Development of Electrochemical and Colorimetric Biosensors for Detection of Dopamine
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instruments
2.3. Preparation of Gold Nanoparticles (AuNPs)
2.4. Preparation of Silver Nanoparticles (AgNPs)
2.5. Preparation of Paper-Based Biosensor
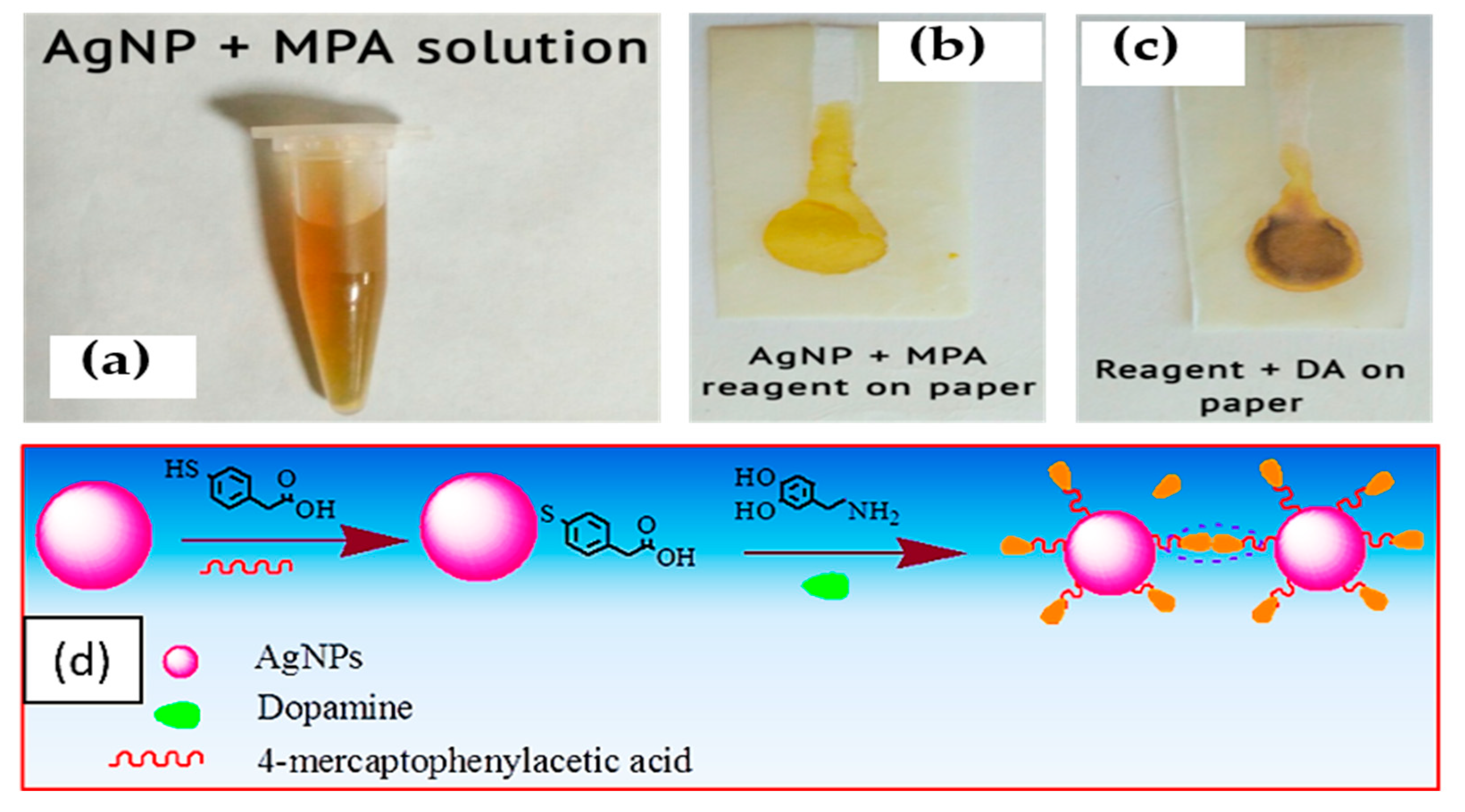
2.6. Colorimetric Detection Using AgNP and MPA as Reagent Solution
2.7. Colorimetric Detection Using Nanocomposite of AgNP+AuNP
2.8. Electrochemical Detection
3. Results
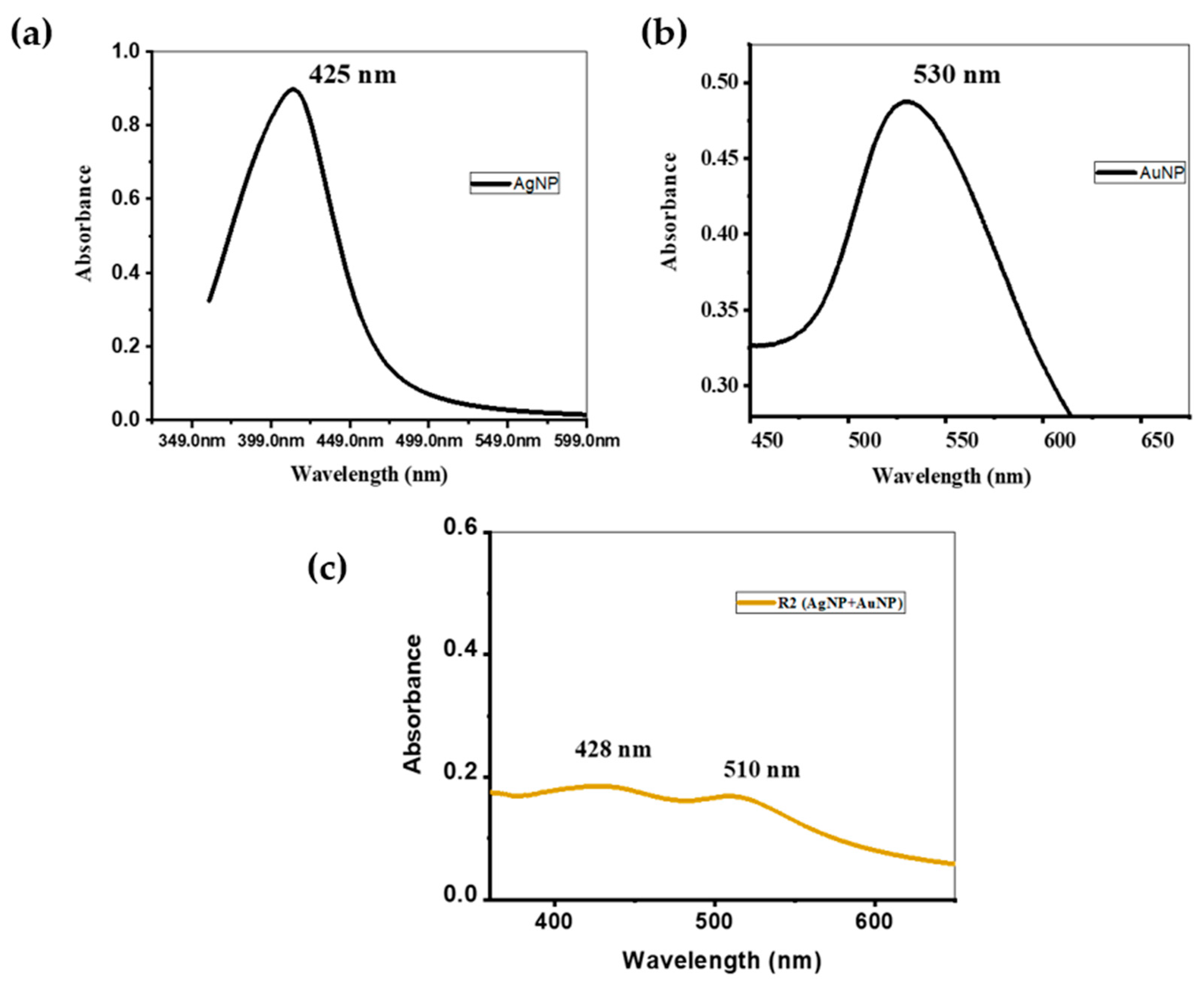
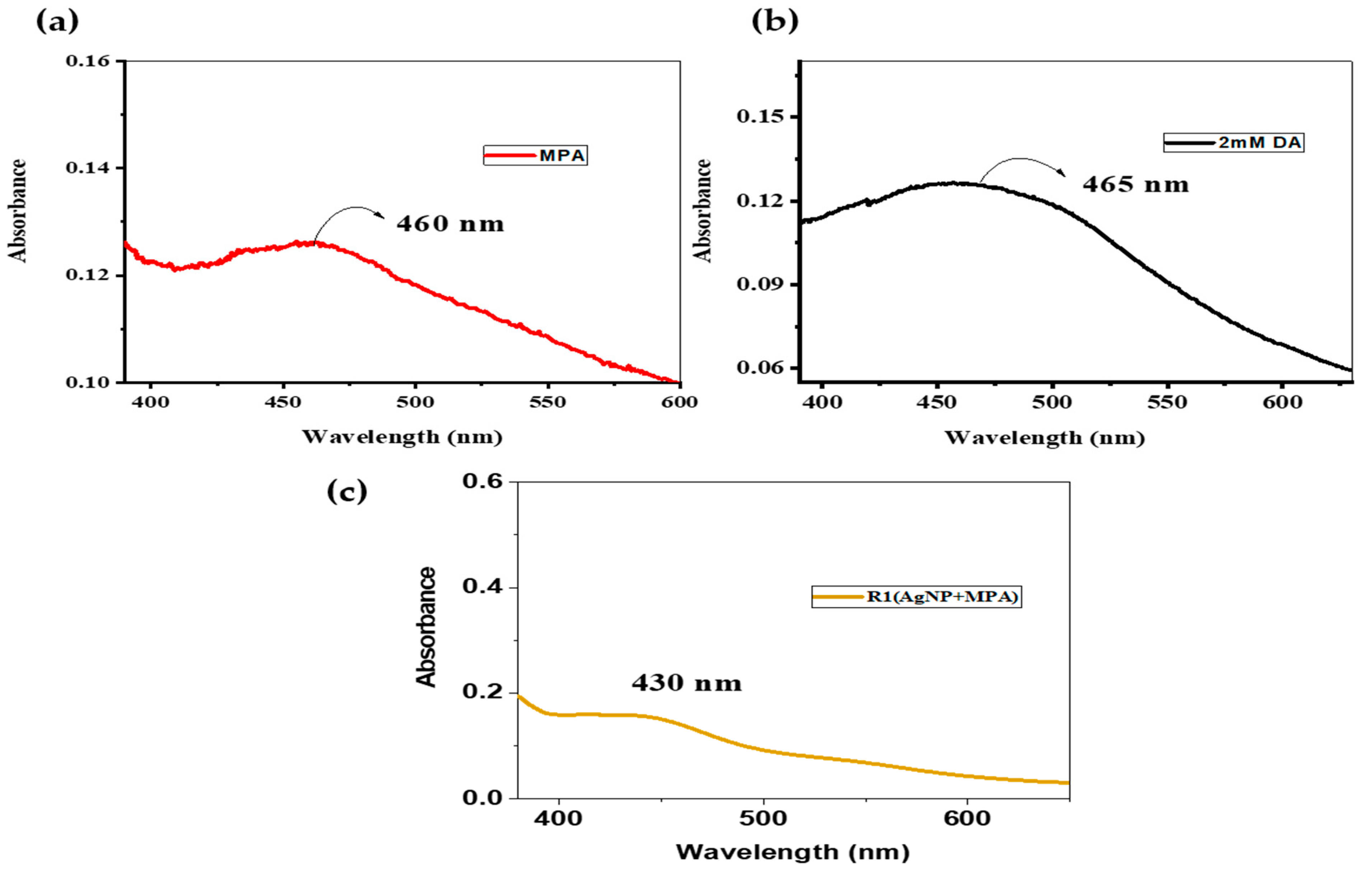
3.1. Characterization
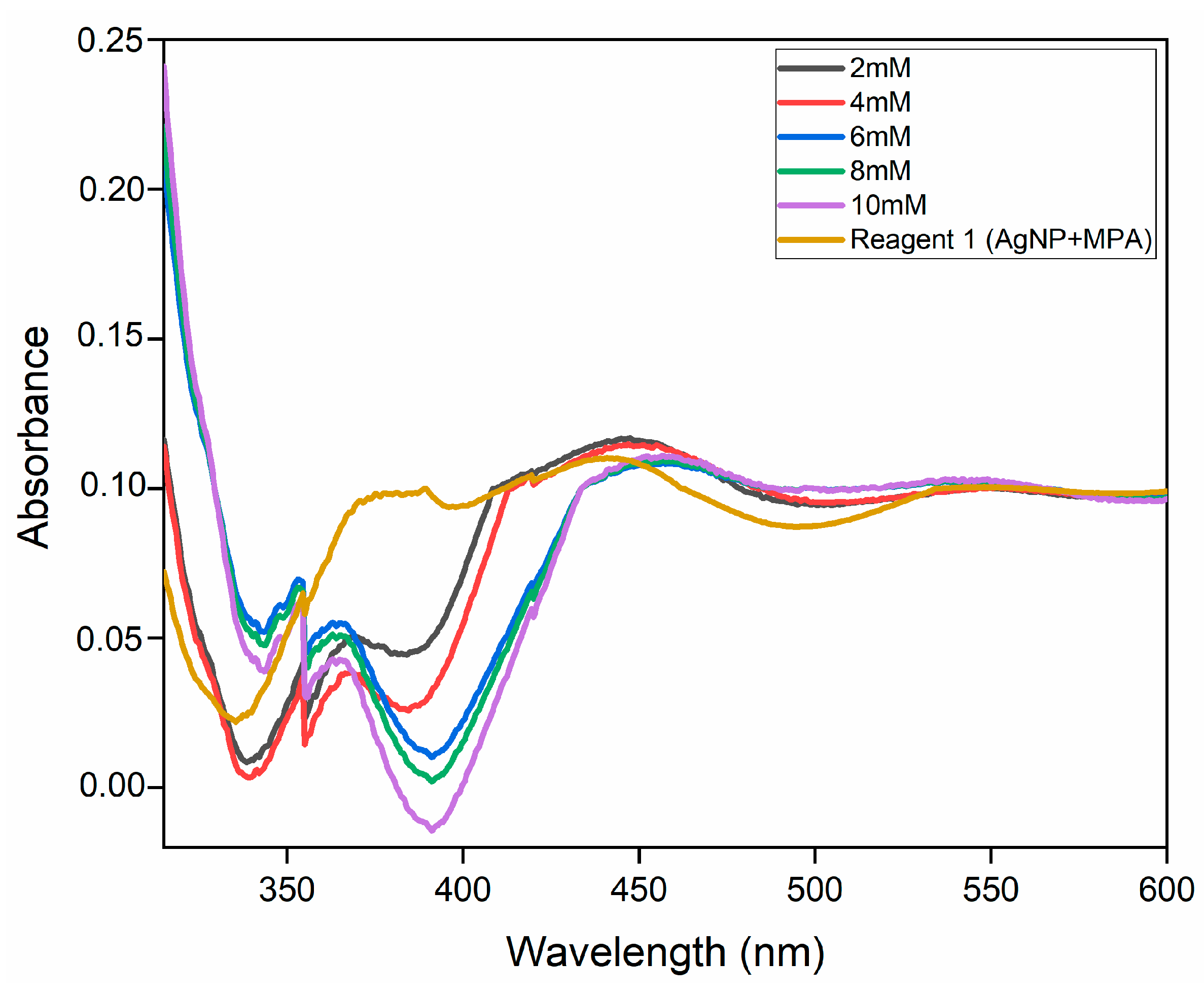
3.2. Interaction of AgNP+MPA with Different Concentrations of DA
3.3. Interaction of Ag/Au Composite with Different Concentrations of DA
3.4. Colorimetric Detection of Dopamine
3.4.1. Reagent Solution (AgNP and MPA)
3.4.2. Colorimetric Detection of Dopamine on Paper-Based Biosensor Using AgNP and MPA as Reagent Solution
3.4.3. Ag/Au Nanocomposite Solution
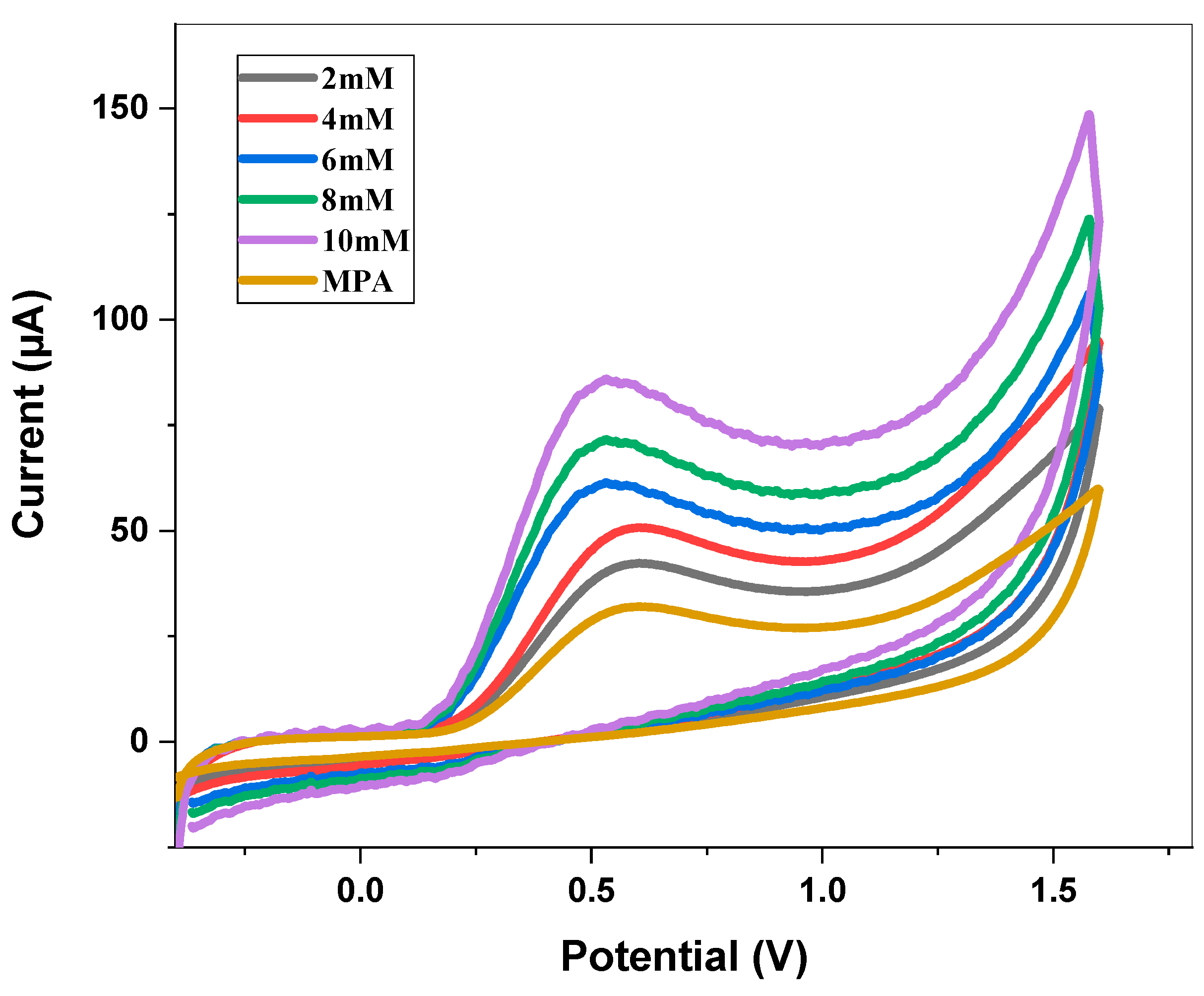
3.5. Electrochemical Detection of Dopamine and Selectivity and Sensitivity for Dopamine Sensing
3.6. Electrochemical Detection with Different Concentrations of DA
3.7. Interference Effect of Ascorbic Acid and Aric Acid
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schultz, W. Multiple dopamine functions at different time courses. Annu. Rev. Neurosci. 2007, 30, 259–288. [Google Scholar] [CrossRef]
- Deng, H.; Zhao, J.; Zhao, S.; Jiang, S.; Cui, G. A graphene-based electrochemical flow analysis device for simultaneous determination of dopamine, 5-hydroxytryptamine, and melatonin. Analyst 2022, 147, 1598–1610. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Tang, L.; Lu, J.; Li, J. Application of graphene-modified electrode for selective detection of dopamine. Electrochem. Commun. 2009, 11, 889–892. [Google Scholar] [CrossRef]
- Jayaraman, S.; Rajarathinam, T.; Chang, S.-C. Disposable Sensor with Copper-Loaded Carbon Nanospheres for the Simultaneous Determination of Dopamine and Melatonin. Chemosensors 2023, 11, 254. [Google Scholar] [CrossRef]
