Abstract
TiO2 nanoparticles (NPs) doped with W (W:TiO2), double-doped with W and V (W&V:TiO2), and loaded with noble metals (W:TiO2 @Pt/Pd/Ag and W&V:TiO2@Pt/Pd/Ag) were synthesized by laser pyrolysis followed by chemical impregnation and reduction. Due to its exceptional properties, TiO2 is considered a key material being used in a wide range of applications. To improve its detection activity, the increase in the specific surface of the material, and the presence of defects in its structure play a decisive role. Doped and double-doped TiO2 nanoparticles with dimensions in the range of 25–30 nm presented a mixture of phases corresponding to titania, with the anatase phase accounting for the majority (95%). By loading these nanoparticles with small particles of noble metals, a significant increase in the specific surface area by three or even five times the original values was achieved. Sensitive thin films for surface acoustic wave (SAW) sensors were made with the NPs, embedded in polyethyleneimine (PEI) polymer and deposited by spin-coating. Each sensor was tested at CH4 concentrations between 0.4 and 2%, at room temperature, and the best results were obtained by the sensor with NPs doped with V and decorated with Pd, with a limit of detection (LOD) of 17 ppm, due to the strong catalytic effect of Pd.
1. Introduction
Methane (CH4) is the principal component of natural gas, a gas of major interest for various industries, and a fossil fuel with great applicability potential in many industries [1,2]. It is found in natural CH4 deposits, in coal mines, and as a product of cellulose fermentation in the absence of air [3]. CH4 is one of the gasses with a high greenhouse effect, and, in the last 100 years, the amount of CH4 has increased by 28 times more than carbon dioxide, the effect of which is clearly seen in the global warming phenomenon our planet is going through [1,4]. It is a colorless and odorless gas, which makes it difficult to detect by human senses [5]. Also, at concentrations in the range of 4.9–15.4 vol%, CH4 becomes flammable, with an increased risk of explosion [6,7]. It also imposes problems due to its ability to considerably reduce the amount of oxygen in an environment, the consequence of this being asphyxiation [8].
As such, there is a real and practical need to develop sensors capable of detecting CH4 at concentrations as low as possible and in the shortest possible time.
Several types of sensors for CH4 detection have been developed over time: metal oxide semiconductor sensors, electrochemical sensors, chemoresistive sensors, optical sensors, calorimetric sensors, pyroelectric sensors, conductive sensors, and surface acoustic wave (SAW) sensors [1,6,7,9,10,11,12]. In addition to the specific advantages of each type of sensors, each one of them also poses some disadvantages that limit properties such as sensibility, selectivity, response and recovery times, or detection at room temperature (RT), requiring also high costs and energy consumption [9].
SAW sensors stand out through their good sensitivity at RT, with a low limit of detection (LOD), short response and return times, reversibility, small dimensions, reliability, and the possibility of wireless operation [13,14]. A SAW sensor is composed of a piezoelectric substrate, two pairs of interdigital traductors, and a sensitive layer. Its operating principle consists of changing the oscillation frequency of the acoustic waves that cross the sensor, when mass or acoustic–electrical changes occur at the level of the sensitive layer [15,16]. So far, some SAW sensors for CH4 have been developed [10,17,18]. Most of these sensors do not simultaneously have the possibility to respond to the RT and a response and recovery in the order of ten seconds. These two parameters can be improved by taking into account both the nature of the material used as a sensitive layer and its morphology.
For the categories of CH4 sensors mentioned above, a wide range of sensitive materials have been used: oxide metal semiconductors (SnO2, TiO2, ZnO, WO3, In2O3), metals (Pt, Pd), polymers (polyethyleneimine-PEI, polydimethylsiloxane-PDMS), carbon nanotubes, carbon nanowalls, graphene, or composite materials [6,10,19,20,21].
TiO2 is one of the oxide metal semiconductors with very high potential in the field of sensors, being one of the few oxides with the ability to provide sensing performance at RT due to its high surface reactivity [6,22,23,24]. Comert et al. [25] developed CH4 resistive sensors through a sputtering method, with TiO2-sensitive thin films, demonstrating that increasing the temperature detection leads to better performance of the sensors and also that, at 50 °C, these sensors could obtain a good sensibility of 0.15 Ω/ppm.
Although significant improvements have been achieved in the use of TiO2 nanopowders as sensing materials, various disadvantages, such as sensitivity and selectivity, restrict their practical application. Sensor performance can be significantly increased by modifying the catalytic activity of the sensing layer by doping/co-doping, due to sensor performance depending directly on the sensing layers’ properties [26]. Furthermore, by loading titania-based nanoparticles (NPs) with noble metals, the chemical interactions between the target gas and the surface of the sensitive material are enhanced, leading to an improved sensing performance [27]. Therefore, different types of dopants, such as Nb, W, Al, V, and Ru, have been shown to significantly improve the different functional properties of TiO2 [28,29,30], alongside loading with noble metals like Au, Ag, and Pt or Pd NPs. They have catalytic action on gas molecules, and, through doping, new sites for the adsorption of gas molecules are created, which also involves the alteration of the charge transfer mechanism [1].
