Abstract
Nitroreductase (NTR) is an enzyme expressed at an abnormally high level in solid tumors, which is associated with the hypoxia level in tumors. The establishment of a high-performance and convenient fluorescent platform for the fast monitoring of NTR is of pivotal importance. Herein, a novel host–guest complex was created by encapsulating a fluorescent substrate GP-NTR within a metal–organic capsule Zn-MPB that included a NADH mimic for the detection of hypoxia via responding to nitroreductase (NTR) with fast responsiveness and good fluorescence imaging. Notably, the double-substrate process was streamlined to a single–substrate process by the host–guest supramolecular method in the catalytic process of NTR, which enabled the reaction to be independent of the cofactor NADH supply and shortened the distance between the substrate and the active site of NTR. The increasing fluorescence intensity of Zn-MPB⊃GP-NTR exhibits a linear relationship with NTR concentration and shows a fast response toward NTR in solution in tens of seconds. Zn-MPB⊃GP-NTR also displays high sensitivity to NTR with a low detection limit of 6.4 ng/mL. Cells and in vivo studies have confirmed that Zn-MPB⊃GP-NTR could be successfully applied for the fast imaging of NTR in NTR-overexpressed tumor cells and tumor-bearing animals. The host–guest platform not only provides a new avenue for the design and optimization of a fluorescence detection platform for the rapid and quantitative detection of NTR activity, but also offers an imaging tool for the early diagnosis of hypoxia-related tumors.
1. Introduction
Hypoxia is a common physiological manifestation in the growth of malignant tumors [1,2,3,4,5], and it could result in the overexpression of several intracellular reductase enzymes that typically catalyze redox reactions in the tumor microenvironment with impressive selectivity and specificity [6,7]. As one type of reductive enzyme in hypoxic tumors, nitroreductase (NTR) could effectively reduce nitroaromatic compounds to the corresponding arylamine with reduced nicotinamide adenine dinucleotide (NADH) as the coenzyme [8,9,10]. It is of great significance to monitor the expression of NTR in hypoxic tumors owing to its crucial role in malignant tumor progression, invasion, angiogenesis, and prodrug activation for directed anticancer therapies [11,12,13]. Several analytical methods have been developed for NTR detection, including positron emission tomography (PET) [14], magnetic resonance imaging (MRI) [15], ultrasound imaging [16], colorimetric methods [17], and fluorescence spectroscopy [18]. Among them, numerous fluorescent probes have recently been reported with the ability to visualize NTR owing to their high sensitivity, easy operation, and unrivaled spatiotemporal resolution [19,20]. They mainly depend on the changes in the fluorescence intensity of NTR activity to the fluorescent substrate, and the intensity of its emission may be closely related to the activity of the enzyme and the local concentrations of the probe and the coenzyme. Based on the ‘ping-pong’ mechanism, a stable relationship rarely exists between the fluorescence intensity and enzyme content when the NADH concentration is not fixed. The uncertain levels of enzymes and coenzymes lead to the variability in probe emission over time. In the conventional detection system of NTR, a large excess of NADH is often added to the reaction solution, which could reduce the interference of the concentration of NADH, ensure the reaction reaches fluorescence emission equilibrium quickly, and improve the accuracy of the detection [21,22]. Considering that the expression levels of hypoxia enzymes and coenzymes in living system are indefinite, the in vivo detection of hypoxic enzymes is highly dependent on the corresponding coenzyme; therefore, it is worth paying attention to improving the detection efficiency of hypoxic enzymes [23]. It is challenging to maintain the coenzyme supply via a covalent molecule bond with the coenzyme because of the intricate molecular design and tedious synthesis. Thus, developing a platform with sufficient coenzymes for highly efficient, quantitative, and rapid detection of NTR in biological systems is highly desired.
Supramolecular systems based on host–guest interactions have been evolving immensely in recent years, and they are widely used in various biomedical applications, such as biological imaging, clinical medicine, and drug delivery due to the functional modification features and dynamic properties of host−guest self-assembly [24]. Well-known macrocyclic molecules such as cyclodextrins, crown ethers, calixarenes, and pillararenes have been established as ‘hosts’ to accommodate ‘guests’ inside their cavities through noncovalent interactions to construct host–guest complexes [25]. Metal–organic capsules have attracted extensive attention due to their adjustable cavity and characteristics of easy assembly and could solve the problem of difficult covalent modification [26,27]. A metal–organic capsule incorporated by the active site of NADH and its mimics has been postulated to be an effective host molecule for constructing an artificial catalytic platform, and it could emulate the environment of an enzyme pocket that changes the reactivity of the substrate and achieves a significant increase in the reaction rate [28,29,30]. The host–guest platform consists of a cofactor-modified metal–organic capsule and a substrate through intermolecular interactions, featuring the advantages of strong contact, stimulus response and dynamic characteristics. This platform provides a simple and effective approach for the detection of NTR, which could enable the redox reaction between the substrate, cofactor, and enzyme to occur effectively. When the host–guest platform interacts with NTR, it enables the efficient proton/electron transport of the biological microenvironment, eliminates the diffusion of cofactors during the process, and accelerates the detection efficiency of NTR.
Herein, a host–guest complex was reported by encapsulating a substrate GP-NTR within a NADH mimic-containing metal–organic capsule Zn-MPB for the detection of NTR (Scheme 1). The substrate, GP-NTR, was well established with naphthalimide as the fluorophore and a nitro group as the recognition moiety. Zn-MPB serves not only as a substitute for NADH, but also as a carrier to efficiently transport the substrate to NTR, which accelerates the process of the reaction. Its water solubility and biocompatibility have been improved, which was attributed to the host–guest system. The positively charged Zn-MPB would contribute to the accumulation of the host–guest complex in tumor cells. It is particularly worth noting that the host–guest strategy could simplify the original double–substrate enzymatic process of a substrate-based probe into a simpler pseudo-single-substrate kinetic, with the Michaelis−Menten equation. In accordance with this feature, the enzymatic reaction process was independent of the cofactor NADH supply, which accelerated the reaction rate and shortened the equilibrium time. The fluorescence intensity of Zn-MPB⊃GP-NTR was linearly related to the concentration of NTR. Zn-MPB⊃GP-NTR exhibited superior performance in vitro, including fast NTR response time, low detection limit, and good NTR selectivity. The specific recognition of NTR by Zn-MPB⊃GP-NTR was verified by HPLC. Zn-MPB⊃GP-NTR shows low cytotoxicity and could be used to evaluate the hypoxia levels in both MCF-7 and HepG2 cells. In vitro and in vivo experiments have proven the good NTR detection ability of Zn-MPB⊃GP-NTR. It could serve as a trustworthy platform for the effective bio-tracing of NTR in an hypoxic microenvironment and has great potential for cancer diagnostic applications.
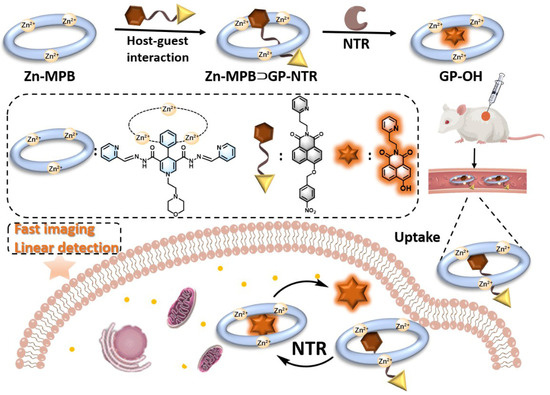
Scheme 1.
Schematic diagram of preparation and application of Zn-MPB⊃GP-NTR for NTR detection.
2. Materials and Methods
2.1. Reagents and Instrumentation
All reagents and solvents were purchased commercially and used as received. Nitroreductase from Escherichia coli and NADH were purchased from Sigma-Aldrich (St. Louis, MO, USA). NMR spectra were acquired using a Bruker AVANCE III 400 MHz spectrometer and a Bruker AVANCE III 500 MHz spectrometer (Bruker, Ferranden, Switzerland). Mass spectrometric data were collected using an LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Titration calorimetry (ITC) was performed on a Nano ITC (TA Instruments Inc. Waters LLC, New Castle County, DE, USA). Fluorescent spectra were obtained with a Steady State, Fluorescence lifetime Spectrometer (Edinburgh Instruments (FLS1000), Livingston, Scotland). The UV–vis spectra were measured on a TU 1900 UV–vis spectrometer (Persee, Shanghai, China). HPLC analyses were performed using Agilent 1100 high-performance liquid chromatography (Agilent, Santa Clara, CA, USA). The BD Accuri C6 Plus flow cytometer was used to sort out specific cells (BD, Franklin Lake, NJ, USA). Confocal fluorescence imaging was performed using an OLYMPUS FV1000 confocal microscopy (OLYMPUS, Tokyo, Japan). The mice were imaged using Berthold Night Owl LB 983 NC100 systems (Berthold Technologies, Baden-wurttemberg, Germany).
