Tropical Weathering Effects on Neat Gasoline: An Analytical Study of Volatile Organic Profiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Gas Chromatography/Mass Spectrometry (GC-MS)
2.3. Data Collection and Analysis
3. Results and Discussion
3.1. Identification of Compounds in Weathered Gasoline
3.2. Effect of Evaporation on Chemical Composition of Weathered Gasoline
3.3. Effect of Environment Temperature on Weathering Rate of Gasoline
3.4. Effect of Evaporation on Association of Weathered Gasoline Using PPMC Coefficients
3.5. Comparing Target Compound Concentration Between Indoor and Outdoor Gasoline Using t-Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACT | Activated Charcoal Tablet |
| ASTM | American Society for Testing and Materials |
| CTIF | Comité Technique International de Prévention et d’Extinction du Feu |
| GCMS | Gas Chromatography Mass Spectrometry |
| ILR | Ignitable Liquid Residue |
| NFPA | National Fire Protection Association |
| NIST | National Institute of Standards and Technology |
| PI | Petrol Indoor |
| PO | Petrol Outdoor |
| PPMC | Pearson product–moment correlation coefficients |
| QDA | Quadratic Discriminant Analysis |
| SVM | Support Vector Machines |
| VOC | Volatile Organic Compound |
References
- Collins, J.; Barnoux, M.; Langdon, P.E. Adults with intellectual disabilities and/or autism who deliberately set fires: A systematic review. Aggress. Violent Behav. 2021, 56, 101545. [Google Scholar] [CrossRef]
- CTIF. World Fire Statistic report. No.29 International Association of Fire and Rescue Services. Center for Fire Statistics World Fire Statistics. 2022. Available online: https://www.ctif.org/sites/default/files/2024-06/CTIF_Report29_ERG.pdf (accessed on 26 June 2025).
- USBDC. Annual Arson Incident Report; United States of Bomb Data Center; Bureau of Alcohol, Tobacco, Firearms and Explosives: Washington, DC, USA, 2021.
- Noumeur, A.; Tohir, M.Z.M.; Said, M.S.M.; Baharudin, M.R.; Yusoff, H.M. 399 Fire incidence and relative risk analysis in Malaysia (2000–2019): Patterns, causes, and implications for safety and prevention. Inj. Prev. 2024, 30, A83. [Google Scholar]
- Osman, K.; Gabriel, G.F.; Hamzah, N.H. Crime scene investigation issues: Present issues and future recom-mendations. J. Undang-Undang Dan Masy. 2021, 28, 3–10. [Google Scholar] [CrossRef]
- Smith, P. Fire science myths: Examining arson and wrongful convictions. SFU Undergrad. Res. Symp. J. 2020, 1, 1. [Google Scholar]
- Lentini, J.J. Fire scene inspection methodology. In Encyclopedia of Forensic Sciences, 2nd ed.; Academic Press: London, UK, 2013; pp. 392–395. [Google Scholar]
- NFPA. NFPA 921 Standard Development. National Fire Protection Association. 2024. Available online: https://www.nfpa.org/codes-and-standards/nfpa-921-standard-development/921 (accessed on 15 August 2025).
- Desa, W.N.S.M.; Daéid, N.N.; Ismail, D.; Savage, K. Application of unsupervised chemometric analysis and self-organizing feature map (SOFM) for the classification of lighter fuels. Anal. Chem. 2010, 82, 6395–6400. [Google Scholar] [CrossRef] [PubMed]
- Low, Y.; Tyrrell, E.; Gillespie, E.; Quigley, C. Review: Recent advancements and moving trends in chemical analysis of fire debris. Forensic Sci. Int. 2023, 345, 111623. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, Y.; Dai, F.; Yan, X.; Hamdalnile, A.; Wu, L. Rapid detection of accelerants in fire debris using a field portable mid-infrared quantum cascade laser-based analyzer. Open J. Appl. Sci. 2023, 13, 746–757. [Google Scholar] [CrossRef]
- Lim, P.W.; Abdullah, A.F.L.; Chang, K.H. Analisis kromatografi gas forensik bagi gasolin yang masanya berlalu dalam penyiasatan tempat kebakaran. Malays. J. Anal. Sci. 2018, 22, 72–79. [Google Scholar]
- Dhabbah, A.M. Detection of petrol residues in natural and synthetic textiles before and after burning using SPME and GC-MS. Aust. J. Forensic Sci. 2020, 52, 194–207. [Google Scholar] [CrossRef]
- Office of Justice Programs. Detection of Gasoline as an Accelerant. Available online: https://ojp.gov/ncjrs/virtual-library/abstracts/detection-gasoline-accelerant (accessed on 19 February 2024).
