rates in 10 mM of [Fe(CN)6]3−/4−/0.1 M KCl electrolyte and (e) linear plot of vs. . (f) Comparison of impedance spectra between MoSe2/rGO and other electrodes in 10 mM of [Fe(CN)6]3−/4−.
To optimize the detection performance of the MoSe2/rGO electrode for UA, several experimental parameters were systematically investigated, including the MoSe2/rGO loading mass, electrolyte pH, and scan rate. The optimal MoSe2/rGO loading mass was determined by adjusting the volume of the dispersion drop-cast onto the GCE surface to achieve the deposition of different amounts (1, 3, 5, 7, and 9 µg) of MoSe2/rGO on the bare GCE, and DPV measurements were taken in PBS electrolyte containing 10 µM of UA. Based on the results shown in Figure 4b, when the loading exceeds 7 μg, the current response shows a downward trend, which may be due to the effect of charge transfer blockage by material stacking. Optimizing the loading amount is critical to balancing active site exposure and mass transport efficiency. Therefore, 7 µg of MoSe2/rGO loading was selected for subsequent electrochemical studies. Since electrolyte pH significantly influences the electrochemical behavior of UA at the electrode interface, its effect on UA electro-oxidation at the MoSe2/rGO electrode was examined across pH values ranging from 5.0 to 9.0 (Figure 4c). The electrochemical response reached its maximum at pH 7.0, which was subsequently selected as the optimal condition for UA detection using the MoSe2/rGO electrode.
To assess the electrochemical behavior of the MoSe2/rGO electrode surface, CV measurements were performed at scan rates ranging between 20 and 200 mV s−1 in 10 mM of [Fe(CN)6]3−/4− electrolyte containing 0.1 M KCl (Figure 4d). The peak current densities for Iox and Ired showed a linear relationship with the square root of the scan rate (Figure 4e), indicating that the redox reactions occurring on the MoSe2/rGO electrode are diffusion-controlled [42]. The electroactive surface area (ESA) of various electrodes was calculated using the Randles–Sevcik equation [43,44]:
In this equation, Ired represents the reduction peak current, n denotes the number of transferred electrons involved the reduction process, A is the ESA of the working electrode, D is the diffusion coefficient of ferricyanide (7.6 × 10−7 cm2 s−1), ν refers to the scan rate, and c is the concentration of ferricyanide in the electrolyte (1 × 10−5 mol cm−3). The results indicated that the ESA of the MoSe2/rGO electrode was 0.092 cm2, while the ESA of the bare GCE was approximated to be its geometric area (0.071 cm2). These findings suggest that the MoSe2/rGO-modified electrode remarkably enhanced the electroactive surface area of the GCE, attributable to the high conductivity and specific surface area of MoSe2/rGO.
The electron transfer capability of the MoSe2/rGO electrode was investigated to assess its electrochemical performance for UA detection. The interfacial charge transfer capabilities of the GCE, rGO, MoSe2, and MoSe2/rGO electrodes were evaluated using EIS in 10 mM of [Fe(CN)6]3−/4− electrolyte solution (Figure 4f). The obtained Nyquist plots for the MoSe2 electrode were analyzed using an equivalent circuit [45]:
where is the series resistance. Notably, represents the charge transfer resistance, a critical indicator of the sensor’s charge transfer capability [46]. The values for the GCE, rGO, MoSe2, and MoSe2/rGO electrodes were 2514.0, 722.6, 350.7, and 194.1 Ω, respectively. The lower value of the MoSe2/rGO electrode compared to the other electrodes indicates improved charge transfer capability.
3.4. Detection Performance and Practical Applications of MoSe2/rGO Sensors
The electrochemical analysis of UA was performed quantitatively using the MoSe2/rGO electrode through DPV measurements, as demonstrated in Figure 5a. The peak current density exhibited a positive correlation with UA concentration across a range spanning from 40 nM to 200 µM. Figure 5b presents the calibration curve constructed from the averaged peak current responses against UA concentrations. Within the concentration range of 40 nM to 200 µM, the linear regression equation for UA was as follows: . Based on the regression results, the LOD was determined to be 28.4 nM (S/N = 3). As presented in Table 1, compared to the other UA sensors based on TMDs, our MoSe2/rGO sensor demonstrates a very low limit of detection (LOD) and is simple to prepare.