2.2. Synthesis of Compound 2
4-Bromo-1,8-naphthalic anhydride (1100 mg, 4 mmol) and 2-(2-aminoethyl)pyridine (526 μL, 4.4 mmol) were dissolved in 15 mL of ethanol [31]. The reaction mixture was heated at 75 °C for 8 h. After cooling to room temperature, the solvent was removed under vacuum, and the obtained solid was washed with ethanol to give 4-bromo-N-2-aminoethylpyridine-1,8-naphthalimide (compound 2) as a white solid product. Yield: 82%. 1H NMR (400 MHz, DMSO) δ 8.51 (d, J = 4.7 Hz, 2H), 8.44 (d, J = 4.3 Hz, 1H), 8.27 (d, J = 6.4 Hz, 1H), 8.21–8.15 (m, 1H), 7.96 (t, J = 7.3 Hz, 1H), 7.70 (t, J = 7.5 Hz, 1H), 7.31 (d, J = 7.7 Hz, 1H), 7.25–7.17 (m, 1H), 4.37 (t, J = 7.4 Hz, 2H), 3.08 (t, J = 7.5 Hz, 2H).
2.3. Synthesis of Compound GP-OH
Compound 2 (343 mg, 0.9 mmol) and N-Hydroxyphthalimide (163 mg, 1 mmol) were dissolved in 5 mL dimethyl sulfoxide, then potassium carbonate (414 mg, 3 mmol) was added, and the mixture was refluxed at 80 °C for 6 h. After cooling to room temperature, the reaction solution was added dropwise to 100 mL of ultrapure water and then stirred. The pH was adjusted to about 3 by concentrated hydrochloric acid. The pure product 4-hydro-N-2-aminoethylpyridine-1,8-naphthalimide (compound GP-OH) was obtained by filtering reaction mixture without further purification. Yield: 76%. 1H NMR (400 MHz, DMSO) δ 11.85 (s, 1H), 8.52 (dd, J = 8.3, 0.8 Hz, 1H), 8.49–8.42 (m, 2H), 8.33 (d, J = 8.2 Hz, 1H), 7.79–7.65 (m, 2H), 7.29 (d, J = 7.8 Hz, 1H), 7.21 (dd, J = 7.0, 5.2 Hz, 1H), 7.15 (d, J = 8.2 Hz, 1H), 4.42–4.32 (m, 2H), 3.10–3.03 (m, 2H). HR–MS m/z: [M+H]+ calculated for [C19H15N2O3]+: 319.1086, found: 319.1093.
2.4. Synthesis of Compound GP-NTR
The synthetic route of GP-NTR was described in Scheme 2. Compound GP-OH (318 mg, 1 mmol) and K2CO3 (420 mg, 3 mmol) were dissolved in 5 mL acetonitrile, and the mixture was stirred for 30 min, and then, 1-(bromomethyl)-4-nitrobenzene (216 mg, 1 mmol) was added and refluxed at 75 °C for 8 h. After cooling to room temperature, the solvent was removed under vacuum; then, the crude product was purified by column chromatography on silica gel using eluent (dichloromethane/methanol = 100/1) to give GP-NTR. Yield: 84%. 1H NMR (400 MHz, CDCl3/CD3OD) δ 8.71 (d, J = 8.3 Hz, 1H), 8.66 (d, J = 7.2 Hz, 1H), 8.58 (d, J = 8.2 Hz, 1H), 8.54 (d, J = 4.1 Hz, 1H), 8.38 (d, J = 8.6 Hz, 2H), 7.82 (dd, J = 12.9, 8.4 Hz, 3H), 7.72 (t, J = 7.6 Hz, 1H), 7.42–7.36 (m, 2H), 7.19 (d, J = 8.3 Hz, 1H), 5.57 (s, 2H), 4.72–4.53 (m, 2H), 3.36–3.21 (m, 2H). 13C NMR (126 MHz, CDCl3/CD3OD) δ 168.30, 167.71, 163.20, 162.55, 152.65, 152.56, 151.83, 146.85, 141.06, 137.31, 137.22, 135.75, 135.68, 133.34, 132.53, 131.85, 130.27, 127.89, 127.57, 127.44, 126.14, 125.78, 119.41, 110.53, 73.40, 43.87, 39.99. HR–MS m/z: [M+H]+ calculated for [C26H20N3O5]+: 454.1402, found: 454.1391.
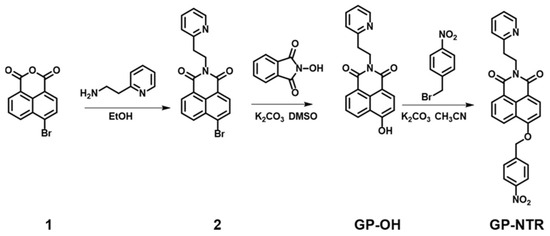
Scheme 2.
The synthetic route of GP-NTR.
2.5. The General Procedure for In Vitro Spectra Measurement
The spectroscopic measurements were performed in Tris–HCl at 25 °C. A stock solution of GP-NTR (2 mM) or Zn-MPB (2 mM) was prepared in DMSO and diluted to the required concentration when it was used in vitro measurement. Other analytes were prepared by dissolving the relevant analytes with the same amount of distilled water to obtain the final concentrations, including NaCl, KCl, L-Tyr, L-Cys, β-Ala, L-Pro, L-Phe, L-Pro, H2O2, AA (Ascorbic Acid), NQO1 (NAD(P)H quinone oxidoreductase 1), GSR (Glutathione Reductase), and Escherichia coli NTR (Nitroreductase) (5 μg/mL in distilled water) (λex = 440 nm, λem = 520 nm). The solution was swiftly combined, and then, the absorbance or fluorescence spectrum was measured by pouring it into a quartz colorimetric dish.
2.6. Kinetic Study of GP-NTR and Zn-MPB⊃GP-NTR Reacting with NTR
The catalytic activity of NTR toward the reduction of Zn-MPB⊃GP-NTR was investigated by fluorescence spectra at 25 °C. By using the Michaelis–Menten equation, the kinetic parameters VmaxZn-MPB⊃GP-NTR/VmaxGP-NTR and kcatZn-MPB⊃GP-NTR/kcatGP-NTR were estimated [32,33]. The catalytic number ratio between GP-NTR and Zn-MPB⊃GP-NTR reacting with NTR is kcatZn-MPB⊃GP-NTR/kcatGP-NTR. The maximum reaction rate ratio between GP-NTR and Zn-MPB⊃GP-NTR reacting with NTR is VmaxZn-MPB⊃GP-NTR/VmaxGP-NTR.
2.7. ITC Experiments
The isothermal titration microcalorimeter was applied in the ITC experiments (at atmospheric pressure and 25 °C) [34], and it provided data for the association constant (K) and thermodynamic parameters. A solution of GP-NTR (1 mM) in a 0.25 mL syringe was sequentially injected with stirring at 250 rpm into a solution of Zn-MPB (0.1 mM) in the sample cell (1.30 mL volume). The experiments were conducted in DMF–H2O solution (DMF/H2O = 98/2, v/v). Using the ‘independent’ model, all of the thermodynamic parameters given in this study were determined.
2.8. Molecular Docking Preparation
The structure of NTR was obtained from the Protein Crystal Structure Database (https://www.rcsb.org/3d-view/1ICR/1 (accessed on July 7, 2024)) (PDB code: 1ICR for NTR). The basic structure of Zn-MPB and GP-NTR were appropriately optimized and adjusted. For the docking calculations, the models of NTR were refined by eliminating water molecules, adding hydrogen atoms, and adding Gasteiger charges, fragmental volumes, and atomic solvation parameters to adhesive through AutoDock Tools (AutoDock Tools-1.5.6) [35]. For the ligand, the molecule was refined by removing and subsequently adding hydrogen atoms in a similar manner. PyMOL 2.5.4 was used to render the AutoDock docking calculation results following optimization.
2.9. HPLC Analysis
The substrate GP-NTR was dissolved in PBS, and NTR was added into the solution of NADH or Zn-MPB for 30 min, and the resultant solution (NTR: 10 μg/mL, GP-NTR or GP-OH: 10 μM) was filtered before sample injection. Mobile phase A was water containing 0.2% acetic acid and 0.2% triethylamine, and phase B was pure methanol. The flow rate was 0.8 mL/min.
2.10. Cell Culture and Cell Cytotoxicity Studies
The Michigan Cancer Foundation-7 (MCF-7) cells and HepG2 cells were cultured in DMEM supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin–streptomycin in a 5% CO2 humidified incubator at 37 °C.
The cytotoxicity of GP-NTR and Zn-MPB to MCF-7 cells and HepG2 cells was measured by the MTT method. In brief, cells were seeded in 96-well microplates and incubated in complete DMEM culture medium for 24 h. Then, the cells were washed with PBS and replaced with a fresh culture medium and then cultured in medium with 0, 1, 2, 5, 10, and 20 µM of GP-NTR and Zn-MPB for 24 h. After that, the medium was removed, and the cells were washed 3 times with fresh PBS. The MTT reagent with the fresh medium was added into each well and then incubated for another 4 h. The culture medium was taken out, and 100 μL DMSO was added to dissolve the precipitate. Finally, the Thermo MK3 ELISA Microplate Reader was used to measure the absorbance at 570 nm, and the cell viability of MCF-7 cells and HepG2 cells was estimated.
