Abstract
A novel material composed of Au@SiO2-(3-Aminopropyl Triethoxysilane) (Au@SiO2-APTES) was successfully synthesised using the sol–gel method, and was used to modify glassy carbon electrodes. Its effectiveness as a molecular recognition element is evaluated in the design of an electrochemical sensor for the precise detection of dopamine. The Au@SiO2-APTES composite was analysed using Fourier transform infrared spectroscopy, scanning electron microscopy, energy-dispersive X-ray spectroscopy, and X-ray diffraction. Elemental analysis verified the presence of oxygen, silicon, and gold, with atomic percentages of around 77.19%, 21.12%, and 1.65%, respectively. The corresponding elemental mapping for Au@SiO2-APTES composite showed that the spatial distribution of all the elements was fairly homogeneous throughout the composite, indicating that the Au NPs are embedded in the silica structures. Traces of dopamine were detected by differential pulse voltammetry with a low limit of detection (S/N = 3) and quantification (S/N = 10) of 1.4 × 10−8 molL−1 and 4.7 × 10−8 molL−1, respectively. The Au@SiO2-APTES composite had two linear ranges: from 4.7 × 10−8 to 1 × 10−7 molL−1 and 1.25 × 10−7 to 8.75 × 10−7 molL−1. Moreover, the sensor showed outstanding selectivity even in the presence of various potential interfering species. It also demonstrated good reusability and signal recovery when tested in human urine and plasma samples spiked with different dopamine concentrations. The electrochemical sensor, constructed using this novel composite material, shows great promise in the selective and sensitive detection of dopamine in the biological matrix. These results underscore the sensor’s capability for practical application in analysing real-world samples.
1. Introduction
Nanomaterials are widely used in many applications, thanks to their unique properties [1]. In this regard, they exhibit a notable change in both chemical and physical properties, highlighting unique electrical, thermal, optical, magnetic, and catalytic characteristics compared to bulk materials [2]. Nanotechnology has numerous uses in medicine [3], agriculture [4], food industries [5], biotechnology [6] and electronics [7], environmental pollution monitoring, and management treatment [8]. The combination of different nanomaterials yields unique properties, notably the use of magnetite nanoparticles (MNPs) with graphene oxide (GO) and silica (SiO2), which provides high electronic conductivity and a relatively large surface area. In addition to these nanocomposites, others such as SnO2-chitosan/GCE [9], Au/RGO/GCE [9], and poly(o-anisidine)/CNT/GCE have also been used for dopamine detection [10]. In addition, gold nanoparticles (Au NPs) have garnered significant attention due to their excellent conductivity, chemical stability, and high catalytic activity. When combined with other nanomaterials, Au NPs show outstanding performance in various applications [11]. One of the most interesting applications focuses on the development of biosensing devices, with a special importance on electrochemical and optical applications, where nanomaterials excel thanks to their catalytic behaviour, high surface area-to-volume ratio, synergistic effects, and the potential to serve as scaffolds for more complex structures and devices [12,13]. The intricate interplay of these features not only enhances the sensitivity, precision, selectivity, and limit of detection (LOD) of the (bio)sensors developed, but also opens up avenues for cutting-edge applications in fields such as environmental monitoring, healthcare, and advanced diagnostics [14,15,16].
Dopamine (DA), 3,4-dihydroxyphenylmethylamine, is a neurotransmitter crucial for regulating the functions of the central nervous, cardiovascular, and endocrine systems, among others. In abnormal levels, it is associated with various diseases such as Parkinson’s disease, depression, anxiety, schizophrenia, autoimmune disorders, and attention deficit hyperactivity disorder (ADHD) [17,18,19,20,21,22]. In addition, DA plays a fundamental role in the experience of pleasure and reward. Thus, the released DA activates the pleasure centres in the brain, providing a sense of well-being and motivation, and it plays a significant role in the illness of addiction [23]. Consequently, the repeated artificial increase in dopamine levels from drug use can lead to alterations in brain plasticity and cognitive functions. Over time, this can lead to lower sensitivity to natural DA, meaning that individuals may seek increasingly intense experiences to achieve the same sense of pleasure.
Elevated DA in adults’ urine has been linked to the use of certain drugs such as intravenous DA, clozapine, methyldopa, L-DOPA, antidepressants, and metoclopramide [18,24]. Therefore, the accurate DA determination in biological samples, and the understanding of its role in various metabolic pathways, is essential for addressing and treating addictions. On the other hand, DA is the key neurotransmitter in both Parkinson’s disease (PD) diagnosis and treatment. There is much interest in developing sensitive and selective methods for its detection, with significant implications for research and clinical applications. Several techniques have been designed to measure DA and other catecholamines in clinical samples such as serum, plasma, urine, and cerebrospinal fluid for diagnostic purposes. Over the past 20 years, a variety of approaches, including colorimetric [25], spectrophotometric [26], and high-performance liquid chromatography (HPLC) methods [27] have been developed for DA detection. Nevertheless, while these methods are widely embraced for their notable attributes, their main limitations are related to the limited sensitivity (colorimetric), resolution (spectrophotometric), presence of interferences that affect sensitivity, and/or the need for expensive and complex equipment, such as that required for chromatographic methods.
Thanks to its electroactive nature, DA can be detected using electrochemical techniques, as demonstrated by several research groups in the early 1980s [28]. Therefore, electrochemical methods for DA detection have attracted much attention because of their high accuracy, fast response, low-cost, and their user-friendly methods and instrumentation [29,30]. In this context, the major challenge in detecting DA arises from (1) its low concentration in body fluids and tissues, ranging from 0.01 to 0.3 nmolL−1 in plasma, 0.02 to 0.07 nmolL−1 in cerebrospinal fluid, and 0.2 to 1.4 µmolL−1 in urine, making its detection and precise measurement a significant challenge in both clinical and research settings [17,31,32,33]; (2) the interference phenomena of its metabolites (dihydroxyphenylacetic acid and homovanillic acid), other neurotransmitters (norepinephrine, serotonin); and (3) the high concentration of other natural electroactive substances (uric acid and ascorbic acid), and artificial substances such as active pharmacological agents, e.g., paracetamol (acetaminophen) [17,25,34,35]. Chemically modified electrodes and modified electrochemical techniques have been proposed to solve low sensitivity and selectivity issues [36,37,38,39]. Over the past decade, various nanomaterial-modified sensors with catalytic activity for dopamine detection, along with the use of permselective polymeric membranes, have been reported in the literature [34,39,40,41].
The present study focuses on the synthesis of nanomaterials based on silica, using a method that encapsulates gold nanoparticles (Au NPs) in a thin silica layer. This process is carried out in the presence of a sodium hydroxide and a silica agent, leading to the formation of a thin film composed of repeating [-(-O-Si-O-)-] units around the Au NPs. Subsequently, this silica-coated gold nanostructure undergoes functionalisation with an organosilane known as APTES (3-aminopropyl triethoxysilane), resulting in the synthesis of Au@SiO2-APTES composite. This choice is supported by the surface properties of silica, including a noteworthy specific surface area and its ability to enhance electron transfer in the presence of gold NPs. Furthermore, the inherent porosity of silica NPs facilitates efficient absorption, ensuring faster responses in the detection of DA. Different characterisation techniques were used to elucidate the nature of the composite, including energy-dispersive X-ray analysis, electron microscopy, infrared spectroscopy, and X-ray diffraction. Electrochemical methods, such as electrochemical impedance spectroscopy cyclic voltammetry and differential pulse voltammetry, were used to evaluate the performance of the prepared Au@SiO2-APTES composite. The investigation meticulously analysed the sensor’s dynamics and selectivity, scrutinising critical factors that influence its performance. The optimal operational parameters were determined by studying experimental factors such as the pH of the buffered solution, incubation time, and the quantity of Au@SiO2-APTES deposited on the electrode. The sensor’s effectiveness in detecting DA in biological samples was subsequently assessed.
2. Materials and Methods
2.1. Materials and Reagents
Tetrachloroauric (III) acid trihydrate (HAuCl4·3H2O, ≥99.0%), (3-aminopropyl) triethoxysilane (APTES, 99%), Tetraethyl orthosilicate (TEOS, ≥99.0%), potassium chloride (KCl), ethanol (EtOH, 96%), hydrochloric acid (HCl), sodium hydroxide (NaOH), sodium borohydride (NaBH4), potassium hexacyanoferrate (III) (K3[Fe(CN)6]), potassium hexacyanoferrate (II) trihydrate (K4[Fe(CN)6]·3H2O), dopamine (DA), ascorbic acid (AA), uric (UA) acid, and glucose (Glu), serotonin, and L-tryptophan were purchased from Sigma-Aldrich through Chimisi and Chimie Tunisie companies (Tunisia). All solutions were prepared with ultrapure water (resistivity 18.2 mΩ cm). Phosphate-buffer solution (PBS, 0.1 molL−1) was prepared by dissolving disodium hydrogen phosphate dodecahydrate (Na2HPO4·12H2O) and monosodium dihydrogen phosphate trihydrate (NaH2PO4·2H2O) in distilled water. The pH value of the supporting electrolytes was modified, if necessary, by adding NaOH (1 molL−1) or HCl (1 molL−1) solutions.
Different glucose (Glu), dopamine (DA), uric acid (UA), ascorbic acid (AA), and hydroquinone (HQ) solutions were prepared in PBS. A fresh dopamine stock solution was prepared daily and used to generate the calibration curves. The experiments for DA detection started with the lowest concentration and proceeded to higher concentrations.