- Kaur, H.; Siwal, S.S.; Saini, R.V.; Singh, N.; Thakur, V.K. Significance of an Electrochemical Sensor and Nanocomposites: Toward the Electrocatalytic Detection of Neurotransmitters and Their Importance within the Physiological System. ACS Nanosci. Au 2022, 3, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Siwal, S.S.; Kumar, V.; Thakur, V.K. Deep Eutectic Solvents toward the Detection and Extraction of Neurotransmitters: An Emerging Paradigm for Biomedical Applications. Ind. Eng. Chem. Res. 2023, 62, 18906–18917. [Google Scholar] [CrossRef]
- Yusoff, N.; Pandikumar, A.; Ramaraj, R.; Lim, H.N.; Huang, N.M. Gold nanoparticle based optical and electrochemical sensing of dopamine. Microchim. Acta 2015, 182, 2091–2114. [Google Scholar] [CrossRef]
- Malenka, R.; Nestler, E.; Hyman, S. Chapter 6: Widely projecting systems: Monoamines, acetylcholine, and orexin. In Molecular Neuropharmacology: A Foundation for Clinical Neuroscience; McGraw Hill: New York, NY, USA, 2009; pp. 147–157. [Google Scholar]
- Sanghavi, B.J.; Wolfbeis, O.S.; Hirsch, T.; Swami, N.S. Nanomaterial-based electrochemical sensing of neurological drugs and neurotransmitters. Microchim. Acta 2015, 182, 1–41. [Google Scholar] [CrossRef]
- Alkasir, R.S.; Rossner, A.; Andreescu, S. Portable colorimetric paper-based biosensing device for the assessment of bisphenol A in indoor dust. Environ. Sci. Technol. 2015, 49, 9889–9897. [Google Scholar] [CrossRef]
- Ornatska, M.; Sharpe, E.; Andreescu, D.; Andreescu, S. Paper bioassay based on ceria nanoparticles as colorimetric probes. Anal. Chem. 2011, 83, 4273–4280. [Google Scholar] [CrossRef]
- Hossain, S.M.Z.; Luckham, R.E.; Smith, A.M.; Lebert, J.M.; Davies, L.M.; Pelton, R.H.; Filipe, C.D.M.; Brennan, J.D. Development of a bioactive paper sensor for detection of neurotoxins using piezoelectric inkjet printing of sol-gel-derived bioinks. Anal. Chem. 2009, 81, 5474–5483. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Ali, M.M.; Filipe, C.D.M.; Li, Y.; Pelton, R. Microgel-based inks for paper-supported biosensing applications. Biomacromolecules 2008, 9, 935–941. [Google Scholar] [CrossRef]