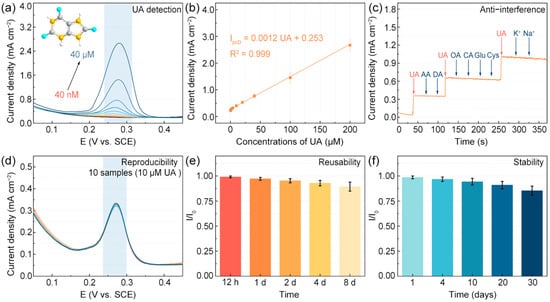
Figure 5.
(a) DPV measurements of MoSe2/rGO electrodes with various UA concentrations. (b) Calibration curve of UA detection. (c) The anti-interference; (d) reproducibility; (e) reusability; and (f) stability of MoSe2/rGO electrodes.

Table 1.
Comparison of LOD and linear range of MoSe2/rGO electrodes with reported TMD nanomodifiers for UA detection.
Additionally, to assess the interference resistance of the MoSe2/rGO sensor, 10 µM of UA was detected in the company of common urine interfering substances, including 1 mM DA, 1 mM AA, 1 mM OA, 1 mM CA, 1 mM Cys, 1 mM Glu, 5 mM Na+, and 5 mM K+. The i–t technique was applied to the MoSe2/rGO electrode, as shown in Figure 5c. The MoSe2/rGO electrode demonstrated remarkable anti-interference capability against various molecular species during electrochemical detection. Reproducibility tests conducted on ten independent samples under identical conditions revealed a minimal relative standard deviation of 1.61% for current density (Figure 5d), confirming the exceptional reliability of the MoSe2/rGO sensor. The sensor’s reusability was systematically evaluated through consecutive daily measurements, yielding consistently satisfactory performance. The electrode retained 89.4% of its initial current density after eight consecutive days of testing (Figure 5e), while long-term stability assessments revealed the retention of 85.3% of the initial current density following one month of storage (Figure 5f). These comprehensive performance metrics substantiate the practical viability of our sensor for UA detection applications.
4. Conclusions
In this study, tumbleweed-structured MoSe2 was synthesized via a hydrothermal method and subsequently integrated with rGO to construct a UA sensing platform. The fabricated MoSe2/rGO electrode demonstrated superior sensitivity toward UA oxidation compared to pristine MoSe2, rGO, and bare GCE electrodes. The sensor exhibited outstanding analytical performance for UA detection across a broad linear range from 40 nM to 200 μM, achieving a detection limit of 28.4 μM. Comprehensive validation studies confirmed that the MoSe2/rGO electrode possessed outstanding anti-interference capabilities, excellent reusability, and long-term stability. These findings establish a promising foundation for developing high-performance electrochemical biosensors based on MoSe2/rGO composite materials.
Author Contributions
Conceptualization, C.-T.L.; methodology, C.-T.L.; validation, P.S., N.Z., Z.S., K.S., and C.Y.; formal analysis, P.S., N.Z., L.F., and Z.S.; investigation, P.S., K.S., W.C., T.C., H.-S.T., and L.X.; resources, L.X., H.-S.T., W.C., L.W., Y.W., N.J., and C.-T.L.; data curation, W.C., L.W., Y.W., T.C., and N.J.; writing—original draft preparation, P.S. and L.F.; writing—review and editing, L.F., and C.-T.L.; visualization, P.S., N.Z., L.W., and Y.W.; supervision, C.Y., W.C., L.W., Y.W., N.J., and C.-T.L.; project administration, C.-T.L.; funding acquisition, C.-T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China (2022YFA1203100), the National Natural Science Foundation of China (52272053, 52102055, 52302120), Ningbo Key Scientific and Technological Project (2021Z120, 2021Z115, 2022Z084, 2022Z191), the Yongjiang Talent Introduction Programme of Ningbo (2021A-037-C, 2021A-108-G), the Youth Fund of Chinese Academy of Sciences (JCPYJ-22030), China Postdoctoral Science Foundation (2022M713243), CAS Youth Innovation Promotion Association (2020301), Science and Technology Major Project of Ningbo (2021ZDYF020196, 2021ZDYF020198), the Project of Chinese Academy of Science (ZDKYYQ2020001), and Ningbo 3315 Innovation Team (2019A-18-C).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are unavailable due to privacy or ethical restrictions.
