Cellulose-Based SERS Substrate for Vapor-Phase Thiol Detection with PCA for Enhanced Chemical Selectivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
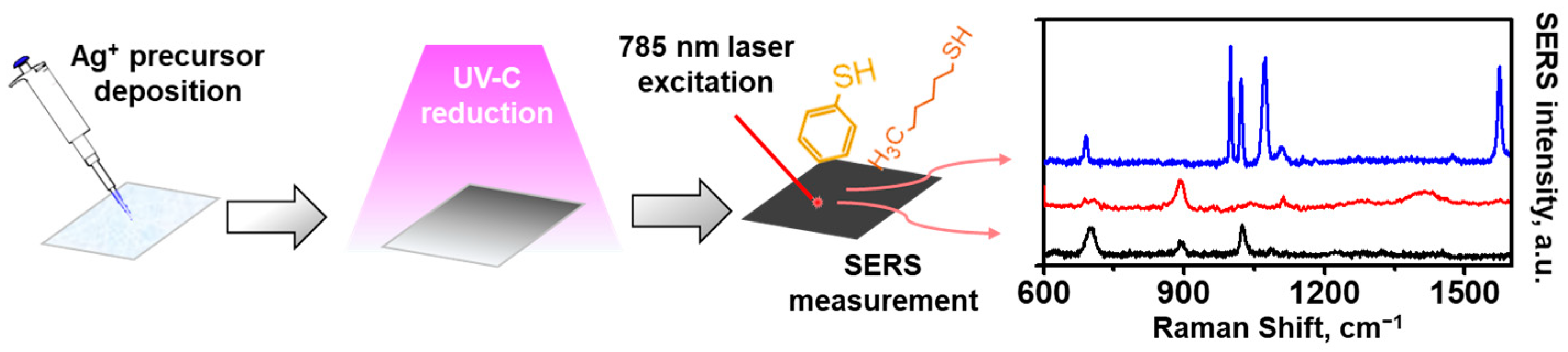
2.2. AgNPs/Cellulose SERS Substrate Fabrication
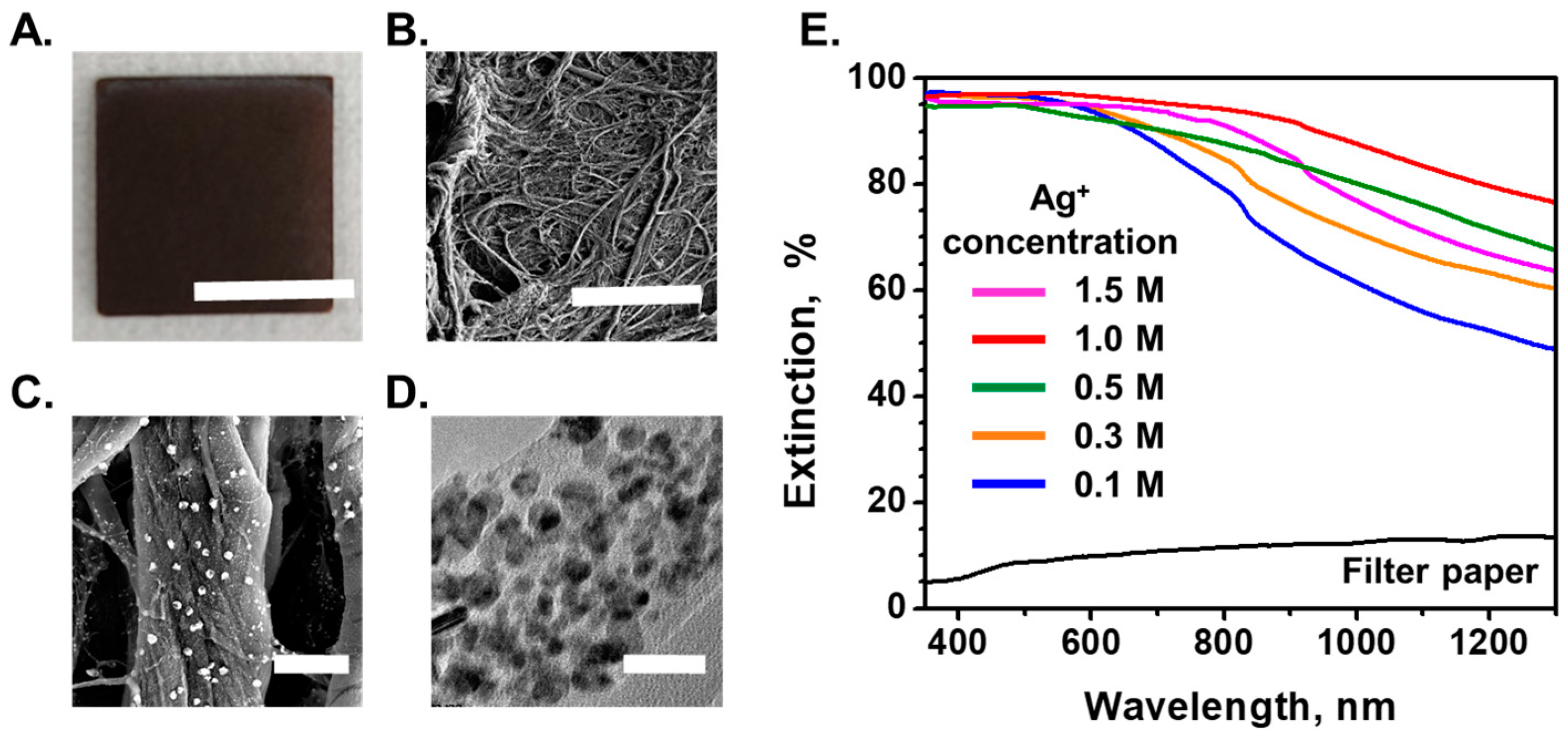
2.3. Surface Morphologies and Optical Properties of AgNPs/Cellulose Substrate
2.4. Liquid Phase Sample Preparation
2.5. Gas Phase Sample Preparation
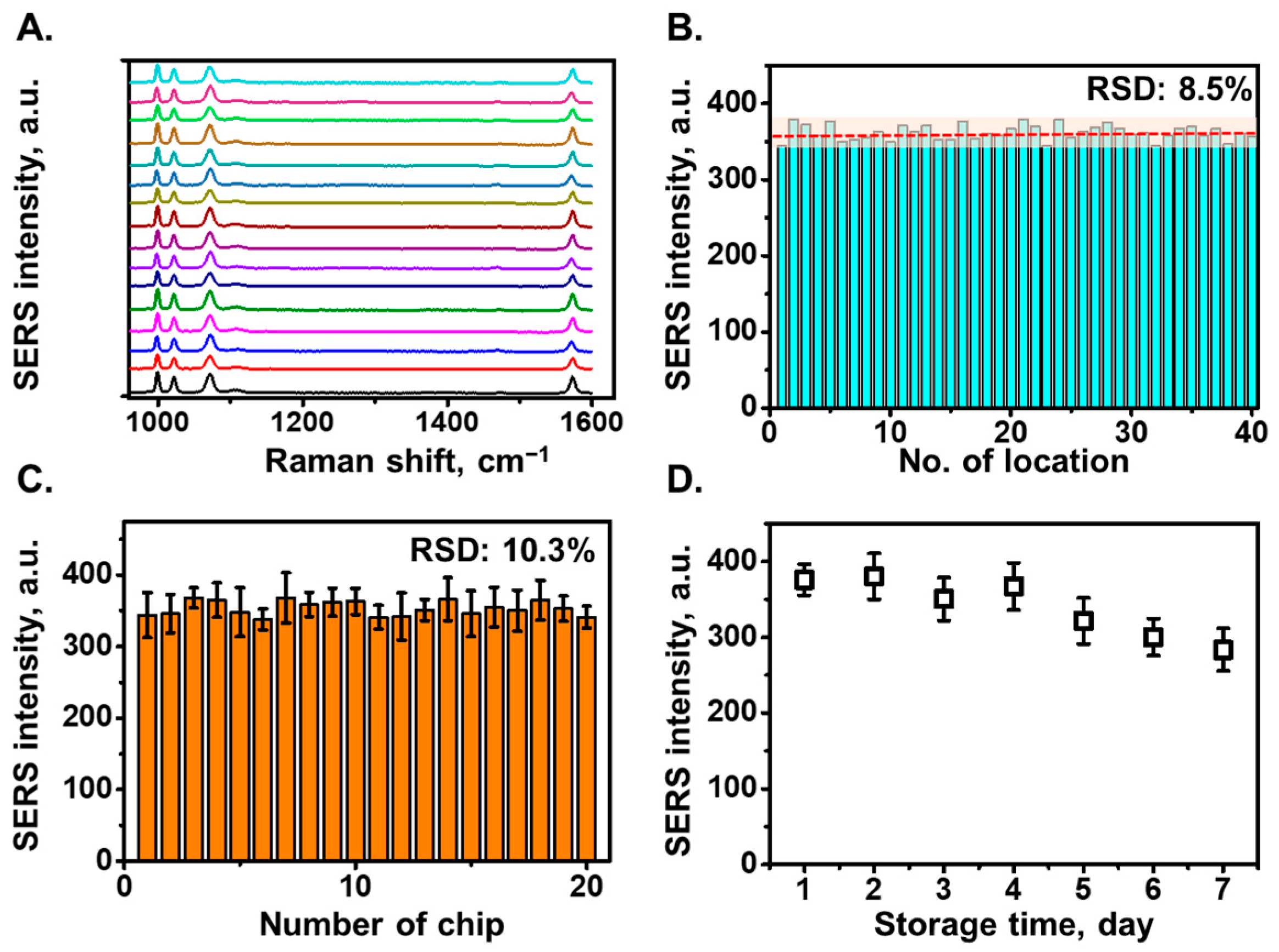
2.6. SERS Measurement and Signal Processing
3. Results and Discussion
3.1. Surface Morphology and Optical Characterization
3.2. SERS Characterization
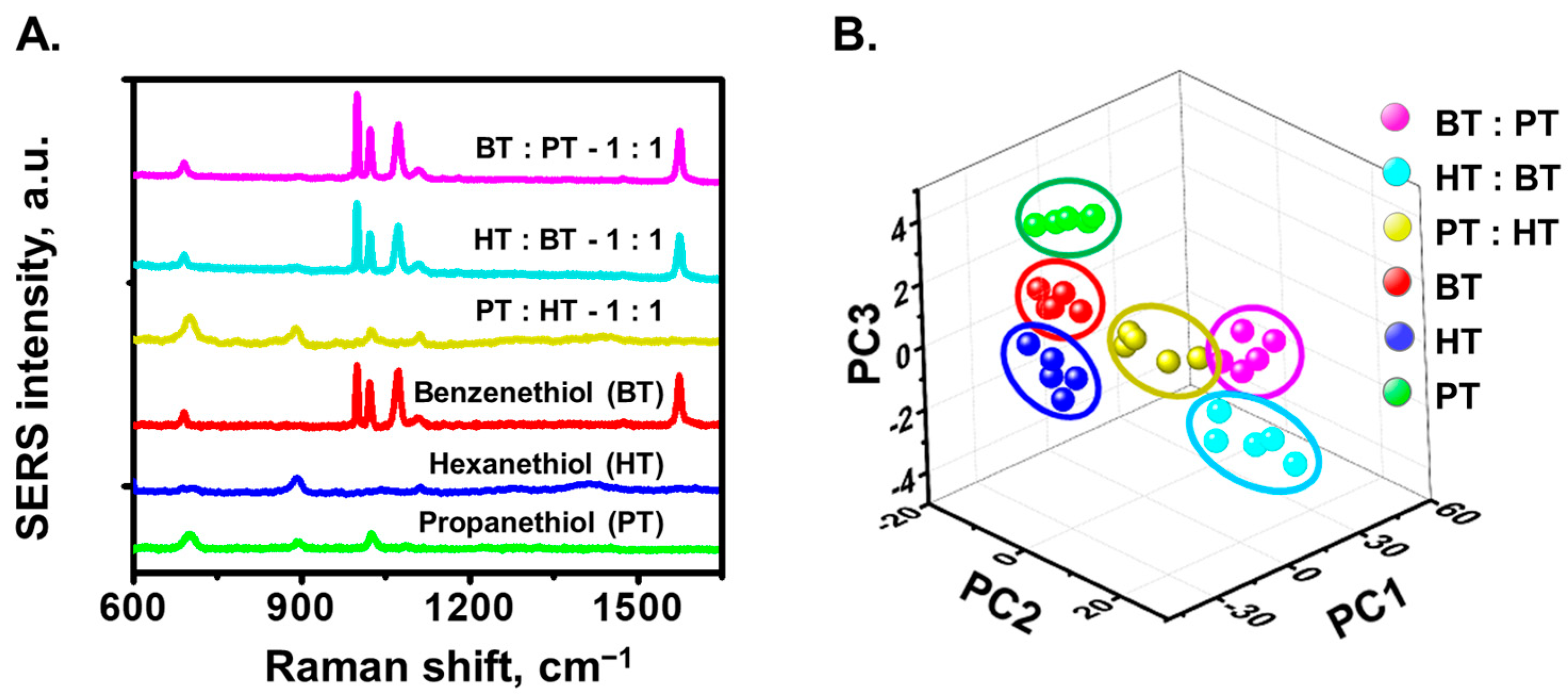
3.3. Vapor-Phase Detection of Thiols Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SERS | Surface-enhanced Raman scattering |
| UVC | Ultraviolet C |
| NP | Nanoparticle |
| EF | Enhancement factor |
| PCA | Principal component analysis |
| BT | Benzenethiol |
| HT | Hexanethiol |
| PT | Propanethiol |
| RSD | Relative standard deviation |
References
- Langer, J.; Jimenez De Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. Acs Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Pan, S.-Q.; Wang, W.-L.; Yue, X.; Xi, X.-H.; Yan, S.; Wu, D.-Y.; Wang, X.; Liu, G.; Ren, B. Surface-Enhanced Raman Spectroscopy: Current Understanding, Challenges, and Opportunities. Acs Nano 2024, 18, 14000–14019. [Google Scholar] [CrossRef] [PubMed]
- Dieringer, J.A.; Mcfarland, A.D.; Shah, N.C.; Stuart, D.A.; Whitney, A.V.; Yonzon, C.R.; Young, M.A.; Zhang, X.; Van Duyne, R.P. Introductory Lecture Surface enhanced Raman spectroscopy: New materials, concepts, characterization tools, and applications. Faraday Discuss. 2006, 132, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Shiohara, A.; Wang, Y.; Liz-Marzán, L.M. Recent approaches toward creation of hot spots for SERS detection. J. Photochem. Photobiol. C Photochem. Rev. 2014, 21, 2–25. [Google Scholar] [CrossRef]