- Gabriel, G.F.; Ismail, A.; Ayuni, A.; Osman, K.; Hamzah, N.H. Analisis produk penguraian haba yang terhasil daripada tisu khinzir yang terdedah kepada pembakaran terbuka. Malays. J. Anal. Sci. 2017, 21, 585–596. [Google Scholar]
- Gabriel, G.F.; Ghazali, N.F.; Kumaraguru, D.; Osman, K.; Hamzah, N.H. Volatile chemical component differences between fully and partially dried merbau (intsia sp.) wood using gas chromatography-mass spectrometry (GC-MS). Malays. J. Sains Kesihat. Malays. 2019, 17, 85–97. [Google Scholar]
- Willis, I.C.; Fan, Z.; Davidson, J.T.; Jackson, G.P. Weathering of ignitable liquids at elevated temperatures: A thermodynamic model, based on laws of ideal solutions, to predict weathering in structure fires. Forensic Chem. 2020, 18, 100215. [Google Scholar] [CrossRef]
- Hodálik, M.; Veľková, V.; Kačíková, D. Comparing the weathering process of gasoline in selected residues by HS-GC-MS method. Delta 2022, 38–48. [Google Scholar]
- Birks, H.L.; Cochran, A.R.; Williams, T.J.; Jackson, G.P. The surprising effect of temperature on the weathering of gasoline. Forensic Chem. 2017, 4, 32–40. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Ferreiro-González, M.; Barbero, G.F.; Ayuso, J.; Palma, M.; Barroso, C.G. Study of the weathering process of gasoline by eNose. Sensors 2018, 18, 139. [Google Scholar] [CrossRef]
- ASTM E1618-19; Standard Test Method for Ignitable Liquid Residues in Extracts from Fire Debris Samples by Gas Chromatography-Mass Spectrometry. American Society of Testing & Materials: West Conshohocken, PA, USA, 2019. Available online: https://store.astm.org/e1618-19.html (accessed on 15 January 2025).
- Okamoto, K.; Watanabe, N.; Hagimoto, Y.; Miwa, K.; Ohtani, H. Changes in evaporation rate and vapor pressure of gasoline with progress of evaporation. Fire Saf. J. 2009, 44, 756–763. [Google Scholar] [CrossRef]
- Hodálik, M.; Veľková, V.; Kačíková, D. The effect of weathering time on the change in the gasoline residues composition. Delta 2021, 15, 7–15. [Google Scholar]
- Falatová, B.; Ferreiro-González, M.; Kačíková, D.; Galla, Š.; Palma, M.; Barosso, C.G. Multivariate statistical analysis in fire debris analysis. Fire Prot. Saf. Sci. J. 2018, 12, 82–90. [Google Scholar]
- Amalina, F.A.S.; Hasan, M.N. Classification of petroleum-based accelerants in fire debris using gas-chromatography mass spectrometry and chemometric technique. In Proceedings of the 2nd International Science Postgraduate, Universiti Teknologi Malaysia, Johor Bahru, Malaysia, 19 February 2014. [Google Scholar]
- Md Ghazi, M.G.; Lee, L.C.; Samsudin, A.S.; Sino, H. Comparison of decision tree and naïve Bayes algorithms in detecting trace residue of gasoline based on gas chromatography–mass spectrometry data. Forensic Sci. Res. 2023, 8, 249–255. [Google Scholar] [CrossRef]
- Lennard, C.J.; Tristan Rochaix, V.; Margot, P.; Huber, K. A GC–MS database of target compound chromatograms for the identification of arson accelerants. Sci. Justice 1995, 35, 19–30. [Google Scholar] [CrossRef]
- ASTM E1412; Standard Practice for Separation of Ignitable liquid Residues from Fire Debris Samples by Passive Headspace Concentration with Activated Charcoal. American Society of Testing & Materials: West Conshohocken, PA, USA, 2019. Available online: https://store.astm.org/e1412-19.html (accessed on 15 August 2025).
