Abstract
The present cancer diagnostic techniques and contrast agents suffer drawbacks, adverse effects, and poor compatibility with patients due to health variations. To improve the detection of breast cancer, this work examined and contrasted the prospective applications of pomegranates, chitosan nanoparticles (Cs NPs), and pomegranate-loaded chitosan nanoparticles (PCs NPs) as contrast agents for breast cancer, based on the diffuse reflectance properties at the following laser frequencies: red (670 nm) and near-infrared (700 and 808 nm) spectrum. Herein, a platform for the detection of breast cancer is proposed, offering a promising pathway for cancer detection. PCs NPs with two different pomegranate contents (2 and 5 g/L) were synthesized using the sol–gel method. The cytotoxicity of the developed nanomaterials on human normal (Vero) and breast cancer (MCF7) cell lines were evaluated in the presence of laser irradiation at 670 nm, and the fluorescent effect of the nanoparticles was observed. The chemical structures of the nanomaterials and pomegranate extract were analyzed using FTIR, and they were then further analytically characterized using dynamic light scattering, zeta potential, and field-emission scanning electron microscopy. Results confirmed the structural stability of the nanomaterials. Cytotoxicity measurements revealed that the nanomaterials achieved a selective cytotoxic effect toward tumor cells. Results also showed significant wavelength-dependent changes in diffuse reflectance characteristics between malignant and normal cells. PCs NPs, at a content of 5 g/L, enhanced the reflectance in malignant cells compared to normal cells of more than three folds. These findings indicate the potential of PCs NPs to distinguish between healthy and malignant cells based on the reflection measurements.
1. Introduction
Breast cancer is a serious health problem throughout the world, particularly for women. Based on the recent statistics, breast cancer is the most common cancer in women in the United States, accounting for roughly 30% of new female cancer diagnoses each year. According to the current estimates, around 310,720 women are expected to be diagnosed with invasive breast cancer in 2024, with an additional 56,500 cases of ductal carcinoma in situ (DCIS) also anticipated to be diagnosed in the same year. In addition, it is projected that 42,250 deaths will result from breast cancer [1]. The latest data indicate that the rate of breast cancer diagnoses has been steadily increasing, with a slight upward trend of 0.6% annually, while the rate among women under 50 has shown a more pronounced rise at 1.0% per year [2]. Importantly, the detection and treatment of breast cancer in its early stages significantly improves survival rates [3,4,5]. Therefore, increasing public awareness about breast cancer, offering accessible and cost-effective early detection methods like routine screenings, and actively researching new treatment options can significantly decrease the prevalence and mortality rates of this disease [6,7,8].
The primary methods for diagnosing breast tumors currently involve a combination of imaging techniques, such as mammograms [9], ultrasound scans [10], and magnetic resonance imaging (MRI) [11], with a definitive diagnosis usually requiring a tissue sample obtained through a core biopsy or a vacuum-assisted biopsy [12], which is often guided by one of the imaging methods. However, each of these methods has inherent limitations. While mammograms are a primary tool for breast cancer detection, their limitation in identifying tumors within dense breast tissue can lead to missed cancers, potentially delaying the diagnosis and treatment of women with this condition, highlighting the need for additional imaging methods like ultrasounds or MRIs in high-risk cases [13]. The accuracy of ultrasound scans heavily depends on the skills of the technician or radiologist performing the procedure. Inexperienced operators may overlook anomalies or mistakenly identify normal tissue as suspicious, which can lead to missed or false positive findings, potentially delaying diagnosis and treatment [14]. MRI scans are significantly more expensive than other imaging techniques, and they are not suitable for routine screening [15]. Biopsies carry risks such as bleeding, infection, and potential damage to nearby tissues or organs. Although these risks are relatively minor, they should be carefully considered, particularly for individuals with underlying medical conditions [16]. As a result, there is an increasing need for alternative or modified procedures to facilitate the safe and cost-effective early identification of breast cancer.
Optical imaging is a non-invasive diagnostic method that utilizes light to visualize and analyze biological tissues, making it a key tool in cancer detection and monitoring due to its ability to identify specific molecular markers within tumors, often with high sensitivity and specificity when combined with fluorescent probes [17]. By leveraging the interaction of light with tissues, optical imaging provides high-resolution, real-time insights into cellular and molecular structures, which in turn enables the distinction between healthy and cancerous tissues, providing valuable information for diagnosis and treatment monitoring. Techniques such as diffuse reflectance, fluorescence imaging, and photoacoustic imaging are commonly employed to evaluate tumor characteristics, blood vessel networks, and metabolic processes within the body [18,19]. To further improve the specificity and sensitivity of optical imaging, contrast agents like nanoparticles or fluorescent dyes can be introduced to precisely pinpoint cancerous cells [20]. Previous studies have demonstrated the potential of gold nanoparticles in detecting squamous cell carcinoma of the tongue by leveraging their unique laser-induced fluorescence and diffuse reflectance properties through in vitro experiments [21]. Ongoing research is focused on the development of materials that can both diagnose and treat diseases while minimizing toxicity [22,23,24]. For instance, iodinated contrast agents, often used in CT scans, can cause kidney toxicity or allergic reactions, while gadolinium-based agents used in MRIs have been linked to gadolinium retention in tissues and nephrogenic systemic fibrosis in patients with kidney dysfunction [25]. Additionally, certain fluorescent dyes, such as indocyanine green, and radioactive tracers, such as fluorodeoxyglucose (FDG), pose minimal but notable risks of hypersensitivity and radiation exposure, respectively [15,26,27]. The are attempts to replace these with safer and more efficient alternatives, such as non-toxic nanomaterials.
Nanotechnology has emerged as a promising approach for cancer detection and surveillance. It has demonstrated efficacy in identifying and diagnosing the various types of malignancies, including cervical, lung, breast [28], gastric [29], nasopharyngeal [30], colon [31], and oral cancer [32]. By taking advantage of the unique properties and capabilities of nanotechnology such as targeted drug delivery, enhanced imaging, and sensitive detection methods, nanotechnology has improved the accuracy and efficiency of cancer diagnostics and treatment by enabling the detection of cancer-related molecules and the precise delivery of therapies [33]. This opens new possibilities for the development of innovative approaches that can improve early detection and ultimately lead to an improvement in cancer management [34,35]. Chitosan nanoparticles (Cs NPs) are gaining significant interest in cancer treatment, especially for breast cancer, because of their unique properties like biocompatibility, biodegradability, low toxicity, and the ability to enhance the delivery and accumulation of chemotherapeutic drugs at the tumor site through the “enhanced permeability and retention (EPR)” effect, allowing for more targeted treatment with reduced side effects [36,37]. Their primary application lies in acting as drug delivery systems, where Cs NPs encapsulate chemotherapy drugs, improving their solubility, stability, and bioavailability, enabling targeted distribution to cancer cells and thus minimizing the required dosages and reducing the side effects associated with conventional chemotherapy [38]. Additionally, Cs NPs can be engineered to carry photosensitizers or photothermal agents, enabling photodynamic or photothermal therapy where light activation triggers localized damage to cancer cells by generating heat or reactive oxygen species [39,40], providing a minimally invasive therapeutic option; the ability to conjugate Cs NPs with imaging agents also allows for theranostic applications, enhancing contrast in imaging modalities like MRI, CT scans, and optical imaging to facilitate diagnosis and the monitoring of treatment response during surgery [41]. This improves the ability to detect and monitor tumor growth and response to therapy. Moreover, Cs NPs can be employed in post-surgical treatment to deliver medications directly to the operative site. This approach aims to eliminate any remaining cancer cells and reduce the risk of recurrence. These applications are currently at various stages of research, development, and clinical testing. The flexibility of Cs NPs offers numerous opportunities for more effective, targeted, and personalized non-toxic treatments [42,43]. Also, green synthesis eliminates the use of hazardous chemicals, promoting the production of nanoparticles that are safer for biological applications and exhibit minimal risk to human tissues and cells [44,45].
Pomegranates are native to the Middle East and Mediterranean regions, and they have been traditionally used in various countries to treat acidosis, dysentery, microbial infections, diarrhea, helminthiasis, bleeding, and respiratory illnesses. Studies have shown that this plant has high concentrations of bioactive components such as tannins, phenolic acids, flavonoids, anthocyanins, and steroids, which contribute to its numerous health innervations [46,47,48]. Scientific research has confirmed the positive effects of the pomegranate fruit, highlighting its antioxidant, anti-inflammatory, anticancer, antibacterial, and hepatoprotective qualities [49,50,51,52,53]. Also, pomegranates have been extensively studied for their potential applications in cancer treatment [54,55,56,57]. It is strongly believed that developing natural nanomaterials with chitosan and pomegranate can advance non-toxic cancer detection.
