SnO2-Based CMOS-Integrated Gas Sensor Optimized by Mono-, Bi-, and Trimetallic Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
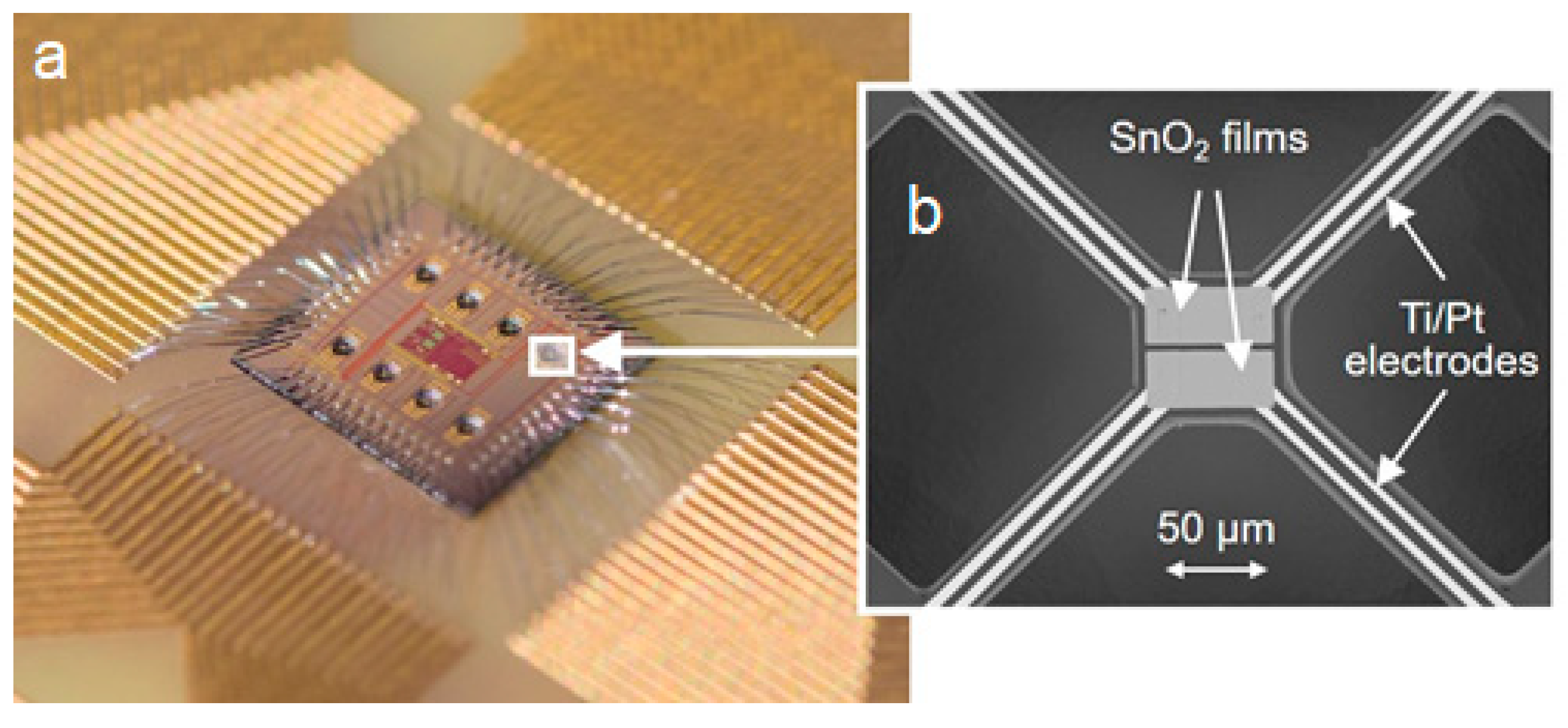
2.1. Fabrication of Sensor Devices
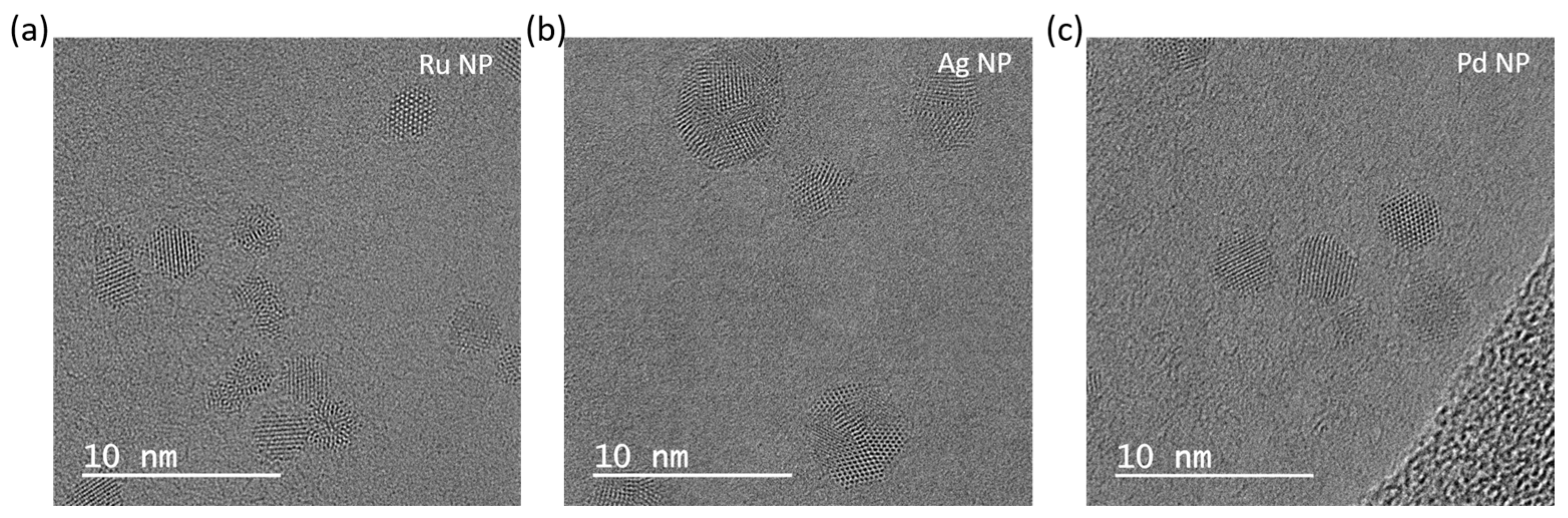
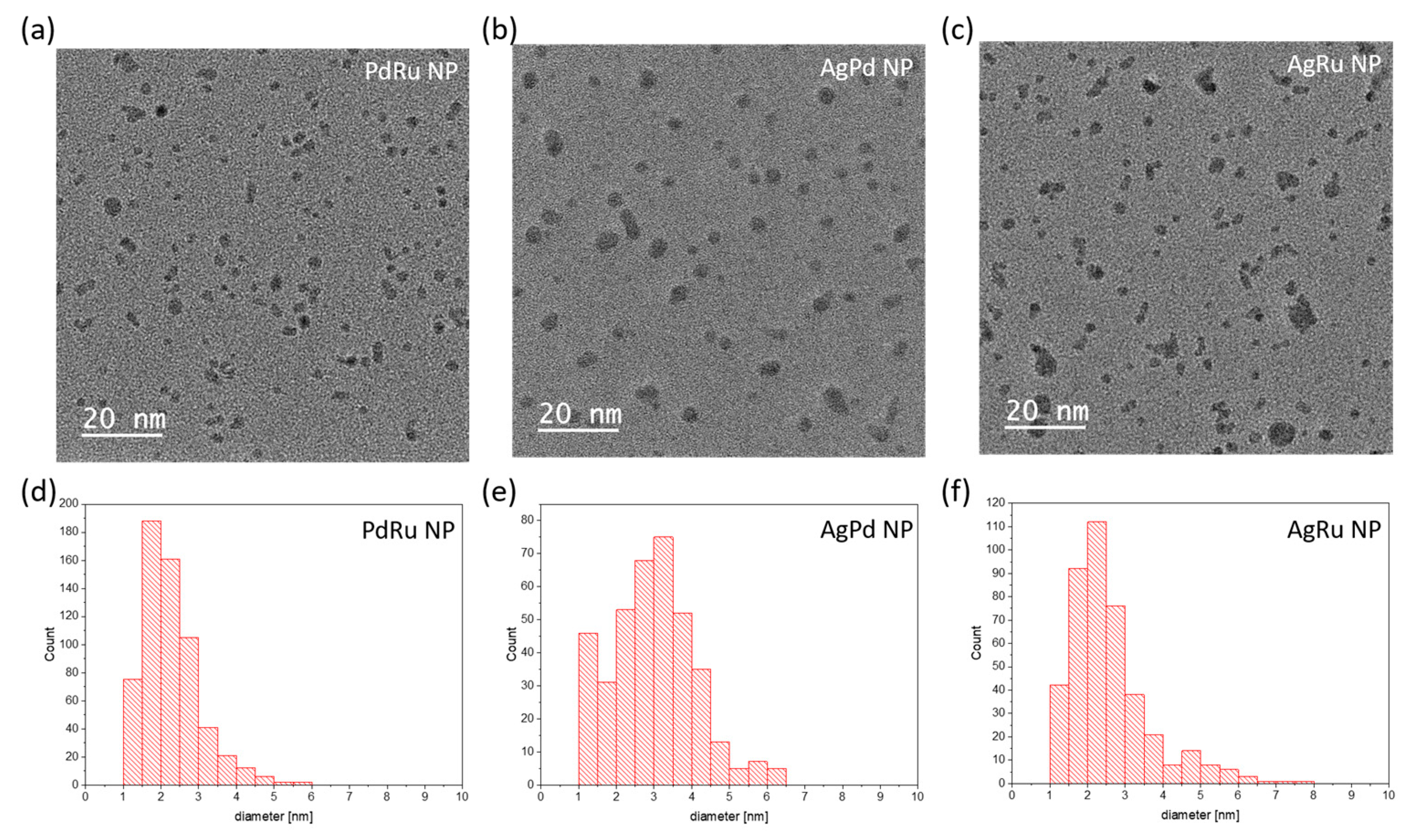
- The size distribution for bimetallic PdRu-NPs, as determined from 613 NPs, is in the range from 1 to 5 nm, with an average size of 2 nm, as shown in Figure 4d;
- The size distribution of bimetallic AgPd-NPs, as determined from 390 NPs, is in the range from 1 to 5 nm, with an average size of 3.5 nm, as shown in Figure 4e;
- The size distribution of bimetallic AgRu-NPs, as determined from 423 NPs, is in the range from 1 to 6 nm, with an average size of 2.5 nm, as shown in Figure 4f;
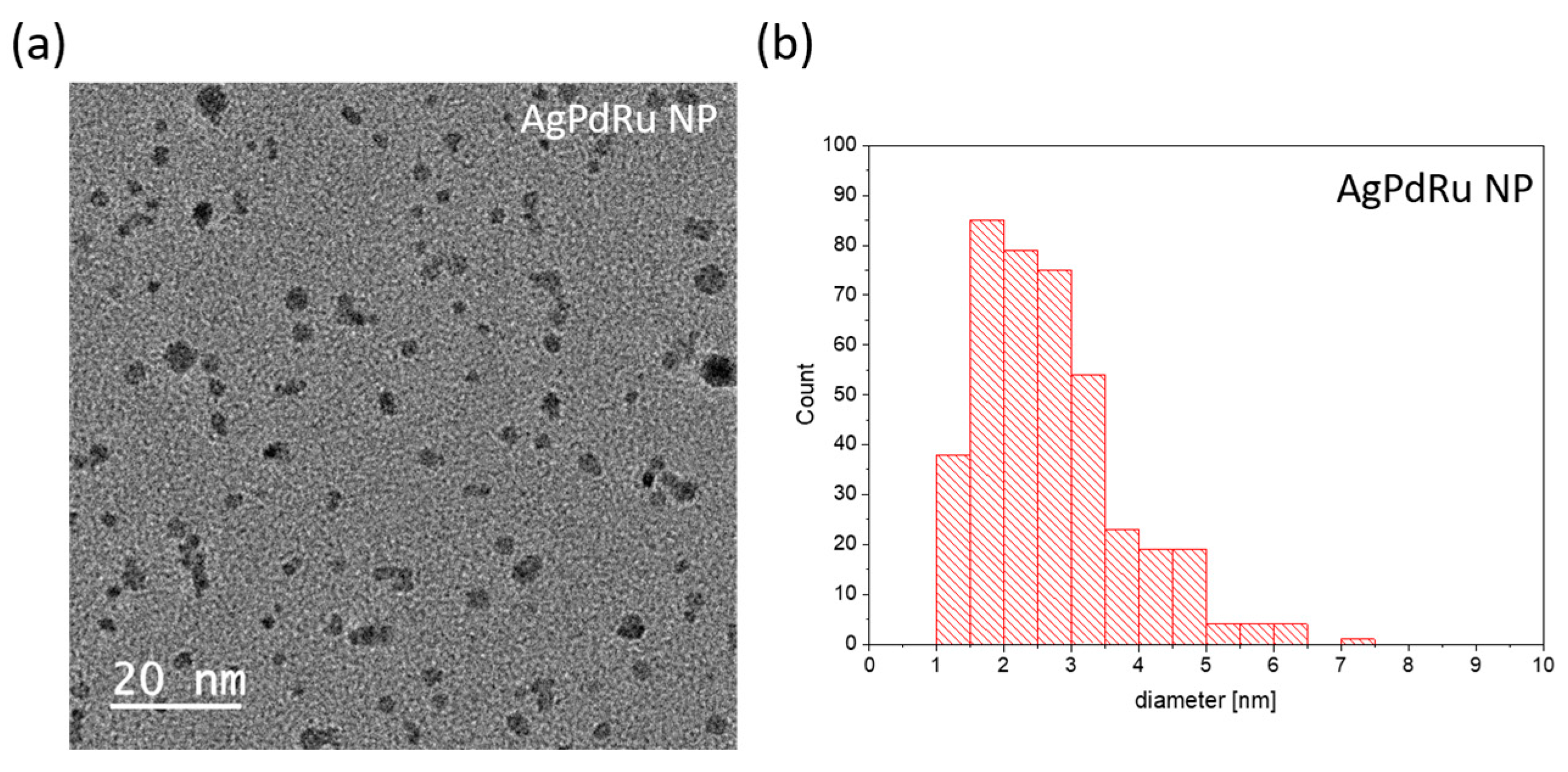
- The size distribution of trimetallic AgRuPd-NPs, as determined from 423 NPs, is in the range from 1 to 6 nm, with an average size of 2.5 nm, as shown in Figure 5b.
2.2. Characterization of the CMOS-Based Sensors
3. Results and Discussion
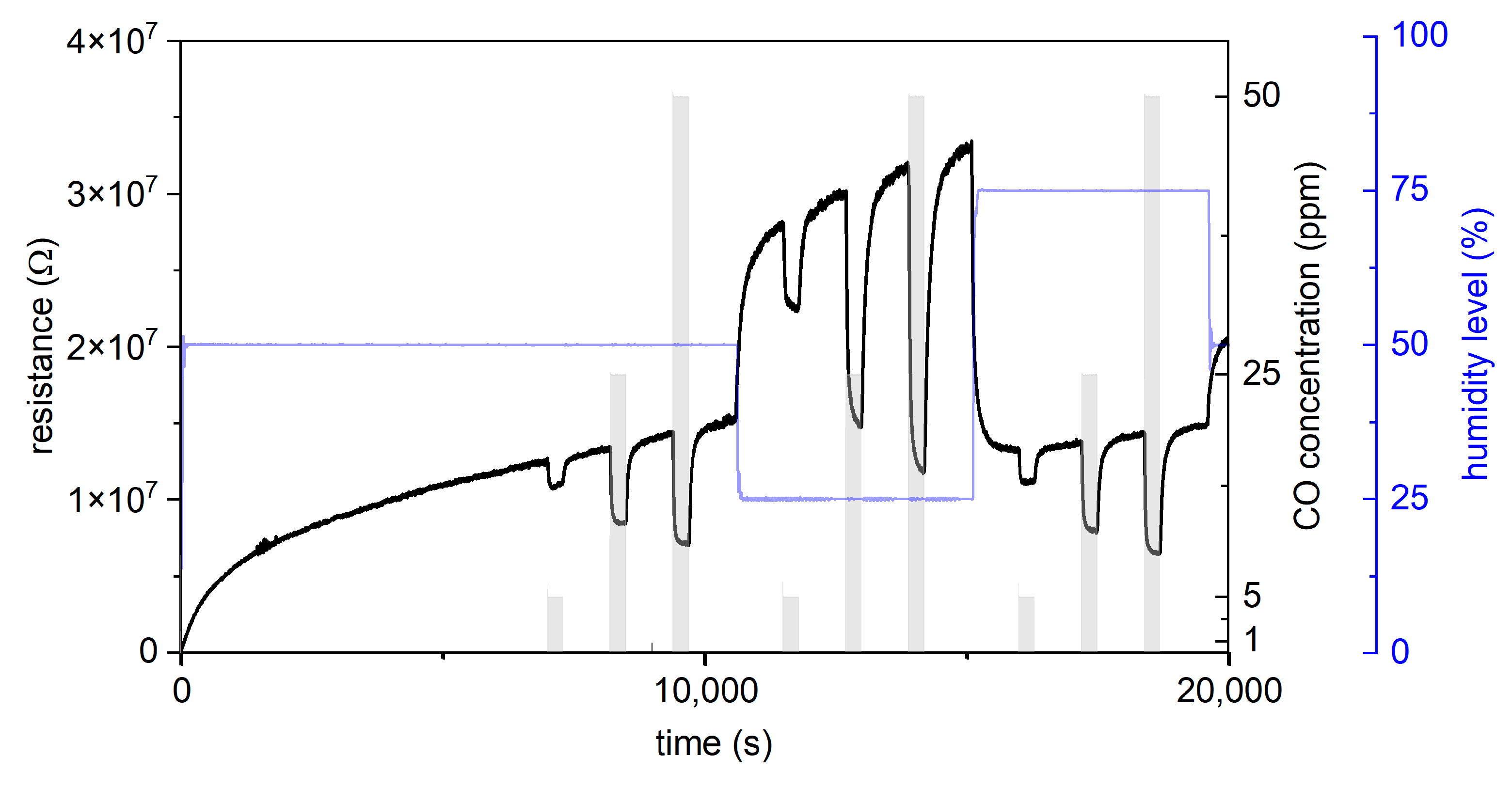
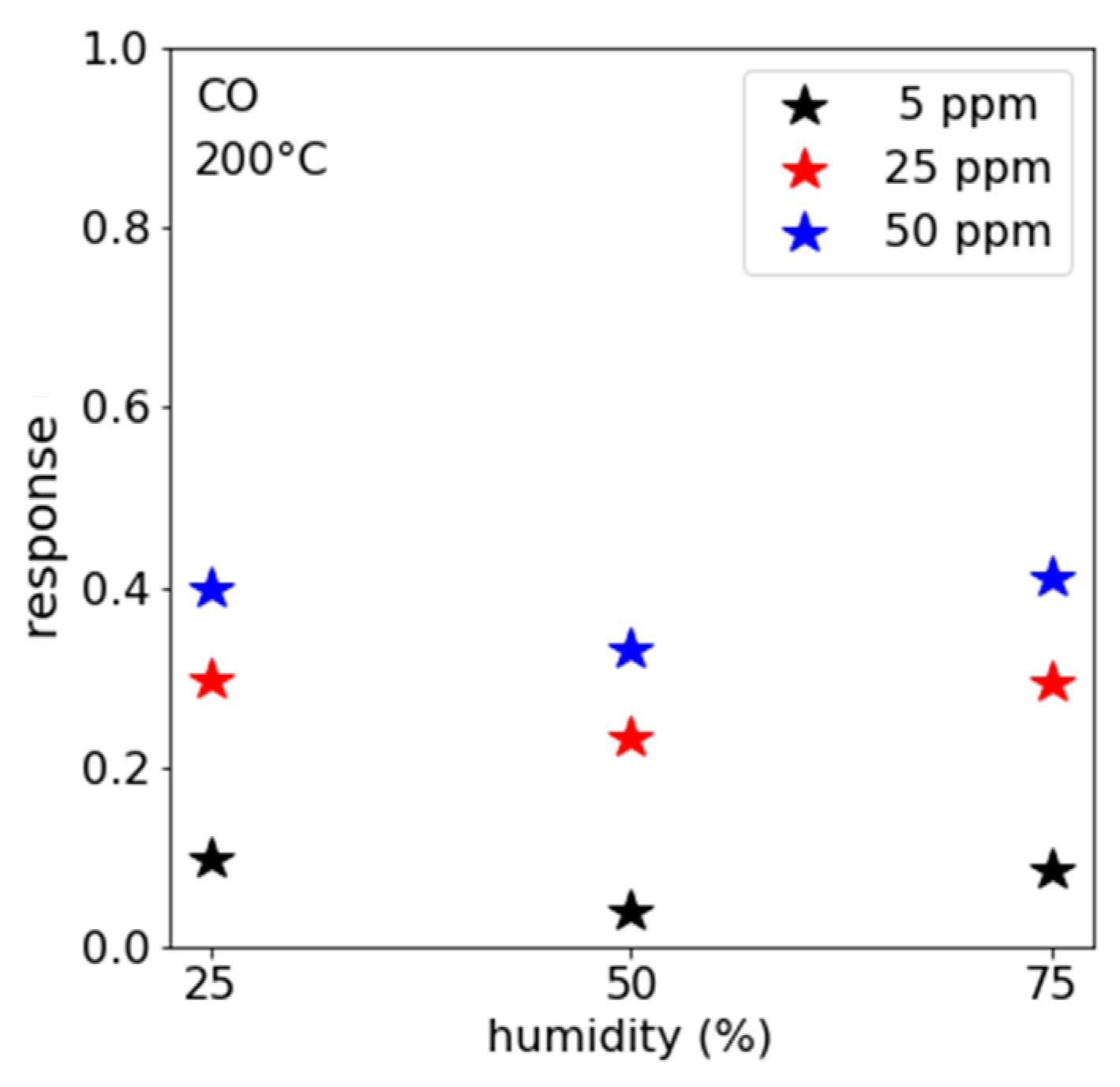
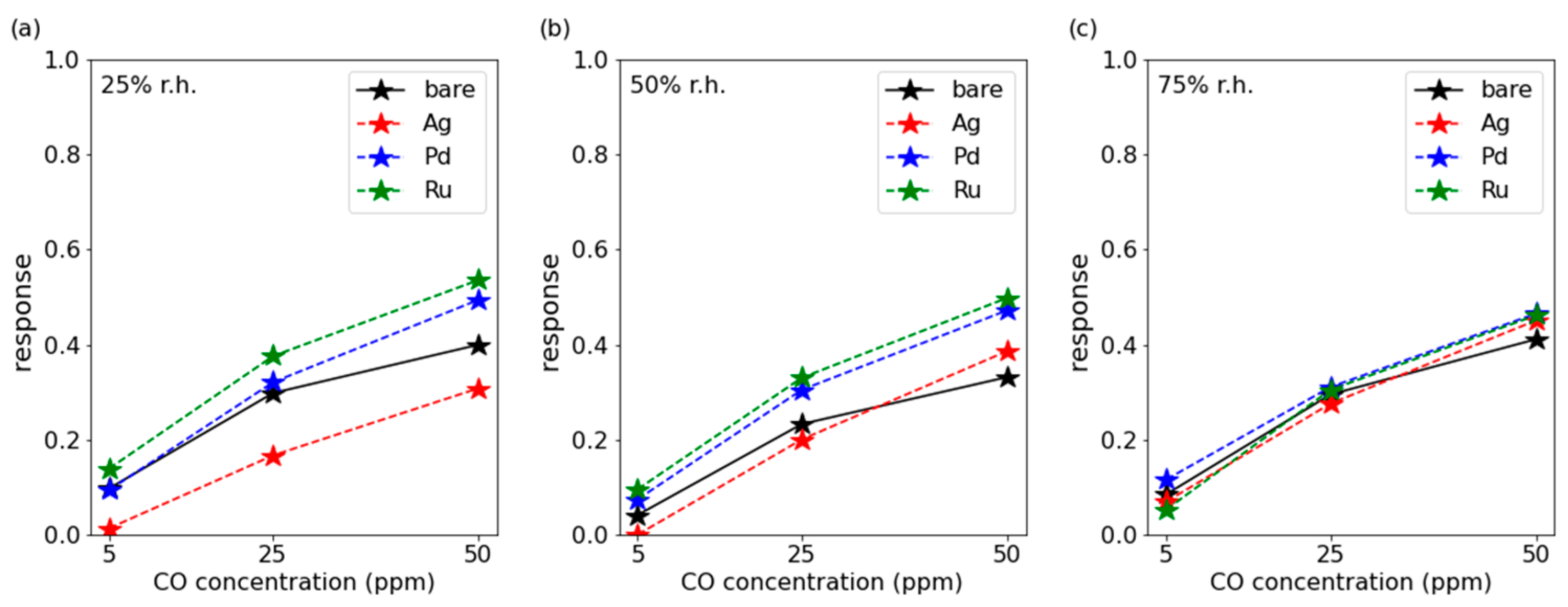
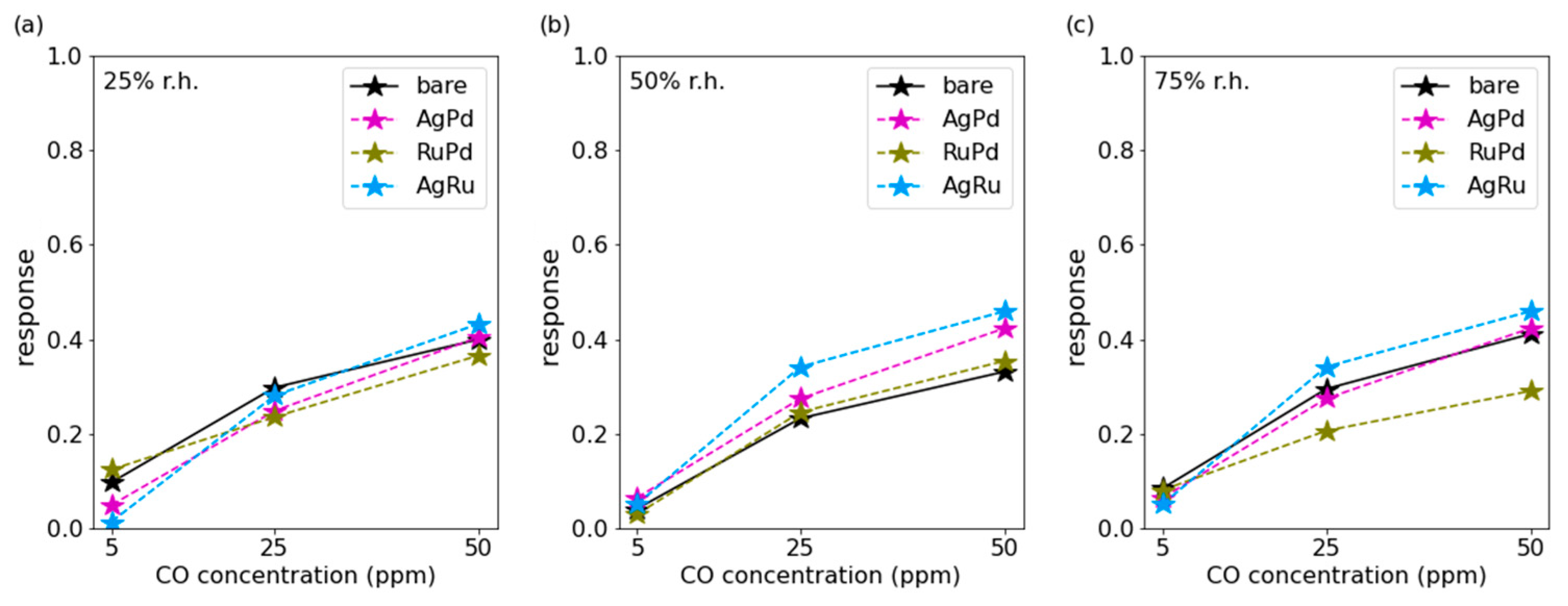
