Electrochemical Pretreatment and Functionalization of Pencil Graphite Electrodes for Enhanced Transducer Performance in Biosensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Virus and Antibodies Samples
2.3. Electrochemical Measurements
2.4. PGE Activation
2.5. Electrochemical Analysis
2.6. Electrodeposition of the Polymeric Film
2.7. Morphological Analysis
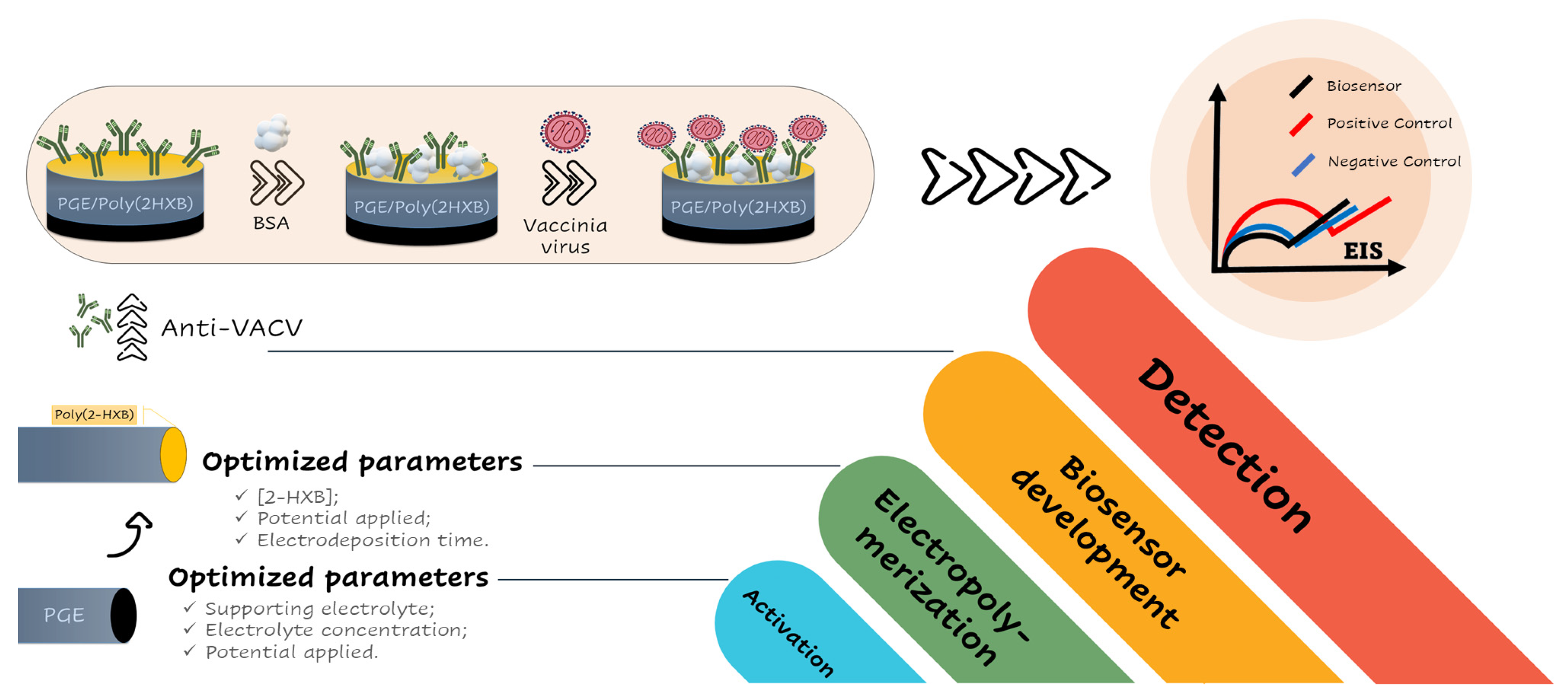
2.8. Immunosensors Fabrication Protocol
3. Results and Discussion
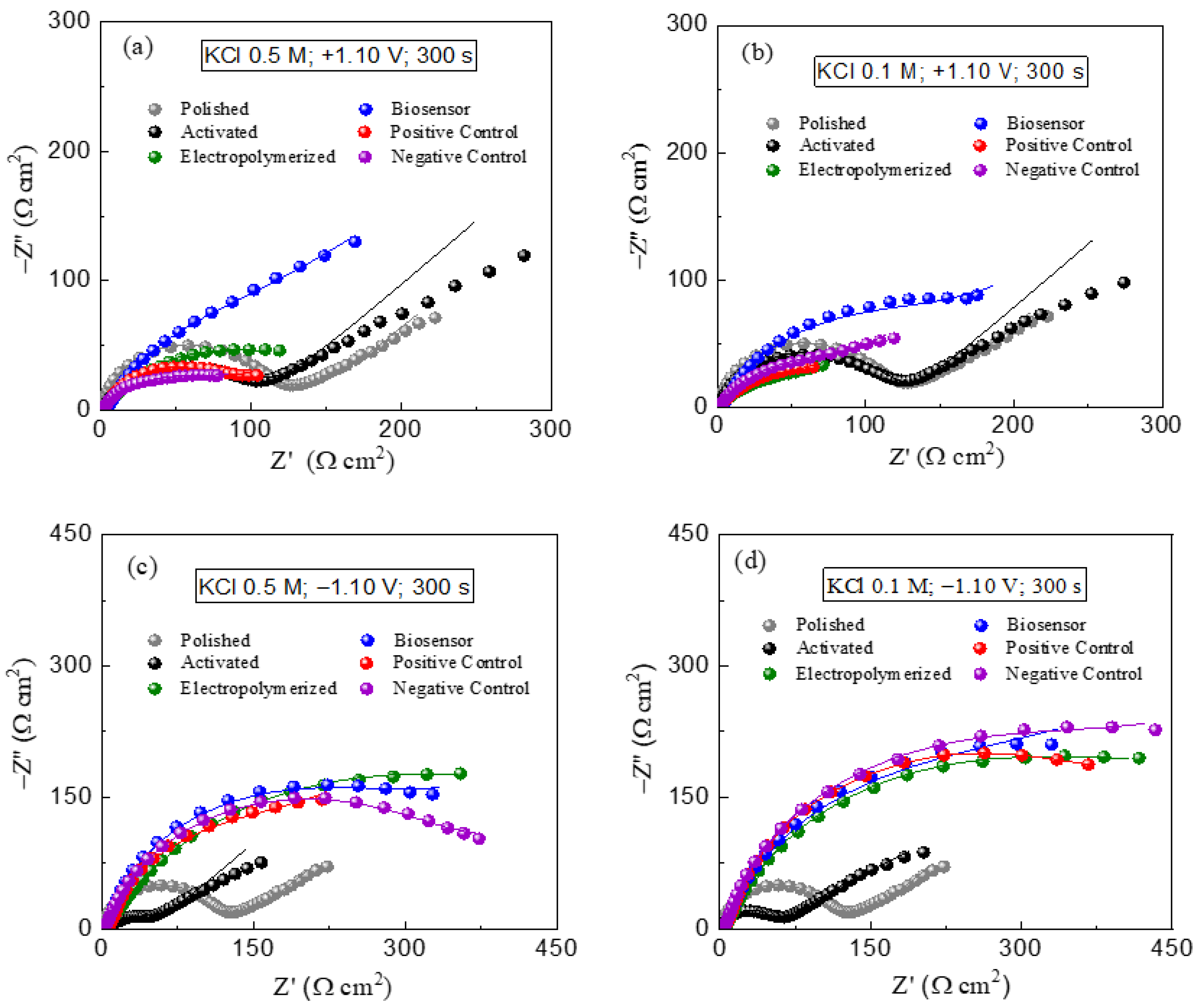
3.1. Investigation of Electrochemical Pretreatment of PGEs on Imunosensor Response
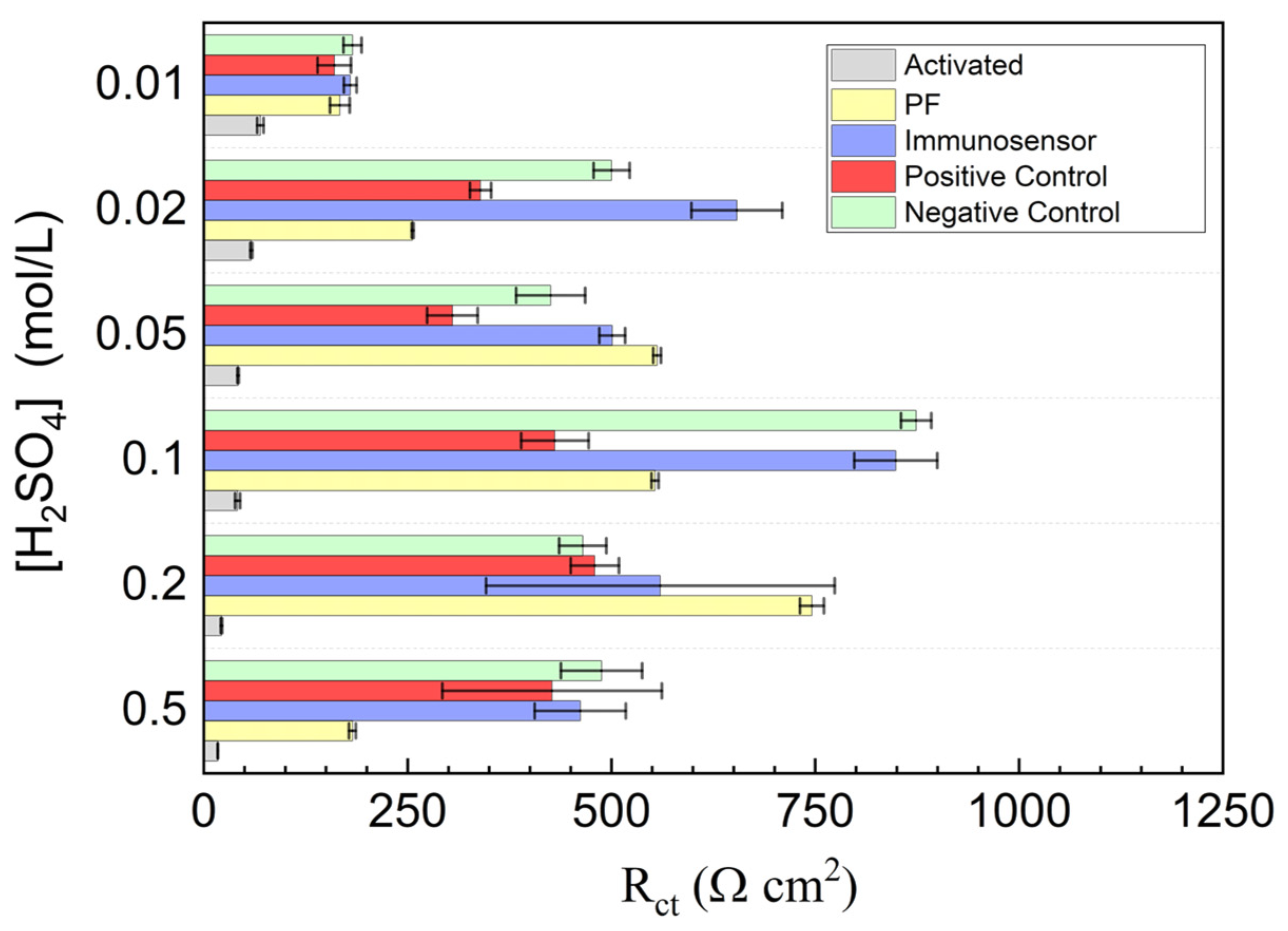
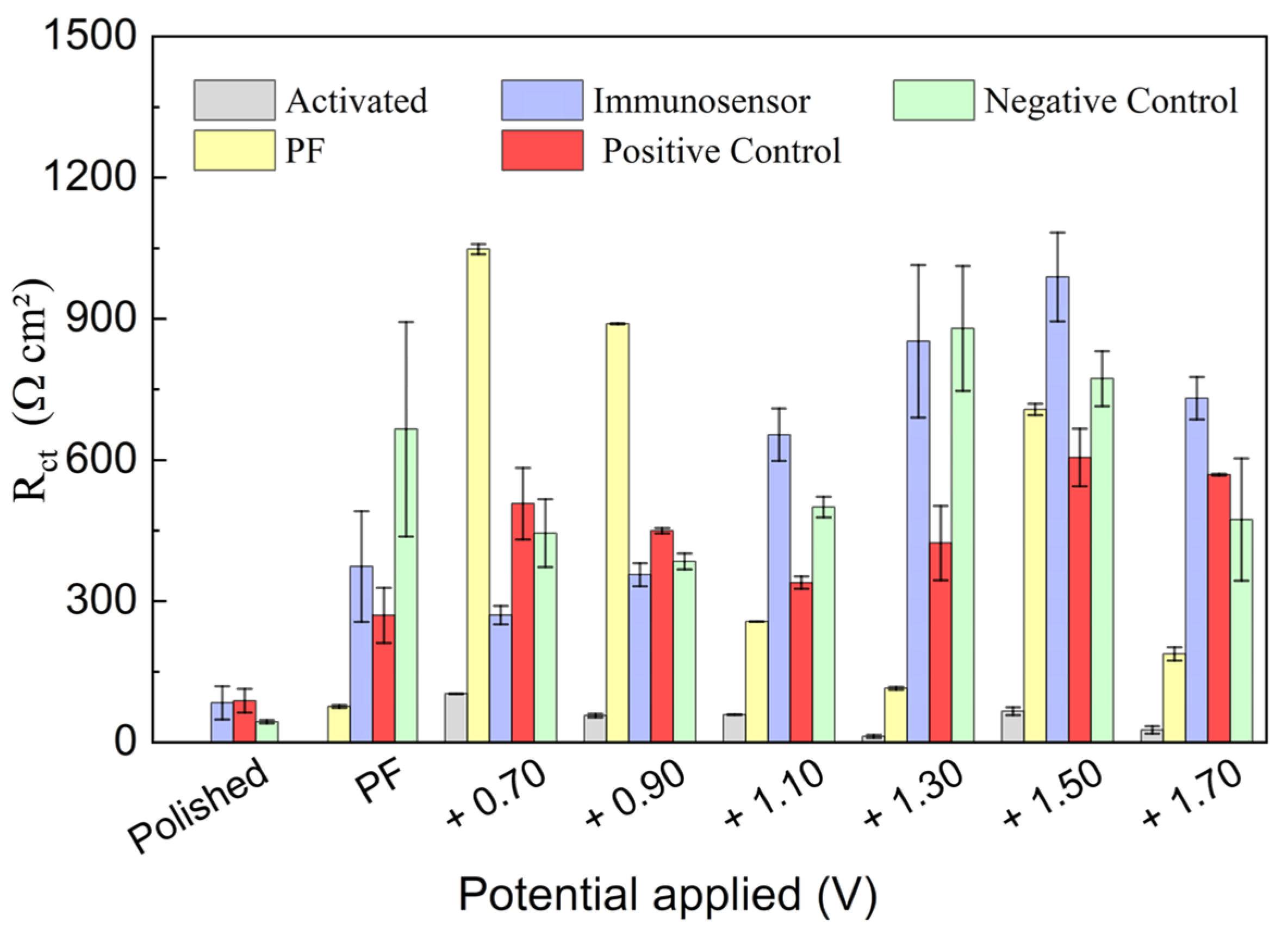
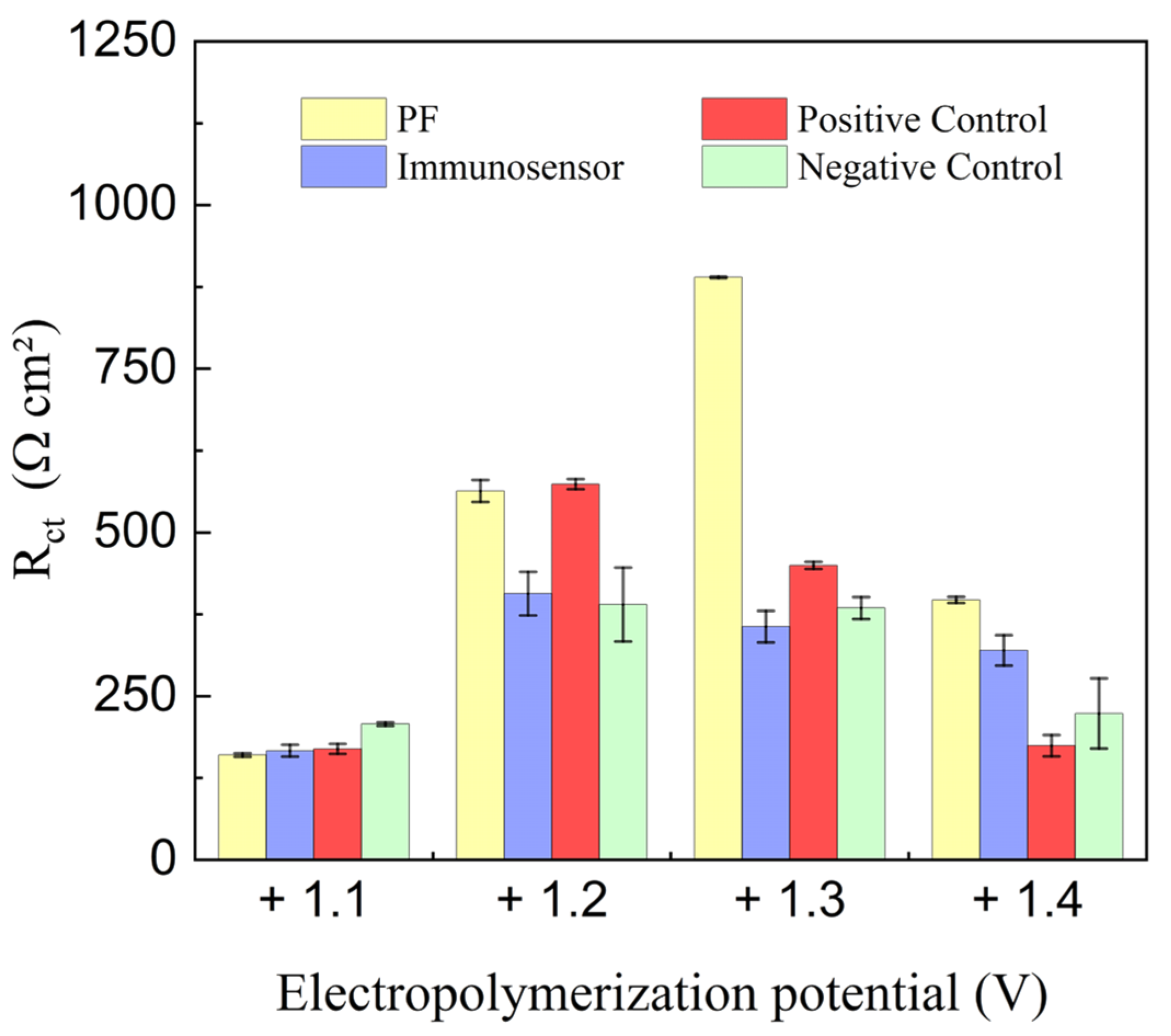
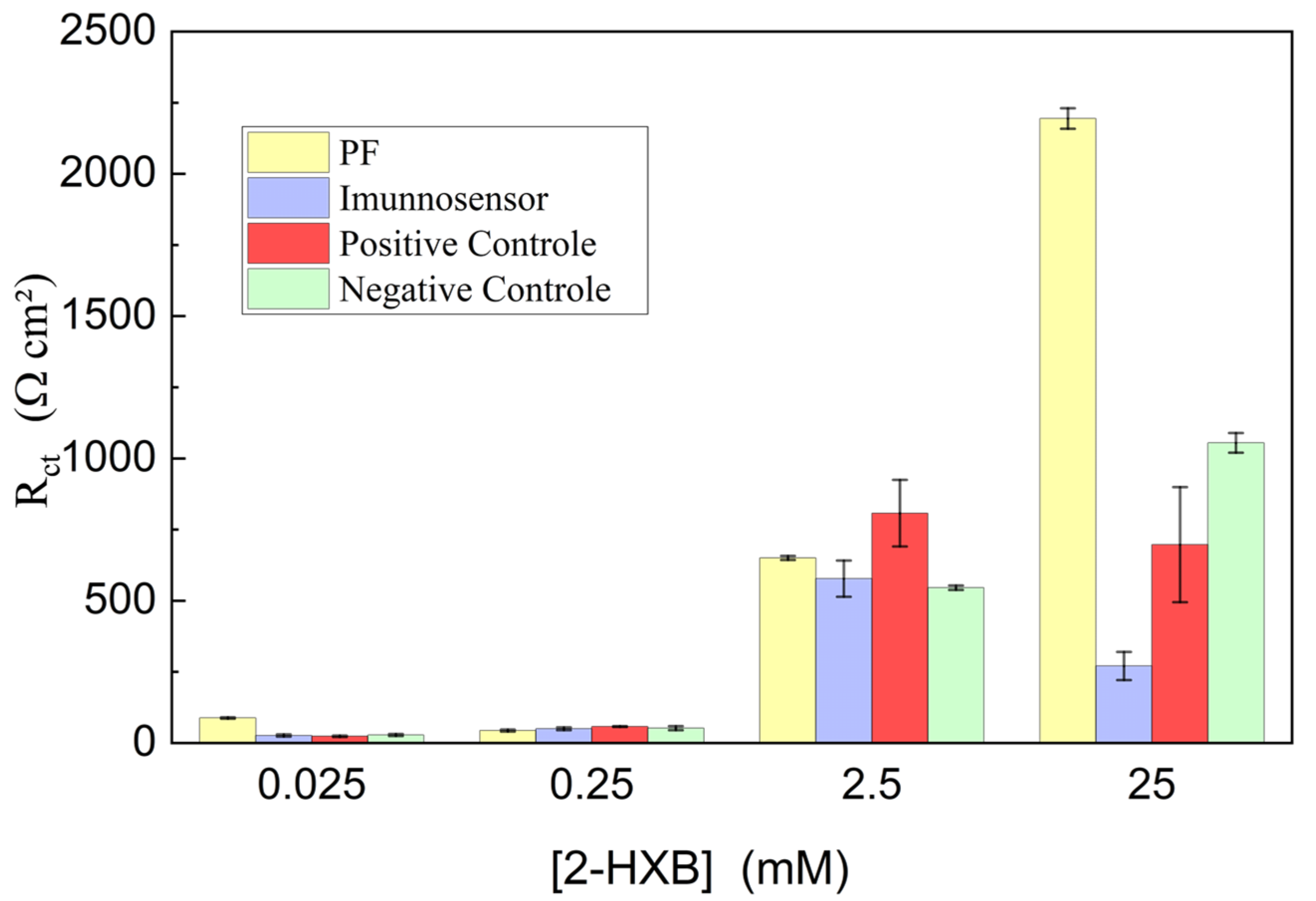
3.2. Investigation of PGE Functionalization Conditions in the Immunosensor Response
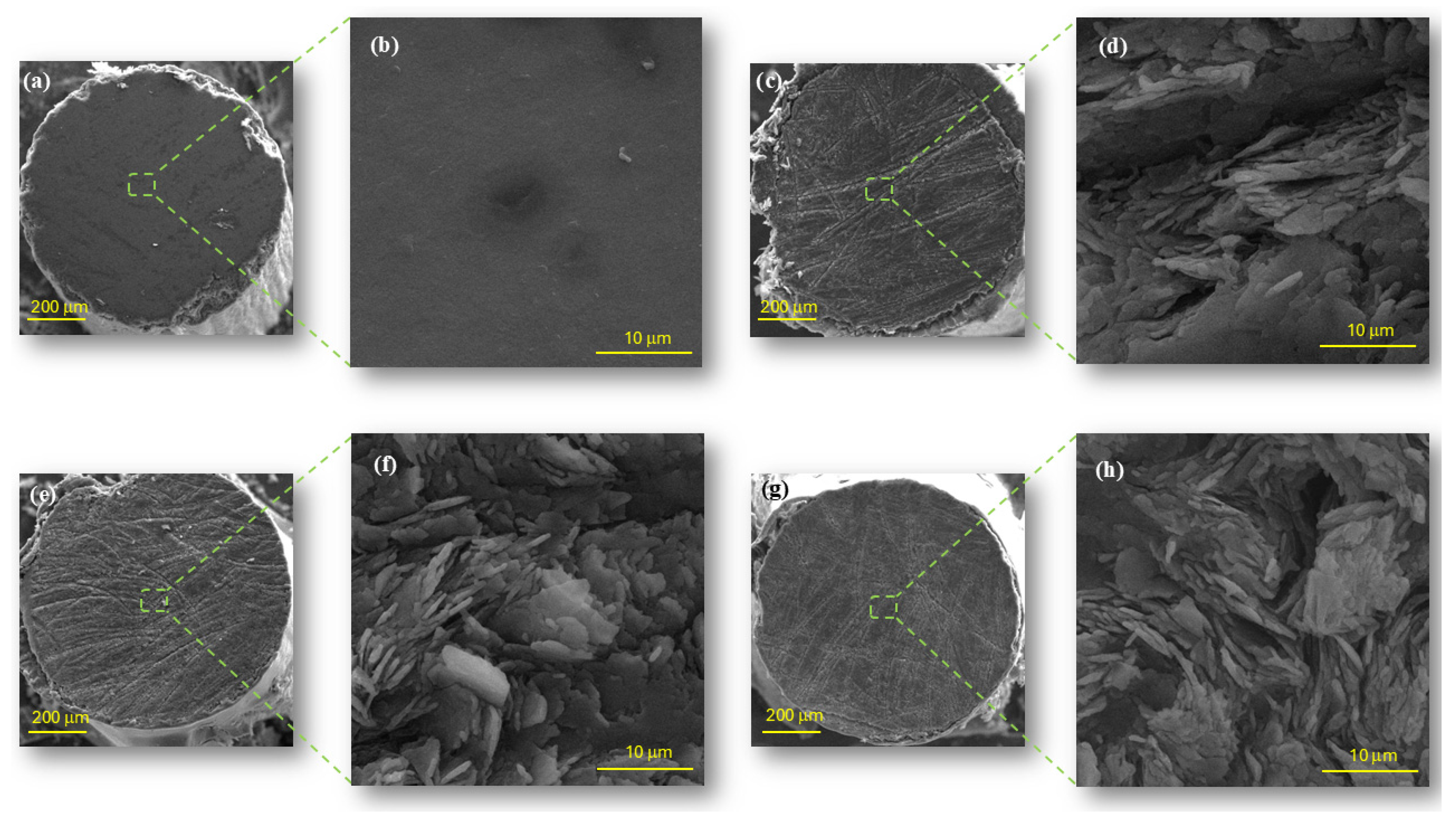
