Response Time Dynamics of a Membrane-Based Microfluidic Gas Sensor
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
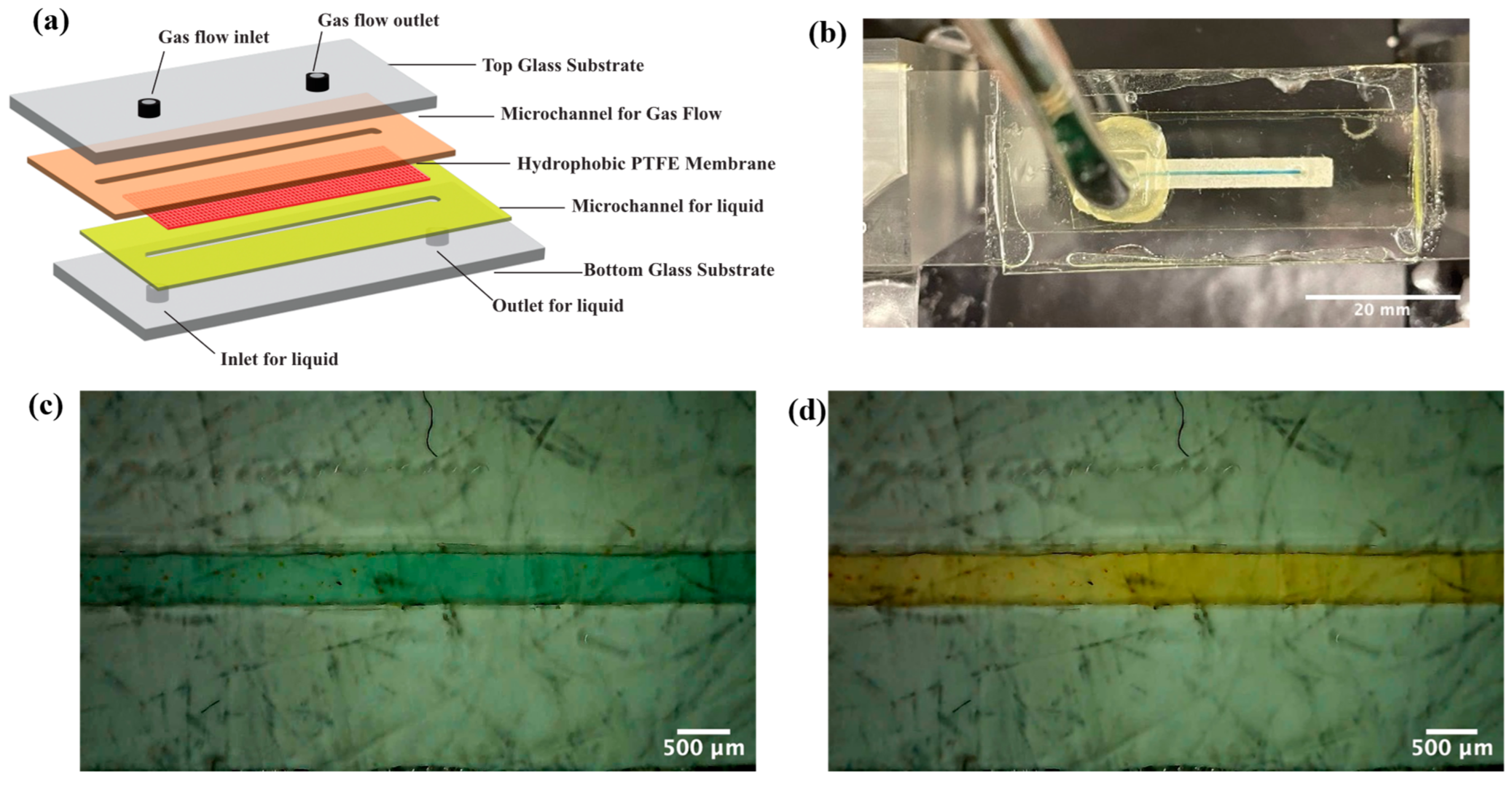
2.2. Structure and Fabrication of Membrane-Based Microfluidic Platform
2.2.1. Fabrication Using Double-Sided Tape
2.2.2. Fabrication Using Parafilm
2.3. Leak Test and Gas Permeation Study
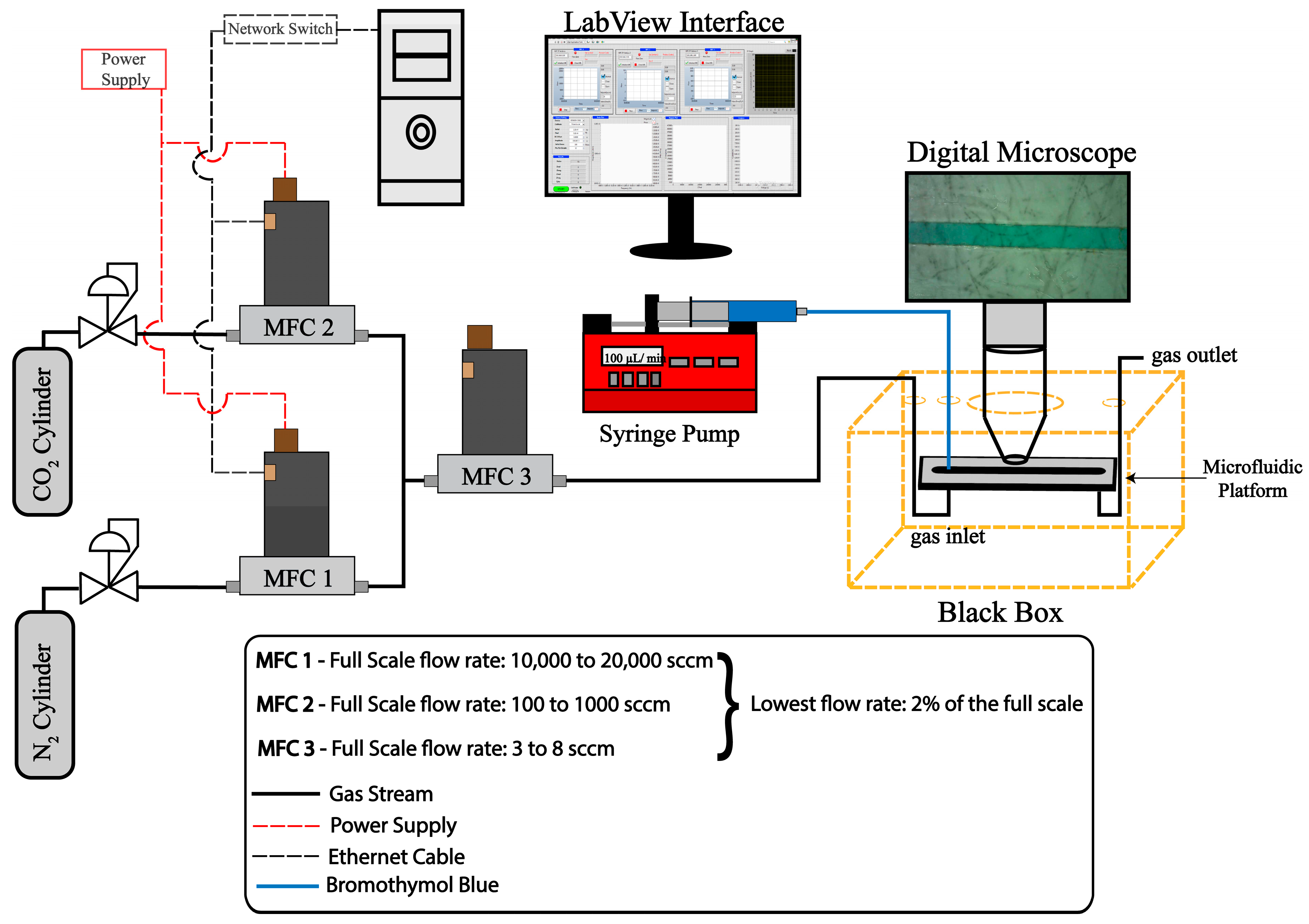
2.4. Response Characteristics
2.4.1. Mass Flow Controller and Digital Microscope Setup
2.4.2. Video and Image Analysis
3. Results and Discussion
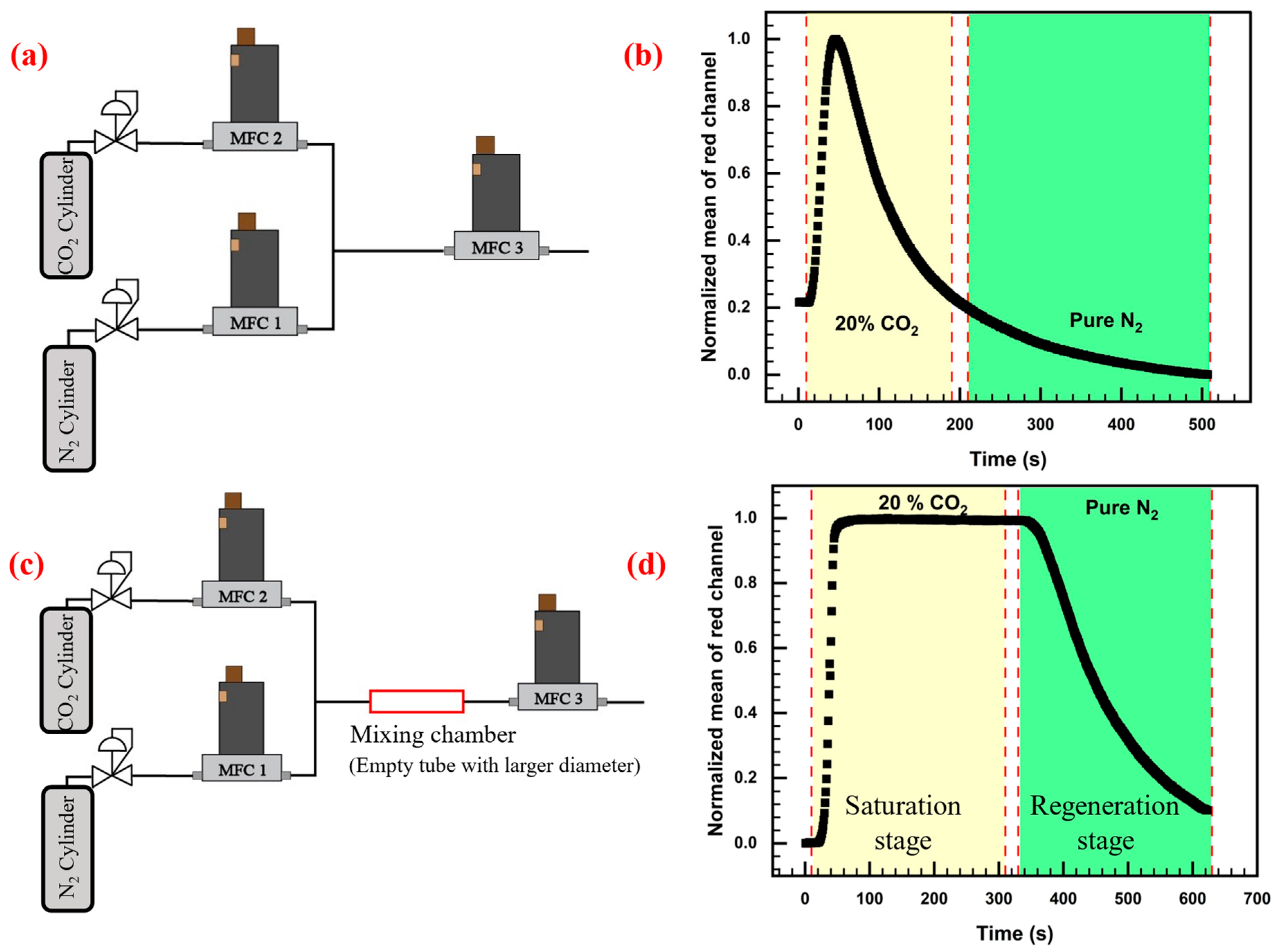
3.1. Gas Mixing Problem
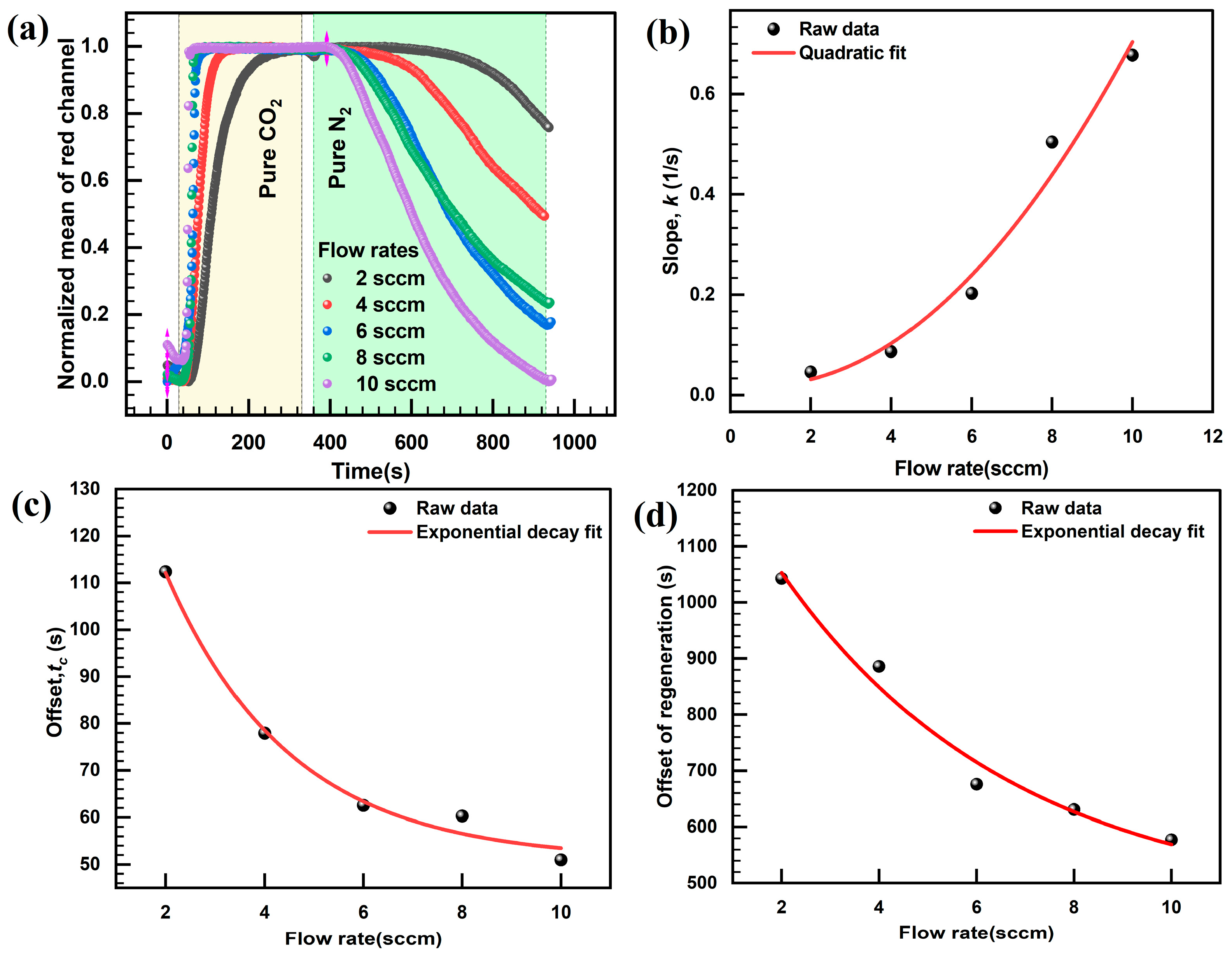
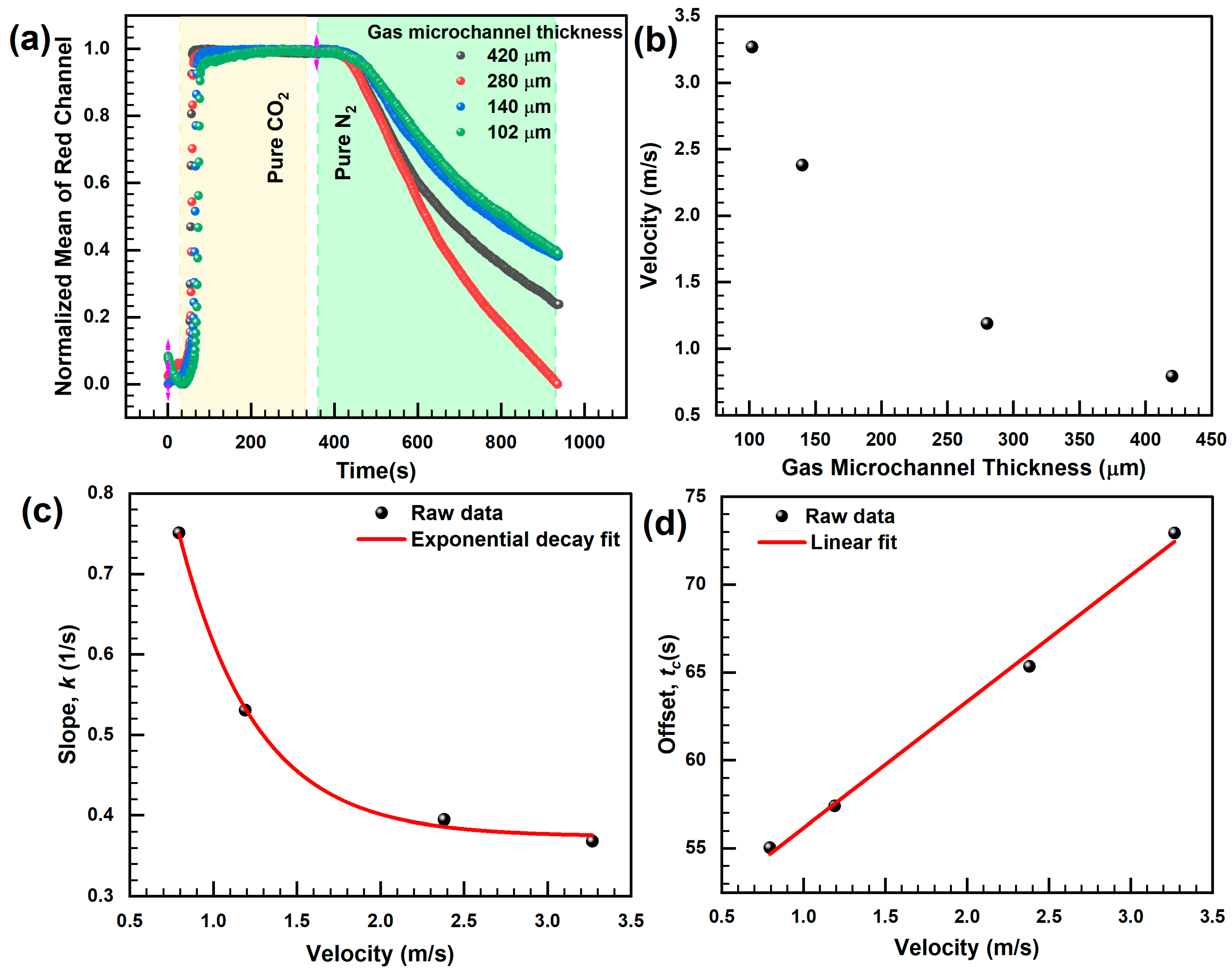
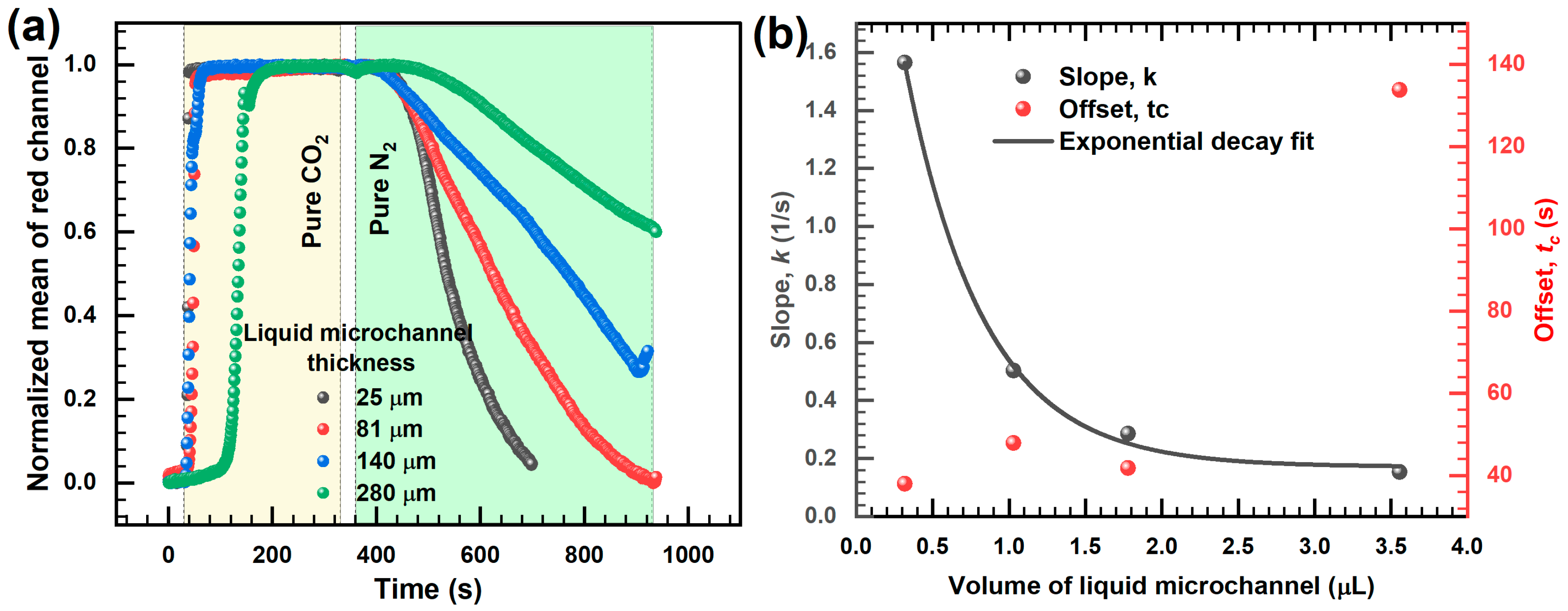
3.2. Response Characteristics of the Sensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, Y.-H.; Kargupta, R.; Ghoshal, D.; Li, Z.; Chande, C.; Feng, L.; Chatterjee, S.; Koratkar, N.; Motkuri, R.K.; Basuray, S. ESSENCE–A rapid, shear-enhanced, flow-through, capacitive electrochemical platform for rapid detection of biomolecules. Biosens. Bioelectron. 2021, 182, 113163. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-H.; Chande, C.; Zhenglong, L.; Kaaliveetil, S.; Basuray, S. Sensitive and Selective Determination of multiple Diagnostic Targets using a Modular, ASSURED POC Platform called ESSENCE. In Proceedings of the 2022 IEEE Healthcare Innovations and Point of Care Technologies (HI-POCT), Houston, TX, USA, 10–11 March 2022. [Google Scholar]
- Ferguson, B.S.; Buchsbaum, S.F.; Swensen, J.S.; Hsieh, K.; Lou, X.; Soh, H.T. Integrated microfluidic electrochemical DNA sensor. Anal. Chem. 2009, 81, 6503–6508. [Google Scholar] [CrossRef] [PubMed]
