Microcontact-Printed Flexible Electrodes for Label-Free Electrochemical Detection of Lung Cancer Biomarker
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication Process of the SU-8 Master Mold and Replica Molding
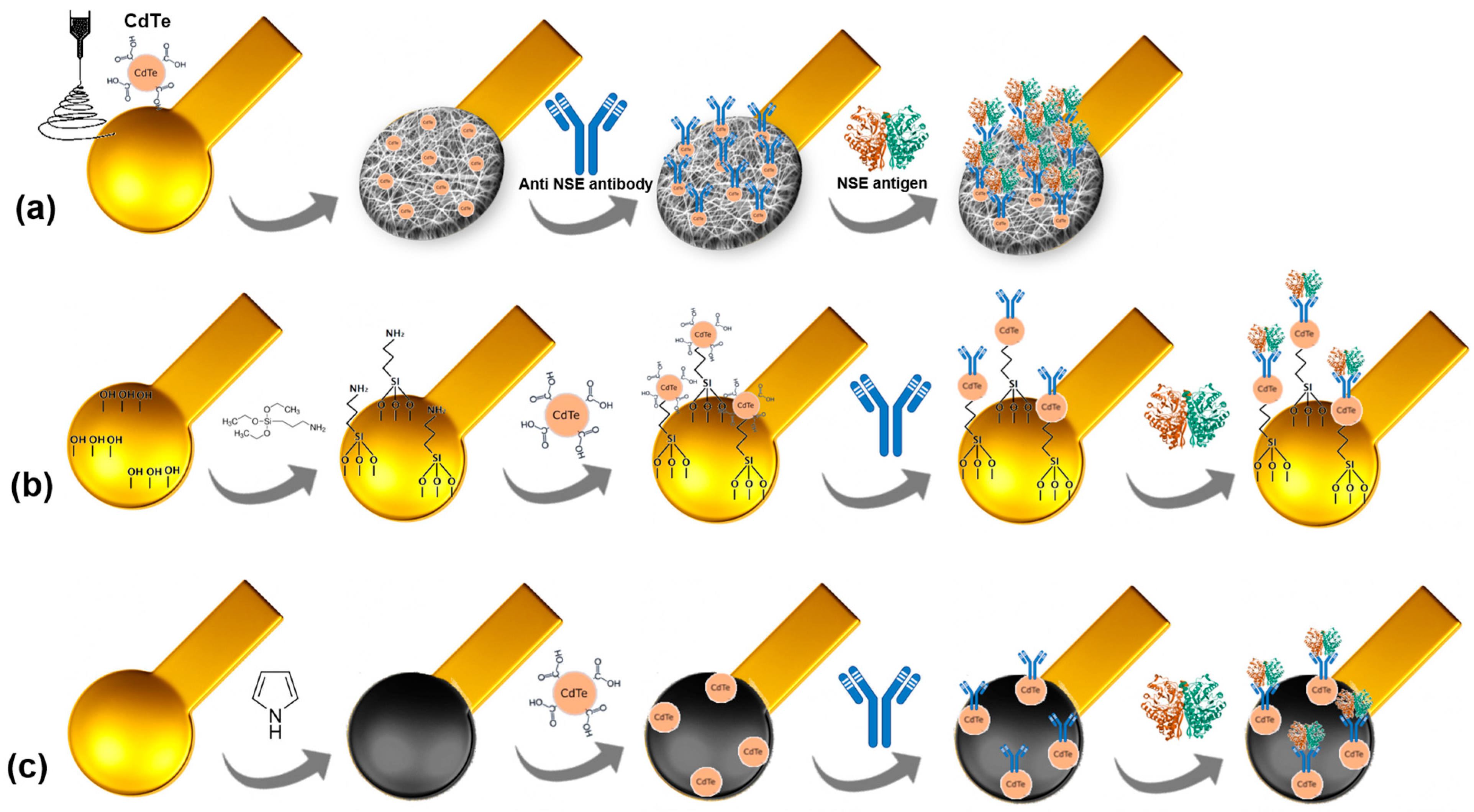
2.3. Electrode Fabrication Through Microcontact Printing
2.4. Immunosensor Platform Assembly Process and Bioactivity Assays
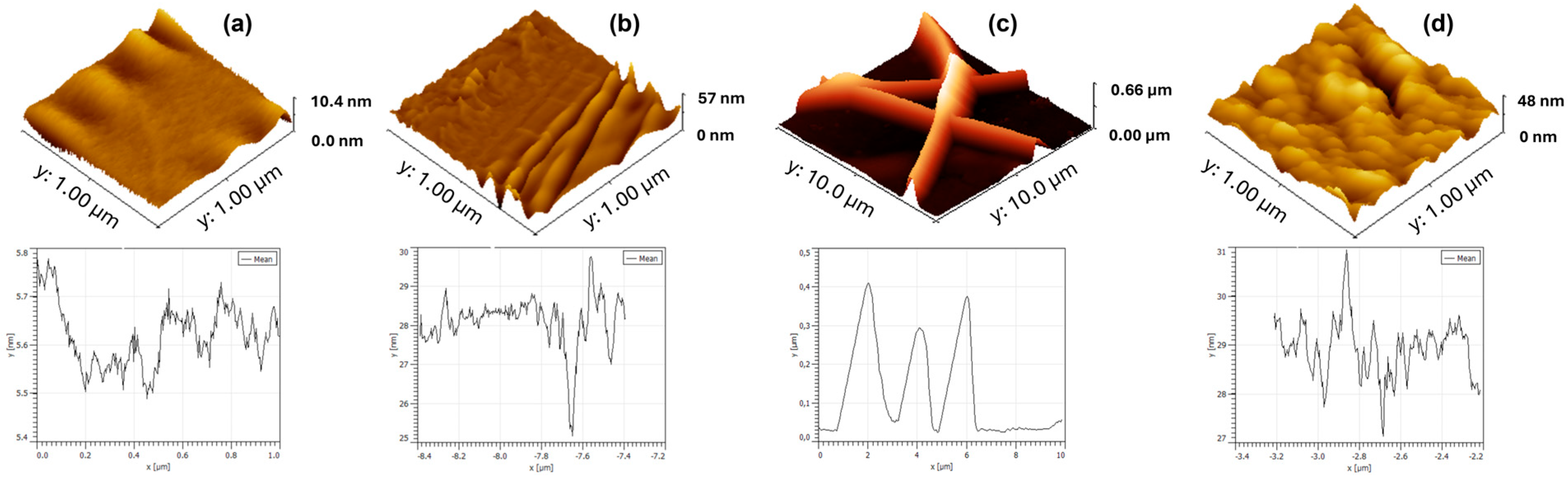
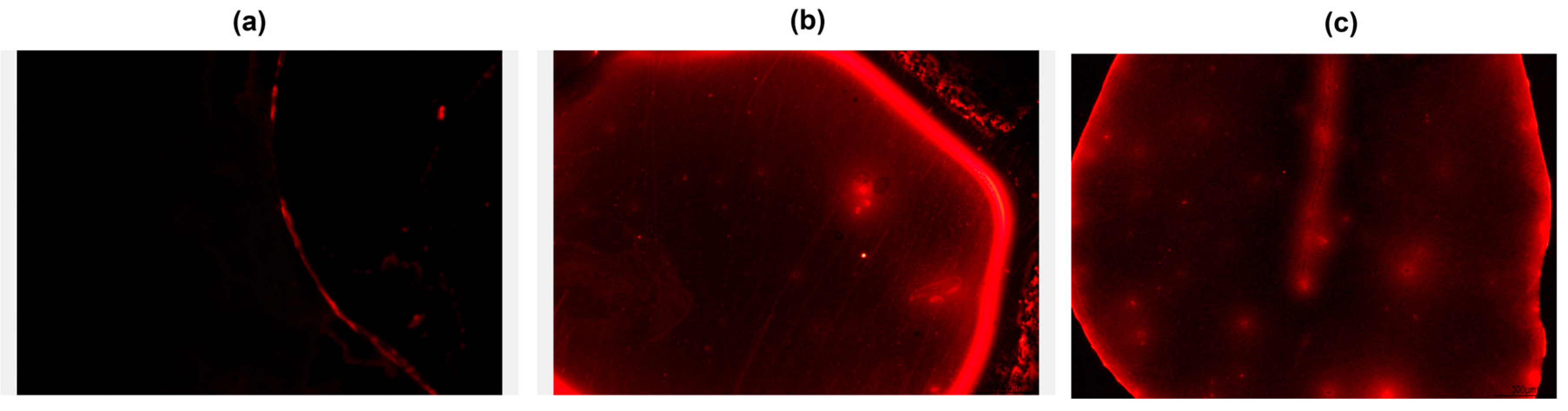
2.5. Optical, Topographical, and Physicochemical Analyses
2.6. Electrochemical Characterization
3. Results and Discussion
3.1. Morphological and Fluorescence Characterization
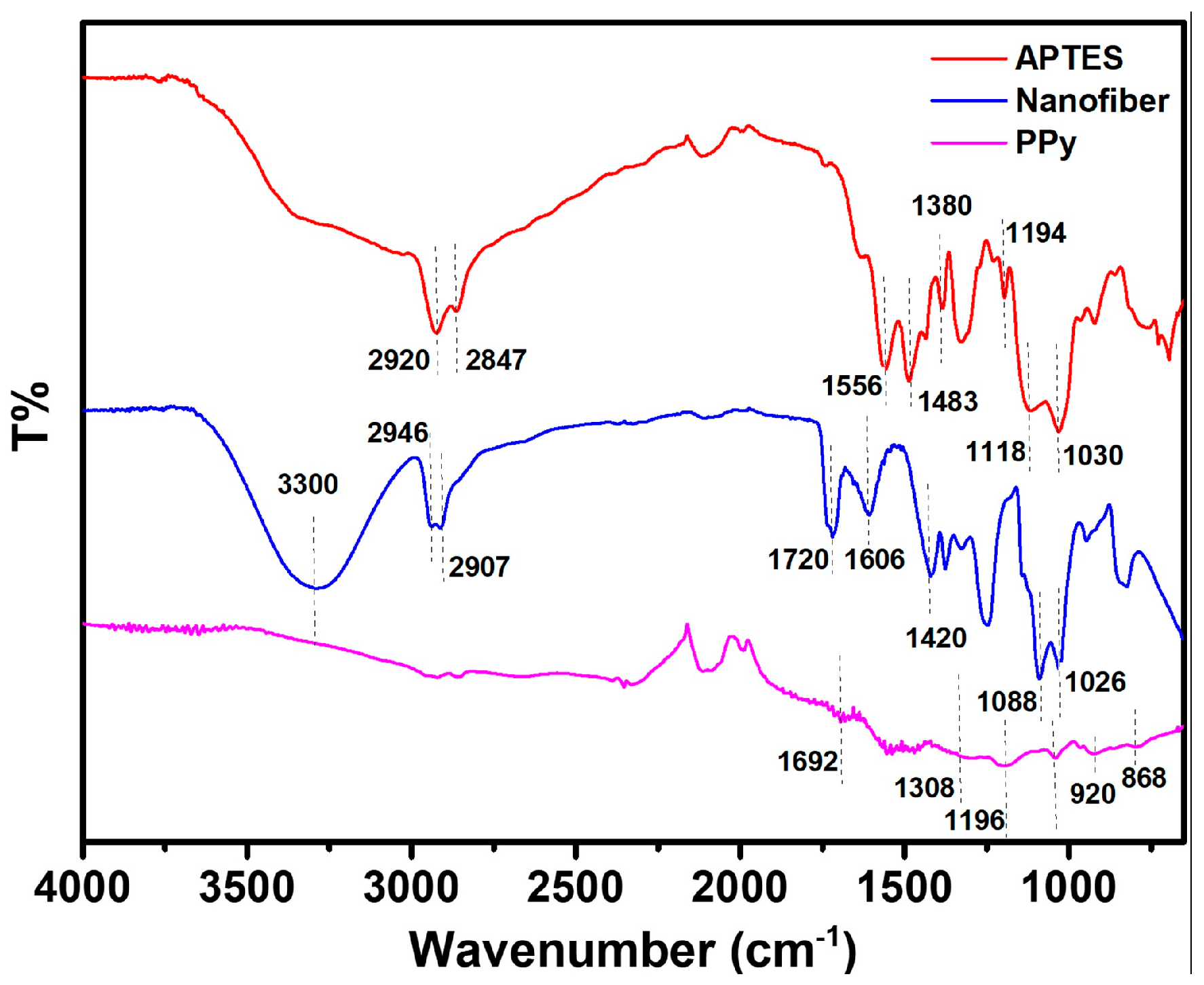
3.2. Wettability and ATR-FTIR Measurements
3.3. XRD and TGA Analysis
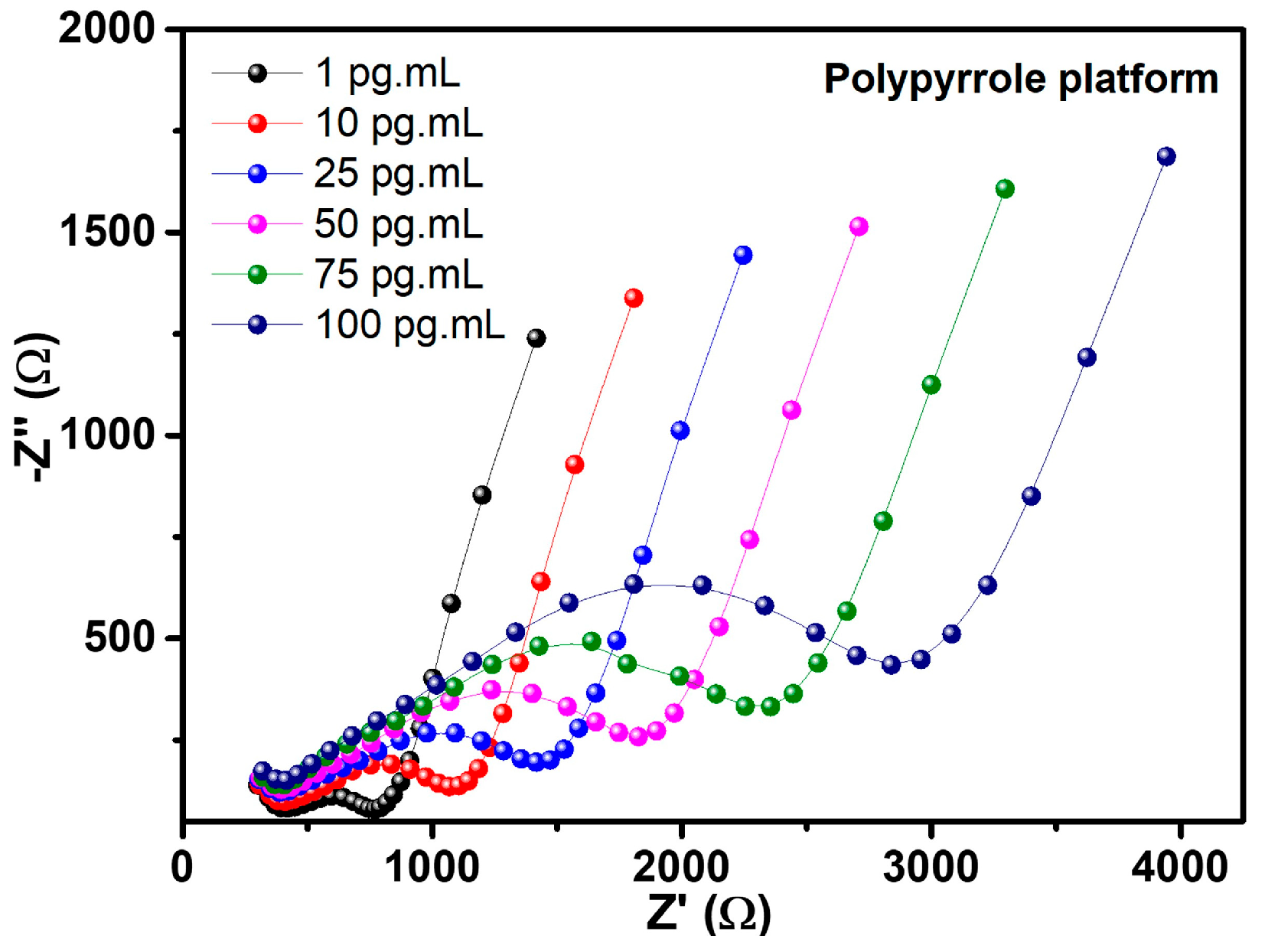
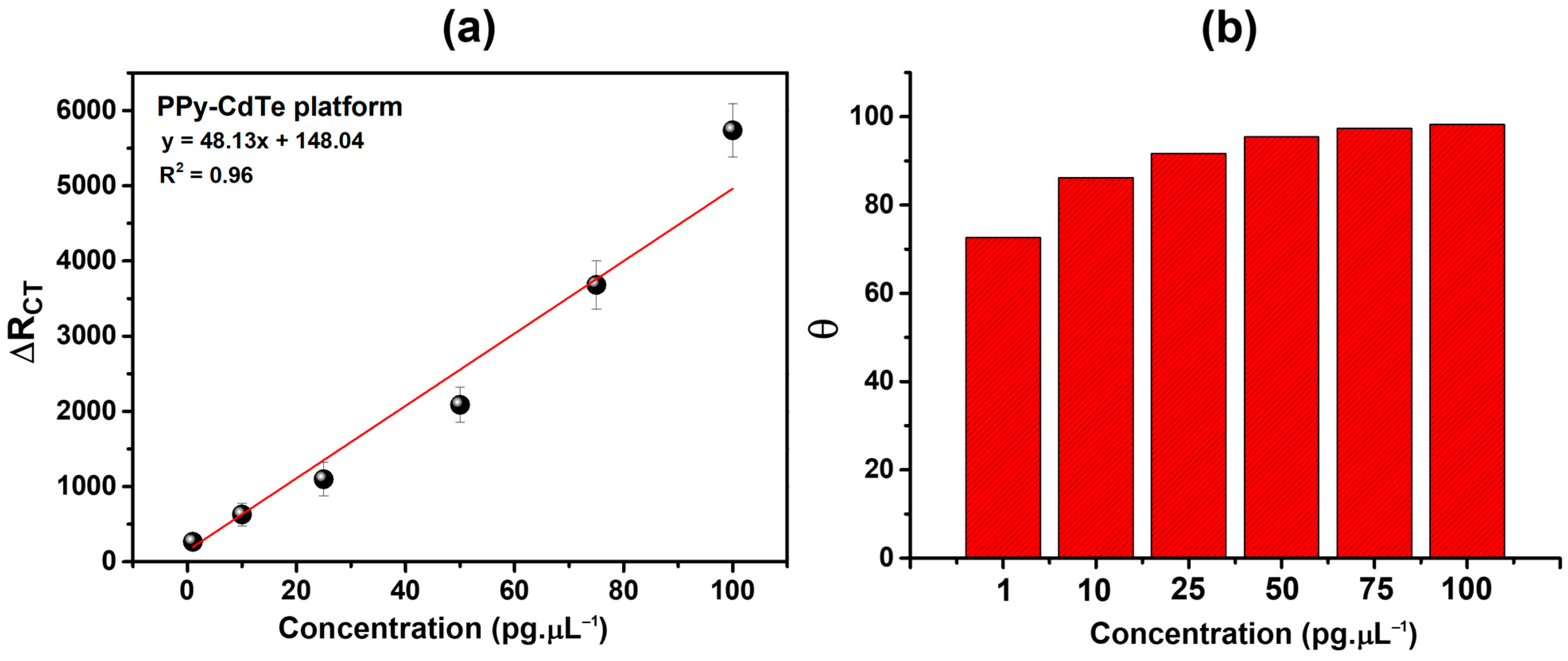
3.4. Electrochemical Investigation of the Biosensing Platform
3.5. Bioanalytical Performance of the Biosensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of Lung Cancer. Contemp. Oncol. 2021, 25, 45. [Google Scholar] [CrossRef]
- Gierada, D.S.; Black, W.C.; Chiles, C.; Pinsky, P.F.; Yankelevitz, D.F. Low-Dose Ct Screening for Lung Cancer: Evidence from 2 Decades of Study. Radiol. Imaging Cancer 2020, 2, e190058. [Google Scholar] [CrossRef]
- Bhatia, D.; Paul, S.; Acharjee, T.; Ramachairy, S.S. Biosensors and Their Widespread Impact on Human Health. Sens. Int. 2024, 5, 100257. [Google Scholar] [CrossRef]
- Hasan, M.R.; Ahommed, M.S.; Daizy, M.; Bacchu, M.S.; Ali, M.R.; Al-Mamun, M.R.; Saad Aly, M.A.; Khan, M.Z.H.; Hossain, S.I. Recent Development in Electrochemical Biosensors for Cancer Biomarkers Detection. Biosens. Bioelectron. X 2021, 8, 100075. [Google Scholar] [CrossRef]
- Tian, Z.; Liang, C.; Zhang, Z.; Wen, H.; Feng, H.; Ma, Q.; Liu, D.; Qiang, G. Prognostic Value of Neuron-Specific Enolase for Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. World J. Surg. Oncol. 2020, 18, 116. [Google Scholar] [CrossRef]
- Mehta, D.; Gupta, D.; Kafle, A.; Kaur, S.; Nagaiah, T.C. Advances and Challenges in Nanomaterial-Based Electrochemical Immunosensors for Small Cell Lung Cancer Biomarker Neuron-Specific Enolase. ACS Omega 2024, 9, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Sanjayan, C.G.; Ravikumar, C.H.; Balakrishna, R.G. Perovskite QD Based Paper Microfluidic Device for Simultaneous Detection of Lung Cancer Biomarkers—Carcinoembryonic Antigen and Neuron Specific Enolase. Chem. Eng. J. 2023, 464, 142581. [Google Scholar] [CrossRef]