- Scida, K.; Li, B.; Ellington, A.D.; Crooks, R.M. DNA detection using origami paper analytical devices. Anal. Chem. 2013, 85, 9713–9720. [Google Scholar] [CrossRef]
- Costa, M.N.; Veigas, B.; Jacob, J.M.; Santos, D.S.; Gomes, J.; Baptista, P.V.; Martins, R.; Inácio, J.; Fortunato, E. A low cost, safe, disposable, rapid and self-sustainable paper-based platform for diagnostic testing: Lab-on-paper. Nanotechnology 2014, 25, 094006. [Google Scholar] [CrossRef] [PubMed]
- Grau, G. Low-cost fabrication of paper-based systems: Microfluidics, sensors, electronics and deployment. In Proceedings of the 2017 IEEE 60th International Midwest Symposium on Circuits and Systems (MWSCAS), Medford, MA, USA, 6–9 August 2017; IEEE: Piscataway, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Park, T.S.; Li, W.; McCrackena, K.E.; Yoon, J.-Y. Smartphone quantifies Salmonella from paper microfluidics. Lab A Chip 2013, 13, 4832–4840. [Google Scholar] [CrossRef]
- Cate, D.M.; Adkins, J.A.; Mettakoonpitak, J.; Henry, C.S. Recent developments in paper-based microfluidic devices. Anal. Chem. 2014, 87, 19–41. [Google Scholar] [CrossRef]
- Alkasir, R.S.; Ornatska, M.; Andreescu, S. Colorimetric paper bioassay for the detection of phenolic compounds. Anal. Chem. 2012, 84, 9729–9737. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Wiley, B.J.; Gupta, M.; Whitesides, G.M. FLASH: A rapid method for prototyping paper-based microfluidic devices. Lab A Chip 2008, 8, 2146–2150. [Google Scholar] [CrossRef] [PubMed]
- Liana, D.D.; Raguse, B.; Gooding, J.J.; Chow, E. Recent advances in paper-based sensors. Sensors 2012, 12, 11505–11526. [Google Scholar] [CrossRef]
- Wu, Y.; Xue, P.; Kang, Y.; Hui, K.M. Paper based microfluidic electrochemical immunodevice integrated with nanobioprobes onto graphene film for ultrasensitive multiplexed detection of cancer biomarkers. Anal. Chem. 2013, 85, 8661–8668. [Google Scholar] [CrossRef]