- Robin, P.; Barnabei, L.; Marocco, S.; Pagnoncelli, J.; Nicolis, D.; Tarantelli, C.; Tavilla, A.C.; Robortella, R.; Cascione, L.; Mayoraz, L.; et al. A DNA biosensors-based microfluidic platform for attomolar real-time detection of unamplified SARS-CoV-2 virus. Biosens. Bioelectron. X 2023, 13, 100302. [Google Scholar] [CrossRef] [PubMed]
- Chittuam, K.; Jampasa, S.; Vilaivan, T.; Tangkijvanich, P.; Chuaypen, N.; Avihingsanon, A.; Sain, M.; Panraksa, Y.; Chailapakul, O. Electrochemical capillary-driven microfluidic DNA sensor for HIV-1 and HCV coinfection analysis. Anal. Chim. Acta 2023, 1265, 341257. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cheng, Y.-H.; Chande, C.; Chatterjee, S.; Basuray, S. A highly sensitive, easy-and-rapidly-fabricable microfluidic electrochemical cell with an enhanced three-dimensional electric field. Anal. Chim. Acta 2022, 1232, 340488. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ma, M.; Zhang, F.; Liu, F.; Lu, T.; Liu, Z.; Li, Y. Wireless microfluidic sensor for metal ion detection in water. ACS Omega 2021, 6, 9302–9309. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.-L.; Zhang, G.-R.; Li, W.; Biesalski, M.; Etzold, B.J. Modifier-free microfluidic electrochemical sensor for heavy-metal detection. ACS Omega 2017, 2, 4593–4603. [Google Scholar] [CrossRef]
- Filippidou, M.K.; Kanaris, A.I.; Aslanidis, E.; Rapesi, A.; Tsounidi, D.; Ntouskas, S.; Skotadis, E.; Tsekenis, G.; Tsoukalas, D.; Tserepi, A. Integrated Plastic Microfluidic Device for Heavy Metal Ion Detection. Micromachines 2023, 14, 1595. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Li, J.; Yuan, S.; Yang, X.; Lu, J.; Jiang, B. A centrifugal microfluidic system for automated detection of multiple heavy metal ions by aptamer-based colorimetric assay. Sens. Actuators B Chem. 2024, 403, 135210. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Barpaga, D.; Soltis, J.A.; Shutthanandan, V.; Kargupta, R.; Han, K.S.; McGrail, B.P.; Motkuri, R.K.; Basuray, S.; Chatterjee, S. Metal–organic framework-based microfluidic impedance sensor platform for ultrasensitive detection of perfluorooctanesulfonate. ACS Appl. Mater. Interfaces 2020, 12, 10503–10514. [Google Scholar] [CrossRef]
