Electrospun WO3/TiO2 Core–Shell Nanowires for Triethylamine Gas Sensing
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Sensitive Materials
2.3. Characterization
2.4. Gas Sensor Fabrication and Test
3. Results and Discussion
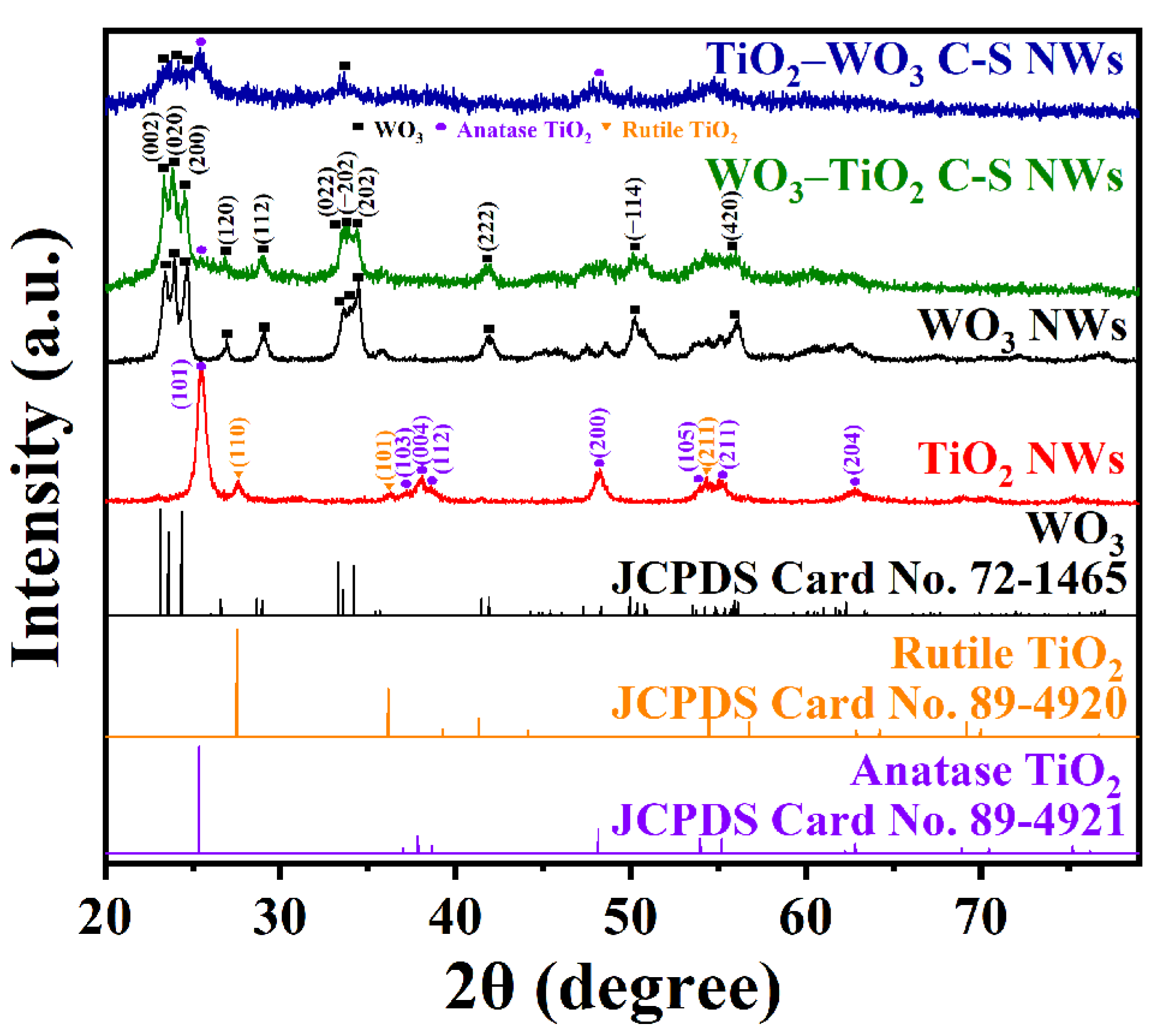
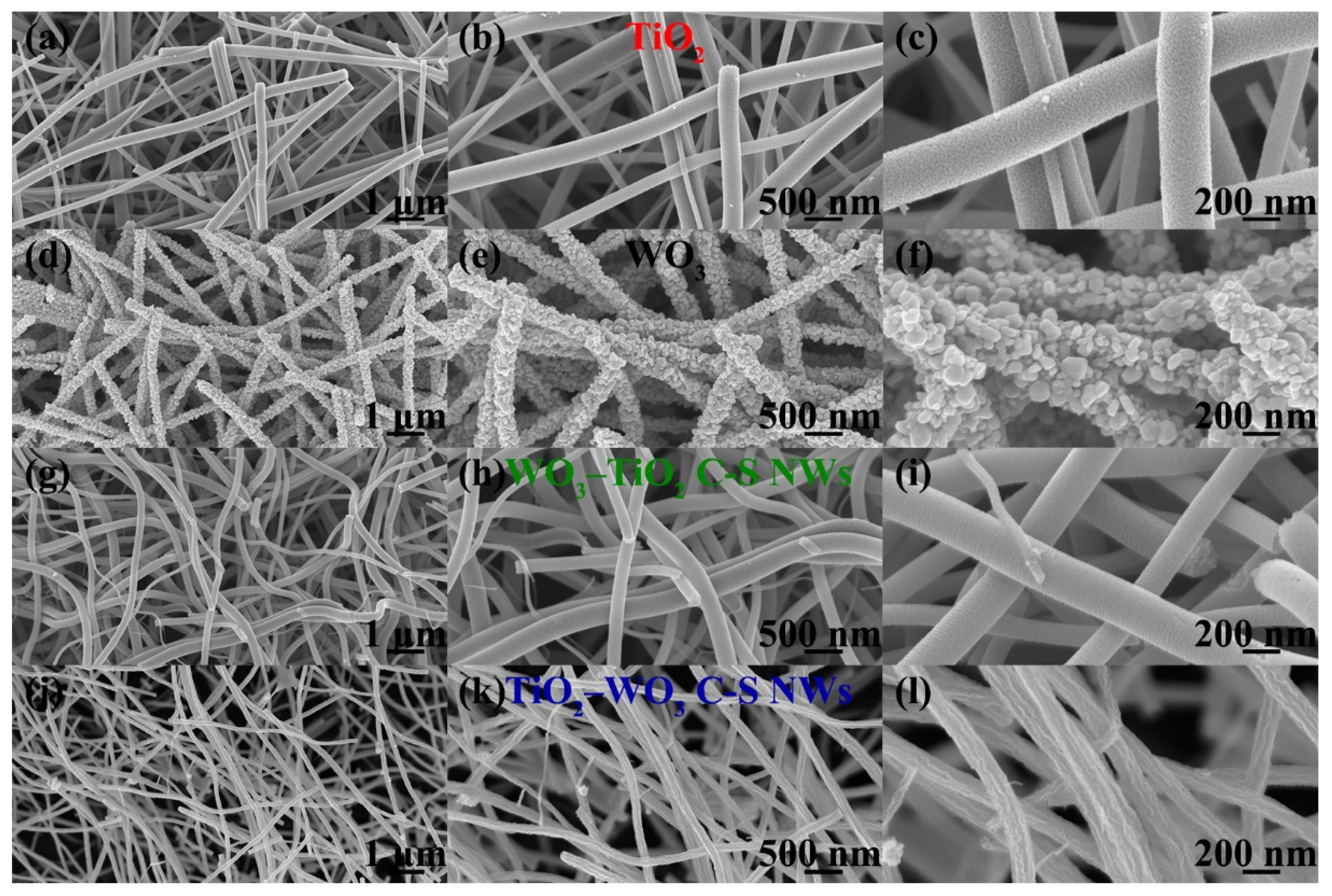
3.1. Structural and Morphological Features
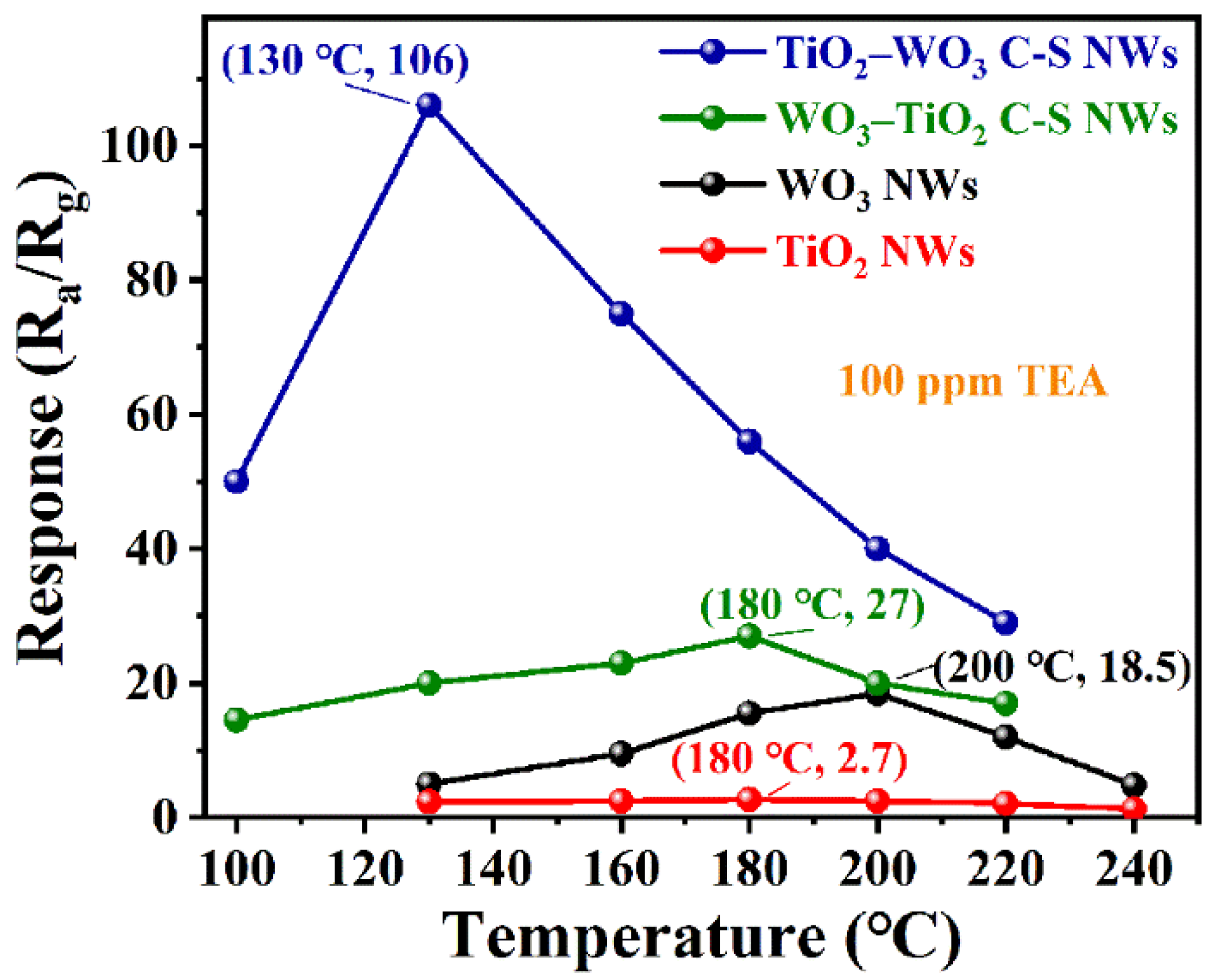
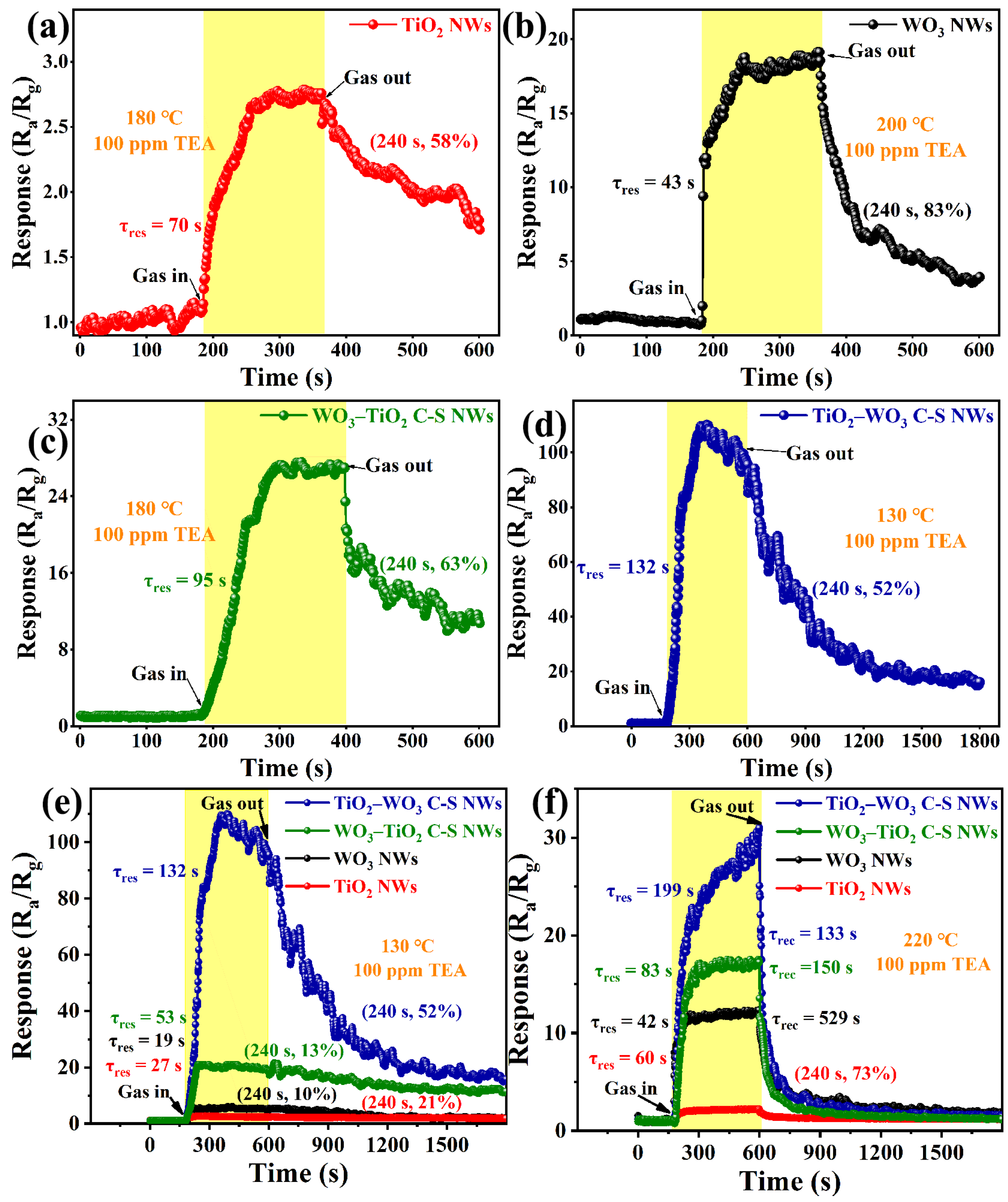
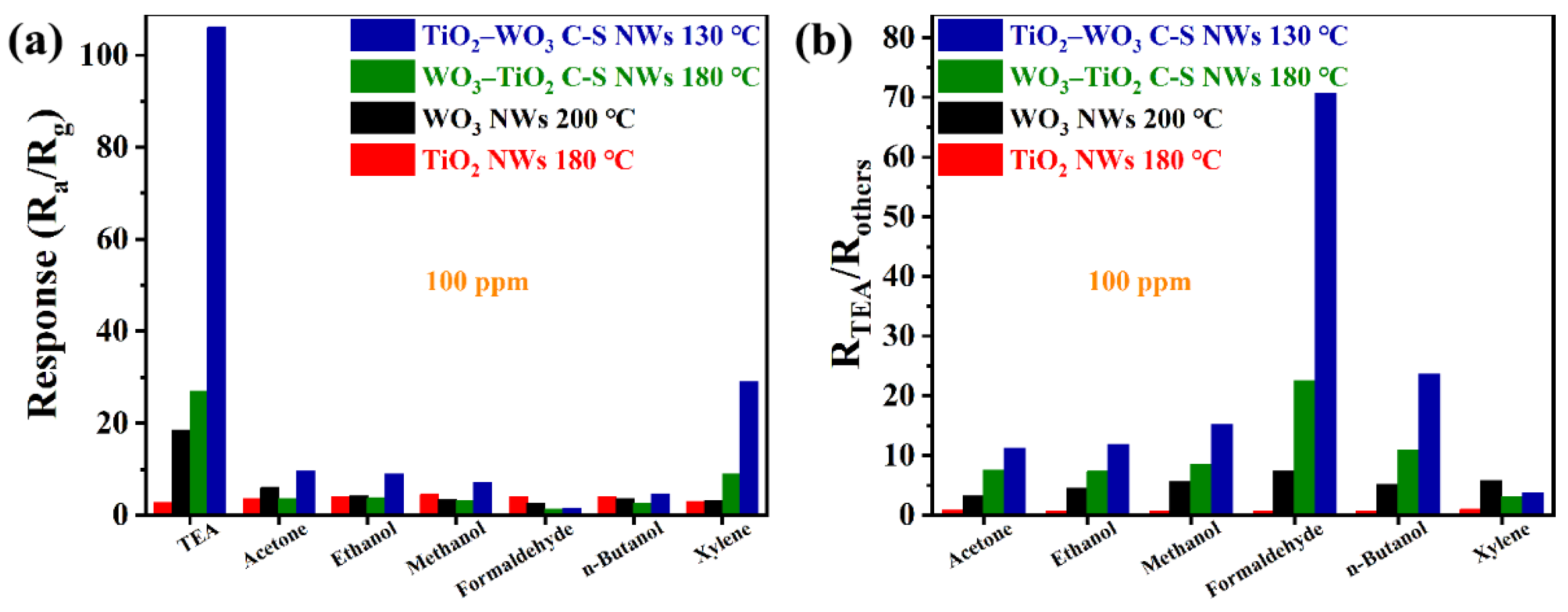
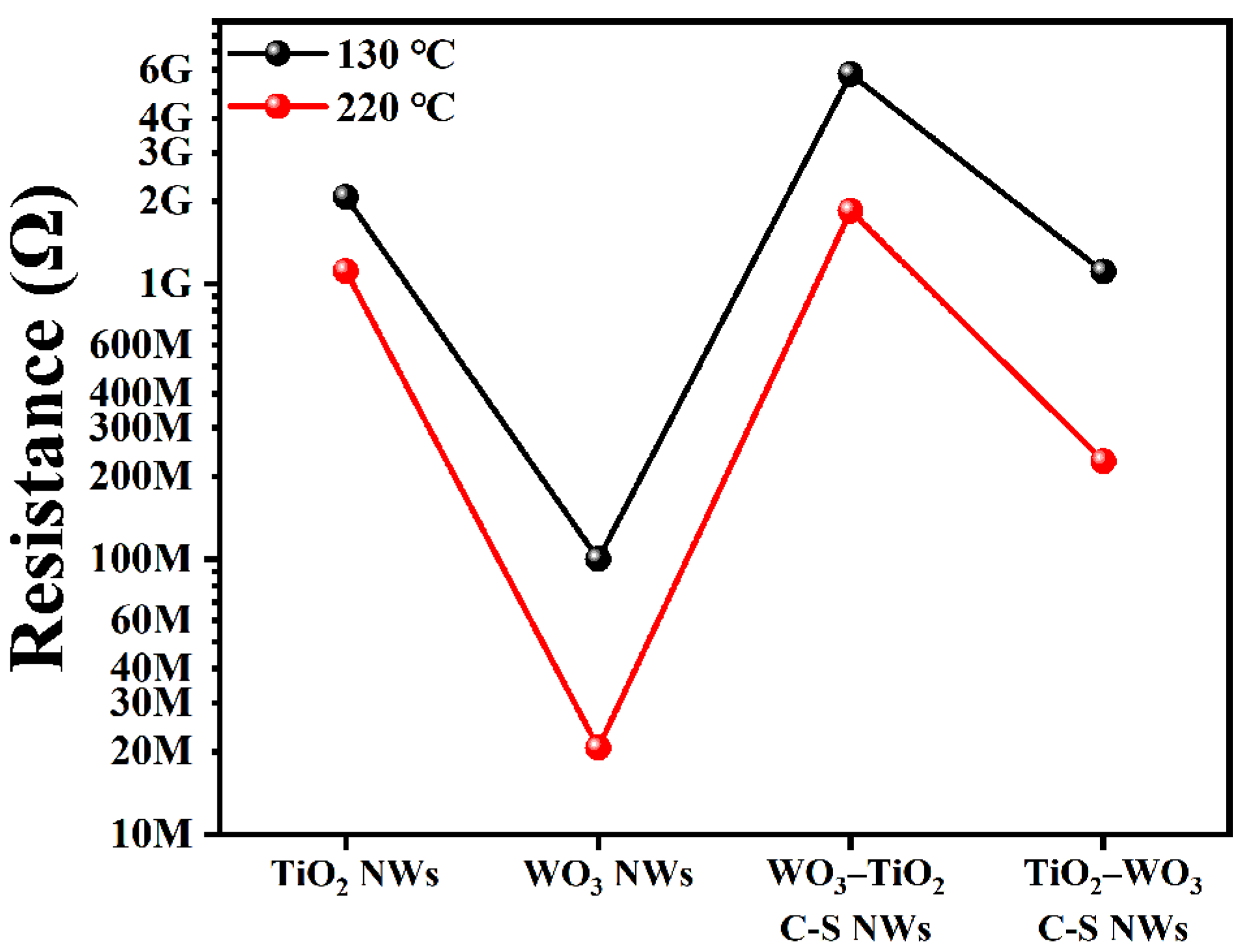
3.2. Gas Sensing Properties
3.3. Gas Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, L.Q.; Zhao, Z.H.; Chen, C.; Shao, Z.G.; Wu, L. Fabrication of Bi2S3/ZnO nanocomposites for TEA gas sensors operating at room temperature: Towards seafood freshness detection during storage. Microchem. J. 2024, 207, 112239. [Google Scholar] [CrossRef]
- Li, X.B.; Hu, X.; Yang, B.; Zheng, L.H.; Ren, Y.Y.; Wang, F.P.; Sun, S.; Wang, Y.J.; Liu, D.N.; Xu, H.H.; et al. Heterostructure engineering of In2O3/ErVO4 hairy curd for high-performance TEA gas sensors. Ceram. Int. 2024, 50, 29590–29602. [Google Scholar] [CrossRef]
- Ma, Y.T.; Chen, X.W.; Shi, J.; Zhao, Y.; Wang, Y. The preparation of Bi3+ quantum dots modified SnS nanosheets gas sensor for detecting TEA. Vacuum 2023, 207, 111594. [Google Scholar] [CrossRef]
- Liu, J.J.; Zhu, B.C.; Zhang, L.Y.; Fan, J.J.; Yu, J.G. 0D/2D CdS/ZnO composite with n-n heterojunction for efficient detection of triethylamine. J. Colloid Interface Sci. 2021, 600, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Zhai, C.B.; Zhu, M.M.; Jiang, L.N.; Yang, T.Y.; Zhao, Q.; Luo, Y.; Zhang, M.Z. Fast triethylamine gas sensing response properties of nanosheets assembled WO3 hollow microspheres. Appl. Surf. Sci. 2019, 463, 1078–1084. [Google Scholar] [CrossRef]
- Wang, X.; Han, W.J.; Yang, J.Q.; Jiang, B.; Cheng, P.F.; Wang, Y.L.; Sun, P.; Zhang, H.; Sun, Y.F.; Lu, G.Y. Facet effect on diverse WO3 morphologies and ideal work function for ppb-level triethylamine detection. Sens. Actuators B 2022, 363, 131849. [Google Scholar] [CrossRef]
- Wang, D.; Chu, X.F.; Gong, M.L. Gas-sensing properties of sensors based on single-crystalline SnO2 nanorods prepared by a simple molten-salt method. Sens. Actuators B 2006, 117, 183–187. [Google Scholar] [CrossRef]