2.11. Confocal Fluorescence Imaging for Cells
The MCF-7 cells and HepG2 cells were treated under normoxic (20% O2) or different hypoxic conditions (O2 concentration of 8% or 0.1%) for 6 h at 37 °C. After culture, the cells were further incubated with GP-NTR (2 μM) and Zn-MPB⊃GP-NTR (2 μM) biomimetic catalytic platform for 1 min. Kinetic fluorescence imaging was carried out by incubating MCF-7 cells and HepG2 cells with GP-NTR (2 μM) and Zn-MPB⊃GP-NTR (2 μM) for 0, 1, 5, 10, or 20 min after treatment under hypoxic conditions (0.1% O2) for 6 h at 37 °C. The cells were rinsed three times with PBS. An Olympus FV1000 fluorescent microscope was used to take fluorescence photographs of the cells. The wavelength of the laser source was 488 nm, and the cell fluorescence signals were collected at 510–600 nm.
2.12. Flow Cytometry Analyses
MCF-7 cells were cultured with different degrees of hypoxia (20% O2 and 0.1% O2) for 6 h and treated with 5 μM Zn-MPB⊃GP-NTR for 15 min. The cells were washed 3 times with PBS buffer, and then trypsinized, centrifugated, and resuspended in 1 mL PBS medium, and subjected to flow cytometric analysis. In another experiment, the MCF-7 cells and HepG2 cells were incubated under 0.1% O2 condition for 6 h. Then, they were treated with 5 μM GP-NTR or 5 μM Zn-MPB⊃GP-NTR at 37 °C for 15 min and washed 3 times with PBS buffer, and then trypsinized, centrifugated, and resuspended in 1 mL PBS medium, and subjected to flow cytometric analysis.
2.13. Establishment of Mice Model
A Berthold Night Owl LB 983 NC100 system was employed for in vivo imaging. Mice were anesthetized before fluorescence imaging. Then, the mice were placed in the in vivo imaging system for fluorescence imaging after giving injection of GP-NTR (100 μL,100 μM) or Zn-MPB⊃GP-NTR (100 μL,100 μM) in saline (λex = 450 nm, λem = 520 nm).
3. Results and Discussion
3.1. Preparation and Characterization of Zn-MPB⊃GP-NTR
The well-known NTR-sensitive moiety, p-nitrobenzyl, was attached to the hydroxyl part of naphthalimide to construct the substrate, GP-NTR. The metal–organic capsule Zn-MPB consists of three Zn2+ ion and three alternating connected ligands, and each Zn2+ ion was combined with two tridentate chelators from two different ligands. The NADH mimics was located on the surface of Zn-MPB, and it could make the capsule function as the coenzyme beta-nicotinamide adenine dinucleotide (NADH) that receives and transfers protons and electrons directly from enzymatic processes when the capsule enters the catalytic domain of the enzyme and then provides adequate electrons and protons to reduce the nitro group of GP-NTR [36,37]. The cyclic voltammogram experiment of Zn-MPB has been performed in CH3CN (Figure S33), confirming that the redox activity of Zn-MPB was enhanced compared to the ligand H2MPB [29]. It is noteworthy that the morpholine moieties in Zn-MPB increase the water solubility and biocompatibility of the supramolecular system, and the positively charged Zn-MPB contributes to the accumulation of host–guest complex in the tumor. GP-NTR and H2MPB were easily synthesized and purified by column chromatography. The detailed synthetic rote of GP-NTR was laid out in Scheme 2. The synthesis of Zn-MPB was reported in the supporting information. The chemical structures of GP-NTR and Zn-MPB were characterized by 1H NMR and mass spectrometry.
The assembly of Zn-MPB⊃GP-NTR was monitored via ESI–MS, 1H NMR spectroscopy, 1H diffusion ordered spectroscopy (1H DOSY), and 1H–1H NOESY spectroscopy. Results from the ESI–MS analysis of Zn-MPB in acetonitrile revealed an intense peak at m/z = 942.2663 that was attributed to [H2Zn3(MPB)3]2+ species (Figure 1a), showing the integrity of Zn-MPB [38]. After adding an equimolar amount of GP-NTR to Zn-MPB, a H2Zn3(MPB)3(GP-NTR)]2+ species appeared at m/z = 1169.8331 (Figure 1b). The establishment of a 1:1 host–guest species Zn-MPB⊃GP-NTR in solution was proposed by a comparison of the experimental peaks and those obtained via simulation based on natural isotopic abundances. With the 1H NMR spectra of the free GP-NTR, the free Zn-MPB, and the mixture of GP-NTR and Zn-MPB, the encapsulation of GP-NTR in Zn-MPB could be signaled by the proton chemical shift changes in peaks for the host and guest molecules with respect to the corresponding signal of the starting material (Figure 1c and Figure S10) [39,40]. The peak related to the protons (H12 and H15) of Zn-MPB moved ~0.05 ppm upfield, which indicated the encapsulation of GP-NTR into Zn-MPB [28,41]. The diffusion-ordered NMR spectroscopy (DOSY) spectra of the 1:1 mixture of GP-NTR with Zn-MPB measured in DMSO-d6 indicated that GP-NTR and Zn-MPB could belong to a single assembly species with a weight average diffusion coefficient of 2.104 × 10−10 m2 s−1 (Figure 1c) [42]. The formation of Zn-MPB⊃GP-NTR species was demonstrated by NOESY spectroscopy of the phenyl ring Hc of GP-NTR with the H12 and H14 of Zn-MPB (Figure S12). These results suggest that the host–guest system forms steadily. The formation of host–guest complexation species was examined by UV–vis spectra [43,44]. Job’s plot between Zn-MPB and GP-NTR with different molar ratios clearly showed a 1:1 stoichiometry between Zn-MPB and GP-NTR (Figure 2a and Figure S15). Isothermal titration calorimetry (ITC) measurements were conducted in DMF-H2O solution (DMF/H2O = 98/2, v/v) to further characterize the host–guest combination (Figure 2c). The titration results showed that, upon the addition of GP-NTR, Zn-MPB exhibited the absolute values of enthalpic and entropic changes of ΔH = 11.16 kJ mol−1 and TΔS = 43.26 kJ mol−1, respectively [45,46]. The free energy of binding between Zn-MPB and GP-NTR was calculated as −7.66 kcal mol−1, and the binding constant (Ka) was calculated as 4.22 × 105 M−1, indicating the favored formation and steady existence of the 1:1 host–guest system [28,41]. As shown in Figure S32, the solubility of the host–guest system was also improved due to the self-assembly of the host and guest, which made Zn-MPB⊃GP-NTR more suitable for biological detection.
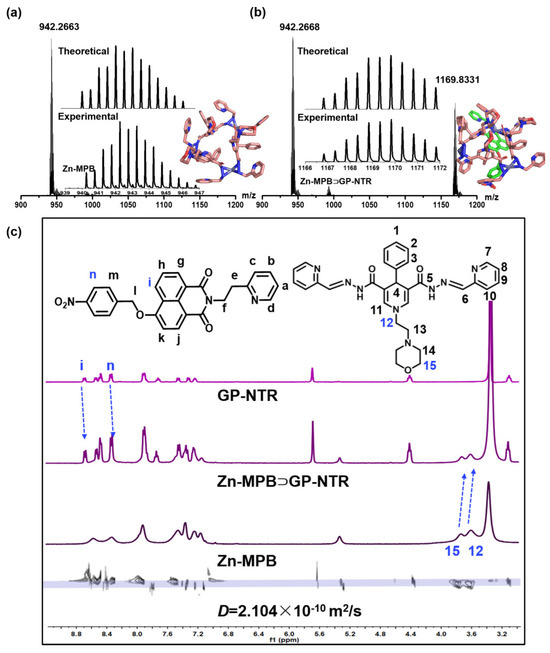
Figure 1.
Characterization of the interactions between Zn–MPB and GP-NTR. ESI–MS spectra of Zn-MPB (a) and Zn-MPB following the addition of 1.0 equiv of GP-NTR (b) in CH3CN solution. (c) 1H DOSY spectra of Zn-MPB⊃GP-NTR (DMSO-d6, 298 K).
