Nanostructured CeO2-C Derived from Ce-BDC Precursors for Room-Temperature Ammonia Sensing
Abstract
1. Introduction
2. Experiment Section
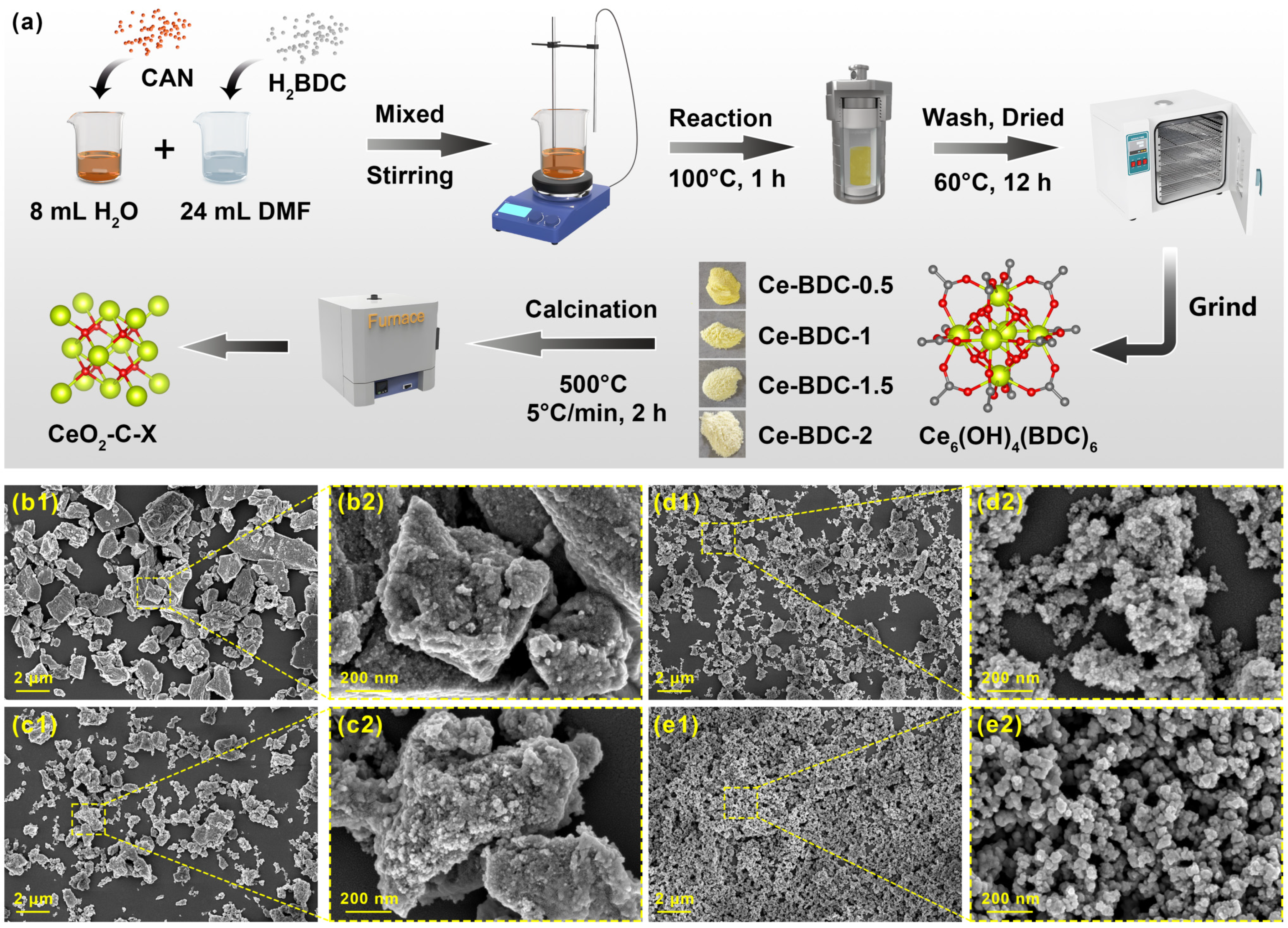
2.1. Fabrication of CeO2-C-X Gas Sensing Materials
2.2. Characterization and Gas Sensing Properties
3. Results and Discussion
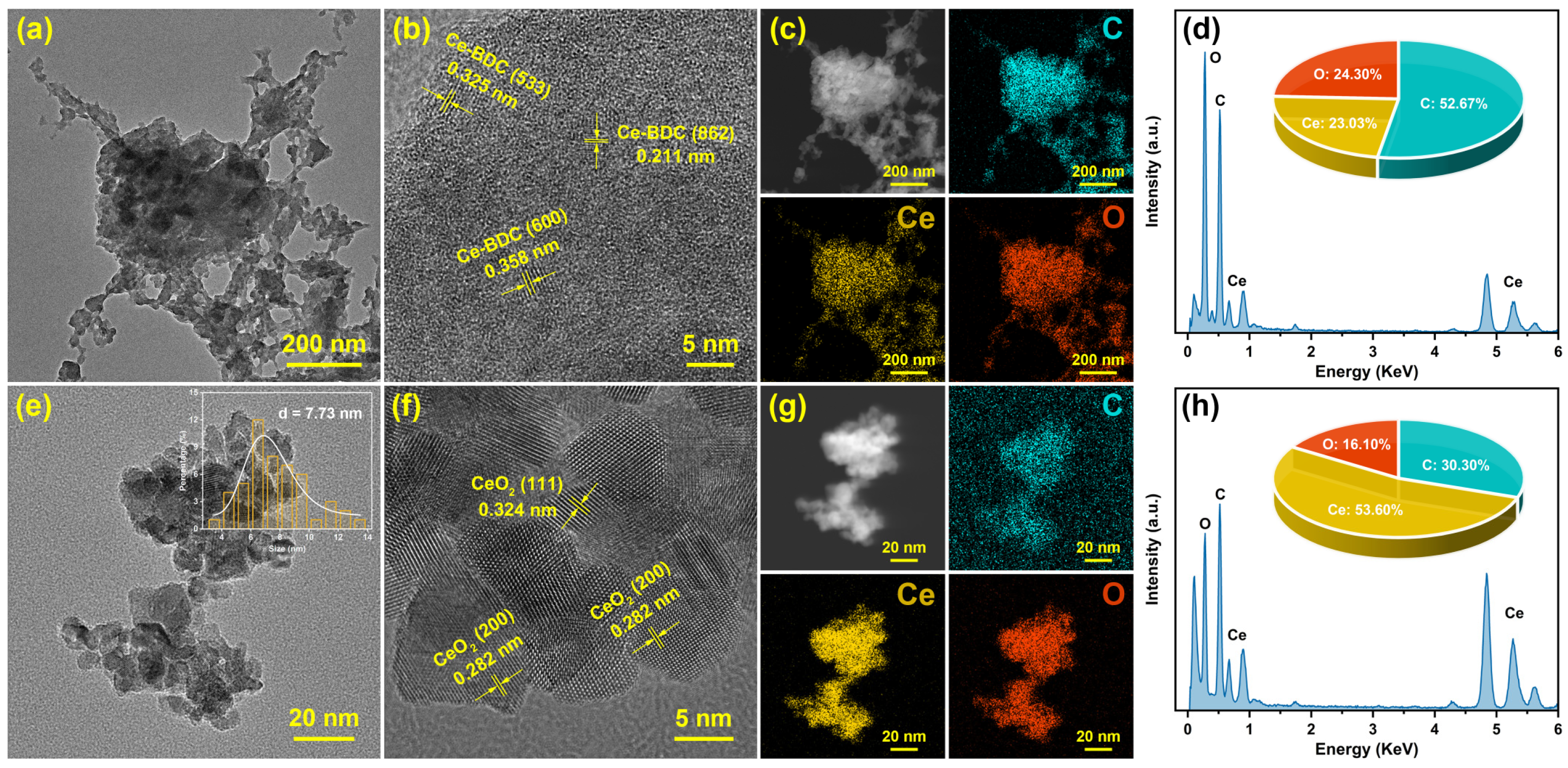
3.1. Morphology Characterization and Physical Properties
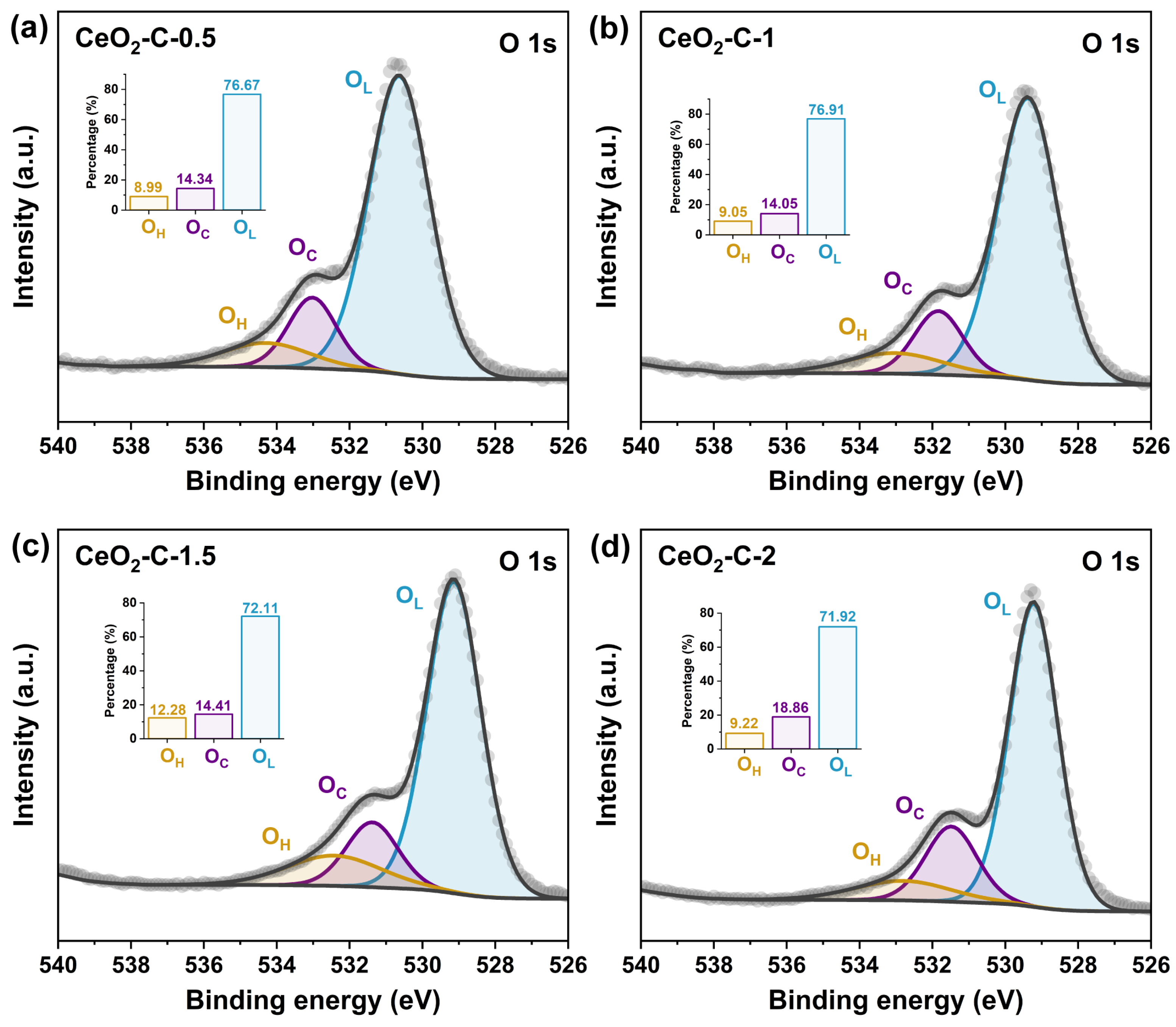
3.2. XPS Analysis
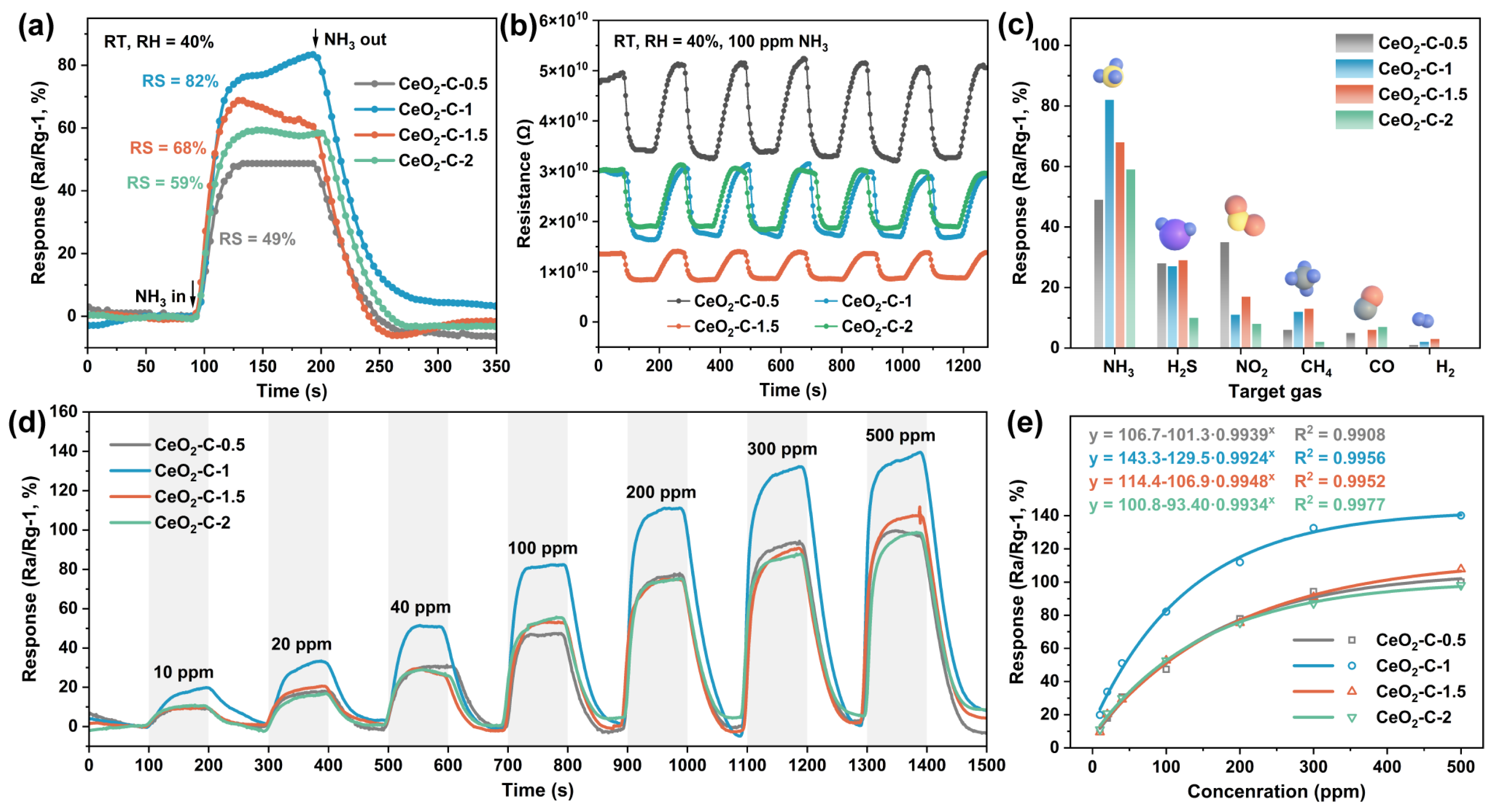
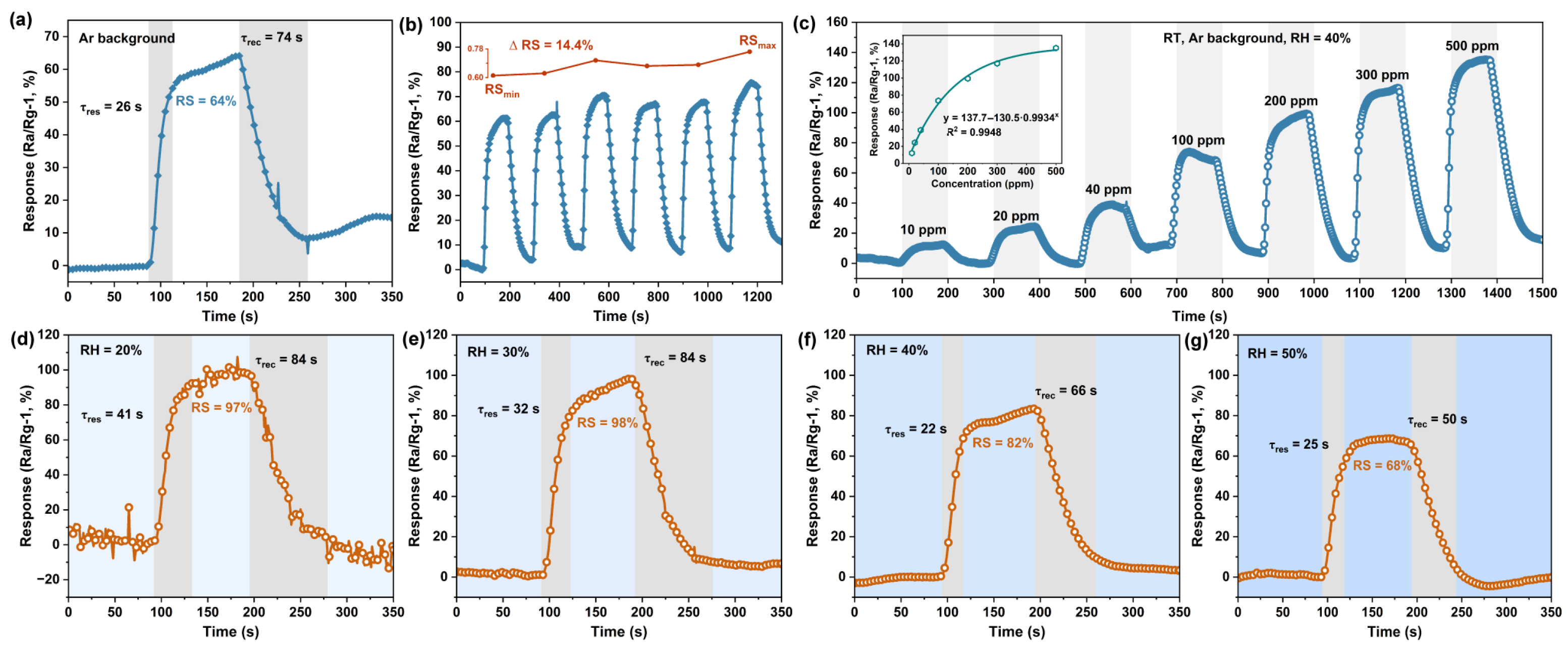
3.3. Gas Sensing Properties
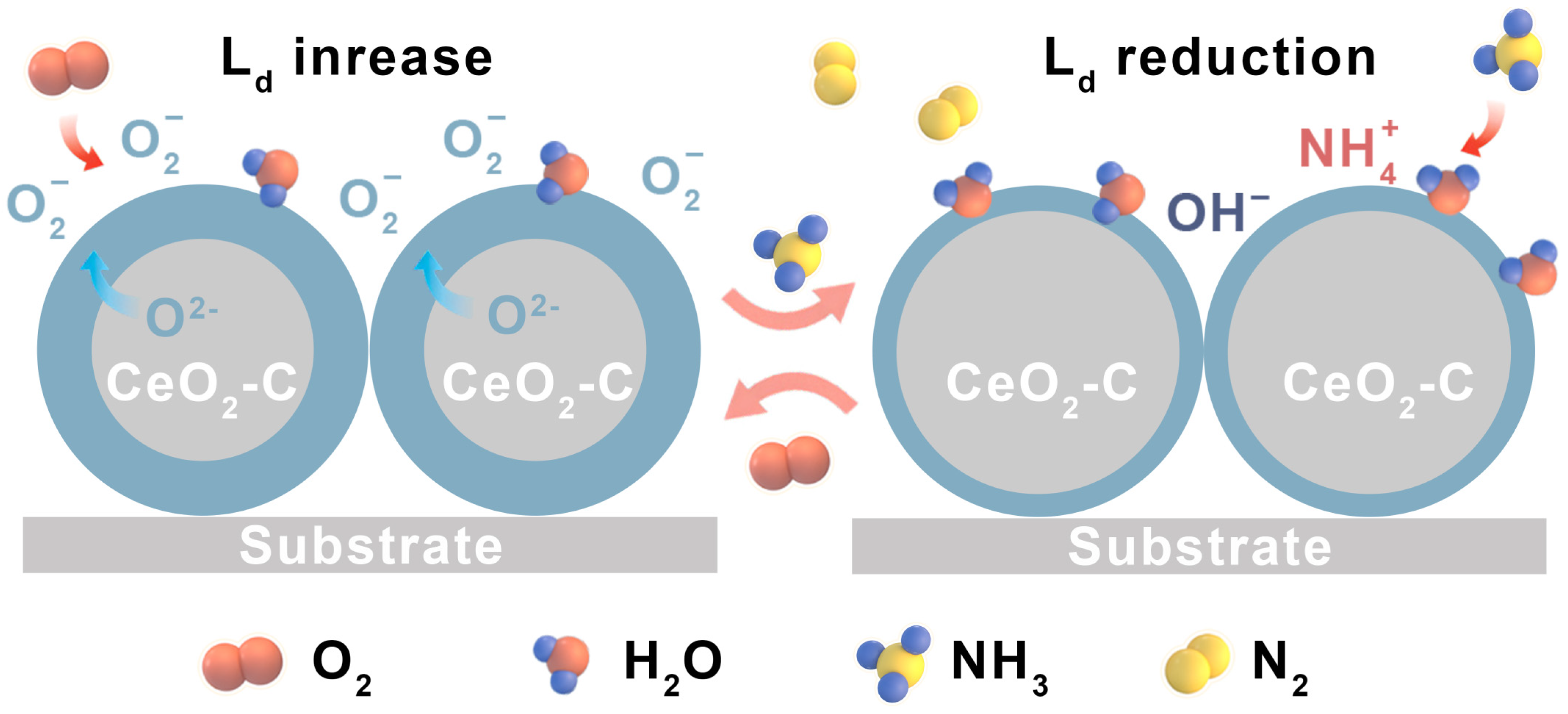
3.4. Gas-Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, M.; Wang, L.; Ren, S.; Bai, B.; Chai, S.; He, C.; Zheng, C.; Yin, X.; Li, F. Preparation, improvement, and application of metal-organic framework-based sensing materials for gas leakage and emission: A review. Nano Mater. Sci. 2025, in press. [Google Scholar] [CrossRef]
- Yue, C.; Xu, J.; Liu, Z.; Yang, Z.; Mu, Y.; Chen, Q.; Chen, X.; Tan, X.; Wang, F.; Wang, X.; et al. Construction of Cr2O3-CdS heterojunction for enhancing the response of n-butanol gas. Sens. Actuators B Chem. 2025, 444, 138433. [Google Scholar] [CrossRef]
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.Y.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective Catalytic Reduction of NOx with NH3 by Using Novel Catalysts: State of the Art and Future Prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef]
- Chen, L.; Guan, X.; Fei, Z.; Asakura, H.; Zhang, L.; Wang, Z.; Su, X.; Yao, Z.; Keenan, L.L.; Hayama, S.; et al. Tuning the selectivity of NH3 oxidation via cooperative electronic interactions between platinum and copper sites. Nat. Commun. 2025, 16, 26. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Cherepanov, P.V.; Choi, J.; Suryanto, B.H.R.; Hodgetts, R.Y.; Bakker, J.M.; Ferrero Vallana, F.M.; Simonov, A.N. A Roadmap to the Ammonia Economy. Joule 2020, 4, 1186–1205. [Google Scholar] [CrossRef]