- Li, S.M.; Zhang, W.L.; Wang, C.W.; Li, G. Au nanoparticles decorated ZnIn2S4/WO3 core-shell heterostructures as highly sensitive and selective ethylene glycol gas sensors. J. Alloys Compd. 2024, 998, 175027. [Google Scholar] [CrossRef]
- Yan, S.; Yang, F.; Dong, X.H.; Zhang, C.; Xu, X.D.; Chi, Z.T.; Yang, W.; Xie, W.F. Rational construction of α-MoO3@WO3 nanosheets and its triethylamine sensing performances. Ceram. Int. 2024, 50, 14238–14246. [Google Scholar] [CrossRef]
- Zhang, S.M.; Zhang, H.; Yao, H.Y.; Wang, P.J.; Zhu, M.; Shi, X.R.; Xu, S.S. Recent advances in metal oxide semiconductor heterojunctions for the detection of volatile organic compounds. Chemosensors 2024, 12, 244. [Google Scholar] [CrossRef]
- Hu, K.C.; Cai, Y.H.; Wang, Z.R.; Zhang, Z.W.; Xian, J.Y.; Zhang, C. A review on metal oxide semiconductor-based chemo-resistive ethylene sensors for agricultural applications. Chemosensors 2024, 12, 13. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Y.; Wang, Z.H.; Shu, Y.Q.; He, Z.W.; Zhang, F.; Gao, L.F.; Li, C.; Wang, C.; Song, Y.F.; et al. Tellurium@Selenium core-shell hetero-junction: Facile synthesis, nonlinear optics, and ultrafast photonics applications towards mid-infrared regime. Appl. Mater. Today 2020, 20, 100657. [Google Scholar] [CrossRef]
- Li, D.D.; Ren, Z.X.; Fan, Q.; Wang, C.; She, J.B. NIR dual-wavelengths excited upconversion photoluminescence of the core–shell luminescent material for anti-counterfeiting recognition. Microchem. J. 2023, 193, 109213. [Google Scholar] [CrossRef]
- Lys, A.; Zabolotnii, V.; Čaplovičová, M.; Tepliakova, I.; Berzins, A.; Sahul, M.; Čaplovič, Ľ.; Pogrebnjak, A.; Iatsunskyi, I.; Viter, R. Core-shell nanofibers of ZnFe2O4/ZnO for enhanced visible-light photoelectrochemical performance. J. Alloys Compd. 2024, 984, 173885. [Google Scholar] [CrossRef]
- Covaliu, C.I.; Jitaru, I.; Paraschiv, G.; Vasile, E.; Biriş, S.-Ş.; Diamandescu, L.; Ionita, V.; Iovu, H. Core–shell hybrid nanomaterials based on CoFe2O4 particles coated with PVP or PEG biopolymers for applications in biomedicine. Powder Technol. 2013, 237, 415–426. [Google Scholar] [CrossRef]