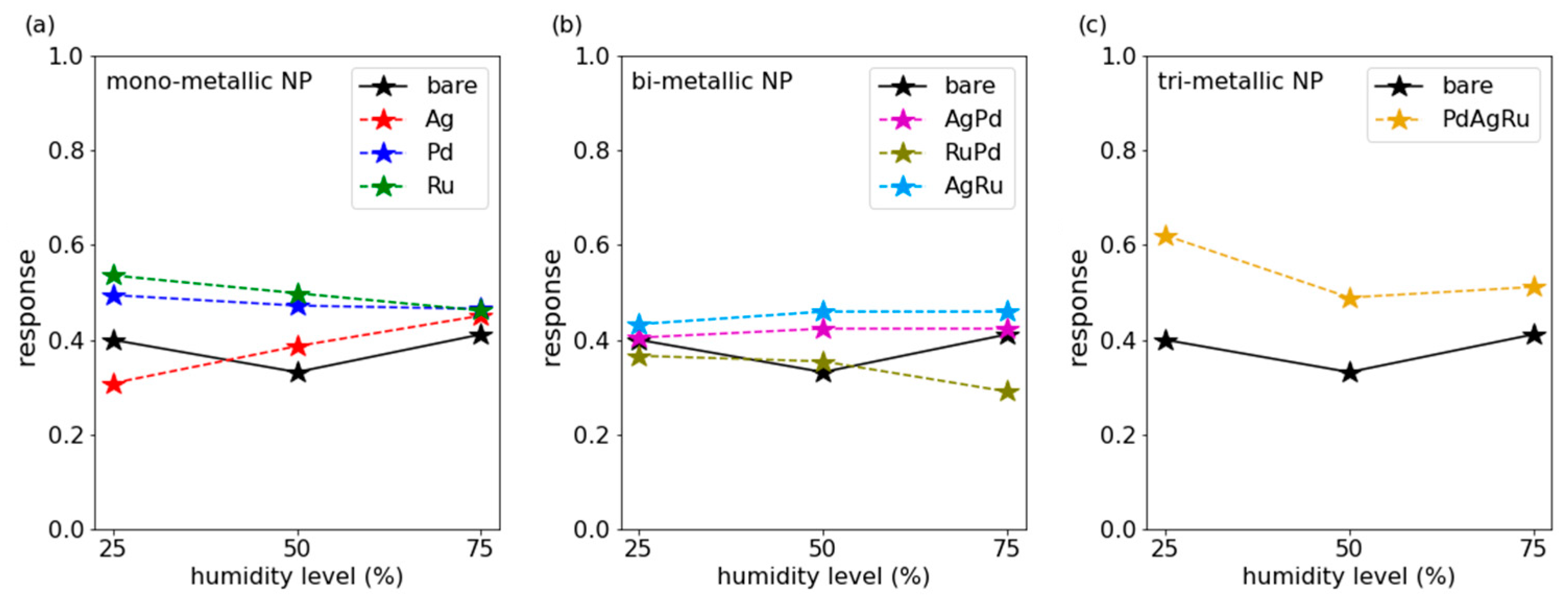
3.1. CO Measurements
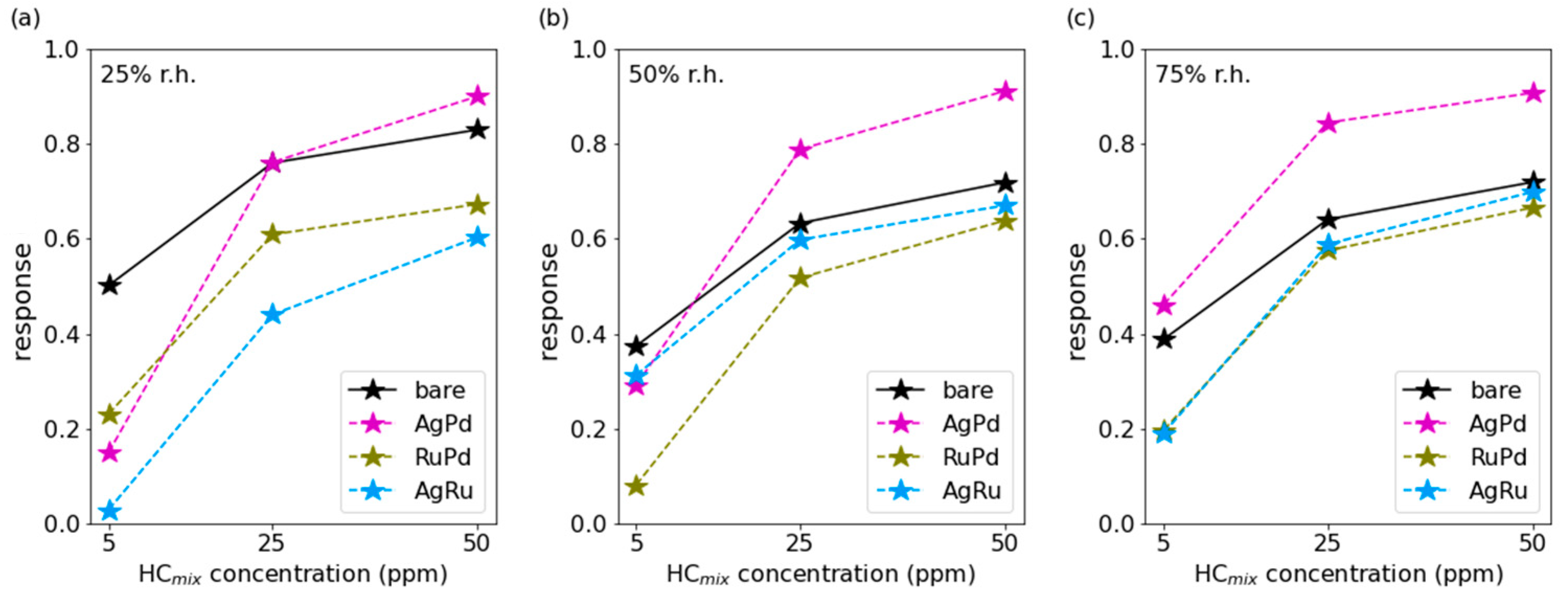
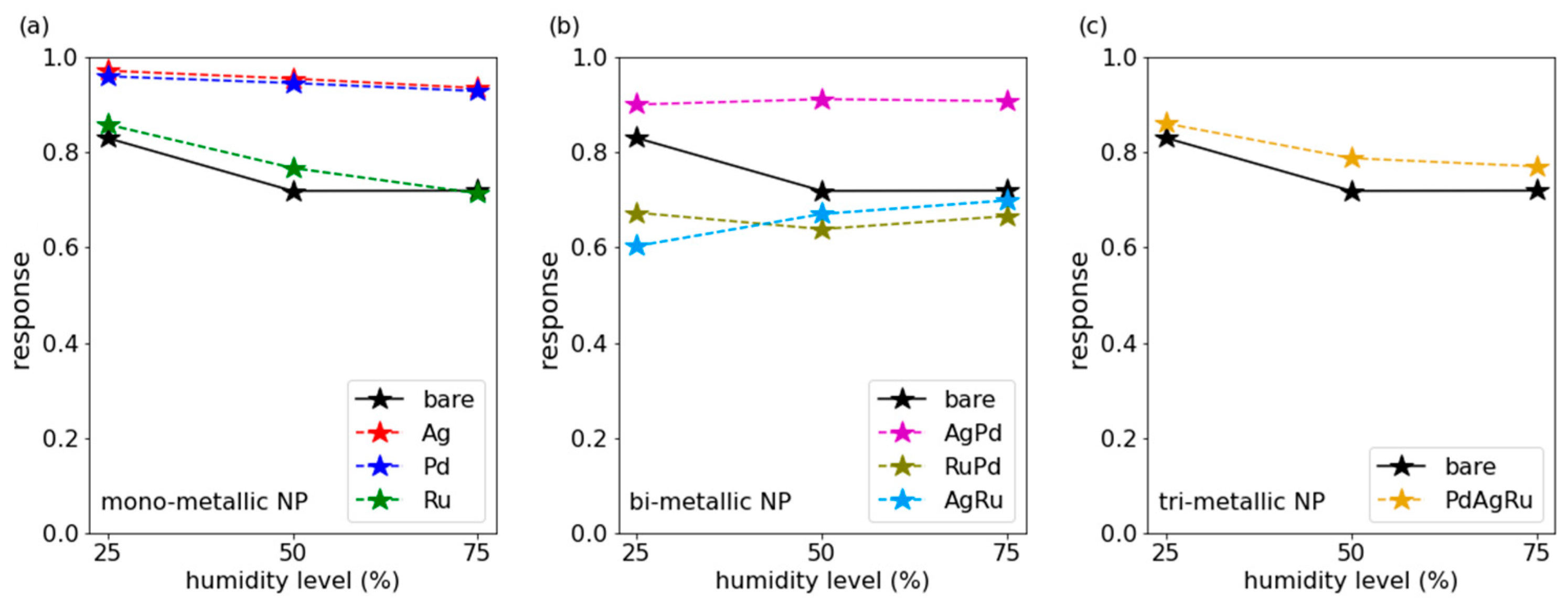
3.2. HCmix Measurements
3.3. Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- González-Martín, J.; Kraakman, N.J.R.; Pérez, C.; Lebrero, R.; Muñoz, R. A State–of–the-Art Review on Indoor Air Pollution and Strategies for Indoor Air Pollution Control. Chemosphere 2021, 262, 128376. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization (WHO) Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Lara-Ibeas, I.; Rodríguez Cuevas, A.; Le Calvé, S. Recent Developments and Trends in Miniaturized Gas Preconcentrators for Portable Gas Chromatography Systems: A Review. Sens. Actuators B Chem. 2021, 346, 130449. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor Gas Sensors: Materials, Technology, Design, and Application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.Y.; Lo, K.M.; Mak, T.; Leung, K.S.; Leung, Y.; Meng, M.L. A Survey of Wireless Sensor Network Based Air Pollution Monitoring Systems. Sensors 2015, 15, 31392–31427. [Google Scholar] [CrossRef]
- Pathania, A.; Dhanda, N.; Verma, R.; Sun, A.-C.A.; Thakur, P.; Thakur, A. Metal Oxide Chemoresistive Gas Sensing Mechanism, Parameters, and Applications. ECS Sens. Plus 2024, 3, 13401. [Google Scholar] [CrossRef]