- Portarapillo, M.; Bellucci Sessa, A.; Russo, D.; di Benedetto, A. Ammonia as a Hydrogen Carrier: Energetic Assessment of Processes Integrated with Fuel Cells for Power Generation. Energy Fuels 2025, 39, 2843–2853. [Google Scholar] [CrossRef]
- Jonson, J.E.; Fagerli, H.; Scheuschner, T.; Tsyro, S. Modelling changes in secondary inorganic aerosol formation and nitrogen deposition in Europe from 2005 to 2030. Atmos. Chem. Phys. 2022, 22, 1311–1331. [Google Scholar] [CrossRef]
- Li, D.; Han, D.; Chen, Y.; Hong, Y.; Duan, Q.; Wang, H.; He, X.; Zhao, L.; Wang, W.; Sang, S. GaN/rGO nanocomposite gas sensor for enhanced NH3 sensing performances at room temperature. Sens. Actuators B Chem. 2024, 403, 135209. [Google Scholar] [CrossRef]
- Wang, W.; Zhen, Y.; Zhang, J.; Li, Y.; Zhong, H.; Jia, Z.; Xiong, Y.; Xue, Q.; Yan, Y.; Alharbi, N.S.; et al. SnO2 nanoparticles-modified 3D-multilayer MoS2 nanosheets for ammonia gas sensing at room temperature. Sens. Actuators B Chem. 2020, 321, 128471. [Google Scholar] [CrossRef]
- Bulemo, P.M.; Kim, D.H.; Shin, H.; Cho, H.J.; Koo, W.T.; Choi, S.J.; Park, C.; Ahn, J.; Guntner, A.T.; Penner, R.M.; et al. Selectivity in Chemiresistive Gas Sensors: Strategies and Challenges. Chem. Rev. 2025, 125, 4111–4183. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Zeng, W.; Bai, B.; Ren, S. Regulating surface terminals and interlayer structure of Ti3C2Tx for superior NH3 sensing. Talanta 2025, 283, 127107. [Google Scholar] [CrossRef]
- Wang, Z.; Bu, M.; Hu, N.; Zhao, L. An overview on room-temperature chemiresistor gas sensors based on 2D materials: Research status and challenge. Compos. Part B Eng. 2023, 248, 110378. [Google Scholar] [CrossRef]
- Yi, S.; Shi, W.; Yang, X.; Yao, Z. Engineering sensitive gas sensor based on MOF-derived hollow metal-oxide semiconductor heterostructures. Talanta 2023, 258, 124442. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Hou, L.; Cui, X.; Zhan, P.; He, P.; Dai, C.; Li, R.; Dong, J.; Zou, Y.; Liu, G.; et al. Self-condensation-assisted chemical vapour deposition growth of atomically two-dimensional MOF single-crystals. Nat. Commun. 