- Kang, T.; Yoo, S.M.; Yoon, I.; Lee, S.Y.; Kim, B. Patterned Multiplex Pathogen DNA Detection by Au Particle-on-Wire SERS Sensor. Nano Lett. 2010, 10, 1189–1193. [Google Scholar] [CrossRef]
- Eom, G.; Kim, H.; Hwang, A.; Son, H.-Y.; Choi, Y.; Moon, J.; Kim, D.; Lee, M.; Lim, E.-K.; Jeong, J.; et al. Nanogap-Rich Au Nanowire SERS Sensor for Ultrasensitive Telomerase Activity Detection: Application to Gastric and Breast Cancer Tissues Diagnosis. Adv. Funct. Mater. 2017, 27, 1701832. [Google Scholar] [CrossRef]
- Abu Bakar, N.; Shapter, J.G. Silver nanostar films for surface-enhanced Raman spectroscopy (SERS) of the pesticide imidacloprid. Heliyon 2023, 9, e14686. [Google Scholar] [CrossRef]
- Lu, J.; Cai, Z.; Zou, Y.; Wu, D.; Wang, A.; Chang, J.; Wang, F.; Tian, Z.; Liu, G. Silver Nanoparticle-Based Surface-Enhanced Raman Spectroscopy for the Rapid and Selective Detection of Trace Tropane Alkaloids in Food. ACS Appl. Nano Mater. 2019, 2, 6592–6601. [Google Scholar] [CrossRef]
- Taylor, R.W.; Esteban, R.; Mahajan, S.; Aizpurua, J.; Baumberg, J.J. Optimizing SERS from Gold Nanoparticle Clusters: Addressing the Near Field by an Embedded Chain Plasmon Model. J. Phys. Chem. C 2016, 120, 10512–10522. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.; Ko, H. Transparent and Flexible Surface-Enhanced Raman Scattering (SERS) Sensors Based on Gold Nanostar Arrays Embedded in Silicon Rubber Film. Acs Appl. Mater. Interfaces 2017, 9, 44088–44095. [Google Scholar] [CrossRef]