For the process of loading TiO2 NPs with noble metals, the method of wet impregnation followed by reduction is commonly used [31], while, for doped TiO2 NP synthesis, a wide range of techniques have been reported, including laser pyrolysis, hydrothermal, chemical vapor deposition, ion implantation, sol–gel methods, etc. [32,33,34,35,36]. In general, different doping technologies can affect the physicochemical properties of doped TiO2 due to variations in different experimental parameters, such as the type of Ti precursors, the chemicals used, the concentration and pH of the solutions, and the sintering temperature. Impurities may also appear in the samples if the obtaining process involves several steps. Among all the methods listed above, laser pyrolysis offers the advantage of obtaining pure TiO2 NPs or NPs doped/co-doped with two or more dopants via single-step synthesis. This process takes place continuously in the flow of gasses, without quantitative limitations, and can be scaled at an industrial level [37]. Using this synthesis method, in our previous study, we obtained TiO2 NPs double-doped with W and V and loaded with Pt and Pd, which showed significant photocatalytic degradation of CH3OH [38]. These samples will also be examined for sensor applications in the current study, together with novel synthesized materials.
In the present study, the CH4 gas-sensing properties of Ag-loaded W:TiO2/W&V:TiO2 NPs are presented for the first time, to our knowledge, in comparison with Pt/Pd-loaded W:TiO2/W&V:TiO2, thus highlighting the influence of the noble metal type on the CH4 gas-sensing efficiency. These NPs were used in the sensitive layers of SAW sensors, in a PEI polymer matrix. The development of CH4 sensors with good sensitivity at RT leads to low energy consumption and the simplification of the test system, provided that performances are maintained.
2. Materials and Methods
2.1. Synthesis of W:V:TiO2@Ag Nanopowders
In our previous study, TiO2 samples doped with W and double-doped with W and V were obtained using the laser pyrolysis technique [30]. This method has the benefit of producing materials in the form of nanopowders, and the morphological and structural properties of the produced particles can be tailored by modifying the experimental settings. This technique can produce a wide range of nanomaterials, including oxides, which can be simple, mixed, doped, or co-doped with two or more dopants. The remarkable fact is that these complex materials can be produced directly, in a single step of synthesis, and the quantity is not limited, because the reaction occurs under continuous gas flow. This method, which has been detailed in our previous papers, is based on the resonance of laser radiation (CW CO2, wavelengths 10.6 μm) with at least one of the reactants or a sensitizer [38]. The precursors used to obtain the desired materials can be gasses or volatile liquids with vapor pressure at room temperature. The following materials were utilized to synthesize TiO2 nanoparticles doped with W or co-doped with W and V: TiCl4, titanium tetrachloride (as a precursor for Ti); synthetic air (as an oxidizing agent); C2H4, ethylene (as a sensitizer); WCl6 (as a precursor for W); and VOCl3 (as a precursor for V). Thus, W:TiO2 nanoparticles (sample named TWV-7-0) and W&V:TiO2 (sample named TWV-7-1) were obtained and then loaded with noble metal particles of Pt and Pd using the precursors H2PtCl6 × 6H2O and K2PdCl4, respectively, leading to W:TiO2@Pt/Pd-type nanoparticles (samples named TWV-7-0@Pt and TWV-7-0@Pd) and W&V:TiO2@Pt/Pd-type nanoparticles (samples named TWV-7-1 @Pt and TWV-7-1@Pd). Details of the synthesis parameters required to produce these nanomaterials as well as the results of their morphostructural characterization are already reported in our previous research [30], and their sensing properties are investigated in this work, compared to those of samples coated with Ag, obtained during the current study as follows.
Starting from the previously reported W&V:TiO2 NPs [38], the process of impregnation followed by reduction was used to load TiO2-based samples with Ag, using Ag NO3 (Aldrich, 97% purity). After the impregnation of NPs in a titanium-based suspension with a noble metal (NM), complex ions in an aqueous solution (90 mL, 10 g/L) were reduced (10 mL, 1.5 g/L) with NaBH4 (Aldrich, purity 98%).
Metal loading occurred on the TiO2 surface by the chemical process detailed below [39]:
2 × AgNO3 + 2 × NaBH4 + 6 × H2O → 2 × Ag0 + 7 × H2 + 2 × B(OH)3 + 2 × NaNO3
The post-treatment synthesis process involved drying and heating at 450 °C under atmospheric air. Using TWV-7-0 and TWV-7-1 nanopowders as the standards, they were impregnated with Ag to produce two different samples, named TWV-7-0-Ag and TWV-7-1-Ag. These samples were subsequently characterized. The synthesis methods used are described in detail in our previous works [38,40].
Figure 1 shows all the obtained nanopowders to be tested regarding their properties as CH4 gas sensors. The color of the samples changed from white (undecorated TiO2-based samples) to different colors depending on the type of noble metal NPs used for loading: light gray for Pt, dark gray for Pd, and light brown coffee for Ag.
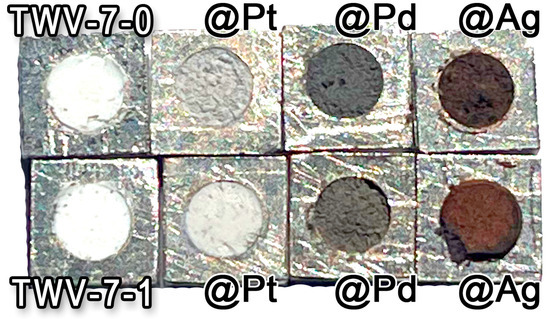
Figure 1.
Images for W:TiO2@NM (up) and W&V:TiO2@NM (down) nanopowders.