- Degler, D.; Weimar, U.; Barsan, N. Current Understanding of the Fundamental Mechanisms of Doped and Loaded Semiconducting Metal-Oxide-Based Gas Sensing Materials. ACS Sens. 2019, 4, 2228–2249. [Google Scholar] [CrossRef]
- Schröder, S.; Ababii, N.; Brînză, M.; Magariu, N.; Zimoch, L.; Bodduluri, M.T.; Strunskus, T.; Adelung, R.; Faupel, F.; Lupan, O. Tuning the Selectivity of Metal Oxide Gas Sensors with Vapor Phase Deposited Ultrathin Polymer Thin Films. Polymers 2023, 15, 524. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yin, W.; Gao, S.; Sun, Y.; Xu, P.; Wu, S.; Kong, H.; Yang, G.; Wei, G. The Combination of Two-Dimensional Nanomaterials with Metal Oxide Nanoparticles for Gas Sensors: A Review. Nanomaterials 2022, 12, 982. [Google Scholar] [CrossRef] [PubMed]
- Dadkhah, M.; Tulliani, J.-M. Green Synthesis of Metal Oxides Semiconductors for Gas Sensing Applications. Sensors 2022, 22, 4669. [Google Scholar] [CrossRef] [PubMed]
- Korotcenkov, G. Metal Oxides for Solid-State Gas Sensors: What Determines Our Choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Xavier, R.; Sivaperuman, K. Review on the of Physical Vapor Deposition on Imminent Chemiresistive Metal Oxide Gas Sensors and Their Future Scope. Mater. Today Commun. 2024, 38, 107831. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured Materials for Room-Temperature Gas Sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yang, Z.; Yu, S.; Mi, Q.; Pan, Q. Diversiform Metal Oxide-Based Hybrid Nanostructures for Gas Sensing with Versatile Prospects. Coord. Chem. Rev. 2020, 413, 213272. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Engineering Approaches for the Improvement of Conductometric Gas Sensor Parameters: Part 1. Improvement of Sensor Sensitivity and Selectivity (Short Survey). Sens. Actuators B Chem. 2013, 188, 709–728. [Google Scholar] [CrossRef]
- Kolmakov, A. Some Recent Trends in the Fabrication, Functionalisation and Characterisation of Metal Oxide Nanowire Gas Sensors. Int. J. Nanotechnol. 2008, 5, 450–474. [Google Scholar] [CrossRef]
- Kim, K.W.; Cho, P.S.; Kim, S.J.; Lee, J.H.; Kang, C.Y.; Kim, J.S.; Yoon, S.J. The Selective Detection of C2H5OH Using SnO2-ZnO Thin Film Gas Sensors Prepared by Combinatorial Solution Deposition. Sens. Actuators B Chem. 2007, 123, 318–324. [Google Scholar] [CrossRef]