- Liang, P.; Yu, H.; Guntupalli, B.; Xiao, Y. Paper based device for rapid visualization of NADH based on dissolution of gold nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 15023–15030. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Deng, Z.; Chen, H.; Zheng, Z.; Ji, L.; Chen, Y.; Sun, M.; Ouyang, S.; Yuan, Z.; Zhao, P. Dual-Signal Colorimetric and Electrochemical Sensor of Dopamine Based on Nanocomposite of Cobalt Oxyhydroxide/Carbon Black. J. Electrochem. Soc. 2023, 170, 017503. [Google Scholar] [CrossRef]
- Ge, L.; Yan, J.; Song, X.; Yan, M.; Ge, S.; Yu, J. Three-dimensional paper-based electrochemiluminescence immunodevice for multiplexed measurement of biomarkers and point-of-care testing. Biomaterials 2012, 33, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fang, X.; Cao, H.; Kong, J. Paper based fluorescence resonance energy transfer assay for directly detecting nucleic acids and proteins. Biosens. Bioelectron. 2016, 80, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.B.; Son, S.E.; Seong, G.H. Apta-sensor for selective determination of dopamine using chitosan-stabilized Prussian blue nanoparticles. J. Mater. Chem. B 2023, 11, 7217–7227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhao, J.; Xu, H.; Li, Y.; Ji, J.; Liu, B. Multifunctional paper strip based on self-assembled interfacial plasmonic nanoparticle arrays for sensitive SERS detection. ACS Appl. Mater. Interfaces 2015, 7, 16767–16774. [Google Scholar] [CrossRef]
- Dungchai, W.; Sameenoi, Y.; Chailapakul, O.; Volckens, J.; Henry, C.S. Determination of aerosol oxidative activity using silver nanoparticle aggregation on paper-based analytical devices. Analyst 2013, 138, 6766–6773. [Google Scholar] [CrossRef] [PubMed]