- Yoon, I.; Kang, T.; Choi, W.; Kim, J.; Yoo, Y.; Joo, S.-W.; Park, Q.H.; Ihee, H.; Kim, B. Single Nanowire on a Film as an Efficient SERS-Active Platform. J. Am. Chem. Soc. 2009, 131, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Díez, A.G.; Rincón-Iglesias, M.; Lanceros-Méndez, S.; Reguera, J.; Lizundia, E. Multicomponent magnetic nanoparticle engineering: The role of structure-property relationship in advanced applications. Mater. Today Chem. 2022, 26, 101220. [Google Scholar] [CrossRef]
- Mercadal, P.A.; Encina, E.R.; Coronado, E.A. Colloidal SERS Substrate for the Ultrasensitive Detection of Biotinylated Antibodies Based on Near-Field Gradient within the Gap of Au Nanoparticle Dimers. J. Phys. Chem. C 2019, 123, 23577–23585. [Google Scholar] [CrossRef]
- Zeng, F.; Duan, W.; Zhu, B.; Mu, T.; Zhu, L.; Guo, J.; Ma, X. Paper-Based Versatile Surface-Enhanced Raman Spectroscopy Chip with Smartphone-Based Raman Analyzer for Point-of-Care Application. Anal. Chem. 2019, 91, 1064–1070. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, H.; Zhang, L.; Zhu, P.; Ge, S.; Yu, J. Paper-Based SERS Sensing Platform Based on 3D Silver Dendrites and Molecularly Imprinted Identifier Sandwich Hybrid for Neonicotinoid Quantification. ACS Appl. Mater. Interfaces 2020, 12, 8845–8854. [Google Scholar] [CrossRef] [PubMed]
- Trinh, B.-T.; Akter, R.; Cho, H.; Omelianovych, O.; Jo, K.; Kim, H.; Kang, T.; Nguyen, H.-Q.; Lee, J.; Seo, K.; et al. Rapid and sensitive melamine detection via paper-based surface-enhanced Raman scattering substrate: Plasma-assisted in situ growth of closely packed gold nanoparticles on cellulose paper. Appl. Surf. Sci. Adv. 2025, 26, 100717. [Google Scholar] [CrossRef]