3.3. Morphological Analysis by Scanning Electron Microscopy (SEM) and Energy Dispersive Spectrometry (EDS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bragazzi, N.L.; Lehr, T. Big Epidemiology: The Birth, Life, Death, and Resurgence of Diseases on a Global Timescale. Epidemiologia 2024, 5, 669–691. [Google Scholar] [CrossRef] [PubMed]
- Barouki, R.; Kogevinas, M.; Audouze, K.; Belesova, K.; Bergman, A.; Birnbaum, L.; Boekhold, S.; Denys, S.; Desseille, C.; Drakvik, E.; et al. The COVID-19 Pandemic and Global Environmental Change: Emerging Research Needs. Environ. Int. 2021, 146, 106272. [Google Scholar] [CrossRef]
- Leik, N.K.O.; Ahmedy, F.; Mac Guad, R.; Baharuddin, D.M.P. COVID-19 Vaccine and Its Consequences in Pregnancy: Brief Review. Ann. Med. Surg. 2021, 72, 103103. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global Trends in Emerging Infectious Diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.J. One Health: Zoonoses in the Exotic Animal Practice. Vet. Clin. N. Am. Exot. Anim. Pract. 2011, 14, 421–426. [Google Scholar] [CrossRef]
- Rahman, M.T.; Sobur, M.A.; Islam, M.S.; Ievy, S.; Hossain, M.J.; Zowalaty, M.E.E.; Rahman, A.M.M.T.; Ashour, H.M. Zoonotic Diseases: Etiology, Impact, and Control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef]
- Chomel, B.B. Zoonoses. In Encyclopedia of Microbiology; Elsevier: Amsterdam, The Netherlands, 2009; p. 820. [Google Scholar] [CrossRef]
- Borremans, B.; Faust, C.; Manlove, K.R.; Sokolow, S.H.; Lloyd-Smith, J.O. Cross-Species Pathogen Spillover across Ecosystem Boundaries: Mechanisms and Theory. Philos. Trans. R. Soc. B 2019, 374, 20180344. [Google Scholar] [CrossRef]
- Morens, D.M.; Fauci, A.S. Emerging Pandemic Diseases: How We Got to COVID-19. Cell 2020, 182, 1077–1092. [Google Scholar] [CrossRef]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The Changing Epidemiology of Human Monkeypox—A Potential Threat? A Systematic Review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef]