- Turner, D.A.; Williams, M.; Sigman, M.A.; Goodpaster, J.V. A comprehensive study of the alteration of ignitable liquids by weathering and microbial degradation. J. Forensic Sci. 2018, 63, 58–65. [Google Scholar] [CrossRef]
- Upadhyay, R.; Patel, K.; Upadhya, U. A review article on advancements in GC-MS. Int. J. Pharm. Res. Appl. 2023, 8, 54–59. [Google Scholar]
- Prajapati, P.; Tailor, P.; Shahi, A.; Acharya, A.; Shah, S. Application of taguchi oa and box–behnken design for the implementation of DoE-based AQbDapproach to HPTLC method for simultaneous estimation of Azilsartan and Cilnidipine. J. Chromatogr. Sci. 2023, 61, 725–736. [Google Scholar] [CrossRef]
- Sousa, P.F.M.; de Waard, A.; Åberg, K.M. Elucidation of chromatographic peak shifts in complex samples using a chemometrical approach. Anal. Bioanal. Chem. 2018, 410, 5229–5235. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Li, L. Effects of solvent evaporation methods and short-term room temperature storage on high-coverage cellular metabolome analysis. Metabolites 2023, 13, 1052. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.; Williams, M.R.; Thurn, N.A.; Sigman, M.E. Model distribution effects on likelihood ratios in fire debris analysis. Separations 2018, 5, 44. [Google Scholar] [CrossRef]

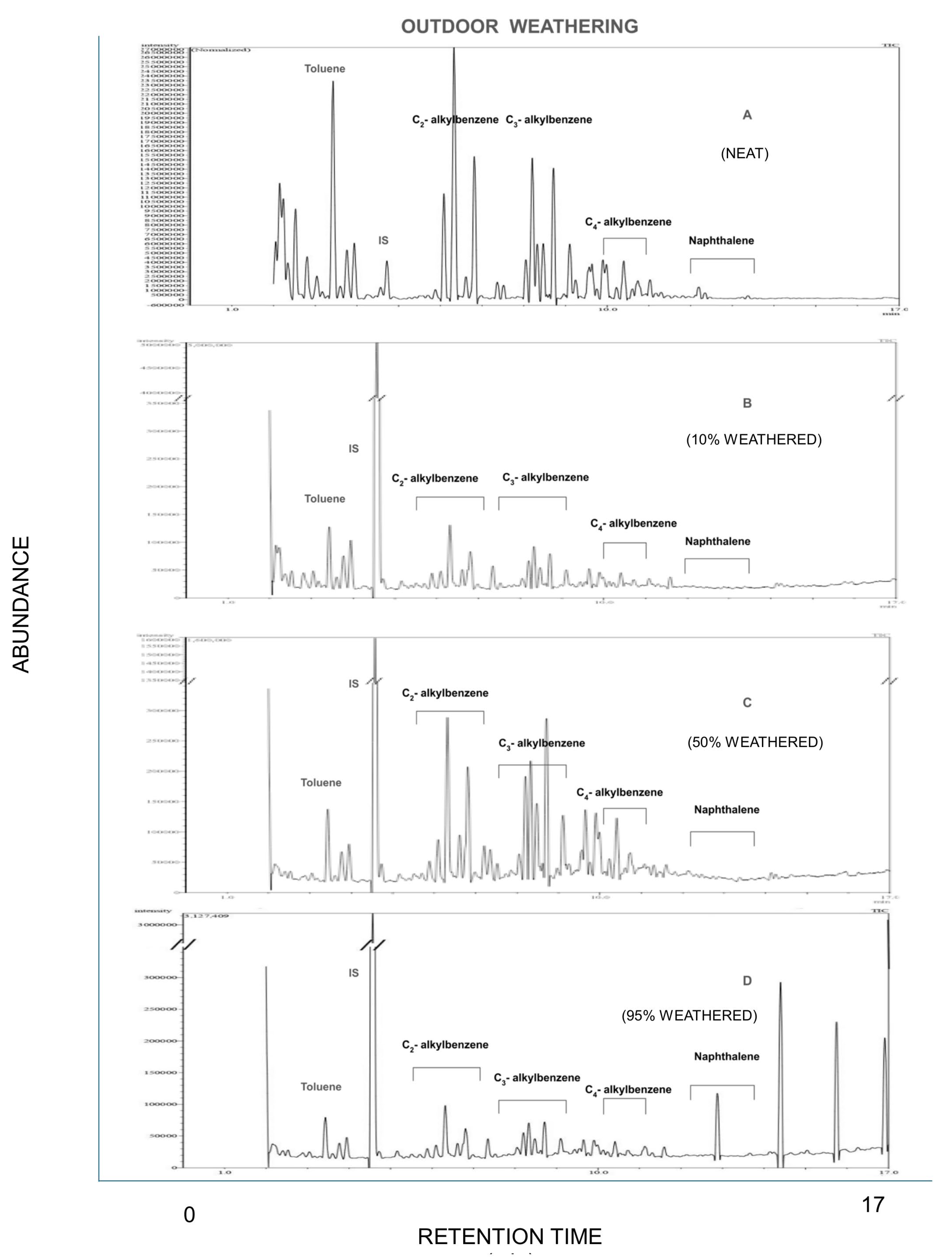

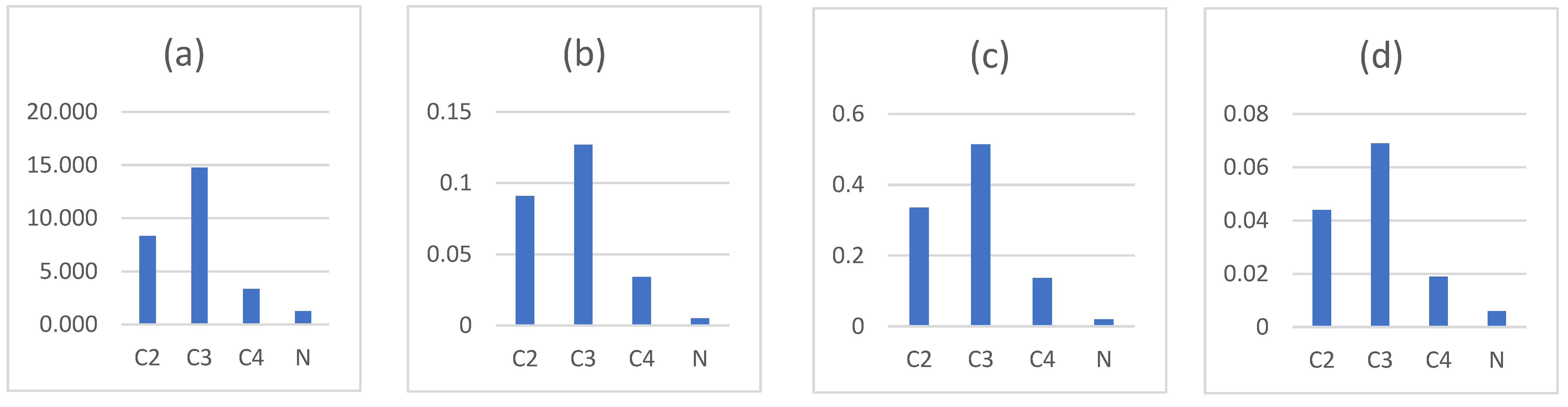
| Retention Time | Target Compound | Chemical Group |
|---|---|---|
| 3.429 | toluene | C1-alkylbenzene |
| 6.091 | ethylbenzene | C2-alkylbenzene |
| 6.313 | p-xylene | C2-alkylbenzene |
| 6.818 | o-xylene | C2-alkylbenzene |
| 8.056 | propylbenzene | C3-alkylbenzene |