Acknowledgments
The authors gratefully acknowledge Houmeng Yang for his guidance on this work and sincerely thank the support of the projects mentioned in the Funding Section above.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fathallah-Shaykh, S.A.; Cramer, M.T. Uric acid and the kidney. Pediatr. Nephrol. 2014, 29, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Kang, D.H.; Feig, D.; Kivlighn, S.; Kanellis, J.; Watanabe, S.; Tuttle, K.R.; Rodriguez-Iturbe, B.; Herrera-Acosta, J.; Mazzali, M. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension 2003, 41, 1183–1190. [Google Scholar] [CrossRef]
- Usman Ali, S.M.; Alvi, N.H.; Ibupoto, Z.; Nur, O.; Willander, M.; Danielsson, B. Selective potentiometric determination of uric acid with uricase immobilized on ZnO nanowires. Sensor. Actuat. B Chem. 2011, 152, 241–247. [Google Scholar] [CrossRef]
- Johnson, R.J.; Nakagawa, T.; Jalal, D.; Sánchez-Lozada, L.G.; Kang, D.-H.; Ritz, E. Uric acid and chronic kidney disease: Which is chasing which? Nephrol. Dial. Transplant. 2013, 28, 2221–2228. [Google Scholar] [CrossRef]
- Riches, P.L.; Wright, A.F.; Ralston, S.H. Recent insights into the pathogenesis of hyperuricaemia and gout. Hum. Mol. Genet. 2009, 18, R177–R184. [Google Scholar] [CrossRef]
- Alderman, M.; Aiyer, K.J.V. Uric acid: Role in cardiovascular disease and effects of losartan. Curr. Med. Res. Opin. 2004, 20, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Mateos, E.A.; Puig, J.G. Purine metabolism in Lesch-Nyhan syndrome versus Kelley-Seegmiller syndrome. J. Inherit. Metab. Dis. 1994, 17, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Iranmanesh, T.; Foroughi, M.M.; Jahani, S.; Shahidi Zandi, M.; Hassani Nadiki, H. Green and facile microwave solvent-free synthesis of CeO2 nanoparticle-decorated CNTs as a quadruplet electrochemical platform for ultrasensitive and simultaneous detection of ascorbic acid, dopamine, uric acid and acetaminophen. Talanta 2020, 207, 120318. [Google Scholar] [CrossRef]
- Liberopoulos, E.; Christides, D.; Elisaf, M. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricemia and gout. J. Hypertens. 2002, 20, 347. [Google Scholar] [CrossRef]
- Zhao, F.Y.; Wang, Z.H.; Wang, H.; Zhao, R.; Ding, M.Y. Determination of uric acid in human urine by ion chromatography with conductivity detector. Chin. Chem. Lett. 2011, 22, 342–345. [Google Scholar] [CrossRef]
- Galbán, J.; Andreu, Y.; Almenara, M.J.; Marcos, S.D.; Castillo, J.R. Direct determination of uric acid in serum by a fluorometric-enzymatic method based on uricase. Talanta 2001, 54, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.L.; Rocha, F.R.P. A flow-based procedure with solenoid micro-pumps for the spectrophotometric determination of uric acid in urine. Microchem. J. 2010, 94, 53–59. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, J.; Ye, F.; Liu, Y.M. Determination of uric acid in human urine and serum by capillary electrophoresis with chemiluminescence detection. Anal. Biochem. 2008, 378, 127–131. [Google Scholar] [CrossRef]