- Covaliu, C.I.; Paraschiv, G.; Biriş, S.-Ş.; Jitaru, I.; Vasile, E.; Diamandescu, L.; Velickovic, T.C.; Krstic, M.; Ionita, V.; Iovu, H.; et al. Maghemite and poly-dl-alanine based core–shell multifunctional nanohybrids for environmental protection and biomedicine applications. Appl. Surf. Sci. 2013, 285, 86–95. [Google Scholar] [CrossRef]
- Rashidi Dafeh, S.; Iranmanesh, P.; Salarizadeh, P. Fabrication, optimization, and characterization of ultra-small superparamagnetic Fe3O4 and biocompatible Fe3O4@ZnS core/shell magnetic nanoparticles: Ready for biomedicine applications. Mater. Sci. Eng. C 2019, 98, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Eurov, D.A.; Kirilenko, D.A.; Sharonova, L.V.; Shvidchenko, A.V.; Smirnov, A.N.; Tomkovich, M.V.; Yagovkina, M.A.; Kurdyukov, D.A. Formation of stable microporous core-shell V2O5/SiO2 colloidal particles potential for heterogeneous catalysis. Mater. Today Commun. 2023, 35, 106047. [Google Scholar] [CrossRef]
- Ma, Y.S.; Liu, X.L.; Zhao, M.Y.; Du, K.F.; Yin, H.Y.; Mao, X.H.; Wang, D.H. New insights into engineering the core size and carbon shell thickness of Co@C core–shell catalysts for efficient and stable Fenton-like catalysis. J. Colloid Interface Sci. 2023, 634, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, K.; Chen, Z.; Chen, A.; Ma, F.; Zhu, M.Q.; Chen, Y.F. Fe-Nx sites coupled with core-shell FeS@C nanoparticles to boost the oxygen catalysis for rechargeable Zn-air batteries. J. Energy Chem. 2024, 90, 565–577. [Google Scholar] [CrossRef]
- Kour, P.; Deeksha; Kour, S.; Sharma, A.L.; Yadav, K. MoS2-based core-shell nanostructures: Highly efficient materials for energy storage and conversion applications. J. Energy Storage 2023, 66, 107393. [Google Scholar] [CrossRef]
- Nair, A.R.; Vetrikarasan, B.T.; Shinde, S.K.; Kim, D.-Y.; Sawant, S.N.; Jagadale, A.D. Bifunctional CuO@CoV layered double hydroxide (LDH) core–shell heterostructure for electrochemical energy storage and electrocatalysis. Fuel 2024, 358, 130217. [Google Scholar] [CrossRef]
- Nanda, O.P.; Veeralingam, S.; Badhulika, S. Fabrication of ZnSnO3@In2O3 core-shell based 1D microfiber heterostructure for high energy density asymmetric supercapacitors. J. Alloys Compd. 2023, 969, 172338. [Google Scholar] [CrossRef]