2.2. Morphostructural Characterization
To determine the shape, size, and morphology of the NPs, the samples were first evaluated using Transmission Electron Microscopy (TEM) and High-Resolution Transmission Electron Microscopy (HRTEM) with a JEM ARM 200F analytical microscope. The BET Specific Surface Area is the most widely recognized technique for determining the surface area of powders. These measurements were made using BET Flowing Gas Surface Area Analyzers, Horiba SA-9600, with a 30% N2 and 70% He gas mixture. To identify and quantify the elemental composition of the materials, we employed Energy-Dispersive X-ray Spectroscopy (EDX) as an analytical technique. The EDX analysis was conducted within a Scanning Electron Microscope (SEM), specifically the FEI Co. Quanta Inspect S model, operating at 15 kV under high vacuum conditions. The X-ray diffraction patterns of the W&V:TiO2@NM nanopowders were obtained at RT using a PANalytical X-Pert PRO MPD diffractometer with a monochromatic Cu Kα (λ = 1.5418 Å) radiation source in the 2θ range of 20° ≤ 2θ ≤ 65° with 0.02° step size/20 s-time/step, 40 kV, and 30 mA. The acquired data were subsequently processed and analyzed using the PANalytical X’Pert High-Score Plus software, version 4.8. X-ray photoelectron spectroscopy (ESCALAB Xi+, Thermo Fisher, Waltham, MA, USA) was used to measure the XPS spectra, utilizing an Al Kα radiation source at 1486.6 eV. To remove physical and chemisorbed molecules, the samples were outgassed at RT to a pressure below 2 × 10−8 Torr. The binding energies were calibrated to the C 1s peak at 284.6 eV. Using the Avantage software (version 6.0), the surface chemical composition and oxidation states were estimated from the XPS spectra by the integral fitting of each peak after applying an “S-shaped” Shirley-type background subtraction.
2.3. SAW Sensor Structure and Testing
According to the image in Figure 2, a SAW sensor is made up of a piezoelectric substrate, two pairs of interdigital transducers (IDTs), and a sensitive layer placed between the two pairs of transducers [15,16]. In our laboratory, we use a quartz piezoelectric substrate due to the stability of the temperature coefficient, thus eliminating an external factor which could affect the quality of the sensor signal [15].

Figure 2.
Experimental setup for SAW sensor measurements.
The IDTs were made of gold, and they were deposited on an initial chrome layer, for good adhesion on the quartz substrate. The deposition was carried out by photolithography, with the thickness of the chromium layer being ~10 nm and that of gold ~150 nm. The configuration of the IDTs is that of a double comb and consists of 50 pairs of transducers. The sensitive layer is positioned between the two pairs of IDTs (Figure 2).
The sensitive films were made from a 20 µL suspension of PEI polymer solution and NPs, deposited on the sensor substrate by spin-coating. The polymer solution concentration was 10 mg PEI/1 mL ethanol, and the suspension concentration was 8 mg NP/1 mL PEI solution. Thus, the sensors described in Table 1 were obtained. The PEI polymer was used as a deposition matrix, its concentration being the same in all the sensors. Therefore, the discussion in this paper will not focus on the contribution brought about by the use of PEI, but on the influence of the properties of each type of NP.

Table 1.
Characteristics of SAW sensors deposited by spin-coating.
The signal obtained by SAW sensors is based on the change in the oscillation frequency of the acoustic waves crossing the sensors and generated after the electric current is applied on the quartz piezoelectric substrate. The shift in the oscillation frequency takes place due to the interaction between the gas molecules and the sensitive material after adsorption and mass accumulation, but also due to acoustic–electrical interactions.
A delay line-type sensor was used, with a parallelogram geometry, to reduce the effect of wave reflections. The dimensions of the sensor were the following: 38 mm long, 10 mm wide, and 0.5 mm thick.
Figure 2 presents the SAW sensor test system. The gas concentration reaching the SAW test chamber was controlled by a gas controller connected to two mass flow meters in order to homogenize the CH4 with the synthetic air in the necessary quantities. The mass flow gas was kept constant at 0.5 L/min for all the gas concentrations tested. The SAW sensor was connected to an amplifier to compensate for the amplitude losses and to a frequency meter connected to a computer.
All sensor tests were carried out at RT, and the CH4 concentrations varied between 0.4 and 2%.
3. Results and Discussion
3.1. Morphology and Structure
3.1.1. TEM
A representative TEM image is shown in Figure 3a. Some particles have irregular polyhedral shapes, bonded together and slightly aggregated. The images show anatase crystallites ranging in size from 10 to 50 nm, with the average size being around 25–30 nm. The HRTEM images reveal aggregates of anatase crystallites with Ag particles on the surface. In the case of the TWV-7-0@Ag sample, the Ag particles are about 3–5 nm in size and are frequently observed (Figure 3b). In contrast, in the case of the TWV-7-1@Ag sample, the Ag particles are rarely observed and measure between 3 and 4 nm (Figure 3c). The EDX spectrum shows the presence of noble metal NPs and reveals the material composition (Figure 3d). The particle sizes determined by TEM are generally congruent with the XRD results (see below).