2024, 15, 3618. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.T.; Mirzaei, A.; Kim, J.Y.; Phan, T.B.; Tran, L.D.; Wu, K.C.; Kim, H.W.; Kim, S.S.; Doan, T.L.H. Advancements in MOF-based resistive gas sensors: Synthesis methods and applications for toxic gas detection. Nanoscale Horiz. 2025, 10, 1025–1053. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Li, Y.; Sui, C.; Liu, Y.; Liu, Y.; Zhao, Y.; Liang, X.; Liu, F.; Lu, G. Bimetallic MOF derived mesoporous structure of Ru doped SnO2 enable high-sensitivity gas sensors for triethylamine in high humidity. Sens. Actuators B Chem. 2024, 405, 135275. [Google Scholar] [CrossRef]
- Liu, M.; Wang, L.; Ren, S.; Bai, B.; Chai, S.; He, C.; Zheng, C.; Li, X.; Yin, X.; Xu, C.C. Effect of CTAB on the Morphology of Sn-MOF and the Gas Sensing Performance of SnO2 with Different Crystal Phases for H2 Detection. Chemosensors 2025, 13, 192. [Google Scholar] [CrossRef]
- Song, Z.; Liu, Y.; Wang, Y.; Chen, Y.; Li, J.; Li, L.; Yao, J. Polycrystalline hollow MOF derived Co3O4 semiconductor to achieve room-temperature ammonia detection in human exhaled breath. Sens. Actuators B Chem. 2024, 411, 135701. [Google Scholar] [CrossRef]
- Ren, X.; Xu, Z.; Liu, D.; Li, Y.; Zhang, Z.; Tang, Z. Conductometric NO2 gas sensors based on MOF-derived porous ZnO nanoparticles. Sens. Actuators B Chem. 2022, 357, 131384. [Google Scholar] [CrossRef]
- Montoro, C.; Kim, J.-Y.; Mirzaei, A.; Lee, J.-H.; Sayegh, S.; Makhoul, E.; Iatsunskyi, I.; Coy, E.; Bechelany, M.; Kim, H.W.; et al. MOF-derived metal oxide (Cu, Ni, Zn) gas sensors with excellent selectivity towards H2S, CO and H2 gases. Compos. Part B Eng. 2024, 283, 111637. [Google Scholar] [CrossRef]