- Tahirbegi, I.B.; Ehgartner, J.; Sulzer, P.; Zieger, S.; Kasjanow, A.; Paradiso, M.; Strobl, M.; Bouwes, D.; Mayr, T. Fast pesticide detection inside microfluidic device with integrated optical pH, oxygen sensors and algal fluorescence. Biosens. Bioelectron. 2017, 88, 188–195. [Google Scholar] [CrossRef]
- Campaña, A.L.; SFlorez, L.; Noguera, M.J.; Fuentes, O.P.; Puentes, P.R.; Cruz, J.C.; Osma, J.F. Enzyme-based electrochemical biosensors for microfluidic platforms to detect pharmaceutical residues in wastewater. Biosensors 2019, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Lin, Y.; Xu, J.; Lu, X. Inertial microfluidic-assisted rapid detection of chemical contaminant in foods using quantum dots-based molecularly imprinted sensor. Sens. Actuators B Chem. 2023, 394, 134342. [Google Scholar] [CrossRef]
- Li, T.; Guo, G.; Xing, H.; Tang, S.; Hu, H.; Wang, L.; Qian, X.; Chen, D. Construction of fluorescent sensor array and three-dimensional microfluidic paper based analytical device for specific identification and visual determination of antibiotics in food. Food Chem. 2023, 429, 136947. [Google Scholar] [CrossRef]
- Azhdary, P.; Janfaza, S.; Fardindoost, S.; Tasnim, N.; Hoorfar, M. Highly selective molecularly imprinted polymer nanoparticles (MIP NPs)-based microfluidic gas sensor for tetrahydrocannabinol (THC) detection. Anal. Chim. Acta 2023, 1278, 341749. [Google Scholar] [CrossRef]
- Ollé, E.P.; Farré-Lladós, J.; Casals-Terré, J.; Martínez, J.A.L. Enhanced selectivity of a 3D-printed microfluidic gas detector towards different volatile organic compounds (VOCs) for the effective monitoring of indoor air quality in vehicles. Environ. Technol. Innov. 2024, 33, 103481. [Google Scholar] [CrossRef]