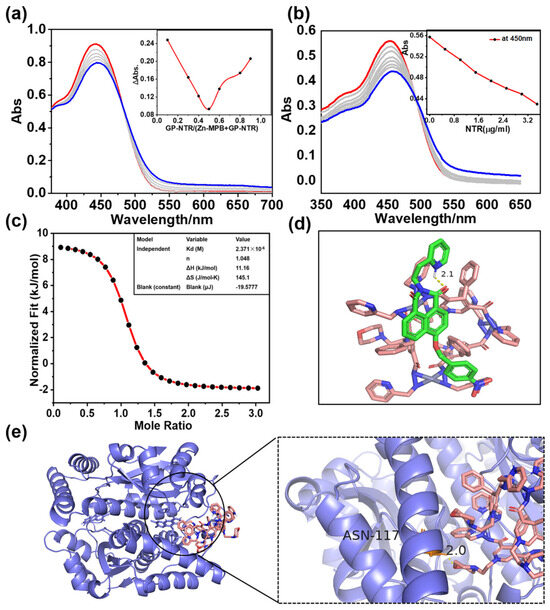
Figure 2.
(a) UV titration spectra of Zn-MPB (10 μM) with GP-NTR (0–20 μM) in DMSO/Tris–HCl solution (red line: the initial titration curve; blue line: the final titration curve). The inset shows a Job’s plot curve of GP-NTR/Zn-MPB mixtures with different molar ratios. (b) UV titration spectra of Zn-MPB (10 μM) with NTR (0–3.5 μg/mL) in Tris–HCl solution (red line: the initial titration curve; blue line: the final titration curve). The inset shows the changes at 450 nm. (c) ITC experiments of Zn-MPB upon the addition of GP-NTR in the DMF/H2O (98:2) solution. (d) Molecular docking results of GP-NTR with Zn-MPB. (e) Molecular docking results of Zn-MPB with NTR.
3.2. Molecular Docking
In molecular design and the simulation of chemical and protein binding processes, molecular docking is a helpful technique for predicting the binding mode of receptors and ligands [47,48,49]. Small molecule ligands and receptor molecules were docked with a semi-flexible docking method. The docking results demonstrated that a possible hydrogen bond was formed between the N atom of the pyridine ring of GP-NTR and the O atom of Zn-MPB (labeled in yellow), and the distance of hydrogen bonding was 2.1 Å. The free energy of binding between Zn-MPB and GP-NTR was about −8.3 kcal mol−1, which indicated the stable binding behavior of Zn-MPB with GP-NTR (Figure 2d). The free energy of binding between GP-NTR and NTR was about −6.47 kcal mol−1 (Figure S16). As shown in Figure 2e, the docking results revealed that strong hydrogen bonds were formed between the O atom of the pyridine ring of Zn-MPB and the nearby arginine residues (ASN-117) of NTR, which further enabled Zn-MPB to enter the hydrophobic protein pocket of NTR and bind to it closely. The binding free energy between Zn-MPB and NTR was about −3.34 kcal mol−1. The above results demonstrated that Zn-MPB may have good binding ability to NTR.
3.3. Fluorescence Responses of Zn−MPB⊃GP-NTR toward NTR
The spectroscopic responses of Zn-MPB⊃GP-NTR or GP-NTR were investigated in vitro to verify the response ability of Zn-MPB⊃GP-NTR toward NTR under model conditions (Tris–HCl buffer, pH 6.5, and 25 °C) [50]. Upon the addition of NTR to Zn-MPB⊃GP-NTR, there was a noticeable increase in fluorescence intensity at 520 nm within 40 s. The fluorescence intensity and NTR content (0–5 μg/mL) exhibited a good linear relationship, which ensures the quantitative analysis of NTR (Figure 3a,b,e). According to the slope of the curve, the limit of detection (LOD) value of Zn-MPB⊃GP-NTR could be obtained by using the formula 3σ/k. And the LOD was calculated to be 6.4 ng/mL (R2 = 0.9936) (Figure S17). In comparison, traditional probe GP-NTR was investigated by varying the amount of NTR added to the mixture of GP-NTR and NADH, which displayed similar emission peaks to those of the above-mentioned solution (Figure 3c). However, GP-NTR needed over 10 min to reach equilibrium, which was an over 20-fold longer reaction time than that of Zn-MPB⊃GP-NTR (Figure 3d). Due to the plots and slopes of the intensity versus NTR content change over time during the slower reaction of GP-NTR with NTR, it is difficult for a linear relationship to form between the enhancement in fluorescence intensity and the concentration of enzyme (Figure S18b). For better understanding the reduction progress of Zn-MPB⊃GP-NTR to NTR, HPLC analysis was performed (Figure S27). A new chromatographic peak (retention time, TR = 5.13 min/5.15 min) was observed after GP-NTR was incubated with NTR for 20 min in the presence of NADH or Zn-MPB, which was exactly matched with the resultant GP-OH fluorophore. In addition, principal component analysis (PCA) was applied to further distinguish the reaction modes of GP-NTR and Zn-MPB⊃GP-NTR with NTR. As shown in Figure 3f, there was little overlap between the two sets of data. The projection data of Zn-MPB⊃GP-NTR on the third component axis were narrowly clustered between about −0.4 and −0.7, while the data of GP-NTR on the third component axis were scattered between −0.3 and 1.2. It was further confirmed that the intensity of the host–guest complex changed little after the reaction equilibrium was obtained rapidly from 30 s to 100 s. The host–guest complex significantly improved NTR detection with a substantially shorter response time than that of the conventional probe, which indicated that the host–guest complex can be applied for the fast detection of NTR [51]. Further investigation exemplified that GP-NTR reduction by NTR was significantly influenced by the increased concentration of NADH (Figure S19a), implying that the supply of NADH was necessary for NTR detection. Little difference was seen in the reaction between Zn-MPB⊃GP-NTR and NTR with or without the addition of NADH before or after the reaction (Figure S20). It could be seen from the fluorescence comparison experiments that Zn-MPB⊃GP-NTR could improve the fluorescence response rate of NTR and shorten the equilibrium time, and the fluorescence intensity showed a linear response to the concentration of NTR. In addition, we tested the photostability of Zn-MPB⊃GP-NTR and Zn-MPB⊃GP-NTR coexisting with NTR (5 μg/mL) and the influence of temperature change on the efficiency of the host–guest system. The results proved that the photostability of the host–guest system was better, and the influence of temperature on the system was relatively small in 0–40 °C before and after the addition of NTR (Figures S21 and S22). When the host–guest platform was compared with some published NTR probes, the fluorescence response time of Zn-MPB⊃GP-NTR was relatively short, and the detection limit was also relatively low (Table S1). These results showed that the host–guest platform Zn-MPB⊃GP-NTR could be a suitable turn-on biosensor for the quantitative detection of NTR with a significantly shorter response time.
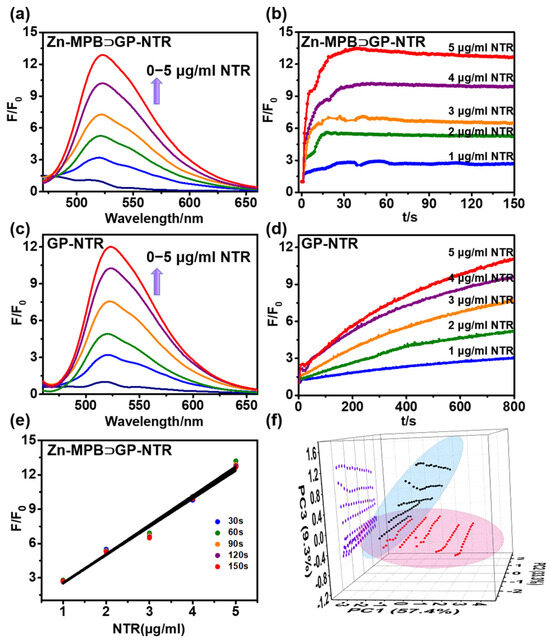
Figure 3.
Fluorescence spectra of GP-NTR (5 μM) in response to different concentrations of NTR with Zn-MPB (5 μM) (a) or NADH (15 μM) (c). (b,d) Time-dependent intensity variation in GP-NTR (5 μM) with Zn-MPB (5 μM) or NADH (15 μM) with different concentrations of NTR (1–5 μg/mL). (e) Intensity vs. NTR levels in 5 μM Zn-MPB⊃GP-NTR at different times. (f) 3D PCA plot of 5 μM Zn-MPB⊃GP-NTR (red) and 5 μM GP-NTR (black) reaction with 1–5 μg/mL NTR in 30–100 s and their YZ plane projection (purple). 95% confidence ellipses surround each sample cluster. λex = 440 nm, λem = 520 nm.
Encouraged by the excellent fluorescence capability of Zn-MPB⊃GP-NTR, the kinetic parameters of the NTR-catalyzed cleavage reaction were also determined (Figures S23 and S24). It is well known that the reduction of nitro group by NADH and NTR is a typical enzyme process involving two substrates [52]. However, the response of Zn-MPB⊃GP-NTR to NTR concentration was not affected by NADH, and the pseudo-intramolecular signal communication reduced the complexity of the double-substrate mechanism into a single-substrate free collision process. By using the Michaelis−Menten equation, the kinetic parameter value of VmaxZn-MPB⊃GP-NTR/VmaxGP-NTR and kcatZn-MPB⊃GP-NTR/kcatGP-NTR is about 28 [32,33]. In comparison to a natural catalytic system, Zn-MPB⊃GP-NTR enhanced catalytic efficiency by changing the catalytic kinetics. These above-mentioned results demonstrated that Zn-MPB⊃GP-NTR was capable of the rapid detection of NTR with great potential in biological applications.