- Pundir, L.C.S. Discrete analysis of serum uric acid with immobilized uricase and peroxidase. J. Biochem. Bioph. Meth. 1999, 39, 125–136. [Google Scholar] [CrossRef]
- Wang, J.; Golden, T.; Peng, T. Poly(4-vinylpyridine)-coated glassy carbon flow detectors. Anal. Chem. 1987, 59, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, B.; Zhong, J.; Yan, B.; Zhang, K.; Zhai, C.; Shiraishi, Y.; Du, Y.; Yang, P. Dopamine and uric acid electrochemical sensor based on a glassy carbon electrode modified with cubic Pd and reduced graphene oxide nanocomposite. J. Colloid Interface Sci. 2017, 497, 172–180. [Google Scholar] [CrossRef]
- Immanuel, S.; Aparna, T.K.; Sivasubramanian, R. A facile preparation of Au–SiO2 nanocomposite for simultaneous electrochemical detection of dopamine and uric acid. Surf. Interfaces 2019, 14, 82–91. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Chen, J.; Wang, L.; Bian, L.; Ye, J.; Weng, L.; Zhao, X.; Lin, C.-T.; Li, S.; et al. A biosensor using semi-DNA walker and CHA -FRET loop for ultrasensitive detection of single nucleotide polymorphism. Sensor. Actuat. B Chem. 2024, 400, 134908. [Google Scholar] [CrossRef]
- Li, H.; Zhou, K.; Cao, J.; Wei, Q.; Lin, C.-T.; Pei, S.E.; Ma, L.; Hu, N.; Guo, Y.; Deng, Z.; et al. A novel modification to boron-doped diamond electrode for enhanced, selective detection of dopamine in human serum. Carbon 2021, 171, 16–28. [Google Scholar] [CrossRef]
- Loan, P.T.K.; Wu, D.; Ye, C.; Li, X.; Tra, V.T.; Wei, Q.; Fu, L.; Yu, A.; Li, L.-J.; Lin, C.-T. Hall effect biosensors with ultraclean graphene film for improved sensitivity of label-free DNA detection. Biosens. Bioelectron. 2018, 99, 85–91. [Google Scholar] [CrossRef]
- Zhou, M.; Zhai, Y.; Dong, S. Electrochemical Sensing and Biosensing Platform Based on Chemically Reduced Graphene Oxide. Anal. Chem. 2009, 81, 5603–5613. [Google Scholar] [CrossRef]
- Kalambate, P.K.; Dhanjai; Huang, Z.; Li, Y.; Shen, Y.; Xie, M.; Huang, Y.; Srivastava, A.K. Core@shell nanomaterials based sensing devices: A review. TrAC Trend. Anal. Chem. 2019, 115, 147–161. [Google Scholar] [CrossRef]
- Sha, R.; Badhulika, S. Facile green synthesis of reduced graphene oxide/tin oxide composite for highly selective and ultra-sensitive detection of ascorbic acid. J. Electroanal. Chem. 2018, 816, 30–37. [Google Scholar] [CrossRef]
- Zhao, J.; He, C.; Wu, W.; Yang, H.; Peng, L.; Wen, L.; Hu, Z.; Hou, C.; Huo, D. MXene-MoS2 carbon-fiber-based flexible electrochemical interface for multiple bioanalysis in biofluids. Chem. Eng. J. 2022, 446, 136841. [Google Scholar] [CrossRef]
- Ambrosi, A.; Chua, C.K.; Latiff, N.M.; Loo, A.H.; Wong, C.H.A.; Eng, A.Y.S.; Bonanni, A.; Pumera, M. Graphene and its electrochemistry—An update. Chem. Soc. Rev. 2016, 45, 2458–2493. [Google Scholar] [CrossRef] [PubMed]
- Kuila, T.; Bose, S.; Khanra, P.; Mishra, A.K.; Kim, N.H.; Lee, J.H. Recent advances in graphene-based biosensors. Biosens. Bioelectron. 2011, 26, 4637–4648. [Google Scholar] [CrossRef]