- Golubewa, L.; Klimovich, A.; Timoshchenko, I.; Padrez, Y.; Fetisova, M.; Rehman, H.; Karvinen, P.; Selskis, A.; AdomavičiūTė-Grabusovė, S.; Matulaitienė, I.; et al. Stable and Reusable Lace-like Black Silicon Nanostructures Coated with Nanometer-Thick Gold Films for SERS-Based Sensing. ACS Appl. Nano Mater. 2023, 6, 4770–4781. [Google Scholar] [CrossRef]
- Pham, X.-H.; Seong, B.; Bock, S.; Hahm, E.; Huynh, K.-H.; Kim, Y.-H.; Kim, W.; Kim, J.; Kim, D.-E.; Jun, B.-H. Nonenzymatic Hydrogen Peroxide Detection Using Surface-Enhanced Raman Scattering of Gold–Silver Core–Shell-Assembled Silica Nanostructures. Nanomaterials 2021, 11, 2748. [Google Scholar] [CrossRef]
- Tarannum, N.; Khatoon, S.; Yadav, A.; Yadav, A.K. SERS-based molecularly imprinted polymer sensor for highly sensitive norfloxacin detection. J. Food Compos. Anal. 2023, 119, 105281. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, H.; Park, E.; Kim, T.; Chung, D.R.; Choi, Y.-M.; Kang, M. Paper-Based Multiplex Surface-Enhanced Raman Scattering Detection Using Polymerase Chain Reaction Probe Codification. Anal. Chem. 2021, 93, 3677–3685. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, A.K.; Tarannum, N.; Kumar, D. Paper-Based Flexible Nanoparticle Hybrid Substrate for Qualitative and Quantitative Analysis of Melamine in Powder Milk by SERS. ACS Omega 2024, 9, 2687–2695. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Lee, S.H.; Kim, J.H.; Ahn, Y.J.; Kim, Y.-H.; Yu, J.S.; Choi, S. Paper-Based Surface-Enhanced Raman Spectroscopy for Diagnosing Prenatal Diseases in Women. Acs Nano 2018, 12, 7100–7108. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Kim, H.J.; Lee, S.H.; Noh, H. Paper-Based Substrate for a Surface-Enhanced Raman Spectroscopy Biosensing Platform—A Silver/Chitosan Nanocomposite Approach. Biosensors 2022, 12, 266. [Google Scholar] [CrossRef]