| 8.193 | 1-ethyl-3-methylbenzene | C3-alkylbenzene |
| 8.224 | 1-ethyl-4-methylbenzene | C3-alkylbenzene |
| 8.325 | 1,3,5-trimethylbenzene | C3-alkylbenzene |
| 8.469 | 1-ethyl-2-methylbenzene | C3-alkylbenzene |
| 8.708 | 1,2,4-trimethylbenzene | C3-alkylbenzene |
| 9.104 | 1,2,3-trimethylbenzene | C3-alkylbenzene |
| 9.25 | indane | benzocyclopentane |
| 9.56 | 1-methyl-4-propylbenzene | C4-alkylbenzene |
| 9.61 | 1-methyl-3-propylbenzene | C4-alkylbenzene |
| 9.754 | 1-methyl-2-propylbenzene | C4-alkylbenzene |
| 10.227 | 1,2,3,4-tetramethylbenzene | C4-alkylbenzene |
| 10.378 | 1,2,4,5-tetramethylbenzene | C4-alkylbenzene |
| 10.418 | 1,2,3,5-tetramethylbenzene | C4-alkylbenzene |
| 11.01 | naphthalene | naphthalene |
| 11.476 | dodecane | alkane |
| 11.641 | tridecane | alkane |
| 12.199 | 1-methylnaphthalene | methylnaphthalene |
| 12.352 | 2-methylnaphthalene, | methylnaphthalene |
| 13.376 | 2,3-dimethylnaphthalene, | dimethylnaphthalene |
| 13.537 | 1,3-dimethylnaphthalene | dimethylnaphthalene |
| Environment Condition | Indoor | Outdoor | ||||||||||
| Weathering Percentage (%) | 10 | 25 | 50 | 75 | 90 | 95 | 10 | 25 | 50 | 75 | 90 | 95 |
| Heat Index (°C) | 25 | 25 | 24 | 24 | 24 | 24 | 48 | 36 | 36 | 25 | 33 | 28 |
| Temperature (°C) | 24.5 | 24.5 | 23.5 | 23.5 | 23.5 | 24 | 36.5 | 30 | 29.5 | 24 | 27.5 | 26 |
| Humidity (%rh) | 78 | 78 | 77.5 | 82.5 | 78.5 | 77.5 | 57.5 | 74 | 77.5 | 95 | 90 | 92 |
| Dewing Point (°C) | 20.4 | 20.4 | 19.3 | 20.3 | 19.5 | 19.8 | 26.7 | 24.9 | 25.1 | 23.1 | 25.7 | 24.6 |
| Sampling Day | Day 1 | Day 2 | Day 1 | Day 2 | Day 5 | Day 9 | Day 11 | |||||