- Chen, B.; Guo, H.; Liu, C.J.; Shang, L.; Ye, X.; Chen, L.; Feng, C.H.; Hayashi, K. Molecularly imprinted sol-gel/Au@Ag core-shell nano-urchin localized surface plasmon resonance sensor designed in reflection mode for detection of organic acid vapors. Biosens. Bioelectron. 2020, 169, 112639. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Liu, Y.; Ren, J.; Yang, W.Z.; Shang, E.; Ma, K.X.; Zhang, L.H.; Jiang, J.; Sun, X.H. Conformable core-shell fiber tactile sensor by continuous tubular deposition modeling with water-based sacrificial coaxial writing. Mater. Des. 2020, 190, 108567. [Google Scholar] [CrossRef]
- Güler, M.; Zengin, A.; Alay, M. Fabrication of glucose bioelectrochemical sensor based on Au@Pd core-shell supported by carboxylated graphene oxide. Anal. Biochem. 2023, 667, 115091. [Google Scholar] [CrossRef]
- Katowah, D.F.; Hussein, M.A.; Alam, M.M.; Ismail, S.H.; Osman, O.I.; Sobahi, T.R.; Asiri, A.M.; Ahmed, J.; Rahman, M.M. Designed network of ternary core-shell PPCOT/NiFe2O4/C-SWCNTs nanocomposites. A Selective Fe3+ ionic sensor. J. Alloys Compd. 2020, 834, 155020. [Google Scholar] [CrossRef]
- Rafique, N.; Hannan Asif, A.; Hirani, R.a.K.; Wu, H.; Shi, L.; Zhang, S.; Sun, H.Q. Binder free 3D core–shell NiFe layered double hydroxide (LDH) nanosheets (NSs) supported on Cu foam as a highly efficient non-enzymatic glucose sensor. J. Colloid Interface Sci. 2022, 615, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.Z.; Li, C.C.; Zhou, Y.F.; Liu, Z.X.; Gao, X.; Wang, X.Q.; Jiang, L.; Tian, M.W.; Zhou, F.L.; Jerrams, S.; et al. A flexible dual-mode pressure sensor with ultra-high sensitivity based on BTO@MWCNTs core-shell nanofibers. Compos. Sci. Technol. 2022, 224, 109478. [Google Scholar] [CrossRef]
- Shan, L.X.; Li, Y.; Wang, R.C.; Lian, X.X. Sensing properties of ZnS@ZnO core-shell based triethylamine sensor fabricated using solvothermal and sintering methods. J. Alloys Compd. 2023, 944, 169223. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Wu, R.; Chen, X.T.; Mu, Y.; Yang, Z.G.; Liu, Z.Y.; Yue, C.; Dastan, D.; Yin, X.T.; Liu, T. Enhancement of TEA gas-sensitive properties by Mn2O3@In2O3 core-shell structured p-n heterojunction. J. Alloys Compd. 2024, 976, 173340. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; San, X.; Zhang, L.; Wang, N.; Wang, G.; Meng, D.; Shen, Y. Highly selective gas sensors for formaldehyde detection based on ZnO@ZIF-8 core-shell heterostructures. Sens. Actuators B 2024, 398, 134689. [Google Scholar] [CrossRef]