- Rattanarat, P.; Dungchai, W.; Cate, D.M.; Siangproh, W.; Volckens, J.; Chailapakul, O.; Henry, C.S. A microfluidic paper-based analytical device for rapid quantification of particulate chromium. Anal. Chim. Acta 2013, 800, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Byrne, L.; Barker, J.; Pennarun-Thomas, G.; Diamond, D.; Edwards, S. Digital imaging as a detector for generic analytical measurements. TrAC Trends Anal. Chem. 2000, 19, 517–522. [Google Scholar] [CrossRef]
- Sicard, C.; Glen, C.; Aubie, B.; Wallace, D.; Jahanshahi-Anbuhi, S.; Pennings, K.; Daigger, G.T.; Pelton, R.; Brennan, J.D.; Filipe, C.D.M.; et al. Tools for water quality monitoring and mapping using paper-based sensors and cell phones. Water Res. 2015, 70, 360–369. [Google Scholar] [CrossRef]
- Sharpe, E.; Bradley, R.; Frasco, T.; Jayathilaka, D.; Marsh, A.; Andreescu, S. Metal oxide based multisensor array and portable database for field analysis of antioxidants. Sens. Actuators B: Chem. 2014, 193, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Sher, M.; Zhuang, R.; Demirci, U.; Asghar, W. Paper based analytical devices for clinical diagnosis: Recent advances in the fabrication techniques and sensing mechanisms. Expert Rev. Mol. Diagn. 2017, 17, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Ellerbee, A.K.; Phillips, S.T.; Siegel, A.C.; Mirica, K.A.; Martinez, A.W.; Striehl, P.; Jain, N.; Prentiss, M.; Whitesides, G.M. Quantifying colorimetric assays in paper-based microfluidic devices by measuring the transmission of light through paper. Anal. Chem. 2009, 81, 8447–8452. [Google Scholar] [CrossRef] [PubMed]
- Busa, L.S.A.; Mohammadi, S.; Maeki, M.; Ishida, A.; Tani, H.; Tokeshi, M. Advances in microfluidic paper-based analytical devices for food and water analysis. Micromachines 2016, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Kim, K.; Park, C.W.; Lee, G.J.; Choi, S. A low-cost ion detecting device with paper-based disposal sensor. In Proceedings of the 2015 15th International Conference on Control, Automation and Systems (ICCAS), Busan, Republic of Korea, 13–16 October 2015; IEEE: Piscataway, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Chen, S.; Liu, T.-L.; Jia, Y.; Li, J. Recent advances in bio-integrated electrochemical sensors for neuroengineering. Fundam. Res. 2023, in press. [CrossRef]