- Sharma, S.; Madou, M. Review Article: A New Approach to Gas Sensing with Nanotechnology. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2012, 370, 2448–2473. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Li, K.H.H.; Tan, O.K. Microhotplates for Metal Oxide Semiconductor Gas Sensor Applications—Towards the CMOS-MEMS Monolithic Approach. Micromachines 2018, 9, 557. [Google Scholar] [CrossRef] [PubMed]
- Köck, A.; Wimmer-Teubenbacher, R.; Sosada-Ludwikovska, F.; Rohracher, K.; Wachmann, E.; Herold, M.; Welden, T.V.; Kim, J.M.; Ali, Z.; Poenninger, A.; et al. 3D-Integrated Multi-Sensor Demonstrator System for Environmental Monitoring. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (Transducers & Eurosensors XXXIII), Berlin, Germany, 23–27 June 2019; pp. 1136–1139. [Google Scholar]
- Mallires, K.R.; Wang, D.; Tipparaju, V.V.; Tao, N. Developing a Low-Cost Wearable Personal Exposure Monitor for Studying Respiratory Diseases Using Metal--Oxide Sensors. IEEE Sens. J. 2019, 19, 8252–8261. [Google Scholar] [CrossRef] [PubMed]
- Bhati, V.S.; Hojamberdiev, M.; Kumar, M. Enhanced Sensing Performance of ZnO Nanostructures-Based Gas Sensors: A Review. Energy Rep. 2020, 6, 46–62. [Google Scholar] [CrossRef]