2.2. Preparation of Au@SiO2-APTES
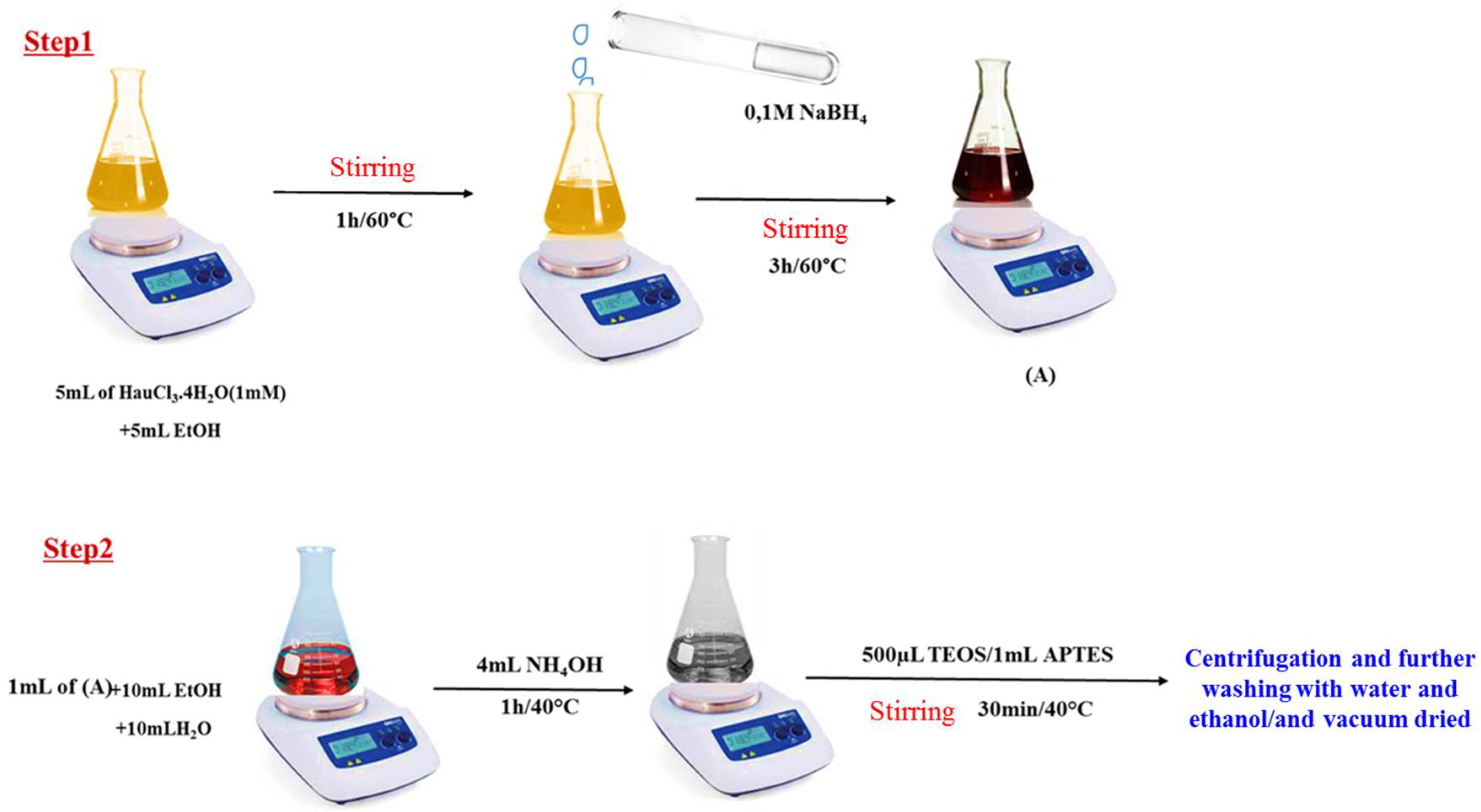
The synthesis of Au@SiO2-APTES nanocomposites was performed in two steps. Firstly, Au NPs were prepared using a solution containing 5 mL of 1 mmolL−1 HAuCl3·4H2O and 5 mL of EtOH. The solution was heated at 60 °C and stirred for 1 h, after which 0.1 molL−1 NaBH4 (2 mL) was added, and the mixture was stirred for a another 3 h at the same temperature. During this time, the colour changed from yellow to wine red, confirming the reduction of Au3+ ions and the formation of the Au NPs (Figure 1, step 1).
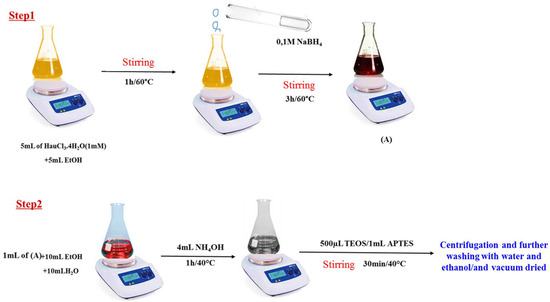
Figure 1.
Schematic illustration of the preparation of the Au NPs (solution A) and Au@SiO2-APTES composite.
The second stage consisted of adding 1 mL of solution as prepared to an Erlenmeyer flask containing 10 mL of EtOH and 10 mL of H2O. After a few minutes, 4 mL of NH4OH (1 molL−1) was added as a basic catalyst, followed by the simultaneous addition of TEOS (500 µL) and APTES (1 mL). The reaction was conducted at 40 °C with stirring for 30 min. The solid material was then collected by centrifugation, washed twice with water and ethanol, and vacuum dried at 60 °C (for details see Figure 1, step 2).
2.3. Assembly of Au@SiO2-APTES/GCEs
Glassy carbon electrodes (GCEs) were cleaned prior to any modifications with alumina slurry, sonicated in ethanol for 5 min, and dried at room temperature. The preparation procedure of the sensor surface was as follows: A quantity of 1 mg of Au@SiO2-APTES was dispersed in 1 mL of ethanol and sonicated for 30 min to obtain a uniform suspension. Subsequently, 7 µL of the prepared suspension was applied to the polished surface of the GCE. The coated electrode (Au@SiO2-APTES/GCE) was allowed to air-dry at room temperature (25 °C) before use.
2.4. Characterisation and Electrochemical Measurements
The morphology and microstructure of the sample were analysed using scanning electron microscopy (SEM) with a JEOL JEM-2000 FX microscope. X-ray diffraction (XRD) patterns of the powder samples were obtained using a Philips Panalytical X’Pert powder diffractometer equipped with CuKα radiation (λ = 1.540 Å) over a 2θ range of 5° to 80°. Additional characterisation was performed by recording infrared (IR) spectra with a PerkinElmer spectrometer.
Electrochemical tests were conducted using a computer-controlled Autolab PG potentiostat/galvanostat (AUT 83965). These measurements were conducted in a conventional three-electrode cell configuration, where the modified GCE was the working electrode and a platinum wire and an Ag/AgCl (KClsat) electrode was used as the counter and reference electrodes, respectively. The differential pulse voltammetry (DPV) approach for DA measurement involved two sequential phases: (1) the polarisation of the working electrode (Au@SiO2-APTES/GCE) for 5 min at a potential of −0.2 V to pre-concentrate cationic DA on the sensor surface, and (2) the use of DPV over a potential range from −0.2 V to 0.8 V to facilitate the oxidation of the accumulated DA into dopamine-o-quinone.
3. Results and Discussion
3.1. Material Characterisation
3.1.1. Morphology and Structure of Au@SiO2-APTES
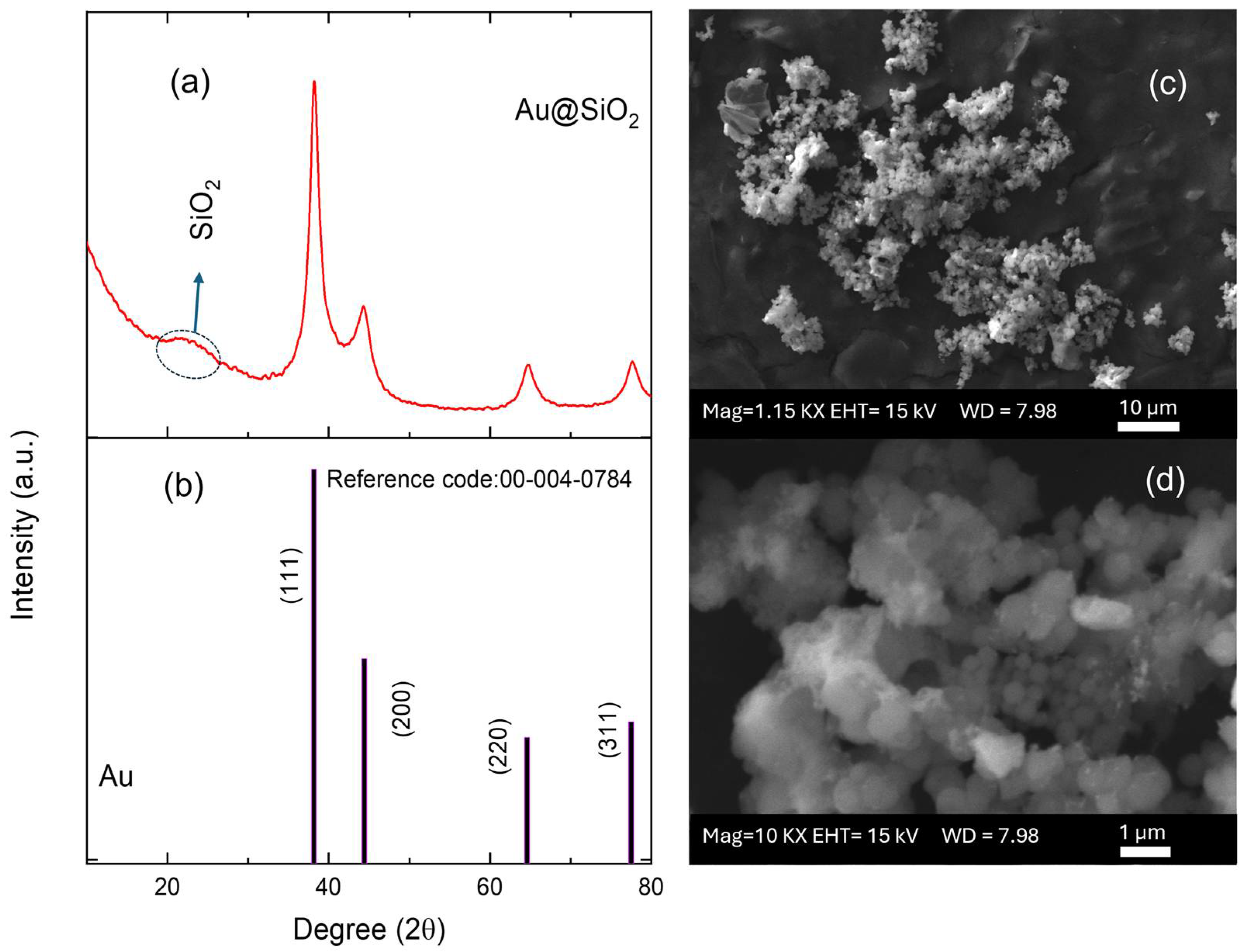
X-ray diffraction analysis of Au@SiO2-APTES composite revealed distinct peaks corresponding to two different components: gold and silica (Figure 2a). The peaks identified at 2θ values of approximately 38.2°, 44.4°, 64.6°, and 77.5° were assigned to the (111), (200), (220), and (311) diffraction planes of polycrystalline face-centred cubic (fcc) [42] Au nanoparticles (JCPDS card number 00-004-0784, Figure 2b). The presence of broad bands in the diffractogram suggests the existence of small-sized Au NPs. The full-width at half maximum (FWHM) of the (111) XRD line (2θ = 38.2°) of the Au NPs and the well-known Scherrer equation were used to estimate the average crystallite size, whose value was about 6 nm [43]. Furthermore, the broad and diffuse peak around 22° was attributed to the amorphous phase of SiO2 and confirmed the presence of silica in the composite.
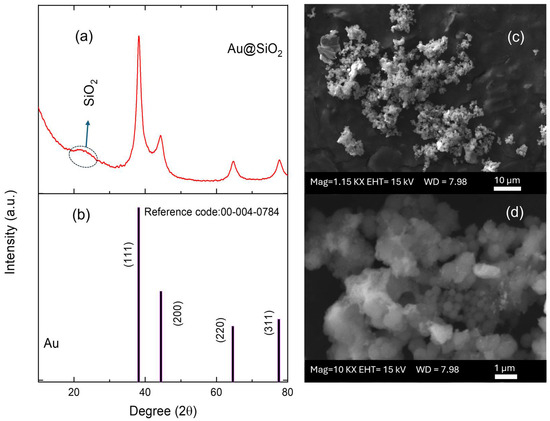
Figure 2.