- Montes-García, V.; Squillaci, M.A.; Diez-Castellnou, M.; Ong, Q.K.; Stellacci, F.; Samorì, P. Chemical sensing with Au and Ag nanoparticles. Chem. Soc. Rev. 2021, 50, 1269–1304. [Google Scholar] [CrossRef] [PubMed]
- Le, T.-V.; Lee, S.-W. Core–shell Au–Ag nanoparticles as colorimetric sensing probes for highly selective detection of a dopamine neurotransmitter under different pH conditions. Dalton Trans. 2022, 51, 15675–15685. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; She, Y.; Zhu, Y.; Dai, D.; Shi, M.; Chu, W.; Cai, T.; Tsai, H.-S.; Li, H.; Jiang, N.; et al. Highly Sensitive and Selective Dopamine Determination in Real Samples Using Au Nanoparticles Decorated Marimo-like Graphene Microbead-Based Electrochemical Sensors. Sensors 2023, 23, 2870. [Google Scholar] [CrossRef]
- Qi, W.; Zhao, J.; Zhang, W.; Liu, Z.; Xu, M.; Anjum, S.; Majeed, S.; Xu, G. Visual and surface plasmon resonance sensor for zirconium based on zirconium-induced aggregation of adenosine triphosphate-stabilized gold nanoparticles. Anal. Chim. Acta 2013, 787, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Sun, R.; Yu, S.; Zhang, Z.; Zhou, L.; Huang, H.; Du, R. Size-controlled preparation of silver nanoparticles by a modified polyol method. Colloids Surf. A Physicochem. Eng. Asp. 2010, 366, 197–202. [Google Scholar] [CrossRef]
- Umashankari, J.; Inbakandan, D.; Ajithkumar, T.T.; Balasubramanian, T. Mangrove plant, Rhizophora mucronata (Lamk, 1804) mediated one pot green synthesis of silver nanoparticles and its antibacterial activity against aquatic pathogens. Aquat. Biosyst. 2012, 8, 11. [Google Scholar] [CrossRef]
- Weng, C.H.; Huang, C.C.; Yeh, C.S.; Lee, G.B. Synthesis of Gold Nanoparticles Using Microfluidic Reaction Systems. In Proceedings of the 2007 7th IEEE Conference on Nanotechnology (IEEE NANO), Hong Kong, China, 2–5 August 2007; pp. 462–466. [Google Scholar] [CrossRef]