| Neat | PO10 | PO125 | PO50 | PO75 | PO90 | PO95 | PI10 | PI25 | PI50 | PI75 | PI90 | PI95 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neat | 1.000 | ||||||||||||
| PO10 | 0.928 | 1.000 | |||||||||||
| PO125 | 0.911 | 0.995 | 1.000 | ||||||||||
| PO50 | 0.872 | 0.951 | 0.957 | 1.000 | |||||||||
| PO75 | 0.906 | 0.993 | 0.993 | 0.966 | 1.000 | ||||||||
| PO90 | 0.898 | 0.993 | 0.995 | 0.961 | 0.996 | 1.000 | |||||||
| PO95 | 0.906 | 0.994 | 0.996 | 0.967 | 0.997 | 0.998 | 1.000 | ||||||
| PI10 | 0.907 | 0.977 | 0.983 | 0.970 | 0.988 | 0.988 | 0.986 | 1.000 | |||||
| PI25 | 0.919 | 0.982 | 0.987 | 0.972 | 0.990 | 0.990 | 0.989 | 0.999 | 1.000 | ||||
| PI50 | 0.874 | 0.950 | 0.961 | 0.970 | 0.970 | 0.968 | 0.965 | 0.993 | 0.990 | 1.000 | |||
| PI75 | 0.741 | 0.837 | 0.867 | 0.922 | 0.877 | 0.874 | 0.870 | 0.925 | 0.916 | 0.960 | 1.000 | ||
| PI90 | 0.346 | 0.522 | 0.595 | 0.646 | 0.580 | 0.588 | 0.583 | 0.631 | 0.616 | 0.682 | 0.827 | 1.000 | |
| PI95 | 0.187 | 0.393 | 0.476 | 0.505 | 0.450 | 0.463 | 0.458 | 0.490 | 0.474 | 0.537 | 0.695 | 0.975 | 1.000 |
| Compound | p-Value | Mwt (g/mol) |
|---|---|---|
| Toluene | 0.0265 | 92.41 |
| Ethylbenzene | 0.2026 | 106.17 |
| p-xylene | 0.4437 | 106.17 |
| o-xylene | 0.3441 | 106.17 |
| Propylbenzene | 0.5647 | 120.19 |
| 1-ethyl-3-methylbenzene | 0.0183 | 120.19 |
| 1-ethyl-4-methylbenzene | 0.0136 | 120.19 |
| 1,3,5-trimethylbenzene | 0.0016 | 120.19 |
| 1-ethyl-2-methylbenzene | 0.0407 | 120.19 |
| 1,2,4-trimethylbenzene | 0.0316 | 120.19 |
| 1,2,3-trimethylbenzene | 0.3441 | 120.19 |
| Indane | 0.1846 | 118.18 |
| 1-methyl-4-propylbenzene | 0.1042 | 134.22 |
| 1-methyl-3-propylbenzene | 0.1471 | 134.22 |
| 1-methyl-2-propylbenzene | 0.1309 | 134.22 |