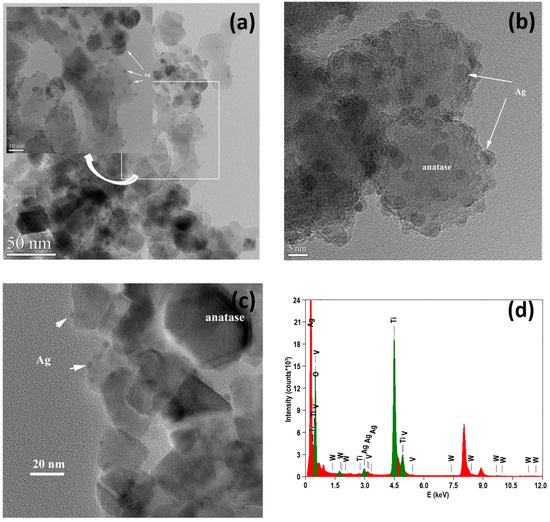
Figure 3.
TEM/HRTEM images of the TWV-7-0@Ag (a,b) and TWV-7-1@Ag (c) samples, alongside the corresponding EDX spectrum (d).
3.1.2. BET Analysis
As mentioned in our previous investigation [38], the specific surface area of TWV-7-0 is 9.8062 m2/g, and it slightly increases with the introduction of vanadium atoms to 16.3626 m2/g (Table 2). The decoration of both W:TiO2 and W&V:TiO2 NPs with Ag atoms leads to an increase in the surface area up to 53.5350 m2/g and 56.4137 m2/g, respectively (Table 2). This multiplication could be explained with the addition of the Ag NPs, whose dimensions are between 3 and 5 nm, measured by TEM.

Table 2.
BET/EDX/XRD measurements for the TiO2-based samples loaded with Ag, compared to the corresponding references (without Ag).
3.1.3. EDX Analysis
EDX was used to verify the compositions of the obtained NPs. Thus, the presence of selective elements such as Ti and O in an almost stoichiometric ratio as well as the dopants W and V or Ag loading could be clearly observed for the TVW-7-1-Ag sample (Figure 4). The elemental compositions of the prepared samples are shown in Table 1. The Ag-modified titania samples have a lower impurity content than the standard samples (TWV-7-0 and TWV-7-1), which decreases following impregnation. Impurities such as C, Cl, and Na, resulting from the laser pyrolysis and impregnation processes, lead to the formation of AgCl as an impurity in the TWV-7-1-Ag sample. This sample contains a slightly larger quantity of adsorbed Cl due to the use of the VOCl3 precursor (in the TWV-7-1 samples with vanadium content).

Figure 4.
EDX spectrum corresponding to the TWV-7-1-Ag sample.
3.1.4. XRD Analysis
The XRD analysis detected only the TiO2 phases (anatase and rutile). The main phase in the samples was anatase, accounting for 82% (JPCDS file 00-021-1272), and the rest were rutile (JCPDS file 00-021-1276) (Table 1). The phase ratio did not change with the introduction of the vanadium atoms. The crystallite sizes were 21 ± 1 nm and 32 ± 1 nm for the anatase and rutile phases, respectively.
The TEM and XPS investigations revealed silver NPs that should crystalize in a cubic structure. The diffraction pattern of the Ag cubic phase (JCPDS file 00-004-0783) is shown in Figure 5. However, the peaks corresponding to the Ag cubic phase are very low in intensity and in the range of background noise, because of the small dimensions and low concentration of the Ag NPs, as confirmed by TEM (3–6 nm) and EDS (<1 at.%). At the same time, the diffraction pattern of the TWV-7-1-Ag powder shows peaks at 2θ = 27.83°, 32.24°, and 46.25°, corresponding to the AgCl phase (JCPDS file 00-006-0480). Chlorine gas may have remained on the surface of the W&V:TiO2 particles as a result of the increased number of chlorine atoms obtained from the vanadium precursor (VOCl3). During the decoration process, the chlorine interacts with the Ag atoms, creating points of nucleation. As explained in our previous investigation [38], the chlorine atoms in the powder result in the formation of new compounds, like NaCl, during the decoration process.
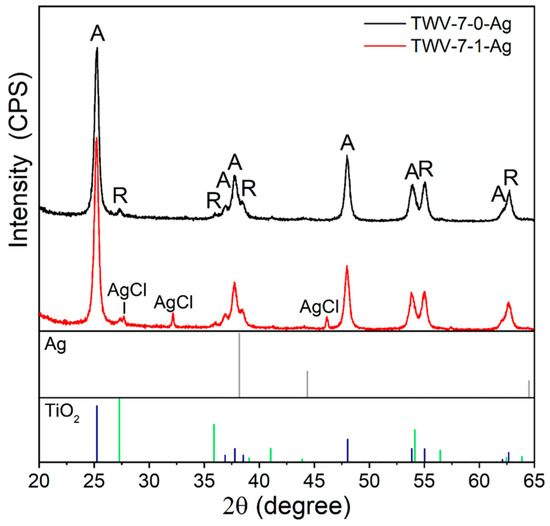
Figure 5.
Diffractograms of the W&V:TiO2 powders decorated with Ag at RT.
3.1.5. XPS Analysis
The elemental composition and chemical status of the TWV-7-0-Ag and TWV-7-1-Ag composites were analyzed using XPS. The survey scan spectra shown in Figure 6a indicate that the samples primarily consist of the elements Ti, O, and C, with a small amount of Cl detected in the TWV-7-1-Ag sample. To determine the chemical states of silver, high-resolution XPS scan spectra of the Ag 3d region were recorded for both samples. The Ag 3d3/2 and Ag 3d5/2 peaks identified at 374.2 eV and 368.2 eV, respectively, correspond to metallic Ag (Ag⁰) [41], as depicted in Figure 6b, while the peaks at 373.5 eV and 367.5 eV correspond to silver chloride (AgCl) [42], as shown in Figure 6c. Furthermore, the surface ratio of Ag⁺ to Ag⁰ in sample TWV-7-1-Ag is approximately 1:0.47. Sample TWV-7-0-Ag is composed exclusively of metallic silver, indicating a pure metallic state. In contrast, sample TWV-7-1-Ag consists of a combination of Ag⁰ and AgCl, suggesting the presence of both elemental silver and its chloride compound within the same sample. By comparing these results and considering the small amounts of Ag present in both samples, we can suggest that this explains why the XRD analysis detected the presence of AgCl rather than metallic silver.