- Huang, B.Y.; Zhang, Z.X.; Zhao, C.H.; Cairang, L.M.; Bai, J.L.; Zhang, Y.X.; Mu, X.M.; Du, J.W.; Wang, H.; Pan, X.J.; et al. Enhanced gas-sensing performance of ZnO@In2O3 core@shell nanofibers prepared by coaxial electrospinning. Sens. Actuators B 2018, 255, 2248–2257. [Google Scholar] [CrossRef]
- Wang, L.L.; Lou, Z.; Deng, J.N.; Zhang, R.; Zhang, T. Ethanol gas detection using a yolk-shell (core-shell) α-Fe2O3 nanospheres as sensing material. ACS Appl. Mater. Interfaces 2015, 7, 13098–13104. [Google Scholar] [CrossRef]
- Liao, Y.L.; Que, W.X.; Jia, Q.Y.; He, Y.C.; Zhang, J.; Zhong, P. Controllable synthesis of brookite/anatase/rutile TiO2 nanocomposites and single-crystalline rutile nanorods array. J. Mater. Chem. 2012, 22, 7937–7944. [Google Scholar] [CrossRef]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Cao, S.; Sui, N.; Zhang, P.; Zhou, T.T.; Tu, J.C.; Zhang, T. TiO2 nanostructures with different crystal phases for sensitive acetone gas sensors. J. Colloid Interface Sci. 2022, 607, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, Y.Y.; Wu, B.Z.; Zhang, X.T. Different crystalline phases of aligned TiO2 nanowires and their ethanol gas sensing properties. Phys. E Low-Dimens. Syst. Nanostruct. 2019, 114, 113601. [Google Scholar] [CrossRef]
- Li, F.; Gao, X.; Wang, R.; Zhang, T. Design of WO3-SnO2 core-shell nanofibers and their enhanced gas sensing performance based on different work function. Appl. Surf. Sci. 2018, 442, 30–37. [Google Scholar] [CrossRef]
- Li, F.; Gao, X.; Wang, R.; Zhang, T.; Lu, G.Y. Study on TiO2-SnO2 core-shell heterostructure nanofibers with different work function and its application in gas sensor. Sens. Actuators B 2017, 248, 812–819. [Google Scholar] [CrossRef]
- Zhang, B.; Bao, N.; Wang, T.; Xu, Y.; Dong, Y.; Ni, Y.; Yu, P.P.; Wei, Q.F.; Wang, J.; Guo, L.L.; et al. High-performance room temperature NO2 gas sensor based on visible light irradiated In2O3 nanowires. J. Alloys Compd. 2021, 867, 159076. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Yu, P.P.; Guo, L.L.; Xu, Y.; Dong, Y.; Ni, Y.; Ao, J.P.; Wei, Q.F.; Xia, Y. In/Fe cospinning nanowires for triethylamine gas sensing. ACS Appl. Nano Mater. 2022, 5, 9554–9566. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.Y.; Li, J.; Duan, Z.H.; Yang, Y.J.; Yuan, Z.; Jiang, Y.D.; Tai, H.L. Pd-decorated ZnO hexagonal microdiscs for NH3 sensor. Chemosensors 2024, 12, 43. [Google Scholar] [CrossRef]