- Hossein-Babaei, F.; Zare, A.H. The selective flow of volatile organic compounds in conductive polymer-coated microchannels. Sci. Rep. 2017, 7, 42299. [Google Scholar] [CrossRef]
- Paknahad, M.; Mcintosh, C.; Hoorfar, M. Selective detection of volatile organic compounds in microfluidic gas detectors based on “like dissolves like”. Sci. Rep. 2019, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, M.; Janfaza, S.; Tahmooressi, H.; Tasnim, N.; Hoorfar, M. Selective detection of VOCs using microfluidic gas sensor with embedded cylindrical microfeatures coated with graphene oxide. J. Hazard. Mater. 2022, 424, 127566. [Google Scholar] [CrossRef]
- Piorek, B.D.; Lee, S.J.; Moskovits, M.; Meinhart, C.D. Free-surface microfluidics/surface-enhanced Raman spectroscopy for real-time trace vapor detection of explosives. Anal. Chem. 2012, 84, 9700–9705. [Google Scholar] [CrossRef]
- Piorek, B.D.; Lee, S.J.; Santiago, J.G.; Moskovits, M.; Banerjee, S.; Meinhart, C.D. Free-surface microfluidic control of surface-enhanced Raman spectroscopy for the optimized detection of airborne molecules. Proc. Natl. Acad. Sci. USA 2007, 104, 18898–18901. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zong, S.; Zhang, Y.; Qian, Z.; Liu, Y.; Zhu, K.; Li, L.; Li, N.; Wang, Z.; Cui, Y. Array-assisted SERS microfluidic chips for highly sensitive and multiplex gas sensing. ACS Appl. Mater. Interfaces 2019, 12, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Ge, M.; Zhao, C.; Silvester, D.S. Fast responding hydrogen gas sensors using platinum nanoparticle modified microchannels and ionic liquids. Anal. Chim. Acta 2019, 1072, 35–45. [Google Scholar] [PubMed]