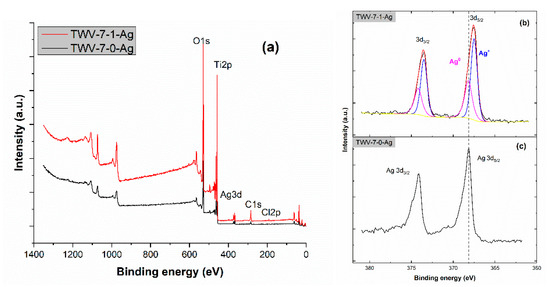
Figure 6.
X-ray photoelectron spectroscopy (XPS) spectra of the as-prepared samples. (a) Survey and (b,c) Ag 3d of the indicated samples.
3.2. Characterization of Gas-Sensing Performance
All the SAW sensors with the NPs studied as sensitive layers tested at CH4 concentrations between 0.4% and 2% responded at RT. The noise level (n) was 10 Hz for all the measurements, and the oscillation frequency was about 69 MHz.
The frequency shifts in Figure 7 demonstrate the improvement in sensitivity, brought by the use of NPs in the sensitive layer of the sensors. The smallest frequency shift was recorded by the S1-PEI sensor, with a single sensitive layer of PEI.

Figure 7.
The frequency shift of the SAW sensors at different CH4 concentrations.
The following results were obtained by the sensors with a sensitive layer of PEI and W&V:TiO2@NM without metal decoration: S2-TWV-7-0-PEI and S3-TWV-7-1-PEI. The difference between the two sensors S2-TWV-7-0-PEI and S3-TWV-7-1-PEI is due to the V doping of the NPs in the sensitive film of the S3 sensor. The higher frequency shift of the S3 sensor, despite not being a major difference, demonstrates the fact that the doping creates additional areas of interaction with the gas molecules. The BET analyses confirmed a higher specific surface area for TWV-7-1 NPs [38]. Moreover, the BET analyses indicated that doping with V led to a larger specific surface area for all W&V:TiO2@NM, an aspect which also emerges in all sensor results: in each pair of sensors, the one double-doped with V and W and with metallic decorations had a more pronounced frequency shift. Introducing metals such as Pd, Pt, and Ag led to an obvious improvement in the sensor’s sensibility, indicated by the increased frequency shifts in Figure 7.
The detection mechanism involved in this work started with the general mechanism of interaction of the oxide metal semiconductors with hydrocarbons, with the added catalytic effect brought about by the presence of metals [20]. Thus, oxide metal semiconductors with metal decoration have the ability to dissociate oxygen molecules from the air, which form an oxygen ion layer on the surface of oxide NPs. Also, metallic NPs act as hydrogen adsorption centers that can lead to the dissociation of C-H bonds from CH4, leading to the decrease in the activation energy which is needed for the sensing reaction [19].
CH4 molecules interact with the surface of metal nanoparticles, where H−- and CH3+-type species are generated. They spill over to the oxide surface, where the interaction with the oxygen species takes place, forming water molecules and free electrons, which reach the oxide phase. In this way, the frequency oscillation of the surface acoustic waves also changes, obtaining the sensor signal faster than usual and at RT.
This mechanism is applied to most interactions between noble metals and hydrocarbons, especially for Pd and CH4. It is known that Pd is a material that, in combination with other oxides, has a very good sensitivity for CH4 [20,43]. This was also demonstrated in the present work, where the sensors with NPs decorated with Pd (S8-TWV-7-0-Pd-PEI and S9-TWV-7-1-Pd-PEI) had the most pronounced frequency shifts (Figure 7).
Although most noble metals interact with CH4 and other hydrocarbons according to the mechanism presented above, Pt is an exception when it comes to the interaction with CH4. It has a low catalytic efficiency for CH4 but a high catalytic efficiency for many other reducing gasses [7,20]. Therefore, Pt is often used as a selective filter material for CH4 detection [20,44]. Because it interacts with other reducing gasses from the atmosphere, it allows CH4 molecules to penetrate unaltered to the sensitive layer. Thus, an upper layer of Pt can become a selective filter for CH4 detection. However, the sensors with Pt had lower frequency shifts than those with Pd, due to the stronger catalytic activity of Pd compared to Pt. To develop a sensor with high sensibility and selectivity to CH4, the simultaneous use of Pd as a catalyst and Pt as a filter, deposited on an oxide substrate, should be investigated in a future research study.
Regarding the effect of NP decoration with Ag on the sensor results, it can be observed that their sensitivity is lower, which denotes a lower activity of Ag on CH4 compared to Pd and Pt.
The results discussed above are also confirmed by the calculated results regarding the sensitivity and LOD of the studied sensors. Sensitivity is defined as the frequency shift in Hz per unit analyte concentration in ppm [15]. The LOD is defined as three times the noise level divided by the sensitivity [15]. The results presented in Table 3 represent the average sensitivity and the LOD, respectively, obtained by each sensor at each tested concentration. The best values were obtained by the S9-TWV-7-1-Pd-PEI sensor, with 1.79 Hz/ppm sensitivity and 17 ppm LOD. These results are remarkable because the SAW sensor tests were carried out at RT.