- Luques, M.N.; Oliveira, R.L.; Hir, S.; Nunes, D.d.S.; Higa, L.M.; Mendonça, A.F.; Pereira, L.A.; Sousa, F.; Castiñeiras, T.M.P.P.; Tanuri, A.; et al. Co-Circulation of Vaccinia and Monkeypox Viruses in Rural Areas of Brazil: Importance of Differential Molecular Diagnosis. Travel Med. Infect. Dis. 2023, 53, 102578. [Google Scholar] [CrossRef]
- Alcamí, A. Pathogenesis of the Circulating Mpox Virus and Its Adaptation to Humans. Proc. Natl. Acad. Sci. USA 2023, 120, e2301662120. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.L.; Smith, G.L. Vaccinia Virus Morphogenesis and Dissemination. Trends Microbiol. 2008, 16, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Pan, Z.; Li, X.; Zhao, M.; Li, Z.; Zhang, L. Narciclasine Inhibits Vaccinia Virus Infection by Activating the RhoA Signaling Pathway. Biosaf. Health 2024, 6, 341–349. [Google Scholar] [CrossRef]
- Trindade, G.S.; Emerson, G.L.; Carroll, D.S.; Kroon, E.G.; Damon, I.K. Brazilian Vaccinia Viruses and Their Origins. Emerg. Infect. Dis. 2007, 13, 965–972. [Google Scholar] [CrossRef]