- Kuladeep, R.; Jyothi, L.; Alee, K.S.; Deepak, K.L.N.; Rao, D.N. Laser-assisted synthesis of Au-Ag alloy nanoparticles with tunable surface plasmon resonance frequency. Opt. Mater. Express 2012, 2, 161–172. [Google Scholar] [CrossRef]
- El-Zohry, A.M.; Hashem, E. Environmental method to determine dopamine and ascorbic acid simultaneously via derivative spectrophotometry. J. Spectrosc. 2013, 2013, 260376. [Google Scholar] [CrossRef]
- Meenakshi, S.; Pandian, K. Simultaneous voltammetry detection of dopamine and uric acid in pharmaceutical products and urine samples using ferrocene carboxylic acid primed Nanoclay modified glassy carbon electrode. J. Electrochem. Soc. 2016, 163, B543–B555. [Google Scholar] [CrossRef]
- Lin, J.; Huang, B.; Dai, Y.; Wei, J.; Chen, Y. Chiral ZnO nanoparticles for detection of dopamine. Mater. Sci. Eng. C 2018, 93, 739–745. [Google Scholar] [CrossRef]
- Kim, Y.-R.; Bong, S.; Kang, Y.-J.; Yang, Y.; Mahajan, R.K.; Kim, J.S.; Kim, H. Electrochemical detection of dopamine in the presence of ascorbic acid using graphene modified electrodes. Biosens. Bioelectron. 2010, 25, 2366–2369. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, C.; Wang, C.; Pu, F.; Ren, J.; Qu, X. Silver nanoprobe for sensitive and selective colorimetric detection of dopamine via robust Ag–catechol interaction. Chem. Commun. 2011, 47, 1181–1183. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Y.; Yang, X. Aptamer-based colorimetric biosensing of dopamine using unmodified gold nanoparticles. Sens. Actuators B Chem. 2011, 156, 95–99. [Google Scholar] [CrossRef]
- Yao, Z.; Yang, X.; Niu, Y.; Wu, F.; Hu, Y.; Yang, Y. Voltammetric dopamine sensor based on a gold electrode modified with reduced graphene oxide and Mn3O4 on gold nanoparticles. Microchim. Acta 2017, 184, 2081–2088. [Google Scholar] [CrossRef]
- Vasanthi, S.; Kumar, K.K.; Narayanan, S.S. An amperometric sensor for the determination of dopamine using poly zincon film modified electrode. IJSRST 2018, 4, 751–757. [Google Scholar]