- Cha, W.; Tung, Y.-C.; Meyerhoff, M.E.; Takayama, S. Patterned electrode-based amperometric gas sensor for direct nitric oxide detection within microfluidic devices. Anal. Chem. 2010, 82, 3300–3305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaaliveetil, S.; Lee, Y.-Y.; Li, Z.; Cheng, Y.-H.; Menon, N.H.; Dongare, S.; Gurkan, B.; Basuray, S. Ionic Liquid-Packed Microfluidic Device with Non-Planar Microelectrode as a Miniaturized Electrochemical Gas Sensor. J. Electrochem. Soc. 2023, 170, 087508. [Google Scholar] [CrossRef]
- Lee, S.H.; Lim, J.H.; Park, J.; Hong, S.; Park, T.H. Bioelectronic nose combined with a microfluidic system for the detection of gaseous trimethylamine. Biosens. Bioelectron. 2015, 71, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Zeng, X. Methods and approaches of utilizing ionic liquids as gas sensing materials. RSC Adv. 2015, 5, 58371–58392. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Yin, H.; Mason, A.J. Rapid measurement of room temperature ionic liquid electrochemical gas sensor using transient double potential amperometry. Sens. Actuators B Chem. 2017, 242, 658–666. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Edgehouse, K.; Klemm, A.; Mao, H.; Pentzer, E.; Gurkan, B. Capsules of reactive ionic liquids for selective capture of carbon dioxide at low concentrations. ACS Appl. Mater. Interfaces 2020, 12, 19184–19193. [Google Scholar] [CrossRef]
- Bulbul, A.; Kim, H. A bubble-based microfluidic gas sensor for gas chromatographs. Lab A Chip 2015, 15, 94–104. [Google Scholar] [CrossRef]
- Tirandazi, P.; Hidrovo, C.H. An integrated gas-liquid droplet microfluidic platform for digital sampling and detection of airborne targets. Sens. Actuators B Chem. 2018, 267, 279–293. [Google Scholar] [CrossRef]