- Li, Y.; Chen, P.; Zeng, W.; Li, X.; Wang, Q. MOF-derived Co3O4 decorated SnO2 nanosheets for NH3 sensor fabricated by carrier gas regulation. Chem. Eng. J. 2025, 519, 165001. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; Zhang, J.; Zeng, F.; Bao, J.; Liu, L.; Tian, G. Cocatalyst Embedded Ce-BDC-CeO2 S-Scheme Heterojunction Hollowed-Out Octahedrons With Rich Defects for Efficient CO2 Photoreduction. Small 2024, 20, e2406487. [Google Scholar] [CrossRef] [PubMed]
- Hirschle, P.; Preiß, T.; Auras, F.; Pick, A.; Völkner, J.; Valdepérez, D.; Witte, G.; Parak, W.J.; Rädlerb, J.O.; Wuttke, S. Exploration of MOF nanoparticle sizes using various physical characterization methods-is what you measure what you get? CrystEngComm 2016, 18, 4359–4368. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.; Pei, W.; Dang, J.; Zhang, Y.; Liu, X.; Maqsood, A.; He, Q.; Li, M. Ultraefficient Phosphate Adsorption via Monocarboxylic Acid-Regulated Defective Ce-MOFs: Pore Size Regulation, Valence State Modulation, and Unsaturated Center Design. ACS Appl. Mater. Interfaces 2025, 17, 36751–36762. [Google Scholar] [CrossRef]
- Matemb Ma Ntep, T.J.; Reinsch, H.; Liang, J.; Janiak, C. Acetylenedicarboxylate-based cerium(IV) metal-organic framework with fcu topology: A potential material for air cleaning from toxic halogen vapors. Dalton Trans. 2019, 48, 15849–15855. [Google Scholar] [CrossRef]
- Lammert, M.; Wharmby, M.T.; Smolders, S.; Bueken, B.; Lieb, A.; Lomachenko, K.A.; Vos, D.D.; Stock, N. Cerium-based metal organic frameworks with UiO-66 architecture: Synthesis, properties and redox catalytic activity. Chem. Commun. 2015, 51, 12578–12581. [Google Scholar] [CrossRef]
- Prieur, D.; Bonani, W.; Popa, K.; Walter, O.; Kriegsman, K.W.; Engelhard, M.H.; Guo, X.; Eloirdi, R.; Gouder, T.; Beck, A.; et al. Size Dependence of Lattice Parameter and Electronic Structure in CeO2 Nanoparticles. Inorg. Chem. 2020, 59, 5760–5767. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, X.; Lv, L.; Zhang, W.; Pan, B. Redox-oriented anchoring of CeO2 over nanostructured MnCo-spinel: Strong oxide-support interaction for enhanced toluene combustion. Appl. Catal. B 2025, 378, 125548. [Google Scholar] [CrossRef]