On account of the complexity of cellular milieux, selectivity experiments were performed via recording the fluorescence spectra changes after treatment with various potential interfering species (NaCl, KCl, L-Tyr, L-Cys, β-Ala, L-Pro, L-Phe, L-Pro, H2O2, AA, NQO1, GSR) to verify the specificity of Zn-MPB⊃GP-NTR for detecting NTR (Figure S25) [53]. Upon the addition of analytes to the Tris–HCl solution with GP-NTR or Zn-MPB⊃GP-NTR, only NTR caused an obvious change in fluorescence spectra, whereas the variations with the other reactive species were negligible. It is worth noting that the addition of NTR to the solution containing interfering analytes and Zn-MPB⊃GP-NTR quickly produced a fluorescence response identical to that of the Zn-MPB⊃GP-NTR solution, suggesting that NTR could be detected effectively even in complex situations. This feature is extremely beneficial for monitoring hypoxic regions in vivo.
3.4. Imaging NTR in Cancer Cells by Zn-MPB⊃GP-NTR
The cytotoxicity of GP-NTR and Zn-MPB to living cells was evaluated with MCF-7 and HepG2 cells by the standard MTT assay (Figure S28) [54]. It is demonstrated that, after 24 h of incubation at various concentrations, the viability levels of MCF-7 cells and HepG2 cells maintained above 85%, indicating that both GP-NTR and Zn-MPB were suitable for NTR detection in living cells. No detectable toxicity was observed in the case of MCF-7 cell line and HepG2 cell line. These results suggest that Zn-MPB⊃GP-NTR has acceptable toxicological properties and excellent biocompatibility for NTR detection in cancer cells and mice models.
The ability of Zn-MPB⊃GP-NTR was evaluated to detect hypoxia by monitoring NTR in MCF-7 and HepG2 cells after incubating under normoxic (20% O2) and different hypoxic (8% and 0.1% O2) conditions for 6 h, respectively, followed by treatment with GP-NTR or Zn-MPB⊃GP-NTR for 1 min under the respective conditions (Figure 4a and Figure S29a) [55]. For the Zn-MPB⊃GP-NTR group, negligible fluorescence was observed in MCF-7 cells and HepG2 cells under the normoxic condition. Significantly enhanced fluorescence was observed under hypoxia conditions, and the signal became stronger when the O2 concentration decreased. In the comparison group, it could be seen that cells incubated with GP-NTR had much lower fluorescence intensity than that of the group incubated with Zn-MPB⊃GP-NTR at the same oxygen content. These findings revealed that Zn-MPB⊃GP-NTR was suitable for the detection of NTR under different hypoxia levels in a short incubation time. It is confirmed that the host–guest system was highly responsive to intracellular NTR changes and could be used to monitor the hypoxia in living cells.
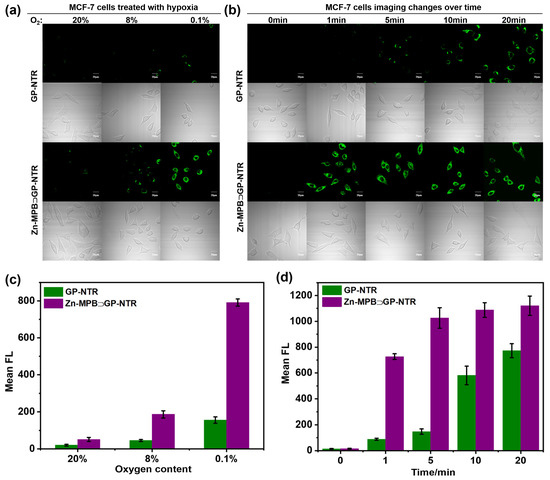
Figure 4.
(a) Imaging of MCF-7 cells under different conditions. Cells were treated with 2 μM GP-NTR or 2 μM Zn-MPB⊃GP-NTR for 1 min after 6 h incubation under normoxic (20% O2) and different hypoxic (8% and 0.1% O2) conditions. (b) Imaging of MCF-7 cells under 0.1% O2 condition incubated with 2 μM GP-NTR or 2 μM Zn-MPB⊃GP-NTR for different times (0, 1, 5, 10, 20 min). Scale bar: 20 µm. (c) Relative fluorescence intensity of the corresponding images of MCF-7 cells after being incubated with 2 μM GP-NTR or 2 μM Zn-MPB⊃GP-NTR under different oxygen contents for 1 min. (d) Relative fluorescence intensity of the corresponding images of MCF-7 cells at varied time points after being incubated with 2 μM GP-NTR or 2 μM Zn-MPB⊃GP-NTR under hypoxia conditions. The results were presented as mean ± SE with replicates, n = 3.
Inspired by the superiority of Zn-MPB⊃GP-NTR as a new molecular tool for NTR imaging in living cells, the tests were conducted for MCF-7 and HepG2 cells regarding the capacity of intracellular NTR imaging with various incubation times (Figure 4b and Figure S29b). The cells were incubated in a hypoxic environment (0.1% O2) for 6 h and then treated with Zn-MPB⊃GP-NTR or GP-NTR for different times (0–20 min). The MCF-7 and HepG2 cells exhibited no fluorescence when not incubated with Zn-MPB⊃GP-NTR or GP-NTR. The fluorescence intensity of MCF-7 and HepG2 cells in the red channel became stronger with a longer incubation time. In particular, the GP-NTR group took longer to reach equilibrium (approximately 20 min), while the fluorescence intensity of cells in the Zn-MPB⊃GP-NTR groups reached equilibrium quickly in 5 min after incubation and exhibited no significant change even after a longer period (until 20 min). In comparison, the fluorescence intensity of MCF-7 and HepG2 cells treated with GP-NTR was weaker than the Zn-MPB⊃GP-NTR group after reaching fluorescence equilibrium. These results illustrated that Zn-MPB⊃GP-NTR could rapidly monitor NTR in cells with the improvement of the double-substrate mechanism to a single-substrate one. Hence, Zn-MPB⊃GP-NTR was applicable for the rapid and effective detection of NTR in hypoxic cancer cells.
Flow cytometry analysis has been regarded as a high-throughput assay technique that is frequently employed for the analysis of various samples [56,57]. Flow cytometry analysis was preferred to verify the fluorescence imaging results of the host−guest complex. The fluorescence intensity of MCF-7 cells treated with Zn-MPB⊃GP-NTR under hypoxic conditions (0.1% O2) for 6 h was significantly higher than that of the control group (Figure 5c). Then, a comparative experiment between the Zn-MPB⊃GP-NTR group and the GP-NTR group was performed under hypoxic conditions (0.1% O2). As shown in Figure 5d and Figure S31, the fluorescence intensity of the Zn-MPB⊃GP-NTR group and the GP-NTR group treated with 0.1% O2 for 6 h in MCF-7 and HepG2 cells was significantly increased than that of the control group, while the fluorescence intensity of Zn-MPB⊃GP-NTR group was slightly higher. These results indicate that the host–guest complex Zn-MPB⊃GP-NTR could achieve the good detection of NTR with fast imaging. The flow cytometry results were consistent with cell confocal imaging results, demonstrating that Zn-MPB⊃GP-NTR could be applied to monitoring NTR in hypoxia cancer cells.
3.5. In Vivo Fluorescence Imaging of NTR
Based on the good selectivity and rapid response to NTR, the ability of Zn-MPB⊃GP-NTR for the in vivo real-time tracking imaging of NTR was studied. In consideration of the adaptability of Zn-MPB⊃GP-NTR, MCF-7 tumor-bearing model was used to trace the NTR catalytic kinetics in vivo due to the NTR overexpression in hypoxic tumors. The mice injected with GP-NTR were selected as the control group. As shown in Figure 5a,b, the mice injected with GP-NTR or Zn-MPB⊃GP-NTR showed no obvious background fluorescence. Mice treated with GP-NTR showed a small fluorescence signal initially, and this signal increased with the time. By contrast, after the intratumoral injection of Zn-MPB⊃GP-NTR was administered to mice, a certain fluorescence response appeared in a relatively short time and showed no obvious alterations over a longer incubation time. These findings revealed that the fluorescence signal of Zn-MPB⊃GP-NTR in the tumor area was strong, and the rapid reaction of Zn-MPB⊃GP-NTR with NTR occurred in the tumor site. Taken together, the interaction of Zn-MPB⊃GP-NTR with NTR could lead to the rapid detection of NTR, making it appropriate for the in vivo imaging of NTR in hypoxic tumors.