The developed nanomaterials can help in avoiding the side effects of the current breast cancer detection approaches. The present work presents a novel approach utilizing pomegranate, chitosan nanoparticles (Cs NPs), and pomegranate-loaded chitosan nanoparticles (PCs NPs) as contrast agents for breast cancer, based on diffuse reflectance properties at specific laser wavelengths (670, 700, and 808 nm). This study observed the fluorescent effect of PCs NPs (at a pomegranate content of 5 g/L) when irradiated with 670 nm. By leveraging the diffuse reflectance properties of cells at these wavelengths, the study explored the optical and cytotoxic effects of these nanoparticles on normal (Vero) and breast cancer (MCF 7) cell lines. The research characterized all synthesized nanoparticles using zeta potential, dynamic light scattering, scanning electron microscopy, and Fourier transform infrared spectroscopy. The results demonstrate that pomegranate-loaded Cs NPs significantly enhanced the ability to distinguish between healthy and malignant cells, particularly at the specified laser wavelengths, highlighting their potential as a novel tool for improving breast cancer detection techniques.
2. Materials and Methods
2.1. Materials
Low molecular weight chitosan (Nanogate Co., Cairo, Egypt) and sodium triphosphate (TPP, 85%) were obtained from Sigma-Aldrich, St. Louis, MI, USA. Glacial acetic acid (≥99.7%) was purchased from Thermo Fisher Scientific (Norcross, GA, USA). Polyacrylonitrile (PAN), with an average molecular weight of 150,000 g/mol, and dimethyl sulfoxide (DMSO) of ACS reagent grade were acquired from Sigma-Aldrich, USA. N, N-Dimethylformamide (DMF) of at least 99% purity, classified as laboratory reagent grade, was procured from Fisher Chemical, Waltham, MA, USA. N, N-Dimethylacetamide (DMAc) of analytical research grade was sourced from Alpha Chemika, India. The Punica granatum fruits were obtained from a local market in Egypt (30.0654905, 31.3334145) in January 2023 and were manually peeled. A total of 200 g of air-dried pomegranate peels were pulverized and macerated in 80% methanol for three days before being filtered. The extract was concentrated using a rotary evaporator (Hei-VAP Value, Heidolph coupled with Buchi Vac. V-500 pump, Geneva, Switzerland) to obtain 88 g of dried extract, which was kept at −20 °C until further analysis.
2.2. Nanoparticles Formations
In this study, three distinct nanoparticle formulations were developed using the sol–gel method [58]. To synthesize Cs NPs, a chitosan solution was first obtained by dissolving 0.5 mg of chitosan into 1% acetic acid and distilled water. Next, the chitosan solution was neutralized by the dropwise addition of 0.16 gm of TPP dissolved in 100 mL DI H2O under magnetic stirring (700 rpm, one hour) at room temperature. Figure 1 shows a schematic diagram of the nanoparticle formation steps using chitosan. The formation of nanoparticles (NPs) occurred after continuous magnetic stirring for 20 h. Finally, the solution containing nanoparticles was dialyzed by centrifugation at 15,000× g rpm for 20 min to attain the nanoparticles.
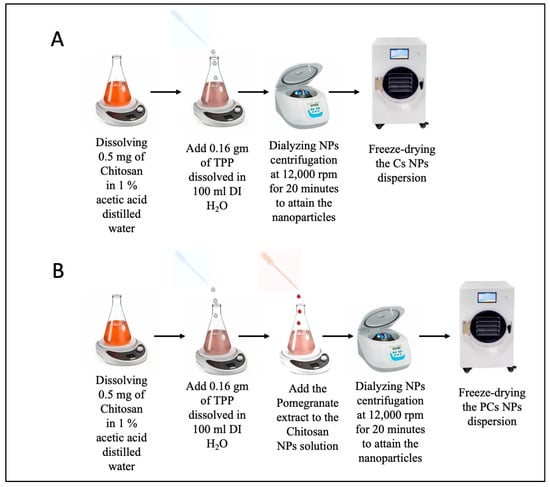
Figure 1.
Schematic diagram of the nanoparticle formation steps showing (A) chitosan nanoparticles (Cs NPs), and (B) pomegranate-loaded chitosan nanoparticles (PCs NPs) using sol gel method.
Pomegranate-loaded chitosan nanoparticles (PCs NPs) were synthesized in two different concentrations of pomegranate extract (2 and 5 g/L); a chitosan solution and a TPP solution were prepared as mentioned above. Pomegranate-loaded chitosan nanoparticles at a pomegranate extract content of 5 g/L (PCs NPs 0.5) were formed by adding 0.5 mg of pomegranate extract to the chitosan NPs solution. Likewise, (PCs NPs 0.2) were formed by adding 0.2 mg of the pomegranate extract to the chitosan NPs solution. Both nano solutions were formed under continuous magnetic stirring for 20 h. The nanoparticle solutions underwent dialysis using a centrifuge at 15,000× g rpm for 20 min to obtain the nanoparticles. The formation steps for Cs NPs and PCs NPs are illustrated in Figure 1A and Figure 1B, respectively.
2.3. Nanoparticle Characterization
The molecular structure of the nanoparticles were analyzed utilizing Fourier transform infrared spectroscopy (FT/IR, Bruker, Leipzig, Germany); the hydrodynamic particle size was determined using dynamic light scattering (DLS) on ZS-ZEN (Malvern Instruments Co., Malvern, UK); the zeta potential (ZP) was used to assess nanoparticle stability (by determining whether they would remain dispersed or aggregate together), and insights into the morphology, crystallography, and composition of the nanoparticles were obtained using scanning electronic microscopy (SEM, Thermo Fisher Scientific, Norcross, GA, USA).
2.3.1. Hydrodynamic Size and Stability Evaluation
Dynamic light scattering (DLS) was used to determine the hydrodynamic particle size. Zetasizer (Malvern, UK) analyzed both the particle diameter size distribution and the surface charge of the nanoforms. Dynamic light scattering was conducted with count rates spanning from 50 to 300 Kcps and zeta potential measurements within the range of −200 to 200 mV at a scattering angle of 173°. The specimens were immersed in distilled water, and measurements were conducted at room temperature with a precision of ±0.1 °C.
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
The FT-Raman Module, produced by Bruker in Germany, covers a wavelength range from 400 to 7500 cm−1 and provides a high spectral resolution of 0.1 cm−1. It displays scans at a minimum rate of 50 scans per second, achieving a spectral resolution of 16 cm−1. The FTIR spectroscopy technique was employed to perform infrared (IR) spectral analysis on the nanoforms. This allowed for the detection of their chemical functional groups and the analysis of their capping ligands.
2.3.3. Scanning Electron Microscopy
The scanning electron microscope (SEM) used was provided by Thermo Fisher Scientific (Norcross, GA, USA). It was supplied with a Quattro S Felid Emission Gun and equipped with the following detectors: ETD: Everhart–Thornley Secondary Electron Detector (SED); LVD: Low Vacuum Secondary Electron Detector (20–200 Pa); GSED: Gausses Secondary Electron Detector (200–2000 Pa); and BSD: Back Scattered Detector (multiple array detector).
2.4. Cytotoxicity and Fluorescent Effect Measurements
The Vero and MCF 7 cell lines were used as models for normal and breast cancer cells, respectively. These cell lines were obtained from the American Tissue Culture Collection (ATCC, Manassas, VA, USA). They were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Lonza, Belgium), supplemented with 10% fetal bovine serum (FBS, Gibco, Rio de Janeiro, Brazil), 2% penicillin/streptomycin (Lonza, Verviers, Belgium), 1% sodium pyruvate (100 mM, Lonza, Belgium), and 1% L-glutamine (200 mM, Lonza, Belgium). The cells were maintained at 37 °C with 5% CO2 and 85–95% humidity. To evaluate the cellular toxicities of pomegranate extract and the synthesized nanoparticles, MTT dye (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) was used after 48 h of incubation with the Vero and MCF7 cells. Cytotoxicity was assessed with seven serial dilutions, starting at 200 µg/mL. Cytotoxicity concentration (IC50) values were determined using GraphPad® Prism software v.9.5.1. The two study groups were compared using the ANOVA test with Bonferroni post hoc multiple two-group comparisons as a statistical analysis. The fluorescent properties of the PCs NPs 0.5 were examined under a fluorescent microscope with an excitation wavelength of 670 nm, and emission signals were captured in the red fluorescence channel.