- Gobi, R.; Babu, R.S.; Rani, M.U.; Gothandam, K.M.; Prakash, J.; Venkatasubbu, G.D. Investigating the effect of antibacterial cerium oxide nanoparticles embedded in biodegradable PVA/chitosan films for wound healing application: An in-vitro study. Emergent Mater. 2024, 8, 1813–1833. [Google Scholar] [CrossRef]
- Zuas, O.; Abimanyu, H.; Wibowo, W. Synthesis and characterization of nanostructured CeO2 with dyes adsorption property. Process. Appl. Ceram. 2014, 8, 39–46. [Google Scholar] [CrossRef]
- Sartoretti, E.; Novara, C.; Giorgis, F.; Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. In situ Raman analyses of the soot oxidation reaction over nanostructured ceria-based catalysts. Sci. Rep. 2019, 9, 3875. [Google Scholar] [CrossRef] [PubMed]
- Albu, Z.; Abass, N.A.; Sharma, P.K.; Qahtan, T.; Huang, S.; Rashid, N.; Sanfo, G.; Pineda, M.; Al-Sayoud, A.; AlOtaibi, B.; et al. Transition metal doping of CeO2 boosts photo-assisted electrocatalytic oxygen evolution performance. J. Energy Chem. 2025, 110, 973–985. [Google Scholar] [CrossRef]
- Wang, H.; Cui, G.; Lu, H.; Li, Z.; Wang, L.; Meng, H.; Li, J.; Yan, H.; Yang, Y.; Wei, M. Facilitating the dry reforming of methane with interfacial synergistic catalysis in an Ir@CeO2-x catalyst. Nat. Commun. 2024, 15, 3765. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, J.; Sohn, H.Y.; Chen, C.; Yang, J.; Liu, X.; Lan, Y.; Zhang, W.; Wang, Q.; Liu, L. Redox on Mn-Ce interface and its effects on low temperature selective catalytic reduction for NO removal. Fuel 2023, 350, 128806. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Chen, L.; Zhang, S.; Li, W.; Li, S. Unveiling the role of surface hydroxyl oxygen in modulating the ‘seesaw effect’ of NH3-SCR over amorphous FeMnTi-AM catalysts. Appl. Catal. B 2025, 370, 125191. [Google Scholar] [CrossRef]