- Drumond, B.P.; Leite, J.A.; da Fonseca, F.G.; Bonjardim, C.A.; Ferreira, P.C.P.; Kroon, E.G. Brazilian Vaccinia Virus Strains Are Genetically Divergent and Differ from the Lister Vaccine Strain. Microbes Infect. 2008, 10, 185–197. [Google Scholar] [CrossRef]
- Gubser, C.; Hué, S.; Kellam, P.; Smith, G.L. Poxvirus Genomes: A Phylogenetic Analysis. J. Gen. Virol. 2004, 85, 105–117. [Google Scholar] [CrossRef]
- Dunlop, L.R.; Oehlberg, K.A.; Reid, J.J.; Avci, D.; Rosengard, A.M. Variola Virus Immune Evasion Proteins. Microbes Infect. 2003, 5, 1049–1056. [Google Scholar] [CrossRef]
- Diaz, J.H. The Disease Ecology, Epidemiology, Clinical Manifestations, Management, Prevention, and Control of Increasing Human Infections with Animal Orthopoxviruses. Wilderness Environ. Med. 2021, 32, 528–536. [Google Scholar] [CrossRef]
- Fiocruz, F.O.C. Pesquisadores Explicam a Atual Importância Do Vírus Vaccinia. Available online: https://agencia.fiocruz.br/pesquisadores-explicam-a-atual-importância-do-vírus-vaccinia (accessed on 24 February 2022).