- Lin, C.; Wang, Y.; Peng, T.; Liu, P.; Liang, Y.; Kang, W.; Yu, X.; Song, Y.; Shentu, X. Absolute Quantification of Neuron-Specific Enolase Based on Surface Plasmon Resonance. SLAS Discov. 2025, 30, 100205. [Google Scholar] [CrossRef]
- Chen, W.; Hu, S.; Zhou, B.; Ren, Y.; Xue, Y.; Yang, R. Label-Free Immunoassay Based on Chemiluminescence-Functionalized Magnetic Mesoporous Nanoparticles for Rapid Detection of Neuron-Specific Enolase. Microchim. Acta 2025, 192, 638. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Feng, S.; Nag, A.; Afsarimanesh, N.; Han, T.; Mukhopadhyay, S.C. Recent Progress in 3D Printed Mold-Based Sensors. Sensors 2020, 20, 703. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.D.; Hussain, Z.; Yang, K.-L. Aptamer-Based Gold Nanoparticles–PDMS Composite Stamps as a Platform for Micro-Contact Printing. Biosensors 2022, 12, 1067. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, H.; Luo, W.; Yuan, R.; Zhao, Y.; Huang, J.-A.; Yang, X. Microcontact Printing of Gold Nanoparticle at Three-Phase Interface as Flexible Substrate for SERS Detection of MicroRNA. Anal. Chim. Acta 2022, 1229, 340380. [Google Scholar] [CrossRef]
- Song, Y.; Ya, Y.; Cen, X.; Tang, D.; Shi, J.; Wu, Y.; Luo, H.; Huang, K.-J.; Tan, X.; Yan, F. Multiple Signal Amplification Strategy Induced by Biomarkers of Lung Cancer: A Self-Powered Biosensing Platform Adapted for Smartphones. Int. J. Biol. Macromol. 2024, 264, 130661. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Kumar, Y.; Sharma, N.; Chandra, R.; Kumar, S. Disposable Zirconium Trisulfide-Reduced Graphene Oxide Modified Conducting Thread Based Electrochemical Biosensor for Lung Cancer Diagnosis. Bioelectrochemistry 2024, 160, 108801. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.; Aligayev, A.; Jiang, H.; Muhammad; Wang, S.; Yu, X.; Tao, J.; Wang, J.; Jastrzębska, A.; et al. Dual-Mode SERS and Colorimetric Sensor for Lung Cancer VOC-Biomarker Detection Using Hydrogel Patches. Chem. Eng. J. 2025, 523, 168343. [Google Scholar] [CrossRef]
- Ullah, S.; Hashmi, M.; Hussain, N.; Ullah, A.; Sarwar, M.N.; Saito, Y.; Kim, S.H.; Kim, I.S. Stabilized Nanofibers of Polyvinyl Alcohol (PVA) Crosslinked by Unique Method for Efficient Removal of Heavy Metal Ions. J. Water Process Eng. 2020, 33, 101111. [Google Scholar] [CrossRef]