2.5. Diffuse Reflectance Optical Setup
Six-well plates were seeded with normal and cancerous cells to facilitate reflectance measurements. After 24 h of incubation, pomegranate extract and the synthesized nanomaterials were added to their respective wells. Laser wavelengths in the visible to near-infrared spectral bands (670, 700, and 808 nm) were then used to measure the optical diffuse reflectance of the cell lines. CW laser diodes with a power density of 10 mW/cm2 (laser land company, Wuhan, Wuhan, China) were employed in the experimental measurements. The reflected beam was then sent to a digital spectrometer (STDFSM, Touptek Photonics Co. Ltd., Hangzhou, China) via an optical fiber cable. The spectrometer was connected to the computer via a USB cable. The measuring detector (Toshiba TCD1304AP Linear CCD array (Sony ILX511 2048 Linear CCD array)) had a response range of 200–1100 wavelengths, 3648 pixels, and 8 × 200 µm2 pixel size. The detector was positioned at a slight angle (typically between 5 and 10 degrees) relative to the incoming laser beam to minimize the impact of unwanted specular reflections and distortion in the light spot, thereby improving the accuracy of the measurement being taken. Data processing and analysis were carried out using the spectrometer software (Toup Spm) and MATLAB R2018b. The optical setup is presented in Figure 2.
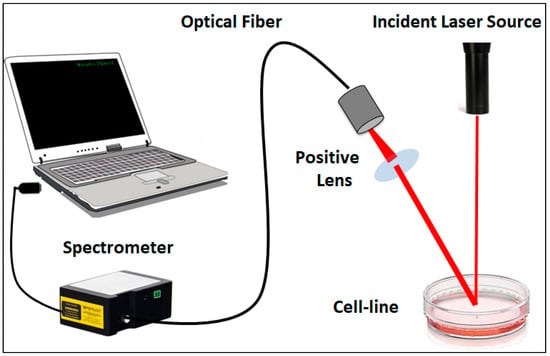
Figure 2.
Schematic diagram of the experimental setup utilized for diffuse reflectance measurement.
3. Results and Discussion
Current cancer detection techniques and contrast agents have limitations. This study utilized pomegranate extract, Cs NPs, and PCs NPs to improve the diagnosis of breast cancer by investigating their diffuse reflectance properties at specific laser wavelengths. Air-dried pomegranate peels were extracted using 80% methanol. The following three different nanoparticles were synthesized using the sol–gel method: (i) Cs NPs, (ii) PCs NPs 0.2, and (iii) PCs NPs 0.5, by dissolving 0.2 mg and 0.5 mg of pomegranate peel extract, respectively, in 100 mL of Cs NPs. The optical characteristics and behavior of normal and malignant cells were studied by examining the changes in diffuse reflectance of two cell lines using the three different nanoparticles at the following three laser wavelengths: 670 nm, 700 nm, and 808 nm. The diffuse reflectance of the samples was recorded using a compact spectrometer to capture the response. Diffuse optical imaging (DOI) via reflectance measurements is a powerful and non-invasive tool for the functional imaging of tissues, offering valuable insights into biological structures and supporting the early diagnosis and monitoring of various health conditions.
3.1. Nanoparticles Characterization Assessment
FTIR analysis was performed to reveal the characteristic peak values and identify the functional groups within the methanol extracts of pomegranate, Cs NPs, PCs NPs 0.2, and PCs NPs 0.5, as shown in Figure 3. The vibrational modes of the functional groups that controlled the chemical interactions of the nanoparticles are summarized in Table 1. The FT-IR analysis of Cs NPs reveals the following key absorption peaks: A peak at 3290 cm−1 indicates N-H functional group, and it overlaps the O–H group. At 2870 cm−1, the C-H stretching vibration is assigned, with amide II (C=O) stretching at 1640 cm−1 and N-H bending at 1541 cm−1. The P=O stretch at 1212 cm−1 signifies chitosan’s cross-linking with TPP, as well as a C-O-C group stretch at 1072 cm−1. The pomegranate extract exhibits distinct peaks, including an O-H stretch at 3291 cm−1 and a C-H stretch at 2931 cm−1, with a C=O stretch at 1720 cm−1, a phenolic O-H stretch at 1347 cm−1, and an O-C-O stretch at 1006 cm−1. Loading pomegranate onto chitosan NPs broadens the O-H stretch at 3290 cm−1 and shifts the amide II and N-H bending vibrations, indicating hydrogen bonding and the successful loading of pomegranate onto the chitosan NPs.
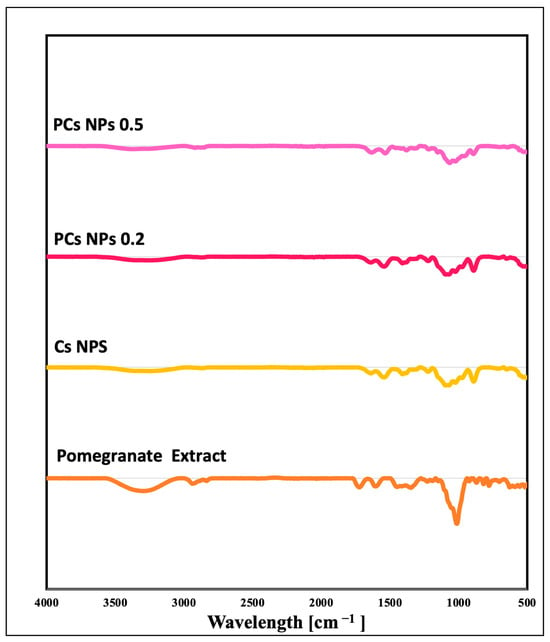
Figure 3.
Fourier transform infrared spectra for pomegranate extract, Cs NPs, PCs NPs 0.2, and PCs NPs 0.5.

Table 1.
Fourier transform infrared spectroscopy analysis of the pomegranate extract and the synthesized nanoparticles.
The dynamic light scattering (DLS) and zeta potential measurements for the pomegranate extract, Cs NPs, and PCs NPs were conducted using a Nano ZS apparatus, as shown in Figure 4a,b, respectively. The Zeta potential distribution measurements for Cs NPs, pomegranate extract, PCs NPs 0.2, and PCs NPs 0.5 are represented in Figure S2A,B,C,D, respectively (Supplementary Materials). The zeta potential for pomegranate extract and Cs NPs was initially recorded as −17.2 mV and 29.2 mV, respectively, as shown Figure 4a. Upon binding the Cs NPs with two different concentrations of pomegranate extract (PCs NPs 0.2 and PCs NPs 0.5), the zeta potential values shifted to 17.2 mV and 14.2 mV, respectively, as shown in Figure 4a, indicating the attachment of negatively charged pomegranate extract to the positively charged Cs NPs surface. The DLS measurements revealed particle sizes of approximately 210 nm, 607 nm, 476 nm, and 493 nm for Cs NPs, pomegranate extract, PCs NPs 0.2, and PCs NPs 0.5, respectively, as represented in Figure 4b. The nanoparticle size distribution measurements conducted by the DSL for the Cs NPs, pomegranate extract, PCs NPs 0.2, and PCs NPs 0.5 are represented in Figure S1A,B,C,D, respectively (SI). The CS sample has the smallest particle size (210 nm) with the lowest PdI (0.176), indicating high uniformity and a monodisperse system, as shown in Figure S1A. In contrast, the pomegranate extract sample has the largest particles (607 nm) and the highest PdI (0.504), as shown in Figure S1B, suggesting a broad distribution and significant heterogeneity. The PCs NPs 0.2 sample has a moderate size (476 nm) with some aggregation, as shown in Figure S1C, while the PCs NPs 0.5 sample exhibits similar characteristics with a slightly larger average size (493 nm), as shown in Figure S1C. Overall, the CS sample is the most uniform, whereas the pomegranate extract sample shows the greatest variation in particle size distribution caused by the aggregation in the pomegranate extract.
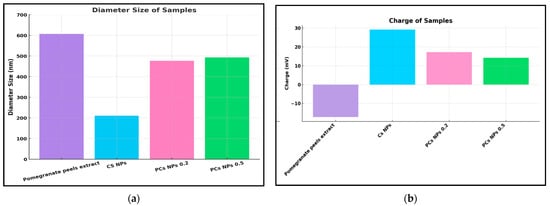
Figure 4.
Measurements of (a) Dynamic Light Scattering and (b) Zeta Potential Measurements.
The morphology and distribution of the nanoparticles are represented in the field emission scanning electron Microscopy (FE-SEM) images shown in Figure 5. The analysis of the FE-SEM images reveals distinct characteristics of the nanoparticles. Cs NPs have a mean diameter of 17.66 nm, as shown in Figure 5a. PCs NPs 0.2 exhibit a mean diameter of 21.10 nm, and the largest maximum diameter (1257.52 nm), indicating extreme aggregation due to the interactions with the pomegranate extract, as shown in Figure 5b. In contrast, PCs 0.5 NPs have a mean diameter of 15.04 nm, and a maximum diameter of 346.47 nm, suggesting smaller particles with reduced aggregation and better stability, as shown in Figure 5c. This trend highlights the impact of pomegranate concentration, with PCs NPs 0.5 showing a more uniform size distribution and improved stability. The increase in size for PCs NPs 0.2 and PCs NPs 0.5 following the addition of pomegranate extract further confirms the successful attachment of the extract to the Cs NPs surface. The discrepancies between the DLS and SEM measurements are attributed to their differing methodologies, as follows: DLS measures the hydrodynamic diameter, encompassing surface-bound molecules and solvent layers, which often results in larger particle sizes. In contrast, SEM measures the dry core size under vacuum conditions. Additionally, DLS detects particle aggregation in suspension, while SEM captures individual particles, explaining the observed size variations between the two techniques [59].