- Trinh, B.T.; Cho, H.; Lee, D.; Omelianovych, O.; Kim, T.; Nguyen, S.K.; Choi, H.-S.; Kim, H.; Yoon, I. Dual-Functional Solar-to-Steam Generation and SERS Detection Substrate Based on Plasmonic Nanostructure. Nanomaterials 2023, 13, 1003. [Google Scholar] [CrossRef] [PubMed]
- Khinevich, N.; Bandarenka, H.; Zavatski, S.; Girel, K.; Tamulevičienė, A.; Tamulevičius, T.; Tamulevičius, S. Porous silicon—A versatile platform for mass-production of ultrasensitive SERS-active substrates. Microporous Mesoporous Mater. 2021, 323, 111204. [Google Scholar] [CrossRef]
- Pham, X.-H.; Hahm, E.; Kang, E.; Ha, Y.N.; Lee, S.H.; Rho, W.-Y.; Lee, Y.-S.; Jeong, D.H.; Jun, B.-H. Gold-silver bimetallic nanoparticles with a Raman labeling chemical assembled on silica nanoparticles as an internal-standard-containing nanoprobe. J. Alloys Compd. 2019, 779, 360–366. [Google Scholar] [CrossRef]
- Biggs, K.B.; Camden, J.P.; Anker, J.N.; Duyne, R.P.V. Surface-Enhanced Raman Spectroscopy of Benzenethiol Adsorbed from the Gas Phase onto Silver Film over Nanosphere Surfaces: Determination of the Sticking Probability and Detection Limit Time. J. Phys. Chem. A 2009, 113, 4581–4586. [Google Scholar] [CrossRef]
- Kim, H.; Trinh, B.T.; Kim, K.H.; Moon, J.; Kang, H.; Jo, K.; Akter, R.; Jeong, J.; Lim, E.-K.; Jung, J.; et al. Au@ZIF-8 SERS paper for food spoilage detection. Biosens. Bioelectron. 2021, 179, 113063. [Google Scholar] [CrossRef]
- Lafuente, M.; Sanz, D.; Urbiztondo, M.; Santamaría, J.; Pina, M.P.; Mallada, R. Gas phase detection of chemical warfare agents CWAs with portable Raman. J. Hazard. Mater. 2020, 384, 121279. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Z.; Li, H.; Yin, H.; Hao, P.; Dai, X.; Jiang, K.; Liu, C.; Zhang, T.; Yin, J.; et al. Ultrasensitive SERS Analysis of Liquid and Gaseous Putrescine and Cadaverine by a 3D-Rosettelike Nanostructure-Decorated Flexible Porous Substrate. Anal. Chem. 2022, 94, 5273–5283. [Google Scholar] [CrossRef]
- Lafuente, M.; Pellejero, I.; Sebastián, V.; Urbiztondo, M.A.; Mallada, R.; Pina, M.P.; Santamaría, J. Highly sensitive SERS quantification of organophosphorous chemical warfare agents: A major step towards the real time sensing in the gas phase. Sens. Actuators B Chem. 2018, 267, 457–466. [Google Scholar] [CrossRef]
- Lafuente, M.; Almazán, F.; Bernad, E.; Urbiztondo, M.A.; Santamaría, J.; Mallada, R.; Pina, M.P. SERS Detection of Neurotoxic Agents in Gas Phase Using Microfluidic Chips Containing Gold-Mesoporous Silica as Plasmonic-Sorbent. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII), Berlin, Germany, 23–27 June 2019; pp. 1313–1316. [Google Scholar] [CrossRef]
- Lafuente, M.; De Marchi, S.; Urbiztondo, M.; Pastoriza-Santos, I.; Pérez-Juste, I.; Santamaría, J.; Mallada, R.; Pina, M. Plasmonic MOF Thin Films with Raman Internal Standard for Fast and Ultrasensitive SERS Detection of Chemical Warfare Agents in Ambient Air. ACS Sens. 2021, 6, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- Hou, A.; Feng, G.; Zhuo, J.; Sun, G. UV Light-Induced Generation of Reactive Oxygen Species and Antimicrobial Properties of Cellulose Fabric Modified by 3,3′,4,4′-Benzophenone Tetracarboxylic Acid. Acs Appl. Mater. Interfaces 2015, 7, 27918–27924. [Google Scholar] [CrossRef]