- Lal, M.; Sharma, P.; Ram, C. Calcination temperature effect on titanium oxide (TiO2) nanoparticles synthesis. Optik 2021, 241, 166934. [Google Scholar] [CrossRef]
- Liu, N.; Bu, W.Y.; Zhou, Y.; Zhang, Y.; Chuai, X.H.; Lu, G.Y. Hetero-interface enabled highly responsive acetone gas sensor based on MOF-derived Co3O4/In2O3 composite. Vacuum 2025, 233, 113974. [Google Scholar] [CrossRef]
- Kong, D.L.; Hong, B.; Xu, J.C.; Peng, X.L.; Li, J.; Chen, H.W.; Qiu, S.; Zhang, N.; Wang, X.Q. P–n heterojunction construction and interfacial interaction mechanism: NiO/In2O3 formaldehyde gas sensors with excellent sensitivity and selectivity. Appl. Surf. Sci. 2025, 688, 162442. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Tao, X.W.; Yi, L.N.; Zhao, Z.G.; Yang, Q. Transamidation and decarbonylation of n-phthaloyl-amino acid amides enabled by palladium-catalyzed selective C–N bond cleavage. J. Org. Chem. 2022, 87, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gu, D.; Li, X.G. Detection of Ppb-level NO2 using mesoporous ZnSe/SnO2 core-shell microspheres based chemical sensors. Sens. Actuators B 2020, 320, 128365. [Google Scholar] [CrossRef]
- Wu, J.; Feng, S.L.; Li, Z.; Tao, K.; Chu, J.; Miao, J.M.; Norford, L.K. Boosted sensitivity of graphene gas sensor via nanoporous thin film structures. Sens. Actuators B 2018, 255, 1805–1813. [Google Scholar] [CrossRef]
- Sun, C.X.; Liu, H.Y.; Shao, J.K.; Pan, G.F.; Yang, X.L.; Wang, M.J.; Dong, J.Y.; Zhu, M.Y.; Qi, Y.H. Au-loaded Zn2SnO4/SnO2/ZnO nanosheets for fast response and highly sensitive TEA gas sensors. Sens. Actuators B 2023, 376, 132951. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, D.K.; Zhang, D.X.; Liu, Y.M.; Sun, M.L.; Sun, M.J.; Li, J. Construction of Ag2O/WO3-based hollow microsphere p-n heterojunction for continuous detection of ppb-level H2S gas sensor. J. Alloys Compd. 2024, 1003, 175735. [Google Scholar] [CrossRef]
- Duan, X.H.; Jiang, Y.D.; Liu, B.H.; Duan, Z.H.; Zhang, Y.J.; Yuan, Z.; Tai, H.L. Enhancing the carbon dioxide sensing performance of LaFeO3 by Co doping. Sens. Actuators B 2024, 402, 135136. [Google Scholar] [CrossRef]