(a) XRD pattern of the Au@SiO2-APTES composite and (b) JCPDS reference pattern. (c,d) SEM micrographs for the Au@SiO2-APTES composite deposited on screen-printed electrodes.
3.1.2. Electron Microscopy Analysis
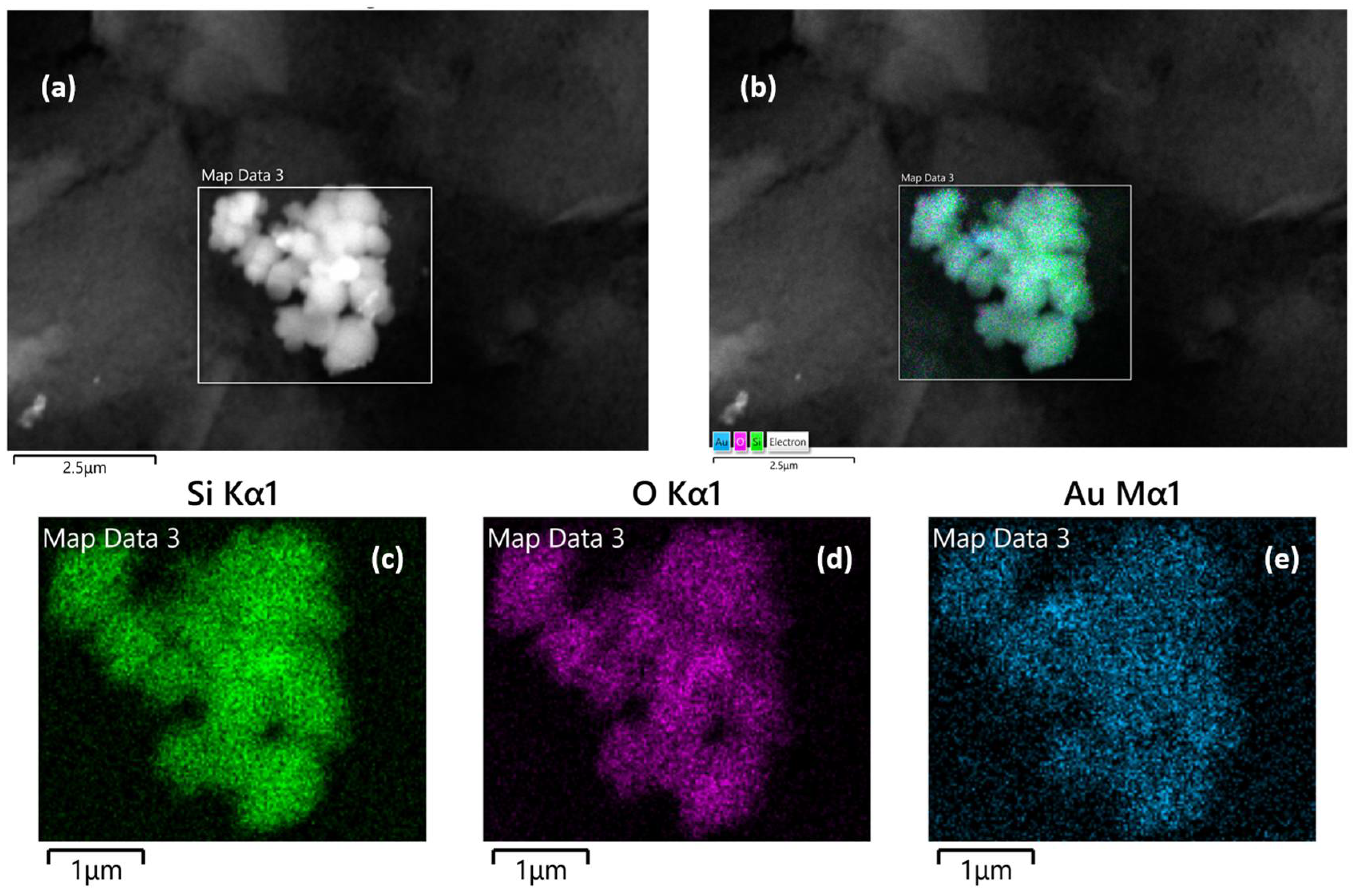
SEM, TEM, and energy-dispersive X-ray (EDX) spectroscopy were used to better understand the composition and structure of the Au@SiO2-APTES composite. To evaluate the surface morphology of the SiO2 support and Au@SiO2-APTES, TEM analysis was performed. According to the obtained results, SiO2 (Figure S1) exhibits a uniform spherical morphology. Following the insertion of gold nanoparticles and functionalization with APTES (Figure S1b–d), a swelling of the spheres is observed, along with the appearance of an outer layer, confirming the presence of gold nanoparticles (Au NPs) within the SiO2 matrix. These observations also highlight a strong interaction between NH2 groups and the gold nanoparticles, while preventing their aggregation on the support. Figure 2c,d show the surface of the SPE modified with the Au@SiO2-APTES composite. These images reveal the presence of noticeable particle aggregates, highlighting the distribution and clustering of the composite material on the electrode surface. NPs tend to form aggregates of different shapes and size due to several phenomena such as covalent bonds, Van der Waals forces, hydrogen bonds, or electrostatic interactions. In addition, this behaviour may be amplified by functionalising the particle surface by specific groups. In this case, the presence of aminopropyl groups from the APTES molecules on the surface of the silica particles could contribute to this effect. Figure 3 shows the composition of these aggregates, which is made up of spherical silica NPs ranging from approximately 200 to 500 nm, where Au NPs are inserted in the structure of the silica particles. EDX analysis confirmed the chemical composition, their proportions, and spatial distribution of the nanomaterials in the composite. Elemental analysis confirmed the presence of oxygen (O), silicon (Si), and gold atoms, with atomic percentages of approximately 77.19% (O), 21.12% (Si), and 1.65% (Au). In addition, the corresponding elemental mapping for Au@SiO2-APTES NPs is also shown in Figure 3. The spatial distribution for all elements is fairly homogenous in the composite, suggesting the Au NPs are inserted in the silica structures, and may improve the conductive and catalytic properties of the Au-modified silica material.
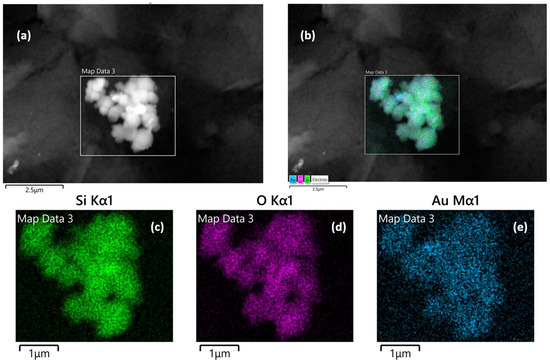
Figure 3.
Energy-dispersive X-ray mapping analysis of Au@SiO2-APTES NPs. (a) Selected region of interest for mapping, (b) overall elemental composition mapping, accompanied by individual elemental mapping images for (c) silicon (Si), (d) oxygen (O), and (e) gold (Au).
3.1.3. Infrared Spectrum Characterisation
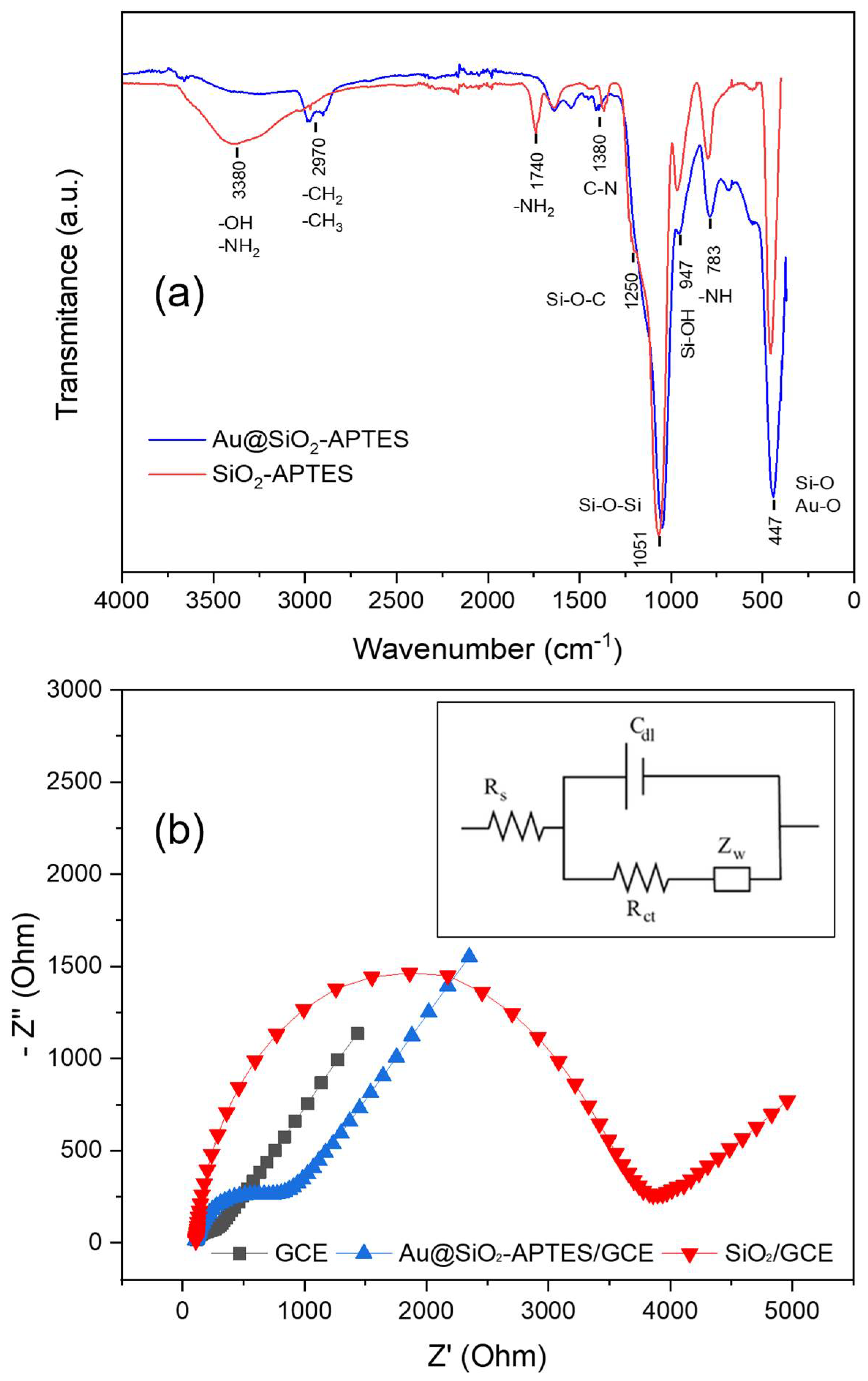
Figure 4a depicts the FT-IR spectra for both SiO2-APTES and Au@SiO2-APTES nanocomposites. The SiO2-APTES spectrum shows the characteristic bands at 447 cm−1 and 1051 cm−1 due to the deformation of Si-O bonds [44]. Furthermore, the wide peak at 3380 cm−1 was attributed to the O-H and N-H vibrations. Lastly, the peaks at 950 and 783 cm−1 were assigned to the stretching vibration of Si-OH groups and -NH bonds, respectively. The spectrum for Au@SiO2-APTES nanocomposite has a similar shape, with some qualitative differences. For example, the two characteristic bands located at 2970 cm−1, ascribed to the stretching of C-H bonds, are more evident [45]. This behaviour can be attributed to the considerable reduction in peak intensity in the hydroxyl and amine region. (3400–3600 cm−1) [46], attributed to a combination of stretching vibrations of silanol groups or hydrogen bonding with Au NPs. Finally, the peak ratio between the bands assigned to Si-O-Si (1051 cm−1) and Si-O (447 cm−1) vibration modes shows, once again, a significant change, attributed to a new contribution at 447 cm−1 from the Au-O bonding.