- Wu, H.; Yu, J.; Yao, G.; Li, Z.; Zou, W.; Li, X.; Zhu, H.; Huang, Z.; Tang, Z. Room temperature NH3 sensing properties and humidity influence of Ti3C2Tx and Ag-Ti3C2Tx in an oxygen-free environment. Sens. Actuators B Chem. 2022, 369, 132195. [Google Scholar] [CrossRef]
- Burgues, J.; Jimenez-Soto, J.M.; Marco, S. Estimation of the limit of detection in semiconductor gas sensors through linearized calibration models. Anal. Chim. Acta 2018, 1013, 13–25. [Google Scholar] [CrossRef]
- Elavarasan, T.; Amalarani, A.; Ernest, S.; Mohammed Gazzali, P.M.; Fairose, S. Structural, morphological and ammonia sensing properties of spray deposited CeO2 films. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, J.; Lei, X.; Sun, H.; Ai, T.; Ma, F.; Chu, P.K. Edge-enriched SnS2 nanosheets on graphene for chemiresistive room temperature NH3 sensors. Sens. Actuators B Chem. 2025, 433, 137565. [Google Scholar] [CrossRef]
- Choudhary, V.S.; Gangwar, S.; Yadav, C.S.; Sharma, S.; Almeida, M.A.P.; Sharma, S.K. Vanadium doping as a key factor for superior NH3 sensing at room temperature in MoSe2/TiO2 composites. Sens. Actuators A Phys. 2025, 388, 116501. [Google Scholar] [CrossRef]
- Wang, P.; Tang, C.; Zhang, L.; Lu, Y.; Huang, F. Hierarchical 0D/1D/2D Au/PANI/WS2 ternary nanocomposite NH3 sensor with high performance and fast response/recovery for food spoilage detection. Chem. Eng. J. 2024, 496, 153998. [Google Scholar] [CrossRef]
- Sinha, S.K.; Kumar, B.; Kumar, R.; Ganguly, S.; Hazra, A. Synthesis and characterization of hybrid NiO/CeO2 p-n heterojunction nanofibers for room temperature ammonia sensing application. Surf. Interfaces 2024, 51, 104568. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, J.; Wang, D.; Lei, X.; Sun, H.; Huang, Y.; Ma, F.; Ai, T.; Chu, P.K. Enhanced Room-Temperature NH3-Sensing Performance of Ti3C2Tx MXene Decorated with CeO2 Nanoparticles. ACS Appl. Nano Mater. 2025, 8, 12090–12099. [Google Scholar] [CrossRef]