- Bulbul, A.; Hsieh, H.C.; Kim, H. Microfluidic bubble-based gas sensor. In Proceedings of the 2014 IEEE 27th International Conference on Micro Electro Mechanical Systems (MEMS), San Francisco, CA, USA, 26–30 January 2014. [Google Scholar]
- Huang, S.; Connolly, J.; Khlystov, A.; Fair, R.B. Digital microfluidics for the detection of selected inorganic ions in aerosols. Sensors 2020, 20, 1281. [Google Scholar] [CrossRef] [PubMed]
- Bulbul, A.; Hsieh, H.C.; Kim, H. Droplet based microfluidics. Rep. Prog. Phys. 2011, 75, 016601. [Google Scholar]
- Femmer, T.; Eggersdorfer, M.L.; Kuehne, A.J.C.; Wessling, M. Efficient gas–liquid contact using microfluidic membrane devices with staggered herringbone mixers. Lab A Chip 2015, 15, 3132–3137. [Google Scholar] [CrossRef] [PubMed]
- Malankowska, M.; Martins, C.; Rho, H.; Neves, L.; Tiggelaar, R.; Crespo, J.; Pina, M.; Mallada, R.; Gardeniers, H.; Coelhoso, I. Microfluidic devices as gas–Ionic liquid membrane contactors for CO2 removal from anaesthesia gases. J. Membr. Sci. 2018, 545, 107–115. [Google Scholar] [CrossRef]
- Jani, J.M.; Wessling, M.; Lammertink, R.G.H. A microgrooved membrane based gas–liquid contactor. Microfluid. Nanofluidics 2012, 13, 499–509. [Google Scholar] [CrossRef]
- Malankowska, M.; Julian, I.; Pellejero, I.; Rho, H.; Schlautmann, S.; Tiggelaar, R.; Pina, M.; Gardeniers, H.; Mallada, R. Understanding blood oxygenation in a microfluidic meander double side membrane contactor. Sens. Actuators B Chem. 2019, 288, 414–424. [Google Scholar] [CrossRef]
- Li, Z.; Haridas, N.; Kaaliveetil, S.; Cheng, Y.-H.; Chande, C.; Perez, V.; Miri, A.K.; Basuray, S. Low-Cost Rapid Prototyping for Microfluidics Using Parafilm®-based Microchannels For Low Resource Settings. Sens. Actuators B Chem. 2023, 404, 135212. [Google Scholar] [CrossRef]
- Li, Z.; Haridas, N.; Rahman, M.; Kaaliveetil, S.; Cheng, Y.-H.; Chande, C.; Basuray, S. Flow-Based Compact Microfluidic Electrochemical Cell with Parafilm® Channels. Electrochim. Acta 2023, 470, 143349. [Google Scholar] [CrossRef]