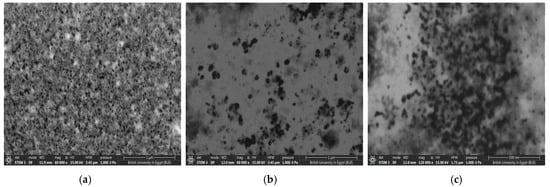
Figure 5.
FE-SEM images: (a) Cs NPs, (b) PCs NPs 0.2, and (c) PCs NPs 0.5.
3.2. Cytotoxicity and Fluorescent Measurements Assessment
As routine work, the cellular cytotoxicity of pomegranate extract and the synthesized nanoparticles were evaluated using the MTT dye against the 48 h incubated Vero and MCF7 cells, as the models for normal and breast cancer cell lines, respectively. The cytotoxic effects for pomegranate extract and the nanoparticles (CS NPs, PCs NPs 0.2, and PCs NPs 0.5) on the two cell lines (MCF-7 and Vero cells), are shown in Figure 6a and Figure 6b, respectively, under standard conditions after laser irradiation at a wavelength of 670 nm for 3 min. Cell survival decreased consistently with the increase in the concentrations for all proposed materials. Laser irradiation significantly enhanced the effects of all the nanomaterials. MCF-7 cells are slightly more sensitive to nanomaterials compared to Vero cells, and that indicates that the materials are safe on healthy tissue. The pomegranate-loaded chitosan nanoparticles (PCs NPs) offer several significant advantages in targeted cancer compared to unloaded chitosan nanoparticles (Cs NPs). One of the key benefits is their enhanced selectivity towards cancer cells, as evidenced by their lower IC50 values for the MCF-7 cells and reduced toxicity to the normal cells (VERO), making them safer and more effective as a contrast agent option. The bioactive compounds in pomegranate extract, including tannins, flavonoids, and phenolic acids, contribute synergistically to apoptosis induction, the inhibition of cell proliferation, and metastasis prevention, complementing the intrinsic anticancer properties of chitosan [6,46,60]. Additionally, the presence of pomegranate polyphenols enhances the biocompatibility of the nanoparticles, reducing the potential side effects and making them more suitable for long-term detection applications [60].
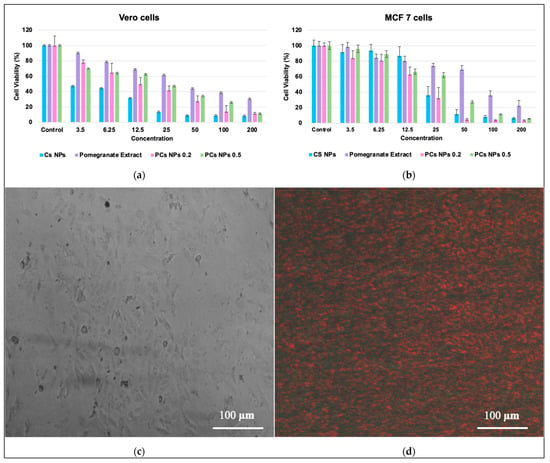
Figure 6.
The cell viability of pomegranate extract, Cs NPs, PCs NPs 0.2, and PCs NPs 0.5 was assessed against (a) Vero cell lines with laser irradiation, (b) MCF-7 cell lines with laser irradiation across different concentrations (The data represented the ± SD, (p > 0.0001)), (c) MCF-7 cells injected with PCs NPs 0.5 without laser irradiation, (d) MCF-7 cells injected with PCs NPs 0.5 through laser irradiation (670 nm).
The cells that were injected with nanoparticles but were not exposed to laser irradiation did not exhibit a fluorescence effect, as shown in Figure 6c. However, the cells that were injected with the nanoparticles and irradiated with the laser at a wavelength at 670 nm displayed fluorescence, as shown in Figure 6d, demonstrating their potential application in bioimaging for cells injected with PCs NPs 0.5 and furthering their tolerance to being photographed under a fluorescent microscope. The observed fluorescence in laser-irradiated cells confirmed the successful uptake and activation of the nanoparticles, while the absence of fluorescence in the non-irradiated cells further supported their laser-responsive properties due to the polyphenols [61,62]. These findings align with the existing literature, which highlights the use of chitosan nanoparticles as contrast agents when combined with natural compounds for cancer theranostics [41,63,64]. The results demonstrate the potential of PCs NPs as effective contrast agents for cancer detection under laser irradiation.
Table 2 presents the IC50 values (µg/mL) of pomegranate extract, Cs NPs, PCs NPs 0.2, and PCs NPs 0.5 against the MCF-7 and Vero—cancerous and normal—cell lines, respectively, after 3 min of laser irradiation. For the MCF-7 cell line, the pomegranate extract exhibited the highest IC50 value of 91.17 µg/mL, indicating the lowest cytotoxicity, while Cs NPs demonstrated the strongest cytotoxicity with the lowest IC50 value of 11.33 µg/mL. PCs NPs 0.2 and PCs NPs 0.5 showed IC50 values of 14.889 µg/mL and 17.111 µg/mL, respectively, reflecting moderate effectiveness, with PCs NPs 0.2 being slightly more effective. In the Vero cells, the pomegranate extract again had the highest IC50 value of 99.77 µg/mL, suggesting minimal toxicity to normal cells. Cs NPs showed an IC50 of 13.67 µg/mL, indicating significant toxicity, while PCs NPs 0.2 and PCs NPs 0.5 demonstrated IC50 values of 18.889 µg/mL and 24.667 µg/mL, respectively, showing reduced toxicity in normal cells. These results highlight that PCs NPs 0.5 offer the best balance between cytotoxicity against cancer cells and safety for normal cells, making them a promising candidate for targeted cancer detection under laser irradiation. The two study groups were compared using the ANOVA test with Bonferroni post hoc multiple two-group comparisons. p-value measurements of less than 0.0001 were considered statistically significant.

Table 2.
The IC 50 values (µg/mL) of the pomegranate extract, Cs NPs, PCs NPs 0.2, and PCs NPs 0.5, after laser irradiation at a wavelength of (670 nm).
The rationale behind preparing and using pomegranate-loaded chitosan nanoparticles lies in their enhanced selectivity and potential synergistic effects. While the unloaded chitosan nanoparticles demonstrated notable cytotoxic activity, the presence of pomegranate polyphenols enhanced antioxidant properties and introduced multiple anticancer mechanisms, such as apoptosis induction and metastasis inhibition, which may not be as pronounced in chitosan alone. These nanoparticles offer great potential in cancer detection without the side effects of other toxic materials.
3.3. Diffuse Reflectance Analysis
The variations in the recorded diffuse reflectance for the normal and cancerous cells were measured after the addition of pomegranate extract and all the proposed nanomaterials (at half concentration of the IC50), at wavelengths of 670 nm, 700 nm, and 808 nm, as presented in Figure 7, Figure 8 and Figure 9, respectively. The measurements of the diffuse reflectance at a wavelength of 670 nm revealed distinct differences in the performance of PCs NPs (PCs NPs 0.2 and PCS NPS 0.5), as shown in Figure 7. CNPs exhibited moderate specificity for cancer cell detection; however, the separation between normal and cancer cells was not highly pronounced, limiting their diagnostic potential, as shown in Figure 7a. The pomegranate extract showed a more noticeable difference in spectral peaks, with MCF7 cancer cells displaying a sharper and slightly higher peak, indicating better interaction with cancer cells, as shown in Figure 7b. PCs NPs 0.2 showed moderate reflectance intensity, with a spectral peak intensity ratio (Normal/MCF7) of approximately 1.5. This reflects a reasonable level of differentiation between normal and cancerous cells, as shown in Figure 7c. The lower concentration reduces the likelihood of nanoparticle aggregation, maintaining uniform optical properties and ensuring stable interaction with cell surfaces. Additionally, this concentration may minimize potential interference from excessive nanoparticle density, which can sometimes mask subtle optical differences between cell types. In contrast, PCs NPs at 0.5 concentration demonstrated a significantly higher spectral peak intensity ratio (approximately 3), as shown in Figure 7d. This suggests enhanced reflectance contrast and improved differentiation between normal and cancer cells. Higher concentration likely increases the interaction between the nanoparticles and cellular components, leading to more pronounced optical changes. However, the increased nanoparticle density at this concentration may introduce potential challenges, such as aggregation or altered optical properties. These effects could reduce the uniformity of reflectance signals and potentially compromise the specificity of the measurements in certain conditions. Overall, while PCs NPs at 0.5 concentration provided superior reflectance contrast and differentiation, the potential for aggregation and altered optical behavior must be carefully considered. PCs NPs at 0.2 concentration, although offering lower contrast, ensured greater stability and specificity under the experimental conditions. This highlights the importance of optimizing nanoparticle concentration to balance sensitivity, specificity, and practical applicability in diffuse reflectance-based diagnostics.