- Dhanawade, R.N.; Pawar, N.S.; Chougule, M.A.; Hingangavkar, G.M.; Jadhav, Y.M.; Nimbalkar, T.M.; Navale, Y.H.; Chavan, G.T.; Jeon, C.-W.; Patil, V.B. Highly sensitive and selective PAni-CeO2 nanohybrid for detection of NH3 biomarker at room temperature. J. Mater. Sci. Mater. Electron. 2023, 34, 781. [Google Scholar] [CrossRef]
- Singh, S.; Shin, K.Y.; Moon, S.; Kim, S.S.; Kim, H.W. Phase-Engineered MoSe2/CeO2 Composites for Room-Temperature Gas Sensing with a Drastic Discrimination of NH3 and TEA Gases. ACS Sens. 2024, 9, 3994–4006. [Google Scholar] [CrossRef]
- Wang, B.R.; Hu, Y.; Pan, Z.; Wang, J. MOF-derived manganese oxide/carbon nanocomposites with raised capacitance for stable asymmetric supercapacitor. RSC Adv. 2020, 10, 34403–34412. [Google Scholar] [CrossRef]
- Lim, H.; Kwon, H.; Kang, H.; Jang, J.E.; Kwon, H.J. Laser-Induced and MOF-Derived Metal Oxide/Carbon Composite for Synergistically Improved Ethanol Sensing at Room temperature. Nano-Micro Lett. 2024, 16, 113. [Google Scholar] [CrossRef]
- Isaac, N.A.; Pikaar, I.; Biskos, G. Metal oxide semiconducting nanomaterials for air quality gas sensors: Operating principles, performance, and synthesis techniques. Mikrochim. Acta 2022, 189, 196. [Google Scholar] [CrossRef]
- Liu, J.; Dai, M.; Wang, T.; Sun, P.; Liang, X.; Lu, G.; Shimanoe, K.; Yamazoe, N. Enhanced Gas Sensing Properties of SnO2 Hollow Spheres Decorated with CeO2 Nanoparticles Heterostructure Composite Materials. ACS Appl. Mater. Interfaces 2016, 8, 6669–6677. [Google Scholar] [CrossRef]
- Yoon, J.W.; Kim, J.S.; Kim, T.H.; Hong, Y.J.; Kang, Y.C.; Lee, J.H. A New Strategy for Humidity Independent Oxide Chemiresistors: Dynamic Self-Refreshing of In2O3 Sensing Surface Assisted by Layer-by-Layer Coated CeO2 Nanoclusters. Small 2016, 12, 4229–4240. [Google Scholar] [CrossRef]
- Esmaeili, C.; Ashtiani, S.; Regmi, C.; Laposa, A.; Voves, J.; Kroutil, J.; Friess, K.; Povolny, V.; Lotfian, S. Preparation and characterisation of NH3 gas sensor based on PANI/Fe-doped CeO2 nanocomposite. Heliyon 2024, 10, e34801. [Google Scholar] [CrossRef]