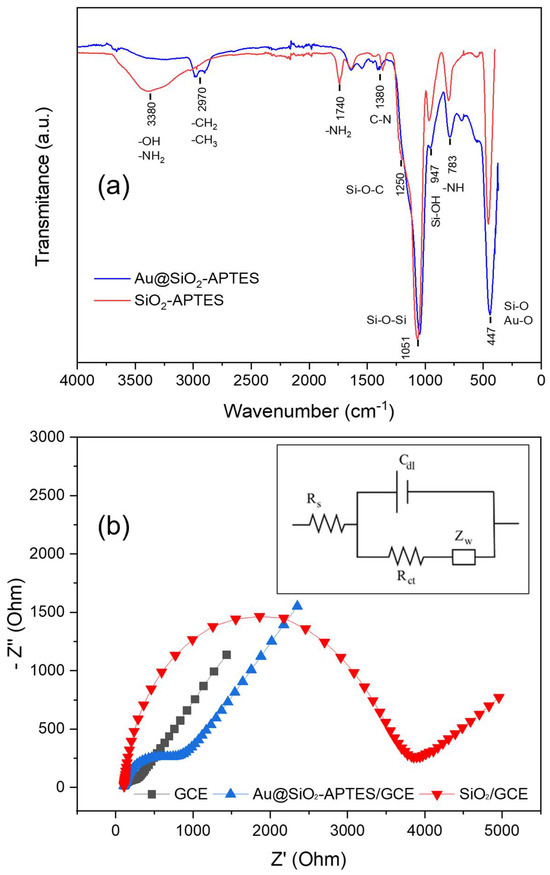
Figure 4.
(a) FT-IR spectra of Au@SiO2-APTES and SiO2-APTES nanocomposites. (b) EIS spectra of bare/GCE, SiO2-APTES/GCE and Au@SiO2-APTES/GCE in 0.10 molL−1, KCl with 5.0 mmolL−1 [Fe(CN)6]3−/4− solution (frequency range: 0.1–105 Hz).
3.1.4. Electrochemical Characterisation of SiO2-APTES and Au@SiO2-APTES Nanocomposites
The correct functionalisation of the silica particles and their electroactivity were studied by means of electrochemical impedance analysis (EIS) in the presence of ferro/ferricyanide redox pair. EIS is an efficient technique for studying the electrochemical properties of different materials, allowing the electron transfer processes on the surface of the electrode/material to be better understood. The Nyquist plot (Figure 4b) contains a semicircular and a straight part. The first segment is associated with a restricted electron transfer process at high frequencies, while its diameter represents the charge transfer resistance (Rct), which governs the electron transfer process at the electrode interface. The straight part on a Nyquist plot, at low frequencies, is associated with a Warburg element (Zw), which represents the diffusion of reactants to the electrode surface. Figure 4b shows the Nyquist plot for different configurations: bare glassy carbon electrode (GCE), silica-modified GCE (SiO2/GCE), and nanocomposite-modified electrode (Au@SiO2-APTES/GCE). The Rct values during different construction stages were calculated using the Randles circuit and are shown in Figure 4b (inset). This equivalent circuit comprises an active electrolyte resistance (Rs) connected in series with a parallel arrangement that includes the double-layer capacitance (Cdl), a Warburg element (Zw), and a charge transfer resistance (Rct). (Figure 4b). After fitting, the obtained Rct values were the following: 74 Ω (GCE), 3.687 Ω (SiO2/GCE) and 943 Ω (Au@SiO2-APTES/GCE). These findings verify the outstanding conductivity properties of the bare GCE and the insulating properties of the silica particles in the SiO2/GCE configuration. On the other hand, the introduction of amino groups through APTES enhances the interaction with electron donors, improving the conductivity of the modified silica composite (Au@SiO2-APTES). Furthermore, the incorporation of Au NPs into the silica structure further enhances the composite’s electronic properties. The good chemical reactivity, conductivity, and large surface area of Au NPs boost charge transport and electron transfer rates in the nanocomposite, leading to improved overall reactivity/conductivity. The above results suggest that this nanocomposite may be effective for oxidation/reduction in different electroactive substances, highlighting its potential for different applications, including catalysis, analytical chemistry, electrochemical sensors, energy storage devices, and environmental remediation.
3.2. Analytical and Electrochemical Optimisations
3.2.1. Optimisation of Experimental Conditions
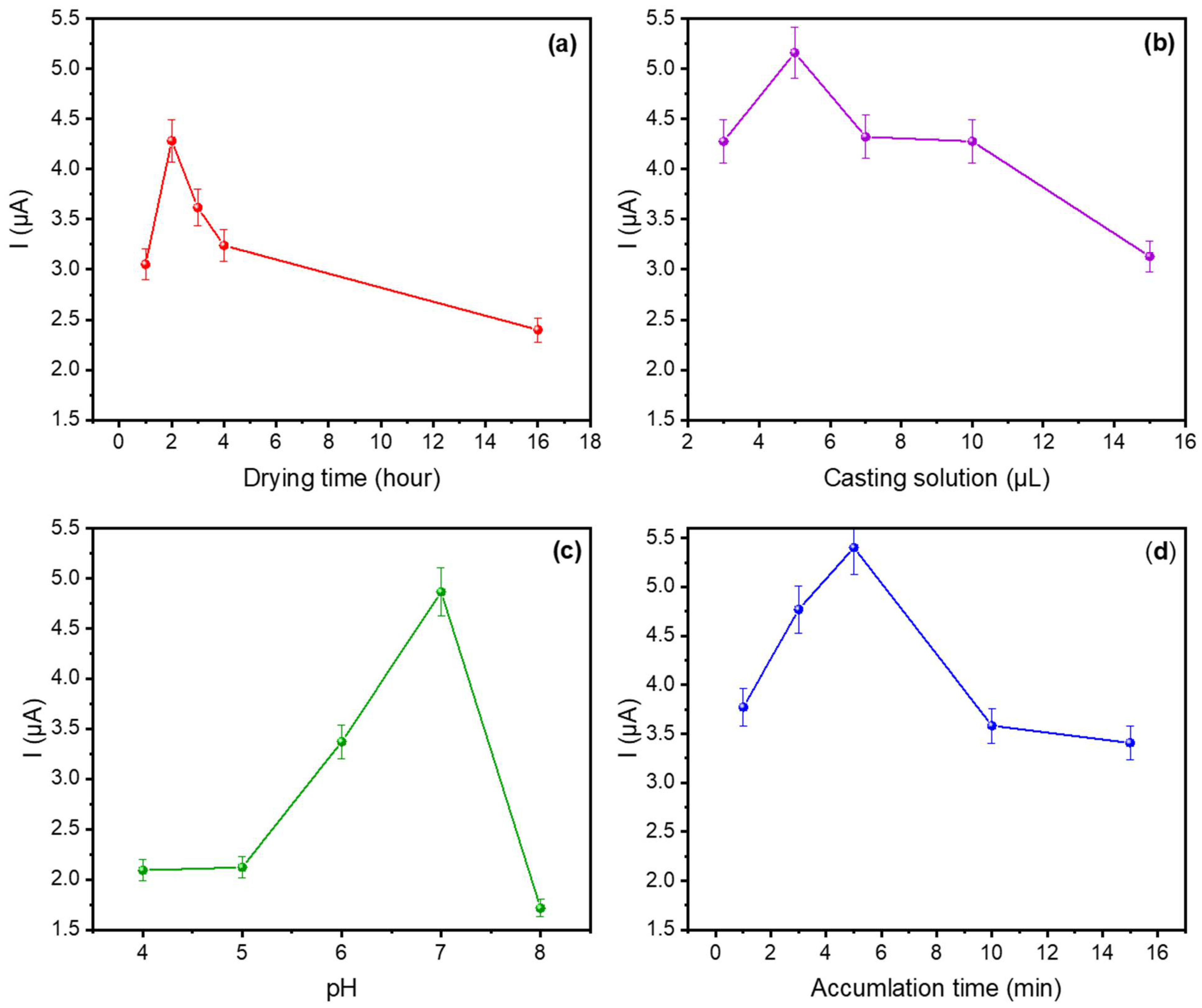
The electrocatalytic response of the Au@SiO2-APTES/GCE was optimised by taking into consideration several factors that can influence the sensor response such as the amount of nanocomposite deposited on the electrode surface, the pH of the buffer solution, the drying time after casting, and the accumulation time during the adsorption step. Differential pulse voltammetry (DPV) was used for DA detection and the optimisation of the above parameters. The initial studies started by using 10−5 molL−1 DA in phosphate-buffer solution (PBS, pH 7). Therefore, DA was preconcentrated on the sensor surface, polarising the working electrode at a fixed potential of −0.2 V. Since the isoelectric point (IEP) of DA is 9.8, under pH 7 conditions and with a negative polarisation of the working electrode, the positive charged DA will exhibit an attractive electrostatic interaction with the electrode surface and will be adsorbed onto it. The potential was held constant for 1 to 15 min (Figure 5), after which the stripping step started increasing the working potential until +0.8 V, a voltage that is high enough to induce the oxidation of the adsorbed dopamine (DA).
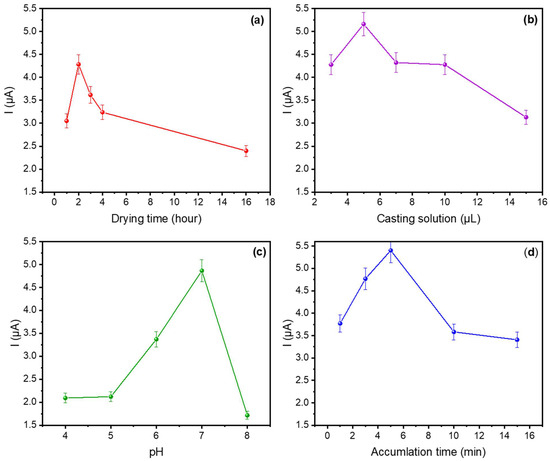
Figure 5.
Influence of (a) drying time, (b) Au@SiO2-APTES amount deposited on the sensor; (c) pH value of the buffer solution, and (d) accumulation time on the voltametric response of Au@SiO2-APTES/GCE in PBS (0.1 molL−1) containing 10−5 molL−1 of DA.