- Pasternak, N. Varíola é a Única Doença Erradicada No Mundo−Saúde−Estadão. Available online: https://saude.estadao.com.br/noticias/geral,variola-e-a-unica-doenca-erradicada-no-mundo,70003870535 (accessed on 26 February 2022).
- Tegnell, A.; Wahren, B.; Elgh, F. Smallpox—Eradicated, but a Growing Terror Threat. Clin. Microbiol. Infect. 2002, 8, 504–509. [Google Scholar] [CrossRef]
- Xiang, Y.; Lane, R.K. Vaccinia Virus (Poxviridae). Encycl. Virol. 2021, 1–5, 854–859. [Google Scholar] [CrossRef]
- Fonseca, F.G.; Kroon, E.G.; Nogueira, M.L.; de Souza Trindade, G. Zoonotic Vaccinia Virus Outbreaks in Brazil. Futur. Virol. 2011, 6, 697–707. [Google Scholar] [CrossRef]
- Kleo, K.; Kapp, A.; Ascher, L.; Lisdat, F. Detection of Vaccinia Virus DNA by Quartz Crystal Microbalance. Anal. Biochem. 2011, 418, 260–266. [Google Scholar] [CrossRef]
- Donaldson, K.A.; Kramer, M.F.; Lim, D.V. A Rapid Detection Method for Vaccinia Virus, the Surrogate for Smallpox Virus. Biosens. Bioelectron. 2004, 20, 322–327. [Google Scholar] [CrossRef]
- Trindade, G.D.S.; Emerson, G.L.; Sammons, S.; Frace, M.; Govil, D.; Mota, B.E.F.; Abrahão, J.S.; de Assis, F.L.; Olsen-Rasmussen, M.; Goldsmith, C.S.; et al. Serro 2 Virus Highlights the Fundamental Genomic and Biological Features of a Natural Vaccinia Virus Infecting Humans. Viruses 2016, 8, 328. [Google Scholar] [CrossRef] [PubMed]
- Quixabeira-Santos, J.C.; Medaglia, M.L.G.; Pescador, C.A.; Damaso, C.R. Animal Movement and Establishment of Vaccinia Virus Cantagalo Strain in Amazon Biome, Brazil. Emerg. Infect. Dis. 2011, 17, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Sasso, E.; D’Alise, A.M.; Zambrano, N.; Scarselli, E.; Folgori, A.; Nicosia, A. New Viral Vectors for Infectious Diseases and Cancer. Semin. Immunol. 2020, 50, 101430. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.B.; Borges, I.A.; Alves, P.A.; Miranda, J.B.; Luiz, A.P.M.F.; Ferreira, P.C.P.; Abrahão, J.S.; Moreno, E.C.; Kroon, E.G.; Trindade, G.d.S. Alternative Routes of Zoonotic Vaccinia Virus Transmission, Brazil. Emerg. Infect. Dis. 2015, 21, 2244–2246. [Google Scholar] [CrossRef]
- Costa, G.B.; Moreno, E.C.; Trindade, G.d.S. Neutralizing Antibodies Associated with Exposure Factors to Orthopoxvirus in Laboratory Workers. Vaccine 2013, 31, 4706–4709. [Google Scholar] [CrossRef]
- Costa, G.B.; Augusto, L.T.S.; Leite, J.A.; Ferreira, P.C.P.; Bonjardim, C.A.; Abrahão, J.S.; Kroon, E.G.; Moreno, E.C.; Trindade, G.d.S. Seroprevalence of Orthopoxvirus in Rural Brazil: Insights into Anti-OPV Immunity Status and Its Implications for Emergent Zoonotic OPV. Virol. J. 2016, 13, 1–8. [Google Scholar] [CrossRef]
- Fedorko, D.P.; Preuss, J.C.; Fahle, G.A.; Li, L.; Fischer, S.H.; Hohman, P.; Cohen, J.I. Comparison of Methods for Detection of Vaccinia Virus in Patient Specimens. J. Clin. Microbiol. 2005, 43, 4602–4606. [Google Scholar] [CrossRef]
- Generalov, V.M.; Naumova, O.V.; P’yankov, S.A.; Kolosova, I.V.; Safatov, A.S.; Zaitsev, B.N.; Zaitseva, E.G.; Buryak, G.A.; Cheremiskina, A.A.; Filatova, N.A.; et al. Identifying the Vaccinia Virus with the Use of a Nanowire Silicon-on-Insulator Biosensor. Optoelectron. Instrum. Data Process. 2021, 57, 37–43. [Google Scholar] [CrossRef]
- Rakhimbekova, A.; Kudaibergenov, B.; Seitkamal, K.; Bellone, A.; Dauletova, A.; Sypabekova, M.; Olivero, M.; Perrone, G.; Radaelli, A.; Zanotto, C.; et al. Rapid detection of vaccinia virus using biofunctionalized fiber-optic ball-tip biosensors. Sci. Rep. 2023, 13, 17470. [Google Scholar] [CrossRef]
- Li, Z.; Liu, R. Construction and Isolation of Recombinant Vaccinia Virus by Homologous Recombination Using Fluorescent Protein Markers. In Vaccinia, Mpox, and Other Poxviruses: Methods and Protocols, 1st ed.; Springer Protocols; Yang, Z., Satheshkumar, P.S., Eds.; Humana Press: New York, NY, USA, 2025; Volume 2860, pp. 83–95. [Google Scholar] [CrossRef]
- Coelho, R.M.; de Oliveira Almeida, A.; Soares, P.I.; Rocha, K.L.S.; de Oliveira, D.B.; Pereira, A.C.; Franco, D.L.; Ferreira, L.F. A Low-Cost Electrochemical Biosensor for Vaccinia Virus Using Pencil Graphite Electrodes Modified with Poly(Hydroxybenzamide). Chem. Pap. 2023, 77, 7563–7575. [Google Scholar] [CrossRef]
- Cohen, J.I.; Hohman, P.; Preuss, J.C.; Li, L.; Fischer, S.H.; Fedorko, D.P. Detection of Vaccinia Virus DNA, but Not Infectious Virus, in the Blood of Smallpox Vaccine Recipients. Vaccine 2007, 25, 4571–4574. [Google Scholar] [CrossRef] [PubMed]
- Que, H.; Zhang, D.; Guo, B.; Wang, T.; Wu, H.; Han, D.; Yan, Y. Label-Free and Ultrasensitive Electrochemical Biosensor for the Detection of EBV-Related DNA Based on AgDNCs@DNA/AgNCs Nanocomposites and Lambda Exonuclease-Assisted Target Recycling. Biosens. Bioelectron. 2019, 143, 111610. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to Biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef]