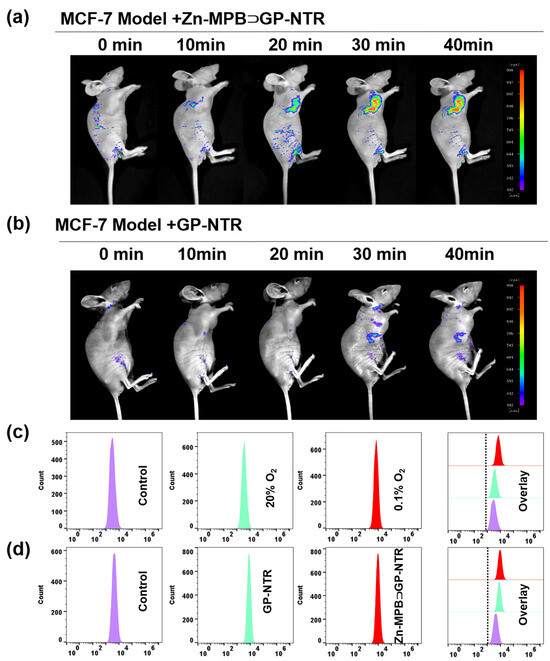
Figure 5.
Real-time in vivo fluorescence imaging of hypoxia in MCF-7 tumor-bearing mice after an injection of Zn-MPB⊃GP-NTR (a) or GP-NTR (b). (c) Flow cytometry analyses of MCF-7 cells treated with 5 μM Zn-MPB⊃GP-NTR under hypoxic or normoxic conditions. (d) Flow cytometry analysis of MCF-7 cells treated with 5 μM GP-NTR or 5 μM Zn-MPB⊃GP-NTR under hypoxic conditions.
4. Conclusions
In summary, a host–guest complex, Zn-MPB⊃GP-NTR, was developed for quantitative, sensitive, and rapid bio-tracking of NTR. Zn-MPB⊃GP-NTR was encapsulated by the inclusion of a fluorescent substrate GP-NTR in a NADH mimic-containing metal–organic capsule Zn-MPB to improve its responsiveness to NTR. The resulting host–guest platform made the detection of NTR independent of NADH, while the response process was switched from the original complex double-substrate process to a single-substrate one. It not only demonstrated a fast response toward NTR with a low detection limit, but also could been employed for the quantitative detection of NTR. Confocal microscopy, flow cytometry analysis, and tumor imaging in the tumor-bearing mouse model demonstrated that Zn-MPB⊃GP-NTR showed an excellent detection performance for NTR. The host–guest platform described here might present significant opportunities for the development of molecular tools for in vivo hypoxia monitoring and could be used for the early detection of cancers associated with hypoxia.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemosensors12080145/s1, Figure S1: 1H NMR spectrum of compound 2 (400 MHz, 298 K, DMSO-d6); Figure S2: 1H NMR spectrum of GP-OH (400 MHz, 298 K, DMSO-d6); Figure S3: 1H NMR spectrum of GP-NTR (400 MHz, 298 K, CDCl3/CD3OD (v/v = 6/1)); Figure S4: 13C NMR spectrum of GP-NTR (400 MHz, 298 K, CDCl3/CD3OD (v/v = 6/1)); Figure S5: 1H NMR spectrum of H2MPB (400 MHz, 298 K, DMSO-d6); Figure S6: 1H NMR spectrum of Zn-MPB (400 MHz, 298 K, DMSO-d6); Figure S7: HR–MS spectrum of GP-OH; Figure S8: HR–MS spectrum of GP-NTR; Figure S9: HR–MS spectrum of H2MPB; Figure S10: (a) 1H NMR spectra of the free GP-NTR, Zn-MPB, and an equimolar mixture of Zn-MPB and GP-NTR in DMSO-d6 solution. (b) Uphill of the protons of subtract GP-NTR (Hi, Hd, Hg, Hj, and Hn). (c) Uphill of the protons of the molecular square (H12 and H15); Figure S11: 1H COSY spectroscopy of intermolecular H-H interaction of GP-NTR with equimolar Zn-MPB (v/v, 1/1) in a DMSO-d6 solution; Figure S12: 1H NOESY spectroscopy of intermolecular H-H interaction of GP-NTR with equimolar Zn-MPB (v/v, 1/1) in a DMSO-d6 solution; Figure S13: (a) UV titration experiments of Zn-MPB with GP-NTR in DMSO/Tris–HCl solution. (b) UV titration experiments of Zn-MPB with NTR in Tris–HCl solution; Figure S14: (a) Time-dependent UV tests for the stability of GP-NTR (5 μM) in Tris–HCl. (b) Time-dependent UV tests for the stability of Zn-MPB⊃GP-NTR (5 μM) in Tris–HCl; Figure S15: (a) UV–vis difference spectrometry of guest inclusion in DMSO/Tris–HCl solution. (b) Job–plot of changes in the absorbance of host–guest complex at 370 nm in DMSO/Tris–HCl solution; Figure S16: Theoretical docking study optimized model of GP-NTR with NTR; Figure S17: The Linear fitting curve of the fluorescence intensity of Zn-MPB⊃GP-NTR at 520 nm versus the concentration of NTR from 0 to 5 μg/mL; Figure S18: Intensity vs. NTR concentration after reacting with (a) Zn-MPB⊃GP-NTR (5 μM) or (b) GP-NTR (5 μM) and NADH (15 μM) for different times; Figure S19: Solvent kinetics tests of Zn-MPB⊃GP-NTR. (a) NTR (1 μg/mL) reacted with GP-NTR (5 μM) at various concentrations of NADH (1 and 15 μM). (b) NTR (1 μg/mL) reacted with Zn-MPB⊃GP-NTR (5 μM) at various concentrations of NADH (1, 15, or 30 μM); Figure S20: The reaction kinetics between Zn-MPB⊃GP-NTR (5 μM) and NTR (1 μg/mL) with/without the addition of NADH (15 μM) before or after the reaction; Figure S21: The photostability of Zn-MPB⊃GP-NTR and Zn-MPB⊃GP-NTR coexisting with NTR (5 μg/mL), λex = 440 nm, λem = 520 nm; Figure S22: The fluorescence intensity of Zn-MPB⊃GP-NTR and Zn-MPB⊃GP-NTR coexisting with NTR (5 μg/mL) toward different temperatures, λex = 440 nm, λem = 520 nm; Figure S23: (a) Kinetics of NTR (1 μg/mL) with various concentrations of Zn-MPB⊃GP-NTR. (b) Kinetics of NTR (1 μg/mL) with NADH (15 μM) and various concentrations of GP-NTR; Figure S24: Plot of 1/v against 1/S according to the Michaelis–Menten equation, where the black line was derived from Zn-MPB⊃GP-NTR (0-5 μM) reacting with NTR (1 μg/mL), and the blue and red lines were obtained from the kinetic curves of NTR reacting with various concentrations of GP-NTR in 1 or 15 μM NADH; Figure S25: Fluorescence response of 5 μM GP-NTR (with 15 μM NADH, black) or Zn-MPB⊃GP-NTR (blue) in the presence of different analytes for 10 min, until GP-NTR groups reached equilibrium. Then, adding NTR (5 μg/mL) to the Zn-MPB⊃GP-NTR (5 μM) in the presence of various interfering analyte mixture solutions, data recorded equilibrium in seconds (red); Figure S26: Fitting results of lifetime for Zn-MPB⊃GP-NTR in DMSO; Figure S27: HPLC analysis to confirm the reaction mechanism of GP-NTR towards NTR detection; Figure S28: Cell viability of MCF-7 cells treated with various concentrations of GP-NTR (a) and Zn-MPB (b) (from 0 to 20 μM) for 24 h. Cell viability of HepG2 cells treated with various concentrations of GP-NTR (c) and Zn-MPB (d) (from 0 to 20 μM) for 24 h; Figure S29: (a) Imaging of HepG2 cells under different conditions. (b) Imaging of HepG2 cells under 0.1% O2 condition incubated with 2 μM GP-NTR or 2 μM Zn-MPB⊃GP-NTR for different times (0, 1, 5, 10, 20 min); Figure S30: (a) Relative fluorescence intensity of the corresponding images of HepG2 cells after being incubated with 2 μM GP-NTR or 2 μM Zn-MPB⊃GP-NTR under different oxygen contents for 1 min. (b) Relative fluorescence intensity of the corresponding images of HepG2 cells at varied time points after being incubated with 2 μM GP-NTR or 2 μM Zn-MPB⊃GP-NTR under hypoxia conditions; Figure S31: Flow cytometry analysis of HepG2 cells treated with 5 μM GP-NTR or 5 μM Zn-MPB⊃GP-NTR under hypoxic conditions; Figure S32: The corresponding image of GR-NTR, Zn-MPB⊃GP-NTR, and Zn-MPB in water; Figure S33: Cyclic voltammogram of Zn-MPB (0.1 mM) and H2MPB (0.2 mM) in CH3CN containing TBAPF6 (0.1 M). Table S1: Comparison of fluorescent probes for NTR detection. References [33,48,58,59] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, W.S., Y.J. and C.D.; validation, W.S., Y.J., M.G., Y.G. and L.Z.; investigation, W.S., X.J., M.G. and Y.Z.; resources, X.J., Y.Z. and Y.G.; data curation, W.S. and X.J.; formal analysis, W.S., X.J. and L.Z.; writing—original draft preparation, W.S.; writing—review and editing, Y.J.; visualization, Y.G., Y.Z. and L.Z.; supervision, Y.J. and C.D.; project administration, C.D.; funding acquisition, Y.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (No. 21977015) and the Fundamental Research Funds for the Central Universities (No. DUT23YG208).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Janczy-Cempa, E.; Mazuryk, O.; Kania, A.; Brindell, M. Significance of Specific Oxidoreductases in the Design of Hypoxia-Activated Prodrugs and Fluorescent Turn off–on Probes for Hypoxia Imaging. Cancers 2022, 14, 2686–2711. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Radons, J.; Vaupel, P. Critical Role of Aberrant Angiogenesis in the Development of Tumor Hypoxia and Associated Radioresistance. Cancers 2014, 6, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Kheshtchin, N.; Hadjati, J. Targeting hypoxia and hypoxia-inducible factor-1 in the tumor microenvironment for optimal cancer immunotherapy. J. Cell. Physiol. 2022, 237, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, J.S.; Kaur, N.; Singh, N. Trends in small organic fluorescent scaffolds for detection of oxidoreductase. Biosens. Bioelectron. 2021, 191, 113441. [Google Scholar] [CrossRef] [PubMed]