Table 3.
Sensitivity and limit of detection (LOD) of the sensors during CH4 tests. Legend: Δf—frequency shift; c—CH4 concentration; and n—noise level of ~10 Hz.
The dynamic response in Figure 8 demonstrates the reversible nature of the sensors, with an average response time of 30–60 s and a recovery time of 50–90 s.
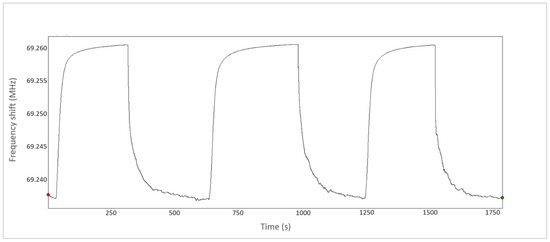
Figure 8.
Dynamic response of the S9-TWV-7-1-Pd-PEI sensor at 2% CH4 concentration.
4. Conclusions
In conclusion, we successfully produced SAW sensors with composite thin films: TiO2 double-doped with W and V and decorated with Pd, Pt, and Ag were embedded in a PEI polymer. The composite thin films were deposited by spin-coating under the same conditions.
W&V:TiO2@Ag NPs, obtained by laser pyrolysis followed by chemical impregnation and reduction, were structurally and morphologically analyzed, exhibiting features similar to those previously observed for Pt- and Pd-loaded nanoparticles [30]. Thus, the TiO2 particles were doped with 0.1 at.% V and W and loaded with 0.3 at.% Ag NPs. The titania NPs were predominantly in the anatase phase (95%), with average sizes between 25 and 30 nm, and loaded with small Ag NPs, with sizes between 3 and 5 nm. The combined effects of the doping and loading processes on the TiO2 NPs resulted in an increase in the specific surface area from 9.8062 m2g−1 (TWV-7-0 sample) to 59.623 m2g−1 (TWV-7-0-Ag sample).
SAW sensors were tested at different CH4 concentrations (0.4–2%), at RT. Results were obtained for all the tested sensors, highlighting the strong catalytic effect of Pd, with a sensitivity of 1.79 Hz/ppm and an LOD of 17 ppm. Also, the ability of Pt to develop selective sensors for CH4 was underlined. Sensors with Ag-decorated NPs also demonstrated their ability to be used as sensitive materials for CH4 detection.
TiO2 NPs double-doped with W and V and decorated with metals, especially Pd and Pt, demonstrated great potential for developing sensors with both high sensitivity and selectivity for CH4 detection.
Author Contributions
M.S., conceptualization, methodology, writing—review and editing, and supervision; I.C., conceptualization, methodology, writing—review and editing, and investigation; E.G., formal analysis and investigation; I.P.M., formal analysis and investigation; V.S.T., formal analysis and investigation; and C.V., conceptualization, methodology, writing—review and editing, and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
This research was supported (or financed) by the Romanian Ministry of Research, Innovation and Digitalization under the Romanian National Nucleu Program LAPLAS VII, with contract no. 30N/2023.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aldhafeeri, T.; Tran, M.-K.; Vrolyk, R.; Pope, M.; Fowler, M. A Review of Methane Gas Detection Sensors: Recent Developments and Future Perspectives. Inventions 2020, 5, 28. [Google Scholar] [CrossRef]
- Asadzadeh, S.; de Oliveira, W.J.; de Souza Filho, C.R. UAV-Based Remote Sensing for the Petroleum Industry and Environmental Monitoring: State-of-the-Art and Perspectives. J. Pet. Sci. Eng. 2022, 208, 109633. [Google Scholar] [CrossRef]
- Ramsden, A.E.; Ganesan, A.L.; Western, L.M.; Rigby, M.; Manning, A.J.; Foulds, A.; France, J.L.; Barker, P.; Levy, P.; Say, D.; et al. Quantifying fossil fuel methane emissions using observations of atmospheric ethane and an uncertain emission ratio. Atmos. Chem. Phys. 2022, 22, 3911–3929. [Google Scholar] [CrossRef]
- Nagahage, I.S.P.; Nagahage, E.A.A.D.; Fujino, T. Assessment of the Applicability of a Low-Cost Sensor–Based Methane Monitoring System for Continuous Multi-Channel Sampling. Environ. Monit. Assess. 2021, 193, 509. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.P.; Wang, Y.; Zhang, Z.Y.; Cao, J.L. Porous In2O3 nanospheres with high methane sensitivity: A combined experimental and first-principle study. Sens. Actuators A Phys. 2020, 305, 111944. [Google Scholar] [CrossRef]
- Jiao, M.-Z.; Chen, X.-Y.; Hu, K.-X.; Qian, D.-Y.; Zhao, X.-H.; Ding, E.-J. Recent developments of nanomaterials-based conductive type methane sensors. Rare Met. 2021, 40, 1515–1527. [Google Scholar] [CrossRef]
- Lu, N.; Fan, S.; Zhao, Y.; Yang, B.; Hua, Z.; Wu, Y. A selective methane gas sensor with printed catalytic films as active filters. Sens. Actuators B Chem. 2021, 347, 130603. [Google Scholar] [CrossRef]