Figure 5a shows the effect of different drying times at 5 µL of matrix (from 1 to 16 h) on the oxidation current response. The results obtained show that the current response increases from 1 to 2 h and then decreases after 2 h, suggesting that a uniform, stable, and well-adhered nanoparticle coating is obtained on the GCE surface after the second drying hour. The latter loss of response (at higher drying times) could be attributed to uneven coatings, reduction in the active surface area (aggregation/agglomeration effects), and the hindering of the electrochemical reactions. Consequently, 2 h was identified as the optimal drying time.
Different volumes of the Au@SiO2-APTES dispersion in EtOH (1 mg mL−1) were deposited on GCE to study the effect of the amount of Au@SiO2-APTES deposited on the sensor surface and the electrochemical response against DA. The sensor’s electrochemical response improved as the volume of Au@SiO2-APTES deposited on the electrode surface was increased from 3 to 5 μL (Figure 5b), suggesting improved electron transfer and a greater availability of active sites due to the presence of Au nanoparticles. However, beyond 5 μL, the oxidation peak current starts to decrease. This decline is likely due to excessive deposition, which may result in higher resistance, limited access to active sites, or partial aggregation of the material (as CV curves shown in Figure S2). Additionally, an overly thick layer could block electron transfer pathways, reducing the overall efficiency. Therefore, 5 μL of 1 mg/mL Au@SiO2-APTES dispersion was used for further experiments.
The effect of the solution’s pH on the adsorption process was analysed in PBS over a pH range of 4 to 8. As illustrated in Figure 5c, the peak current showed a rapid increase as the pH shifted from 4 to 7, followed by a decline at pH values above 7. This phenomenon can be ascribed to the mechanism described above and the different IEPs of the materials and compounds involved in the redox reaction. APTES-modified silica materials have an IEP of approximately 7 [47], becoming negatively charged at higher pH levels. Besides which, the zeta potential of DA at a pH of approximately 8–9 approaches zero and becomes negative around pH 9. Consequently, the interaction between APTES-modified silica and DA is primarily governed by electrostatic attraction/repulsion. This phenomenon suggests an operational pH window close to pH 7. At pH values higher or lower than this value, the zeta potential of both elements become equivalent, leading to electrostatic repulsion.
Figure 5d illustrates the relationship between the peak current and the accumulation time for DA. The sensor response increased from 1 to 5 min, whereas for longer periods, the analytical current decreased. This finding can be attributed to the presence of secondary reactions, most likely stemming from the natural oxidation of DA at neutral or slightly alkaline pH values. Under these conditions, the by-products of the natural DA oxidation (dopaminoquinone, 5,6-dihydroxyindoline, and aminochrome) may be passive on the sensor surface. On the other hand, the formation of an insulating layer of polydopamine on the sensor surface [48] may hinder the diffusion and arrival of DA to the surface. Consequently, 5 min was set as the accumulation time for analytical applications.
3.2.2. DA Detection
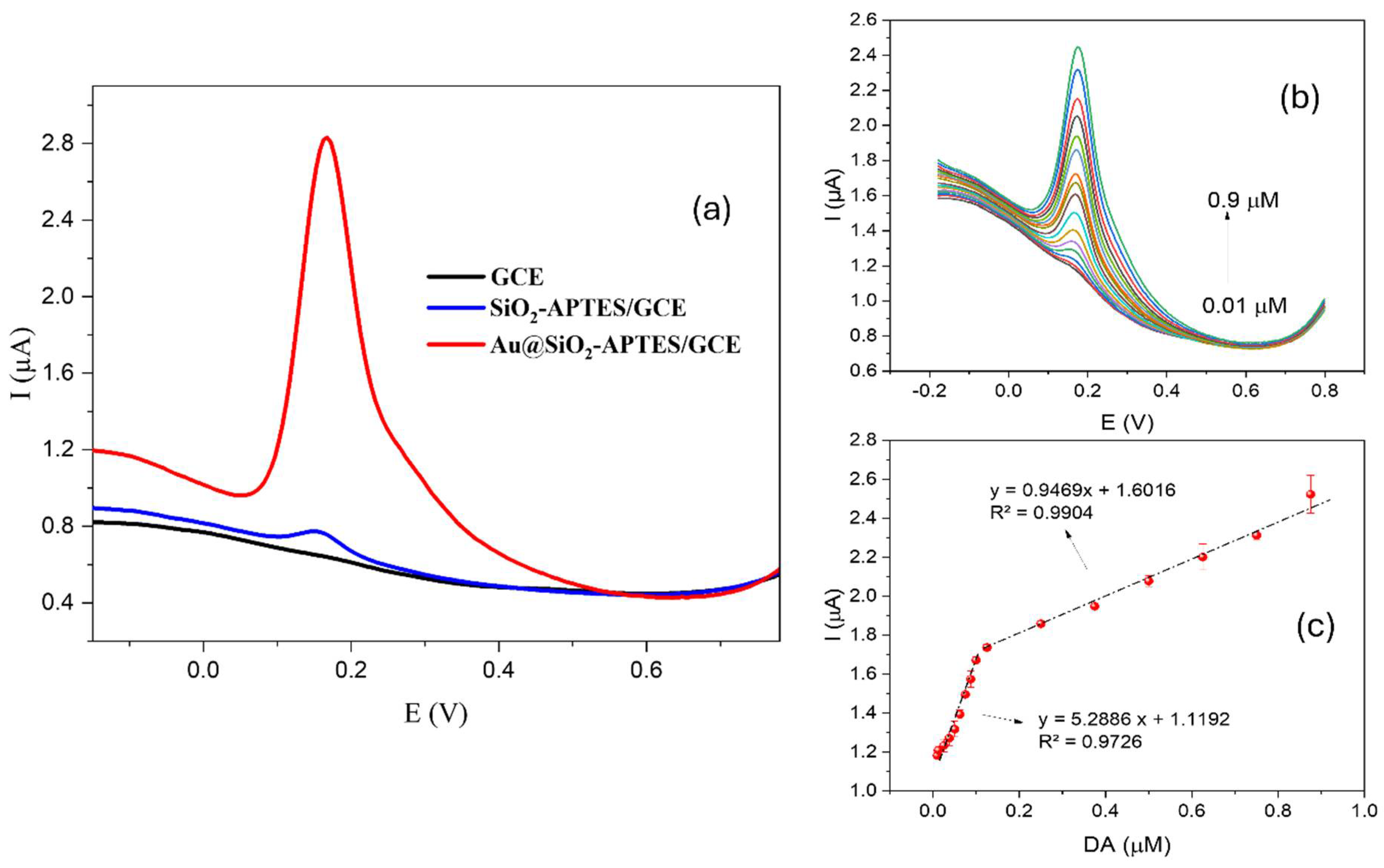
To assess the sensor’s effectiveness, the response to 1 × 10−5 molL−1 DA was tested across different sensor configurations, ranging from the simplest (bare GCE) to the proposed sensor (Au@SiO2-APTES/GCE). The response of an electrode modified with APTES (SiO2-APTES/GCE) in the absence of Au NPs was also evaluated to demonstrate the catalytic effect of the proposed hybrid structure. As shown in Figure 6a, the GCE showed no response to the tested concentration under optimal experimental conditions. In contrast, the SiO2-APTES/GCE configuration showed a positive response to the tested concentration with a peak current of about 0.08 µA. Finally, the hybrid configuration proposed (Au@SiO2-APTES/GCE) here showed a significant improvement, reaching a peak current of about 2 µA, which is 25 times higher than the intermediate configuration. This notable enhancement can be ascribed to the incorporation of gold nanoparticles within the hybrid composite, which probably facilitates electron transfer and increases the electroactive surface area, thereby improving the sensitivity and catalytic efficiency of the sensor.
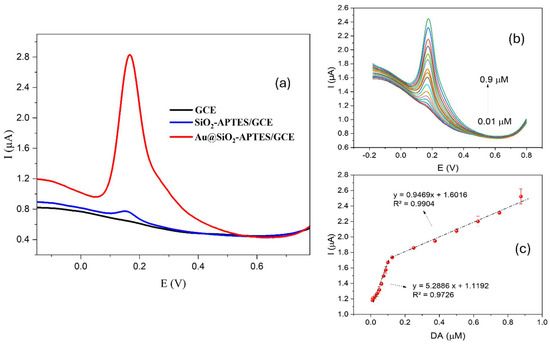
Figure 6.
(a) DPV response of different sensor configurations for DA under optimal conditions (b) DPV of DA on the Au@SiO2-APTES/GCE. (c) Calibration curve for DA in (b). Data were recorded at a casting solution of 5 µL, a drying time of 2 h, and an accumulation time of 5 min in 0.1 molL−1 PBS at pH 7.
After optimising the main experimental variables, the detection capability of the electrode modified with Au@SiO2-APTES for DA detection was also investigated. This included evaluations of sensitivity, operating range, and linearity. Figure 6b shows the raw DPV response of different DA concentrations on the Au@SiO2-APTES/GCE. As can be seen in Figure 6c, the peak current presented a linear increase with rising DA concentrations, observed across two separate concentration ranges. This response is likely due to variations in the detection mechanism, where different limiting factors dominate at specific concentration thresholds. In the first range, up to 1 × 10−7 molL−1, the oxidation process appears to follow a more straightforward kinetic pathway, probably due to a lower surface coverage of DA on the electrode. Beyond this concentration, from 1.25 × 10−7 molL−1 to 8.75 × 10−7 molL−1, a second linear region is observed, which could be explained by changes in the adsorption or diffusion dynamics of DA molecules on the electrode surface as the concentration increases. This transition suggests that, at higher concentrations, the electrochemical response might be influenced by factors such as saturation of surface sites or changes in the electron transfer kinetics, leading to the observed shift in the linear range of the peak current. The response above 8.75 × 10−7 molL−1 exhibited saturation; therefore. The limit of detection (S/N = 3) and the limit of quantification were found to be 1.4 × 10−8 molL−1 (0.014 µmolL−1) and 4.7 × 10−8 molL−1 (0.047 µmolL−1), respectively.
Table 1 provides a summary of the sensing characteristics of DA sensors fabricated with various materials and composites reported in the literature. The authors found that Au@SiO2-APTES offers superior or comparable performance in DA measurements, while the material used in the present study is also easier to prepare. Similar approaches have been proposed, such as the nanocomposite described by Immanuela et al. [11], which utilised gold nanoparticles combined with SiO2 for dopamine detection. However, their methodology differs significantly in terms of fabrication and surface functionalization techniques. In our case, the incorporation of APTES introduces amine groups that enhance the interaction between the electrode surface and dopamine molecules, facilitating better immobilisation of gold nanoparticles and improving electron transfer kinetics. This modification results in a highly responsive and sensitive electrochemical interface. Additionally, the fabrication process employed in our study ensures uniform dispersion of the nanoparticles and a stable nanostructure, effectively minimising aggregation and further improving the sensor’s performance. These advancements contribute to enhanced sensitivity, selectivity, and long-term stability, making our sensor more suitable for real-world applications compared to previously reported materials.

Table 1.
Comparison with other electrochemical sensors for the determination of dopamine.