- Dias, G.G.; King, A.; de Moliner, F.; Vendrell, M.; da Silva Júnior, E.N. Quinone-based fluorophores for imaging biological processes. Chem. Soc. Rev. 2018, 47, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Pitsawong, W.; Hoben, J.P.; Miller, A.F. Understanding the Broad Substrate Repertoire of Nitroreductase Based on Its Kinetic Mechanism. J. Biol. Chem. 2014, 289, 15203–15214. [Google Scholar] [CrossRef]
- Liu, Y.F.; Li, J.Y.; Huang, H.J.; Shu, Y. A fluorescent probe for imaging nitroreductase with signal amplification in high-viscosity environments. J. Mater. Chem. B 2023, 11, 9509–9515. [Google Scholar] [CrossRef]
- Li, H.D.; Kim, D.Y.; Yao, Q.C.; Ge, H.Y.; Chung, J.W.; Fan, J.L.; Wang, J.Y.; Peng, X.J.; Yoon, J. Activity-Based NIR Enzyme Fluorescent Probes for the Diagnosis of Tumors and Image-Guided Surgery. Angew. Chem. Int. Ed. 2021, 60, 17268–17289. [Google Scholar] [CrossRef]
- Qi, Y.L.; Guo, L.; Chen, L.L.; Li, H.; Yang, Y.S.; Jiang, A.Q.; Zhu, H.L. Recent progress in the design principles, sensing mechanisms, and applications of small-molecule probes for nitroreductases. Coord. Chem. Rev. 2020, 421, 213460. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, S.; Li, D.; Yuan, J.; Xu, J.; Shao, S. A mitochondria-targeting nitroreductase fluorescent probe with large Stokes shift and long-wavelength emission for imaging hypoxic status in tumor cells. Anal. Chim. Acta 2020, 1103, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.B.; Hu, D.H.; Yin, J.M.; Sun, K.S.; Chen, L.J.; Liu, S.J.; Li, F.Y.; Zhao, Q. An iridium complex-based probe for phosphorescent lifetime-elongated imaging of nitroreductase in living cells. Sens. Actuators B Chem. 2024, 401, 134960. [Google Scholar] [CrossRef]
- Xu, Y.M.; Hu, B.; Cui, Y.J.; Li, L.; Nian, F.; Zhang, Z.X.; Wang, W.T. A highly selective ratio-metric fluorescent sensor for visualizing nitroreductase in hypoxic cells. Chem. Commun. 2024, 60, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, B.; Wang, Q.; Zhang, Q.; Hu, H.Y.; Nazare, M. An Activatable Lanthanide Luminescent Probe for Time-Gated Detection of Nitroreductase in Live Bacteria. Angew. Chem. Int. Ed. 2020, 59, 8512–8516. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Lee, H.; Ryu, H.G.; Singha, S.; Lee, Y.M.; Reo, Y.J.; Jun, Y.W.; Kim, K.H.; Kim, W.J.; Ahn, K.H. A Study on Hypoxia Susceptibility of Organ Tissues by Fluorescence Imaging with a Ratiometric Nitroreductase Probe. ACS Sens. 2021, 6, 148–155. [Google Scholar] [CrossRef]
- Li, T.; Gu, Q.S.; Chao, J.J.; Liu, T.; Mao, G.J.; Li, Y.F.; Li, C.Y. An intestinal-targeting near-infrared probe for imaging nitroreductase in inflammatory bowel disease. Sens. Actuators B Chem. 2024, 403, 135181. [Google Scholar] [CrossRef]
- Li, M.R.; Zhang, Y.; Ren, X.J.; Niu, W.C.; Yuan, Q.; Cao, K.; Zhang, J.C.; Gao, X.Y.; Su, D.D. Activatable fluorogenic probe for accurate imaging of ulcerative colitis hypoxia in vivo. Chem. Commun. 2022, 58, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Chai, X.H.; He, X.P.; Kim, H.J.; Yoon, J.; Tian, H. Fluorogenic probes for disease-relevant enzymes. Chem. Soc. Rev. 2019, 48, 683–722. [Google Scholar] [CrossRef]
- Meng, T.J.; Ma, W.B.; Fan, M.Y.; Tang, W.; Duan, X.R. Enhancing the Contrast of Tumor Imaging for Image-Guided Surgery Using a Tumor-Targeting Probiotic with the Continuous Expression of a Biomarker. Anal. Chem. 2022, 94, 10109–10117. [Google Scholar] [CrossRef]
- Fu, Y.X.; Guo, W.Y.; Wang, N.; Dai, Y.J.; Zhang, Z.Y.; Sun, X.L.; Yang, W.C.; Yang, G.F. Diagnosis of Bacterial Plant Diseases via a Nitroreductase-Activated Fluorescent Sensor. Anal. Chem. 2022, 94, 17692–17699. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; New, E.J. Bioinspired Small-Molecule Tools for the Imaging of Redox Biology. Acc. Chem. Res. 2019, 52, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.W.; Freinkman, E.; Wang, T.; Birsoy, K.; Sabatini, D.M. Absolute quantification of matrix metabolites reveals the dynamics of mitochondrial metabolism. Cell 2016, 166, 1324–1337. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.H.; Yang, J.; Yang, Y.W. Supramolecular Assemblies with Aggregation-Induced Emission Properties for Sensing and Detection. Chem. Eur. J. 2022, 28, e202103185. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Lei, Q.; Zhong, H.C.; Ren, T.B.; Sun, Y.; Zhang, X.B.; Yuan, L. Fluorophore-based host–guest assembly complexes for imaging and therapy. Chem. Commun. 2023, 59, 3024–3039. [Google Scholar] [CrossRef]
- Tarzia, A.; Jelfs, K.E. Unlocking the computational design of metal–organic cages. Chem. Commun. 2022, 58, 3717–3730. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Slappendel, L.; Nguyen, B.T.; von Krbek, L.K.S.; Ronson, T.K.; Castilla, A.M.; Nitschke, J.R. Light-Powered Reversible Guest Release and Uptake from Zn4L4 Capsules. J. Am. Chem. Soc. 2023, 145, 3828–3832. [Google Scholar] [CrossRef]
- Zhang, L.; Jiao, Y.; Yang, H.; Jia, X.C.; Li, H.Y.; He, C.; Si, W.; Duan, C.Y. Supramolecular Host–Guest Strategy for the Accelerating Detection of Nitroreductase. ACS Appl. Mater. Interfaces 2023, 15, 21198–21209. [Google Scholar] [CrossRef]
- Zhao, L.; Cai, J.K.; Li, Y.N.; Wei, J.W.; Duan, C.Y. A host–guest approach to combining enzymatic and artificial catalysis for catalyzing biomimetic monooxygenation. Nat. Commun. 2020, 11, 2903. [Google Scholar] [CrossRef]
- Wei, J.W.; Zhao, L.; Zhang, Y.; Han, G.; He, C.; Wang, C.; Duan, C.Y. Enzyme Grafting with a Cofactor-Decorated Metal-Organic Capsule for Solar-to-Chemical Conversion. J. Am. Chem. Soc. 2023, 145, 6719–6729. [Google Scholar] [CrossRef]
- Zang, S.P.; Shu, W.; Shen, T.J.; Gao, C.C.; Tian, Y.; Jing, J.; Zhang, X.L. Palladium-triggered ratiometric probe reveals CO’s cytoprotective effects in mitochondria. Dye. Pigment. 2020, 173, 107861. [Google Scholar] [CrossRef]