- Kaya, H.O.; Cetin, A.E.; Azimzadeh, M.; Topkaya, S.N. Pathogen Detection with Electrochemical Biosensors: Advantages, Challenges and Future Perspectives. J. Electroanal. Chem. 2021, 882, 114989. [Google Scholar] [CrossRef]
- Evtugyn, G. Biosensors: Essentials; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; Kazan, Rússia, 2014; Volume 1, ISBN 9783642402418. [Google Scholar]
- Blum, L.J.; Coulet, P.R. Biosensor Principles and Applications; Biotechnology and Bioprocessing; Pierre, R., Coulet, L.J.B., Eds.; CRC: Boca Raton, FL, USA, 1991; Volume 15, ISBN 9780824785468. [Google Scholar]
- David, I.G.; Popa, D.-E.; Buleandra, M. Pencil Graphite Electrodes: A Versatile Tool in Electroanalysis. J. Anal. Methods Chem. 2017, 2017, 1–22. [Google Scholar] [CrossRef]
- Torrinha, Á.; Amorim, C.G.; Montenegro, M.C.B.S.M.; Araújo, A.N. Biosensing Based on Pencil Graphite Electrodes. Talanta 2018, 190, 235–247. [Google Scholar] [CrossRef]
- de Oliveira Almeida, A.; Coelho, R.M.; Machado, Â.R.; Martins, H.R.; Pereira, A.C.; Santos, F.L.N.; Celedon, P.A.F.; Ferreira, L.F. Low-Cost Immunosensing Approach for Chagas Disease: Exploiting Modified Pencil Graphite Electrodes with Polymer Films. J. Solid State Electrochem. 2024, 29, 935–949. [Google Scholar] [CrossRef]
- e Silva, T.S.; e Freitas, G.R.O.; Ferreira, L.F.; Franco, D.L. Development of a Label-Free Impedimetric Immunosensor for the Detection of Respiratory Syncytial Virus. J. Solid State Electrochem. 2024, 28, 4015–4027. [Google Scholar] [CrossRef]
- Labib, M.; Zamay, A.S.; Muharemagic, D.; Chechik, A.V.; Bell, J.C.; Berezovski, M.V. Aptamer-Based Viability Impedimetric Sensor for Viruses. Anal. Chem. 2012, 84, 1813–1816. [Google Scholar] [CrossRef]
- Madiyar, F.R.; Haller, S.L.; Farooq, O.; Rothenburg, S.; Culbertson, C.; Li, J. AC Dielectrophoretic Manipulation and Electroporation of Vaccinia Virus Using Carbon Nanoelectrode Arrays. Electrophoresis 2017, 38, 1515–1525. [Google Scholar] [CrossRef]
- Buleandra, M.; Rabinca, A.A.; Badea, I.A.; Balan, A.; Stamatin, I.; Mihailciuc, C.; Ciucu, A.A. Voltammetric Determination of Dihydroxybenzene Isomers Using a Disposable Pencil Graphite Electrode Modified with Cobalt-Phthalocyanine. Microchim. Acta 2017, 184, 1481–1488. [Google Scholar] [CrossRef]
- Buleandră, M.; Popa, D.E.; David, I.G.; Ciucu, A.A. A Simple and Efficient Cyclic Square Wave Voltammetric Method for Simultaneous Determination of Epinephrine and Norepinephrine Using an Activated Pencil Graphite Electrode. Microchem. J. 2021, 160, 105621. [Google Scholar] [CrossRef]
- Kamyabi, M.; Hajari, N. Low Potential and Non-Enzymatic Hydrogen Peroxide Sensor Based on Copper Oxide Nanoparticle on Activated Pencil Graphite Electrode. J. Braz. Chem. Soc. 2016, 28, 808–818. [Google Scholar] [CrossRef]
- Kamyabi, M.A.; Hajari, N. Easy Activation of Pencil Graphite Electrode as Sensing Platform for Determination of Bisphenol A. J. Anal. Chem. 2019, 74, 286–295. [Google Scholar] [CrossRef]
- Patrascu, D.; David, I.; David, V.; Mihailciuc, C.; Stamatin, I.; Ciurea, J.; Nagy, L.; Nagy, G.; Ciucu, A.A. Selective Voltammetric Determination of Electroactive Neuromodulating Species in Biological Samples Using Iron(II) Phthalocyanine Modified Multi-Wall Carbon Nanotubes Paste Electrode. Sens. Actuators B Chem. 2011, 156, 731–736. [Google Scholar] [CrossRef]
- Sajeevan, B.; MG, G.; Sreelekshmi; Wilson, A.; Narayan Pillai, A.; Saraswathyamma, B. Poly-5-Amino-1-Naphthol Modified Pencil Graphite Electrode for the Simultaneous Electrochemical Determination of Pyridoxine and Riboflavin. Mater. Today Proc. 2023, 80, 570–576. [Google Scholar] [CrossRef]
- Srinivas, S.; Senthil Kumar, A. Surface-Activated Pencil Graphite Electrode for Dopamine Sensor Applications: A Critical Review. Biosensors 2023, 13, 353. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.; Zhang, Q.; Xu, Y.; Kunte, H.J.; De Marco, R. Hydrogen Release from Carbon Steel in Chloride Solution under Anodic Polarization. Int. J. Hydrogen Energy 2020, 45, 3307–3315. [Google Scholar] [CrossRef]
- Jürgen, D.; Steckhan, E. Influence of the Supporting Electrolyte and the PH on the Electrooxidative Activation of Glassy Carbon Electrodes. J. Electroanal. Chem. 1992, 333, 177–193. [Google Scholar] [CrossRef]
- Yagi, I.; Notsu, H.; Kondo, T.; Tryk, D.A.; Fujishima, A. Electrochemical Selectivity for Redox Systems at Oxygen-Terminated Diamond Electrodes. J. Electroanal. Chem. 1999, 473, 173–178. [Google Scholar] [CrossRef]
- Skoog, D.A.; Holler, F.J.; Crouch, S.R. Principles of Instrumental Analysis; Cengage Learning: Boston, MA, USA, 2002; ISBN 9781305577213. [Google Scholar]