3.2.3. Interference Study
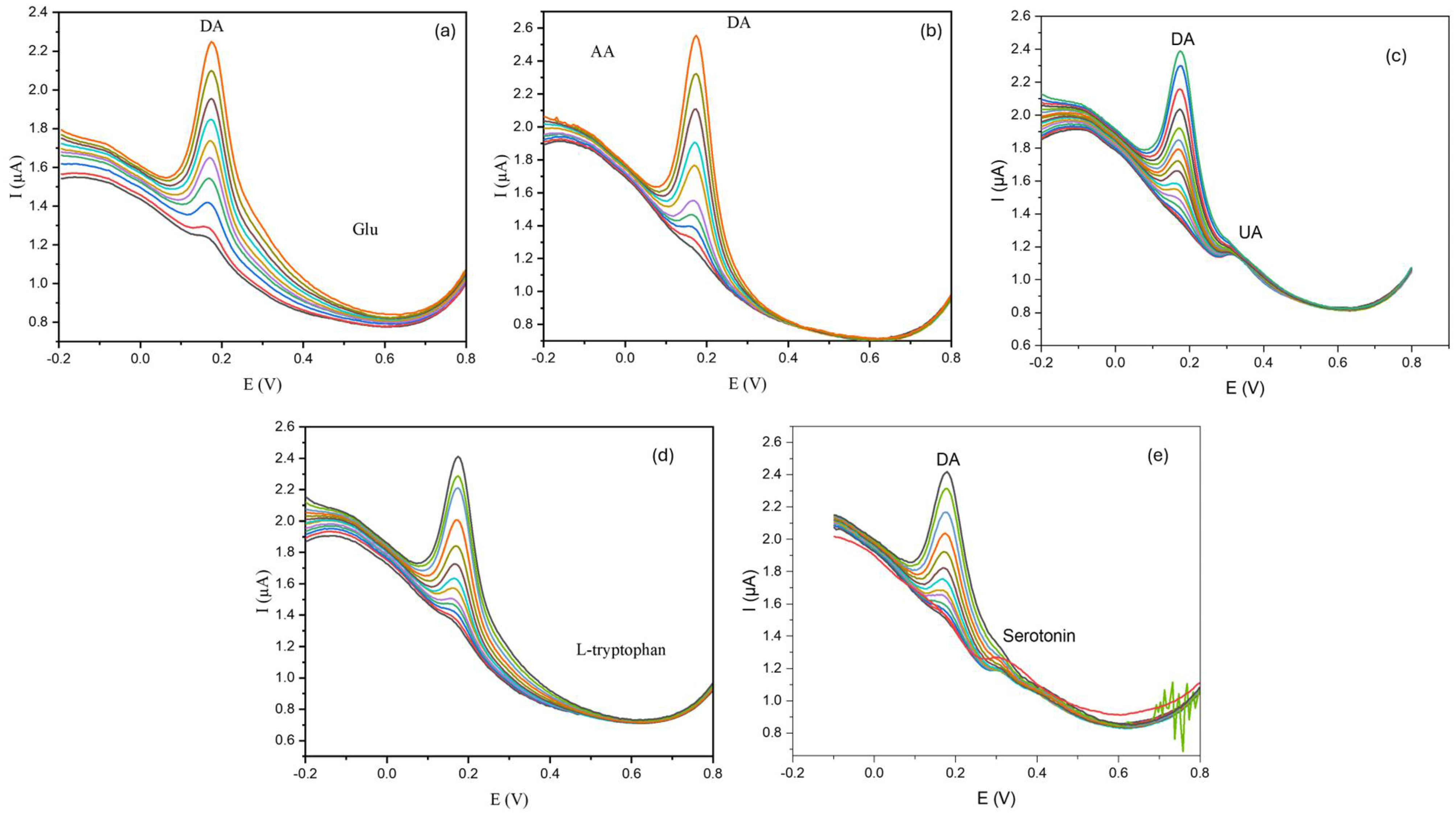
The selectivity of the sensor was assessed using the DPV technique. Interfering species, including uric acid, ascorbic acid, and glucose were chosen for the selectivity experiment on the dopamine sensor presented here. Moreover, a selectivity test in the presence of amine-containing molecules was also evaluated using serotonin and L-tryptophan as biogenic amines and amino acid interferent examples, respectively (see Figure 7). Each interference substance was used at different concentrations (see Figure 7) to ensure that the interfering species were present in the same range found in biological matrixes such as blood and urine. During the oxidation of dopamine, the presence of interfering species produced no significant modification in peak intensity and shape, underscoring the marked selectivity to DA. However, a slight change in the baseline occurs. These deviations can be attributed to the existence of the matrix effect. To correct these variations and ensure precise and consistent detection of dopamine, baseline correction can be applied to the analysis. This correction is essential for improving the practical applicability of the sensor. The combination of its inherent selectivity (without overlapping peaks), baseline correction, and the use of the standard addition method in real samples can enhance the sensor’s potential use as a robust tool for the accurate monitoring of dopamine in real-world applications (see below). To evaluate the matrix’s suitability for dopamine detection in practical applications, we examined the influence of 10−3 M heavy metal ions too, such as Hg2+, Co2+, Cu2+, and Mn2+. Such ions do not show an overlapping effect in the detection of DA. The results of these experiments are displayed in Figure S3.
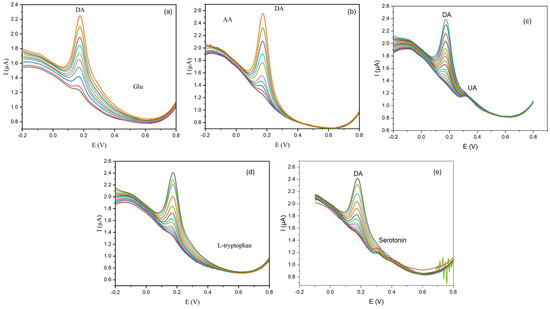
Figure 7.
Raw DPV curves of Au@SiO2-APTES/GCE for dopamine (DA) at different concentrations ranging from 0.375 to 0.875 µmolL−1 of DA (accumulation time = 5 min) in the presence of different potential interferences at a fixed concentration: (a) glucose (Glu) (10−3 molL−1), (b) ascorbic acid (AA) (10−4 molL−1), (c) uric acid (UA) (10−3 molL−1), (d) L-tryptophan (10−4 molL−1), (e) serotonin (10−3 molL−1).
3.2.4. Reproducibility and Repeatability Test
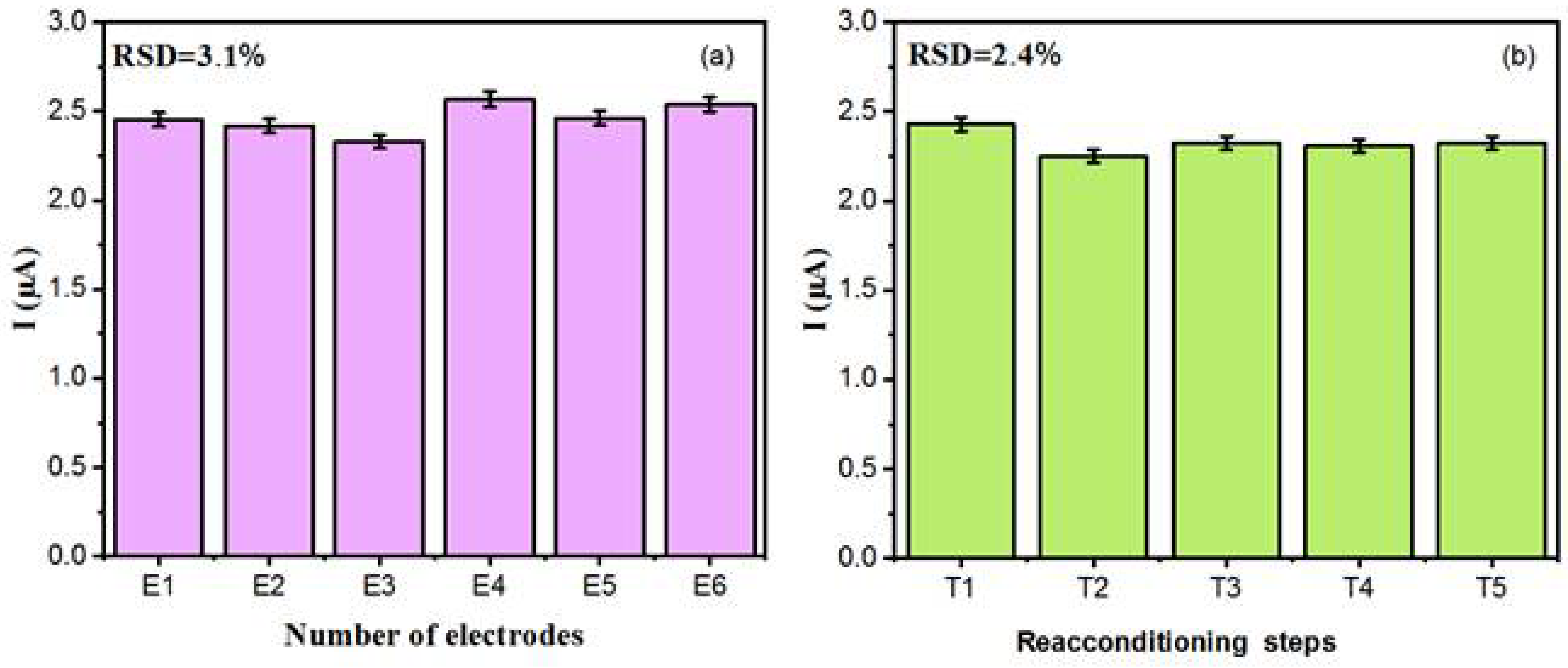
The sensor’s reproducibility and repeatability were assessed by performing DPV measurements for the detection of 0.875 µmolL−1 DA within 0.1 molL−1 PBS (pH 7.0). Reproducibility studies, Figure 8a, were conducted with six different sensors prepared under identical conditions. The sensor response yielded an average peak current value of ca. 2.5 µA at the tested concentration. Notably, the six electrodes demonstrated satisfactory reproducibility in their current response, with a relative standard deviation (RSD) value of about 3.1%, confirming good sensor-to-sensor consistency. A repeatability test, after regeneration in PBS, was conducted using the same sensor. Therefore, such a sensor was tested five times with a solution containing 0.875 µmolL−1 DA to study the stability of this composite for DA detection. After each test, the sensor was regenerated by immersing it in a 0.1 molL−1 PBS buffer solution for 20 min, to eliminate DA molecules absorbed on the electrode surface. After each regeneration step, the sensors were rinsed (three times) with water and dried for 1 h at room temperature. The sensor response, Figure 8b, after five measurements (with four regenerations) in 0.875 µmolL−1 DA showed a well stabilised response with a (RSD) value of 2.4%. Finally, all these results confirmed the outstanding performance (repeatability and reproducibility capacity) in DA detection, making the sensor ideal for accurate analytical measurements.