- Bechu, A.; Liao, J.; Huang, C.; Ahn, C.; McKeague, M.; Ghoshal, S.; Moores, A. Cadmium-Containing Quantum Dots Used in Electronic Displays: Implications for Toxicity and Environmental Transformations. ACS Appl. Nano Mater. 2021, 4, 8417–8428. [Google Scholar] [CrossRef]
- Santos, L.G.T.; Silva-Junior, A.G.; Costa, M.P.; Avelino, K.Y.P.S.; Lucena-Silva, N.; Andrade, C.A.S.; Oliveira, M.D.L. Nanostructured Genosensor Platform Based on Polypyrrole Film and Graphene Quantum Dots for the Detection of High-Risk HPV. J. Pharm. Biomed. Anal. 2025, 263, 116920. [Google Scholar] [CrossRef]
- Yang, X.M.; Zhong, Z.W.; Diallo, E.M.; Wang, Z.H.; Yue, W.S. Silicon Wafer Wettability and Aging Behaviors: Impact on Gold Thin-Film Morphology. Mater. Sci. Semicond. Process. 2014, 26, 25–32. [Google Scholar] [CrossRef]
- Vashist, S.K.; Lam, E.; Hrapovic, S.; Male, K.B.; Luong, J.H.T. Immobilization of Antibodies and Enzymes on 3-Aminopropyltriethoxysilane-Functionalized Bioanalytical Platforms for Biosensors and Diagnostics. Chem. Rev. 2014, 114, 11083–11130. [Google Scholar] [CrossRef]
- Yang, J.M.; Yang, J.H.; Tsou, S.C.; Ding, C.H.; Hsu, C.C.; Yang, K.C.; Yang, C.C.; Chen, K.S.; Chen, S.W.; Wang, J.S. Cell Proliferation on PVA/Sodium Alginate and PVA/Poly(γ-Glutamic Acid) Electrospun Fiber. Mater. Sci. Eng. C 2016, 66, 170–177. [Google Scholar] [CrossRef]
- Mahmoodian, M.; Pourabbas, B.; Mohajerzadeh, S. Effect of Anionic Dopants on Thickness, Morphology and Electrical Properties of Polypyrrole Ultra-Thin Films Prepared by in Situ Chemical Polymerization. Thin Solid Films 2015, 583, 255–263. [Google Scholar] [CrossRef]
- Siva, N.; Gunda, K.; Singh, M.; Norman, L.; Kaur, K.; Mitra, S.K. Applied Surface Science Optimization and Characterization of Biomolecule Immobilization on Silicon Substrates Using (3-Aminopropyl) Triethoxysilane (APTES) and Glutaraldehyde Linker. Appl. Surf. Sci. 2014, 305, 522–530. [Google Scholar] [CrossRef]
- Pasternack, R.M.; Rivillon Amy, S.; Chabal, Y.J. Attachment of 3-(Aminopropyl)Triethoxysilane on Silicon Oxide Surfaces: Dependence on Solution Temperature. Langmuir 2008, 24, 12963–12971. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Zhang, L.; Ning, C.; Liu, X.; Liao, J. Preparation and Characterization of APTES Films on Modification Titanium by SAMs. Thin Solid Films 2011, 519, 4997–5001. [Google Scholar] [CrossRef]