| 1,2,3,4-tetramethylbenzene | 0.3037 | 134.22 |
| 1,2,4,5-tetramethylbenzene | 0.1446 | 134.22 |
| 1,2,3,5-tetramethylbenzene | 0.1853 | 134.22 |
| Dodecane | 0.2779 | 170.34 |
| Tridecane | 0.1982 | 184.37 |
| Naphthalene | 0.4471 | 128.17 |
| 1-methylnaphthalene | 0.3110 | 142.20 |
| 2-methylnaphthalene, | 0.5097 | 142.20 |
| 2,3-dimethylnaphthalene, | 1.783 | 156.22 |
| 1,3-dimethylnaphthalene | 1.814 | 156.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, K.; Ahmad Mazlani, N.; Gabriel, G.F.; Hamzah, N.H.; Abu Hassan, R.; Ismail, D.; Mat Desa, W.N.S. Tropical Weathering Effects on Neat Gasoline: An Analytical Study of Volatile Organic Profiles. Chemosensors 2025, 13, 363. https://doi.org/10.3390/chemosensors13100363
Osman K, Ahmad Mazlani N, Gabriel GF, Hamzah NH, Abu Hassan R, Ismail D, Mat Desa WNS. Tropical Weathering Effects on Neat Gasoline: An Analytical Study of Volatile Organic Profiles. Chemosensors. 2025; 13(10):363. https://doi.org/10.3390/chemosensors13100363
Chicago/Turabian StyleOsman, Khairul, Naadiah Ahmad Mazlani, Gina Francesca Gabriel, Noor Hazfalinda Hamzah, Rogayah Abu Hassan, Dzulkiflee Ismail, and Wan Nur Syuhaila Mat Desa. 2025. "Tropical Weathering Effects on Neat Gasoline: An Analytical Study of Volatile Organic Profiles" Chemosensors 13, no. 10: 363. https://doi.org/10.3390/chemosensors13100363
APA StyleOsman, K., Ahmad Mazlani, N., Gabriel, G. F., Hamzah, N. H., Abu Hassan, R., Ismail, D., & Mat Desa, W. N. S. (2025). Tropical Weathering Effects on Neat Gasoline: An Analytical Study of Volatile Organic Profiles. Chemosensors, 13(10), 363. https://doi.org/10.3390/chemosensors13100363