- De-Giorgio, F.; Grassi, V.M.; Vetrugno, G.; Rossi, R.; Fucci, N.; d’Aloja, E.; Pascali, V.L. Homicide by methane gas. Forensic Sci. Int. 2012, 221, e1–e3. [Google Scholar] [CrossRef]
- Bezdek, M.J.; Luo, S.X.L.; Ku, K.H.; Swager, T.M. A chemiresistive methane sensor. Proc. Natl. Acad. Sci. USA 2021, 118, e2022515118. [Google Scholar] [CrossRef]
- Vizireanu, S.; Constantinoiu, I.; Satulu, V.; Stoica, D.S.; Viespe, C. High-Sensitivity H2 and CH4 SAW Sensors with Carbon Nanowalls and Improvement in Their Performance after Plasma Treatment. Chemosensors 2023, 10, 566. [Google Scholar] [CrossRef]
- Shi, J.; Jiang, Y.; Duan, Z.; Li, J.; Yuan, Z.; Tai, H. Designing an optical gas chamber with stepped structure for non-dispersive infrared methane gas sensor. Sens. Actuators A Phys. 2024, 367, 115052. [Google Scholar] [CrossRef]
- Dong, W.; Sugai, Y.; Wang, Y.; Zhang, H.; Zhang, X.; Sasaki, K. Experimental Study on Enhanced Methane Detection Using an MEMS-Pyroelectric Sensor Integrated with a Wavelet Algorithm. ACS Omega 2024, 9, 19956–19967. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; You, S.; Li, G.; Li, X.; Fan, Z. Application of Semiconductor Metal Oxide in Chemiresistive Methane Gas Sensor: Recent Developments and Future Perspectives. Molecules 2023, 28, 6710. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, S.; Yuan, W.; Fan, S.; Hua, Z.; Wu, Y.; Tian, X. Selective detection of methane by Pd-In2O3 sensors with a catalyst filter film. Sens. Actuators B Chem. 2021, 328, 129030. [Google Scholar] [CrossRef]
- Cao, R.; Ding, H.; Kim, K.-J.; Peng, Z.; Wu, J.; Culp, J.T.; Ohodnicki, P.R.; Beckman, E.; Chen, K.P. Metal-organic framework functionalized polymer coating for fiber optical methane sensors. Sens. Actuators B Chem. 2020, 324, 128627. [Google Scholar] [CrossRef]
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Gas sensors based on TiO2 nanostructured materials for the detection of hazardous gases: A review. Proc. Natl. Acad. Sci. USA 2021, 3, 390–403. [Google Scholar] [CrossRef]
- Parrino, F.; Pomilla, F.R.; Camera-Roda, G.; Loddo, V.; Palmisano, L. Titanium Dioxide (TiO2) and Its Applications, Metal Oxides; Parrino, F., Palmisano, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Chapter 2; pp. 13–66. [Google Scholar]
- Ramanavicius, S.; Jagminas, A.; Ramanavicius, A. Gas Sensors Based on Titanium Oxides. Coatings 2022, 12, 699. [Google Scholar] [CrossRef]
- Comert, B.; Akin, N.; Donmez, M.; Saglam, S.; Ozcelik, S. Titanium Dioxide Thin Films as Methane Gas Sensors. IEEE Sens. J. 2016, 16, 8890–8896. [Google Scholar] [CrossRef]
- Tong, X.; Shen, W.; Chen, X. Enhanced H2S sensing performance of cobalt doped free-standing TiO2 nanotube array film and theoretical simulation based on density functional theory. Appl. Surf. Sci. 2019, 469, 414–422. [Google Scholar] [CrossRef]
- Saruhan, B.; Fomekong, R.L.; Nahirniak, S. Review: Influences of Semiconductor Metal Oxide Properties on Gas Sensing Characteristics. Front. Sens. 2021, 2, 657931. [Google Scholar] [CrossRef]
- Santos, E.; Catto, A.C.; Peterline, A.F.; Avansi, W.J. Transition metal (Nb and W) doped TiO2 nanostructures: The role of metal doping in their photocatalytic activity and ozone gas-sensing performance. Appl. Surf. Sci. 2022, 579, 152146. [Google Scholar] [CrossRef]
- Li, Z.; Ding, D.; Ning, C. p-Type hydrogen sensing with Al- and V-doped TiO2 nanostructures. Nanoscale Res. Lett. 2013, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Al-Shomar, S.M. Investigation the effect of doping concentration in Ruthenium- TiO2 doped thin films for solar cells and sensors applications. Mater. Res. Express 2020, 7, 036409. [Google Scholar] [CrossRef]
- Conte, F.; Rossetti, I.; Ramis, G.; Vaulot, C.; Hajjar-Garreau, S.; Bennici, S. Low Metal Loading (Au, Ag, Pt, Pd) Photo-Catalysts Supported on TiO2 for Renewable Processes. Materials 2022, 15, 2915. [Google Scholar] [CrossRef] [PubMed]
- Belchi, R.; Habert, A.; Foy, E.; Gheno, A.; Vedraine, S.; Antony, R.; Ratier, B.; Bouclé, J.; Herlin-Boime, N. One-Step Synthesis of TiO2/Graphene Nanocomposites by Laser Pyrolysis with Well-Controlled Properties and Application in Perovskite Solar Cells. ACS Omega 2019, 4, 11906–11913. [Google Scholar] [CrossRef] [PubMed]