- Al Obaidi, A.; Karaca, I.M.; Ayhan, Z.; Haskaraca, G.; Gultekin, E. Fabrication and validation of CO2-sensitive indicator to monitor the freshness of poultry meat. Food Packag. Shelf Life 2022, 34, 100930. [Google Scholar] [CrossRef]
- Pantaleão, I.; Portugal, A.F.; Mendes, A.; Gabriel, J. Carbon dioxide absorption in a membrane contactor with color change. J. Chem. Educ. 2010, 87, 1377–1379. [Google Scholar] [CrossRef]
- Pocker, Y.; Bjorkquist, D.W. Stopped-flow studies of carbon dioxide hydration and bicarbonate dehydration in water and water-d2. Acid-base and metal ion catalysis. J. Am. Chem. Soc. 1977, 99, 6537–6543. [Google Scholar]
- Dreybrodt, W.; Lauckner, J.; Zaihua, L.; Svensson, U.; Buhmann, D. The kinetics of the reaction CO2+ H2O→ H+ + HCO3− as one of the rate limiting steps for the dissolution of calcite in the system H2O–CO2–CaCO3. Geochim. Cosmochim. Acta 1996, 60, 3375–3381. [Google Scholar] [CrossRef]
- Ge, M.; Hussain, G.; Hibbert, D.B.; Silvester, D.S.; Zhao, C. Ionic Liquid-based Microchannels for Highly Sensitive and Fast Amperometric Detection of Toxic Gases. Electroanalysis 2019, 31, 66–74. [Google Scholar] [CrossRef]
- Paknahad, M.; Bachhal, J.S.; Ahmadi, A.; Hoorfar, M. Characterization of channel coating and dimensions of microfluidic-based gas detectors. Sens. Actuators B Chem. 2017, 241, 55–64. [Google Scholar] [CrossRef]
- Scott, K. Absorption, desorption, and extraction with membranes. In Handbook of Industrial Membranes; Scott, K., Ed.; Elsevier Science: Amsterdam, The Netherlands, 1995; pp. 633–639. [Google Scholar]
- Sebők, B.; Schülke, M.; Réti, F.; Kiss, G. Diffusivity, permeability and solubility of H2, Ar, N2, and CO2 in poly (tetrafluoroethylene) between room temperature and 180 C. Polym. Test. 2016, 49, 66–72. [Google Scholar] [CrossRef]