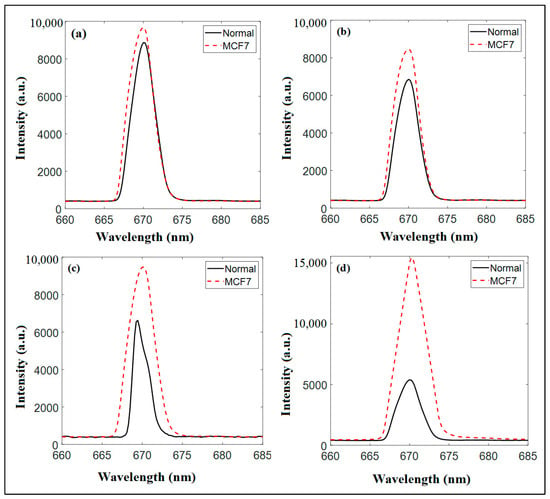
Figure 7.
The diffuse reflectance measurements at a wavelength of 670 nm and at half of the IC50 for (a) Cs NPs., (b) pomegranate extract, (c) PCs NPs 0.2, and (d) PCS NPS 0.5.
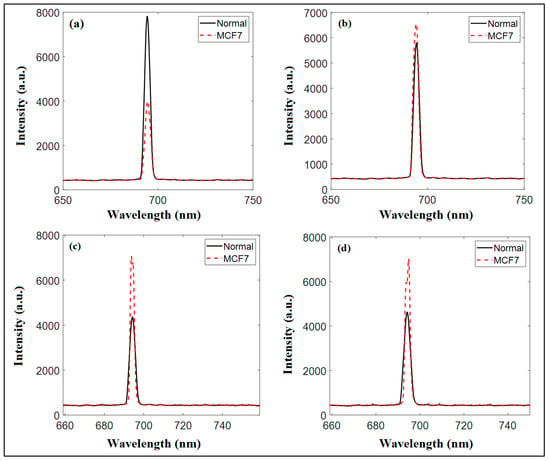
Figure 8.
The diffuse reflectance measurements at a wavelength of 700 nm and at half of the IC50 for (a) Cs NPs, (b) pomegranate extract, (c) PCs NPs 0.2, and (d) PCS NPS 0.5.
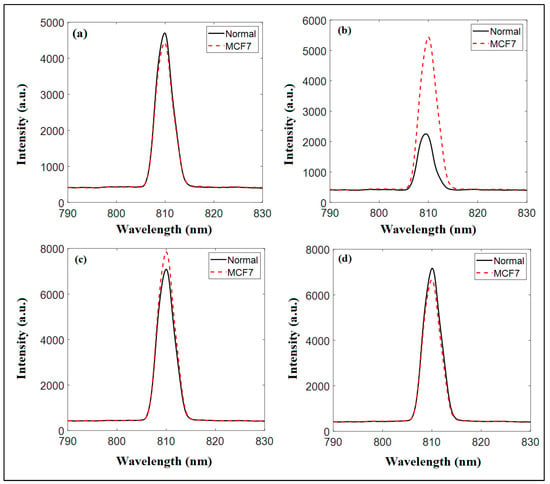
Figure 9.
The diffuse reflectance measurements at a wavelength of 808 nm and at half of the IC50 for (a) Cs NPs, (b) pomegranate extract, (c) PCs NPs 0.2, and (d) PCS NPS 0.5.
Figure 8 illustrates the diffuse reflectance spectra at a wavelength of 700 nm, highlighting the measurements for the normal and cancer cell lines after being treated with pomegranate extracts and the synthesized nanoparticles (Cs NPs, PCs NPs 0.2, and PCs NPs 0.5). For chitosan nanoparticles (Cs NPs), the spectral peak for the normal cells is approximately one fold stronger than that of the MCF-7 cells, indicating moderate differentiation and some level of specificity for cancer cells at this wavelength, as shown in Figure 8a. Pomegranate extract demonstrates a slightly higher reflectance for MCF7 cells compared to normal cells, suggesting some interaction potential with cancer cells, though the distinction remains modest, as shown in Figure 8b. Significant improvement is observed with PCs NPs 0.2, where a clear separation in peaks between normal and MCF7 cells is evident. The enhanced sensitivity and differentiation at this concentration highlight its diagnostic promise, as shown in Figure 8c. At a higher content of pomegranate extract, PCS NPS 0.5, as shown in Figure 8d, differentiation remains significantly similar to PCS NPS 0.2, likely due to broader peaks caused by aggregation or saturation effects, which compromise specificity. The comparative analysis underscores that PCs NPs at a concentration of 0.2 exhibit the best differentiation ability at 700 nm, surpassing the effectiveness of Cs NPs and pomegranate extract alone.
Figure 9 presents the diffuse reflectance spectra at 808 nm for both the normal and MCF7 cancer cells, showcasing intensity variations and peak shifts that reveal the interactions between nanoparticles and cells. Chitosan nanoparticles exhibit overlapping spectra for normal and cancer cells, indicating minimal differentiation and limited specificity, as shown in Figure 9a. Pomegranate extract shows a slight difference in intensity, with the MCF7 cells having a marginally higher reflectance peak, suggesting some interaction but modest discrimination, as shown in Figure 9b. In contrast, PCs NPs 0.2 display significantly higher reflectance intensity for MCF7 cells compared to normal cells, with a clear peak shift, highlighting improved sensitivity and strong differentiation potential, as shown in Figure 9c. However, at a higher content of pomegranate (PCs NPs 0.5), the spectra nearly converge, showing increased intensity but reduced differentiation due to possible saturation or aggregation effects, as shown in Figure 9d.
After a thorough analysis, it can be concluded that PCs NPs at a content of 5 g/L of pomegranate (PCs NPs 0.5) demonstrated the greatest capability to differentiate between the normal and cancer cells, surpassing standalone chitosan NPs and pomegranate extract. These findings emphasize the potential of pomegranate-loaded chitosan nanoparticles as effective diagnostic agents at an optimal pomegranate content of 5 g/L, with further studies needed to optimize their properties for enhanced accuracy.
When light interacts with biological materials such as cells and tissues, it undergoes absorption and scattering. Absorption occurs when photons are absorbed by sample components, such as chromophores or pigments, resulting in a decrease in the intensity of reflected light. Scattering, on the other hand, occurs when photons deviate from their original path due to interactions with cellular structures and components, causing changes in the direction of light propagation. In this study, laser wavelengths within the red to near-infrared (NIR) spectral range were utilized to probe the samples. By measuring light reflectance at different laser wavelengths, the mechanism of the interaction between the samples and light at specific frequencies were analyzed. Diffuse reflectance analysis revealed the distinct absorption and scattering characteristics exhibited by malignant and normal cell lines after the introduction of nanoparticles. Several factors influence the optical properties of cells and tissues, including structural composition, cellular shape, and biochemical composition. It is important to note that these optical properties, such as absorption and scattering, are dependent on the wavelength of the laser used. Consequently, certain wavelengths provided more significant insights for discrimination purposes than others [17,65,66].
When PCs NPs were introduced, the malignant cells had a greater intensity reflectance peak at 670 nm than the normal cell line. This shows that malignant cells absorbed more light at this wavelength, indicating the existence of particular chromophores or biomolecules associated with carcinogenic processes. Malignant cells frequently exhibit different scattering properties than normal cells, owing to the changes in cellular shape and structural composition. In the present investigation, lower reflectance in malignant cells with Cs NPs using a 700 nm incident laser was detected. This shows that light scattering in cancerous cells was reduced at this specific wavelength, indicating alterations in the cellular architecture or scattering interactions within the malignant cells. Accordingly, the synthesized pomegranate-loaded Cs NPs were used as contrast agents in the present study and had an impact on the way that cells absorb and scatter light. Cs NPs and PCs NPs can provide more absorbent components to the cellular context. These nanoparticles have distinct surface properties and can transport a variety of functional groups that interact with cellular structures or biomolecules, potentially affecting absorption characteristics. When these nanoparticles enter cells, they interact with chromophores or pigments that are natively present [67]. These interactions can improve or change the absorption of light within the cells. The chemical characteristics of Cs NPs and PCs NPs, such as surface charge, functional groups, and composition, influence their affinity and binding interactions with cellular components, thereby affecting absorption characteristics. The inclusion of nanoparticles can also alter the refractive index or size distribution of cellular components, causing variations in scattering behavior. Nanoparticles can interact with cellular membranes, organelles, and other subcellular structures, altering their scattering properties and resulting in different light scattering patterns. Additionally, the injection of nanoparticles can alter cellular shape or structure. Changes in cellular shape, size, or refractive index can all have an impact on light scattering [68]. Moreover, the introduction of nanoparticles can modify the local cellular milieu, causing changes in pH, ionic concentration, or molecular crowding. These modifications can have an indirect impact on the quality of cell absorption by affecting chromophores’ behavior or their surroundings. It is important to highlight that cell absorption and scattering properties are complicated and can change depending on several factors, including cancer type and stage, cellular heterogeneity, and microenvironmental factors.