- Lertthanaphol, N.; Pienutsa, N.; Chusri, K.; Sornsuchat, T.; Chanthara, P.; Seeharaj, P.; Kim-Lohsoontorn, P.; Srinives, S. One-Step Hydrothermal Synthesis of Precious Metal-Doped Titanium Dioxide–Graphene Oxide Composites for Photocatalytic Conversion of CO2 to Ethanol. ACS Omega 2021, 6, 35769–35779. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, S.; Bhachu, D.S.; Lu, Y.; Chadwick, N.; Althabaiti, S.A.; Alyoubi, A.O.; Basahel, S.N.; Carmalt, C.J.; Parkin, I.P. Doped TiO2 with Enhanced Photocatalytic and Optoelectrical Properties via Aerosol Assisted Chemical Vapor Deposition. Sci. Rep. 2015, 5, 10952. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Sun, C.; Duan, Y.; Li, H.; Guo, H. Surface metal ion doped TiO2 nanowire arrays by low energy ion implantation for en-hanced photoelectrochemical performance. Ceram. Int. 2023, 49, 30395–30402. [Google Scholar] [CrossRef]
- Ngoc, T.A.N.; Ly, T.Q.T.; Vo, V.Q.G.; Nguyen, N.B.; Nguyen, Q.T.; Doan, H.V.; Le, T.K. Sol–gel synthesis of Al-doped TiO2 nanoparticles as UV filters with diminished photocatalytic activity for the application in sunscreen products. J. Sol-Gel Sci. Technol. 2023, 108, 900–911. [Google Scholar] [CrossRef]
- Spreafco, C.; Russo, D.; Degl’Innocenti, R. Laser pyrolysis in papers and patents. J. Intell. Manuf. 2022, 33, 353–385. [Google Scholar] [CrossRef]
- Goncearenco, E.; Morjan, I.P.; Dutu, E.; Scarisoreanu, M.; Fleaca, C.; Gavrila-Florescu, L.; Dumitrache, F.; Banici, A.M.; Teodorescu, V.S.; Anastasescu, C.; et al. The effect of noble metal addition on the properties of oxide semiconductors nanoparticles. J. Solid State Chem. 2022, 307, 122817. [Google Scholar] [CrossRef]
- Constantinoiu, I.; Viespe, C. ZnO Metal Oxide Semiconductor in Surface Acoustic Wave Sensors: A Review. Sensors 2020, 20, 5118. [Google Scholar] [CrossRef] [PubMed]
- Constantinoiu, I.; Miu, D.; Viespe, C. SAW Hydrogen Sensors with Pd/SnO2 Layers. Materials 2022, 15, 8012. [Google Scholar] [CrossRef] [PubMed]
- Ballantine, D.S.; White, R.M.; Martin, S.J.; Ricco, A.J.; Zellers, E.T.; Frye, G.C.; Wohtjen, H. Acoustic Wave Sensors, Theory, Design, and Physico-Chemical Applications; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Mandal, D.; Banerjee, S. Surface Acoustic Wave (SAW) Sensors: Physics, Materials, and Applications. Sensors 2022, 22, 820. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hu, H.; Liu, X.; He, S.; Pan, Y.; Zhang, C.; Dong, C. Development of a Room Temperature SAW Methane Gas Sensor Incorporating a Supramolecular Cryptophane A Coating. Sensors 2016, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shen, B.; Jiang, L.; Yang, H.; Jin, C.; Zhou, T. Study on SAW Methane Sensor Based on Cryptophane-A Composite Film. Micromachines 2023, 14, 266. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Khan, S.B.; Asiri, A.M. Enhanced H2 generation from NaBH4 hydrolysis and methanolysis by cellulose micro-fibrous cottons as metal templated catalyst. Int. J. Hydrogen Energy 2018, 43, 6539–6550. [Google Scholar] [CrossRef]
- Goncearenco, E.; Morjan, I.P.; Fleaca, C.; Dutu, E.; Criveanu, A.; Viespe, C.; Galca, A.C.; Maraloiu, A.V.; Stan, M.S.; Fort, C.I.; et al. The Influence of SnO2 and Noble Metals on the Properties of TiO2 for Environmental Sustainability. Sustainability 2024, 16, 2904. [Google Scholar] [CrossRef]
- Dong, P.; Hou, G.; Liu, C.; Zhang, X.; Tian, H.; Xu, F.; Xi, X.; Shao, R. Origin of Activity and Stability Enhancement for Ag3PO4 Photocatalyst after Calcination. Materials 2016, 9, 968. [Google Scholar] [CrossRef]
- Wang, J.; An, C.; Zhang, M.; Qin, C.; Ming, X.; Zhang, Q. Photochemical conversion of AgCl nanocubes to hybrid AgCl–Ag nanoparticles with high activity and long-term stability towards photocatalytic degradation of organic dyes. Can. J. Chem. 2012, 90, 858. [Google Scholar] [CrossRef]
- Chu, S.; Wang, E.; Feng, F.; Zhang, C.; Jiang, J.; Zhang, Q.; Wang, F.; Bing, L.; Wang, G.; Han, D. A Review of Noble Metal Catalysts for Catalytic Removal of VOCs. Catalysts 2022, 12, 1543. [Google Scholar] [CrossRef]
- Wu, R.; Tian, L.; Li, H.; Liu, H.; Luo, J.; Tian, X.; Hua, Z.; Wu, Y.; Fan, S. A selective methane gas sensor based on metal oxide semiconductor equipped with an on-chip microfilter. Sens. Actuators B Chem. 2022, 359, 131557. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).