- Kharat, H.J.; Kakde, K.P.; Savale, P.A.; Datta, K.; Ghosh, P.; Shirsat, M.D. Synthesis of Polypyrrole Films for the Development of Ammonia Sensor. Polym. Adv. Technol. 2007, 18, 397–402. [Google Scholar] [CrossRef]
- Arora, K.; Chaubey, A.; Singhal, R.; Singh, R.P.; Pandey, M.K.; Samanta, S.B.; Malhotra, B.D.; Chand, S. Application of Electrochemically Prepared Polypyrrole–Polyvinyl Sulphonate Films to DNA Biosensor. Biosens. Bioelectron. 2006, 21, 1777–1783. [Google Scholar] [CrossRef]
- Mohamad, R.; Jamil, N.A.; Wee, M.F.M.R.; Mohamed, M.A.; Azam, M.A.; Hamzah, A.A.; Menon, P.S. Deposition of Polypyrrole-Carboxylated Multi-Walled Carbon Nanotube Nanohybrid Thin Film for Surface Plasmon Resonance Sensor. Int. J. Nanoelectron. Mater. 2023, 16, 305–315. [Google Scholar] [CrossRef]
- Islam, M.S.; Karim, M.R. Fabrication and Characterization of Poly(Vinyl Alcohol)/Alginate Blend Nanofibers by Electrospinning Method. Colloids Surf. A Physicochem. Eng. Asp. 2010, 366, 135–140. [Google Scholar] [CrossRef]
- Jayasekara, R.; Harding, I.; Bowater, I.; Christie, G.B.Y.; Lonergan, G.T. Preparation, Surface Modification and Characterisation of Solution Cast Starch PVA Blended Films. Polym. Test. 2004, 23, 17–27. [Google Scholar] [CrossRef]
- Aloma, K.K.; Sukaryo, S.; Fahlawati, N.I.; Dahlan, K.; Oemar, S. Synthesis of Nanofibers from Alginate-Polyvinyl Alcohol Using Electrospinning Methods. Macromol. Symp. 2020, 391, 1900199. [Google Scholar] [CrossRef]
- Rafiq, M.; Hussain, T.; Abid, S.; Nazir, A.; Masood, R. Development of Sodium Alginate/PVA Antibacterial Nanofibers by the Incorporation of Essential Oils. Mater. Res. Express 2018, 5, 35007. [Google Scholar] [CrossRef]
- Fahmy, A.; Badry, R.; Mabied, A.F.; Wassel, A.R.; Khafagy, R.M.; Ibrahim, M.A. Application of Electrospun Polyvinyl Alcohol/Sodium Alginate Nanofibers as Biosensor. J. Inorg. Organomet. Polym. Mater. 2025, 1–21. [Google Scholar] [CrossRef]
- Mansur, H.S.; Oréfice, R.L.; Mansur, A.A.P. Characterization of Poly(Vinyl Alcohol)/Poly(Ethylene Glycol) Hydrogels and PVA-Derived Hybrids by Small-Angle X-Ray Scattering and FTIR Spectroscopy. Polymer 2004, 45, 7193–7202. [Google Scholar] [CrossRef]