- Gond, R.; Shukla, P.; Prakash, B.; Rawat, B. Vertically Aligned MoS2/ZnO Heterostructure for Highly Selective NH3 Sensing at Room Temperature. ACS Appl. Electron. Mater. 2024, 6, 2728–2738. [Google Scholar] [CrossRef]
- Maier, K.; Helwig, A.; Muller, G.; Hille, P.; Eickhoff, M. Effect of Water Vapor and Surface Morphology on the Low Temperature Response of Metal Oxide Semiconductor Gas Sensors. Materials 2015, 8, 6570–6588. [Google Scholar] [CrossRef]

| Materials | RH (%) | Conc. (ppm) | RS (%) | τres/τrec (s) | LOD | Ref |
|---|---|---|---|---|---|---|
| CeO2 film | 0 | 100 | 35 | 19/66 | NA | [38] |
| SnS2/graphene | NA | 100 | 62.36 | 63/586 | 0.1 ppm | [39] |
| MoSe2/VTiO2 | 50 | 6 | 2.78 | 94/50 | 0.3 ppm | [40] |
| Au/PANI/WS2 | 51 | 100 | 286.1 | 24/26 | 13.8 ppb | [41] |
| NiO@CeO2 | 0 | 400 | 97 | 79/21 | NA | [42] |
| CeO2/Ti3C2Tx | 0 | 10 | 28.36 | 145/875 | NA | [43] |
| PAni-CeO2 | 0 | 100 | 80 | 9.31/531.60 | NA | [44] |
| CeO2-C-1 | 40 | 100 | 82 | 22/66 | 0.31 ppm | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Liu, M.; Ren, S.; Zhong, X.; Bai, B.; Chai, S.; He, C.; Li, X. Nanostructured CeO2-C Derived from Ce-BDC Precursors for Room-Temperature Ammonia Sensing. Chemosensors 2025, 13, 362. https://doi.org/10.3390/chemosensors13100362
Wang L, Liu M, Ren S, Zhong X, Bai B, Chai S, He C, Li X. Nanostructured CeO2-C Derived from Ce-BDC Precursors for Room-Temperature Ammonia Sensing. Chemosensors. 2025; 13(10):362. https://doi.org/10.3390/chemosensors13100362
Chicago/Turabian StyleWang, Liang, Manyi Liu, Shan Ren, Xiankang Zhong, Bofeng Bai, Shouning Chai, Chi He, and Xinzhe Li. 2025. "Nanostructured CeO2-C Derived from Ce-BDC Precursors for Room-Temperature Ammonia Sensing" Chemosensors 13, no. 10: 362. https://doi.org/10.3390/chemosensors13100362
APA StyleWang, L., Liu, M., Ren, S., Zhong, X., Bai, B., Chai, S., He, C., & Li, X. (2025). Nanostructured CeO2-C Derived from Ce-BDC Precursors for Room-Temperature Ammonia Sensing. Chemosensors, 13(10), 362. https://doi.org/10.3390/chemosensors13100362