| Probe Material | Technique | Linear Range | Detection Limit | Reference |
|---|---|---|---|---|
| Procaterol hydrochloride | Electrochemical | 1.0–100 µmol/L | 0.3 µmol | [48] |
| Chiral ZnO nanoparticles | Fluorescent | 5–13 μg/mL | 0.15 μg/mL | [49] |
| Graphene modified electrodes | Electrochemical | 4–100 µmol/L | 2.64 µmol | [50] |
| Unmodified silver nanoparticles | Colorimetric | 0 to 0.6 mM | 60 nM | [51] |
| Aptamer and unmodified citrate-capped gold nanoparticles | Colorimetric | 5.4 × 10−7 M to 5.4 × 10−6 M | 3.6 × 10−7 M | [52] |
| Mn3O4 and graphene oxide in a nafion film, along with gold nanoparticles | Electrochemical | 1.0 µmol/L to 1.45 µmol/L | 0.25 µmol/L | [53] |
| Poly zincon layer | Electrochemical | 1.16 to 401 μM | 0.38 μM | [54] |
| Present method | Colorimetric andElectrochemical | 1 to 10 μM | 2.51 μM | Recent work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, R.; Anjum, S.; Fatima, N.; Farooq, N.; Shaheen, A.; Fernandez Garcia, J.; Khan, M.I.; Shanableh, A. Development of Electrochemical and Colorimetric Biosensors for Detection of Dopamine. Chemosensors 2024, 12, 126. https://doi.org/10.3390/chemosensors12070126
Khan R, Anjum S, Fatima N, Farooq N, Shaheen A, Fernandez Garcia J, Khan MI, Shanableh A. Development of Electrochemical and Colorimetric Biosensors for Detection of Dopamine. Chemosensors. 2024; 12(7):126. https://doi.org/10.3390/chemosensors12070126
Chicago/Turabian StyleKhan, Rimsha, Saima Anjum, Nishat Fatima, Nosheen Farooq, Aqeela Shaheen, Javier Fernandez Garcia, Muhammad Imran Khan, and Abdallah Shanableh. 2024. "Development of Electrochemical and Colorimetric Biosensors for Detection of Dopamine" Chemosensors 12, no. 7: 126. https://doi.org/10.3390/chemosensors12070126
APA StyleKhan, R., Anjum, S., Fatima, N., Farooq, N., Shaheen, A., Fernandez Garcia, J., Khan, M. I., & Shanableh, A. (2024). Development of Electrochemical and Colorimetric Biosensors for Detection of Dopamine. Chemosensors, 12(7), 126. https://doi.org/10.3390/chemosensors12070126