- Helmiyati; Aprilliza, M. Characterization and Properties of Sodium Alginate from Brown Algae Used as an Ecofriendly Superabsorbent. IOP Conf. Ser. Mater. Sci. Eng. 2017, 188, 12019. [Google Scholar] [CrossRef]
- Sionkowska, A. Current Research on the Blends of Natural and Synthetic Polymers as New Biomaterials: Review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Ozturk, M.K.; Nergis, B.; Candan, C. Thermal Analysis of PVA Nanofibrous Membranes. IOP Conf. Ser. Mater. Sci. Eng. 2018, 460, 12048. [Google Scholar] [CrossRef]
- Qin, X.; Dou, G.; Jiang, G.; Zhang, S. Characterization of Poly (Vinyl Alcohol) Nanofiber Mats Cross-Linked with Glutaraldehyde. J. Ind. Text. 2012, 43, 34–44. [Google Scholar] [CrossRef]
- Yamada, H.; Yoshii, K.; Asahi, M.; Chiku, M.; Kitazumi, Y. Cyclic Voltammetry Part 1: Fundamentals. Electrochemistry 2022, 90, 102005. [Google Scholar] [CrossRef]
- Saxena, R.; Srivastava, S. An Insight into Impedimetric Immunosensor and Its Electrical Equivalent Circuit. Sens. Actuators B Chem. 2019, 297, 126780. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Park, E.-J.; Lee, C.-J.; Kim, S.-W.; Pak, J.J.; Min, N.K. Flexible Electrochemical Biosensors Based on O2 Plasma Functionalized MWCNT. Thin Solid Films 2009, 517, 3883–3887. [Google Scholar] [CrossRef]
- Lucena, R.P.S.; Silva-Junior, A.G.; Frías, I.A.M.; Gil, L.H.V.; Cordeiro, M.T.; El Salhi, A.E.; Andrade, C.A.S.; Oliveira, M.D.L. Microcontact Printing of Lectin Self-Assembled Monolayers for Arbovirus Detection. Biotechnol. Prog. 2025, 41, e70008. [Google Scholar] [CrossRef] [PubMed]
- Ekici, R.; Bozdoğan, B.; Denkbaş, E.B. Development of Electrochemical Biosensor Platforms for Determination of Environmental Viral Structures. Appl. Sci. 2022, 12, 12971. [Google Scholar] [CrossRef]
- Brett, C.M.A. Electrochemical Impedance Spectroscopy in the Electrochemical Sensors and Biosensors. Molecules 2022, 27, 1497. [Google Scholar] [CrossRef] [PubMed]
- Pajkossy, T. Voltammetry Coupled with Impedance Spectroscopy. J. Solid State Electrochem. 2020, 24, 2157–2159. [Google Scholar] [CrossRef]
- Couto, M.T.T.; Silva Júnior, A.G.; Avelino, K.Y.P.d.S.; Gil, L.H.V.G.; Cordeiro, M.T.; Oliveira, M.D.L.; Souza de Andrade, C.A.A.S. Development of Optical and Electrochemical Immunodevice for the Dengue Virus Detection. Anal. Methods 2024, 16, 3539–3550. [Google Scholar] [CrossRef]