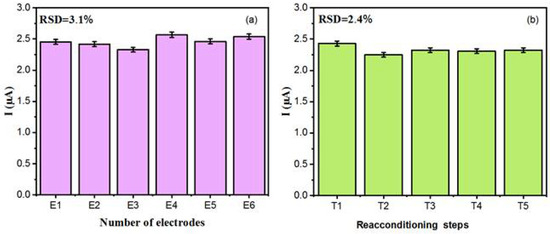
Figure 8.
(a) Reproducibility test for six different electrodes. (b) Repeatability for a single modified electrode after undergoing regeneration (four cycles, from T2 to T5) in 0.1 molL−1 PBS (pH 7.0). T1 denotes the initial current measured by the sensor at a DA concentration of 0.875 µmol L−1.
3.2.5. Applications in Biological Samples
The sensor was applied to the determination of DA in selected real human samples, such as blood and urine, to demonstrate its feasibility. The standard addition method was used to mitigate potential matrix effects during DA analysis. All samples were diluted (1:99) with 0.1 molL−1 PBS (pH 7.0) before measurement. Diluting the analyte is crucial in electrochemical detection. High concentrations can saturate electrodes, affecting accuracy and linearity. Dilution helps prevent this saturation. In addition, urine contains various components that can interfere with DA detection. Diluting the sample reduces these interferences, enhancing selectivity and accuracy. The consistent results obtained further validate the accuracy of the measurements. The results (Table 2) showed acceptable recovery rates of the spiked samples in the range from 90% to 102%. Therefore, the proposed method can provide a feasible tool for the determination of DA in real-world samples.

Table 2.
Quantification of DA in human blood and urine samples.
4. Conclusions
This study presents a novel electrochemical sensor using APTES-modified silica-doped Au NPs for the determination of dopamine (DA). This modification aims to enhance DA detection by improving both sensitivity and selectivity. The Au@SiO2-APTES electrode offers several advantages for DA measurement. Firstly, APTES-functionalised silica introduces amino groups to the electrode surface, which enhances the interaction between DA molecules and the sensor, promoting their adsorption and facilitating greater participation in the electrochemical reaction. Secondly, the incorporation of Au NPs enhances the electrocatalytic properties of the material, boosting the sensor’s ability to detect DA. Thirdly, the synergistic effects of Au NPs and APTES-functionalised silica improve the signal-to-interference ratio, effectively minimising the impact of common biological electroactive substances such as ascorbic acid, hydroquinone, glucose, and uric acid (UA). The sensor demonstrated excellent peak resolution, high selectivity, sensitivity, and recoveries for DA detection in the presence of substantial levels of interferents in real-world samples. Finally, we concluded with these results its suitability for applications in neuroscience research, clinical diagnostics, and environmental monitoring.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemosensors13030087/s1, Figure S1: TEM images obtained for SiO2 (a), and Au@SiO2-APTES samples (b–d). Figure S2: Raw voltammetric curves (CV) related to the amount of Au@SiO2-APTES deposited on the electrode surface (a), and the CV peak current response obtained in the presence of a redox couple ([Fe(CN)6]3−/4− solution implemented with 0.1 M KCl with 5.0 mmolL−1 (b). Figure S3: Raw DPV current obtained in the presence of 10−5 M dopamine (DA) and various interfering ions at a concentration of 10−3 M. Measurements were performed under controlled conditions, including the application of a 5 μL casting solution, a drying period of 2 h, and an accumulation time of 5 min in PBS buffer (pH 7).
Author Contributions
A.D.: Investigation, Methodology, Formal analysis, Writing-Original Draft. P.A.S.-C.: Methodology, Investigation, Writing-Original Draft, Supervision. S.C.: Methodology, Writing-Original Draft. M.H.: Methodology, Formal analysis. B.V.M.R.: Methodology, Formal analysis. H.B.: Methodology, Formal analysis, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in compliance with the Declaration of Helsinki and approved by the Research Ethics and Animal Welfare Committee of the University of La Laguna (CEIBA2024-3528, 10 January 2025).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Mekuye, B.; Abera, B. Nanomaterials: An overview of synthesis, classification, characterization, and applications. Nano Sel. 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Wang, P.; Lombi, E.; Zhao, F.-J.; Kopittke, P.M. Nanotechnology: A New Opportunity in Plant Sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Dimkić, I.; Stanković, S.; Nišavić, M.; Petković, M.; Ristivojević, P.; Fira, D.; Berić, T. Corrigendum: The Profile and Antimicrobial Activity of Bacillus Lipopeptide Extracts of Five Potential Biocontrol Strains. Front. Microbiol. 2017, 8, 1500. [Google Scholar] [CrossRef]
- Yang, D.; Hartman, M.R.; Derrien, T.L.; Hamada, S.; An, D.; Yancey, K.G.; Cheng, R.; Ma, M.; Luo, D. DNA Materials: Bridging Nanotechnology and Biotechnology. Acc. Chem. Res. 2014, 47, 1902–1911. [Google Scholar] [CrossRef]
- Cao, G.; Wang, Y. Nanostructures and Nanomaterials: Synthesis, Properties, and Applications; World Scientific: Singapore, 2011; Volume 2, p. 596. [Google Scholar]
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef]
- Selvarajan, S.; Suganthi, A.; Rajarajan, M. A facile approach to synthesis of mesoporous SnO2/chitosan nanocomposite modified electrode for simultaneous determination of ascorbic acid, dopamine and uric acid. Surf. Interfaces 2017, 7, 146–156. [Google Scholar] [CrossRef]
- Sangamithirai, D.; Munusamy, S.; Narayanan, V.; Stephen, A. Fabrication of neurotransmitter dopamine electrochemical sensor based on poly(o-anisidine)/CNTs nanocomposite. Surf. Interfaces 2016, 4, 27–34. [Google Scholar] [CrossRef]
- Immanuel, S.; Aparna, T.K.; Sivasubramanian, R. A facile preparation of Au—SiO2 nanocomposite for simultaneous electrochemical detection of dopamine and uric acid. Surf. Interfaces 2019, 14, 82–91. [Google Scholar] [CrossRef]
- Ramya, M.; Senthil Kumar, P.; Rangasamy, G.; Uma Shankar, V.; Rajesh, G.; Nirmala, K.; Saravanan, A.; Krishnapandi, A. A recent advancement on the applications of nanomaterials in electrochemical sensors and biosensors. Chemosphere 2022, 308, 136416. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Wang, Z.; Zhang, W.; Liu, X.; Li, M.; Li, G.; Zhang, B.; Singh, R. Optically Active Nanomaterials and Its Biosensing Applications & mdash;A Review. Biosensors 2023, 13, 85. [Google Scholar]
- Pirzada, M.; Altintas, Z. Nanomaterials for Healthcare Biosensing Applications. Sensors 2019, 19, 5311. [Google Scholar] [CrossRef]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2, 63. [Google Scholar] [CrossRef]
- Su, S.; Wu, W.; Gao, J.; Lu, J.; Fan, C. Nanomaterials-based sensors for applications in environmental monitoring. J. Mater. Chem. 2012, 22, 18101–18110. [Google Scholar] [CrossRef]
- Kujawska, M.; Bhardwaj, S.K.; Mishra, Y.K.; Kaushik, A. Using Graphene-Based Biosensors to Detect Dopamine for Efficient Parkinson’s Disease Diagnostics. Biosensors 2021, 11, 433. [Google Scholar] [CrossRef]
- Nichkova, M.; Wynveen, P.M.; Marc, D.T.; Huisman, H.; Kellermann, G.H. Validation of an ELISA for urinary dopamine: Applications in monitoring treatment of dopamine-related disorders. J. Neurochem. 2013, 125, 724–735. [Google Scholar] [CrossRef]
- Ryding, E.; Lindström, M.; Träskman-Bendz, L. The role of dopamine and serotonin in suicidal behaviour and aggression. In Progress in Brain Research; Di Giovann, G., Di Matteo, V., Esposito, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 172, pp. 307–315. [Google Scholar]
- Dong, M.-X.; Chen, G.-H.; Hu, L. Dopaminergic System Alteration in Anxiety and Compulsive Disorders: A Systematic Review of Neuroimaging Studies. Front. Neurosci. 2020, 14, 608520. [Google Scholar] [CrossRef]
- Post, M.R.; Sulzer, D. The chemical tools for imaging dopamine release. Cell Chem. Biol. 2021, 28, 748–764. [Google Scholar] [CrossRef]
- Juárez Olguín, H.; Calderón Guzmán, D.; Hernández García, E.; Barragán Mejía, G. The Role of Dopamine and Its Dysfunction as a Consequence of Oxidative Stress. Oxidative Med. Cell. Longev. 2016, 2016, 9730467. [Google Scholar] [CrossRef]
- Wise, R.A.; Jordan, C.J. Dopamine, behavior, and addiction. J. Biomed. Sci. 2021, 28, 83. [Google Scholar] [CrossRef] [PubMed]
- Davidson, D.F. Elevated urinary dopamine in adults and children. Ann. Clin. Biochem. 2005, 42, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, C.; Wang, C.; Pu, F.; Ren, J.; Qu, X. Silver nanoprobe for sensitive and selective colorimetric detection of dopaminevia robust Ag–catechol interaction. Chem. Commun. 2011, 47, 1181–1183. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, A.; Khajehzadeh, A.; Ghaffarinejad, A. A simple and cost-effective method, as an appropriate alternative for visible spectrophotometry: Development of a dopamine biosensor. Analyst 2009, 134, 1692–1698. [Google Scholar] [CrossRef]
- Abrantes Dias, A.S.; Amaral Pinto, J.C.; Magalhães, M.; Mendes, V.M.; Manadas, B. Analytical methods to monitor dopamine metabolism in plasma: Moving forward with improved diagnosis and treatment of neurological disorders. J. Pharm. Biomed. Anal. 2020, 187, 113323. [Google Scholar] [CrossRef]
- He, C.; Tao, M.; Zhang, C.; He, Y.; Xu, W.; Liu, Y.; Zhu, W. Microelectrode-Based Electrochemical Sensing Technology for in Vivo Detection of Dopamine: Recent Developments and Future Prospects. Crit. Rev. Anal. Chem. 2022, 52, 544–554. [Google Scholar] [CrossRef]
- Anuar, N.S.; Basirun, W.J.; Shalauddin, M.; Akhter, S. A dopamine electrochemical sensor based on a platinum–silver graphene nanocomposite modified electrode. RSC Adv. 2020, 10, 17336–17344. [Google Scholar] [CrossRef]