- Ahemad, M.J.; Le, T.D.; Kim, D.-S.; Yu, Y.-T. Bimetallic AgAualloy@ ZnO Core-Shell Nanoparticles for Ultra-High Detection of Ethanol: Potential Impact of Alloy Composition on Sensing Performance. Sens. Actuators B Chem. 2022, 359, 131595. [Google Scholar] [CrossRef]
- Isaac, N.A.; Pikaar, I.; Biskos, G. Metal Oxide Semiconducting Nanomaterials for Air Quality Gas Sensors: Operating Principles, Performance, and Synthesis Techniques. Microchim. Acta 2022, 189, 196. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.Y.; Ou, L.X.; Mao, L.W.; Wu, X.Y.; Liu, Y.P.; Lu, H.L. Advances in Noble Metal-Decorated Metal Oxide Nanomaterials for Chemiresistive Gas Sensors: Overview; Springer Nature: Singapore, 2023; Volume 15, ISBN 0123456789. [Google Scholar]
- Ren, Y.; Xie, W.; Li, Y.; Ma, J.; Li, J.; Liu, Y.; Zou, Y.; Deng, Y. Noble Metal Nanoparticles Decorated Metal Oxide Semiconducting Nanowire Arrays Interwoven into 3D Mesoporous Superstructures for Low-Temperature Gas Sensing. ACS Cent. Sci. 2021, 7, 1885–1897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, M.; Guo, Y. Highly Sensitive Ethanol Gas Sensor Based on Ag Nanoparticles Decorated In2O3. Chem. Res. Chin. Univ. 2024, 40, 1033–1040. [Google Scholar] [CrossRef]