- Tabish, T.A.; Hayat, H.; Abbas, A.; Narayan, R.J. Graphene Quantum Dots-Based Electrochemical Biosensing Platform for Early Detection of Acute Myocardial Infarction. Biosensors 2022, 12, 77. [Google Scholar] [CrossRef]
- Tabish, T.A.; Hayat, H.; Abbas, A.; Narayan, R.J. Graphene Quantum Dot-Based Electrochemical Biosensing for Early Cancer Detection. Curr. Opin. Electrochem. 2021, 30, 100786. [Google Scholar] [CrossRef]
- Nunez, F.A.; de Oliveira, V.L.; Remuzgo, C.; Silva, M.R.d.A.; Daher, I.; Oliveira, J.R.; Silva, T.L.; Cunha-Neto, E.; Kalil, J.; Santos, K.S.; et al. Electrochemical Immunosensor for Antibody Recognition against SARS-CoV-2 B-Cell Epitope: Impact of RBD Mutations on Antigen–Antibody Binding. J. Mater. Chem. B 2025, 13, 9925–9936. [Google Scholar] [CrossRef] [PubMed]
- Dantas, H.B.; Silva-Junior, A.G.; Silva, N.L.C.L.; Errachid, A.; Oliveira, M.D.L.; Andrade, C.A.S. Genosensor Based on Polypyrrole and Dendrimer-Coated Gold Nanoparticles for Human Papillomavirus Detection. Biochem. Eng. J. 2025, 213, 109551. [Google Scholar] [CrossRef]
- Avelino, K.Y.P.S.; Silva-junior, A.G.; Pitta, M.G.R.; Errachid, A.; Oliveira, M.D.L.; Andrade, A.S. Nanoimmunosensor for the Electrochemical Detection of Oncostatin M Receptor and Monoclonal Autoantibodies in Systemic Sclerosis. Talanta 2023, 256, 124285. [Google Scholar] [CrossRef] [PubMed]
- Moulahoum, H.; Ghorbanizamani, F. The LOD Paradox: When Lower Isn’t Always Better in Biosensor Research and Development. Biosens. Bioelectron. 2024, 264, 116670. [Google Scholar] [CrossRef]
- Genet, S.A.A.M.; Visser, E.; van den Borne, B.E.E.M.; Soud, M.Y.-E.; Belderbos, H.N.A.; Stege, G.; de Saegher, M.E.A.; Eduati, F.; Broeren, M.A.C.; van Dongen, J.; et al. Correction of the NSE Concentration in Hemolyzed Serum Samples Improves Its Diagnostic Accuracy in Small-Cell Lung Cancer. Oncotarget 2020, 11, 2660–2668. [Google Scholar] [CrossRef]
- Liu, C.-C.; Wang, H.; Wang, J.-H.; Wang, L.; Geng, Q.-R.; Chen, X.-Q.; Lu, Y. Serum Neuron-Specific Enolase Levels Are Upregulated in Patients with Acute Lymphoblastic Leukemia and Are Predictive of Prognosis. Oncotarget 2016, 7, 55181–55190. [Google Scholar] [CrossRef][Green Version]
- Silva Junior, A.G.; Frias, I.A.M.; Lima-neto, R.G.; Franco, O.L.; Oliveira, D.L.; Andrade, C.A.S. ELECTROCHEMICAL DETECTION OF GRAM-NEGATIVE BACTERIA THROUGH MASTOPARAN-CAPPED MAGNETIC NANOPARTICLE. Enzyme Microb. Technol. 2022, 160, 110088. [Google Scholar] [CrossRef]