- Crofts, T.S.; Sontha, P.; King, A.O.; Wang, B.; Biddy, B.A.; Zanolli, N.; Gaumnitz, J.; Dantas, G. Discovery and characterization of a nitroreductase capable of conferring bacterial resistance to chloramphenicol. Cell Chem. Biol. 2019, 26, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, S.; Nayek, A.; Roy, B.; Dey, A. Induction of enzyme-like peroxidase activity in an iron porphyrin complex using second sphere interactions. Inorg. Chem. 2019, 58, 2954–2964. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.K.; Zhao, L.; Li, Y.N.; He, C.; Wang, C.; Duan, C.Y. Binding of Dual-Function Hybridized Metal–Organic Capsules to Enzymes for Cascade Catalysis. JACS Au 2022, 2, 1736–1746. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Wang, H.L.; Nie, G. Ultrasensitive Fibroblast Activation Protein-α-Activated Fluorogenic Probe Enables Selective Imaging and Killing of Melanoma In Vivo. ACS Sens. 2022, 7, 1837–1846. [Google Scholar] [CrossRef]
- Fang, Y.; Powell, J.A.; Li, E.; Wang, Q.; Perry, Z.; Kirchon, A.; Yang, X.; Xiao, Z.; Zhu, C.; Zhang, L.; et al. Catalytic reactions within the cavity of coordination cages. Chem. Soc. Rev. 2019, 48, 4707–4730. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jing, X.; Shi, Y.P.; Wu, Y.C.; Duan, C.Y. Modifying Enzymatic Substrate Binding within a Metal–Organic Capsule for Supramolecular Catalysis. J. Am. Chem. Soc. 2023, 145, 10136–10148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Saha, M.L.; Wang, M.; Zhou, Z.X.; Song, B.; Lu, C.J.; Yan, X.Z.; Li, X.P.; Huang, F.H.; Yin, S.C.; et al. Multicomponent Platinum(II) Cages with Tunable Emission and Amino Acid Sensing. J. Am. Chem. Soc. 2017, 139, 5067–5074. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Bai, S.; Han, Y.F. Water-Soluble Self-Assembled Cage with Triangular Metal–Metal-Bonded Units Enabling the Sequential Selective Separation of Alkanes and Isomeric Molecules. J. Am. Chem. Soc. 2022, 144, 16191–16198. [Google Scholar] [CrossRef]
- Mei, Y.X.; Zhang, Q.W.; Gu, Q.Y.; Liu, Z.C.; He, X.; Tian, Y. Pillar[5]arene-Based Fluorescent Sensor Array for Biosensing of Intracellular Multi-neurotransmitters through Host–Guest Recognitions. J. Am. Chem. Soc. 2022, 144, 2351–2359. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, J.; Sun, J.F.; Yang, J. Host–guest interactions of a twisted cucurbit[15]uril with paraquat derivatives and bispyridinium salts. Tetrahedron Lett. 2019, 60, 151022. [Google Scholar] [CrossRef]
- Sobiech, T.A.; Zhong, Y.L.; Miller, D.P.; McGrath, J.K.; Scalzo, C.T.; Redington, M.C.; Zurek, E.; Gong, B. Ultra-Tight Host-Guest Binding with Exceptionally Strong Positive Cooperativity. Angew. Chem. Int. Ed. 2022, 61, e202213467. [Google Scholar] [CrossRef]
- Bobylev, E.O.; Poole, D.A.; Bruin, B.; Reek, J.N.H. M6L12 Nanospheres with Multiple C70 Binding Sites for 1O2 Formation in Organic and Aqueous Media. J. Am. Chem. Soc. 2022, 144, 15633–15642. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.Y.; Yue, Y.X.; Ying, A.K.; Hu, X.Y.; Li, H.B.; Cai, K.; Guo, D.S. An Antitumor Dual-Responsive Host-Guest Supramolecular Polymer Based on Hypoxia-Cleavable Azocalix[4]arene. Angew. Chem. Int. Ed. 2023, 62, e202213578. [Google Scholar] [CrossRef] [PubMed]
- Demers, J.; Mittermaier, A. Binding Mechanism of an SH3 Domain Studied by NMR and ITC. J. Am. Chem. Soc. 2009, 131, 4355–4367. [Google Scholar] [CrossRef] [PubMed]
- Altmann, P.J.; Pöthig, A. Pillarplexes: A Metal–Organic Class of Supramolecular Hosts. J. Am. Chem. Soc. 2016, 138, 13171–13174. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Liu, R.R.; Zhai, H.L.; Meng, Y.J.; Han, L.; Ren, C.L. The binding mechanism of nitroreductase fluorescent probe: Active pocket deformation and intramolecular hydrogen bonds. Int. J. Biol. Macromol. 2020, 150, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.L.; Wang, H.R.; Kang, Q.J.; Chen, L.L.; Qi, P.F.; He, Z.X.; Yang, Y.S.; Zhu, H.L. A versatile fluorescent probe for simultaneously detecting viscosity, polarity and nitroreductases and its application in bioimaging. Sens. Actuators B Chem. 2022, 352, 130989. [Google Scholar] [CrossRef]
- Chen, S.J.; Ma, X.D.; Wang, L.; Wu, Y.Y.; Wang, Y.P.; Hou, S.C.; Fan, W.K. Construction of an intelligent fluorescent probe that can accurately track β-galactosidase activity in fruits and living organisms. Sens. Actuators B Chem. 2023, 387, 133787. [Google Scholar] [CrossRef]
- Guo, H.W.; Yang, K.P.; Fan, X.P.; Chen, M.; Ke, G.L.; Ren, T.B.; Yuan, L.; Zhang, X.B. Designing a brightness-restored rhodamine derivative by the ortho-compensation effect for assessing drug-induced acute kidney injury. Anal. Chem. 2023, 95, 6863–6870. [Google Scholar] [CrossRef]
- Tang, Z.X.; Yan, Z.; Gong, L.L.; Zhang, L.; Yin, X.M.; Sun, J.; Wu, K.; Yang, W.J.; Fan, G.W.; Li, Y.L.; et al. Precise Monitoring and Assessing Treatment Response of Sepsis-Induced Acute Lung Hypoxia with a Nitroreductase-Activated Golgi-Targetable Fluorescent Probe. Anal. Chem. 2022, 94, 14778–14784. [Google Scholar] [CrossRef] [PubMed]
- Race, P.R.; Lovering, A.L.; Green, R.M.; Ossor, A.; White, S.A.; Searle, P.F.; Wrighton, C.J.; Hyde, E.I. Structural and Mechanistic Studies of Escherichia coli Nitroreductase with the Antibiotic Nitrofurazone. J. Biol. Chem. 2005, 280, 13256–13264. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Zhao, Z.X.; Weng, Y.R.; Gardner, S.H.; Brady, C.J.; Peguero, O.D.P.; Chan, J. Hydrolysis-Resistant Ester-Based Linkers for Development of Activity-Based NIR Bioluminescence Probes. J. Am. Chem. Soc. 2023, 145, 1460–1469. [Google Scholar] [CrossRef]
- Du, W.; Wang, J.Q.; Fang, H.X.; Ji, W.H.; Liu, Y.; Qu, Y.W.; Zhang, D.T.; Shao, T.; Hou, X.Y.; Wu, Q.; et al. Mitochondria-specific two-photon fluorogenic probe for simultaneously visualizing nitroreductase and viscosity in cancer cells. Sens. Actuators B Chem. 2022, 370, 132456. [Google Scholar] [CrossRef]
- Chen, S.Z.; Xiao, L.; Li, Y.; Qiu, M.S.; Yuan, Y.P.; Zhou, R.; Li, C.G.; Zhang, L.; Jiang, Z.X.; Liu, M.L.; et al. In Vivo Nitroreductase Imaging via Fluorescence and Chemical Shift Dependent 19F NMR. Angew. Chem. Int. Ed. 2022, 61, e202213495. [Google Scholar] [CrossRef]
- Zwicker, V.E.; Oliveira, B.L.; Yeo, J.H.; Fraser, S.T.; Bernardes, G.J.L.; New, E.J.; Jolliffe, K.A. A Fluorogenic Probe for Cell Surface Phosphatidylserine Using an Intramolecular Indicator Displacement Sensing Mechanism. Angew. Chem. Int. Ed. 2019, 58, 3087–3091. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Li, Z.; Hao, Y.T.; Zhang, Y.; Zhang, C.X. Near-Infrared Fluorescence Probe with a New Recognition Moiety for Specific Detection and Imaging of Aldehyde Dehydrogenase Expecting the Identification and Isolation of Cancer Stem Cells. Anal. Chem. 2022, 94, 17328–17333. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, L.; Gao, X.; Si, W.; Duan, C.Y. Cofactor-substrate-based Reporter for Enhancing Signaling Communications towards Hypoxia Enzyme Expression. Angew. Chem. Int. Ed. 2020, 59, 6021–6027. [Google Scholar] [CrossRef]
- Zhou, J.; Fang, S.J.; Li, J.; Du, W.; Wu, Q. A novel pyrimidine-based two-photon fluorogenic probe for rapidly visualizing nitroreductase activity in hypoxic cancer cells and in vivo. Sens. Actuators B Chem. 2023, 390, 134015. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).