- Li, S.; Ma, Y.; Liu, Y.; Xin, G.; Wang, M.; Zhang, Z.; Liu, Z. Electrochemical sensor based on a three dimensional nanostructured MoS2 nanosphere-PANI/reduced graphene oxide composite for simultaneous detection of ascorbic acid, dopamine, and uric acid. RSC Adv. 2019, 9, 2997–3003. [Google Scholar] [CrossRef]
- Tian, Q.; She, Y.; Zhu, Y.; Dai, D.; Shi, M.; Chu, W.; Cai, T.; Tsai, H.S.; Li, H.; Jiang, N. Highly Sensitive and Selective Dopamine Determination in Real Samples Using Au Nanoparticles Decorated Marimo-like Graphene Microbead-Based Electrochemical Sensors. Sensors 2023, 23, 2870. [Google Scholar] [CrossRef]
- Guo, X.; Yue, H.; Song, S.; Huang, S.; Gao, X.; Chen, H.; Wu, P.; Zhang, T.; Wang, Z. Simultaneous electrochemical determination of dopamine and uric acid based on MoS2 nanoflowers-graphene/ITO electrode. Microchem. J. 2020, 154, 104527. [Google Scholar] [CrossRef]
- Torrinha, Á.; Morais, S. Electrochemical (bio)sensors based on carbon cloth and carbon paper: An overview. TrAC Trend. Anal. Chem. 2021, 142, 116324. [Google Scholar] [CrossRef]
- Aparna, T.K.; Sivasubramanian, R.; Dar, M.A. One-pot synthesis of Au-Cu2O/rGO nanocomposite based electrochemical sensor for selective and simultaneous detection of dopamine and uric acid. J. Alloys Compd. 2018, 741, 1130–1141. [Google Scholar] [CrossRef]
- Darabi, R.; Karimi-Maleh, H.; Akin, M.; Arikan, K.; Zhang, Z.; Bayat, R.; Bekmezci, M.; Sen, F. Simultaneous determination of ascorbic acid, dopamine, and uric acid with a highly selective and sensitive reduced graphene oxide/polypyrrole-platinum nanocomposite modified electrochemical sensor. Electrochim. Acta 2023, 457, 142402. [Google Scholar] [CrossRef]
- Yue, H.Y.; Wu, P.F.; Huang, S.; Gao, X.; Song, S.S.; Wang, W.Q.; Zhang, H.J.; Guo, X.R. Electrochemical determination of dopamine in the presence of uric acid using WS2 nanospheres-carbon nanofibers. J. Electroanal. Chem. 2019, 833, 427–432. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Qian, L.; Durairaj, S.; Prins, S.; Chen, A. Nanomaterial-based electrochemical sensors and biosensors for the detection of pharmaceutical compounds. Biosens. Bioelectron. 2021, 175, 112836. [Google Scholar] [CrossRef]
- Tajik, S.; Dourandish, Z.; Garkani Nejad, F.; Beitollahi, H.; Jahani, P.M.; Di Bartolomeo, A. Transition metal dichalcogenides: Synthesis and use in the development of electrochemical sensors and biosensors. Biosens. Bioelectron. 2022, 216, 114674. [Google Scholar] [CrossRef]
- Cogal, S.; Bhethanabotla, V.R. Electrochemical Sensor Based on Carbon-Incorporated WSe2 Nanosheets for Simultaneous Detection of Ascorbic Acid, Dopamine, and Uric Acid. IEEE Sens. J. 2022, 22, 14952–14958. [Google Scholar] [CrossRef]
- Sha, R.; Vishnu, N.; Badhulika, S. MoS2 based ultra-low-cost, flexible, non-enzymatic and non-invasive electrochemical sensor for highly selective detection of Uric acid in human urine samples. Sens. Actuat. B Chem. 2019, 279, 53–60. [Google Scholar] [CrossRef]