- Rehan, M.; Barhoum, A.; Van Assche, G.; Dufresne, A.; Gätjen, L.; Wilken, R. Towards multifunctional cellulosic fabric: UV photo-reduction and in-situ synthesis of silver nanoparticles into cellulose fabrics. Int. J. Biol. Macromol. 2017, 98, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.-J.; Jeong, K.-H. Glass Nanopillar Arrays with Nanogap-Rich Silver Nanoislands for Highly Intense Surface Enhanced Raman Scattering. Adv. Mater. 2012, 24, 2234–2237. [Google Scholar] [CrossRef]
- Cha, H.; Kim, H.; Joung, Y.; Kang, H.; Moon, J.; Jang, H.; Park, S.; Kwon, H.-J.; Lee, I.-C.; Kim, S.; et al. Surface-enhanced Raman scattering-based immunoassay for severe acute respiratory syndrome coronavirus 2. Biosens. Bioelectron. 2022, 202, 114008. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinh, B.-T.; Nguyen, S.K.; Kim, D.; Nguyen, H.-Q.; Lee, J.; Sohn, Y.; Yoon, I. Cellulose-Based SERS Substrate for Vapor-Phase Thiol Detection with PCA for Enhanced Chemical Selectivity. Chemosensors 2025, 13, 101. https://doi.org/10.3390/chemosensors13030101
Trinh B-T, Nguyen SK, Kim D, Nguyen H-Q, Lee J, Sohn Y, Yoon I. Cellulose-Based SERS Substrate for Vapor-Phase Thiol Detection with PCA for Enhanced Chemical Selectivity. Chemosensors. 2025; 13(3):101. https://doi.org/10.3390/chemosensors13030101
Chicago/Turabian StyleTrinh, Ba-Thong, Sy Khiem Nguyen, Dayeon Kim, Huu-Quang Nguyen, Jaebeom Lee, Youngku Sohn, and Ilsun Yoon. 2025. "Cellulose-Based SERS Substrate for Vapor-Phase Thiol Detection with PCA for Enhanced Chemical Selectivity" Chemosensors 13, no. 3: 101. https://doi.org/10.3390/chemosensors13030101
APA StyleTrinh, B.-T., Nguyen, S. K., Kim, D., Nguyen, H.-Q., Lee, J., Sohn, Y., & Yoon, I. (2025). Cellulose-Based SERS Substrate for Vapor-Phase Thiol Detection with PCA for Enhanced Chemical Selectivity. Chemosensors, 13(3), 101. https://doi.org/10.3390/chemosensors13030101