- Pasternak, R.A.; Christensen, M.V.; Heller, J. Diffusion and permeation of oxygen, nitrogen, carbon dioxide, and nitrogen dioxide through polytetrafluoroethylene. Macromolecules 1970, 3, 366–371. [Google Scholar] [CrossRef]
- Volkov, K.N.; Karpenko, A.G. Preconditioning of gas dynamics equations in compressible gas flow computations at low mach numbers. Comput. Math. Math. Phys. 2015, 55, 1051–1067. [Google Scholar] [CrossRef]
- Darbandi, M.; Schneider, G.E. Analogy-based method for solving compressible and incompressible flows. J. Thermophys. Heat Transf. 1998, 12, 239–247. [Google Scholar] [CrossRef]
- Kraushaar, M. Application of the Compressible and Low-Mach Number Approaches to Large-Eddy Simulation of Turbulent Flows in Aero-Engines. Doctoral Dissertation, Institut National Polytechnique de Toulouse, Toulouse, France, 2011. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaaliveetil, S.; Khaja, N.N.; Haridas Menon, N.; Basuray, S. Response Time Dynamics of a Membrane-Based Microfluidic Gas Sensor. Chemosensors 2024, 12, 127. https://doi.org/10.3390/chemosensors12070127
Kaaliveetil S, Khaja NN, Haridas Menon N, Basuray S. Response Time Dynamics of a Membrane-Based Microfluidic Gas Sensor. Chemosensors. 2024; 12(7):127. https://doi.org/10.3390/chemosensors12070127
Chicago/Turabian StyleKaaliveetil, Sreerag, Najamuddin Naveed Khaja, Niranjan Haridas Menon, and Sagnik Basuray. 2024. "Response Time Dynamics of a Membrane-Based Microfluidic Gas Sensor" Chemosensors 12, no. 7: 127. https://doi.org/10.3390/chemosensors12070127
APA StyleKaaliveetil, S., Khaja, N. N., Haridas Menon, N., & Basuray, S. (2024). Response Time Dynamics of a Membrane-Based Microfluidic Gas Sensor. Chemosensors, 12(7), 127. https://doi.org/10.3390/chemosensors12070127