While this study primarily explores the mechanisms by which nanoparticles influence light absorption and scattering in cells, as well as the role of pomegranate extract in improving differentiation, from a theoretical standpoint without direct experimental validation, the discussion draws upon the established principles of light–tissue interaction and the previous research on the optical behavior of nanoparticles and pomegranate compounds. Chitosan nanoparticles have been proposed to influence light scattering and absorption due to their size, surface charge, and interactions with cellular structures, potentially altering the refractive index contrast and enhancing diffuse reflectance signals [69]. Pomegranate extract is rich in bioactive components such as polyphenols, and it may interact with cell membranes and intracellular components thus modifying their biochemical properties and enhancing the optical contrast between normal and cancerous cells when it is synthesized in nanoform [61,62,70]. Consequently, further research using advanced methods like quantitative light scattering analysis or spectroscopy is essential to provide direct evidence for these mechanisms.
Table 3 shows a comparison between the different nanoparticle contrast agents using imaging modality. Gold nanoparticles (AuNPs) and gold nanorods show high stability and effectiveness in imaging modalities like diffuse reflectance, Raman spectroscopy, and photoacoustic imaging, but they pose kidney toxicity risks. Pomegranate and ashwagandha-loaded chitosan nanoparticles offer a low-cost, non-toxic alternative for cancer detection.

Table 3.
Comparison between Difference Contrast Agent using Difference Imaging Modality (IM).
The current study focuses on in vitro experiments to assess the optical and cytotoxic properties of the proposed nanoparticles. While such studies have limitations on fully replicating the complex biological environment found in living systems, the observed optical characteristics and selective cytotoxicity provide a solid foundation for future research. However, to bridge the gap between in vitro findings and real-world clinical applications, it is recommended that future work incorporate animal model studies. These studies should explore aspects such as biodistribution, clearance, and long-term effects of the nanoparticles, which are crucial for assessing their practical application as contrast agents.
4. Conclusions
The current in vitro study explored the potential use of pomegranate extract, chitosan nanoparticles, and pomegranate-loaded chitosan nanoparticles as contrast agents to enhance breast cancer detection. The proposed nanomaterials were successfully synthesized, and their morphology and stability were thoroughly evaluated, confirming their suitability for medical applications. By leveraging the distinctive diffuse reflectance characteristics at selected laser wavelengths, the present work aimed to improve the sensitivity and specificity of breast cancer detection methods. The present experimental results demonstrated that the incorporation of nanoparticles significantly enhanced cancer diagnosis, particularly when irradiated at 670 nm. The measured light reflectance of cancerous and normal cells provided valuable measurements for distinguishing malignant tissues from healthy ones. Pomegranate-loaded chitosan NPs at content of pomegranate of 5 g/L (PCs NPs 0.5) consistently provided the best differentiation at 670 nm. By utilizing the unique characteristics of chitosan-based nanoparticles, such as their stability and increased diameter when loaded with pomegranate, this work paves the way for potential advancements in breast cancer detection. These nanoparticles show promise as contrast agents for improving the accuracy and reliability of diagnostic techniques. Further in vivo studies are necessary to validate the clinical potential of chitosan and pomegranate-loaded chitosan nanoparticles in breast cancer detection. These findings contribute to the growing body of knowledge in this field and provide a foundation for future research aimed at optimizing and translating nanoparticle-based approaches into clinical practice for improved breast cancer management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemosensors13020073/s1, Figure S1: Dynamic light scattering (DLS) distribution measurements for (A) Cs NPs, (B) pomegranate extract, (C) PCs NPs 0.2 and (D) PCs NPs 0.5; Figure S2: Zeta potential distribution measurements for (A) Cs NPs, (B) pomegranate extract, (C) PCs NPs 0.2 and (D) PCs NPs 0.5.
Author Contributions
H.S.A.: Supervision, Data Analysis and Resources, Project Administration, Methodology, and Software; M.A.S.A.: Conceptualization, Methodology, Characterization, Formal Analysis, Supervision, Writing—Original Draft Preparation, Writing—Review and Editing; S.H.A.: Nanoparticle Synthesis, Formal Analysis, Writing—Review and Editing; S.A.: Characterization, Writing—Original Draft Preparation; M.S.A.M.: Conceptualization, Writing—Original Draft Preparation, Writing—Review and Editing; O.H.: Conceptualization, Supervision, Methodology, Writing—Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
The authors thank Mayar Khaleed and Mina Emad for their assistance during the synthesis of the nanoparticles.
Conflicts of Interest
All the authors declare that there are no relevant financial or non-financial competing interests associated with the present research.
References
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E. Cancer-related fatigue—Mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Jiang, G.; Hu, X.; Yang, D.; Tan, T.; Gao, Z.; Chen, Z.; Xiang, C.; Li, S.; Ouyang, Z.; et al. Punicalin Attenuates Breast Cancer-Associated Osteolysis by Inhibiting the NF-κB Signaling Pathway of Osteoclasts. Front. Pharmacol. 2021, 12, 789552. [Google Scholar] [CrossRef] [PubMed]
- Barba, D.; León-Sosa, A.; Lugo, P.; Suquillo, D.; Torres, F.; Surre, F.; Trojman, L.; Caicedo, A. Breast cancer, screening and diagnostic tools: All you need to know. Crit. Rev. Oncol. Hematol. 2021, 157, 103174. [Google Scholar] [CrossRef]
- Sturgeon, S.R.; Ronnenberg, A.G. Pomegranate and breast cancer: Possible mechanisms of prevention. Nutr. Rev. 2010, 68, 122–128. [Google Scholar] [CrossRef]
- Naser, Z.; Weli, S.; Ramadhan, S. In Silico Molecular Classification of Breast and Prostate Cancers using Back Propagation Neural Network. Cancer Biol. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Champeau, M.; Vignoud, S.; Mortier, L.; Mordon, S. Photodynamic therapy for skin cancer: How to enhance drug penetration? J. Photochem. Photobiol. B 2019, 197, 111544. [Google Scholar] [CrossRef]
- Shetty, M.K. Screening for Breast Cancer with Mammography: Current Status and An Overview. Indian J. Surg. Oncol. 2010, 1, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Alabd, O.L.; Alwarraky, M.S.; Taei, D.M.; Eid, B.; Gomaa, M.E. Correlation between ultrasound-guided percutaneous breast biopsy and diffusion-weighted magnetic resonance imaging of the breast for evaluation of solid breast lesions. Egypt. J. Radiol. Nucl. Med. 2020, 51, 106. [Google Scholar] [CrossRef]