- Sosada-Ludwikowska, F.; Reiner, L.; Egger, L.; Lackner, E.; Krainer, J.; Wimmer-Teubenbacher, R.; Singh, V.; Steinhauer, S.; Grammatikopoulos, P.; Köck, A. Adjusting Surface Coverage of Pt Nanocatalyst Decoration for Selectivity Control in CMOS-Integrated SnO2 Thin Film Gas Sensors. Nanoscale Adv. 2024, 6, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, J. Advancements in Improving Selectivity of Metal Oxide Semiconductor Gas Sensors Opening New Perspectives for Their Application in Food Industry. Sensors 2023, 23, 9548. [Google Scholar] [CrossRef] [PubMed]
- Lackner, E. Optimization of CMOS Integrated Tin Oxide Gas Sensors Using Metallic and Bimetallic Nanoparticles; TU Graz: Styria, Austria, 2017. [Google Scholar]
- Deutsche Forschungsgemeinschaft. List of MAK and BAT Values 2022 (Maximum Concentrations and Biological Tolerance Values at the Workplace); Deutsche Forschungsgemeinschaft: Bonn, Germany, 2022; Report 58. [Google Scholar]
- Grammatikopoulos, P.; Steinhauer, S.; Vernieres, J.; Singh, V.; Sowwan, M. Nanoparticle Design by Gas-Phase Synthesis. Adv. Phys. X 2016, 1, 81–100. [Google Scholar] [CrossRef]