- Pan, M.Q.; Shi, Z.M.; Liu, J.Z.; Wang, Y.J. Study on the process of micro-arc oxidation TiO2 film for rapid detection of triethylamine. Mater. Sci. Semicond. Process. 2022, 146, 106679. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.W.; Luo, N.; Xu, X.T.; Sun, G.; Wang, Y.; Cao, J.L. TiO2/ZnCo2O4 porous nanorods: Synthesis and temperature-dependent dual selectivity for sensing HCHO and TEA. Sens. Actuators B 2020, 321, 128461. [Google Scholar] [CrossRef]
- Zhai, C.B.; Luo, Z.B.; Liang, X.; Song, X.Y.; Zhang, M.Z. A superior selective and anti-jamming performance triethylamine sensing sensor based on hierarchical WO3 nanoclusters. J. Alloys Compd. 2021, 857, 157545. [Google Scholar] [CrossRef]
- Ma, Q.; Li, H.; Liu, Y.; Liu, M.L.; Fu, X.H.; Chu, S.S.; Li, H.; Guo, J. Facile synthesis of flower-like α-Fe2O3/ZnFe2O4 architectures with self-assembled core-shell nanorods for superior TEA detection. Curr. Appl. Phys. 2021, 21, 161–169. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. How shell thickness can affect the gas sensing properties of nanostructured materials: Survey of literature. Sens. Actuators B 2018, 258, 270–294. [Google Scholar] [CrossRef]
- Hu, Q.; He, J.Q.; Chang, J.Y.; Gao, J.M.; Huang, J.H.; Feng, L. Needle-shaped WO3 nanorods for triethylamine gas sensing. ACS Appl. Nano Mater. 2020, 3, 9046–9054. [Google Scholar] [CrossRef]
- Li, F.; Gao, X.; Wang, R.; Zhang, T.; Lu, G.Y.; Barsan, N. Design of core–shell heterostructure nanofibers with different work function and their sensing properties to trimethylamine. ACS Appl. Mater. Interfaces 2016, 8, 19799–19806. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Lee, D.; Chae, M.; Kim, H.D. Advanced recovery and enhanced humidity tolerance of CNTs gas sensor using a filament heater. Chem. Eng. J. 2024, 496, 154014. [Google Scholar] [CrossRef]
- Vidiš, M.; Plecenik, T.; Moško, M.; Tomašec, S.; Roch, T.; Satrapinskyy, L.; Grančič, B.; Plecenik, A. Gasistor: A memristor based gas-triggered switch and gas sensor with memory. Appl. Phys. Lett. 2019, 115, 093504. [Google Scholar] [CrossRef]
- Ali, M.; Lee, D.; Ahmad, I.; Kim, H.D. Recent progress in memristor-based gas sensors (Gasistor; gas sensor + memristor): Device modeling, mechanisms, performance, and prospects. Sens. Actuators Rep. 2025, 9, 100269. [Google Scholar] [CrossRef]