- Carneiro, G.d.A.C.; Pereira, F.P.A.; Lopes, F.P.P.L.; Calas, M.J.G. Magnetic resonance imaging-guided vacuum-assisted breast biopsy: Experience and preliminary results of 205 procedures. Radiol. Bras. 2018, 51, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Bick, U.; Trimboli, R.M.; Athanasiou, A.; Balleyguier, C.; Baltzer, P.A.T.; Bernathova, M.; Borbély, K.; Brkljacic, B.; Carbonaro, L.A.; Clauser, P.; et al. Image-guided breast biopsy and localisation: Recommendations for information to women and referring physicians by the European Society of Breast Imaging. Insights Imaging 2020, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zou, L.; Geng, X.; Zheng, S. Limitations of mammography in the diagnosis of breast diseases compared with ultrasonography: A single-center retrospective analysis of 274 cases. Eur. J. Med. Res. 2015, 20, 49. [Google Scholar] [CrossRef] [PubMed]
- Oglat, A.A.; Abukhalil, T. Ultrasound Elastography: Methods, Clinical Applications, and Limitations: A Review Article. Appl. Sci. 2024, 14, 4308. [Google Scholar] [CrossRef]
- Camps-Herrero, J.; Pijnappel, R.; Balleyguier, C. MR-contrast enhanced mammography (CEM) for follow-up of breast cancer patients: A “pros and cons” debate. Eur. Radiol. 2024, 34, 6264–6270. [Google Scholar] [CrossRef] [PubMed]
- Stibbards-Lyle, M.; Malinovska, J.; Badawy, S.; Schedin, P.; Rinker, K.D. Status of breast cancer detection in young women and potential of liquid biopsy. Front. Oncol. 2024, 14, 1398196. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Mitbander, R.; Tang, Y.; Azimuddin, A.; Carns, J.; Schwarz, R.A.; Richards-Kortum, R.R. Optical imaging technologies for in vivo cancer detection in low-resource settings. Curr. Opin. Biomed. Eng. 2023, 28, 100495. [Google Scholar] [CrossRef] [PubMed]
- Ottobrini, L.; Martelli, C.; Lucignani, G. Optical Imaging Agents. In Molecular Imaging: Principles and Practice; Academic Press: Cambridge, MA, USA, 2021; pp. 603–625. [Google Scholar] [CrossRef]
- Cho, N.; Shokeen, M. Changing landscape of optical imaging in skeletal metastases. J. Bone Oncol. 2019, 17, 100249. [Google Scholar] [CrossRef]
- Cressoni, C.; Malandra, S.; Milan, E.; Boschi, F.; Nicolato, E.; Negri, A.; Veccia, A.; Bontempi, P.; Mangiameli, D.; Pietrobono, S.; et al. Injectable Thermogelling Nanostructured Ink as Simultaneous Optical and Magnetic Resonance Imaging Contrast Agent for Image-Guided Surgery. Biomacromolecules 2024, 25, 3741–3755. [Google Scholar] [CrossRef]
- Abdel Halim, A.S.; Abdel-Salam, Z.; Abdel-Harith, M.; Hamdy, O. Investigating the effect of changing the substrate material analyzed by laser-induced breakdown spectroscopy on the antenna performance. Sci. Rep. 2024, 14, 1964. [Google Scholar] [CrossRef] [PubMed]
- Szymaszek, P.; Tyszka-Czochara, M.; Ortyl, J. Application of Photoactive Compounds in Cancer Theranostics: Review on Recent Trends from Photoactive Chemistry to Artificial Intelligence. Molecules 2024, 29, 3164. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, H.; Tang, C.; Cheng, Y.; Cheng, L. Exploration of Ultrasound-Sensitive Biomaterials in Cancer Theranostics. Adv. Funct. Mater. 2024, 34, 2313454. [Google Scholar] [CrossRef]
- Semenov, K.N.; Shemchuk, O.S.; Ageev, S.V.; Andoskin, P.A.; Iurev, G.O.; Murin, I.V.; Kozhukhov, P.K.; Maystrenko, D.N.; Molchanov, O.E.; Kholmurodova, D.K.; et al. Development of Graphene-Based Materials with the Targeted Action for Cancer Theranostics. Biochemistry 2024, 89, 1362–1391. [Google Scholar] [CrossRef] [PubMed]
- Iyad, N.; S.Ahmad, M.; Alkhatib, S.G.; Hjouj, M. Gadolinium contrast agents- challenges and opportunities of a multidisciplinary approach: Literature review. Eur. J. Radiol. Open 2023, 11, 100503. [Google Scholar] [CrossRef]
- Sun, J.-X.; Xu, J.-Z.; An, Y.; Ma, S.-Y.; Liu, C.-Q.; Zhang, S.-H.; Luan, Y.; Wang, S.-G.; Xia, Q.-D. Future in precise surgery: Fluorescence-guided surgery using EVs derived fluorescence contrast agent. J. Control. Release 2023, 353, 832–841. [Google Scholar] [CrossRef]
- Korposh, S.; Lee, S.-W. A Preliminary Study for Tunable Optical Assessment of Exhaled Breath Ammonia Based on Ultrathin Tetrakis(4-sulfophenyl)porphine Nanoassembled Films. Chemosensors 2021, 9, 269. [Google Scholar] [CrossRef]
- Faid, A.H.; Hussein, F.E.Z.; Mostafa, E.M.; Shouman, S.A.; Badr, Y.A.; Sliem, M.A. Hybrid chitosan gold nanoparticles for photothermal therapy and enhanced cytotoxic action of 6-mercaptopurine on breast cancer cell line. Beni Suef Univ. J. Basic Appl. Sci. 2023, 12, 83. [Google Scholar] [CrossRef]
- Wei, Y.; Zhu, Y.Y.; Wang, M.L. Surface-enhanced Raman spectroscopy of gastric cancer serum with gold nanoparticles/silicon nanowire arrays. Optik 2016, 127, 7902–7907. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Gao, X.; Chen, Y.; Liu, T. Nanotechnology in cancer diagnosis: Progress, challenges and opportunities. J. Hematol. Oncol. 2019, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, R.Y.; Cakir, R. In vitro anticancer efficacy of Calendula Officinalis extract-loaded chitosan nanoparticles against gastric and colon cancer cells. Drug Dev. Ind. Pharm. 2024, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Poonia, M.; Ramalingam, K.; Goyal, S.; Sidhu, S. Nanotechnology in oral cancer: A comprehensive review. J. Oral Maxillofac. Pathol. 2017, 21, 407. [Google Scholar] [CrossRef] [PubMed]
- Steinhauer, S. Gas Sensors Based on Copper Oxide Nanomaterials: A Review. Chemosensors 2021, 9, 51. [Google Scholar] [CrossRef]
- Leibl, N.; Haupt, K.; Gonzato, C.; Duma, L. Molecularly Imprinted Polymers for Chemical Sensing: A Tutorial Review. Chemosensors 2021, 9, 123. [Google Scholar] [CrossRef]
- Cohen, S.; Pellach, M.; Kam, Y.; Grinberg, I.; Corem-Salkmon, E.; Rubinstein, A.; Margel, S. Synthesis and characterization of near IR fluorescent albumin nanoparticles for optical detection of colon cancer. Mater. Sci. Eng. C 2013, 33, 923–931. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Butnariu, M.; Rotariu, L.S.; Sytar, O.; Sestito, S.; Rapposelli, S.; Akram, M.; Iqbal, M.; Krishna, A.; et al. Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell Int. 2021, 21, 318. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, J.; Raichura, Z.; Khan, T.; Momin, M.; Omri, A. Chitosan nanoparticles-insight into properties, functionalization and applications in drug delivery and theranostics. Molecules 2021, 26, 272. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, F.; Qiu, H.; Liu, J.; Qin, S.; Hou, Y.; Wang, C. Preparation and Characterization of Chitosan Nanoparticles for Chemotherapy of Melanoma Through Enhancing Tumor Penetration. Front. Pharmacol. 2020, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- Salem, D.S.; Sliem, M.A.; El-Sesy, M.; Shouman, S.A.; Badr, Y. Improved chemo-photothermal therapy of hepatocellular carcinoma using chitosan-coated gold nanoparticles. J. Photochem. Photobiol. B 2018, 182, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Marpu, S.B.; Benton, E.N. Shining light on chitosan: A review on the usage of chitosan for photonics and nanomaterials research. Int. J. Mol. Sci. 2018, 19, 1795. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Fontana, F.; Tapeinos, C.; Shahbazi, M.-A.; Han, H.; Santos, H.A. Nanoparticles-based phototherapy systems for cancer treatment: Current status and clinical potential. Bioact. Mater. 2023, 23, 471–507. [Google Scholar] [CrossRef]
- Youssef, F.S.; Mohamed, G.G.; Ismail, S.H.; Elzorba, H.Y.; Galal, A.M.; Elbanna, H.A. Synthesis, characterization and in vitro antimicrobial activity of florfenicol-chitosan nanocomposite. Egypt. J. Chem. 2021, 64, 941–948. [Google Scholar] [CrossRef]
- Taherian, A.; Esfandiari, N.; Rouhani, S. Breast cancer drug delivery by novel drug-loaded chitosan-coated magnetic nanoparticles. Cancer Nanotechnol. 2021, 12, 15. [Google Scholar] [CrossRef]