- Lakard, S.; Pavel, I.A.; Lakard, B. Electrochemical Biosensing of Dopamine Neurotransmitter: A Review. Biosensors 2021, 11, 179. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Holmes, C.; Sharabi, Y. Cerebrospinal fluid biomarkers of central catecholamine deficiency in Parkinson’s disease and other synucleinopathies. Brain 2012, 135, 1900–1913. [Google Scholar] [CrossRef]
- Andersen, A.D.; Blaabjerg, M.; Binzer, M.; Kamal, A.; Thagesen, H.; Kjaer, T.W.; Stenager, E.; Gramsbergen, J.B.P. Cerebrospinal fluid levels of catecholamines and its metabolites in Parkinson’s disease: Effect of l-DOPA treatment and changes in levodopa-induced dyskinesia. J. Neurochem. 2017, 141, 614–625. [Google Scholar] [CrossRef]
- Beatto, T.G.; Gomes, W.E.; Etchegaray, A.; Gupta, R.; Mendes, R.K. Dopamine levels determined in synthetic urine using an electrochemical tyrosinase biosensor based on ZnO@Au core–shell. RSC Adv. 2023, 13, 33424–33429. [Google Scholar] [CrossRef] [PubMed]
- Ping, J.; Wu, J.; Wang, Y.; Ying, Y. Simultaneous determination of ascorbic acid, dopamine and uric acid using high-performance screen-printed graphene electrode. Biosens. Bioelectron. 2012, 34, 70–76. [Google Scholar] [CrossRef]
- Huang, K.-J.; Jing, Q.-S.; Wu, Z.-W.; Wang, L.; Wei, C.-Y. Enhanced sensing of dopamine in the present of ascorbic acid based on graphene/poly(p-aminobenzoic acid) composite film. Colloids Surf. B Biointerfaces 2011, 88, 310–314. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Meng, W.; Han, C.; Leng, C. Electrochemical Dopamine Detection using a Fe/Fe3O4@C Composite derived from a Metal-Organic Framework. ChemistrySelect 2022, 7, e202201534. [Google Scholar] [CrossRef]
- Kim, D.-S.; Kang, E.-S.; Baek, S.; Choo, S.-S.; Chung, Y.-H.; Lee, D.; Min, J.; Kim, T.-H. Electrochemical detection of dopamine using periodic cylindrical gold nanoelectrode arrays. Sci. Rep. 2018, 8, 14049. [Google Scholar] [CrossRef]
- Castagnola, E.; Robbins, E.M.; Wu, B.; Pwint, M.Y.; Garg, R.; Cohen-Karni, T.; Cui, X.T. Flexible Glassy Carbon Multielectrode Array for In Vivo Multisite Detection of Tonic and Phasic Dopamine Concentrations. Biosensors 2022, 12, 540. [Google Scholar] [CrossRef]
- Demuru, S.; Nela, L.; Marchack, N.; Holmes, S.J.; Farmer, D.B.; Tulevski, G.S.; Lin, Q.; Deligianni, H. Scalable Nanostructured Carbon Electrode Arrays for Enhanced Dopamine Detection. ACS Sens. 2018, 3, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.-H.; Zheng, X.-Q.; Xu, J.-Y.; Bao, W.-J.; Wang, F.-B.; Xia, X.-H. Electrochemical sensor based on nitrogen doped graphene: Simultaneous determination of ascorbic acid, dopamine and uric acid. Biosens. Bioelectron. 2012, 34, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Kan, X.; Zhao, Y.; Geng, Z.; Wang, Z.; Zhu, J.-J. Composites of Multiwalled Carbon Nanotubes and Molecularly Imprinted Polymers for Dopamine Recognition. J. Phys. Chem. C 2008, 112, 4849–4854. [Google Scholar] [CrossRef]
- Muddapur, U.M.; Alshehri, S.; Ghoneim, M.M.; Mahnashi, M.H.; Alshahrani, M.A.; Khan, A.A.; Iqubal, S.M.S.; Bahafi, A.; More, S.S.; Shaikh, I.A.; et al. Plant-Based Synthesis of Gold Nanoparticles and Theranostic Applications: A Review. Molecules 2022, 27, 1391. [Google Scholar] [CrossRef]
- Patel, K.N.; Trivedi, P.G.; Thakar, M.S.; Prajapati, K.V.; Prajapati, D.K.; Sindhav, G.M. Gold nanoparticles synthesis using Gymnosporia montana L. and its biological profile: A pioneer report. J. Genet. Eng. Biotechnol. 2023, 21, 71. [Google Scholar] [CrossRef] [PubMed]
- Dhaffouli, A.; Salazar-Carballo, P.A.; Carinelli, S.; Holzinger, M.; Barhoumi, H. Improved electrochemical sensor using functionalized silica nanoparticles (SiO2-APTES) for high selectivity detection of lead ions. Mater. Chem. Phys. 2024, 318, 129253. [Google Scholar] [CrossRef]
- Rao, X.; Abou Hassan, A.; Guyon, C.; Zhang, M.; Ognier, S.; Tatoulian, M. Plasma Polymer Layers with Primary Amino Groups for Immobilization of Nano- and Microparticles. Plasma Chem. Plasma Process. 2020, 40, 589–606. [Google Scholar] [CrossRef]
- Dhaffouli, A.; Salazar-Carballo, P.A.; Mabrouk, C.; Carinelli, S.; Holzinger, M.; Barhoumi, H. Synthesis, characterization, and application of ZnO@SiO2-APTES core-shell composite for selective electrochemical detection of Pb2+ ions. Sens. Actuators A Phys. 2024, 373, 115416. [Google Scholar] [CrossRef]
- Yamada, K.; Yoshii, S.; Kumagai, S.; Fujiwara, I.; Nishio, K.; Okuda, M.; Matsukawa, N.; Yamashita, I. High-Density and Highly Surface Selective Adsorption of Protein–Nanoparticle Complexes by Controlling Electrostatic Interaction. Jpn. J. Appl. Phys. 2006, 45, 4259. [Google Scholar] [CrossRef]
- Carinelli, S.; Fernández, I.; Luis González-Mora, J.; Salazar-Carballo, P.A. Hemoglobin-modified nanoparticles for electrochemical determination of haptoglobin: Application in bovine mastitis diagnosis. Microchem. J. 2022, 179, 107528. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, R.; Chai, Y.; Li, W.; Zhong, X.; Zhong, H. Simultaneous voltammetric determination for DA, AA and NO2− based on graphene/poly-cyclodextrin/MWCNTs nanocomposite platform. Biosens. Bioelectron. 2011, 26, 3977–3980. [Google Scholar] [CrossRef]
- Tan, L.; Zhou, K.-G.; Zhang, Y.-H.; Wang, H.-X.; Wang, X.-D.; Guo, Y.-F.; Zhang, H.-L. Nanomolar detection of dopamine in the presence of ascorbic acid at β-cyclodextrin/graphene nanocomposite platform. Electrochem. Commun. 2010, 12, 557–560. [Google Scholar] [CrossRef]
- Liu, B.; Lian, H.T.; Yin, J.F.; Sun, X.Y. Dopamine molecularly imprinted electrochemical sensor based on graphene–chitosan composite. Electrochim. Acta 2012, 75, 108–114. [Google Scholar] [CrossRef]
- Fan, Y.; Lu, H.-T.; Liu, J.-H.; Yang, C.-P.; Jing, Q.-S.; Zhang, Y.-X.; Yang, X.-K.; Huang, K.-J. Hydrothermal preparation and electrochemical sensing properties of TiO2–graphene nanocomposite. Colloids Surf. B Biointerfaces 2011, 83, 78–82. [Google Scholar] [CrossRef]
- Ma, X.; Chao, M.; Wang, Z. Electrochemical detection of dopamine in the presence of epinephrine, uric acid and ascorbic acid using a graphene-modified electrode. Anal. Methods 2012, 4, 1687–1692. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Hou, H.; You, T. Simultaneous electrochemical determination of dopamine, uric acid and ascorbic acid using palladium nanoparticle-loaded carbon nanofibers modified electrode. Biosens. Bioelectron. 2008, 24, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-F.; Kumar, S.A.; Chen, S.-M. Zinc oxide/redox mediator composite films-based sensor for electrochemical detection of important biomolecules. Anal. Biochem. 2008, 380, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.-S.; Chen, Y.-L.; Lee, C.-Y.; Chiu, H.-T. Gold Nanostructures on Flexible Substrates as Electrochemical Dopamine Sensors. ACS Appl. Mater. Interfaces 2012, 4, 5570–5575. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhao, Q.; Tan, F.; Wang, X.; Gao, J. Simultaneous detection of dopamine, uric acid, and ascorbic acid using SnO2 nanoparticles/multi-walled carbon nanotubes/carbon paste electrode. Anal. Methods 2012, 4, 3283–3289. [Google Scholar] [CrossRef]
- Peik-See, T.; Pandikumar, A.; Nay-Ming, H.; Hong-Ngee, L.; Sulaiman, Y. Simultaneous Electrochemical Detection of Dopamine and Ascorbic Acid Using an Iron Oxide/Reduced Graphene Oxide Modified Glassy Carbon Electrode. Sensors 2014, 14, 15227–15243. [Google Scholar] [CrossRef]
- Sun, H.; Chao, J.; Zuo, X.; Su, S.; Liu, X.; Yuwen, L.; Fan, C.; Wang, L. Gold nanoparticle-decorated MoS2 nanosheets for simultaneous detection of ascorbic acid, dopamine and uric acid. RSC Adv. 2014, 4, 27625–27629. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Y.; Xu, Z.; Wang, S.; Chen, B.; Zhang, D.; Fang, Y. Simultaneous Detection of Ascorbic Acid, Dopamine, and Uric Acid Using a Novel Electrochemical Sensor Based on Palladium Nanoparticles/Reduced Graphene Oxide Nanocomposite. Int. J. Anal. Chem. 2020, 2020, 8812443. [Google Scholar] [CrossRef]
- Sun, C.-L.; Lee, H.-H.; Yang, J.-M.; Wu, C.-C. The simultaneous electrochemical detection of ascorbic acid, dopamine, and uric acid using graphene/size-selected Pt nanocomposites. Biosens. Bioelectron. 2011, 26, 3450–3455. [Google Scholar] [CrossRef]
- Murali, A.; Lan, Y.P.; Sarswat, P.K.; Free, M.L. Synthesis of CeO2/reduced graphene oxide nanocomposite for electrochemical determination of ascorbic acid and dopamine and for photocatalytic applications. Mater. Today Chem. 2019, 12, 222–232. [Google Scholar] [CrossRef]
- Vinay, M.M.; Arthoba Nayaka, Y. Iron oxide (Fe2O3) nanoparticles modified carbon paste electrode as an advanced material for electrochemical investigation of paracetamol and dopamine. J. Sci. Adv. Mater. Devices 2019, 4, 442–450. [Google Scholar] [CrossRef]
- Aparna, T.K.; Sivasubramanian, R.; Dar, M.A. One-pot synthesis of Au-Cu2O/rGO nanocomposite based electrochemical sensor for selective and simultaneous detection of dopamine and uric acid. J. Alloys Compd. 2018, 741, 1130–1141. [Google Scholar] [CrossRef]
- Maseed, H.; Reddy Yenugu, V.M.; Devarakonda, S.S.; Petnikota, S.; Gajulapalli, M.; Srikanth, V.V.S.S. Peroxidase-like Fe3O4 Nanoparticle/Few-Layered Graphene Composite for Electrochemical Detection of Dopamine, Ascorbic Acid, and Uric Acid. ACS Appl. Nano Mater. 2023, 6, 18531–18538. [Google Scholar] [CrossRef]
- Tan, C.; Zhao, J.; Sun, P.; Zheng, W.; Cui, G. Gold nanoparticle decorated polypyrrole/graphene oxide nanosheets as a modified electrode for simultaneous determination of ascorbic acid, dopamine and uric acid. New J. Chem. 2020, 44, 4916–4926. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).