- Aguado, A.; Jarrold, M.F. Melting and Freezing of Metal Clusters. Annu. Rev. Phys. Chem. 2011, 62, 151–172. [Google Scholar] [CrossRef][Green Version]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egger, L.; Sosada-Ludwikowska, F.; Steinhauer, S.; Singh, V.; Grammatikopoulos, P.; Köck, A. SnO2-Based CMOS-Integrated Gas Sensor Optimized by Mono-, Bi-, and Trimetallic Nanoparticles. Chemosensors 2025, 13, 59. https://doi.org/10.3390/chemosensors13020059
Egger L, Sosada-Ludwikowska F, Steinhauer S, Singh V, Grammatikopoulos P, Köck A. SnO2-Based CMOS-Integrated Gas Sensor Optimized by Mono-, Bi-, and Trimetallic Nanoparticles. Chemosensors. 2025; 13(2):59. https://doi.org/10.3390/chemosensors13020059
Chicago/Turabian StyleEgger, Larissa, Florentyna Sosada-Ludwikowska, Stephan Steinhauer, Vidyadhar Singh, Panagiotis Grammatikopoulos, and Anton Köck. 2025. "SnO2-Based CMOS-Integrated Gas Sensor Optimized by Mono-, Bi-, and Trimetallic Nanoparticles" Chemosensors 13, no. 2: 59. https://doi.org/10.3390/chemosensors13020059
APA StyleEgger, L., Sosada-Ludwikowska, F., Steinhauer, S., Singh, V., Grammatikopoulos, P., & Köck, A. (2025). SnO2-Based CMOS-Integrated Gas Sensor Optimized by Mono-, Bi-, and Trimetallic Nanoparticles. Chemosensors, 13(2), 59. https://doi.org/10.3390/chemosensors13020059