- Gao, C.; Zheng, P.; Liu, Q.; Han, S.; Li, D.; Luo, S.; Temple, H.; Xing, C.; Wang, J.; Wei, Y.; et al. Recent Advances of Upconversion Nanomaterials in the Biological Field. Nanomaterials 2021, 11, 2474. [Google Scholar] [CrossRef]
- Park, J.; Lee, Y.-K.; Park, I.-K.; Hwang, S.R. Current Limitations and Recent Progress in Nanomedicine for Clinically Available Photodynamic Therapy. Biomedicines 2021, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- El-Hadary, A.E.; Ramadan, M.F. Phenolic profiles, antihyperglycemic, antihyperlipidemic, and antioxidant properties of pomegranate (Punica granatum) peel extract. J. Food Biochem. 2019, 43, e12803. [Google Scholar] [CrossRef]
- Prakash, C.V.S.; Prakash, I. Bioactive chemical constituents from pomegranate (Punica granatum) juice, seed and peel—A review. Int. J. Res. Chem. Environ. 2011, 1, 1–18. [Google Scholar]
- Machado, T.D.; Leal, I.C.; Amaral, A.C.; Santos, K.; Silva, M.G.; Kuster, R.M. Antimicrobial ellagitannin of Punica granatum fruits. J. Braz. Chem. Soc. 2002, 13, 606–610. [Google Scholar] [CrossRef]
- Eroglu Ozkan, E.; Seyhan, M.F.; Kurt Sirin, O.; Yilmaz-Ozden, T.; Ersoy, E.; Hatipoglu Cakmar, S.D.; Goren, A.C.; Yilmaz Aydogan, H.; Ozturk, O. Antiproliferative effects of Turkish pomegranate (Punica granatum L.) extracts on MCF-7 human breast cancer cell lines with focus on antioxidant potential and bioactive compounds analyzed by LC-MS/MS. J. Food Biochem. 2021, 45, e13904. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD-ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef] [PubMed]
- El Newehy, N.M.; Abd-Alhaseeb, M.M.; Omran, G.A.; Harraz, F.M.; Shawky, E. Comparative metabolomics reveal intraspecies variability in bioactive compounds of different cultivars of pomegranate fruit (Punica granatum L.) and their waste by-products. J. Sci. Food Agric. 2022, 102, 5891–5902. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, H.R.; Arastoo, M.; Ostad, S.N. A comprehensive review of Punica granatum (Pomegranate) properties in toxicological, pharmacological, cellular and molecular biology researches. Iran J. Pharm. Res. 2012, 11, 385–400. [Google Scholar] [PubMed]
- Jain, V.; Murugananthan, G.; Deepak, M.; Viswanatha, G.L.; Manohar, D. Isolation and standardization of various phytochemical constituents from methanolic extracts of fruit rinds of Punica granatum. Chin. J. Nat. Med. 2011, 9, 414–420. [Google Scholar] [CrossRef]
- Yu, M.; Gouvinhas, I.; Chen, J.; Zhu, Y.; Deng, J.; Xiang, Z.; Oliveira, P.; Xia, C.; Barros, A. Unlocking the therapeutic treasure of pomegranate leaf: A comprehensive review on phytochemical compounds, health benefits, and future prospects. Food Chem. X 2024, 23, 101587. [Google Scholar] [CrossRef] [PubMed]
- Monika, P.; Chandraprabha, M.N.; Hari Krishna, R.; Vittal, M.; Likhitha, C.; Pooja, N.; Chaudhary, V. Recent advances in pomegranate peel extract mediated nanoparticles for clinical and biomedical applications. Biotechnol. Genet. Eng. Rev. 2024, 40, 3379–3407. [Google Scholar] [CrossRef]
- Pantiora, P.D.; Balaouras, A.I.; Mina, I.K.; Freris, C.I.; Pappas, A.C.; Danezis, G.P.; Zoidis, E.; Georgiou, C.A. The Therapeutic Alliance between Pomegranate and Health Emphasizing on Anticancer Properties. Antioxidants 2023, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; McClees, S.; Afaq, F. Pomegranate for Prevention and Treatment of Cancer: An Update. Molecules 2017, 22, 177. [Google Scholar] [CrossRef]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Souza, T.G.F.; Ciminelli, V.S.T.; Mohallem, N.D.S. A comparison of TEM and DLS methods to characterize size distribution of ceramic nanoparticles. J. Phys. Conf. Ser. 2016, 733, 012039. [Google Scholar] [CrossRef]
- Magangana, T.P.; Makunga, N.P.; Fawole, O.A.; Stander, M.A.; Opara, U.L. Antioxidant, Antimicrobial, and Metabolomic Characterization of Blanched Pomegranate Peel Extracts: Effect of Cultivar. Molecules 2022, 27, 2979. [Google Scholar] [CrossRef]
- Yang, P.; Zhou, X.; Zhang, J.; Zhong, J.; Zhu, F.; Liu, X.; Gu, Z.; Li, Y. Natural polyphenol fluorescent polymer dots. Green Chem. 2021, 23, 1834–1839. [Google Scholar] [CrossRef]
- Cao, L.; Yu, H.; Shao, S.; Wang, S.; Guo, Y. Evaluating the antioxidant capacity of polyphenols with an off–on fluorescence probe and the mechanism study. Anal. Methods 2014, 6, 7149–7153. [Google Scholar] [CrossRef]
- Abuelmakarem, H.S.; Hamdy, O.; Sliem, M.A.; El-Azab, J.; Ahmed, W.A. Early cancer detection using the fluorescent Ashwagandha chitosan nanoparticles combined with near-infrared light diffusion characterization: In vitro study. Lasers Med. Sci. 2023, 38, 37. [Google Scholar] [CrossRef]
- Abuelmakarem, H.; Sliem, M.; El-Azab, J.; Farghaly, M.; Ahmed, W. Toward Highly Efficient Cancer Imaging and Therapy Using the Environment-Friendly Chitosan Nanoparticles and NIR Laser. Biosensors 2019, 9, 28. [Google Scholar] [CrossRef]
- Kim, H.L. Optical imaging in oncology. Urol. Oncol. Semin. Orig. Investig. 2009, 27, 298–300. [Google Scholar] [CrossRef]
- Optical Imaging—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/optical-imaging (accessed on 23 January 2025).
- Aibani, N.; Cuddihy, G.; Wasan, E.K. Chitosan Nanoparticles at the Biological Interface: Implications for Drug Delivery. Pharmaceutics 2021, 13, 1686. [Google Scholar] [CrossRef]
- Kashani, A.S.; Packirisamy, M. Cancer-Nano-Interaction: From Cellular Uptake to Mechanobiological Responses. Int. J. Mol. Sci. 2021, 22, 9587. [Google Scholar] [CrossRef]
- Abuelmakarem, H.S.; Hamdy, O.; Sliem, M.A.; El-Azab, J.; Om-Hashem, M.A.; Ahmed, W.A. Colonic Carcinoma Diagnosis using Chitosan Nanoparticles Based on the Optical Properties. J. Phys. Conf. Ser. 2020, 1472, 012001. [Google Scholar] [CrossRef]
- Das, S.; Sarmah, S.; Singha Roy, A. Monitoring fluorescence emission behaviors of dietary polyphenols in a serum albumin environment. N. J. Chem. 2020, 44, 299–302. [Google Scholar] [CrossRef]
- Rahmani, A.A.; Jia, Q.; Bahti, H.H.; Fauzia, R.P.; Wyantuti, S. Recent advances in lanthanide-based nanoparticle contrast agents for magnetic resonance imaging: Synthesis, characterization, and applications. OpenNano 2025, 21, 100226. [Google Scholar] [CrossRef]
- Hamdy, O.; Nour, M.; Kamel, S.S.; Eltayeb, E.A.; Zaky, A.A.; Faid, A.H. Enhanced laser-induced fluorescence and Raman spectroscopy with gold nanoparticles for the diagnosis of oral squamous cell carcinoma. Discov. Appl. Sci. 2024, 6, 157. [Google Scholar] [CrossRef]
- Nour, M.; Hamdy, O.; Faid, A.H.; Eltayeb, E.A.; Zaky, A.A. Utilization of gold nanoparticles for the detection of squamous cell carcinoma of the tongue based on laser-induced fluorescence and diffuse reflectance characteristics: An in vitro study. Lasers Med. Sci. 2022, 37, 3551–3560. [Google Scholar] [CrossRef] [PubMed]
- Jakic, K.; Selc, M.; Razga, F.; Nemethova, V.; Mazancova, P.; Havel, F.; Sramek, M.; Zarska, M.; Proska, J.; Masanova, V.; et al. Long-Term Accumulation, Biological Effects and Toxicity of BSA-Coated Gold Nanoparticles in the Mouse Liver, Spleen, and Kidneys. Int. J. Nanomed. 2024, 19, 4103–4120. [Google Scholar] [CrossRef]
- Sun, J.-P.; Ren, Y.-T.; Wei, K.; He, M.-J.; Gao, B.-H.; Qi, H. Photoacoustic response optimization of gold nanorods in the near-infrared region. Results Phys. 2022, 34, 105209. [Google Scholar] [CrossRef]
- Reynders, H.; Van Zundert, I.; Silva, R.; Carlier, B.; Deschaume, O.; Bartic, C.; Rocha, S.; Basov, S.; Van Bael, M.J.; Himmelreich, U.; et al. Label-Free Iron Oxide Nanoparticles as Multimodal Contrast Agents in Cells Using Multi-Photon and Magnetic Resonance Imaging. Int. J. Nanomed. 2021, 16, 8375–8389. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).