- Autsavapromporn, N.; Duangya, A.; Klunklin, P.; Chitapanarux, I.; Kranrod, C.; Jaikang, C.; Monum, T.; Tokonami, S. Combined Serum IL-6 and CYFRA 21-1 as Potential Biomarkers for Radon-Associated Lung Cancer Risk: A Pilot Study. Biomedicines 2025, 13, 2145. [Google Scholar] [CrossRef]
- Avelino, K.Y.P.S.; dos Santos, G.S.; Frías, I.A.M.; Silva-Junior, A.G.; Pereira, M.C.; Pitta, M.G.R.; de Araújo, B.C.; Errachid, A.; Oliveira, M.D.L.; Andrade, C.A.S. Nanostructured Sensor Platform Based on Organic Polymer Conjugated to Metallic Nanoparticle for the Impedimetric Detection of SARS-CoV-2 at Various Stages of Viral Infection. J. Pharm. Biomed. Anal. 2021, 206, 114392. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.; Mohanty, P.; Dash, P.P.; Mohapatra, P.; Shubhadarshinee, L.; Behura, R.; Barick, A.K.; Mohapatra, P.; Jali, B.R. Selective Binding of Bovine Serum Albumin (BSA): A Comprehensive Review. Biointerface Res. Appl. Chem. 2023, 13, 555. [Google Scholar] [CrossRef]
- Aydın, M. An Ultrasensitive Immunosensor Based on Tri-Armed Star Poly(Glycidyl Methacrylate) Polymer-Coated ITO-PET Electrode for Detection of Neuron-Specific Enolase in Human Serum. Int. J. Environ. Anal. Chem. 2020, 100, 492–506. [Google Scholar] [CrossRef]
- Mehta, D.; Thakur, N.; Nagaiah, T.C. Label-Free Assessment of Neuron-Specific Enolase via Polydopamine over a Carbon-Nanotube-Based Flexible Immunosensor. ACS Appl. Bio Mater. 2024, 7, 4702–4709. [Google Scholar] [CrossRef] [PubMed]
- Zu, J.; Xuan, X.; Zhang, W.; Li, M.; Jiang, D.; Li, H. Wireless Gold/Boron–Nitrogen-Codoped Graphene-Based Antenna Immunosensor for the Rapid Detection of Neuron-Specific Enolase. Anal. Chem. 2024, 96, 6826–6835. [Google Scholar] [CrossRef] [PubMed]

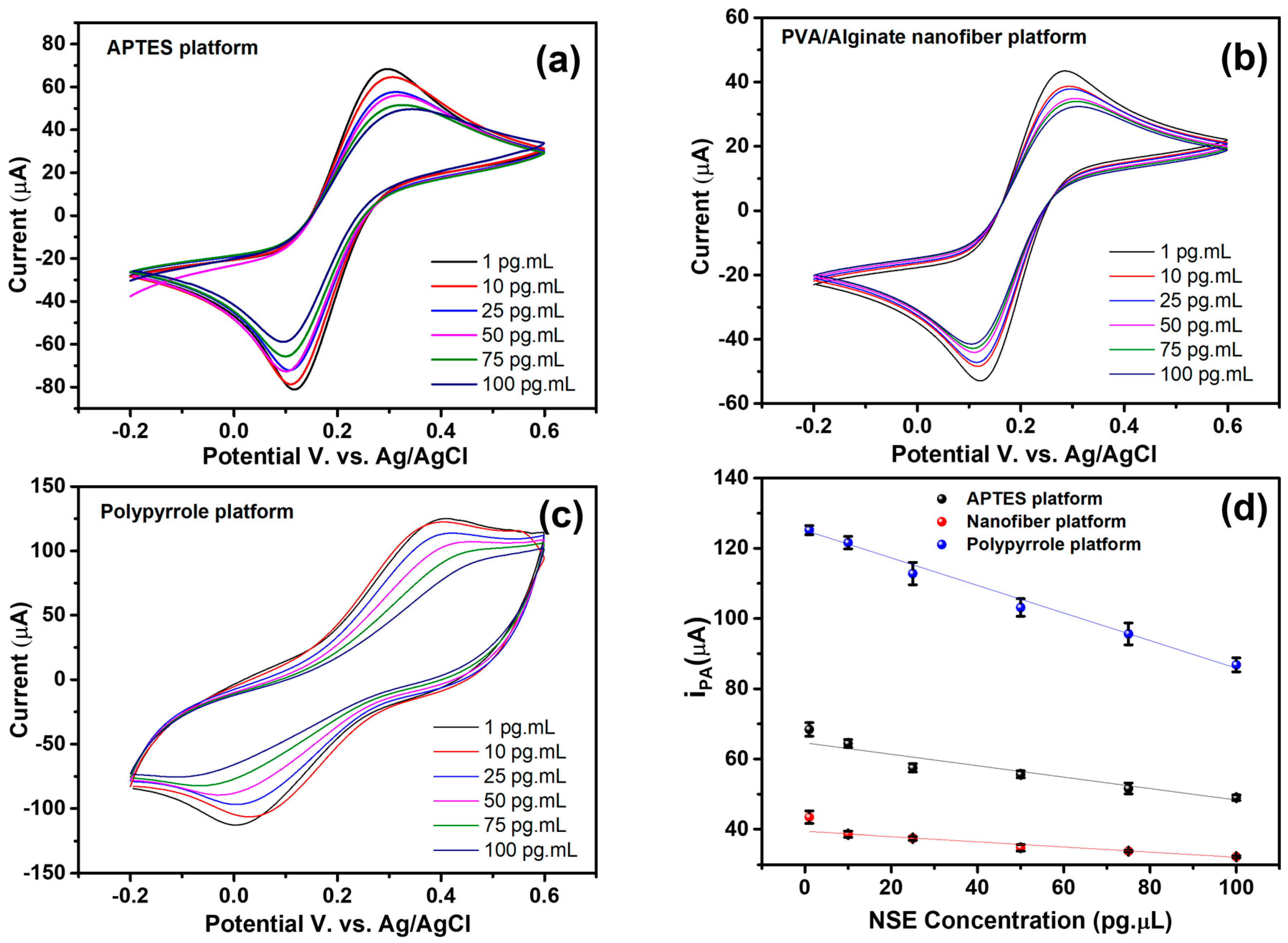
| Analyte Concentration (pg·µL−1) | Platform IPA (µA) | IPA (µA) | ∆I (%) | |
|---|---|---|---|---|
| APTES platform | 75.77 | |||
| 1 | 68.4 ± 0.66 | 9.72 ± 0.24 | ||
| 10 | 64.42 ± 0.94 | 14.97 ± 1.12 | ||
| 25 | 57.48 ± 0.30 | 24.13 ± 0.62 | ||
| 50 | 55.63 ± 0.92 | 26.58 ± 0.71 | ||
| 75 | 51.60 ± 1.13 | 31.89 ± 1.24 | ||
| 100 | 49.01 ± 0.96 | 35.33 ± 1.06 | ||
| Nanofiber platform | 44.91 | |||
| 1 | 43.44 ± 0.45 | 3.27 ± 0.50 | ||
| 10 | 38.64 ± 0.19 | 13.96 ± 0.28 | ||