- Annu; Sharma, S.; Jain, R.; Raja, A.N. Review—Pencil Graphite Electrode: An Emerging Sensing Material. J. Electrochem. Soc. 2020, 167, 037501. [Google Scholar] [CrossRef]
- Santos, C.D.C.; Pimenta, T.C.; Thomasini, R.L.; Verly, R.M.; Franco, D.L.; Ferreira, L.F. Electropolymerization of Phenol and Aniline Derivatives: Synthesis, Characterization and Application as Electrochemical Transducers. J. Electroanal. Chem. 2019, 846, 113163. [Google Scholar] [CrossRef]
- Navratil, R.; Kotzianova, A.; Halouzka, V.; Opletal, T.; Triskova, I.; Trnkova, L.; Hrbac, J. Polymer Lead Pencil Graphite as Electrode Material: Voltammetric, XPS and Raman Study. J. Electroanal. Chem. 2016, 783, 152–160. [Google Scholar] [CrossRef]
- Sajeevan, B.; Gopika, M.G.; Murali, A.S.; Saraswathyamma, B. An Electrochemical Sensor Based on a Pencil Graphite Electrode Modified with Poly-Riboflavin for 4-Nitrophenol Quantification. Mater. Chem. Phys. 2023, 301, 127568. [Google Scholar] [CrossRef]
- Li, X.; Čechal, J.; Spanhel, L.; Toscani, S.; Martinik, J.; Oborilova, R.; Trnkova, L. Intriguing Properties of Graphite/Polysiloxane Composite-Based Pencil Electrodes. Electrochim. Acta 2024, 475, 143615. [Google Scholar] [CrossRef]
- Sahana, H.R.; Arthoba Nayaka, Y.; Pradeepa, E. Development of Simple and Effective Poly Methyl Orange Modified Pencil Graphite Electrode for the Voltammentric Investigation of Aminotriazole in Presence of 2,4-Dichlorophenoxyacetic Acid. Microchem. J. 2024, 199, 109959. [Google Scholar] [CrossRef]

| Activation Method | (Ω cm2) | |||||
|---|---|---|---|---|---|---|
| Polished | Activated | PF | IM | IM + PC | IM + NC | |
| KCl 0.50 M (+1.10 V) | 104.3 | 99.9 | 140.6 | 134.2 | 75.7 | 72.3 |
| KCl 0.50 M (−1.10 V) | 54.1 | 500.5 | 258.9 | 178.1 | 205.4 | |
| KCl 0.10 M (+1.10 V) | 123.4 | 66.8 | 160.9 | 87.6 | 90.5 | |
| KCl 0.10 M (−1.10 V) | 51.5 | 444.0 | 376.5 | 340.3 | 356.2 | |
| Activation Method | (Ω cm2) | |||||
|---|---|---|---|---|---|---|
| Polished | Activated | PF | IM | IM + PC | IM + NC | |
| H2SO4 0.50 M (+1.10 V) | 104.3 | 17.2 | 182.5 | 461.7 | 427.9 | 431.2 |
| H2SO4 0.50 M (−1.10 V) | 80.7 | 238.5 | 550.8 | 505.0 | 501.4 | |
| H2SO4 0.10 M (+1.10 V) | 41.3 | 553.3 | 849.1 | 430.9 | 843.5 | |
| H2SO4 0.10 M (−1.10 V) | 48.9 | 367.0 | 398.7 | 382.2 | 374.1 | |
| [H2SO4] (mol L−1) | (Ω cm2) | |||||
|---|---|---|---|---|---|---|
| Polished | Activated | PF | IM | IM + PC | IM + NC | |
| 0.50 | 104.3 | 17.2 | 182.5 | 461.7 | 427.9 | 431.2 |
| 0.20 | 21.6 | 745.8 | 559.9 | 479.6 | 464.8 | |
| 0.10 | 41.3 | 553.3 | 849.1 | 430.9 | 843.5 | |
| 0.05 | 41.9 | 556.0 | 500.9 | 305.0 | 425.5 | |
| 0.02 | 58.2 | 256.1 | 653.8 | 339.3 | 500.2 | |
| 0.01 | 69.3 | 166.8 | 179.7 | 160.1 | 182.4 | |
| Electropolymerization Time (s) | (Ω cm2) | |||||
|---|---|---|---|---|---|---|
| Polished | Activated | PF | IM | IM + PC | IM + NC | |
| 100 | 104.3 | 56.8 | 355.9 | 420.1 | 177.8 | 388.3 |
| 200 | 559.3 | 380.0 | 387.7 | 562.5 | ||
| 300 | 650.8 | 577.8 | 807.7 | 546.0 | ||
| 400 | 949.6 | 1402.3 | 1065.3 | 1497.8 | ||
| 500 | 903.9 | 1081.2 | 1186.1 | 1033.5 | ||
| Element | In natura | Polished | Activated | PF |
|---|---|---|---|---|
| Atomic % | ||||
| Carbon | 95 | 95 | 94 | 92 |
| Oxygen | 4 | 4 | 5 | 8 |
| Silicon | <1 | <1 | <1 | <1 |
| Nitrogen | ≅0 | ≅0 | ≅0 | <1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, R.M.; da Silva, A.R.M.; Dias, G.F.; de Oliveira, D.B.; Pereira, A.C.; Franco, D.L.; Ferreira, L.F. Electrochemical Pretreatment and Functionalization of Pencil Graphite Electrodes for Enhanced Transducer Performance in Biosensing. Chemosensors 2025, 13, 84. https://doi.org/10.3390/chemosensors13030084
Coelho RM, da Silva ARM, Dias GF, de Oliveira DB, Pereira AC, Franco DL, Ferreira LF. Electrochemical Pretreatment and Functionalization of Pencil Graphite Electrodes for Enhanced Transducer Performance in Biosensing. Chemosensors. 2025; 13(3):84. https://doi.org/10.3390/chemosensors13030084
Chicago/Turabian StyleCoelho, Rafael Mendes, Alexandre Rafael Moraes da Silva, Geycson Figueiredo Dias, Danilo Bretas de Oliveira, Arnaldo César Pereira, Diego Leoni Franco, and Lucas Franco Ferreira. 2025. "Electrochemical Pretreatment and Functionalization of Pencil Graphite Electrodes for Enhanced Transducer Performance in Biosensing" Chemosensors 13, no. 3: 84. https://doi.org/10.3390/chemosensors13030084
APA StyleCoelho, R. M., da Silva, A. R. M., Dias, G. F., de Oliveira, D. B., Pereira, A. C., Franco, D. L., & Ferreira, L. F. (2025). Electrochemical Pretreatment and Functionalization of Pencil Graphite Electrodes for Enhanced Transducer Performance in Biosensing. Chemosensors, 13(3), 84. https://doi.org/10.3390/chemosensors13030084