- Dogra, N.; Agrawal, P.; Pathak, S.; Saini, R.; Sharma, S. Hydrothermally synthesized MoSe2/ZnO composite with enhanced hydrogen evolution reaction. Int. J. Hydrogen Energy 2023, 48, 26210–26220. [Google Scholar] [CrossRef]
- Ahmad, K.; Kim, H. Hydrothermally synthesized MoSe2/rGO composite as electrode modifier for the construction of non-enzymatic urea sensor. Mater. Chem. Phys. 2022, 286, 126206. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.; Li, P.; Pang, M.; Xue, Q. Flexible self-powered high-performance ammonia sensor based on Au-decorated MoSe2 nanoflowers driven by single layer MoS2-flake piezoelectric nanogenerator. Nano Energy 2019, 65, 103974. [Google Scholar] [CrossRef]
- Baikeli, Y.; Mamat, X.; He, F.; Xin, X.; Li, Y.; Aisa, H.A.; Hu, G. Electrochemical determination of chloramphenicol and metronidazole by using a glassy carbon electrode modified with iron, nitrogen co-doped nanoporous carbon derived from a metal-organic framework (type Fe/ZIF-8). Ecotoxicol. Environ. Saf. 2020, 204, 111066. [Google Scholar] [CrossRef]
- Randles, J.E.B. A cathode ray polarograph. Part II—The current-voltage curves. Trans. Faraday Soc. 1948, 44, 327–338. [Google Scholar] [CrossRef]
- Ševčík, A. Oscillographic polarography with periodical triangular voltage. Collect. Czech. Chem. Commun. 1948, 13, 349–377. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Gharbi, O.; Vivier, V.; Gao, M.; Orazem, M.E. Electrochemical impedance spectroscopy. Nat. Rev. Methods Primers 2021, 1, 41. [Google Scholar] [CrossRef]
- Lvovich, V.F. Electrochemical Impedance Spectroscopy (EIS) Applications to Sensors and Diagnostics; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Durai, L.; Kong, C.Y.; Badhulika, S. One-step solvothermal synthesis of nanoflake-nanorod WS2 hybrid for non-enzymatic detection of uric acid and quercetin in blood serum. Mater. Sci. Eng. C 2020, 107, 110217. [Google Scholar] [CrossRef] [PubMed]
- Sha, R.; Vishnu, N.; Badhulika, S. FeS2 Grown Pencil Graphite as an In-expensive and Non-enzymatic Sensor for Sensitive Detection of Uric Acid in Non-invasive Samples. Electroanalysis 2019, 31, 2397–2403. [Google Scholar] [CrossRef]
- Wu, P.; Huang, Y.; Zhao, X.; Lin, D.; Xie, L.; Li, Z.; Zhu, Z.; Zhao, H.; Lan, M. MnFe2O4/MoS2 nanocomposite as Oxidase-like for electrochemical simultaneous detection of ascorbic acid, dopamine and uric acid. Microchem. J. 2022, 181, 107780. [Google Scholar] [CrossRef]
- Pan, D.; Rong, S.; Zhang, G.; Zhang, Y.; Zhou, Q.; Liu, F.; Li, M.; Chang, D.; Pan, H. Electrochemical Determination of Uric Acid at CdTe Quantum Dot Modified Glassy Carbon Electrodes. J. AOAC Int. 2015, 98, 1260–1266. [Google Scholar] [CrossRef]
- Raju, C.V.; Ramya, R.; Imran, K.; Basha, C.K.; Wilson, J.; Boobalan, T.; Arun, A.; Basu, M.J.; Saravanan, S. Simultaneous electrochemical detection of dopamine and uric acid based on tri-composite of poly-pyrrole and α-Fe2O3 embedded MoS2 sheets modified electrode. Microchem. J. 2024, 198, 110189. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).