| 25 | 37.5 ± 0.08 | 16.49 ± 0.11 | ||
| 50 | 34.84 ± 0.33 | 22.42 ± 0.19 | ||
| 75 | 33.86 ± 0.24 | 24.60 ± 0.09 | ||
| 100 | 32.28 ± 0.14 | 28.12 ± 0.21 | ||
| PPy platform | 158.52 | |||
| 1 | 125.19 ± 0.92 | 21.02 ± 0.83 | ||
| 10 | 121.61 ± 1.35 | 23.68 ± 0.70 | ||
| 25 | 112.8 ± 1.28 | 29.29 ± 1.11 | ||
| 50 | 103.15 ± 1.46 | 34.92 ± 0.99 | ||
| 75 | 95.61 ± 0.94 | 39.68 ± 0.87 | ||
| 100 | 86.80 ± 0.85 | 45.24 ± 0.76 |
| Modified Electrode | Analyte Concentration (pg·µL−1) | RCT (kΩ) | CPE (µT) | RS (kΩ) | ZW (kσ) |
|---|---|---|---|---|---|
| Ppy platform | 1 pg·µL−1 | 0.402 ± 0.021 | 20.96 ± 0.49 | 0.587 ± 0.005 | 1.009 ± 0.006 |
| 10 pg·µL−1 | 0.801 ± 0.032 | 37.02 ± 0.64 | 0.553 ± 0.016 | 1.133 ± 0.012 | |
| 25 pg·µL−1 | 1.318 ± 0.019 | 46.25 ± 0.85 | 0.502 ± 0.014 | 0.824 ± 0.018 | |
| 50 pg·µL−1 | 2.405 ± 0.038 | 58.18 ± 0.94 | 0.307 ± 0.017 | 1.009 ± 0.016 | |
| 75 pg·µL−1 | 4.159 ± 0.045 | 68.82 ± 0.52 | 0.244 ± 0.007 | 1.008 ± 0.015 | |
| 100 pg·µL−1 | 6.421 ± 0.059 | 72.09 ± 0.71 | 0.326 ± 0.009 | 1.001 ± 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Junior, A.G.; Errachid, A.; Zine, N.; Hangouet, M.; Raffin, G.; Pereira, M.C.; Oliveira, M.D.L.; Andrade, C.A.S. Microcontact-Printed Flexible Electrodes for Label-Free Electrochemical Detection of Lung Cancer Biomarker. Chemosensors 2025, 13, 377. https://doi.org/10.3390/chemosensors13110377
Silva-Junior AG, Errachid A, Zine N, Hangouet M, Raffin G, Pereira MC, Oliveira MDL, Andrade CAS. Microcontact-Printed Flexible Electrodes for Label-Free Electrochemical Detection of Lung Cancer Biomarker. Chemosensors. 2025; 13(11):377. https://doi.org/10.3390/chemosensors13110377
Chicago/Turabian StyleSilva-Junior, Alberto G., Abdelhamid Errachid, Nadia Zine, Marie Hangouet, Guy Raffin, Michelly C. Pereira, Maria D. L. Oliveira, and Cesar A. S. Andrade. 2025. "Microcontact-Printed Flexible Electrodes for Label-Free Electrochemical Detection of Lung Cancer Biomarker" Chemosensors 13, no. 11: 377. https://doi.org/10.3390/chemosensors13110377
APA StyleSilva-Junior, A. G., Errachid, A., Zine, N., Hangouet, M., Raffin, G., Pereira, M. C., Oliveira, M. D. L., & Andrade, C. A. S. (2025). Microcontact-Printed Flexible Electrodes for Label-Free Electrochemical Detection of Lung Cancer Biomarker. Chemosensors, 13(11), 377. https://doi.org/10.3390/chemosensors13110377