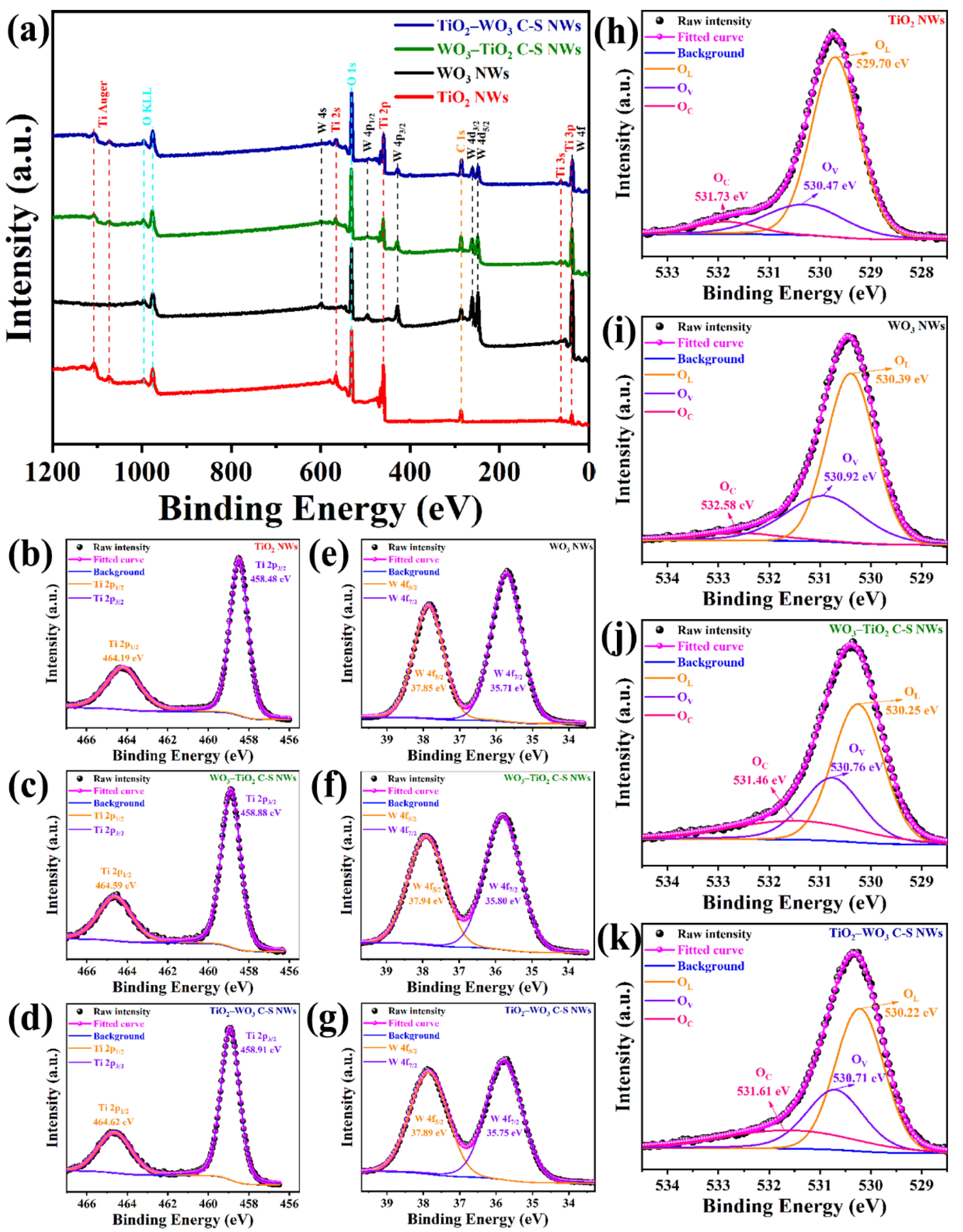



| Mater. | Oxy. | B.E. (eV) | Perc. (%) | OV + OC (%) |
|---|---|---|---|---|
| TiO2 NWs | OL (Ti–O) | 529.7 | 72.76 | 27.24 |
| OV (vacancy) | 530.47 | 21.39 | ||
| OC (chemisorbed) | 531.73 | 5.85 | ||
| WO3 NWs | OL (W–O) | 530.39 | 63.91 | 36.09 |
| OV (vacancy) | 530.92 | 30.49 | ||
| OC (chemisorbed) | 532.58 | 5.60 | ||
| WO3–TiO2 C-S NWs | OL (Ti–O and W–O) | 530.25 | 51.76 | 48.24 |
| OV (vacancy) | 530.76 | 30.05 | ||
| OC (chemisorbed) | 531.46 | 18.19 | ||
| TiO2–WO3 C-S NWs | OL (Ti–O and W–O) | 530.22 | 52.66 | 47.34 |
| OV (vacancy) | 530.71 | 29.07 | ||
| OC (chemisorbed) | 531.61 | 18.27 |
| Mater. | Temp. (°C) | Conc. (ppm) | Res. | τres/τrecov (s) | Ref. | Y. |
|---|---|---|---|---|---|---|
| TiO2 film | 225 | 100 | 7.2 | 5/52 | [53] | 2022 |
| TiO2/ZnCo2O4 porous nanorods | 220 | 100 | 15 | 9/77 | [54] | 2020 |
| WO3 nanoclusters | 280 | 50 | 137 | 1.5/1275 | [55] | 2021 |
| WO3 hollow microspheres | 220 | 50 | 16 | 1.5/22 | [5] | 2019 |
| WO3 microflowers | 325 | 100 | 61.8 | -/- | [6] | 2022 |
| Fe2O3@ZnFe2O4 | 280 | 100 | 141 | -/- | [56] | 2021 |
| ZnS@ZnO | 200 | 100 | 74.65 | 3/176 | [30] | 2023 |
| Mn2O3@In2O3 | 180 | 100 | 47 | 79/24 | [31] | 2024 |
| TiO2–WO3 C-S NWs | 130 | 100 | 106 | 132/>1200 | this work | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Zhang, B.; Dong, X.; Lu, Q.; Shen, H.; Ni, Y.; Liu, Y.; Song, H. Electrospun WO3/TiO2 Core–Shell Nanowires for Triethylamine Gas Sensing. Chemosensors 2025, 13, 45. https://doi.org/10.3390/chemosensors13020045
Li W, Zhang B, Dong X, Lu Q, Shen H, Ni Y, Liu Y, Song H. Electrospun WO3/TiO2 Core–Shell Nanowires for Triethylamine Gas Sensing. Chemosensors. 2025; 13(2):45. https://doi.org/10.3390/chemosensors13020045
Chicago/Turabian StyleLi, Wenhao, Bo Zhang, Xiangrui Dong, Qi Lu, Hao Shen, Yi Ni, Yuechen Liu, and Haitao Song. 2025. "Electrospun WO3/TiO2 Core–Shell Nanowires for Triethylamine Gas Sensing" Chemosensors 13, no. 2: 45. https://doi.org/10.3390/chemosensors13020045
APA StyleLi, W., Zhang, B., Dong, X., Lu, Q., Shen, H., Ni, Y., Liu, Y., & Song, H. (2025). Electrospun WO3/TiO2 Core–Shell Nanowires for Triethylamine Gas Sensing. Chemosensors, 13(2), 45. https://doi.org/10.3390/chemosensors13020045






